Johnson and colleagues reported that cortical retention of tau PET ligand [F-18]AV-1451 (previously [F-18]T807) was higher in mild cognitively impaired (MCI) and Alzheimer’s disease (AD) patients compared to cognitively normal elderly controls (NC), correlated with cognitive impairment, and resembled known progression of tau lesions along neuropathologically-defined stages in AD (1). While these encouraging results suggest [F-18]AV-1451 is a reliable radioligand for imaging AD tau pathology, an unexpected finding was that MCI and AD groups did not have significantly elevated [F-18]AV-1451 retention in the hippocampus, a brain area invariably affected with tau pathology in AD. The authors speculated this was due to “off-target” binding in adjacent structures. This presumed “off-target” binding corresponds to the choroid plexus (CP) adjoining the hippocampus along the ventricle wall, and ligand retention in this structure could result in high background in elderly NC and mask disease-related signal increases in MCI and AD subjects. Similarly high retention of [F-18]AV-1451 in CP was observed in our studies (Fig 1A), and other groups also referred to CP as a site of “off-target” [F-18]AV-1451 binding (2), assuming that CP lacks tau pathology. However, our histology analyses demonstrate that epithelial cells of CP 1) contain tangle-like structures which are labeled with a fluorescent AV-1451 derivative (CN-AV-1451) and X-34, a highly fluorescent derivative of Congo red that is sensitive to beta-pleated sheet protein deposits (Fig 1B–E), and 2) are immunoreactive to pan-tau and phosphorylated tau-specific antibodies (Fig 1F–K), with minimal Aβ immunoreactivity (Fig 1L&M). These structures correspond morphologically to Biondi “ring” tangles, described previously in CP of aged NC and AD brains (3–4). Note that in Fig 3 of Johnson (1), elderly NC have much higher [F-18]AV-1451 retention in the hippocampus than in other brain regions, while in MCI/AD subjects [F-18]AV-1451 retention in the hippocampus is similar to that in entorhinal and parahippocampal cortex, areas with significant elevations compared to NC. Another study reported high CP retention of [F-18]AV-1451 in AD and aged NC, but not young NC (2), consistent with observations that CP tangles are scarce in young NC, increase with age, and are most abundant in AD (4). The course of tau changes in CP, and threshold levels for their detection using PET, should be determined during the clinical progression of AD. In the meantime, retention of [F-18]AV-1451 in the CP should be considered possible “on-target” binding for this (and possibly other) candidate tau imaging radioligands.
Figure 1. In vivo imaging and postmortem histopathology of choroid plexus.
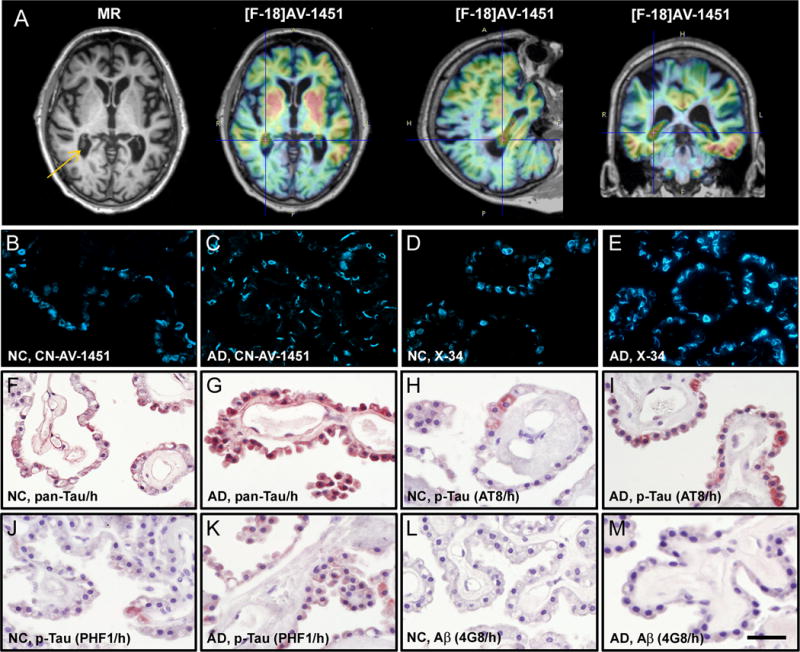
(A) MR and [F-18]AV-1451 PET images of a 91-year old AD patient. Choroid plexus is evident in the MR image (arrow) and shows high [F-18]AV-1451 retention in PET scans as denoted by crosshairs. (B–M) Fluorescence and light microscopy micrographs of choroid plexus from postmortem brains of a 83-year old NC and a 77-year old AD patient, stained with CN-AV-1451 (B,C), X-34 (D,E), total and phosphorylated tau (F–K) or Aβ immunohistochemistry (L,M). B–E: In both NC and AD choroid plexus, CN-AV-1451 and X-34 label tangles which contain fibrillar β-sheet structure. F–M: In NC and more prominently in AD, epithelial cells of the choroid plexus contain immunoreactivity (brown color) with antibodies against pan-Tau (F,G) and p-Tau antibodies AT8 (H,I) and PHF1 (J,K), but only light or no Aβ immunoreactivity (L,M, 4G8 antibody). Sections in F–M were counter-stained with hematoxylin (h, blue color). Scale bar = 35 μm (B–M).
Acknowledgments
This work was supported by NIH grants P01 AG025204 (M.D.I., J.C.P., C.A.M., W.E.K.) and P01 AG14449 (M.D.I.). The authors wish to thank Ms. Lan Shao for technical assistance.
Footnotes
Potential Conflicts of Interest:
The authors have no conflicts of interest relevant to this work.
Author Contributions:
- Conception and design of the study: M.D.I., E.E.A., J.C.P., C.A.M., W.E.K.
- Acquisition and analysis of data: M.D.I., E.E.A., J.C.P., C.A.M., W.E.K.
- Drafting a significant portion of the manuscript or figures: M.D.I., E.E.A., J.C.P., C.A.M., W.E.K.
References
- 1.Johnson KA, Schultz A, Betensky RA, et al. Tau positron emission tomographic imaging in aging and early Alzheimer disease. Ann Neurol. 2016;79(1):110–119. doi: 10.1002/ana.24546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schöll M, Lockhart SN, Schonhaut DR, et al. PET Imaging of Tau Deposition in the Aging Human Brain. Neuron. 2016;89(5):971–982. doi: 10.1016/j.neuron.2016.01.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Miklossy J, Kraftsik R, Pillevuit O, et al. Curly fiber and tangle-like inclusions in the ependyma and choroid plexus–a pathogenetic relationship with the cortical Alzheimer-type changes? J Neuropathol Exp Neurol. 1998;57(12):1202–1212. doi: 10.1097/00005072-199812000-00012. [DOI] [PubMed] [Google Scholar]
- 4.Wen GY, Wisniewski HM, Kascsak RJ. Biondi ring tangles in the choroid plexus of Alzheimer’s disease and normal aging brains: a quantitative study. Brain Res. 1999;832(1–2):40–46. doi: 10.1016/s0006-8993(99)01466-3. [DOI] [PubMed] [Google Scholar]


