Abstract
Apical periodontitis (periapical lesions) is an infection-induced chronic inflammation in the jaw, ultimately resulting in the destruction of apical periodontal tissue. Toll-like receptors (TLRs) are prominent in the initial recognition of pathogens. Our previous study showed that TLR4 signaling is proinflammatory in periapical lesions induced by a polymicrobial endodontic infection. In contrast, the functional role of TLR2 in regulation of periapical tissue destruction is still not fully understood. Using TLR2 deficient (KO), TLR2/TLR4 double deficient (dKO), and wild-type (WT) mice, we demonstrate that TLR2 KO mice are highly responsive to polymicrobial infection-induced periapical lesion caused by over activation of TLR4 signal transduction pathway that resulted in elevation of NF-κB (nuclear factor kappa B) and proinflammatory cytokine production. The altered TLR4 signaling is caused by TLR2 deficiency-dependent elevation of CD14 (cluster of differentiation 14), which is a co-receptor of TLR4. Indeed, neutralization of CD14 strikingly suppresses TLR2 deficiency-dependent inflammation and tissue destruction in vitro and in vivo. Our findings suggest that a network of TLR2, TLR4, and CD14 is a key factor in regulation of polymicrobial dentoalveolar infection and subsequent tissue destruction.
Introduction
A periapical lesion is an inflammatory and immune response caused by anaerobic polymicrobial infection including both Gram-positive and –negative species in the dental root canal system (so called endodontic infection). Endodontic infection elicits immune and inflammatory responses including both innate and adaptive immunity leading to destruction of the alveolar bone surrounding the dental root apex and the formation of granulomatous tissue, which are representative pathologies of this disease. Macrophages is a member of innate immune cells and play a key regulatory role, which is production of the central proinflammatory cytokine IL-1 in the development of periapical lesions (Wang and Stashenko, 1993; Tani-Ishii et al., 1995).
Toll-like receptors (TLRs) play a prominent role in induction of innate immune and inflammatory responses upon recognition of PAMPs (specific pathogen-associated molecular patterns), common structures shared by most pathogens. PAMPs trigger TLR signaling that leads to NF-κB activation and subsequent pro-inflammatory cytokine production. TLR2 and TLR4 are the components most thoroughly investigated TLRs, because TLR2 and TLR4 are critical in recognizing Gram-positive and -negative bacteria, respectively. TLR2 recognizes Gram-positive PAMPs such as lipoteichoic acid, peptidoglycan, and also lipoprotein, while TLR4 is crucial in recognition of LPS (lipopolysaccharide), a representative PAMP of Gram-negative bacteria.
The actual role of TLRs in host defense against bacterial infections has been extensively studied using TLR deficient (KO) mice and monospecies infection in vivo (Gerold et al., 2007). In response to Gram-negative Salmonella typhimurium, TLR4 signaling and TLR2 signaling work in a sequential manner. TLR4 signaling induces early cytokine production and killing of S. typhimurium by macrophages (6 hours post infection), while TLR2 signaling is important for macrophage bactericidal activity at 24 hours post infection (Weiss et al., 2004). Therefore, C3H/HeJ mice (a congenic hypo-TLR4 signal model) are more susceptible to S. typhimurium oral infection compared to C3H/HeOuJ mice exhibiting normal TLR4 signaling (Weiss et al., 2004). In contrast, TLR2 deficient (KO) mice are more susceptible to Gram-positive Staphylococcus aureus infection compared to wild-type controls (Takeuchi et al., 2000), demonstrating that recognition of S. aureus by TLR2 is critical in prevention of S. aureus infection. TLR2 is also important in an early inflammatory response against Staphylococcus pneumonia (Knapp et al., 2004).
Regardless of Gram-stainability, endodontic pathogens induce innate and subsequent adaptive immune response during endodontic infection (Hou et al., 2000b, 2000a). TLRs are critical in the development of periapical lesions caused by polymicrobial endodontic infection. The C3H/HeJ mouse, a hypo TLR4 signal model, exhibits less periapical inflammation and reduced bone loss in response to polymicrobial endodontic infection than normal C3H/HeOuJ mice (Hou et al., 2000b). This finding suggests a detrimental role of TLR4 signal in this disease. By contrast, a recent study demonstrated that the TLR2 signal is unexpectedly protective in the development of periapical lesions (da Silva et al., 2012), although the mechanism of this TLR2-mediated protection is uncertain. Since the extent of periapical lesions in TLR2/TLR4 double deficient mice was similar to that in WT mice in our preliminary study (data not shown), we expected a negative regulatory mechanism between TLR2 and TLR4 signaling in periapical lesions.
Polymicrobial infections including both Gram-positive and negative species cause a variety of disease conditions such as abscesses, respiratory diseases, otitis media, periodontitis, and intestinal disorders via stimulating multiple pattern recognition receptors including TLRs. Such combined activation of TLRs may lead to complementary, synergistic or antagonistic effects that modulate immune response (Trinchieri and Sher, 2007). However, the functional network of TLRs in the regulation of polymicrobial infection and subsequent inflammation is not fully understood. In this report, we demonstrate that the TLR2 signal negatively regulates TLR4-mediated response via suppression of CD14 in periapical lesions induced by polymicrobial infection through morphological/histological and molecular analyses.
Materials and Methods
Animals
Age-matched (6 weeks old) wild-type C57BL/6J (wild-type (WT)), TLR2 KO (B6.129-Tlr2tm1Kir/J; Jackson Laboratory, Bar Harbor, ME), and TLR2/TLR4 double KO mice were employed. The dKO mice were generated by first breeding TLR2 KO and TLR4 KO mice (a generous gift from Dr. Shizuo Akira, Osaka University, Osaka, Japan) to obtain double heterozygote offspring for targeted TLR alleles. Subsequent interbreeding of double-heterozygotes yielded offspring including homozygotes for the targeted alleles. All genetic mutant strains used in this study were on C57BL/6 background. All experimental protocols were approved by The Forsyth Institute’s Institutional Animal Care and Use Committee.
Endodontic infection
Polymicrobial endodontic infection was induced as described (Hou et al., 2000b; Sasaki et al., 2000; Sasaki et al., 2004). Briefly, mandibular first molar dental pulps were microsurgically exposed under anesthesia. Common human endodontic pathogens including two Gram-positive bacteria (Parvimonas micra (formerly Peptostreptococcus micros (ATCC 33270), and Streptococcus intermedius (ATCC 27335)) and two Gram-negative bacteria (Prevotella intermedia (ATCC25611), Fusobacterium nucleatum (ATCC 25586)) were inoculated into the pulp chamber and root canals. These pathogens were equally mixed to yield 1010 cells/ml of PBS containing 2% CMC. A total 10 µl of the mixture was inoculated per tooth. Non-exposed/non-infected animals served as negative controls.
Morphological analyses by micro computed tomography (µCT) and histology
On days 10 and 21 post infection, mice were killed. Mandibles were isolated and hemisected. One hemimandible was fixed and subjected to µCT following histological procedures. Quantification of lesion size and subsequent histological examination on same samples were performed as previously described (AlShwaimi et al., 2013). The lesion size was expressed as square millimeters.
After µCT scanning, the samples were subjected to histology as we previously described (AlShwaimi et al., 2013). In brief, the samples were decalcified using 10% formic acid and sodium citrate, embedded in paraffin following general histology protocol, sectioned in serial sections (6 µm thickness). H&E staining was employed for general observation. Pivotal sections containing patent root canal with localized periapical lesion were stained for neutrophils (anti-Ly6G (lymphocyte antigen 6 complex, locus G); dilution 1:200; Abcam), macrophages (anti-F4/80 protein; dilution 1:50; Santa Cruz Biotechnology), ab3523 (anti–inducible NO synthase (iNOS), dilution 1:400; Abcam, Cambridge, MA), N20 (anti–arginase 1 (Arg1), dilution 1:50; Santa Cruz Biotechnology), and endothelial progenitor cells/endothelial cells (anti-CD34 (a hematopoietic progenitor cell antigen); dilution 1:250; Santa Cruz Biotechnology), respectively. At least three specimens were subjected to each primary antibody. Primary antibodies were detected using Vector Elite ABC kits (Vector Laboratories, Burlingame, CA). Omissions of the primary antibodies were used as negative controls. Observations were carried out either at ×100, ×200 or ×400 magnification. Note that controlled counting and comparisons of staining-positive cells between these two strains were impossible due to their histological appearance.
Real-time RT-PCR
Bone blocks containing periapical lesions were isolated from the other hemimandibles. Total RNA extraction following cDNA synthesis was conducted using TRIzol Reagent (Life Technologies, Carlsbad, CA), Fastprep-24 with matrix A (both MP Biomedicals, Solon, OH), and iScript cDNA Synthesis Kit (Bio-Rad, Waltham, MA). Real-time RT-PCR was performed using the Light Cycler 480 II and Light cycler 480 sybr green I master (both Roche, Indianapolis, IN) in triplicates. Genes examined in the present study are listed in Table 1. All primers used in this study were purchased from Real Time Primers (Elkins Park, PA). The primer sequences are available in Supplemental Table 1. The 2^-ddCT method was used for data analyses.
Table 1.
Genes examined by quantitative RT-PCR
| Symbol | Gene name | Type of gene product | Existing domain |
|---|---|---|---|
| CD14 | Cluster of differentiation 14 | Co-receptor of Toll-like receptor 4 | Extracellular/Membrane |
| TLR4 | Toll-like receptor 4 | Pattern recognition receptor | Extracellular/Membrane |
| MYD88 | Myeloid differentiation primary response gene 88 | Universal TLR adapter protein | Cytoplasmic |
| IRAK1 | Interleukin-1 receptor-associated kinase 1 | TLR signal transduction molecule | Cytoplasmic |
| IRAK2 | Interleukin-1 receptor-associated kinase 2 | TLR signal transduction molecule | Cytoplasmic |
| TRAF6 | TNF receptor associated factor 6 | TLR signal transduction molecule | Cytoplasmic |
| TICAM1 | Toll-Like Receptor Adaptor Molecule 1 | TLR signal transduction molecule | Cytoplasmic |
| NFKB1* | Nuclear factor kappa B p105 subunit | NF-κB family member | Cytoplasmic/Nucleus |
| NFKB2 | Nuclear factor kappa B p100 subunit | NF-κB family member | Cytoplasmic/Nucleus |
| RELA | v-rel reticuloendotheliosis viral oncogene homolog A (avian) |
NF-κB family member | Cytoplasmic/Nucleus |
| RELB* | Avian reticuloendotheliosis viral (v-rel) oncogene related B |
NF-κB family member | Cytoplasmic/Nucleus |
| REL* | v-rel avian reticuloendotheliosis viral oncogene homolog |
NF-κB family member | Cytoplasmic/Nucleus |
| IL1A | Interleukin-1 alpha | Proinflammatory cytokine | Membrane/Extracellular |
| IL12* | Interleukin-12 | Th1 response inducing cytokine | Extracellular |
| IFNG | Interferon gamma | Th1 type inflammatory cytokine | Extracellular |
| IL10 | Interleukin-10 | Anti-inflammatory cytokine | Extracellular |
| YWHAZ | 14-3-3 protein zeta/delta (an internal control gene) |
The sequence of PCR primers is listed in supplemental table 1.
: Data of these genes are not shown.
Cell culture and protein quantifications
Resident peritoneal macrophages were isolated from the mice described above and seeded in 96-well plates (105 cells/well) as previously described (Sasaki et al., 2000). The cells were cultured in the presence or absence of highly purified E. coli LPS (0111:B4; Sigma-Aldrich, St. Louis, MO; 1 µg/ml; a TLR4-specific ligand), Pam2CSK4 (InvivoGen, San Diego, CA; 100 ng/ml; a TLR2-specific ligand), and formalin-fixed whole endodontic pathogens (1×109 cells/ml) for 24 hrs. The level of IL-1α, IL-1β, and soluble CD14 (sCD14) in the culture supernatants was determined by specific ELISA (all R&D Systems, Minneapolis, MN). Cytosolic and membrane CD14 were separately isolated from 3×106 cells using the Mem-PER Plus Membrane Protein Extraction Kit (Thermo Scientific, Waltham, MA).
CD14 neutralization
We neutralized CD14 either in vitro or in vivo using anti-mouse CD14 antibody (clone: 4C1; rat IgG2b, k; BD Biosciences, San Jose, CA). For in vitro neutralization, TLR2 KO macrophages were treated with 5 µg/ml of the neutralizing antibody. Infected TLR2 KO mice received i.v. injection of the neutralizing antibody (25 µg/mouse/time) on days −1, 2, 5, 8, 11, 14, and 17 relative to the pulpal infection. An isotype-matched non-specific antibody was used as a control.
Statistics
Statistical analysis was performed using either one-way or two-way ANOVA and Bonferroni post hoc test.
Results
TLR2 and TLR4 play opposing roles in the development of periapical lesions
We determined the functional interaction between TLR2 and TLR4 signaling in a response to polymicrobial infection using a well-established periapical lesion model in vivo (Hou et al., 2000b; Sasaki et al., 2000; Sasaki et al., 2004) using wild-type (WT), TLR2 KO and TLR2/TLR4 dKO mice.
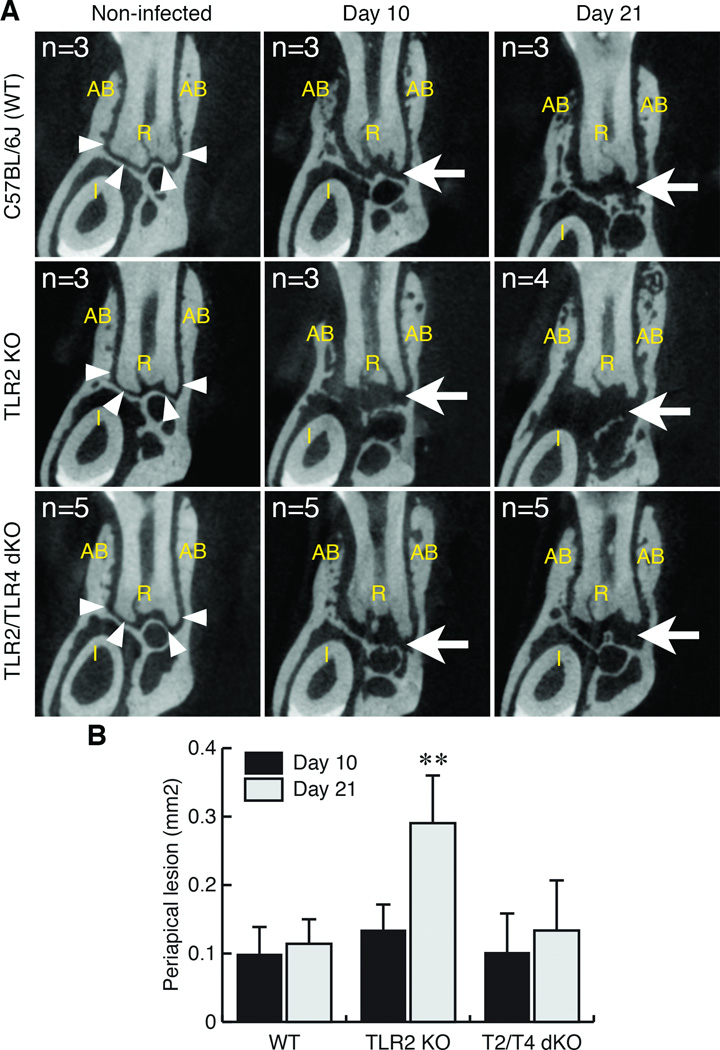
Since TLR2 is a major receptor for pathogen-associated molecular patterns derived from Gram-positive bacteria (Medzhitov, 2001), our initial expectation was that TLR2 deficiency attenuates periapical inflammation and bone loss similar to LPS-hyporesponsive C3H/HeJ mice (Hou et al., 2000). However, as shown in Fig. 1, TLR2 KO mice unexpectedly exhibited enhanced periapical lesions on Day 21 post infection, corroborating a previous report describing susceptibility of TLR2 KO mice to this disease (da Silva et al., 2012). In contrast, the extent of lesions in TLR2/TLR4 dKO (double deficient) mice was strikingly lower than that in TLR2 KO mice and was similar to the extent of lesions in WT mice.
Figure 1. TLR2 KO mice exhibited extended periapical bone destruction compared to wild-type C57BL/6 and TLR2/TLR4 dKO mice.
A. Representative µCT images of periapical lesions in anterior-posterior direction. The effect of TLR2 and TLR2/TLR4 double deficiencies on periapical bone destruction was determined on days 10 and 21 post endodontic infection. Non-infected mice in each strain served as corresponding background controls. The sample number (n) of each group was indicated in each panel. The position of each CT slice was the most central part of mandibular first molar distal root (R). AB: alveolar bone. Arrowheads in the panel of non-infected controls indicate the margin of alveolar bone in periapical region. Arrow points to enlarged radiolucent area indicating the development of periapical lesion. The original length of the side of each square CT image was 2.4 mm.
B. Size of periapical lesions. The extent of periapical lesions was measured as previously described (AlShwaimi et al., 2013). Although the periapical lesion size on day 10 post-infection was similar in all genotypes, TLR2 KO mice exhibited significantly enlarged periapical bone loss compared to wild-type C57BL/6J and TLR2/TLR4 dKO mice on day 21. **: p<0.01 vs. either C57BL/6J or dKO, vertical bar: standard deviation.
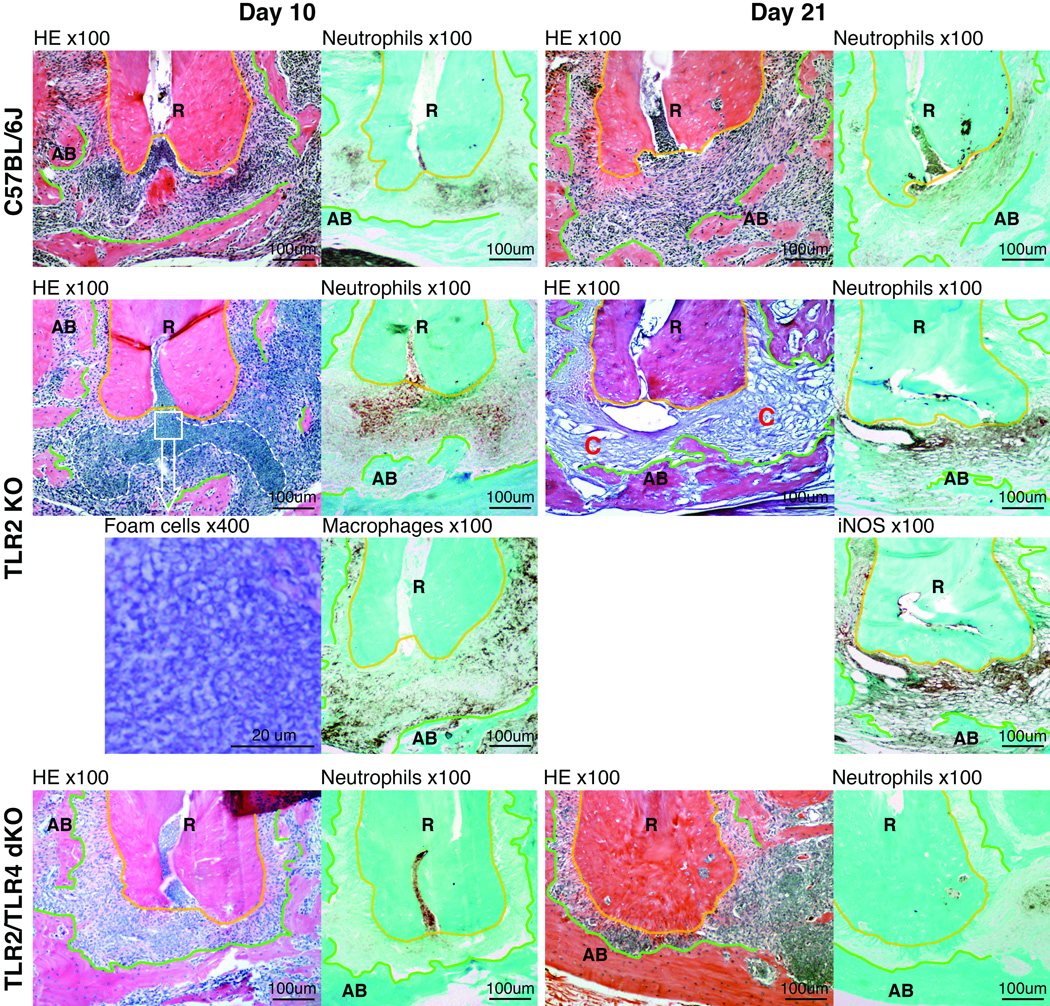
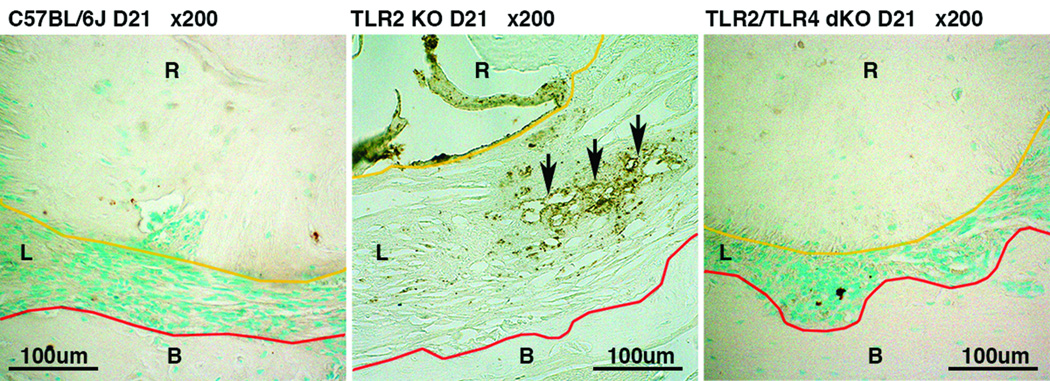
Histologically, deficiency of TLR2 and TLR2/TLR4 did not alter normal structure of periapical periodontal tissue compared to wild-type mice (Fig. 2). The level of tissue resident macrophages (F4/80-positive cells) in periodontal ligament was similar in all strains (data not shown). No Ly6G-positive neutrophil infiltration was observed in the region (data not shown). Upon polymicrobial endodontic infection, TLR2 KO mice exhibited a sign of advanced infection and inflammation, which was cells with a foamy appearance (Malcolm et al., 1979; Liu et al., 1984; Gossett et al., 1985), on day 10 post infection (Fig. 3). These cells seemed to be neutrophils, because these cells were mostly Ly6G-positive but F4/80-negative as shown in Fig. 3. Such cytoplasmic vacuolation of neutrophils was not observed in WT and TLR2/TLR4 dKO mice. On day 21 post infection, the vacuolated cells were resolved in TLR2 KO mice. Instead, the lesion was filled with a capillary-rich tissue consisting of CD34-positive cells (Fig. 4), suggesting de novo angiogenesis (Bettencourt et al., 1998). Moderate to severe Ly6G-positive neutrophil infiltration was observed in the capillary-rich tissue (Fig. 3). The proportion of neutrophils in periapical lesions appeared to be elevated at days 21 compared to day 10 in TLR2 KO mice. In addition, the region of neutrophil infiltration in TLR2 KO on day 21 widely overlapped with the area of iNOS-positive cells (Fig. 3), suggesting that periapical inflammation in TLR2 KO was still active at this observation period. By contrast, infected WT mice exhibited chronic inflammation represented by fibrous periapical granuloma with mild Ly6G-positive neutrophil infiltration on day 21 (Fig. 3). In TLR2/TLR4 dKO mice, Ly6G-positive neutrophil infiltration was notably faint either on day 10 or 21 post infection (Fig. 3), indicating minimal inflammation in the process of wound healing compared to wild-type and TLR2/TLR4 dKO mice. Taken together, these data suggest that the TLR2 signal negatively regulates TLR4-mediated inflammatory responses during the development of periapical lesions.
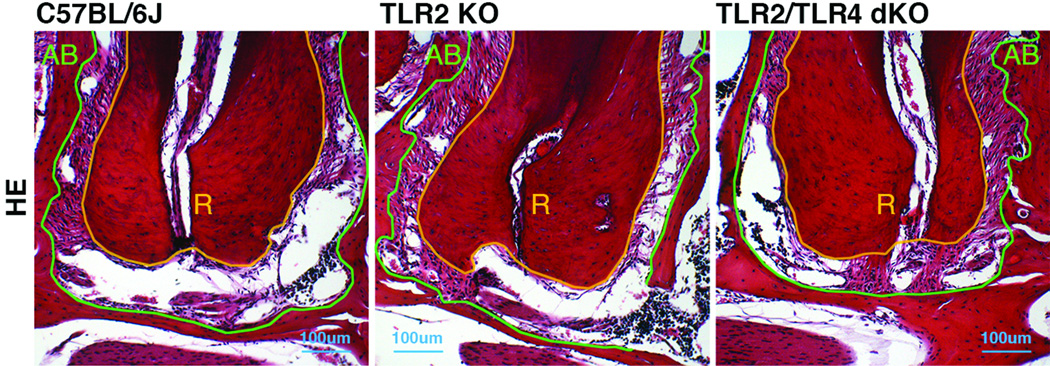
Figure 2. Genotypes did not affect on the structure of normal periapical tissue.
Panels are representative histology of normal (non-infected) periapical tissue in wild-type C57BL/6J, TLR2 KO and TLR2/TLR4 dKO mice. The original magnification was ×100. Polygonal orange and green lines outline the margin of dental root and alveolar bone, respectively. The area lying between these two lines is normal periodontal ligament tissue, which anchors tooth in alveolar bone. The structure of periapical region in non-infected animals was similar in all genotypes, and no visible differences were observed. AB: alveolar bone, R: dental root.
Figure 3. Susceptibility of TLR2 KO mice to periapical inflammation was diminished in TLR2/TLR4 dKO mice.
Panels are representative histological images of periapical lesions on days 10 and 21 in wild-type C57BL/6J, TLR2 KO and TLR2/TLR4 dKO mice. The original magnification is indicated on the top of each panel. The area enclosed by a polygonal orange line is dental root (R). Polygonal green lines outline the margin of residual alveolar bone (AB). The area lying between these lines is periapical lesion. On day 10 post infection, an accumulation of foamy appearance cells was observed in the area enclosed by a white broken line only in TLR2 KO mice. The square specifies the area for high power (×400) observation of the foamy appearance cells. To determine the cell type of the foamy cells, neutrophils and macrophages were visualized using anti-Ly6G and anti-F4/80 antibody, respectively. Most cells in the enclosed area were Ly6G-positve but F4/80-negative, suggesting that these cells were mostly neutrophils. Unlike wild-type and TLR2/TLR4 dKO lesions, on day 21, periapical lesions in TLR2 KO mice were rich in capillaries (C), neutrophils, and iNOS-positive cells.
Figure 4. Capillaries observed in TLR2 KO mice were in part consisting of CD34-positive cells.
Panels are representative images of anti-CD34 (a hematopoietic progenitor cell antigen) immunostaining, visualizing cells/capillaries under de novo angiogenesis, in periapical lesion of wild-type C57BL/6J, TLR2 KO and TLR2/TLR4 dKO mice on day 21 post infection. The original magnification was ×200. Polygonal orange and red lines outline the margin of dental root (R) and alveolar bone (B), respectively. The area lying between these two lines is periapical lesion (L). Arrows indicate the area of capillaries with CD34-positive cells. Capillaries with CD34-positive cells were observed only in TLR2 KO mice, suggesting that active angiogenesis occurred in infected TLR2 KO mice.
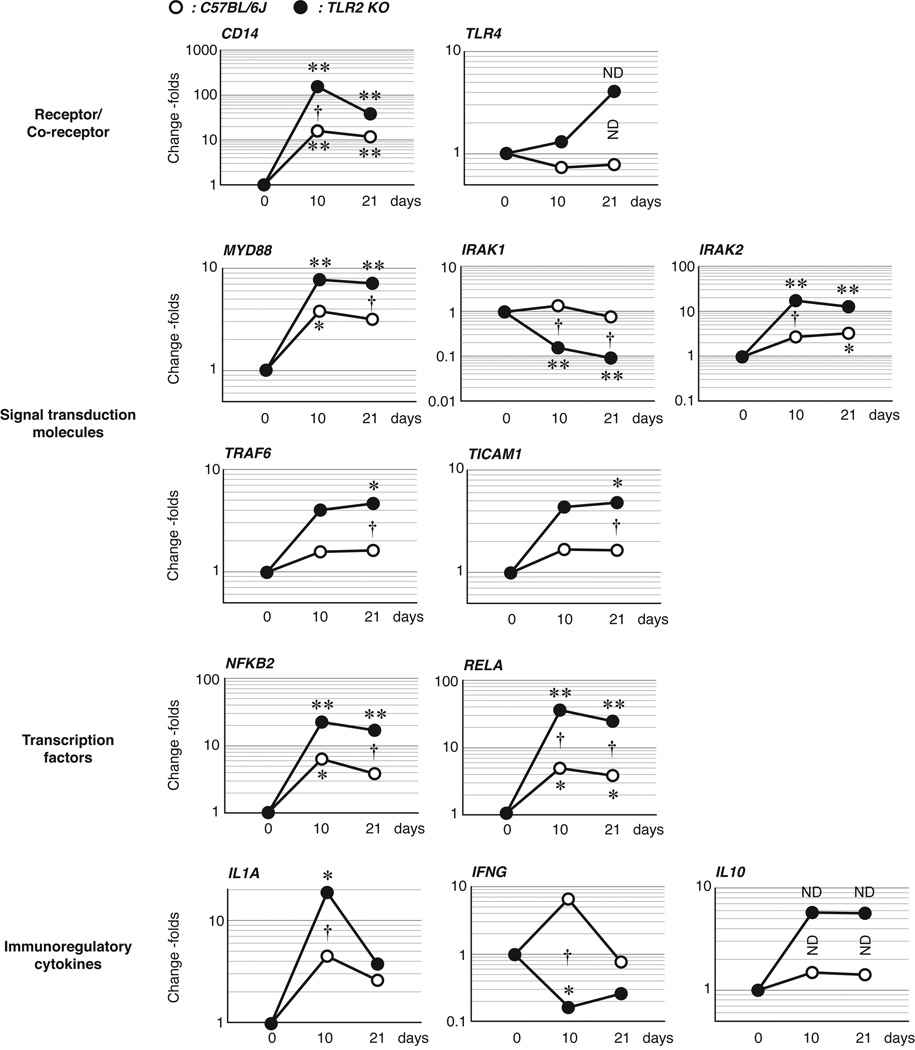
We, therefore, comprehensively examined whether TLR2 deficiency led to an elevated TLR4 signaling in periapical lesions by quantitative RT-PCR. As shown in Figure 5, overall, infected TLR2 KO mice showed a profile indicating elevated TLR4 signaling and subsequent NF-κB activation compared to that in infected wild-type mice. Interestingly, the expression of the TLR4 gene was not affected by either endodontic infection or TLR2 deficiency. In our data, CD14 gene was strikingly up-regulated in TLR2 KO mice on Day 10 post infection. This data suggests that the TLR2 signal appears to be suppressing CD14 expression in wild-type mice. TLR2 KO mice supposed to be utilizing both MyD88- and TICAM1-dependent pathways in up-regulation of NF-κB genes, particularly NFkB2 and RELA. Further, IL1A gene was significantly up-regulated in TLR2 KO mice on Day 10, indicating altered pro-inflammatory response in early phase of periapical inflammation. On the other hand, a central type 1 helper T lymphocyte cytokine interferon gamma (IFNγ seemed not to be involved much in the development of periapical lesion in both strains corroborating a previous study using IFNγ KO mice (Sasaki et al., 2004). The TLR2 signal is involved in expression of an immunosuppressive cytokine interleukin (IL)-10 (Clohisy et al., 2004; Gaddis et al., 2013), which potently suppresses periapical inflammation and bone loss (Sasaki et al., 2000). In the present study, the IL10 gene expression in periapical lesions has not been modulated by TLR2 deficiency. These data lead us to a hypothesis that the TLR2 signaling negatively regulates the TLR4 signaling via CD14 in polymicrobial endodontic infection.
Figure 5. Infected TLR2 KO mice exhibited elevated TLR4 signaling compared to that in wild-type mice.
TLR4 signaling-related gene expression profile in TLR2 KO and wild-type C57BL/6J mice was determined by real-time RT-PCR on days 10 and 21 post infection (n = 3, triplicates per sample). The data set was organized based on the TLR4 signal transduction pathway. Non-infected mice (day 0) in each genotype served as baseline controls to determine infection effect (*: p<0.05; **p<0.01 vs. Day 0). Genotype effect (TLR2 KO vs. C57BL/6J) was determined on days 10 and 21 separately (†: p<0.05). Open dot: C57BL/6J mice, Closed dot: TLR2 KO mice, X-axis: observation periods, Y-axis: fold change value in a semi-logarithmic format. ND in horizontal orientation: no statistical difference vs. Day 0; ND in vertical orientation: no statistical difference between the genotypes.
TLR2 deficiency up-regulates cellular CD14 expression and bacteria-stimulated proinflammatory cytokines by macrophages in vitro
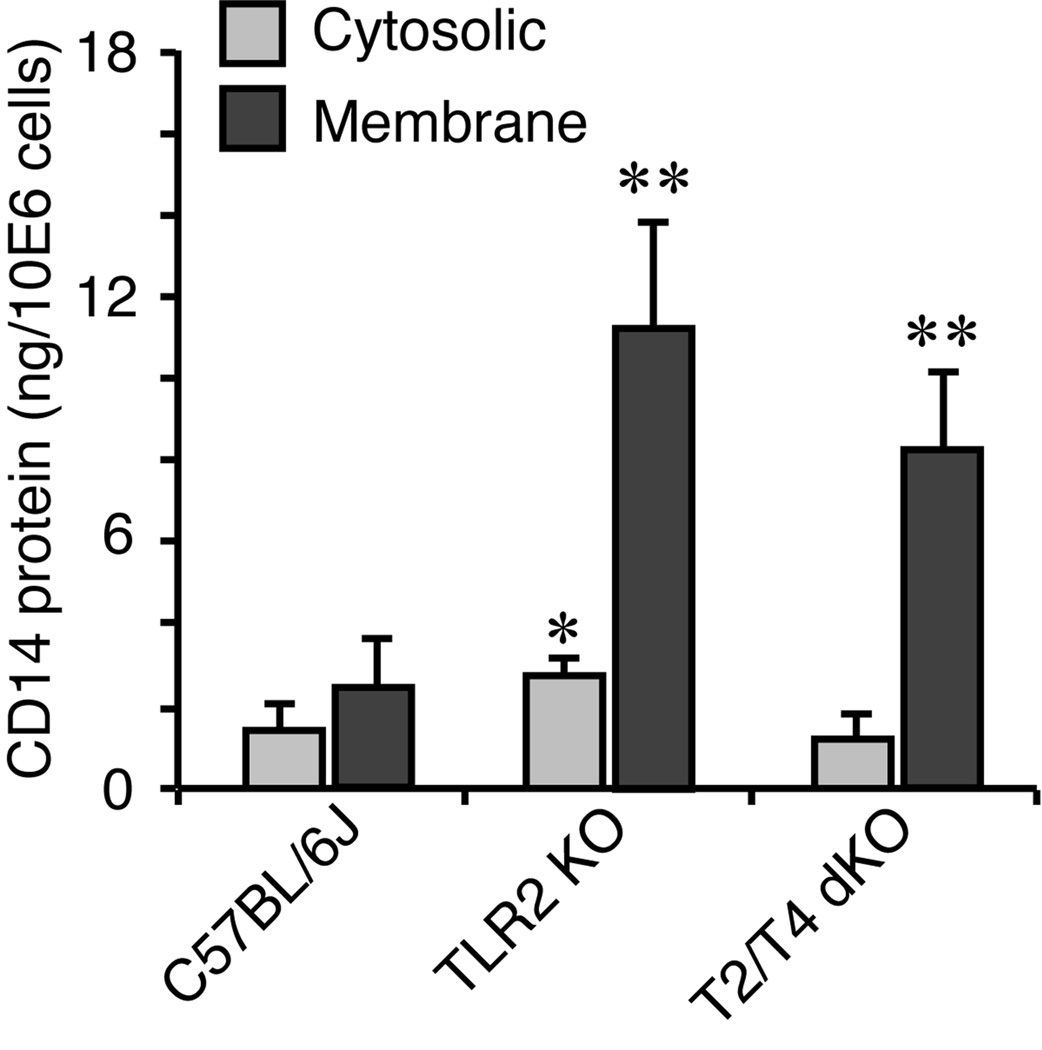
We next examined the impact of TLR2 deficiency on cellular CD14 expression in macrophages, which are prominent cells in the development of periapical lesions (Tani-Ishii et al., 1995). As shown in Fig. 6, membrane-bound form CD14 was largely affected by TLR2 deficiency (4.6-fold higher in TLR2 KO and 3.4-fold higher in TLR2/TLR4 dKO vs. wild-type cells; p<0.01, respectively). The elevation of cytosolic CD14 in TLR2 KO macrophages was 1.9-fold vs. wild-type observed (p<0.05) while cytosolic CD14 was not elevated in TLR2/TLR4 dKO cells. Although CD14 also exists in a soluble form (Kirkland and Viriyakosol, 1998), deficiencies of TLR2 and TLR2/TLR4 did not alter physiological secretion of soluble CD14 in 24-hour observation (data not shown). The elevation of total cellular CD14 (sum of membrane-bound and cytosolic forms) was 3.36-fold in TLR2 KO and 2.2-fold in TLR2/TLR4 KO compared to that in wild-type cells (p<0.01, respectively). There was no statistical significance between TLR2 KO and TLR2/TLR4 dKO cells (p=0.099).
Figure 6. TLR2 deficiency led to elevation of cellular CD14 protein expression in resident peritoneal macrophages.
The level of cytosolic and membrane-bounded CD14 protein expression was quantified by ELISA (n=3 per condition) and compared among wild-type C57BL/6J, TLR2 KO and TLR2/TLR4 dKO strains. The results were expressed in nano grams of CD14 protein per 1×106 cells. TLR2 deficiency (TLR2 KO or TLR2/TLR4 KO cells) resulted in significant elevation of membrane-bounded CD14 protein compared to wild-type cells. TLR2 single deficiency also up-regulated cytosolic CD14 protein expression compared with wild-type cells. Vertical bar: SD, *: p<0.05, **: p<0.01 vs. wild-type.
We further assessed the effect of TLR2 deficiency on macrophage-derived proinflammatory cytokinesIL-1 production in vitro (Tables 2 and 3). Overall, TLR2 KO macrophages exhibited hyper production of IL-1α by E. coli LPS and fixed whole bacteria including Gram-positive species. In contrast, the production of IL-1 by TLR2/TLR4 dKO cells was generally lower than that by TLR2 KO cells and similar to that by wild-type cells. As expected, TLR2 KO and TLR2/TLR4 dKO cells were hyporesponsive to Pam2CSK4 and both LPS and Pam2CSK4, respectively. These data suggest that TLR2 deficiency compels elevation of cellular CD14 expression and overly up-regulates CD14-TLR4 signaling upon infection.
Table 2.
IL-1α production by TLR2 KO and TLR2/TLR4 dKO primary macrophages in vitro.
| Stimuli | C57BL/6J | TLR2 KO | TLR2/TLR4 KO |
|---|---|---|---|
| Negative control | 8.52±8.03 | 42.67±28.10 * | 31.34±5.12 * |
| LPS | 97.37±18.22 | 6544.00±2225.25 * | 25.78±9.17 *† |
| Pam2CSK4 | 59.46±6.87 | 40.00±8.00 * | 12.67±6.21 *† |
| P. intermedia | 134.80±22.69 | 1672.00±444.49 * | 82.32±16.42 *† |
| F. nucleatum | 336.28±87.84 | 7272.00±2056.05 * | 372.94±96.05 † |
| S. intermedius | 228.50±42.54 | 7293.33±1610.85 * | 350.46±16.42 *† |
| P. micra | 32.33±1.59 | 2168.00±1154.86 * | 40.02±9.50 † |
| Mixed | 300.74±137.01 | 6850.67±604.75 * | 218.06±20.09 † |
Each data indicates Mean value ± Standard Deviation in pg cytokine/ml culture supernatant.
: p<0.01 vs. C57BL cells under the same condition.
: p<0.01 vs. TLR2 KO cells under the same condition.
Table 3.
IL-1β production by TLR2 KO and TLR2/TLR4 dKO primary macrophages in vitro.
| Stimuli | C57BL/6J | TLR2 KO | TLR2/TLR4 KO |
|---|---|---|---|
| Negative control | 32.27±6.21 | 24.03±0.05 | 22.48±3.64 |
| LPS | 61.53±6.70 | 381.33±154.78 * | 30.55±23.28 † |
| Pam2CSK4 | 31.09±14.32 | 8.03±0.05 * | 7.42±0.73 * |
| P. intermedia | 61.45±4.09 | 72.00±13.86 | 33.46±6.76 † |
| F. nucleatum | 150.88±42.41 | 957.33±156.84 * | 231.96±18.52 *† |
| S. intermedius | 179.98±20.60 | 579.00±149.50 * | 229.80±61.55 † |
| P. micra | 44.76±9.06 | 213.33±92.72 * | 30.93±4.37 † |
| Mixed | 190.78±112.72 | 922.67±9.24 * | 122.01±22.32 † |
Each data indicates Mean value ± Standard Deviation in pg cytokine/ml culture supernatant.
: p<0.01 vs. C57BL cells under the same condition.
: p<0.01 vs. TLR2 KO cells under the same condition.
CD14 neutralization attenuates TLR2 deficiency-dependent inflammation
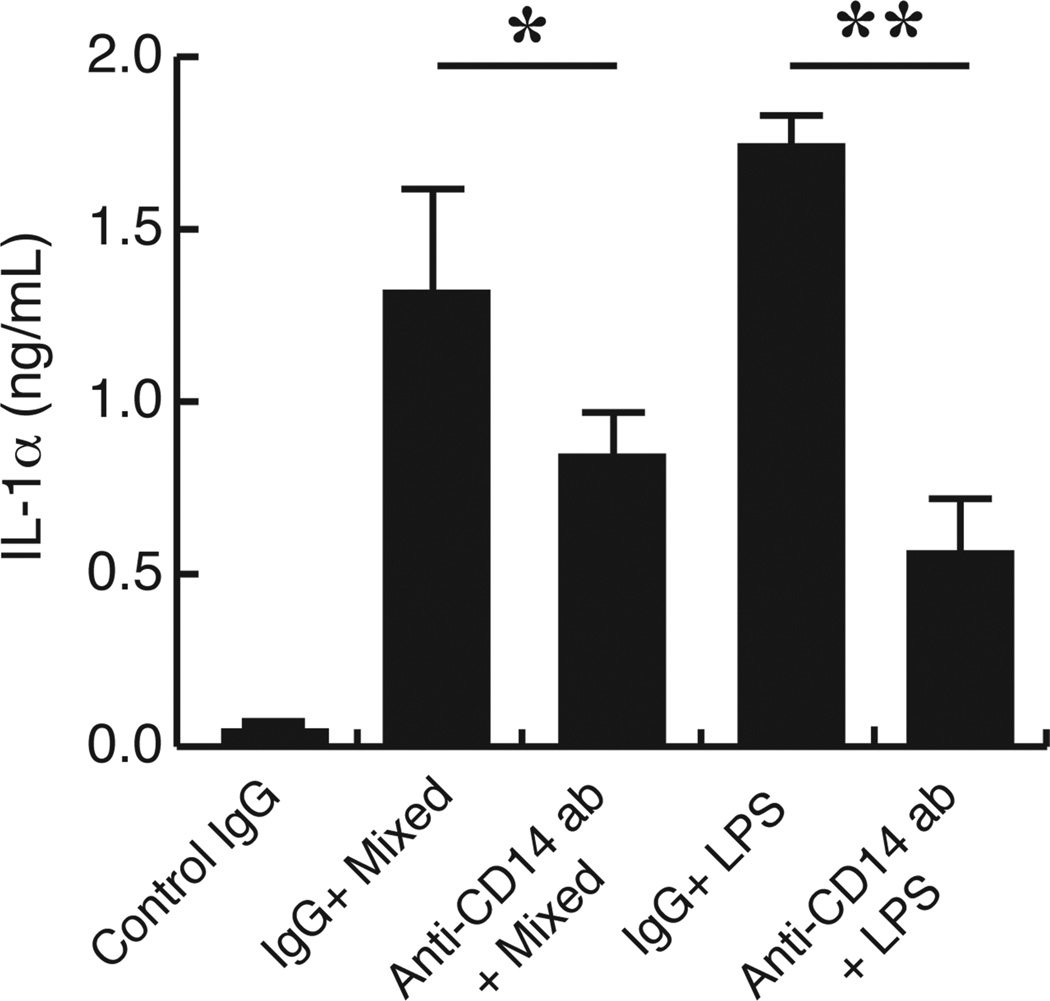
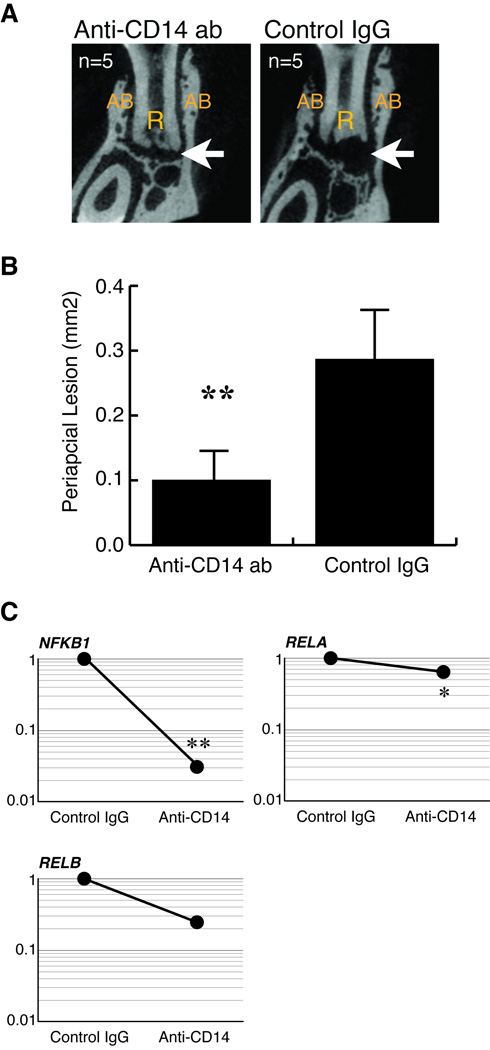
Finally, we tested whether blocking CD14 attenuates TLR4-mediated inflammation under TLR2 deficiency in vitro and in vivo. Resident TLR2 KO peritoneal macrophages were stimulated with whole endodontic pathogens or E. coli LPS in presence or absence of anti-CD14 antibody. As shown in Figure 7, the anti-CD14 antibody treatment potently suppressed bacteria-stimulated IL-1α production by TLR2 deficient macrophages compared to control IgG-treated cells (by 60%). According to these data, we neutralized CD14 in infected TLR2 KO mice as described in the materials and methods. The extent of periapical lesion was determined on day 21 post-infection. As shown in Figures 8A and 8B, anti-CD14 treatments strikingly suppressed the extent of periapical lesion by approximately 35% compared to that in the control IgG-treated animals. As shown in Fig. 8C, CD14 neutralization led to down-regulation of NFKB1 and RELA with statistical significance. We also observed a trend of down-regulation of RELB. These results proved that CD14 is involved in up-regulation of periapical inflammation and bone destruction under TLR2 deficiency.
Figure 7. CD14 neutralization attenuated IL-1α production by in TLR2 KO macrophages in vitro.
Resident peritoneal TLR2 KO macrophages were stimulated with either mixed endodontic pathogens (1×109 cells/ml) or E. coli LPS (1 µg/ml) in presence of anti-CD14 antibody or isotype-matched control IgG (5 µg/ml, respectively) (n = 3 per group). Cells treated with control IgG alone served as negative controls (n = 3). The level of IL-1α in culture supernatants was determined by ELISA, and the results were expressed in nano grams of IL-1α per milliliter supernatant. Vertical bar: SD, *: p<0.05, **: p<0.01.
Figure 8. CD14 neutralization attenuated periapical inflammation in TLR2 KO mice.
A. Representative µCT images of periapical lesions in anterior-posterior direction. The effect of CD14 neutralization on infection-induced periapical bone loss in TLR2 KO mice was determined on day 21 post infection (n = 5). Mice that received isotype-matched control IgG served as disease controls (n = 5). The treatment regimen was described in the materials and methods. The position of each CT slice was the most central part of mandibular first molar distal root (R). The original length of the side of each square CT image was 2.4 mm. Arrow points to radiolucent periapical lesion. AB: alveolar bone.
B. Size of periapical lesions. CD14 neutralization effectively inhibited the development of periapical lesion in TLR2 KO mice. Anti-CD14 antibody treatment significantly suppressed the extent of periapical lesions in TLR2 KO mice. **: p<0.01 vs. control IgG group.
C. Effect of CD14 neutralization on NF-κB genes. The treatment effect on NF-κB gene expressions was determined by real-time RT-PCR (n = 3, triplicates per sample). CD14 neutralization suppressed the expression of NF-κB genes in periapical lesions compared to control IgG group (*: p<0.05; **p<0.01 vs. control IgG group). Y-axis: relative gene expression level (fold change value) in a semi-logarithmic format.
Discussion
In the present study, we have investigated a critical immunoregulatory mechanism involving of TLR2, TLR4, and CD14 in response to poly-microbial endodontic infection. As summarized in Fig. 9, we demonstrated that TLR2 signaling down-regulates TLR4 signaling-mediated periapical inflammation via suppression of CD14 using genetic mutant mouse models and functional neutralization of CD14 in vivo and in vitro.
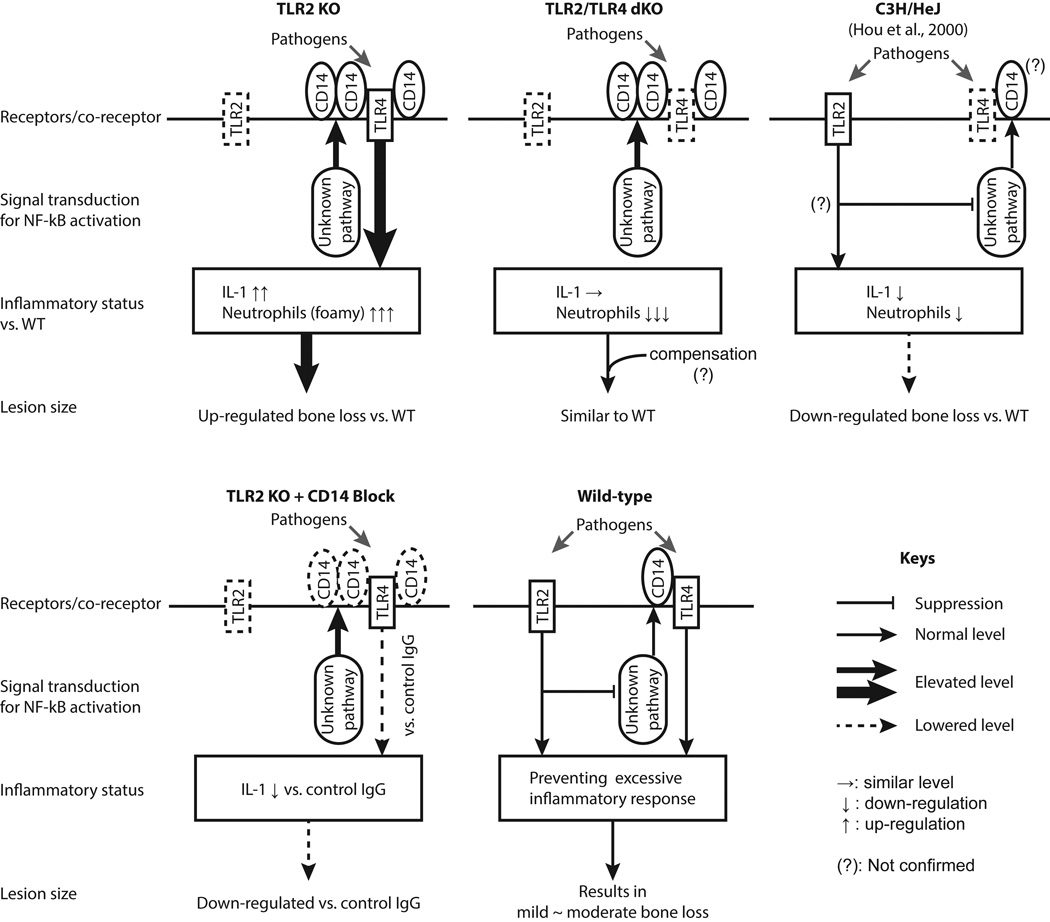
Figure 9. Graphic summary of present findings.
The effect of TLR2 deficiency on the level of TLR4 signaling, subsequent pro-inflammatory response, and bone destruction was graphically summarized. The panel of C3H/HeJ strain, in which TLR4 signaling is missing, is based on a published research article (Hou et al., 2000b). Taken together, our data suggest that TLR2 plays a regulatory role preventing over activation of CD14/TLR4 signaling-mediated inflammatory response in mouse periapical lesions.
TLRs are pattern recognition receptors, and each TLR recognizes specific pathogen-associated molecular patterns such as lipoteichoic acids and lipopolysaccharide at infection. According to this specificity, TLR2 and TLR4 are thought to be important in recognition of Gram-positive and negative pathogens, respectively (Hoshino et al., 1999; Takeuchi et al., 1999). However, regardless of Gram stainability, endodontic pathogens have stimulated proinflammatory cytokine production by primary macrophages in all genotypes. Particularly, in TLR2 KO macrophages, IL-1 production induced by Gram-positive S. intermedius and P. micros was significantly elevated in a range of 3- to 67-fold compared with wild-type cells. In contrast, bacteria-induced hyper cytokine production under TLR2 deficiency became invalid in TLR2/TLR4 dKO cells. Therefore, our data suggest that TLR4 signaling seems to be the most important sensing pathway for both Gram-positive and negative bacteria and subsequent inflammatory responses in polymicrobial endodontic infection.
In our data, the level of IL-1 secreted by TLR2/TLR4 dKO macrophages in vitro was in part similar to that in wild-type cells. In addition, the extent of periapical lesions in TLR2/TL4 dKO mice was similar to that in wild-type controls. We expect that the TLR2/TLR4 double deficiency led to functional compensation by such as other TLRs, resulting in induction of the same level of inflammatory response and bone loss compared to the wild-type. However, details of the functional compensation are unknown and to be determined in follow-up studies.
The result of gene expression analysis (Fig. 5) suggests that the up-regulated TLR4 signaling in infected TLR2 KO mice seems not to be dependent on TLR4 itself. It is widely accepted that the activation of the TLR4 signaling in part depends on other co-receptors including CD14, LBP, and MD2 (Muta and Takeshige, 2001; Gioannini et al., 2004; Akashi-Takamura and Miyake, 2008). In this study, CD14 was strikingly up-regulated by TLR2 deficiency and played a key role in elevation of TLR4 signal activation. The roles of CD14 in TLR4 signaling include: 1) binding with PAMPs; 2) presentation of PAMPs to TLR4; and 3) elevation of TLR4 signaling (Jiang et al., 2000; Muroi et al., 2002). We therefore think that the elevated CD14 expression could make TLR2 KO mice more responsive to polymicrobial infection than wild-type via up-regulation of TLR4 signaling.
Our histological examination revealed that TLR2 deficiency led to toxic change in neutrophils and a pro-angiogenic response. Vacuolization (vacuolation) is a sign of toxic neutrophils, which were frequently observed in acute inflammation and bacterial infections (Malcolm et al., 1979; Liu et al., 1984; Gossett et al., 1985). These toxic neutrophils were iNOS positive cells. In addition, infected TLR2 KO mice exhibited a pro-angiogenic response with continuous accumulation of neutrophils on day 21 post-infection, whereas the importance of TLR2/MyD88 signal in angiogenic response to oxidative stress was previously demonstrated (West et al., 2010). Such TLR2 deficiency-dependent pro-angiogenic response was also observed in an ischemia model (Wagner et al., 2013). In a previous report, ischemia-induced tissue necrosis was observed only in TLR2 KO but not in wild-type and TLR4 KO mice (Sachdev et al., 2012). In the same study, inflammatory response in TLR2 KO mice was mediated by TLR4-MyD88 pathway (Sachdev et al., 2012). On the other hand, Mycobacterium tuberculosis and Toxoplasma gondii were shown to utlize TLR2/MyD88 pathway to induce arginase 1 (ARG1) leading to inhibition of pro-angiogenic iNOS expression (El Kasmi et al., 2008). Although further investigations are necessary in periapical inflammation, these previous observations suggest that imbalance of TLR-mediated ARG1 and iNOS expressions seems to be a potential mechanism of the elevated myeloid inflammation and pro-angiogenic response in infected TLR2 KO mice. Taken together, these findings suggest two important things: 1) the TLR functional network that consists of TLR2, TLR4, CD14, and MyD88 primarily regulates responses of neutrophils and macrophages; and 2) impaired TLR network, caused by TLR2 deficiency in this study, influences not only myeloid cell responses but also pro-angiogenic response in inflammation.
Intracellular signaling derived from pattern recognition receptors constitutes complex interactions including both synergistic and antagonistic pathways in innate immunity, and a considerable number of pathogens are able to alter innate immune signaling for persistent infections (Hajishengallis and Lambris, 2011). To date, the importance of TLR2 has been emphasized particularly in induction of periodontitis. A Gram-negative oral pathogen Porphyromonas gingivalis induces alveolar bone loss via TLR2 but not TLR4 (Burns et al., 2006; Papadopoulos et al., 2013). More specifically, TLR2-mediated tumor necrosis factor alpha (TNFα production by macrophages was suggested as an important mechanism of P. gingivalis-stimulated marginal periodontitis (Papadopoulos et al., 2013) while P. gingivalis potentially antagonize TLR4-meidated antimicrobial responses (Darveau, 2010). Although P. gingivalis is involved in human infected root canals and periapical lesions (de Souza et al., 2005; Lin et al., 2007), the impact of P. gingivalis as a constituent of poly-microbial endodontic infection vs. a P. gingivalis mono-infection has not been precisely examined in endodontic microflora and periapical inflammation.
Since TLR2 naturally expresses on resting/resident macrophages (Trinchieri and Sher, 2007), TLR2 signaling appears to be a key natural endogenous suppressor of cellular CD14 expression. We thus think that this TLR2-mediated suppression of CD14 is an auto-regulatory mechanism that prevents overactive CD14/TLR4-mediated inflammation against poly microbial infection. The mechanism for TLR2-mediated suppression of CD14 needs to be determined by future studies. The proposed immunoregulatory network would impact the understanding of host defense system and the therapeutic strategies not only for oral infections but also for systemic polymicrobial infections such as sepsis.
Supplementary Material
Acknowledgments
Authors thank to Ms. Justine Dobeck and Mr. Subbiah Yoganathan (both the Forsyth Institute) for their assistance in histology and animal care, respectively. We express our thanks to Drs. Martin Taubman, Susan Rittling, and Xiaozhe Han (all Forsyth) for their helpful discussions. We are grateful to Ms. Susan Orlando for her assistance in preparation of this manuscript.
This work was in part supported by grants from The JSPS Strategic Young Researcher Overseas Visits Program for Accelerating Brain Circulation (H.F), RR027553 (H.S) from NCRR/NIH, R21DE023178 (H.S), and R01DE024796 (H.S) from NIDCR/NIH.
Footnotes
The authors have no conflicting financial interests.
Literature Cited
- Akashi-Takamura S, Miyake K. TLR accessory molecules. Curr Opin Immunol. 2008;20:420–425. doi: 10.1016/j.coi.2008.07.001. [DOI] [PubMed] [Google Scholar]
- AlShwaimi E, Berggreen E, Furusho H, Rossall JC, Dobeck J, Yoganathan S, Stashenko P, Sasaki H. IL-17 receptor A signaling is protective in infection-stimulated periapical bone destruction. J Immunol. 2013;191:1785–1791. doi: 10.4049/jimmunol.1202194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bettencourt MC, Bauer JJ, Sesterhenn IA, Connelly RR, Moul JW. CD34 immunohistochemical assessment of angiogenesis as a prognostic marker for prostate cancer recurrence after radical prostatectomy. J Urol. 1998;160:459–465. [PubMed] [Google Scholar]
- Burns E, Bachrach G, Shapira L, Nussbaum G. Cutting Edge: TLR2 is required for the innate response to Porphyromonas gingivalis: activation leads to bacterial persistence and TLR2 deficiency attenuates induced alveolar bone resorption. J Immunol. 2006;177:8296–8300. doi: 10.4049/jimmunol.177.12.8296. [DOI] [PubMed] [Google Scholar]
- Clohisy JC, Hirayama T, Frazier E, Han SK, Abu-Amer Y. NF-kB signaling blockade abolishes implant particle-induced osteoclastogenesis. J Orthop Res. 2004;22:13–20. doi: 10.1016/S0736-0266(03)00156-6. [DOI] [PubMed] [Google Scholar]
- da Silva RA, Ferreira PD, De Rossi A, Nelson-Filho P, Silva LA. Toll-like receptor 2 knockout mice showed increased periapical lesion size and osteoclast number. J Endod. 2012;38:803–813. doi: 10.1016/j.joen.2012.03.017. [DOI] [PubMed] [Google Scholar]
- Darveau RP. Periodontitis: a polymicrobial disruption of host homeostasis. Nature reviews Microbiology. 2010;8:481–490. doi: 10.1038/nrmicro2337. [DOI] [PubMed] [Google Scholar]
- de Souza CA, Teles RP, Souto R, Chaves MA, Colombo AP. Endodontic therapy associated with calcium hydroxide as an intracanal dressing: microbiologic evaluation by the checkerboard DNA-DNA hybridization technique. J Endod. 2005;31:79–83. doi: 10.1097/01.don.0000133157.60731.3f. [DOI] [PubMed] [Google Scholar]
- El Kasmi KC, Qualls JE, Pesce JT, Smith AM, Thompson RW, Henao-Tamayo M, Basaraba RJ, Konig T, Schleicher U, Koo MS, Kaplan G, Fitzgerald KA, Tuomanen EI, Orme IM, Kanneganti TD, Bogdan C, Wynn TA, Murray PJ. Toll-like receptor-induced arginase 1 in macrophages thwarts effective immunity against intracellular pathogens. Nature immunology. 2008;9:1399–1406. doi: 10.1038/ni.1671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaddis DE, Maynard CL, Weaver CT, Michalek SM, Katz J. Role of TLR2-dependent IL-10 production in the inhibition of the initial IFN-gamma T cell response to Porphyromonas gingivalis. J Leukoc Biol. 2013;93:21–31. doi: 10.1189/jlb.0512220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerold G, Zychlinsky A, de Diego JL. What is the role of Toll-like receptors in bacterial infections? Seminars in immunology. 2007;19:41–47. doi: 10.1016/j.smim.2006.12.003. [DOI] [PubMed] [Google Scholar]
- Gioannini TL, Teghanemt A, Zhang D, Coussens NP, Dockstader W, Ramaswamy S, Weiss JP. Isolation of an endotoxin-MD-2 complex that produces Toll-like receptor 4-dependent cell activation at picomolar concentrations. Proc Natl Acad Sci U S A. 2004;101:4186–4191. doi: 10.1073/pnas.0306906101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gossett KA, MacWilliams PS, Cleghorn B. Sequential morphological and quantitative changes in blood and bone marrow neutrophils in dogs with acute inflammation. Canadian journal of comparative medicine Revue canadienne de medecine comparee. 1985;49:291–297. [PMC free article] [PubMed] [Google Scholar]
- Hajishengallis G, Lambris JD. Microbial manipulation of receptor crosstalk in innate immunity. Nat Rev Immunol. 2011;11:187–200. doi: 10.1038/nri2918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoshino K, Takeuchi O, Kawai T, Sanjo H, Ogawa T, Takeda Y, Takeda K, Akira S. Cutting edge: Toll-like receptor 4 (TLR4)-deficient mice are hyporesponsive to lipopolysaccharide: evidence for TLR4 as the Lps gene product. J Immunol. 1999;162:3749–3752. [PubMed] [Google Scholar]
- Hou L, Sasaki H, Stashenko P. B-Cell deficiency predisposes mice to disseminating anaerobic infections: protection by passive antibody transfer. Infect Immun. 2000a;68:5645–5651. doi: 10.1128/iai.68.10.5645-5651.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou L, Sasaki H, Stashenko P. Toll-like receptor 4-deficient mice have reduced bone destruction following mixed anaerobic infection. Infect Immun. 2000b;68:4681–4687. doi: 10.1128/iai.68.8.4681-4687.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang Q, Akashi S, Miyake K, Petty HR. Lipopolysaccharide induces physical proximity between CD14 and toll-like receptor 4 (TLR4) prior to nuclear translocation of NF-kappa. B. J Immunol. 2000;165:3541–3544. doi: 10.4049/jimmunol.165.7.3541. [DOI] [PubMed] [Google Scholar]
- Kirkland TN, Viriyakosol S. Structure-function analysis of soluble and membrane-bound CD14. Progress in clinical and biological research. 1998;397:79–87. [PubMed] [Google Scholar]
- Knapp S, Wieland CW, van 't Veer C, Takeuchi O, Akira S, Florquin S, van der Poll T. Toll-like receptor 2 plays a role in the early inflammatory response to murine pneumococcal pneumonia but does not contribute to antibacterial defense. J Immunol. 2004;172:3132–3138. doi: 10.4049/jimmunol.172.5.3132. [DOI] [PubMed] [Google Scholar]
- Lin S, Sela G, Sprecher H. Periopathogenic bacteria in persistent periapical lesions: an in vivo prospective study. J Periodontol. 2007;78:905–908. doi: 10.1902/jop.2007.060224. [DOI] [PubMed] [Google Scholar]
- Liu CH, Lehan C, Speer ME, Fernbach DJ, Rudolph AJ. Degenerative changes in neutrophils: an indicator of bacterial infection. Pediatrics. 1984;74:823–827. [PubMed] [Google Scholar]
- Malcolm ID, Flegel KM, Katz M. Vacuolization of the neutrophil in bacteremia. Archives of internal medicine. 1979;139:675–676. [PubMed] [Google Scholar]
- Medzhitov R. Toll-like receptors and innate immunity. Nat Rev Immunol. 2001;1:135–145. doi: 10.1038/35100529. [DOI] [PubMed] [Google Scholar]
- Muroi M, Ohnishi T, Tanamoto K. Regions of the mouse CD14 molecule required for toll-like receptor 2- and 4-mediated activation of NF-kappa B. J Biol Chem. 2002;277:42372–42379. doi: 10.1074/jbc.M205966200. [DOI] [PubMed] [Google Scholar]
- Muta T, Takeshige K. Essential roles of CD14 and lipopolysaccharide-binding protein for activation of toll-like receptor (TLR)2 as well as TLR4 Reconstitution of TLR2- and TLR4-activation by distinguishable ligands in LPS preparations. European journal of biochemistry / FEBS. 2001;268:4580–4589. doi: 10.1046/j.1432-1327.2001.02385.x. [DOI] [PubMed] [Google Scholar]
- Papadopoulos G, Weinberg EO, Massari P, Gibson FC, 3rd, Wetzler LM, Morgan EF, Genco CA. Macrophage-specific TLR2 signaling mediates pathogen-induced TNF-dependent inflammatory oral bone loss. J Immunol. 2013;190:1148–1157. doi: 10.4049/jimmunol.1202511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sachdev U, Cui X, McEnaney R, Wang T, Benabou K, Tzeng E. TLR2 and TLR4 mediate differential responses to limb ischemia through MyD88-dependent and independent pathways. PloS one. 2012;7:e50654. doi: 10.1371/journal.pone.0050654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasaki H, Balto K, Kawashima N, Eastcott J, Hoshino K, Akira S, Stashenko P. Gamma interferon (IFN-gamma) and IFN-gamma-inducing cytokines interleukin-12 (IL-12) and IL-18 do not augment infection-stimulated bone resorption in vivo. Clin Diagn Lab Immunol. 2004;11:106–110. doi: 10.1128/CDLI.11.1.106-110.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasaki H, Hou L, Belani A, Wang CY, Uchiyama T, Muller R, Stashenko P. IL-10, but not IL-4, suppresses infection-stimulated bone resorption in vivo. J Immunol. 2000;165:3626–3630. doi: 10.4049/jimmunol.165.7.3626. [DOI] [PubMed] [Google Scholar]
- Takeuchi O, Hoshino K, Akira S. Cutting edge: TLR2-deficient and MyD88-deficient mice are highly susceptible to Staphylococcus aureus infection. J Immunol. 2000;165:5392–5396. doi: 10.4049/jimmunol.165.10.5392. [DOI] [PubMed] [Google Scholar]
- Takeuchi O, Hoshino K, Kawai T, Sanjo H, Takada H, Ogawa T, Takeda K, Akira S. Differential roles of TLR2 and TLR4 in recognition of gram-negative and gram-positive bacterial cell wall components. Immunity. 1999;11:443–451. doi: 10.1016/s1074-7613(00)80119-3. [DOI] [PubMed] [Google Scholar]
- Tani-Ishii N, Wang CY, Stashenko P. Immunolocalization of bone-resorptive cytokines in rat pulp and periapical lesions following surgical pulp exposure. Oral Microbiol Immunol. 1995;10:213–219. doi: 10.1111/j.1399-302x.1995.tb00145.x. [DOI] [PubMed] [Google Scholar]
- Trinchieri G, Sher A. Cooperation of Toll-like receptor signals in innate immune defence. Nat Rev Immunol. 2007;7:179–190. doi: 10.1038/nri2038. [DOI] [PubMed] [Google Scholar]
- Wagner NM, Bierhansl L, Noldge-Schomburg G, Vollmar B, Roesner JP. Toll-like receptor 2-blocking antibodies promote angiogenesis and induce ERK1/2 and AKT signaling via CXCR4 in endothelial cells. Arteriosclerosis, thrombosis, and vascular biology. 2013;33:1943–1951. doi: 10.1161/ATVBAHA.113.301783. [DOI] [PubMed] [Google Scholar]
- Wang CY, Stashenko P. The role of interleukin-1 alpha in the pathogenesis of periapical bone destruction in a rat model system. Oral Microbiol Immunol. 1993;8:50–56. doi: 10.1111/j.1399-302x.1993.tb00543.x. [DOI] [PubMed] [Google Scholar]
- Weiss DS, Raupach B, Takeda K, Akira S, Zychlinsky A. Toll-like receptors are temporally involved in host defense. J Immunol. 2004;172:4463–4469. doi: 10.4049/jimmunol.172.7.4463. [DOI] [PubMed] [Google Scholar]
- West XZ, Malinin NL, Merkulova AA, Tischenko M, Kerr BA, Borden EC, Podrez EA, Salomon RG, Byzova TV. Oxidative stress induces angiogenesis by activating TLR2 with novel endogenous ligands. Nature. 2010;467:972–976. doi: 10.1038/nature09421. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.