SUMMARY
Type III secretion systems (T3SSs) inject bacterial effector proteins into host cells and underlie the virulence of many Gram-negative pathogens. Studies have illuminated bacterial factors required for T3SS function, but the required host processes remain largely undefined. We coupled CRISPR/Cas9 genome editing technology with the cytotoxicity of two Vibrio parahaemolyticus T3SSs (T3SS1 and T3SS2) to identify human genome disruptions conferring resistance to T3SS-dependent cytotoxicity. We identity non-overlapping genes required for T3SS1- and T3SS2-mediated cytotoxicity. Genetic ablation of cell surface sulfation reduces bacterial adhesion and thereby alters the kinetics of T3SS1-mediated cytotoxicity. Cell surface fucosylation is required for T3SS2-dependent killing, and pharmacological or genetic inhibition of fucosylation prevents membrane insertion of the T3SS2 translocon complex. These findings reveal the importance of ubiquitous surface modifications for T3SS function, potentially explaining the broad tropism of V. parahaemolyticus, as well as highlight the utility of genome-wide CRISPR/Cas9 screens to discover processes underlying host-pathogen interactions.
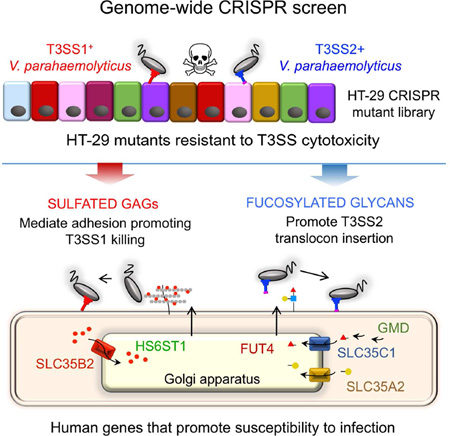
Graphical abstract
eTOC Blurb
Type III Secretion Systems (T3SS) underlie the virulence of many bacterial pathogens, but the required host factors remain largely undefined. Blondel et al. harness CRISPR Cas9 technology to perform genome-wide screens and identify requirements for host cell sulfation and fucosylation in conferring susceptibility to T3SS-mediated killing by vibrio parahaemolyticus.
INTRODUCTION
The virulence of many human, animal, and plant pathogens depends on type III secretion systems (T3SSs). These multicomponent nanomachines enable Gram-negative pathogens to inject a wide repertoire of effector proteins directly into the cytosol of eukaryotic host cells (reviewed in (Portaliou et al., 2016)). T3SSs are composed of a basal body found within the pathogen cell envelope, a needle that extends from the bacterial surface to the host cell, and a tip complex that creates a pore in the host cell membrane. This pore, termed a translocon, consists of several bacterial proteins that form a conduit through which effectors are translocated into the host cytoplasm. T3SSs from different pathogens transfer distinct sets of effectors that can enable pathogen adhesion, internalization, or modulation of diverse host processes, often in a redundant fashion (Shames and Finlay, 2012).
The Gram-negative marine bacterium Vibrio parahaemolyticus is a leading cause worldwide of gastroenteritis linked to the consumption of contaminated seafood and a cause of major economic losses for the aquaculture industry, reviewed in (Letchumanan et al., 2014). All V. parahaemolyticus strains harbor genes encoding a T3SS on their large chromosome, T3SS1, and nearly all clinical isolates produce a second, evolutionarily distinct T3SS, T3SS2 (Abby and Rocha, 2012; Hazen et al., 2015), which is encoded within a pathogenicity island on the small chromosome (Park et al., 2004; Sugiyama et al., 2008).
The two V. parahaemolyticus T3SSs are functionally independent and translocate their own set of effector proteins (Park et al., 2004). Although T3SS1 activity causes marked cytotoxicity in a variety of cultured human cell lines, studies with animal models suggested that this secretion system plays at most a minor role in the pathogenesis of V. parahaemolyticus enteritis (Piñeyro et al., 2010; Ritchie et al., 2012). The universality of T3SS1 among V. parahaemolyticus isolates and the absence of a virulence defect in T3SS1 mutants in oro-gastric animal models suggests that this secretion system may contribute to environmental fitness, perhaps through targeting of predatory marine eukaryotes. In contrast, studies in animal models demonstrate that T3SS2 is essential for V. parahaemolyticus to colonize the intestine and to cause enteritis (Piñeyro et al., 2010; Ritchie et al., 2012). T3SS2 also has cytotoxic activity against predatory protists and thus may promote V. parahaemolyticus survival in the environment (Matz et al., 2011).
Decades of research have led to deep knowledge of T3SS structure, assembly, and function, as well as of the biochemical activities and eukaryotic targets of individual effector proteins (Dean, 2011; Galán et al., 2014). However, our understanding of host factors that enable the targeting of T3SSs to host cells is more rudimentary. To date, most attempts to identify such factors have relied on focused biochemical approaches e.g. (Lafont et al., 2002). Only two genetic screens – employing either siRNA or haploid cell technology – have been carried out to find host factors important for T3SS function (Russo et al., 2016; Sheahan and Isberg, 2015).
The development of CRISPR/Cas9 technology, which allows generation of complete loss-of-function alleles in a variety of cell types, is transforming functional genetic analyses in higher eukaryotes (Shalem et al., 2014a; Shi et al., 2015; Wang et al., 2014; Doudna and Charpentier, 2014). Here, we used CRISPR/Cas9 based screening to identify disruptions in human protein coding genes that confer resistance to the activity of evolutionarily divergent T3SSs. These screens revealed that distinct host cell pathways confer susceptibility to T3SS1 and T3SS2 killing. Cell surface sulfation was important for bacterial adhesion and T3SS1 killing but dispensable for T3SS2 killing. In contrast, T3SS2 killing was dependent on cell surface fucosylation, which was critical for T3SS2 translocon insertion into host cell membranes. Thus, there is significant heterogeneity in the host factors targeted by different T3SSs. Our study demonstrates the utility and potency of CRISPR/Cas9-based genetic screens for investigations of host-pathogen interactions.
RESULTS
CRISPR/Cas9 screens identify host factors required for T3SS1 or T3SS2 cytotoxicity
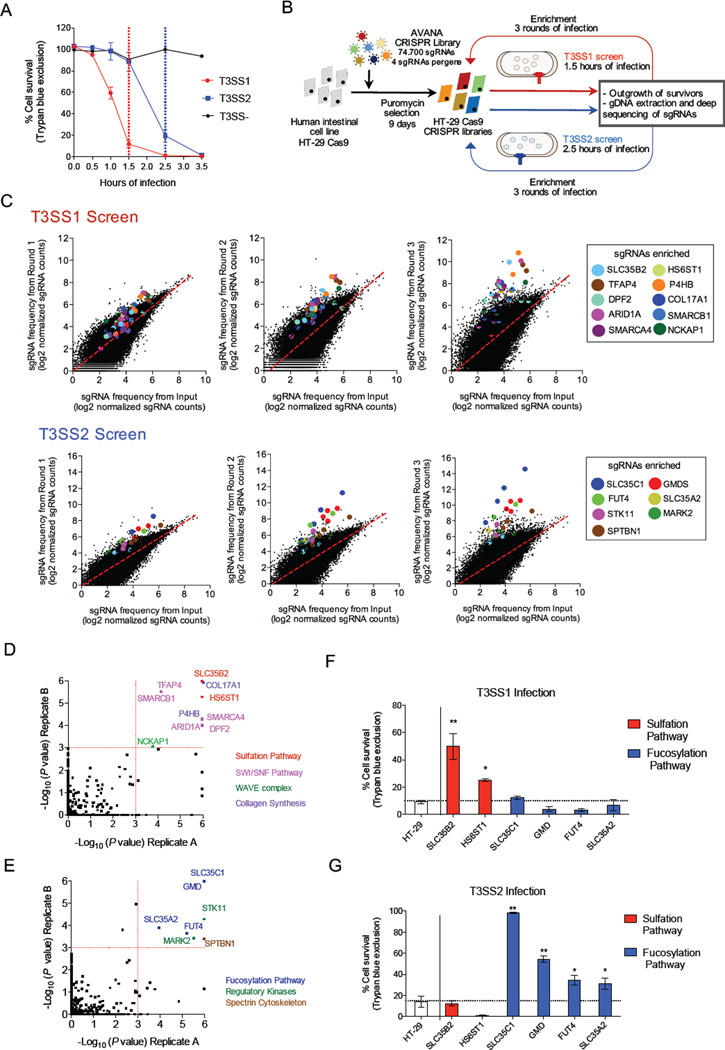
V. parahaemolyticus producing either T3SS1 or T3SS2 induces rapid cell death in human cells (Burdette et al., 2008; Kodama et al., 2007), but with distinct kinetics. To identify phenotypes attributable to an individual T3SS, we generated isogenic V. parahaemolyticus strains with deletions in either the ATPase-encoding component of T3SS1 (ΔvscN1, T3SS2+) or T3SS2 (ΔvscN2, T3SS1+). While T3SS1 kills more than 80% of HT-29 intestinal epithelial cells within 1.5 hr of infection, T3SS2 reaches the same levels after 2.5 hr (Figure 1A). We harnessed the robust selection by V. parahaemolyticus’ two T3SSs to identify host cell targets whose disruption confers resistance to T3SS-mediated cytotoxicity.
Figure 1. CRISPR/Cas9 screen reveals coherent and distinct pathways involved in resistance to T3SS killing.
(A) Kinetics of T3SS-dependent HT-29 cell death. The duration of infection for the T3SS1 (red) and T3SS2 (blue) screens is marked with dotted lines. (B) Workflow and screening strategy for the CRISPR/Cas9 screens. (C) Scatter plots showing enrichment of specific sgRNAs in the T3SS1 and T3SS2 screens after each round of infection. sgRNAs targeting the same gene are highlighted with the same color. The values correspond to log2 of normalized reads (see Table S2 for data). (D, E) Statistical significance of the gene candidates from the T3SS1 (D) and T3SS2 (E) screens in both biological replicates analyzed by STARS. Candidates for follow up had a P < 0.001 in both biological replicates. The colors of spheres indicate the associated biological process. See also Table S3. (F, G) Survival of HT-29 cells and mutant cells following infection with T3SS1+ or T3SS2+ bacteria for 1.5 or 2.5 hr, respectively. See also Figure S3B-C. Data are mean with SEM (n=3). P values (** < 0.001, * < 0.05) are based on one-way ANOVA with Dunnet post test correction.
Using the recently described Avana CRISPR human genome-wide library (Doench et al., 2016), we targeted each protein-coding gene in the human intestinal epithelial cell line HT-29 with 4 different single guide RNAs (sgRNAs) (for a total of 74,700 sgRNAs). Seven days after introduction of the CRISPR library in independent biological replicates (replicates A and B) (Figure S1AB), cells were infected with V. parahaemolyticus producing either T3SS1 or T3SS2. To standardize the magnitude of selection between the different screens, infections were carried out for 1.5 hr (T3SS1 selections) or 2.5 hr (T3SS2 selections) based on the kinetics noted above. Survivor cells were outgrown, and the process was repeated for 3 total rounds of infection to enrich for resistant cells (Figure 1B). Deep sequencing of integrated sgRNAs prior to and after each round of infection revealed that a small subset of sgRNAs became increasingly overrepresented after each round of selection (Figure 1C and Figures S1-S2).
We used the STARS algorithm, which integrates data from independent guides targeting the same gene, to identify the most enriched genotypes. In general, our data display a strong concordance between independent guides within the same library and between independent libraries (Table S3, Figure 1DE). For each T3SS screen, we observed enrichment of sgRNAs targeting multiple genes that contribute to a single biological process. Based on a statistically significant enrichment (P < 0.001) in both biological replicates, T3SS1 resistance was associated with sgRNAs targeting multiple genes important for cell surface sulfation, the SWI/SNF chromatin remodeling complex, and collagen synthesis. T3SS2 resistance was largely associated with sgRNAs targeting genes involved in fucosylation of glycans exported to the cell surface, serine-threonine kinases, and a spectrin subunit. The disparity between hits from T3SS1 and T3SS2 screens suggests that disruption of distinct host factors confers resistance to these 2 T3SSs.
To validate the screening results, HT-29 cells containing disruptions of individual genes were generated (Figure S3A). Disruption of at least one gene from each pathway identified was found to specifically augment resistance to either T3SS1- or T3SS2-mediated death (Figure S3BC). Maximal resistance to T3SS1 and T3SS2 was observed with mutants that disrupt cell surface sulfation and fucosylation, respectively (Figures 1FG), leading us to further investigate the role of these host cell surface modifications in V. parahaemolyticus-mediated cytotoxicity.
Host cell surface sulfation facilitates T3SS1 killing via V. parahaemolyticus adherence
Human cell surface sulfation depends upon transport of a sulfate donor, 3-phosphoadenyl 5-phosphosulphate (PAPs), into the Golgi apparatus by antiporters encoded by the SLC35B2 and SLC35B3 genes (Kamiyama et al., 2011; 2003). Within the Golgi, sulfotransferases (eg, the heparan sulfate 6-O-sulfotransferase HS6ST1) catalyze transfer of a sulfate group from PAPS to glycosaminoglycans (GAGs) (Habuchi et al., 1995) (Figure 2A). SLC35B2 disruption reduces the total sulfation of GAGs (eg heparan sulfate) and N-glycans in human cells (Kamiyama et al., 2011), while individual sulfotransferase mutations are expected to have a more modest effect. In agreement with this, the SLC35B2 mutant cells exhibited a more severe reduction in cell surface binding to a heparan sulfate-specific antibody than HS6ST1 mutant cells (Figure 2B).
Figure 2. Cell surface sulfation promotes T3SS1 cytotoxicity.
(A) Overview of sulfated proteoglycan synthesis, highlighting mutations conferring resistance to T3SS1 cytotoxicity. (B) Flow cytometry profiles of HT-29 and mutant cells bound to a heparan sulfate specific antibody (10E4-FITC); GMFI, geometric mean fluorescence intensity. (C) Survival of HT-29 cells treated with the sulfation inhibitor sodium chlorate prior to T3SS1 and T3SS2 infection. (D) T3SS1 killing and sulfation of SLC35B2 mutant cells expressing an sgRNA resistant SLC35B2 cDNA. Data are mean with SEM (n=3). P values (*** < 0.0001) are based on one-way ANOVA with Dunnet post test correction.
Disruption of SLC35B2 protected against T3SS1-but not T3SS2-mediated killing (Figures 1FG). Killing and partial surface sulfation of the SLC35B2 mutant was restored by introduction of a cDNA encoding a sgRNA-resistant version of SLC35B2 (through a silent mutation at the PAM site) (Figure 2D). Disruption of HS6ST1 also had a significant, though less marked effect on T3SS1-mediated killing (Figures 1FG), and treatment of HT-29 cells with sodium chlorate, an inhibitor of ATP-sulfurylase (a key enzyme in PAPS synthesis) (Safaiyan et al., 1999) resulted in dose-dependent and specific protection against T3SS1-mediated death (Figure 2C). Thus, both genetic and pharmacological data indicate that host cell sulfation promotes T3SS1 cytotoxicity.
Sulfated GAGs (e.g. heparan, chondroitin and dermatan) are the principal sulfated molecules in host cells and exist as membrane-bound or secreted proteoglycans (Sarrazin et al., 2011). Our screen identified factors that contribute to GAG sulfation but did not yield genes for synthesis of specific proteoglycans, suggesting that sulfated GAGs in general, rather than a particular proteoglycan, may confer susceptibility to T3SS1-dependent killing by V. parahaemolyticus. Consistent with this idea, we found that coating the sulfation-deficient SLC35B2 mutant with the sulfated GAGs heparin, dermatan sulfate, or chondroitin sulfate all increased the susceptibility of the SLC35B2 mutant to T3SS1 killing, but treatment with hyaluronic acid, a non-sulfated GAG, did not (Figure 3A). Coating of HT-29 cells with heparin did not increase susceptibility, suggesting that endogenous levels of GAGs are not limiting for T3SS1-dependent cell death (Figure 3A). These data also indicate that structurally diverse sulfated GAGs associated with the surface of host cells can promote V. parahaemolyticus T3SS1 toxicity even in the absence of covalent linkages between GAGs and specific cell surface proteins.
Figure 3. Sulfated GAGs facilitate T3SS1 killing by promoting bacterial adhesion.
(A) T3SS1 cytotoxicity toward HT-29 and SLC35B2 mutant cells without pretreatment (PBS), preincubated then washed (coating) with GAGs (500µg/ml) (sulfated heparin (HS); dermatan sulfate (DS); chondroitin sulfate (CSA), or non-sulfated: hyaluronic acid (HA)), or infected in the presence of heparin (500µg/ml) (blocking). (B) Adherence of T3SS- V. parahaemolyticus to HT-29 and SLC35B2 mutant cells in presence of 500µg/ml sulfated or non-sulfated GAGs. (C) Effect of GAGs on resistance of HT-29 cells to T3SS1 and T3SS2 killing. (D) Survival kinetics of host cells of varying genotypes following infection with T3SS1+ and T3SS-deficient V. parahaemolyticus. (E) Survival kinetics of HT-29 and SLC35B2 mutant cells during infection with either T3SS1+ or T3SS1+ V. parahaemolyticus expressing the Afa-I adhesin or following infection initiated with centrifugation of V. parahaemolyticus onto host cells (spin). (F) Translocation of the T3SS1 effector VopQ fused to adenylate cyclase (CyA) into different host cells by V. parahaemolyticus after a 20-minute infection. Translocation into SLC35B2 cells was evaluated with and without centrifugation (spin) to enhance bacterial attachment. Data are mean with SEM (n=3). P values (* < 0.01, ** < 0.001, *** < 0.0001) are based on one-way ANOVA with Dunnet post test correction.
The dependence of T3SS1 cytotoxicity on host cell sulfation is not restricted to HT-29 cells. Sodium chlorate treatment protected all 4 additional cell lines tested (including CHO cells) from T3SS1 killing (Figure S4A). Furthermore, a CHO cell derivative devoid of GAGs (CHO pgsA-745 cells; (Ludington and Ward, 2016)) exhibited enhanced resistance to T3SS1 killing (Figure S4B). In contrast, a CHO derivative that does not synthesize heparan sulfate but produces high levels of chondroitin sulfate (CHO pgsD-677) was as susceptible as the parental cell line to T3SS1 killing, consistent with the observation that diverse sulfated GAGs can mediate cytotoxicity.
Since heparan sulfate proteoglycans mediate host attachment by a wide variety of bacterial and viral pathogens (Kamhi et al., 2013), we speculated that sulfated GAGs might promote T3SS1-dependent cytotoxicity by facilitating V. parahaemolyticus adhesion to host cells. Consistent with this hypothesis, we found that V. parahaemolyticus adhesion to the SLC35B2 mutant cell line was lower than to the HT-29 cells, based on enumeration of bound colony forming units (Figure 3B) or visualization of bound bacteria (Figure S5). Furthermore, addition of sulfated GAGs to culture media reduced V. parahaemolyticus adhesion and caused dose-dependent reduction in T3SS1- (but not T3SS2-) mediated death, presumably by acting as decoy molecules, competing with endogenous GAGs in binding to adhesins (Figures 3ABC, S4C). Notably, a non-sulfated GAG, hyaluronic acid, had no effect on bacterial adhesion or T3SS1-mediated cytotoxicity (Figures 3ABC). Collectively, these findings are consistent with the hypothesis that sulfated GAGs promote the adhesion of V. parahaemolyticus to host cells and thereby facilitate T3SS1 killing.
SLC35B2 mutant cells were not absolutely resistant to T3SS1 killing, but instead were killed with delayed kinetics compared to WT cells (Figure 3D, S3D), suggesting that adhesion is a rate-limiting precursor to T3SS1 cytotoxicity, rather than an integral component. In support of this idea, we found that survival of the SLC35B2 mutant was markedly reduced when adherence of the T3SS1+ strain was augmented by expression of an exogenous, sulfation-independent adhesin, Afa-1 (Labigne-Roussel et al., 1984) or when centrifugation was used to force contact between V. parahaemolyticus and host cells (Figures 3E and S4D). Centrifugation also abrogated a deficiency in translocation of a T3SS1 effector protein (VopQ) into SLC35B2 cells (Figure 3F). Overall, these data suggest that sulfated GAGs act upstream of T3SS1 to promote V. parahaemolyticus’ initial interaction with host cells, rather than interacting directly with components of the T3SS1 machinery.
We created mutants lacking one or more of the 3 previously described V. parahaemolyticus adhesin-encoding genes (mam7, mshA1 and vpadF) (Boyd, 2014) that have been implicated in T3SS1-killing to test whether they interact with sulfated compounds and contribute to the sulfation-dependent adhesion of the pathogen to host cells. MshA1 has been reported to bind both sulfated and non-sulfated glycans, while MAM7 and VpadF are reported to bind fibrinogen and/or fibronectin. We found that deletion of vpadF resulted in a marked reduction in heparin binding by V. parahaemolyticus, that deletion of mshA1 or mam7 had a less pronounced effect, and that the effects of these mutations were additive (Figure 4A). Thus, all 3 adhesins could contribute to sulfation-dependent cytotoxicity.
Figure 4. MAM7, MshA1, VpadF promote sulfation-dependent adhesion and T3SS1 killing.
(A) Flow cytometry profile of T3SS1+ V. parahaemolyticus and derivatives lacking MAM7, MshA1, VpadF or all of them, bound to heparin-FITC; GMFI, geometric mean fluorescence intensity. (B) Kinetics of T3SS1 killing in bacteria lacking the adhesins MAM7, MshA1 and/or VpadF. (C) Survival of SLC35B2 cells infected with T3SS1+ and adhesin-deficient strains with or without heparin coating. Data are mean with SEM (n=3). P values (* < 0.01) are based on one-way ANOVA with Dunnet post test correction.
To further explore the roles of these adhesins in T3SS1 activity, we measured the survival of HT-29 cells infected with control and adhesin-deficient T3SS1+ strains. No single adhesin mutation significantly reduced host cell killing; however, there was a significant delay in T3SS1 killing kinetics with the mam7/mshA1 double mutant and even more with the mam7/mshA1/vpadF triple mutant (Figure 4B). Strikingly, the cytotoxicity of the triple mutant towards SLC35B2 cells was not increased by coating of host cells with heparin (Figure 4C). Collectively, these data suggest that MAM7, MshA1, and VpadF have partially redundant function, consistent with the overlap that we have demonstrated among their targets. Additionally, these observations provide compelling evidence that surface sulfation is recognized by bacterial adhesins and critical for V. parahaemolyticus’ interaction with host cells prior to T3SS1 cytotoxicity.
Cell surface fucosylation enables T3SS2 cytotoxicity
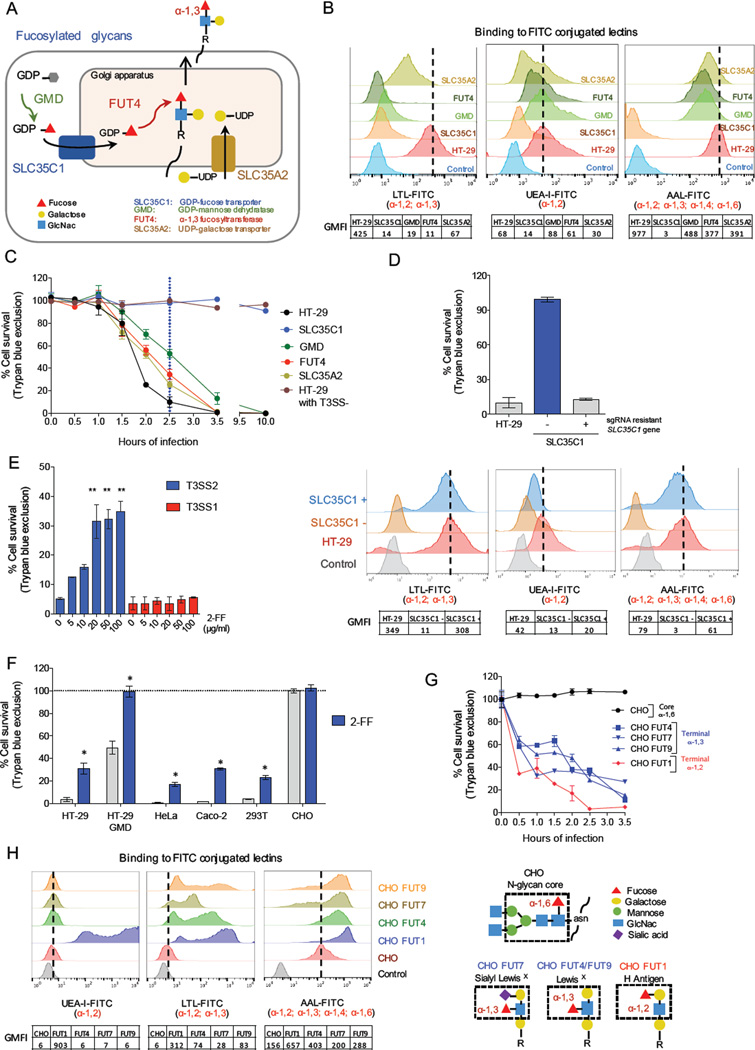
sgRNAs targeting genes critical for fucosylation of host cell surface proteins conferred the greatest degree of resistance to V. parahaemolyticus T3SS2-dependent killing. These included GMD, which encodes a cytosolic enzyme that generates the charged fucose intermediate GDP-fucose, and SLC35C1, which encodes the sole Golgi importer of GDP-fucose (Figures 1E, 5A) (Lühn et al., 2001). Within the Golgi, a variety of fucosyltransferases (FUTs), including FUT4 (Figure 5A), encoded by another hit in the T3SS2 screen) transfer fucose to assembling glycan structures to generate fucosylated glycans that are then exported to the cell surface (Hadley et al., 2014). A UDP-galactose transporter encoded by SLC35A2, which enables synthesis of certain fucosyltransferase substrates (Becker and Lowe, 2003), was also identified (Figure 5A). Thus, the T3SS2 screen strongly suggests that fucosylated glycans created in the Golgi are important for T3SS2-induced cytotoxicity. Additionally, the absence of hits in genes encoding particular fucosylated surface proteins and/or lipids suggests that there may not be a specific surface protein/lipid required for susceptibility to T3SS2 killing; instead, the screen results suggest that multiple fucosylated cell surface glycans could enable killing by T3SS2.
Figure 5. Terminal fucosylation is required for T3SS2 cytotoxicity.
(A) Overview of the steps in the synthesis of fucosylated glycans, highlighting mutations conferring resistance to T3SS2 killing. (B) Flow cytometry profiles of HT-29 and mutant cells bound to fucose-specific FITC-conjugated lectins that recognize distinct fucosylation linkages. Charts below the graphs show geometric mean fluorescence intensity (GMFI). AAL, Aleuria aurantia; LTL, Lotus tetragonolobus; UEA-1, Ulex europaeus Iectin. (C) Kinetics of survival of HT-29 and mutant cells after infection with T3SS2+ or T3SS2− V. parahaemolyticus. (D). T3SS2 killing (top) and fucosylation (bottom) of wt, SLC35C1 mutant cells, and SLC35C1 mutant cells expressing an sgRNA resistant SLC35C1 cDNA. (E) Impact of the fucosylation inhibitor 2-FF on T3SS1 and T3SS2 killing of HT-29 cells. (F) Impact of 2-FF on survival of different cell lines infected with T3SS2+ V. parahaemolyticus. (G) Kinetics of cell survival following T3SS2+ V. parahaemolyticus infection of CHO parental and mutant cells. (H) Lectin binding profiles for CHO cells transduced with FUT genes that generate diverse terminal fucose linkages. Structures of the CHO cell N-glycan core and various terminal fucosylated blood group antigens are shown on the right. Data are mean with SEM (n=3), P values (* < =0.01, ** < 0.001) are based on one-way ANOVA with Dunnet post test correction.
Using individual sgRNAs targeting SLC35C1, GMD, FUT4, or SLC35A2, we confirmed that these factors contribute specifically to T3SS2 susceptibility (Figures 1F-G). Furthermore, analyses of surface fucosylation, using a variety of fucose-specific lectins, revealed a strong correlation between the reduction of glycan fucosylation and the extent of resistance to T3SS2 killing (Figures 5BC). Little or no fucosylation was detected in the SLC35C1 mutant, and these cells were fully resistant to T3SS2 cytotoxicity even after extended infection. In contrast, limited surface fucosylation in the GMD, FUT4, and SLC35A2 mutants was still apparent with a subset of fucose-specific lectins (UEA and AAL, but not LTL), and these mutants exhibited delayed killing following infection. Pharmacological inhibition of glycan fucosylation by the GDP-fucose mimetic compound 2-fluorofucose (2-FF) likewise revealed a correlation between reduced fucosylation and increased resistance to T3SS2 killing; 2-FF treatment was protective for wt and GMD mutant HT-29 cells as well as for a variety of unrelated cell lines (Figures 5EF, S6A). Finally, fucosylation and T3SS2-susceptibility of the HT-29 SLC35C1 mutant were restored by expression of a cDNA encoding a sgRNA resistant version of SLC35C1 (Figure 5D), confirming the genetic link between surface fucosylation and T3SS2 cytotoxicity.
The incomplete resistance of the FUT4 deficient cells, which are thought to lack a subset of α-1,3 fucosylated glycans but to maintain other linkages (e.g., α-1,2) (Figure 5BC), suggested that more than one type of fucosylated glycan might confer susceptibility to T3SS2 killing. We took advantage of CHO cells, which are completely resistant to T3SS2 killing (Figure 5G), to more precisely define the link between surface fucosylation and T3SS2-mediated cytotoxicity. CHO cells only express one fucosyltransferase, FUT8, which generates the α-1,6 core linkage in N-glycans (Xu et al., 2011), leaving them devoid of terminally fucosylated glycans (Figure 5H). Transduction of individual FUT genes into CHO cells yielded cell lines with distinct α-terminal α-fucose linkages, including α-1,2 linkages, such as found in the blood group H antigen (produced by FUT1), and α-1,3 linkages, such as found in the Sialyl LewisX blood group (produced by FUT7) and in the LewisX blood group (produced by FUT4 and FUT9) (Figure 5H). Despite their diverse products, each of the transduced FUTs rendered the corresponding CHO cell lines susceptible to T3SS2 killing (Figure 5G). Thus, terminal fucosylation in a variety of forms and contexts can mediate susceptibility to T3SS2 killing and the specific identification of FUT4 in the screen likely reflects the major contribution of this FUT to fucosylation in HT-29 cells (Giordano et al., 2015).
Host cell surface fucosylation facilitates T3SS2 activity
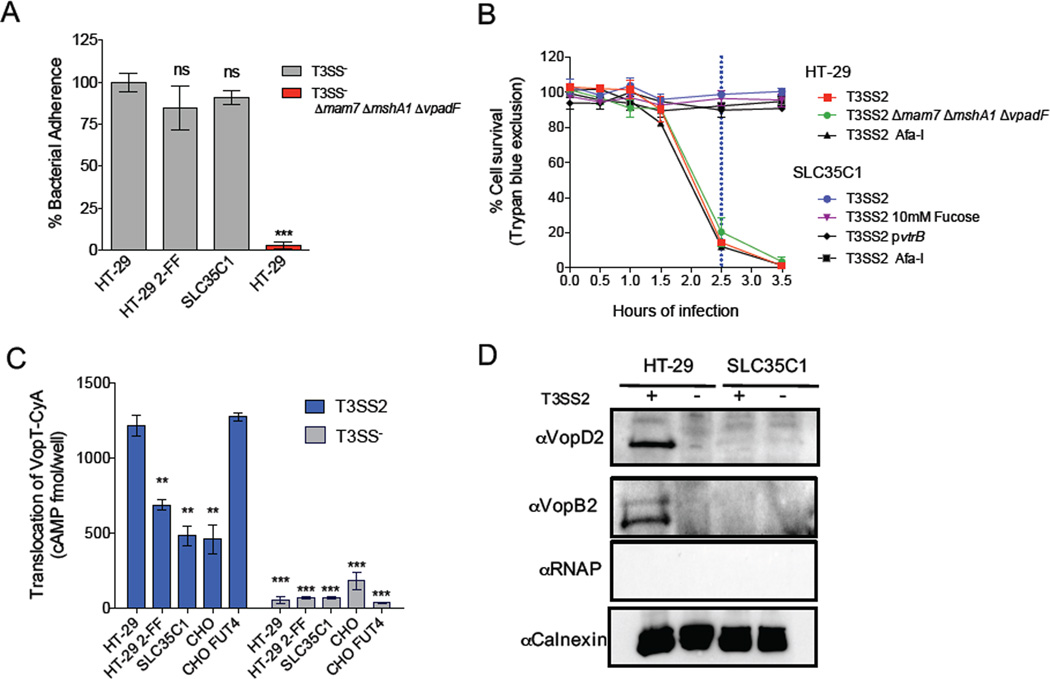
The resistance of sulfation-deficient host cells to T3SS1 cytotoxicity was not absolute, and reflected decreased V. parahaemolyticus adhesion to resistant cells. In contrast, SLC35C1 mutant cells were not susceptible to T3SS2 cytotoxicity even after an extended infection period (Figures 5C, S3E), suggesting that the absence of fucosylation affects a process downstream of the initial adherence of V. parahaemolyticus to host cells. Consistent with this hypothesis, we observed that adherence of T3SS− V. parahaemolyticus to SLC35C1 mutant cells and to 2-FF treated HT-29 cells was indistinguishable from adherence to WT HT-29 cells (Figure 6A). Furthermore, unlike our results with the SCL35B2 mutant and T3SS1 cytotoxicity, death of the SLC35C1 mutant in response to T3SS2 infection was not augmented by expression of the exogenous adhesion Afa-I. Instead, the SLC35C1 cells remained fully resistant to T3SS2 killing (Figure 6B). The presence or absence of bacterial adhesins also did not affect T3SS2 cytotoxicity (Figure 6B), consistent with our observation (Figure 1F) that their sulfated GAG targets are not required for T3SS2-mediated cell death. Early in infection (20 minutes), the absence of sulfation-mediated adhesion moderately reduced T3SS2 effector translocation (Figure S6B). However, T3SS2 itself has been shown to promote bacterial attachment (Zhou et al., 2014), and our data suggests that the sulfation-independent translocation is sufficient to establish and maintain an association between bacteria and host cell.
Figure 6. Fucosylation is required for T3SS2 effector translocation and translocon insertion but not bacterial adhesion.
(A) Adhesion of T3SS− V. parahemolyticus to HT-29 cells alone or treated with 50mM 2-FF or to SLC35C1 mutant cells. The T3SS− Δmam7 ΔmshA1 ΔvpadF strain was included as a negative control. (B) Survival kinetics of HT-29 and SLC35C1 mutant cells infected with T3SS2+ V. parahaemolyticus expressing the T3SS2 regulator VtrB or the adhesin Afa-I, infected in the presence of 10mM fucose, or infected with a V. parahaemolyticus strain lacking MAM7, MshA1 and VpadF. (C) Translocation of the T3SS2 effector VopT fused to adenylate cyclase after a 45-minute infection of HT-29 cells +/− 50mM 2-FF, SLC35C1 mutant cells, or CHO cells +/− FUT4. See also Figure S6B. (D) Immunoblot of T3SS2 translocon components VopB2 and VopD2 in host cell membranes after infection with T3SS2+ V. parahaemolyticus. Blots were also probed with antibodies against the host membrane protein calnexin (loading/fractionation control) and bacterial RNAP, demonstrating absence of contaminating intact bacteria. Data are mean with SEM (n=3). P values (** < 0.001, *** < 0.0001) are based on one-way ANOVA with Dunnet post test correction.
The resistance of the SLC35C1 mutant cells to T3SS2 cytotoxicity does not seem to reflect a failure of this strain to promote T3SS2 expression, although host-derived fucose does regulate expression of T3SS genes in EHEC (Pacheco et al., 2012). Culture media from HT-29 cells and SLC35C1 mutant cells infected with a T3SS2+ translocation reporter strain contained comparable amounts of an epitope tagged effector protein (VopV-CyA; Figure S6C), demonstrating that functional T3SS2 is being produced during both infections. Furthermore, addition of fucose to the media during T3SS2+ infection of SLC35C1 mutant cells did not render them susceptible to T3SS2 cytotoxicity (Figure 6B), nor did induction of T3SS2 gene expression via overexpression of the transcriptional activator VtrB (Kodama et al., 2010) (Figure 6B). Collectively, these observations argue against the idea that deficient expression of T3SS2 during infection of the SLC35C1 mutant accounts for the mutant’s resistance to T3SS2 killing.
Since the resistance of the SLC35C1 mutant cell line did not appear to be attributable to a general adhesion deficit or a reduction in T3SS2 expression, we investigated whether the extent of host cell surface fucosylation affected the translocation of T3SS2 effectors. We observed a marked reduction in translocation of the T3SS2 effector VopT into SLC35C1-deficient cells relative to HT-29 cells (Figure 6C). Translocation of VopT was also lower for HT-29 cells treated with 2-FF compared to untreated cells. Finally, we observed that translocation of VopT was markedly higher into CHO cells expressing FUT4 than into the parental cell line. Collectively, these data provide strong evidence that the extent of terminal glycan α-fucosylation correlates with T3SS2 activity against host cells.
The reduced translocation of T3SS2 effector proteins into cells lacking terminally fucosylated glycans might reflect a requirement for fucosylation in host membrane insertion of the T3SS2 translocon complex. To test this hypothesis, we compared the amounts of hydrophobic T3SS2 translocon components, VopB2 and VopD2, in membranes of HT-29 and SLC35C1 mutant cells after V. parahaemolyticus (T3SS2+) infection. Both translocon proteins were detected in the membranes of infected HT-29 cells but not in SLC35C1 cells (Figure 6D), suggesting that terminally fucosylated glycans are required for T3SS2 killing because they facilitate translocon insertion into host cell membrane and thereby enable effector delivery. Notably, this requirement is specific to the T3SS2 injectisome, since cells lacking fucosylation (e.g., SLC35C1-deficient cells and CHO cells) are highly susceptible to T3SS1 killing. Thus, translocons from diverse T3SSs may require distinct host factors to facilitate their capacity to engage host cell membranes.
DISCUSSION
The advent of CRISPR/Cas9-based technology is revolutionizing our capacity to comprehensively interrogate gene function in higher eukaryotes (Hartenian and Doench, 2015; Shalem et al., 2014b; Doudna and Charpentier, 2014). We used genome-wide CRISPR/Cas9 screens to identify host factors that sensitize human cells to V. parahaemolyticus T3SS-mediated killing. The screens identified distinct host cell surface modifications that enable each of V. parahaemolyticus’ two T3SSs to engage host cells. Sulfation, which contributes to adhesion mediated by several V. parahaemolyticus adhesins, promoted downstream cytotoxicity caused by T3SS1, but not by T3SS2. In contrast, cell surface fucosylation promotes T3SS2 translocon insertion, effector translocation, and associated cytotoxicity, but is not required for T3SS1-mediated cytotoxicity. Thus, critical host factors required for T3SS function are not conserved between the two phylogenetically distinct T3SSs of V. parahaemolyticus.
Sulfated proteoglycans, particularly those containing heparan sulfate, are known to promote host cell recognition and binding by a variety of viral, bacterial, and parasitic pathogens (Kamhi et al., 2013). However, there has been little indication to date that cell surface sulfation mediates V. parahaemolyticus interaction with host cells. We found that genetic or pharmacologic ablation of host cell sulfation reduces V. parahaemolyticus adhesion to HT-29 cells and delays T3SS1-associated cytotoxicity. Sulfated GAGs promoted V. parahaemolyticus adhesion even when GAGs were not covalently bound to host cells; however, bacterial adhesion and associated cytotoxicity were blocked by the presence of excess sulfated GAGs, which likely serve as competitive inhibitors. The bacterial adhesins MAM7, MshA1, and VpadF are known to promote cytotoxicity through recognition of specific host proteins (Krachler and Orth, 2011; Liu and Chen, 2015; O’Boyle et al., 2013). Our data suggest that sulfated host GAGs potentiate bacterial adhesion by promoting early stages of binding between partially redundant adhesins and host cells prior to recognition of specific targets. Adhesins, like sulfation, are not absolutely required for T3SS1 killing; however, cytotoxicity is reduced in their absence, apparently due to delayed bacterial association with host cells. Thus, interactions between sulfated host cell surface molecules and bacterial adhesins are upstream facilitators, rather than intrinsic determinants of T3SS1 activity.
Neither these adhesins nor host cell sulfation are required for cytotoxicity associated with T3SS2. In part, this likely reflects the fact that T3SS2 cytotoxicity was assessed after a longer infection (2.5 hr) than T3SS1 cytotoxicity (1.5 hr), and thus is less dependent upon a rapid establishment of bacterial/host cell contact. Although translocation of T3SS2 effectors into sulfation deficient host cells was reduced early after infection, this reduction did not ultimately impact T3SS2 killing assayed at 2.5hr. T3SS2 cytotoxicity may not require accessory adhesins to stabilize interactions with host cells because this system can itself promote V. parahaemolyticus adhesion to host cells through translocation of VopV (Zhou et al., 2014).
Fucosylation of surface glycans was required for T3SS2 killing. Disruption of the Golgi fucose transporter gene SLC35C1, which eliminated detectable surface fucosylation, protected against T3SS2 but not T3SS1 killing. Selective expression of different FUTs revealed that diverse terminal fucosylation products, but not core fucosylation, sensitize cells to T3SS2-mediated cytotoxicity. Our findings indicate that fucosylation plays a crucial role in insertion of hydrophobic T3SS2 translocon proteins into the host cell membrane, a process required for effector protein translocation and subsequent cytotoxicity. Given T3SS2’s essential role in virulence, our data suggests that the fucosylation status of the intestinal epithelium is a key determinant of the organism’s pathogenicity.
The means by which fucosylation contributes to T3SS2 translocon insertion require further definition; however, parallels with other biological systems are apparent. For example, interactions between fucosylated glycans (such as blood group antigens) and bacterial cholesterol-dependent pore forming toxins (e.g, pneumolysin and streptolysin O) promote insertion of these toxins within the host cell membrane, and binding to fucosylated glycans appears to promote host cell uptake of cholera toxin (Shewell et al., 2014; Wands et al., 2015). Thus, fucosylated surface molecules may serve as receptors for T3SS2 translocon components, whose binding may be an obligatory step in translocon insertion. Alternatively, host molecules may stabilize translocon complexes in the host membrane after insertion (Russo et al., 2016).
Fucosylated glycans are entirely dispensable for the action of T3SS1; thus, V. parahaemolyticus’ two T3SSs depend on distinct factors to engage host cells. Previous studies have also suggested there is heterogeneity in host determinants of T3SS susceptibility; for example, Yersinia pestis T3SS activity was found to depend on CCR5, a chemokine receptor (Sheahan and Isberg, 2015), and the Shigella flexneri translocon protein IpaB interacts with the host receptor CD44 (Skoudy et al., 2000). Given the apparent diversity in potential receptors for T3SSs, we speculate that different T3SSs have evolved distinct adaptations within their respective tip complexes – thought to sense the host cell and enable contact dependent translocon insertion – that are fine tuned to the diverse host cell types and environments where they function.
The fact that V. parahaemolyticus utilizes ubiquitous surface modifications, rather than discrete target proteins, may underlie its T3SS activity in host organisms ranging from single celled eukaryotes to humans. Our screen did not identify genes linked to lipid rafts or intermediate filament formation, which were previously shown to interact with translocon proteins by several enteric pathogens (Hayward et al., 2005; Lafont et al., 2002; Russo et al., 2016). It is possible that roles for these host factors were masked by cell-type specific functional redundancy or essentiality that prevented scoring as hits in our screens.
CRISPR/Cas9-based creation of comprehensive libraries of host cells with null mutations provides an invaluable functional genomics approach to the study of host-pathogen interactions. Identification of host factors that mediate susceptibility or resistance to infection not only yields critical insight into the evolution, emergence, and mechanisms of pathogenicity, but it also provides useful information for the development of therapeutic interventions aimed at blocking the host processes exploited by microbial pathogens.
EXPERIMENTAL PROCEDURES
See supplemental experimental procedures for additional information.
Construction of CRISPR/Cas9 Avana libraries in HT-29 cells
The Avana CRISPR sgRNA library contains 4 guide RNAs targeting each of the annotated human protein coding genes (18,675 genes) as described in (Doench et al., 2016). HT-29 cells constitutively expressing Cas9 (HT-29 Cas9) were transduced by lentiviral spin infection to generate two independent libraries. The following considerations were taken into account during library construction: i) we aimed to achieve 500X coverage (500 cells per perturbation); with 74.700 sgRNAs in the library this translates into ~40*106 infected cells; ii) since an MOI of less than 1 is desired to avoid insertions of multiple sgRNAs per cell, we titrated the Avana lentiviral library to obtain 30% infectivity on HT-29 Cas9 cells, and therefore a total of 135*106 cells were transduced. 100ml of 1.35*106 trypsinized HT-29 Cas9 cells/ml were prepared in DMEM 10% FBS supplemented with polybrene (8µg/ml), and aliquoted (2ml per well) among 4 12-well format plates, along with concentrated virus. Lentiviral transduction was performed using spin infection conditions for 2 hr at 2000rpm. Plates were then incubated at 37°C with 5% CO2 for 6 hr, followed by seeding of 3*106 trypsinized cells in each of 45 T225 flasks. After 2 days incubation, media was replaced with DMEM 10% FBS supplemented with Puromycin 1µg/ml. Following an additional 5–7 days of selection, transduced cells were trypsinized, counted and seeded for the positive selection screen. The procedure was performed in duplicate to obtain two biological replicates of the HT-29 CRISPR/Cas9 library (library A and B).
Positive selection screens using the HT-29 CRISPR Avana libraries
Ten T225 flasks seeded with 8*106 cells each were incubated for 48 hr. HT-29 cells doubled every 48 hr, yielding 1.6*108 cells per experimental condition (2000X coverage per perturbation in each library). Three experimental conditions were seeded: i) cells harvested as the library input, ii) cells used for the T3SS1 screen and iii) cells used for the T3SS2 screen.
The V. parahaemolyticus strains used for the 2 screens were cultured overnight, then diluted 1:100 into LB media and grown for 2.5 hr to an OD600nm of 0.6. Expression of T3SS2 was induced with 0.04% bile (Gotoh et al., 2010). HT-29 cells were infected at an MOI=1 and incubated at 37°C with 5% CO2 for either 1.5 hr (T3SS1 screen) or 2.5 hr (T3SS2 screen). After infection, media was replaced with fresh media supplemented with gentamicin (100µg/ml). Fresh media with gentamicin was added again after overnight incubation. Flasks were evaluated daily to monitor recovery of survivor cells; when 50–60% confluency was achieved, cells were trypsinized and passaged. At least 8*107 passaged cells were seeded, to maintain a coverage of at least 1000X. For the second and third round of infection, cells were propagated until at least 2.4*108 cells were obtained, allowing repetition of the infection procedure with 10 T225 flasks per experimental condition and 2000X coverage.
Genomic DNA preparation, sequencing and analyses of screen results
gDNA was obtained from 8*107 cells after each round of infection as well as from the input cells using the Blood and Cell Culture DNA Maxi Kit from Qiagen. PCR to amplify guide RNA sequences was performed as described (Doench et al., 2016). The read counts were first normalized to reads per million within each condition by the following formula: reads per sgRNA/total reads per condition × 106. Reads per million were then log2-transformed by first adding 1 to all values, in order to take the log of sgRNAs with zero reads (See Table S2). For analyses, the log2 fold-change of each sgRNA was determined relative to the input sample for each biological replicate. The STARS algorithm for CRISPR-based genetic perturbation screens was used to evaluate the rank and statistical significance of the 10% of perturbations from the ranked list, as described (Doench et al., 2016) (See Table S3).
Supplementary Material
Highlights.
Genome-wide CRISPR screens reveal host factors facilitating T3SS cytotoxicity
Distinct host pathways confer susceptibility to V. parahaemolyticus’ T3SS1 and T3SS2
Sulfation promotes bacterial adhesion and downstream T3SS1-associated cytotoxicity
Fucosylated glycans promote insertion of the T3SS2 translocon into host membranes
Acknowledgments
We gratefully acknowledge Drs. Tetsuya Iida, Toshio Kodama, Marcia Goldberg, Judy Lieberman and Honorine Ward for providing reagents and Emma W. Vaimberg and Yijie Ma for technical assistance. We thank members of the Waldor lab for helpful discussions. This work was supported by NIH (R37 AI-042347 to MKW, F31 AI-120665 to TPH, R01 CA085180 to BEG), and HHMI (MKW). JSP is supported by an HHMI medical research fellowship; CJB was supported by the Pew Latin American Fellows Program in the Biomedical Sciences and a CONICYT BecasChile postdoctoral fellowship; JGD is supported by the Next Generation Fund at the Broad Institute and BEG is supported by a Burroughs Wellcome Career award.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
AUTHOR CONTRIBUTIONS
CJB, BEG, JGD and MKW designed and conceived the study, CJB, JSP, ARP, TPH, CJK and MJW performed all experiments, and CJB, JSP, TPH, BEG, JGD, BMD and MKW analyzed data and wrote the manuscript.
References
- Abby SS, Rocha EPC. The non-flagellar type III secretion system evolved from the bacterial flagellum and diversified into host-cell adapted systems. PLoS Genet. 2012;8:e1002983. doi: 10.1371/journal.pgen.1002983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker DJ, Lowe JB. Fucose: biosynthesis and biological function in mammals. Glycobiology. 2003;13:41R–53R. doi: 10.1093/glycob/cwg054. [DOI] [PubMed] [Google Scholar]
- Boyd A. Manipulation of intestinal epithelial cell function by the cell contact-dependent type III secretion systems of Vibrio parahaemolyticus. Front Cell Infect Microbiol. 2014;10(3):114. doi: 10.3389/fcimb.2013.00114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burdette DL, Yarbrough ML, Orvedahl A, Gilpin CJ, Orth K. Vibrio parahaemolyticus orchestrates a multifaceted host cell infection by induction of autophagy, cell rounding, and then cell lysis. Proc. Natl. Acad. Sci. U.S.A. 2008;105:12497–12502. doi: 10.1073/pnas.0802773105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dean P. Functional domains and motifs of bacterial type III effector proteins and their roles in infection. FEMS Microbiol Rev. 2011;35:1100–1125. doi: 10.1111/j.1574-6976.2011.00271.x. [DOI] [PubMed] [Google Scholar]
- Doench JG, Fusi N, Sullender M, Hegde M, Vaimberg EW, Donovan KF, Smith I, Tothova Z, Wilen C, Orchard R, Virgin HW, Listgarten J, Root DE. Optimized sgRNA design to maximize activity and minimize off-target effects of CRISPR-Cas9. Nat. Biotechnol. 2016;34:184–191. doi: 10.1038/nbt.3437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doudna JA, Charpentier E. Genome editing. The new frontier of genome engineering with CRISPR-Cas9. Science. 2014;346(6213):1258096. doi: 10.1126/science.1258096. [DOI] [PubMed] [Google Scholar]
- Galán JE, Lara-Tejero M, Marlovits TC, Wagner S. Bacterial type III secretion systems: specialized nanomachines for protein delivery into target cells. Annu. Rev. Microbiol. 2014;68:415–438. doi: 10.1146/annurev-micro-092412-155725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giordano G, Febbraro A, Tomaselli E, Sarnicola ML, Parcesepe P, Parente D, Forte N, Fabozzi A, Remo A, Bonetti A, Manfrin E, Ghasemi S, Ceccarelli M, Cerulo L, Bazzoni F, Pancione M. Cancer-related CD15/FUT4 overexpression decreases benefit to agents targeting EGFR or VEGF acting as a novel RAF-MEK-ERK kinase downstream regulator in metastatic colorectal cancer. J. Exp. Clin. Cancer Res. 2015;34:108. doi: 10.1186/s13046-015-0225-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gotoh K, Kodama T, Hiyoshi H, Izutsu K, Park K-S, Dryselius R, Akeda Y, Honda T, Iida T. Bile acid-induced virulence gene expression of Vibrio parahaemolyticus reveals a novel therapeutic potential for bile acid sequestrants. PLoS ONE. 2010;5:e13365. doi: 10.1371/journal.pone.0013365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Habuchi H, Habuchi O, Kimata K. Purification and characterization of heparin sulfate 6-sulfotransferase from the culture medium of Chinese hamster ovary cells. Journal of Biological Chemistry. 1995;270:4172–4179. doi: 10.1074/jbc.270.8.4172. [DOI] [PubMed] [Google Scholar]
- Hadley B, Maggioni A, Ashikov A, Day CJ, Haselhorst T, Tiralongo J. Structure and function of nucleotide sugar transporters: Current progress. CSBJ. 2014;10(16):23–32. doi: 10.1016/j.csbj.2014.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartenian E, Doench JG. Genetic screens and functional genomics using CRISPR/Cas9 technology. FEBS J. 2015;282:1383–1393. doi: 10.1111/febs.13248. [DOI] [PubMed] [Google Scholar]
- Hayward RD, Cain RJ, McGhie EJ, Phillips N, Garner MJ, Koronakis V. Cholesterol binding by the bacterial type III translocon is essential for virulence effector delivery into mammalian cells. Molecular Microbiology. 2005;56:590–603. doi: 10.1111/j.1365-2958.2005.04568.x. [DOI] [PubMed] [Google Scholar]
- Hazen TH, Lafon PC, Garrett NM, Lowe TM, Silberger DJ, Rowe LA, Frace M, Parsons MB, Bopp CA, Rasko DA, Sobecky PA. Insights into the environmental reservoir of pathogenic Vibrio parahaemolyticus using comparative genomics. Front Microbiol. 2015;6:204. doi: 10.3389/fmicb.2015.00204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamhi E, Joo EJ, Dordick JS, Linhardt RJ. Glycosaminoglycans in infectious disease. Biol Rev Camb Philos Soc. 2013;88:928–943. doi: 10.1111/brv.12034. [DOI] [PubMed] [Google Scholar]
- Kamiyama S, Ichimiya T, Ikehara Y, Takase T, Fujimoto I, Suda T, Nakamori S, Nakamura M, Nakayama F, Irimura T, Nakanishi H, Watanabe M, Narimatsu H, Nishihara S. Expression and the role of 3“-phosphoadenosine 5-”phosphosulfate transporters in human colorectal carcinoma. Glycobiology. 2011;21:235–246. doi: 10.1093/glycob/cwq154. [DOI] [PubMed] [Google Scholar]
- Kamiyama S, Suda T, Ueda R, Suzuki M, Okubo R, Kikuchi N, Chiba Y, Goto S, Toyoda H, Saigo K, Watanabe M, Narimatsu H, Jigami Y, Nishihara S. Molecular cloning and identification of 3“-phosphoadenosine 5-”phosphosulfate transporter. Journal of Biological Chemistry. 2003;278:25958–25963. doi: 10.1074/jbc.M302439200. [DOI] [PubMed] [Google Scholar]
- Kodama T, Gotoh K, Hiyoshi H, Morita M, Izutsu K, Akeda Y, Park K-S, Cantarelli VV, Dryselius R, Iida T, Honda T. Two regulators of Vibrio parahaemolyticus play important roles in enterotoxicity by controlling the expression of genes in the Vp-PAI region. PLoS ONE. 2010;5:e8678. doi: 10.1371/journal.pone.0008678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kodama T, Rokuda M, Park K-S, Cantarelli VV, Matsuda S, Iida T, Honda T. Identification and characterization of VopT, a novel ADP-ribosyltransferase effector protein secreted via the Vibrio parahaemolyticus type III secretion system 2. Cell Microbiol. 2007;9:2598–2609. doi: 10.1111/j.1462-5822.2007.00980.x. [DOI] [PubMed] [Google Scholar]
- Krachler AM, Orth K. Functional characterization of the interaction between bacterial adhesin multivalent adhesion molecule 7 (MAM7) protein and its host cell ligands. J. Biol. Chem. 2011;286:38939–38947. doi: 10.1074/jbc.M111.291377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labigne-Roussel AF, Lark D, Schoolnik G, Falkow S. Cloning and expression of an afimbrial adhesin (AFA-I) responsible for P blood group-independent, mannose-resistant hemagglutination from a pyelonephritic Escherichia coli strain. Infect. Immun. 1984;46:251–259. doi: 10.1128/iai.46.1.251-259.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lafont F, Tran Van Nhieu G, Hanada K, Sansonetti P, van der Goot FG. Initial steps of Shigella infection depend on the cholesterol/sphingolipid raft-mediated CD44-IpaB interaction. EMBO J. 2002;21:4449–4457. doi: 10.1093/emboj/cdf457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Letchumanan V, Chan K-G, Lee L-H. Vibrio parahaemolyticus: a review on the pathogenesis, prevalence, and advance molecular identification techniques. Front Microbiol. 2014;5:705. doi: 10.3389/fmicb.2014.00705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu M, Chen S. A novel adhesive factor contributing to the virulence of Vibrio parahaemolyticus. Sci Rep. 2015;5:14449. doi: 10.1038/srep14449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ludington JG, Ward HD. The Cryptosporidium parvum C-Type Lectin CpClec Mediates Infection of Intestinal Epithelial Cells via Interactions with Sulfated Proteoglycans. Infect. Immun. 2016;84:1593–1602. doi: 10.1128/IAI.01410-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lühn K, Wild MK, Eckhardt M, Gerardy-Schahn R, Vestweber D. The gene defective in leukocyte adhesion deficiency II encodes a putative GDP-fucose transporter. Nat. Genet. 2001;28:69–72. doi: 10.1038/ng0501-69. [DOI] [PubMed] [Google Scholar]
- Matz C, Nouri B, McCarter L, Martinez-Urtaza J. Acquired type III secretion system determines environmental fitness of epidemic Vibrio parahaemolyticus in the interaction with bacterivorous protists. PLoS ONE. 2011;6:e20275. doi: 10.1371/journal.pone.0020275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Boyle N, Houeix B, Kilcoyne M, Joshi L, Boyd A. The MSHA pilus of Vibrio parahaemolyticus has lectin functionality and enables TTSS-mediated pathogenicity. International Journal of Medical Microbiology. 2013;303:563–573. doi: 10.1016/j.ijmm.2013.07.010. [DOI] [PubMed] [Google Scholar]
- Pacheco AR, Curtis MM, Ritchie JM, Munera D, Waldor MK, Moreira CG, Sperandio V. Fucose sensing regulates bacterial intestinal colonization. Nature. 2012;492:113–117. doi: 10.1038/nature11623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park K-S, Ono T, Rokuda M, Jang M-H, Okada K, Iida T, Honda T. Functional characterization of two type III secretion systems of Vibrio parahaemolyticus. Infect. Immun. 2004;72:6659–6665. doi: 10.1128/IAI.72.11.6659-6665.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piñeyro P, Zhou X, Orfe LH, Friel PJ, Lahmers K, Call DR. Development of two animal models to study the function of Vibrio parahaemolyticus type III secretion systems. Infect. Immun. 2010;78:4551–4559. doi: 10.1128/IAI.00461-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Portaliou AG, Tsolis KC, Loos MS, Zorzini V, Economou A. Type III Secretion: Building and Operating a Remarkable Nanomachine. Trends Biochem. Sci. 2016;41:175–189. doi: 10.1016/j.tibs.2015.09.005. [DOI] [PubMed] [Google Scholar]
- Ritchie JM, Rui H, Zhou X, Iida T, Kodoma T, Ito S, Davis BM, Bronson RT, Waldor MK. Inflammation and disintegration of intestinal villi in an experimental model for Vibrio parahaemolyticus-induced diarrhea. PLoS Pathog. 2012;8:e1002593. doi: 10.1371/journal.ppat.1002593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russo BC, Stamm LM, Raaben M, Kim CM, Kahoud E, Robinson LR, Bose S, Queiroz AL, Herrera BB, Baxt LA, Mor-Vaknin N, Fu Y, Molina G, Markovitz DM, Whelan SP, Goldberg MB. Intermediate filaments enable pathogen docking to trigger type 3 effector translocation. Nature Microbiology. 2016:16025. doi: 10.1038/nmicrobiol.2016.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Safaiyan F, Kolset SO, Prydz K, Gottfridsson E, Lindahl U, Salmivirta M. Selective effects of sodium chlorate treatment on the sulfation of heparan sulfate. Journal of Biological Chemistry. 1999;274:36267–36273. doi: 10.1074/jbc.274.51.36267. [DOI] [PubMed] [Google Scholar]
- Sarrazin S, Lamanna WC, Esko JD. Heparan Sulfate Proteoglycans. Cold Spring Harbor Perspectives in Biology. 2011;3:a004952–a004952. doi: 10.1101/cshperspect.a004952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shalem O, Sanjana NE, Hartenian E, Shi X, Scott DA, Mikkelsen TS, Heckl D, Ebert BL, Root DE, Doench JG, Zhang F. Genome-scale CRISPR-Cas9 knockout screening in human cells. Science. 2014a;343:84–87. doi: 10.1126/science.1247005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shalem O, Sanjana NE, Hartenian E, Shi X, Scott DA, Mikkelsen TS, Heckl D, Ebert BL, Root DE, Doench JG, Zhang F. Genome-Scale CRISPR-Cas9 Knockout Screening in Human Cells. Science. 2014b;343:84–87. doi: 10.1126/science.1247005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shames SR, Finlay BB. Bacterial effector interplay: a new way to view effector function. Trends in Microbiology. 2012;20:214–219. doi: 10.1016/j.tim.2012.02.007. [DOI] [PubMed] [Google Scholar]
- Sheahan K-L, Isberg RR. Identification of mammalian proteins that collaborate with type III secretion system function: involvement of a chemokine receptor in supporting translocon activity. MBio. 2015;6:e02023–e02014. doi: 10.1128/mBio.02023-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shewell LK, Harvey RM, Higgins MA, Day CJ, Hartley-Tassell LE, Chen AY, Gillen CM, James DBA, Alonzo F, Torres VJ, Walker MJ, Paton AW, Paton JC, Jennings MP. The cholesterol-dependent cytolysins pneumolysin and streptolysin O require binding to red blood cell glycans for hemolytic activity. Proc. Natl. Acad. Sci. U.S.A. 2014;111:E5312–E5320. doi: 10.1073/pnas.1412703111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi J, Zhao Y, Wang K, Shi X, Wang Y, Huang H, Zhuang Y, Cai T, Wang F, Shao F. Cleavage of GSDMD by inflammatory caspases determines pyroptotic cell death. Nature. 2015;526:660–665. doi: 10.1038/nature15514. [DOI] [PubMed] [Google Scholar]
- Skoudy A, Mounier J, Aruffo A, Ohayon H, Gounon P, Sansonetti P, Tran Van Nhieu G. CD44 binds to the Shigella IpaB protein and participates in bacterial invasion of epithelial cells. Cell Microbiol. 2000;2:19–33. doi: 10.1046/j.1462-5822.2000.00028.x. [DOI] [PubMed] [Google Scholar]
- Sugiyama T, Iida T, Izutsu K, Park K-S, Honda T. Precise region and the character of the pathogenicity island in clinical Vibrio parahaemolyticus strains. J. Bacteriol. 2008;190:1835–1837. doi: 10.1128/JB.01293-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wands AM, Fujita A, McCombs JE, Cervin J, Dedic B, Rodriguez AC, Nischan N, Bond MR, Mettlen M, Trudgian DC, Lemoff A, Quiding-Järbrink M, Gustavsson B, Steentoft C, Clausen H, Mirzaei H, Teneberg S, Yrlid U, Kohler JJ. Fucosylation and protein glycosylation create functional receptors for cholera toxin. Elife. 2015;4:e09545. doi: 10.7554/eLife.09545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang T, Wei JJ, Sabatini DM, Lander ES. Genetic screens in human cells using the CRISPR-Cas9 system. Science. 2014;343:80–84. doi: 10.1126/science.1246981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu X, Nagarajan H, Lewis NE, Pan S, Cai Z, Liu X, Chen W, Xie M, Wang W, Hammond S, Andersen MR, Neff N, Passarelli B, Koh W, Fan HC, Wang J, Gui Y, Lee KH, Betenbaugh MJ, Quake SR, Famili I, Palsson BO, Wang J. The genomic sequence of the Chinese hamster ovary (CHO)-K1 cell line. Nat. Biotechnol. 2011;29:735–741. doi: 10.1038/nbt.1932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou X, Massol RH, Nakamura F, Chen X, Gewurz BE, Davis BM, Lencer WI, Waldor MK. Remodeling of the intestinal brush border underlies adhesion and virulence of an enteric pathogen. Mbio. 2014;5 doi: 10.1128/mBio.01639-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.