Abstract
The adrenal glands (AGs) are relatively small yet require definitive identification during their resection, or more commonly, their avoidance. To enable image-guided surgery involving the AGs, we have developed novel near-infrared (NIR) fluorophores that target AGs after a single intravenous injection, which provided dual-NIR image-guided resection or avoidance of the AGs during both open and minimally-invasive surgery.
Graphical Abstract
AGs are paired endocrine glands that produce hormones such as epinephrine, norepinephrine, androgens, estrogens, aldosterone, and cortisol. They are also the site of benign and malignant tumors that can lead to Cushing’s syndrome, Conn syndrome, virilization, and feminization.1 Radical adrenalectomy is traditionally performed by conventional open surgery. However, since its initial description in 1992, laparoscopic adrenalectomy has expanded significantly for the resection of functional adrenal tumors and incidentalomas smaller than 8–10 cm.2,3 The advantages of a laparoscopic approach include a better cosmetic result, shorter recovery time, and significantly less pain, while it may cause shorter survival, less complete removal of the tumor, and shorter time to and a greater chance of the tumor returning.4 In addition, laparoscopic instruments could rub against the tumor during surgery and allow tumor cells to spread to other parts of the abdomen. Real-time image guidance during the AG surgery can help guarantee a complete removal of the tumor with preserving vital tissues neighboring the glands, which is also the key to shortening intraoperative time and effort.5–7
The use of near-infrared (NIR; 700 nm to 900 nm) fluorescence emission enables optical imaging of thick tissues by virtue of lower absorbance, scatter, and autofluorescence in the NIR.8 To date, over 1000 patients around the world have been studied with this technology (reviewed in9), however, the only two contrast agents available clinically are methylene blue (MB), which emits ≈ 700 nm NIR, and indocyanine green (ICG), which emits ≈ 800 nm NIR. Neither agent has significant targeting ability to the AGs. Nevertheless, Obermeyer et al. reported that visualizing the AGs after a single intravenous injection of MB shortened the operative time of laparoscopic adrenalectomy.10 However, the use of MB is not efficient because color visualization demands high dose injections, while retention time in the target is too short for surgery. Indeed, the effective dose of MB used for the aforementioned pig study was 7.5 mg/kg, which is 5-fold higher than the typical clinical dose (1.5 mg/kg). In addition, the duration of bluish color changes in the AG was less than 15 min.10
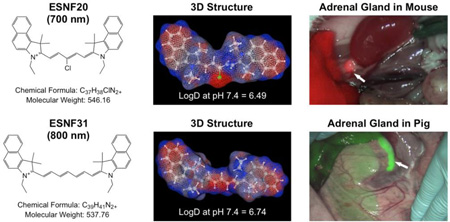
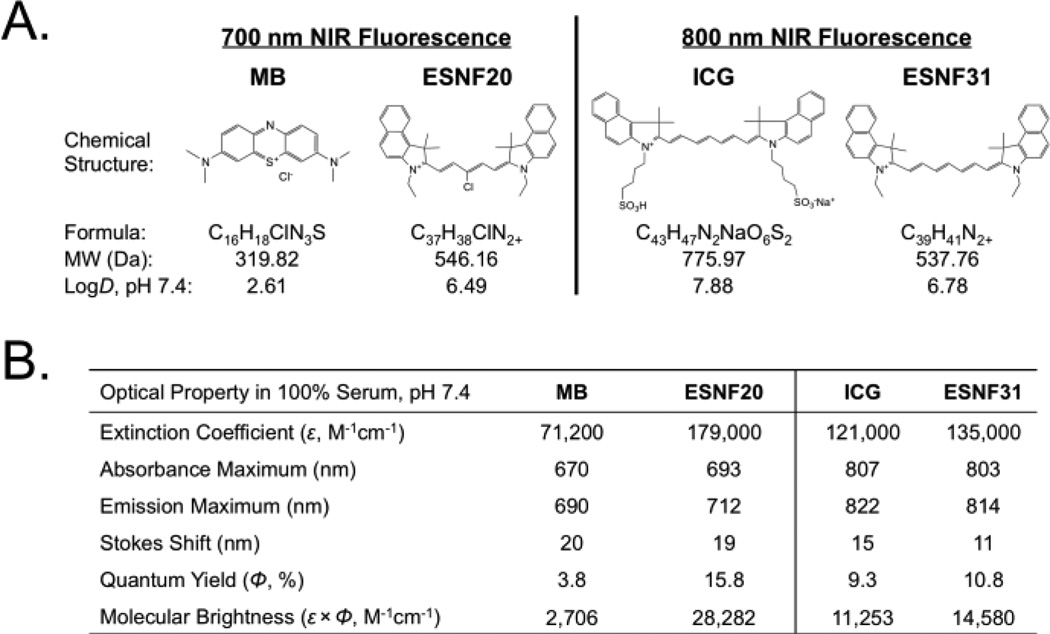
We synthesized two novel, high performance NIR fluorophores that target the AGs, one emitting ≈ 700 nm and the other emitting ≈ 800 nm, and compared their performance, quantitatively, to MB and ICG in both small and large animal models of AG surgery. The chemical synthesis and purification of a family of polymethine cyanine fluorophores named endocrine-specific NIR fluorophore (ESNF) was described in detail previously.11–16 After initial screening, ESNF20 (700 nm NIR) and ESNF31 (800 nm NIR) were identified as AG-targeted agents. The chemical structures and optical properties in neutral buffered serum of all NIR fluorophores used in this study are shown in Fig 1. To summarize, MB and ESNF20 exhibited fluorescence properties compatible with the 700 nm NIR fluorescence channel of the FLARE imaging system while ICG and ESNF31 exhibited fluorescence properties compatible with its 800 nm NIR fluorescence channel. MB had the lowest extinction coefficient and QY of all, consistent with prior reports.17
Fig. 1.
Chemical structures and optical properties of NIR fluorophores: A) Chemical structures of 700 nm (MB, ESNF20) and 800 nm (ICG, ESNF31) NIR fluorophores and B) their optical properties in FBS, pH 7.4.
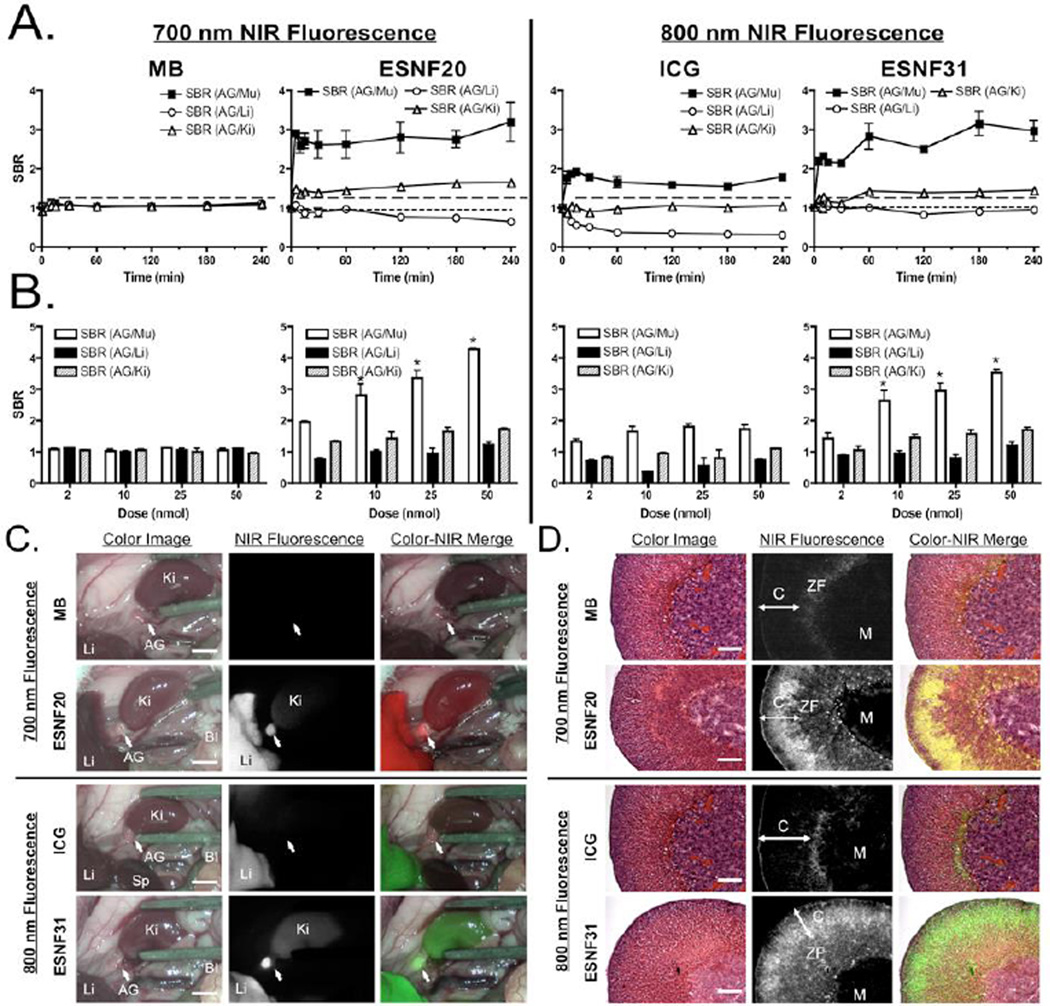
Despite the improved optical properties, both ESNF20 and ESNF31 have poor water solubility (Table S1) because of high logD (Figure S3). To improve the solubility, we dissolved the powder in DMSO (5 mM), and diluted the stock solution into saline containing 10% serum proteins. The final concentration was 200 µM, where both fluorophores were completely dissolved without any precipitates (Figure S4). Then, the injection solution was filtered through a 0.2 µM PTFE filter (Nalgene) prior to injection intravenously into animals. Animals were housed in an AAALAC-certified facility and were studied under the supervision of BIDMC IACUC in accordance with the approved institutional protocol (#057-2014). As shown in Figure 2a, ESNF20 and ESNF31 resulted in significant signal intensity in the AGs (SBR > 2.0 relative to muscle and SBR ≈ 1.5 relative to kidney), which was much higher and more sustained than either MB or ICG. However, in mice, background tissue uptake in the liver of all 4 NIR fluorophores was high, resulting in some cases of an SBR < 1.0. Based on the kinetics of AG uptake, 60 min post-injection was selected as the optimal time point in mice and used for dose ranging. As shown in Figure 2b, there was little dose dependency on either MB or ICG uptake in the AG. On the other hand, ESNF20 and ESNF31 showed a dose-dependent uptake in AGs, and ESNF31 reached the maximum SBR (AG/Mu) > 4.0 when 50 nmol of each compound was injected intravenously in CD-1 mice. A significant difference in the SBR (AG/Mu) was found for injected doses 10 nmol for both ESNF20 and ESNF31. Based on these kinetic and dose ranging studies, intraoperative imaging of AGs (Figure 2c) was performed at 60 min post-injection of a dose of 10 nmol of ESNFs and resulted in distinct highlighting of the glands, and easy differentiation from surrounding muscle and fat, and even kidney in mice. After resection of the AGs, NIR fluorescence microscopy revealed that both ESNF20 and ESNF31 showed significant uptake in adrenal cortex, particularly in the zona fasciculate (Figure 2d).
Fig. 2.
Kinetics, dose dependence, and intraoperative imaging of NIR fluorophores in male CD-1 mice (n = 5): A) Kinetics of AG signal relative to surrounding tissues and organs. SBR of AG compared to surrounding major tissue/organs was measured after intravenous injection of each fluorophore (10 nmol) at indicated time points up to 4 h. B) Dose dependence of AG signal. 2–50 nmol of NIR fluorophores were injected intravenously 1 h prior to quantitation of SBR. C) Intraoperative NIR imaging of AG and surrounding organs (10 nmol at 1 h). Bl, bladder; Ki, kidney; Li, liver; Sp, spleen. Scale bars = 0.5 cm. D) NIR fluorescence microscopy of adrenal glands resected from animals shown in (C). Red pseudo-color was used for 700 nm and lime green for 800 nm NIR in the merged image. C, cortex; M, medulla; ZF, zona fasciculate. Scale bars = 50 µm.
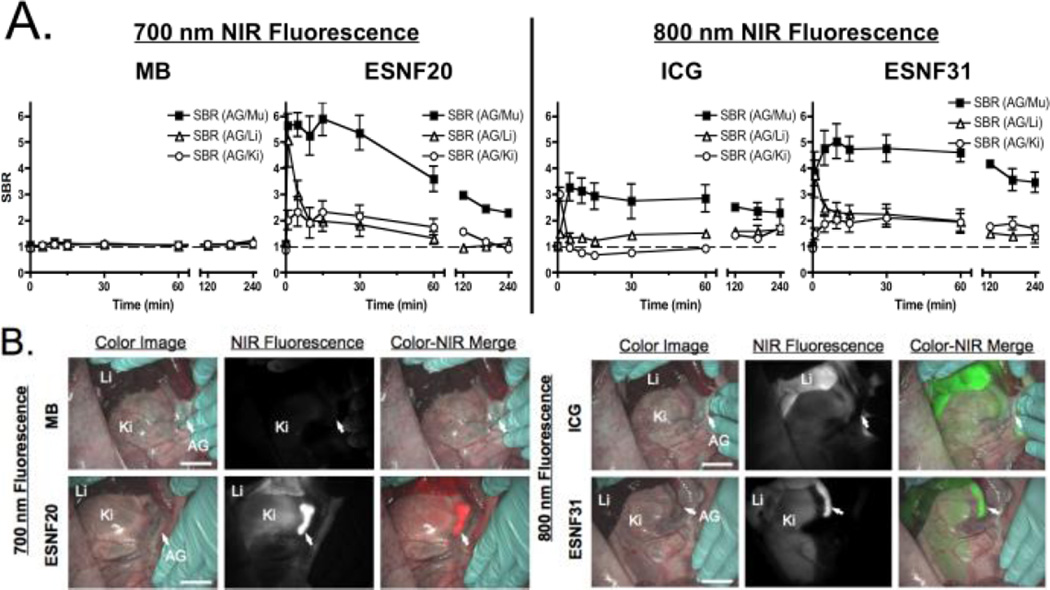
To confirm NIR fluorophore kinetics in a large animal model system approaching the size of humans, 35 kg Yorkshire pigs were injected intravenously with 2 µmol of NIR fluorophore and signal intensity of the AGs and surrounding tissues and organs measured over time. As shown in Figure 3a, MB was not able to generate a SBR > 1.0 at any time point. ICG generated a measurable SBR relative to muscle, but not relative to kidney or liver. Both ESNF20 and ESNF31, however, resulted in a significant SBR relative to all nearby tissues and for an extended period of time. In fact, ESNF31 generated an SBR ≥ 3.0 for 4 h post-injection. No changes in vital signs were observed over the 4 h experiment.
Fig. 3.
Kinetics and intraoperative imaging with 2 µmol intravenous injection of each NIR fluorophore in pigs (n = 3): A) Kinetics of AG signal relative to surrounding tissues and organs. SBR of AG compared to surrounding major tissue/organs was measured at indicated time points up to 4 h. B) Intraoperative NIR imaging of AG and surrounding organs 1 h post-injection. Red pseudo-color was used for 700 nm NIR and lime green for 800 nm NIR in the merged image. AG, adrenal gland; Ki, kidney; Li, liver. Scale bar = 3 cm.
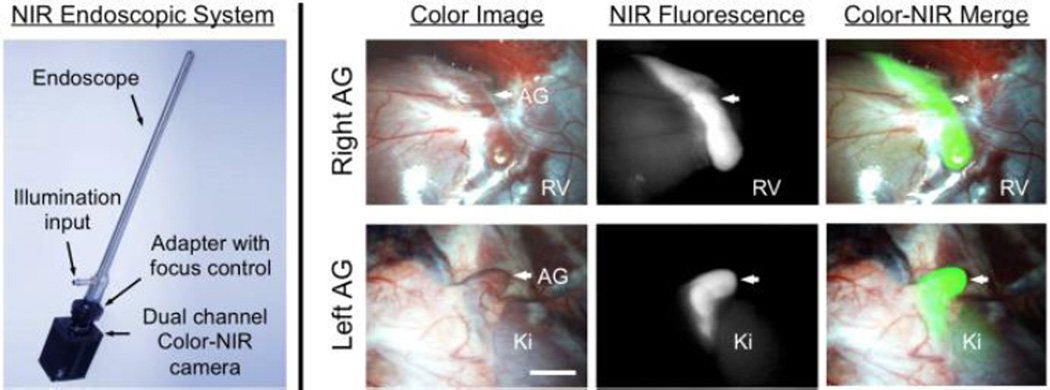
Based on the results during open surgery, 2 µmol of ESNF31 was injected into a 35 kg Yorkshire pig and the right and left AGs imaged using a minimal-invasive fluorescence imaging system (FluoSCOPE). Confirming the results seen previously, AGs could be identified with high sensitivity and specificity, with relatively low non-specific uptake in surrounding tissues and organs (Figure 4).
Fig. 4.
Minimally-Invasive Intraoperative Adrenal Gland Imaging using a Laparoscopic NIR Imaging System: 2 µmol of ESNF31 was injected intravenously into a pig 30 min prior to imaging. Shown are right AG (top) and left AG (bottom) with color image (left), NIR fluorescence (middle), and a merged image of the two (right). NIR fluorescence is pseudo-colored lime green in the merged image. AG, adrenal gland; Ki, kidney; RV, renal vein. Scale bar = 1 cm.
ESNF20 and ESNF31 are the most recent examples of a class of NIR fluorophores with the property of structure-inherent targeting.18–20 That is, there is something, as yet unidentified, in their chemical structure that favors uptake in the AG, while still retaining the property of NIR fluorescence. Such bifunctional molecules provide the most compact possible chemical structure for image-guided surgery because conjugation to a separate targeting ligand is not needed. ESNF20 and ESNF31 were discovered during a biodistribution screen in mice of a large number of NIR fluorophores having varying substituent groups surrounding either a pentamethine (700 nm NIR fluorophores) or heptamethine (800 nm NIR fluorophores) core. The fact that these molecules target the adrenal cortex specifically over the adrenal medulla suggests that they might share structural similarity to precursor molecules used in hormone production in the adrenal cortex, although this hypothesis remains unproven at present.
ESNF20 and ESNF31 appear to perform well for identifying normal AGs across species. At first glance, performance of these molecules in mice appears to be lower than that in pig. However, the lower SBR in mice is likely the result of varying path length. AGs in mice are so thin (≈ 1 mm), and the penetration of NIR light so deep (> 5 mm) that thick organs like the kidney and liver appear much brighter than they are on a per gram basis. In pig and human, however, the AG signal is a true reflection of the relative difference in fluorophore uptake among the various tissues and organs. The kinetics of uptake and clearance also difference among species, with the NIR fluorescence of AGs in mice lasting for up to 4 h and the signal in pig peaking at ≈ 30 min. Because of rapid uptake of both fluorophores in AGs (within 5 min), injections can be repeated intraoperatively, if needed, to keep the signal strong throughout the surgical procedure. What is not known, though, is how they will perform in the setting of adrenal tumors. Tumors in the medulla are less likely to exhibit high uptake based on our histological data. Tumors of the adrenal cortex may exhibit high uptake, in which case these NIR fluorophores could be used to find local and distant metastases. Given the paucity and complexity of mouse models in these diseases,21 and the fact that tissue path length (see above) will confound results, a clinical trial of patients with adrenocortical malignancies may be the most efficient way of evaluating the utility of these agents in tumor resection. Another concern is the potential toxicity. Although we have tested a wide range of doses (2–50 nmol) with a clinically compatible formulation (e.g., 10% serum-containing saline), neither adverse reactions nor in vivo toxicity were observed. This may be because both agents neither cross blood-brain barrier nor accumulate into the reproductive organs in mice and pigs (data not shown). It is also well-known that ICG containing the same polymethine backbone with ESNF20 and ESNF31 has shown only limited adverse reactions in the clinical use.22
Finally, ESNF20 and ESNF31 were chosen for use with the two (700 nm and 800 nm) independent NIR fluorescence channels of the FLARE imaging system. This, in turn, enables mixing and matching of virtually any two different NIR fluorophores for complex surgical procedures. For example, ESNF31 (800 nm emission) could be used to highlight the AGs in conjunction with a 700 nm NIR fluorophore, such as bolus injection of MB, which highlights blood vessels.23 Thus, one channel of FLARE could be used to either find or resect (depending on intention) the AGs while the other channel is used to quickly find feeding arteries. Similarly, ESNF20 (700 nm emission) could be used to find AGs while ICG (800 nm emission) is used to find feeding vessels. As more and more NIR fluorophores are developed, the combinations that can be used together increase substantially, as do the number of complex surgical procedures that might someday benefit from the technology.
Supplementary Material
Acknowledgments
This study was supported by the following grants from the National Institutes of Health: NIBIB grants #R01-EB010022 (JVF), #R01-EB011523 (HSC), and #R01-EB017699 (HSC), and NIDDK grant #K01-DK093603 (SG). This content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Electronic Supplementary Information (ESI) available: See DOI: 10.1039/b000000x/
Author Contributions. YA, AL, MHP, HH, VV, and GP performed the experiments. YA, MH, GEF, SG, JVF, and HSC reviewed, analyzed, and interpreted the data. YA, JVF, and HSC wrote the paper. All authors discussed the results and commented on the manuscript.
Competing Financial Interests. Dr. Frangioni is currently CEO of Curadel, LLC, a for-profit company which has licensed FLARE™ technology from Beth Israel Deaconess Medical Center.
Notes and references
- 1.Farge D, Pagny JY, Chatellier G, Plouin PF, Corvol P. Arch Mal Coeur Vaiss. 1988;81(Spec No):83–87. [PubMed] [Google Scholar]
- 2.Gagner M, Lacroix A, Bolte E. N Engl J Med. 1992;327:1033. doi: 10.1056/NEJM199210013271417. [DOI] [PubMed] [Google Scholar]
- 3.Higashihara E, Tanaka Y, Horie S, Aruga S, Nutahara K, Homma Y, Minowada S, Aso Y. Nihon Hinyokika Gakkai Zasshi. 1992;83:1130–1133. doi: 10.5980/jpnjurol1989.83.1130. [DOI] [PubMed] [Google Scholar]
- 4.Miller BS, Doherty GM. Nat Rev Endocrinol. 2014;10:282–292. doi: 10.1038/nrendo.2014.26. [DOI] [PubMed] [Google Scholar]
- 5.Kazaryan AM, Marangos IP, Rosok BI, Rosseland AR, Edwin B. Surg Innov. 2011;18:358–367. doi: 10.1177/1553350611403772. [DOI] [PubMed] [Google Scholar]
- 6.Suzuki K, Kageyama S, Hirano Y, Ushiyama T, Rajamahanty S, Fujita K. J Urol. 2001;166:437–443. [PubMed] [Google Scholar]
- 7.Naya Y, Nagata M, Ichikawa T, Amakasu M, Omura M, Nishikawa T, Yamaguchi K, Ito H. BJU Int. 2002;90:199–204. doi: 10.1046/j.1464-410x.2002.02845.x. [DOI] [PubMed] [Google Scholar]
- 8.Frangioni JV. Curr Opin Chem Biol. 2003;7:626–634. doi: 10.1016/j.cbpa.2003.08.007. [DOI] [PubMed] [Google Scholar]
- 9.Vahrmeijer AL, Hutteman M, van der Vorst JR, van de Velde CJ, Frangioni JV. Nat Rev Clin Oncol. 2013;10:507–518. doi: 10.1038/nrclinonc.2013.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Obermeyer RJ, Knauer EM, Millie MP, Ojeda H, Peters MB, Jr, Sweeney JF. Am J Surg. 2003;186:531–534. doi: 10.1016/j.amjsurg.2003.07.011. [DOI] [PubMed] [Google Scholar]
- 11.Ashitate Y, Kim SH, Tanaka E, Henary M, Choi HS, Frangioni JV, Flaumenhaft R. J Vasc Surg. 2012;56:171–180. doi: 10.1016/j.jvs.2011.11.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kim SH, Park G, Hyun H, Lee JH, Ashitate Y, Choi J, Hong GH, Owens EA, Henary M, Choi HS. Biomed Mater. 2013;8:014110. doi: 10.1088/1748-6041/8/1/014110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Owens EA, Hyun H, Kim SH, Lee JH, Park G, Ashitate Y, Choi J, Hong GH, Alyabyev S, Lee SJ, Khang G, Henary M, Choi HS. Biomed Mater. 2013;8:014109. doi: 10.1088/1748-6041/8/1/014109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ashitate Y, Hyun H, Kim SH, Lee JH, Henary M, Frangioni JV, Choi HS. Theranostics. 2014;4:693–700. doi: 10.7150/thno.8721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wada H, Hyun H, Vargas C, Gravier J, Park G, Gioux S, Frangioni JV, Henary M, Choi HS. Theranostics. 2015;5:1–11. doi: 10.7150/thno.10259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Owens EA, Hyun H, Tawney JG, Choi HS, Henary M. J Med Chem. 2015;58:4348–4356. doi: 10.1021/acs.jmedchem.5b00475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Matsui A, Tanaka E, Choi HS, Kianzad V, Gioux S, Lomnes SJ, Frangioni JV. Surgery. 2010;148:78–86. doi: 10.1016/j.surg.2009.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hyun H, Owens EA, Wada H, Levitz A, Park G, Park MH, Frangioni JV, Henary M, Choi HS. Angew Chem Int Ed Engl. 2015;54:8648–8652. doi: 10.1002/anie.201502287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hyun H, Park MH, Owens EA, Wada H, Henary M, Handgraaf HJ, Vahrmeijer AL, Frangioni JV, Choi HS. Nat Med. 2015;21:192–197. doi: 10.1038/nm.3728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hyun H, Wada H, Bao K, Gravier J, Yadav Y, Laramie M, Henary M, Frangioni JV, Choi HS. Angew Chem Int Ed Engl. 2014;53:10668–10672. doi: 10.1002/anie.201404930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hantel C, Beuschlein F. Best Pract Res Clin Endocrinol Metab. 2010;24:865–875. doi: 10.1016/j.beem.2010.10.010. [DOI] [PubMed] [Google Scholar]
- 22.R B, Quintana J, Brundage B. Cathet. Cardiovasc. Diagn. 17:231–233. doi: 10.1002/ccd.1810170410. 2989. [DOI] [PubMed] [Google Scholar]
- 23.Tanaka E, Chen FY, Flaumenhaft R, Graham GJ, Laurence RG, Frangioni JV. J Thorac Cardiovasc Surg. 2009;138:133–140. doi: 10.1016/j.jtcvs.2008.09.082. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.