Abstract
Although outcome inequalities for non-Hispanic Black (NHB) kidney transplant recipients are well documented, there is paucity in data assessing the impact of cardiovascular disease (CVD) risk factors on this disparity in kidney transplantation. This was a longitudinal study of a national cohort of veteran kidney recipients transplanted between Jan 2001 and Dec 2007. Data included baseline characteristics acquired through the USRDS linked to detailed clinical follow up information acquired through the VA electronic health records. Analyses were conducted using sequential multivariable modeling (Cox regression), incorporating blocks of variables into iterative nested models; 3,139 patients were included (2,095 NHW [66.7%] and 1,044 NHBs [33.3%]). NHBs had a higher prevalence of hypertension (100% vs. 99%, p<0.01) and post-transplant diabetes (59% vs. 53%, p<0.01) with reduced control of hypertension (BP <140/90, 60% vs. 69% p<0.01), diabetes (A1c <7%, 35% vs. 47%, p<0.01) and LDL (<100 mg/dL, 55% vs. 61%, p<0.01). Adherence to medications used to manage CVD risk was significantly lower in NHBs. In the fully adjusted models, the independent risk of graft loss in NHBs was substantially reduced (unadjusted HR 2.00 vs. adjusted HR 1.49). CVD risk factors and control reduced the influence of NHB race by 9–18%. Similar trends were noted for mortality and estimates were robust across in sensitivity analyses. These results demonstrate that NHB kidney transplant recipients have significantly higher rates of CVD risk factors and reduced CVD risk control. These issues are likely partly related to medication non-adherence and meaningfully contribute to racial disparities for graft outcomes.
Keywords: cardiovascular disease, African American, kidney transplant, medication non-adherence, hypertension, graft loss, racial disparities
INTRODUCTION
Kidney transplantation is a life-prolonging procedure that substantially improves quality of life in those with end stage renal disease (ESRD).1–3 A recent analysis of the United Network for Organ Sharing (UNOS) registry demonstrated that over the past 25 years, kidney transplantation has led to an estimated 1.37 million life-years saved, when compared to patients remaining on the waiting list.2 However, because kidney graft survival is significantly shorter in African-American (AA) recipients and graft function is highly correlated with mortality, this survival advantage is likely substantially lower in AA recipients.4 AA kidney transplant recipients have significantly higher rates of acute rejection and graft loss. The average kidney transplant functions just over half as long in an AA patient.5 Despite 40 years of focused research endeavors into this disparity, little has changed in this racial inequality. In 1977, Opelz et al demonstrated a 10% absolute difference in three-year graft survival rates between AA and Caucasian recipients (25% vs. 35%, p<0.001).6 In the 2012 SRTR annual report, the graft survival inequity between AA and non-AA recipients was 12% at five years post-transplant.5
Racial disparities in kidney transplantation have primarily been attributed to biologic and immunologic differences leading to higher rejection rates,7–10 socioeconomic status (SES) disadvantages,11,12 reduced access to healthcare, medication non-adherence,13,14 and comorbidities.15–17 Research focused on reducing the higher acute rejection rates in AA transplant recipients has been successful, yet graft loss disparities persist. AA renal transplant recipients have disadvantageous immunologic characteristics placing them at higher risk for graft loss.18–23 Therefore, most of the past research trying to eliminate outcome disparities in AA patients was focused on reducing acute rejection rates through immunosuppressant pharmacotherapy.24,25 The impact of SES and medication adherence on racial disparities has been well-studied with conflicting results.12,26–31
A potentially significant but under-studied factor impacting racial disparities in kidney transplant outcomes is cardiovascular disease (CVD) and CVD risk factors. In the general population, AAs have a significantly higher prevalence of diabetes and hypertension, which occur at an earlier age with a more progressive phenotype.32–37 Yet studies assessing the impact of CVD risk factors control on racial disparities in transplantation are quite limited.38,39 Because of this discrepancy in evidence, addressing this area of potential risk is likely to provide strong opportunities to assess modifiable factors that may influence racial disparities in transplant. Thus, the objective of this study was to assess the burden of CVD risk factors and risk control in AA renal transplants and determine the influence of CVD risk factors on racial disparities for long-term post-transplant outcomes.
CONCISE METHODS
Study Design and Patients
This was a longitudinal cohort study of national data obtained through developing a unique dataset by linking the United States Renal Data System (USRDS) and Veterans Affairs (VA) electronic health records. The study population included veteran recipients of solitary kidneys, transplanted between January 1, 2001 and December 31, 2007 (7 years) with longitudinal follow up through December 31, 2010. Pediatrics, non-renal transplant recipients, those that were not NHW or NHB, transplant events that occurred outside the study time frame and those with graft loss or follow up <1 year were excluded. After local IRB, VA HSR&D and USRDS approval, the VA system was queried for eligible kidney transplant recipients using ICD-9 codes (V42.0 or 996.81) without a history of a liver, heart, lung, pancreas or intestine transplant. This list of patients was submitted to the USRDS which linked data through scrambled social security numbers to create the study cohort which contained detailed baseline and clinical follow up data. Further details regarding the data sources to create this cohort have previously been published.40
Outcome Measures
The primary outcome for this analysis was graft loss, which was defined as either a return to chronic dialysis or retransplant. For this outcome, death was considered a competing risk event and accounted for through modeling. The outcome was assessed as a time to event analyses, with the reference time being the date of transplant and the end time being the date of either graft loss (event), death (competing event) or the end of the study period (censored). All-cause mortality was a secondary outcome.
Exposure Measurements
There were two primary exposures for this study, race and CVD risk factors and risk control. The study cohort was restricted to two racial groups, NHWs and NHBs. Race information was gathered from the USRDS dataset and cross-validated with the VA vital records data. CVD risk factors and control was focused on the three predominant risks: hypertension, diabetes and dyslipidemia. Hypertension was defined as a blood pressure of greater than 140/90 mmHg or the use of anti-hypertensive medications. Diabetes was defined as a hemoglobin A1c of >7% or use of anti-glycemic therapy. Dyslipidemia was defined as a LDL of >100 mg/dL, triglyceride level of >150 mg/dL or use of anti-lipemic therapy. These were defined based on national standards and guidelines specific to transplant.41–44
CVD risk factor control assessment was defined as follows: uncontrolled diabetes was classified as a mean follow up A1c of ≥8% (referent group) vs. <8% (also assessed A1c of ≥7% vs. <7% as well). Uncontrolled hypertension control was classified as a mean follow up BP ≥140/90 (referent group) vs BP <140/90 mmHg. For uncontrolled lipids, patients were classified as a mean follow up LDL ≥100 mg/dL or TG ≥150 mg/dL (referent group), vs. LDL<100 mg/dL and TG <150 mg/dL. Medications to treat CVD comorbidities were grouped according to class and patients were categorized based on the prescribing of this agent (yes vs no) and their adherence to the therapy. Medication possession ratios (MPRs) were calculated using patient and medication-specific sums for days’ supply (numerator) divided by total days in the year (denominator) and aggregated by year post-transplant, using previously validated algorithms.45,46 For modeling, MPR was averaged for the entire follow up time and dichotomized as <0.8 vs. ≥0.8 to classify patients as adherent vs. non-adherent; 0.8 has been well established as a valid cut point to define medication adherence as it correlates with outcomes.47 Medications were groups as follows: oral anti-hyperglycemic agents, insulin, HMG CoA reductase inhibitors (statins), other anti-lipid therapy, beta blockers, angiotensin converting enzyme inhibitors [ACE inh] or angiotensin receptor blockers [ARBs], calcium channel blockers (CCBs), diuretics and anti-platelet therapies.
Additional covariates that were included in multivariable models included recipient sociodemographics (age, gender and marital status), recipient comorbidities (history of CAD/angina, previous transplant and years on dialysis), donor characteristics (living donor, expanded criteria donor [ECD] and donor after cardiac death [DCD]), immunologic characteristics (HLA mismatches, current panel reactive antibody level [PRA]), baseline immunosuppression (induction therapy, calcineurin inhibitors, adjunctive agents and corticosteroids) and post-transplant events (delayed graft function [DGF] and acute rejection).
Statistical Analysis
Univariate analyses compared baseline variables and clinical outcomes by race (NHBs vs NHWs), assessed the baseline and follow up prevalence and control of hypertension, diabetes and dyslidemia and determined CVD medication use and adherence using the chi square test, the Student’s t-test or Mann Whitney U test, depending on data type and normality assumptions. Multivariable modeling was performed using Cox proportional hazard regression, using the Fine and Gray competing risk model for graft loss to estimate the cumulative incidence function (CIF) and standard Cox models to estimate the survival function for death.48 Model assumptions of proportionality of hazard over time and linearity for continuous variables were first tested and confirmed, followed by residual assessments to ensure we used the most appropriate data fit and to identify any potential outliers or influential observations.
Sequential forward entry of blocks of variables were added to the model and at each step the overall impact of NHB race on the outcome was assessed by comparing the change in β to the previous model. The Akaike information criterion (AIC) was used to assess and compare models as the goodness of fit measure. Large decreases in the AIC after the addition of a block of variables suggested strong influence of that block on explaining the variability associated with the outcome. Covariance between repeated transplants in the same individual was accounted for during modelling to prevent under-estimating variance. Sensitivity analyses were conducted to ensure the robustness of the estimates, which included standard Cox regression vs. competing risk modeling, varying the entry of variable blocks during sequential modelling, using multiple imputation for missing data and using longitudinal analyses through joint modelling to first estimate individual-level random intercepts and slopes for A1c, BP and lipid levels, followed by entry of these estimates in Cox models. As a final sensitivity analysis, we also assessed the impact of BP control in means and longitudinal models with and without anti-hypertensive therapies as covariates. Effect modification was assessed by entering interaction terms in the model, with the primary variables of interest being race, delayed graft function, rejection and CVD risk factors. All data analyses were conducted using SAS (version 9.4, SAS Institute Inc, Cary, NC). Statistical significance was defined using two-sided tests with α set at 0.05.
RESULTS
There were 5,757 kidney transplants performed between Jan 1, 2001 and Dec 31, 2007 that met inclusion criteria and were capable of being linked between the VA and USRDS databases. Of these, 382 were excluded for being Hispanic, 112 were excluded for being Asian or other races, 345 were excluded for receiving a transplant outside the time period and 275 for excluded for having graft loss or follow up <1 year post-transplant, leaving 4,643 eligible for inclusion; 1,504 were missing data for at least one variable, leaving 3,139 cases with complete data, which included NHWs (66.5%) and NHBs (33.5%, see Figure 1 for the study Consort flow diagram). Baseline sociodemographics, donor criteria and transplant characteristics are summarized and compared between NHWs vs. NHBs in Table 1. At the time of transplant, NHBs were significantly younger (59.5±10.0 vs. 57.5±10.4 years), less likely to be married (57.3% vs. 68.4%), had differences in the level of education (finished high school and attended college), differences in the primary cause of ESRD (hypertension, diabetes, and FSGS) and pre-transplant comorbidities (coronary artery disease, hypertension and previous transplant). There were also significant differences between NHBs and NHWs for donor criteria (age, gender, race, less living donors and more donors after cardiac death), immunologic risks (more HLA mismatches, longer cold ischemic times), baseline immunosuppression and post-transplant outcomes; particularly death censored graft loss (NHBs 14.7% vs. NHWs 8.0%), acute rejection (NHBs 14.8% vs. NHWs 11.4%) and delayed graft function (NHBs 24.0% vs. NHWs 11.7%).
Figure 1.
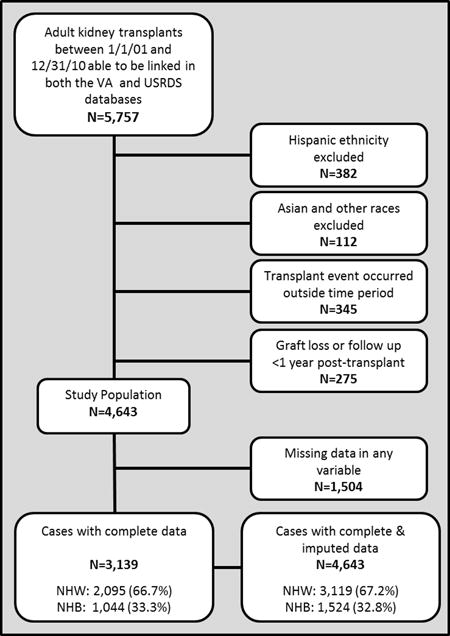
Flowchart summarizing the creation of the study cohort of NHW and NHB adult solitary kidney transplants from Jan 1, 2001 to Dec 31, 2007 with data available within the VA Health System
Table 1.
Baseline and outcomes variables for the entire cohort and stratified by recipient race
| Characteristics | Total Cases with Complete Data (n=3,139) | Non-Hispanic Whites (n=2,095) | Non-Hispanic Blacks (n=1,044) |
|---|---|---|---|
| Age (mean±SD) | 57.5±10.4 | 59.5±10.0 | 53.6±10.1* |
| Male Gender | 98.0% | 98.2% | 97.7% |
| Married | 64.6% | 68.4% | 57.3%* |
| Education, n=2,660 | |||
| Less than high school | 1.6% | 2.1% | 0.6%* |
| High school | 47.0% | 46.7% | 47.6% |
| Attended college/technical school | 29.8% | 28.3% | 32.9%* |
| Received associate/bachelor degree | 16.5% | 17.5% | 14.5% |
| Attended graduate school | 5.1% | 5.4% | 4.5% |
| Primary Cause of ESRD | |||
| Hypertension | 26.8% | 18.5% | 43.4%* |
| Diabetes | 29.0% | 30.2% | 26.4%* |
| Focal Segmental Glomerulosclerosis | 6.7% | 5.3% | 9.6%* |
| Any type of Glomerulonephritis | 13.2% | 12.6% | 14.6% |
| Comorbidities | |||
| Angina or Coronary Artery Disease | 13.0% | 15.3% | 8.5%* |
| Hypertension | 93.7% | 92.3% | 96.4%* |
| Diabetes | 38.5% | 38.4% | 38.8% |
| Previous Kidney Transplant | 3.4% | 4.3% | 1.6%* |
| Donor Information | |||
| Age (mean±SD) | 40.1±14.9 | 40.5±14.9 | 39.1±14.8* |
| Living | 34.5% | 39.7% | 23.9%* |
| Female | 47.4% | 49.3% | 43.6%* |
| Non-Hispanic Black | 15.1% | 4.3% | 36.9%* |
| Expanded Criteria Donor | 13.4% | 13.3% | 13.6% |
| Donor after Cardiac Death | 4.6% | 3.9% | 5.9%* |
| Immunologic Risks | |||
| Cold Ischemic Time (mean hrs±SD), n=2,575 | 14.2±10.4 | 13.3±10.5 | 15.8±10.0* |
| Warm Ischemic Time (mean min±SD), n=1,972 | 40.1±24.6 | 39.5±24.7 | 41.3±24.2 |
| HLA mismatches (mean±SD) | 3.4±1.8 | 3.2±1.8 | 4.0±1.5* |
| Panel Reactive Antibody (mean±SD) | 2.7±11.1 | 2.6±10.9 | 3.0±11.5 |
| Years on Dialysis (mean±SD) | 2.3±2.5 | 1.9±2.1 | 3.3±2.9* |
| Pre-emptive Transplant | 21.0% | 24.5% | 14.1%* |
| Baseline Immunosuppression | |||
| IL-2 Receptor Antagonist Antibody Induction | 31.5% | 34.5% | 25.6%* |
| Cytolytic Induction | 34.1% | 31.6% | 39.2%* |
| Tacrolimus | 71.9% | 70.2% | 75.5%* |
| Cyclosporine | 22.1% | 23.7% | 18.8%* |
| Mycophenolate | 86.6% | 86.7% | 86.2% |
| Azathioprine | 1.5% | 1.8% | 0.9%* |
| mTOR | 10.3% | 10.2% | 10.4% |
| Prednisone | 94.5% | 94.5% | 94.5% |
| Clinical Outcomes | |||
| Death | 19.6% | 21.7% | 15.4%* |
| Overall Graft Loss | 25.1% | 24.9% | 25.6% |
| Death-Censored Graft Loss | 10.2% | 8.0% | 14.7%* |
| Acute Rejection | 12.5% | 11.4% | 14.8%* |
| Delayed Graft Function | 15.8% | 11.7% | 24.0* |
p<0.05 comparing NHB vs. NHW
The prevalence and control of CVD risk factors at baseline and at 1, 3 and 5 years post-transplant significantly differed by race (see Table 2). At baseline, NHBs had a higher prevalence of hypertension (96.4% vs. 92.3%) and lower prevalence of dyslipidemia (40.7% vs. 46.4%). During post-transplant follow-up, the prevalence of hypertension remained significantly higher in NHBs at 1, 3 and 5 years, while the prevalence of diabetes significantly increased in NHBs, as compared to NHWs. Dyslipidemia prevalence substantially increased in both groups, but always remained higher in NHWs until 5 years post-transplant. The baseline and post-transplant control of hypertension, diabetes and LDL was significantly better in NHWs vs. NHBs at most time points. The use of medication classes to treat CVD risk factors and adherence to these medications also differed by race. NHBs were more likely to be prescribed anti-hypertensive therapy but, in general, had lower rates of adherence to these therapies. This was also demonstrated for post-transplant insulin therapy. Post-transplant statin prescribing was similar, but adherence to this therapy was lower in NHBs. Figure S1 and figure S2 display the data from Table 2 in graphical format.
Table 2.
CVD risk factor prevalence and control, compared between NHB and NHW adult kidney transplant recipients
| CVD Variable | Baseline | One Year | Three Years | Five Years | ||||
|---|---|---|---|---|---|---|---|---|
| NHW | NHB | NHW | NHB | NHW | NHB | NHW | NHB | |
| Prevalence of Hypertension† | 92.3% | 96.4%‡ | 97.2% | 99.0%‡ | 98.7% | 99.9%‡ | 99.2% | 100.0%‡ |
| Prevalence of Diabetes§ | 38.4% | 38.8% | 46.9% | 50.3% | 49.4% | 52.9% | 53.3% | 58.9%‡ |
| Prevalence of Dyslipidemia‖ | 46.4% | 40.7%‡ | 69.2% | 63.3%‡ | 81.4% | 77.2%‡ | 86.3% | 83.9% |
| Mean Systolic BP (±SD) | 140±18 | 141±19 | 137±14 | 139±15‡ | 136±12 | 138±12* | 135±11 | 137±12‡ |
| Mean Diastolic BP (±SD) | 75±10 | 79±11‡ | 73±9 | 76±9‡ | 73±8 | 76±8‡ | 73±8 | 77±8‡ |
| Blood Pressure <140/90 mmHg | 51% | 46%* | 60% | 52%‡ | 65% | 58%‡ | 69% | 60%‡ |
| Mean Hemoglobin A1c (±SD) | 6.6±1.3 | 6.5±1.5 | 7.1±1. | 7.8±1.7‡ | 7.2±1.3 | 7.8±1.6‡ | 7.2±1.2 | 7.7±1.6‡ |
| Hemoglobin A1c <7%¶ | 57% | 61% | 53% | 35%‡ | 46% | 31%‡ | 47% | 35%‡ |
| Hemoglobin A1c <8%¶ | 92% | 93% | 87% | 78%‡ | 83% | 74%‡ | 86% | 83% |
| Mean LDL (±SD) | 86±32 | 86±31 | 100±34 | 103±34 | 97±30 | 102±32‡ | 95±28 | 99±30‡ |
| LDL <100 mg/dL | 72% | 70% | 52% | 51% | 56% | 52%* | 61% | 55%‡ |
| Mean TG (±SD) | 200±149 | 164±105‡ | 198±150 | 161±104‡ | 194±126 | 160±98‡ | 187±120 | 154±86‡ |
| TG < 150 mg/dL | 46% | 57%‡ | 44% | 59%‡ | 43% | 57%‡ | 45% | 60%‡ |
| Prescribed ACE inhibitor or ARB# | 48% | 50% | 35% | 38%* | 50% | 54%* | 56% | 61%‡ |
| MPR ≥80% | 23% | 18%* | 56% | 49%* | 53% | 51% | 52% | 40%‡ |
| Prescribed Beta Blocker# | 49% | 51% | 59% | 64%* | 70% | 73%* | 73% | 78%* |
| MPR ≥80% | 34% | 23%‡ | 61% | 56%* | 56% | 47%‡ | 51% | 46% |
| Prescribed Oral Anti-hyperglycemic¶ | 34% | 35% | 30% | 33% | 36% | 38% | 39% | 42% |
| MPR ≥80% | 17% | 19% | 60% | 49%* | 44% | 47% | 49% | 43% |
| Prescribed Insulin¶ | 48% | 44% | 62% | 66% | 70% | 73% | 70% | 74% |
| MPR ≥80% | 69% | 45%‡ | 86% | 78%‡ | 83% | 82% | 84% | 76%* |
| Prescribed Statin** | 84% | 80%* | 74% | 70% | 77% | 76% | 78% | 78% |
| MPR ≥80% | 37% | 22%‡ | 62% | 52%‡ | 57% | 50%* | 58% | 43%‡ |
| Prescribed Other Anti-Lipemic** | 19% | 17% | 13% | 7%‡ | 19% | 12%‡ | 20% | 12%‡ |
| MPR ≥80% | 18% | 7%‡ | 58% | 60% | 46% | 43% | 51% | 40% |
| Prescribed Anti-platelet | 10% | 7%‡ | 7% | 5% | 10% | 7%‡ | 13% | 9%‡ |
| MPR ≥80% | 27% | 21% | 51% | 42% | 51% | 35%* | 44% | 24% |
p<0.05
p<0.01
Defined as documentation, blood pressure >140/90 mmHg or treatment with anti-hypertensive therapy
Defined as documentation, hemoglobin A1c >7% or treatment with anti-glycemic therapy
Defined as a LDL >130 mg/dL, TG >150 mg/dL or treatment with anti-lipemic therapy
Only in those with a diagnosis of diabetes
Only in those with a diagnosis of hypertension
Only in those with a diagnosis of dyslipidemia
The fully adjusted model for the primary outcome of graft loss is displayed in Table 3, demonstrating that after adjusting for all baseline and follow up variables, including CVD risk and control, NHB race continued to be a significant risk factor (HR 1.49, 1.11–1.99, p=0.0072). The fully adjusted model reduced the risk associated with NHB race by 26%. Beyond race, there were seven variables that were significant independent predictors of graft loss. This included receiving an expanded criteria donor kidney (HR 1.39, 1.01–1.91, p=0.0455), receiving and being adherent to ACE inh/ARB therapy (HR 0.45, 0.29–0.68, p=0.0002), being non-adherent to diuretic therapy (HR 1.96, 1.49–2.57, p<0.0001), being non-adherent to insulin therapy (HR 1.59, 1.02–2.47, p=0.0414), receiving and being adherent to statin therapy (HR 0.53, 0.36–0.80, p=0.0022), uncontrolled hypertension (HR 1.64, 1.29–2.07, p<0.0001) and developing acute allograft rejection (HR 1.91, 1.46–2.51, p<0.0001).
Table 3.
Fully adjusted competing risk model for the primary outcome of graft loss
| Variable | Reference | Hazard Ratio | 95% CI | p-Value |
|---|---|---|---|---|
| NHB Race | NHW Race | 1.490 | 1.11–1.99 | 0.0072 |
| Age (per year) | 18 years old | 0.997 | 0.99–1.01 | 0.6683 |
| Female Gender | Male Gender | 1.132 | 0.52–2.48 | 0.7570 |
| Not Married | Married | 0.968 | 0.76–1.23 | 0.7933 |
| Angina or CAD History | No Cardiac History | 0.947 | 0.68–1.33 | 0.7550 |
| Previous Transplant | No Previous Transplant | 1.255 | 0.68–2.33 | 0.4705 |
| Living Donor | Deceased Donor | 0.897 | 0.67–1.20 | 0.4581 |
| Expanded Criteria Donor | Standard Deceased Donor | 1.387 | 1.01–1.91 | 0.0455 |
| Cardiac Death Donor | Brain Dead Donor | 1.150 | 0.62–2.12 | 0.6536 |
| NHB Donor | Any Other Donor Race | 1.087 | 0.79–1.50 | 0.6088 |
| HLA Mismatches – 0 | 1–5 HLA Mismatches | 0.964 | 0.64–1.46 | 0.8626 |
| MLA Mismatches – 6 | 1.204 | 0.86–1.68 | 0.2784 | |
| PRA 0% | PRA 1–20% | 1.056 | 0.73–1.53 | 0.7715 |
| PRA >20% | 0.977 | 0.50–1.91 | 0.9463 | |
| Years on Dialysis | 0 Years | 1.044 | 1.00–1.09 | 0.0739 |
| Preemptive Transplant | On Dialysis | 0.896 | 0.63–1.28 | 0.5474 |
| IL-2 Receptor Antagonist Induction | No Induction Therapy | 0.986 | 0.74–1.31 | 0.9223 |
| Cytolytic Induction | 0.916 | 0.69–1.22 | 0.5525 | |
| Tacrolimus Maintenance Therapy | No Calcineurin Inhibitor Therapy | 0.720 | 0.44–1.17 | 0.1877 |
| Cyclosporine Maintenance Therapy | 0.845 | 0.51–1.41 | 0.5186 | |
| Mycophenolate Maintenance Therapy | No Adjunct Agent | 0.935 | 0.65–1.35 | 0.7213 |
| Azathioprine Maintenance Therapy | 0.762 | 0.29–2.02 | 0.5849 | |
| mTOR Maintenance Therapy | No mTOR | 0.755 | 0.49–1.17 | 0.2046 |
| Corticosteroids Maintenance Therapy | No Corticosteroids | 1.422 | 0.75–2.68 | 0.2764 |
| ACE inh/ARB Therapy MPR 0–79% | No ACE inhibitor or ARB Therapy | 0.959 | 0.74–1.24 | 0.7455 |
| ACE inh/ARB Therapy MPR ≥80% | 0.446 | 0.29–0.68 | 0.0002 | |
| Beta Blocker Therapy MPR 0–79% | No Beta Blocker Therapy | 1.363 | 0.99–1.88 | 0.0599 |
| Beta Blocker Therapy MPR ≥80% | 0.934 | 0.64–1.37 | 0.7276 | |
| Calcium Channel Blocker MPR 0–79% | No Calcium Channel Blocker Therapy | 1.238 | 0.93–1.65 | 0.1488 |
| Calcium Channel Blocker MPR ≥80% | 0.946 | 0.64–1.39 | 0.1488 | |
| Diuretic Therapy MPR 0–79% | No Diuretic Therapy | 1.956 | 1.49–2.57 | <0.0001 |
| Diuretic Therapy MPR ≥80% | 1.025 | 0.63–1.67 | 0.9207 | |
| Anti-Platelet Therapy MPR 0–79% | No Anti-Platelet | 1.198 | 0.83–1.73 | 0.3376 |
| Anti-Platelet Therapy MPR ≥80% | 1.218 | 0.64–2.32 | 0.5486 | |
| Insulin Therapy MPR 0–79% | No Insulin Therapy | 1.585 | 1.02–2.47 | 0.0414 |
| Insulin Therapy MPR ≥80% | 1.220 | 0.80–1.86 | 0.3522 | |
| Oral Anti-Hyperglycemic Therapy MPR 0–79% | No Oral Anti-Hyperglycemic Therapy | 1.191 | 0.85–1.66 | 0.3021 |
| Oral Anti-Hyperglycemic Therapy MPR ≥80% | 0.766 | 0.40–1.48 | 0.4258 | |
| Statin Therapy MPR 0–79% | No Statin Therapy | 0.836 | 0.62–1.14 | 0.2529 |
| Statin Therapy MPR ≥80% | 0.534 | 0.36–0.80 | 0.0022 | |
| Other Dyslipidemia Therapy MPR 0–79% | No Other Dyslipidemia Therapy | 1.240 | 0.89–1.73 | 0.2088 |
| Other Dyslipidemia Therapy MPR ≥80% | 1.464 | 0.80–2.67 | 0.2149 | |
| Diabetes | No Diabetes | 0.657 | 0.43–1.01 | 0.0541 |
| Dyslipidemia | No Dyslipidemia | 0.851 | 0.56–1.29 | 0.4454 |
| Uncontrolled Dyslipidemia | Dyslipidemia Controlled | 1.083 | 0.83–1.42 | 0.5607 |
| Uncontrolled Diabetes | Diabetes Controlled | 0.831 | 0.58–1.19 | 0.3167 |
| Uncontrolled Hypertension | Hypertension Controlled | 1.636 | 1.29–2.07 | <0.001 |
| Delayed Graft Function | No Delayed Graft Function | 0.925 | 0.68–1.27 | 0.6248 |
| Acute Rejection | No Acute Rejection | 1.914 | 1.46–2.51 | <0.001 |
Table 4 summarizes the results for the sequential modelling for the primary outcome of graft loss with varied entry of the blocks of variables through iterative processes. The results demonstrate that donor characteristics (−13.4 to −4.2%), immunologic risks (−9.6 to −4.7%), CVD risks and control (−17.5 to −8.7%), and post-transplant events (−5.5 to −0.7%) had the largest impact on reducing the independent risks associated of NHB race on graft loss. CVD risk factors and risk control produced the largest reduction in the model AIC, suggesting that including these variables in the model substantially improved the model performance in capturing the variability associated with graft loss by enhancing model goodness of fit. Figure 2 displays the cumulative incidence estimates for graft loss, comparing these curves between NHBs vs. NHWs for the unadjusted model (Figure 2A) and the fully adjusted model (Figure 2B). After full adjustment, the estimated incidence curves significantly converge.
Table 4.
Comparison of hazard ratios for NHBs vs. NHWs and assessment of model fit across all sequential competing risk modeling analyses for the primary outcome of graft loss
| Model | Domain | Fixed Entry | Varied Entry* | ||||
|---|---|---|---|---|---|---|---|
| HR for NHB vs. NHW | 95% CI | p-Value | Model AIC | Relative change in NHB HRs vs. NHW as compared to previous model | Change in AICs from previous model | ||
| Model 1 | Race Only | 1.999 | 1.61–2.49 | <0.0001 | 4868 | NA | NA |
| Model 2 | +Sociodemographics | 2.054 | 1.64–2.57 | <0.0001 | 4875 | −2.8 to 2.1% | 5 to 7 |
| Model 3 | +Comorbidities | 2.057 | 1.65–2.57 | <0.0001 | 4877 | 0.1 to 1.3% | 2 to 4 |
| Model 4 | +Donor Characteristics | 1.781 | 1.36–2.32 | <0.0001 | 4870 | −13.4 to −4.2% | −7 to 3 |
| Model 5 | +Immunologic Risks | 1.697 | 1.30–2.22 | 0.0001 | 4878 | −9.6 to −4.7% | 6 to 8 |
| Model 6 | +Immunosuppression | 1.691 | 1.29–2.22 | 0.0001 | 4885 | −0.4 to 0.7% | 7 to 9 |
| Model 7 | +CVD Risk Factors & Control | 1.501 | 1.12–2.01 | 0.0059 | 4773 | −17.5 to −8.7% | −118 to −106 |
| Model 8 | +Post-Transplant Events | 1.490 | 1.11–1.99 | 0.0072 | 4757 | −5.5 to −0.7% | −28 to −16 |
Varied entry was conducted using iterative modeling and changing the introduction of the blocks of variables into the model in order to determine their impact on racial disparities. For all scenarios, race was initially entered into the model (unadjusted risk), followed by entry of blocks of variables which varied across different iterations.
Figure 2.
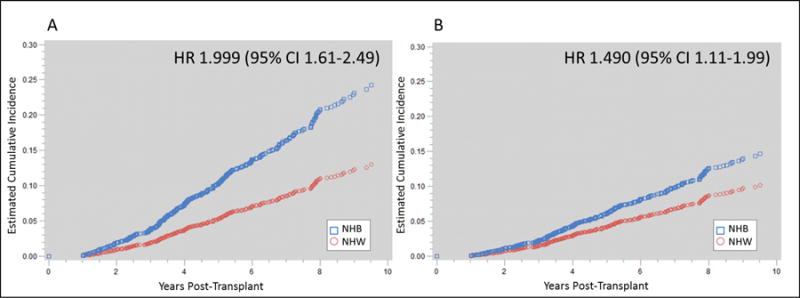
Cumulative incidence function curve estimates for the primary outcome of graft loss in NHB vs. NHW kidney transplants for the unadjusted model (Figure 2A) and the fully adjusted model (Figure 2B) demonstrating a 24% relative reduction in risk for NHBs when controlling for all measured variables
The results for the secondary outcomes of mortality are displayed in table S1 (full model), table S2 (sequential modeling) and figure S3 (unadjusted and fully adjusted survival estimates). The full model for mortality demonstrated that NHB race was a significant protective factor for risk of post-transplant death (HR 0.75, 0.60–0.94, p=0.0132). Additional independent risk factors for death included not being married, deceased donor, mycophenolate therapy, mTOR therapy, non-adherence to CVD medications, the presence of diabetes and uncontrolled hypertension (table S1). Sequential modeling for death demonstrated that sociodemographics (predominantly age) were a significant suppressor, while donor characteristics, immunologic risks and CVD risk factors and control were all significant explanatory variables. Similar to modeling for other outcomes, CVD risk factors and control produced the largest reduction in AIC, suggesting it explains a substantial portion of the variability associated with the risk of death after transplant.
Five sensitivity analyses were performed to ensure robustness of the estimates from the modelling. This included varied entry of blocks of variables into the sequential modelling, using standard Cox regression for the outcome of graft loss, modeling random and fixed effects for CVD risk factor control variables through joint modeling, assessing BP control with and without anti-hypertensives included in the modelling and using multiple imputation for missing data. The results of these analyses demonstrated consistent estimates, trends and results. The full model for the outcome of graft loss with the imputed missing data (n=4,643) is displayed in table S4, with the rate of missingness in table S3. Race continued to be an independent risk factor for graft loss and after adjustment, the reduction in risk for NHBs was similar to the analysis using cases with complete data (25.5% vs. 27.8%); in the imputed model, similar to the model using cases with complete data, CVD risk factors and CVD risk control had the largest impact on model performance and significantly reduced the independent influence of NHB race on graft loss. In the final sensitivity analysis, the estimated impact of uncontrolled hypertension on graft loss was similar in both longitudinal and means models, with and without the inclusion on anti-hypertensive medications (means model with anti-hypertensive medications HR 1.64 (1.29–2.07), without anti-hypertensive medications HR 1.79 (1.42–2.25); longitudinal model with anti-hypertensive medications intercept HR 3.04 (1.73–5.33), slope HR 3.20 (1.07–9.54, without anti-hypertensive medications intercept HR 3.12 (1.80–5.40) slope HR 3.61 (1.24–10.54).
DISCUSSION
The results of this study demonstrate that NHB transplant recipients have a considerably larger burden of CVD risk factors, which meaningfully contribute to racial disparities in graft outcomes. Specifically, NHBs have a higher prevalence of hypertension and post-transplant diabetes with reduced control of hypertension, diabetes and LDL, when compared to NHWs. In addition, adherence to medications used to manage CVD risk factors appears to be significantly lower in NHBs, as compared to NHWs. These data provide novel information to demonstrate that focusing on improving the management of CVD risk factors within kidney transplant recipients offers a promising mechanism to both enhance graft outcomes for the entire population while also potentially impacting racial disparities. To our knowledge, this is the first study to comprehensively assess CVD risk factors and risk control in a large national cohort of contemporary U.S. kidney transplant recipients and specifically assess the impact of CVD risk factors on racial disparities.
Racial disparities for outcomes in kidney transplant recipients have been long-standing and well-documented, with the literature exposing this issue dating back to the late 1970s, when kidney transplantation was still considered an experimental procedure. Recent evidence suggests that over the past 20 years, the absolute disparity in graft survival has slightly improved. Yet, in relative terms, NHBs are still at substantially higher risk of late graft loss (5 year relative risk of graft loss is 2.2 in living donors and 1.8 in deceased donors, comparing NHBs to NHWs).49 Some aspects that contribute to this disparity have been well-studied. It is clear that NHBs have unique immunologic characteristics that increase their risk of acute rejection and have socioeconomic disadvantages which create barriers to access to transplant, access to living donors and access to optimal post-transplant care.12,50 Thus, to date, most of the interventions designed to reduce this disparity have focused on using potent immunosuppression to mitigate immunologic risk and improve access to deceased and living donor transplants.25 There have been some documented successes in this capacity. Recent evidence suggests that acute rejection rate disparities in NHBs have been mostly resolved and access to deceased donor organ transplant is improving in NHBs. However, improving living donation rates within the NHB community has been an area of marginal regional success, with national data demonstrating reduced rates of living donation within NHBs, when compared to NHWs.49,51 Our results corroborate these previous findings, in that immunologic risks and donor characteristics continue to be significant explanatory variables for racial disparities.
There is paucity in the research assessing CVD risk factors and risk factor control as a potential cluster of explanatory factors for racial disparities in kidney transplant outcomes. This is despite the fact that these risks are decidedly mutable and that there is strong evidence to suggest these issues drive disparate outcomes in the general non-transplant population with diabetes and hypertension.52,53 Previous single-center studies from our research group has demonstrated similar results, and the data from this analysis further support these findings.38,39 Hypertension, diabetes and LDL have significantly lower rates of control post-transplant in NHBs, as compared to NHWs. This is despite the increased use of anti-hypertensives in NHBs. The higher rates of medication non-adherence in these patients likely contribute to this issue, and represents an opportunity for future interventions designed to improve CVD risk factor control in NHBs. It is interesting to note that our study only included veterans obtaining medications through the VA system. Thus, the issues surrounding access and cost of medications should not be a significant factor leading to medication non-adherence in this population. Other factors, included regimen complexity, health literacy and self-efficacy may be producing the higher rates of non-adherence that were seen within NHBs in this study.53 We focused on graft loss after the first year post-transplant, because previous studies have demonstrated that racial disparities in transplantation occur later post-transplant and CVD risk factors are most likely to influence these later events.
The prescribing of two medication classes, ACE inh/ARBs and statins, demonstrated a substantial protective benefit for graft loss and death. In our study, only about 50–60% of the population was prescribed ACE inh/ARBs at five years post-transplant; and of these, only 40–50% were adherent (MPR ≥80%). For statins, about three-quarters were prescribed this therapy by five years and 40–60% were adherent. One could argue that due to the prevailing CVD and CVD risks that are highly correlated with CKD, these therapies should be prescribed to nearly all kidney transplant recipients that do not have absolute contraindications.54 Yet, data from this study and other non-VA national and single-center studies of kidney transplant recipients demonstrate the utilization of these therapies is on the rise, but remains substantially below optimal levels.55,56 Given the body of evidence in the general non-transplant population and the inherent CVD risk within the kidney transplant population, these results provide evidence to support future interventional studies designed to optimize utilization and adherence to these therapies in kidney transplant recipients as a mechanism to improve long-term graft and patient outcomes.54–56
It is also noteworthy that non-adherence to a number of medication classes was a significant and independent risk-factor for both graft loss and death. This included diuretics, insulin, beta blockers, calcium channel blockers and anti-platelet therapy. This risk was independent of CVD disease and CVD risk factor control. These results may be a true cause and effect relationship, in that medication non-adherence to these therapies may actually lead to increased rates of graft loss and death, or could also be a proxy for other risks that are highly correlated to medication non-adherence and which were not measured as a part of this study.57 In particular, these include social determinants of health (finances, living conditions, social support), health literacy and self-efficacy.58 Thus, further research is needed to ascertain whether non-adherence to these medication classes truly causes increased rates of graft loss and death in kidney transplant recipients.
There are a number of limitations to this analysis that are worthy of discussion. First, this analysis was confined to a veteran transplant population and the number of female recipients is quite low. Thus, generalizability of these results to female transplant recipients is not warranted. Using a veteran population also restricts the ability of this analysis to assess the impact of insurance coverage and access to care on outcomes. However, previous studies have demonstrated that racial disparities in kidney transplant recipients are similar in magnitude between VA and non-VA populations, and using VA data will allow us to include numerous clinical variables that are missing or not available from previous studies that solely utilized the USRDS or CMS datasets.28 Therefore, for the purposes of this analysis, which focuses on racial disparities as it relates to CVD risk factor control, the benefits provided by the use of VA data greatly outweigh the limitations of solely focusing on veteran patients.
Another limitation of this analysis is its retrospective design, which may increase the risk for confounding and misclassification, potentially biasing the results. However, because we were able to link two databases with unique and overlapping data in a longitudinal format, we dramatically minimized these risks. We validated data elements by comparing them between the two databases for accuracy and were able to include the largest number of covariates ever reported in a transplant racial disparities analysis.40 In addition, retrospectively accurately assessing control of CVD risk factors is a difficult endeavor, and using means during the entire follow up may not truly capture the time dependency of this exposure. However, using the means does allow for a more straightforward analysis and presentation of the data. To assess if longitudinal analyses improve the primary exposure classification, we also conducted a separate analysis, using a time-varying analysis technique to assess for the fixed and random effects (intercept and trajectory) of the BP, A1c, LDL and TG on the outcomes.59 The results of this assessment were similar with respect to direction and magnitude; thus, for ease of display and interpretation, we utilized the mean values, which appear to be an accurate reflection of CVD risk control. Another limitation with this analysis is that we fail to measure and account for biologic and socioeconomic differences between NHB and NHW transplant recipients that may explain some of the disparity. This includes genetic variants that are more prevalent in NHB recipients, including cytochrome P450 3A5 and apolipoprotein L1 and the aforementioned social determinants of health.60–63 Although we recognize there are a number of limitations with using a veteran dataset, the overall objectives of this analysis require the use of longitudinal clinical data that is currently unavailable within any other national dataset accessible at this level of comprehension and detail.
Perspectives
In summary, these results demonstrate that NHB kidney transplant recipients have significantly higher rates of CVD risk factors and reduced CVD risk control, as compared to NHWs. These issues may be partly related to medication non-adherence, meaningfully contribute to disparities for graft outcomes within NHBs and represent a promising area for future interventional studies designed to improve CVD risk factor control as a mechanism to optimize graft outcomes and reduce racial disparities in kidney transplantation.
Supplementary Material
Novelty and Significance.
What is new: the results provide new insights demonstrating that disparities in outcomes for African American kidney transplant recipients are significantly influenced by cardiovascular disease risk factors and risk factor control; in particular, the treatment and control of hypertension and adherence to medications used to manage this condition impact these disparities.
What is relevant: improving the management and control of cardiovascular risk factors, particularly hypertension, is likely to significantly improve outcomes in African American kidney transplant recipients and reduce disparities in graft survival rates.
Summary: these results demonstrate that African American kidney transplant recipients have significantly higher rates of cardiovascular disease risk factors and reduced cardiovascular disease risk control, as compared to Caucasians. These issues may be partly related to medication non-adherence and meaningfully contribute to disparities for graft outcomes.
Acknowledgments
Sources of Funding: Research reported in this publication was supported by the National Institute of Diabetes and Digestive and Kidney Diseases of the National Institutes of Health under Award Number K23DK099440.
Footnotes
Disclosure: The authors have no conflicts of interest to disclose as it relates to the content of this manuscript.
References
- 1.Landreneau K, Lee K, Landreneau MD. Quality of life in patients undergoing hemodialysis and renal transplantation-a meta-analytic review. Nephrol Nurs J. 2010;37:37–44. [PubMed] [Google Scholar]
- 2.Rana A, Gruessner A, Agopian VG, Khalpey Z, Riaz IB, Kaplan B, Halazun KJ, Busuttil RW, Gruessner RW. Survival benefit of solid-organ transplant in the United States. JAMA Surgery. 2015;150:252–259. doi: 10.1001/jamasurg.2014.2038. [DOI] [PubMed] [Google Scholar]
- 3.Tonelli M, Wiebe N, Knoll G, Bello A, Browne S, Jadhav D, Klarenbach S, Gill J. Systematic review: Kidney transplantation compared with dialysis in clinically relevant outcomes. Am J Transplant. 2011;11:2093–2109. doi: 10.1111/j.1600-6143.2011.03686.x. [DOI] [PubMed] [Google Scholar]
- 4.Rao PS, Schaubel DE, Jia X, Li S, Port FK, Saran R. Survival on dialysis post–kidney transplant failure: Results from the scientific registry of transplant recipients. Am J Kid Dis. 2007;49:294–300. doi: 10.1053/j.ajkd.2006.11.022. [DOI] [PubMed] [Google Scholar]
- 5.Matas A, Smith J, Skeans M, Thompson B, Gustafson SK, Schnitzler MA, Stewart DE, Cherikh WS, Wainright JL, Snyder JJ, Israni AK, Kasiske BL. OPTN/SRTR 2012 annual data report: Kidney. Am J Transplant. 2014;14(S1):11–44. doi: 10.1111/ajt.12579. [DOI] [PubMed] [Google Scholar]
- 6.Opelz G, Mickey MR, Terasaki PI. Influence of race on kidney transplant survival. Transplant Proc. 1977;9:137–142. [PubMed] [Google Scholar]
- 7.Ciancio G, Burke GW, Suzart K, Mattiazzi A, Vaidya A, Roth D, Kupin W, Rosen A, Johnson N, Miller J. The use of daclizumab, tacrolimus and mycophenolate mofetil in African-American and Hispanic first renal transplant recipients. Am J Transplant. 2003;3:1010–1016. doi: 10.1034/j.1600-6143.2003.00181.x. [DOI] [PubMed] [Google Scholar]
- 8.Neylan JF. Racial differences in renal transplantation after immunosuppression with tacrolimus versus cyclosporine. FK506 kidney transplant study group. Transplantation. 1998;65:515–523. doi: 10.1097/00007890-199802270-00011. [DOI] [PubMed] [Google Scholar]
- 9.Podder H, Podbielski J, Hussein I, Katz S, Buren C, Kahan BD. Sirolimus improves the two-year outcome of renal allografts in African-American patients. Transplant Int. 2001;14:135–142. doi: 10.1007/s001470100315. [DOI] [PubMed] [Google Scholar]
- 10.Weber M, Deng S, Arenas J, Aradhye S, Grossman R, Shaw L, Najil A, Barker C, Brayman KL. Decreased rejection episodes in African-American renal transplant recipients receiving mycophenolate mofetil/tacrolimus therapy. Transplant Proc. 1997;29:3669–3670. doi: 10.1016/s0041-1345(97)01067-1. [DOI] [PubMed] [Google Scholar]
- 11.Butkus DE, Meydrech EF, Raju SS. Racial differences in the survival of cadaveric renal allografts. Overriding effects of HLA matching and socioeconomic factors. N Engl J Med. 1992;327:840–845. doi: 10.1056/NEJM199209173271203. [DOI] [PubMed] [Google Scholar]
- 12.Curtis JJ. Kidney transplantation: Racial or socioeconomic disparities? Am J Kidney Dis. 1999;34:756–758. doi: 10.1016/S0272-6386(99)70404-X. [DOI] [PubMed] [Google Scholar]
- 13.Kalil R, Heim-Duthoy K, Kasiske B. Patients with a low income have reduced renal allograft survival. Am J Kid Dis. 1992;20:63–69. doi: 10.1016/s0272-6386(12)80318-0. [DOI] [PubMed] [Google Scholar]
- 14.Schweizer RT, Rovelli M, Palmeri D, Vossler E, Hull D, Bartus S. Noncompliance in organ transplant recipients. Transplantation. 1990;49:374–377. doi: 10.1097/00007890-199002000-00029. [DOI] [PubMed] [Google Scholar]
- 15.Cosio FG, Pesavento TE, Kim S, Osei K, Henry M, Ferguson RM. Patient survival after renal transplantation: IV. impact of post-transplant diabetes. Kidney Int. 2002;62:1440–1446. doi: 10.1111/j.1523-1755.2002.kid582.x. [DOI] [PubMed] [Google Scholar]
- 16.Cosio FG, Dillon JJ, Falkenhain ME, Tesi RJ, Henry ML, Elkhammas EA, Davies EA, Bumgardner GL, Ferguson RM. Racial differences in renal allograft survival: The role of systemic hypertension. Kidney Int. 1995;47:1136–1141. doi: 10.1038/ki.1995.162. [DOI] [PubMed] [Google Scholar]
- 17.Cosio FG, Hickson LJ, Griffin MD, Stegall MD, Kudva Y. Patient survival and cardiovascular risk after kidney transplantation: The challenge of diabetes. Am J Transplant. 2008;8:593–599. doi: 10.1111/j.1600-6143.2007.02101.x. [DOI] [PubMed] [Google Scholar]
- 18.Leffell MS, Steinberg AG, Bias WB, Machan CH, Zachary AA. The distribution of HLA antigens and phenotypes among donors and patients in the UNOS registry. Transplantation. 1994;58:1119. [PubMed] [Google Scholar]
- 19.Takemoto S, Terasaki PI, Cecka JM, Cho YW, Gjertson DW. Survival of nationally shared, HLA-matched kidney transplants from cadaveric donors. N Engl J Med. 1992;327:834–839. doi: 10.1056/NEJM199209173271202. [DOI] [PubMed] [Google Scholar]
- 20.Rebellato LM, Arnold AN, Bozik KM, Haisch CE. HLA matching and the united network for organ sharing allocation system: impact of HLA matching on African-American recipients of cadaveric kidney transplants. Transplantation. 2002;74:1634–1636. doi: 10.1097/00007890-200212150-00024. [DOI] [PubMed] [Google Scholar]
- 21.Kerman RH, Kimball P, Van Buren CT, Lewis RM, Kahan BD. Possible contribution of pretransplant immune responder status to renal allograft survival differences of black versus white recipients. Transplantation. 1991;51:338. doi: 10.1097/00007890-199102000-00013. [DOI] [PubMed] [Google Scholar]
- 22.McDaniel DO, Barber WH, Nguyan C, Rhodes SW, May WL, McDaniel LS, Vig PJS, Jemeson LL, Butkus DE. Combined analysis of cytokine genotype polymorphism and the level of expression with allograft function in African-American renal transplant patients. Transpl Immunol. 2003;11:107–119. doi: 10.1016/S0966-3274(02)00171-5. [DOI] [PubMed] [Google Scholar]
- 23.Hutchings A, Purcell WM, Benfield MR. Increased costimulatory responses in African-American kidney allograft recipients. Transplantation. 2001;71:692–695. doi: 10.1097/00007890-200103150-00021. [DOI] [PubMed] [Google Scholar]
- 24.Young CJ, Kew C. Health disparities in transplantation: Focus on the complexity and challenge of renal transplantation in African Americans. Med Clin N Am. 2005;89:1003–1031. doi: 10.1016/j.mcna.2005.05.002. [DOI] [PubMed] [Google Scholar]
- 25.Malat GE, Culkin C, Palya A, Ranganna K, Kumar MS. African American kidney transplantation survival: The ability of immunosuppression to balance the inherent pre- and post-transplant risk factors. Drugs. 2009;69:2045–2062. doi: 10.2165/11318570-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 26.Pallet N, Thervet E, Alberti C, Emal-Aglae V, Bedrossian J, Martinez F, Roy C, Legendre C. Kidney transplant in black recipients: Are African Europeans different from African Americans? Am J Transplant. 2005;5:2682–2687. doi: 10.1111/j.1600-6143.2005.01057.x. [DOI] [PubMed] [Google Scholar]
- 27.Yeates K, Wiebe N, Gill J, Sima C, Schaubel D, Holland D, Hemmelgarn B, Tonelli M. Similar outcomes among black and white renal allograft recipients. Journal of the American Society of Nephrology. 2009;20:172–179. doi: 10.1681/ASN.2007070820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chakkera HA, O’Hare AM, Johansen KL, Hynes D, Stroupe K, Colin PM, Chertow GM. Influence of race on kidney transplant outcomes within and outside the department of Veterans Affairs. J Am Soc Nephrol. 2005;16:269–277. doi: 10.1681/ASN.2004040333. [DOI] [PubMed] [Google Scholar]
- 29.Isaacs RB, Nock SL, Spencer CE, Connors AF, Wang XQ, Sawyer R, Lobo PI. Racial disparities in renal transplant outcomes. Am J Kid Dis. 1999;34:706–712. doi: 10.1016/S0272-6386(99)70397-5. [DOI] [PubMed] [Google Scholar]
- 30.Isaacs RB, Conners A, Nock S, Spencer C, Lobo P. Noncompliance in living-related donor renal transplantation: The united network of organ sharing experience. Transplant Proc. 1999;31(4A):19S–20S. doi: 10.1016/s0041-1345(99)00117-7. [DOI] [PubMed] [Google Scholar]
- 31.Chisholm-Burns MA, Spivey CA, Rehfeld R, Zawaideh M, Roe DJ, Gruessner R. Immunosuppressant therapy adherence and graft failure among pediatric renal transplant recipients. Am J Transplant. 2009;9:2497–2504. doi: 10.1111/j.1600-6143.2009.02793.x. [DOI] [PubMed] [Google Scholar]
- 32.Ferdinand KC, Saunders E. Hypertension-related morbidity and mortality in African Americans-why we need to do better. J Clin Hypertens. 2006;8(1 Suppl 1):21–30. doi: 10.1111/j.1524-6175.2006.05295.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ferdinand KC. Managing cardiovascular risk in minority patients. J Natl Med Assoc. 2005;97:459–466. [PMC free article] [PubMed] [Google Scholar]
- 34.Gadegbeku CA, Lea JP, Jamerson KA. Update on disparities in the pathophysiology and management of hypertension: focus on African Americans. Med Clin North Am. 2005;89:921–933. doi: 10.1016/j.mcna.2005.05.003. [DOI] [PubMed] [Google Scholar]
- 35.Banerji MA. Diabetes in African Americans: Unique pathophysiologic features. Current Diabetes Reports. 2004;4:219–223. doi: 10.1007/s11892-004-0027-3. [DOI] [PubMed] [Google Scholar]
- 36.Crook ED. Diabetic renal disease in African Americans. Am J Med Sci. 2002;323:78–84. doi: 10.1097/00000441-200202000-00004. [DOI] [PubMed] [Google Scholar]
- 37.Egede LE, Dagogo-Jack S. Epidemiology of type 2 diabetes: Focus on ethnic minorities. Med Clin North Am. 2005;89:949–975. doi: 10.1016/j.mcna.2005.03.004. [DOI] [PubMed] [Google Scholar]
- 38.Ozieh MN, Taber DJ, Egede LE. Does African American race impact statin efficacy in renal transplant outcomes? Medicine. 2015;94:e2283. doi: 10.1097/MD.0000000000002283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Taber DJ, Pilch NA, Meadows HB, McGillicuddy JW, Bratton CF, Chavin KD, Baliga PK, Egede LE. The impact of cardiovascular disease and risk factor treatment on ethnic disparities in kidney transplant. J Cardiovasc Pharmacol Ther. 2013;18:243–250. doi: 10.1177/1074248412469298. [DOI] [PubMed] [Google Scholar]
- 40.Taber DJ, Gebregziabher M, Payne EH, Srinivas T, Baliga PK, Egede LE. Overall graft loss versus death-censored graft loss: Unmasking the magnitude of racial disparities in outcomes among US kidney transplant recipients. Transplantation. 2016;100 doi: 10.1097/TP.0000000000001119. epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Anonymous. KDIGO clinical practice guideline for the care of kidney transplant recipients. Am J Transplant. 2009;9(Suppl 3):S1–S155. doi: 10.1111/j.1600-6143.2009.02834.x. [DOI] [PubMed] [Google Scholar]
- 42.Anonymous. American Diabetes Association. Standards of medical care in diabetes-2011. Diabetes Care. 2011;34(Suppl 1):S11–S61. doi: 10.2337/dc11-S011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Anonymous. National Cholesterol Education Program (NCEP) Expert Panel on Detection Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) Third report of the national cholesterol education program (NCEP) expert panel on detection, evaluation, and treatment of high blood cholesterol in adults (adult treatment panel III) final report. Circulation. 2002;106:3143–3421. [PubMed] [Google Scholar]
- 44.Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, Izzo JL, Jones DW, Materson BJ, Oparil S, Wright JT, Roccella EJ. The seventh report of the joint national committee on prevention, detection, evaluation, and treatment of high blood pressure: The JNC 7 report. J Am Med Assoc. 2003;289:2560–2572. doi: 10.1001/jama.289.19.2560. [DOI] [PubMed] [Google Scholar]
- 45.Karve S, Cleves MA, Helm M, Hudson TJ, West DS, Martin BC. An empirical basis for standardizing adherence measures derived from administrative claims data among diabetic patients. Med Care. 2008;46:1125–1133. doi: 10.1097/MLR.0b013e31817924d2. [DOI] [PubMed] [Google Scholar]
- 46.Kim N, Agostini JV, Justice AC. Refill adherence to oral hypoglycemic agents and glycemic control in veterans. Ann Pharmacother. 2010;44:800–808. doi: 10.1345/aph.1M570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chisholm-Burns MA, Spivey CA, Tolley EA, Kaplan EK. Medication therapy management and adherence among US renal transplant recipients. Patient Prefer Adherence. 2016;10:703–709. doi: 10.2147/PPA.S104646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Fine JP, Gray RJ. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc. 1999;94:496–509. [Google Scholar]
- 49.Purnell TS, Luo X, Kucirka LM, Cooper LA, Crews DC, Massie AB, Boulware LE, Segev DL. Reduced racial disparity in kidney transplant outcomes in the United States from 1990 to 2012. J Am Soc Nephrol. 2016 doi: 10.1681/ASN.2015030293. epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Padiyar A, Hricik DE. Immune factors influencing ethnic disparities in kidney transplantation outcomes. Expert Rev Clin Immunol. 2011;7:769–778. doi: 10.1586/eci.11.32. [DOI] [PubMed] [Google Scholar]
- 51.Matas A, Smith J, Skeans M, Thompson B, Gustafson SK, Stewart DE, Cherikh WS, Wainright JL, Boyle G, Snyder JJ, Israni AK, Kasiske BL. OPTN/SRTR 2013 annual data report: kidney. Am J Transplant. 2015;15(S2):1–34. doi: 10.1111/ajt.13195. [DOI] [PubMed] [Google Scholar]
- 52.Kurian AK, Cardarelli KM. Racial and ethnic differences in cardiovascular disease risk factors: A systematic review. Ethnic Dis. 2007;17:143–152. [PubMed] [Google Scholar]
- 53.Peek ME, Cargill A, Huang ES. Diabetes health disparities: a systematic review of health care interventions. Med Care Res Rev. 2007;64(5 Suppl):101S–156S. doi: 10.1177/1077558707305409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sarnak MJ, Levey AS, Schoolwerth AC, Coresh J, Culleton B, Hamm LL, McCullough PA, Kasiske BL, Kelepouris E, Klag MJ, Parfrey P, Pfeffer M, Raij L, Spinosa DJ, Wilson PW. Kidney disease as a risk factor for development of cardiovascular disease: a statement from the American Heart Association councils on kidney in cardiovascular disease, high blood pressure research, clinical cardiology, and epidemiology and prevention. Circulation. 2003;108:2154–2169. doi: 10.1161/01.CIR.0000095676.90936.80. [DOI] [PubMed] [Google Scholar]
- 55.Pilmore HL, Skeans MA, Snyder JJ, Israni AK, Kasiske BL. Cardiovascular disease medications after renal transplantation: Results from the patient outcomes in renal transplantation study. Transplantation. 2011;91:542–551. doi: 10.1097/TP.0b013e31820437bd. [DOI] [PubMed] [Google Scholar]
- 56.Carpenter MA, Weir MR, Adey DB, House AA, Bostom AG, Kusek JW. Inadequacy of cardiovascular risk factor management in chronic kidney transplantation–evidence from the FAVORIT study. Clin Transplant. 2012;26:e438–e446. doi: 10.1111/j.1399-0012.2012.01676.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ho PM, Bryson CL, Rumsfeld JS. Medication adherence: its importance in cardiovascular outcomes. Circulation. 2009;119:3028–3035. doi: 10.1161/CIRCULATIONAHA.108.768986. [DOI] [PubMed] [Google Scholar]
- 58.DiMatteo MR. Social support and patient adherence to medical treatment: A meta-analysis. Health Psychol. 2004;23:207–218. doi: 10.1037/0278-6133.23.2.207. [DOI] [PubMed] [Google Scholar]
- 59.Egede LE, Gebregziabher M, Lynch CP, Gilbert GE, Echols C. Longitudinal ethnic differences in multiple cardiovascular risk factor control in a cohort of US adults with diabetes. Diabetes Res Clin Pract. 2011;94:385–394. doi: 10.1016/j.diabres.2011.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Freedman B, Julian B, Pastan S, Israni AK, Schladt D, Gautreaux MD, Hauptfeld V, Bray RA, Gebel HM, Kirk AD, Gaston RS, Rogers J, Farney AC, Orlando G, Stratta RJ, Mohan S, Ma L, Langefeld CD, Hicks PJ, Palmer ND, Adams PL, Palanisamy A, Reeves-Daniel AM, Divers J. Apolipoprotein L1 gene variants in deceased organ donors are associated with renal allograft failure. Am J Transplant. 2015;15:1615–1622. doi: 10.1111/ajt.13223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Taber DJ, Douglass K, Srinivas T, McGillicuddy JW, Bratton CF, Chavin KD, Baliga PK, Egede LE. Significant racial differences in the key factors associated with early graft loss in kidney transplant recipients. Am J Nephrol. 2014;40:19–28. doi: 10.1159/000363393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Taber DJ, Hamedi M, Rodrigue JR, Gebregziabher MG, Srinivas TR, Baliga PK, Egede LE. Quantifying the race stratified impact of socioeconomics on graft outcomes in kidney transplant recipients. Transplantation. 2015 Oct 1; doi: 10.1097/TP.0000000000000931. Epub ahead of print. http://www.ncbi.nlm.nih.gov/pubmed/26425875. [DOI] [PMC free article] [PubMed]
- 63.Taber DJ, Gebregziabher MG, Srinivas TR, Chavin KD, Baliga PK, Egede LE. African-American race modifies the influence of tacrolimus concentrations on acute rejection and toxicity in kidney transplant recipients. Pharmacotherapy. 2015;35:569–577. doi: 10.1002/phar.1591. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


