Abstract
Importance
Patching has been a mainstay in treating unilateral congenital cataract. However, its efficacy has not been rigorously assessed.
Objective
As a secondary aim of the Infant Aphakia Treatment Study (IATS) we xxamined the relationship between patching and visual acuity in a cohort of children treated for unilateral congenital cataract.
Design
A randomized clinical trial comparing two treatments for unilateral congenital cataract among children born between 2004 and 2009.
Participants
Infants treated for unilateral congenital cataract and followed to age 5.
Intervention
Cataract extraction and randomization to receipt of an intraocular lens (IOL) and being left aphakic for the first five years of life.
Setting
The practices of 13 experienced pediatric ophthalmologists in the US.
Main Outcomes and Measures
Caregivers reported patching in the previous 48-hours in quarterly semi-structured telephone interviews. The average number of hours of patching per day was calculated from surgery to the first birthday (n=92) and between 12 and 48 months of age (n=102). Monocular optotype acuity was assessed at age 4½ years by a traveling examiner using the ATS-HOTV protocol.
Results
Caregivers reported patching their children an average of 3.73 ± 1.47 hours per day in the first year of life and 3.43 ± 2.04 hours per day thereafter. An association between reported patching and treatment was not identified (average difference in 1st year: −0.29 hours per day 95% CI = −0.90, 0.33; average difference between 12 and 48 months of age −0.40 95% CI = −1.20,0.40). Visual acuity was associated with reported hours of patching in the first year of life (rpearson = −0.32 95% CI = −0.49, −0.13) and between 12 and 48 months of age (rpearson = −0.36 95% CI = −0.52, −0.18). However, patching accounted for less than 15% of the variance in log MAR acuity at age 4½ years.
Conclusions and Relevance
These results support the association of occlusion throughout the preschool years with improved visual acuity in infants treated for unilateral congenital cataract. However, similar visual outcomes were achieved with varying amounts of patching. These conclusions are limited given limitations related to generalizability associated with using incomplete data from a clinical trial.
Introduction
Infants born with visually significant unilateral cataracts often have poor visual outcomes. Previous reports suggest that achieving a good outcome requires early surgical removal of the cataract, consistent optical correction, and good adherence to a regimen of occlusion of the fellow eye (1–5).
Chak and colleagues report that poor adherence to occlusion is strongly associated with poor visual acuity in these children (6). However, others note that good outcomes are achievable with good adherence to patching in the first year of life, but lower levels thereafter (7). Adherence to occlusion therapy is likely necessary for achieving achieve good visual acuity, but adherence and visual acuity are may also likely associated because predict adherence, as patching is more difficult in children with poor vision (8). The impact of adherence to occlusion therapy on visual outcome is, therefore, difficult to assess. Further, although the efficacy of occlusion therapy for treating other types of amblyopia has been evaluated (9), its efficacy in children with unilateral congenital cataract has not been rigorously examined (10).
The Infant Aphakia Treatment Study (IATS) is a multi-center, randomized, controlled clinical trial of treatment for unilateral congenital cataract. The objective is to compare visual acuity in children with a unilateral congenital cataract if an intraocular lens (IOL) is implanted at the time of cataract extraction with visual acuity in children left aphakic (8). The IATS has documented that such eyes achieve a wide variety of visual outcomes, but that visual acuity at 4½ years of age does not differ by treatment group (11).
In the IATS, adherence to patching was assessed throughout the first five years of life in order to determine if treatment affects adherence to patching. We previously showed that, in the first year after surgery, adherence to patching was associated with sociodemographic factors, but not IOL implantation (11), and that adherence to patching in the first six months after surgery was associated with grating acuity at 12 months of age (12). However, visual acuity measured behaviorally is not strongly predictive of optotype acuity (13). Further, adherence to patching may become more challenging as children begin to resist patching. Therefore, the IATS provided a unique opportunity to prospectively assess the impact of adherence to occlusion therapy through age four on visual acuity among children treated for unilateral cataract. Specifically, the goals of these analyses are to determine if implanting an IOL at the time of cataract extraction affects adherence to prescribed occlusion, and to assess the association between patching and visual acuity at age 4½ years. We hypothesized that primary IOL implantation would not be associated with adherence to occlusion therapy, but that reported hours of patching would be associated with visual acuity. These questions were included as secondary outcomes in the original design of the IATS and not post-hoc analyses.
Methods
Subjects and Methods
The overall design of the IATS and results of the visual acuity assessment at 4½ years of age have been published (14–16). Briefly, the IATS was a multi-center randomized controlled trial comparing two treatments for visually significant unilateral congenital cataract in children aged six months or younger: removal of the cataractous lens followed by contact lens correction of aphakia versus removal of the cataractous lens and IOL implantation at the time of lens extraction. Children were excluded if they eye had a corneal diameter less than 9 mm; the intraocular pressure was 25 mm Hg or greater; there was persistent fetal vasculature (PFV) causing stretching of the ciliary processes or a tractional retinal detachment; retinal or optic nerve disease, or signs suggestive of uveitis; the child was preterm; the fellow eye had ocular disease that might reduce its visual potential; the child had a medical condition known to limit the ability to obtain visual acuity at 12 months or 4 years of age; or follow-up of the child was not feasible. Written informed consent from caregivers was obtained prior to participation. The study was approved by the institutional review boards of all participating institutions and was in accordance with the tenets of the Declaration of Helsinki.
Prescribed Patching and Visual Correction
Patching was prescribed for all children until age five. Starting the second week after cataract surgery, caregivers were instructed to have the child wear an adhesive occlusive patch over the fellow eye one hour daily per month of age until the child was eight months old. Thereafter, caregivers were told to patch their child 50% of waking hours. Patches were provided to patients at no cost.
Refractive correction was prescribed for all children 100% of waking hours. Within a week after cataract surgery, aphakic patients were fitted with a silicone (Silsoft; Bausch & Lomb, Rochester, New York) or a rigid gas permeable contact lens with a 2.0-D overcorrection to provide a near-point correction. A spare lens was provided to ensure that optical correction was available in the event of loss or damage. Both daily wear and extended wear protocols were acceptable. At age 2, the eye was corrected to emmetropia using a CL, and spectacles were prescribed with +3.0 D bifocal lens for near focus.
For pseudophakic infants, spectacles were prescribed by the 1-month postoperative visit if any of the following conditions existed: hyperopia >1.0 D, myopia > 3.0 D, or astigmatism > 1.5 D. In children younger than 2 years, the aim was to correct the refractive error to 2.0 D of myopia; thereafter the aim was emmetropia at distance with a near correction of +3.0 D. The phakic eye for both groups was corrected with spectacles under any of the following conditions: hyperopia >5.0 D, myopia >5.0 D, astigmatism >1.5 D, or refractive esotropia. The aim was to correct the refractive error to the range of 0 to +3.0.
Assessment of Adherence
Adherence to prescribed patching was reported by caregivers in semi-structured telephone interviews. The interviews were completed quarterly, starting 3 months after surgery, and covered the previous 48 hours. Caregivers were asked to report specific times when the patch was applied and either fell off or was removed. Caregivers were also asked to report use of glasses and/or contact lens, and sleep (see see e-figure 1).
The timing of the interviews was determined using an algorithm that distributed the preferred day of the call evenly throughout the week since patching has been reported to differ on weekdays and weekend days (17). Caregivers were not informed in advance about when they would be contacted to complete an interview. The interviews were conducted in the caregiver’s preferred language (English, Spanish, and Portuguese) by one of three trained interviewers so that the caregiver spoke with the same person on each occasion. The English-speaking interviewer performed the vast majority of interviews (>95%). Interviewers were located centrally and masked to treatment assignment to minimize the possibility that the respondent would exaggerate adherence or that the interviewer’s interpretation of the information would be biased by knowledge of the child’s visual acuity However, it was not possible to ensure that the interviewer remained masked to treatment group over time.
Based on information reported in the interview, we calculated the average number of waking hours each day that the child was occluded over two specific time periods: prior to the first birthday, and between the child’s and 4th birthdays. We selected this dichotomization because the visual acuity assessment at twelve months of age may have affected subsequent patching and because it has previously been observed that patching is higher in the first year of life than thereafter (18). We limited the analysis to children with at least three assessments in the first period and five in the second because, although reported patching is consistent over time (Cronbach’s Alpha >0.75), relatively large intra-individual differences were reported.
Visual Acuity Assessment
Monocular optotype acuity was assessed at 4½ years + 1 month of age by a traveling examiner using the ATS-HOTV test as described in previous reports (19, 20).
Analytic Methods
Statistical analyses were conducted using SPSS 21 (IBM Corp. Released 2012. IBM SPSS Statistics for Windows, Version 21.0. Armonk, NY: IBM Corp) and SAS 9.2 (SAS Institute Inc. Cary, NC). T-tests were used to assess differences between treatment groups. Pearson’s correlation coefficients and linear regression were used to estimate the association between patching and acuity. Statistical significance was defined at α = 0.05. A priori we defined the following as potential confounders because of their presumed association with both vision and patching: treatment, gender, age at surgery (<49 days versus 49–269 days), socioeconomic status (private insurance versus other payment), adverse events (yes/no), and additional surgeries (yes/no) and included these as covariates in regression models.
Results
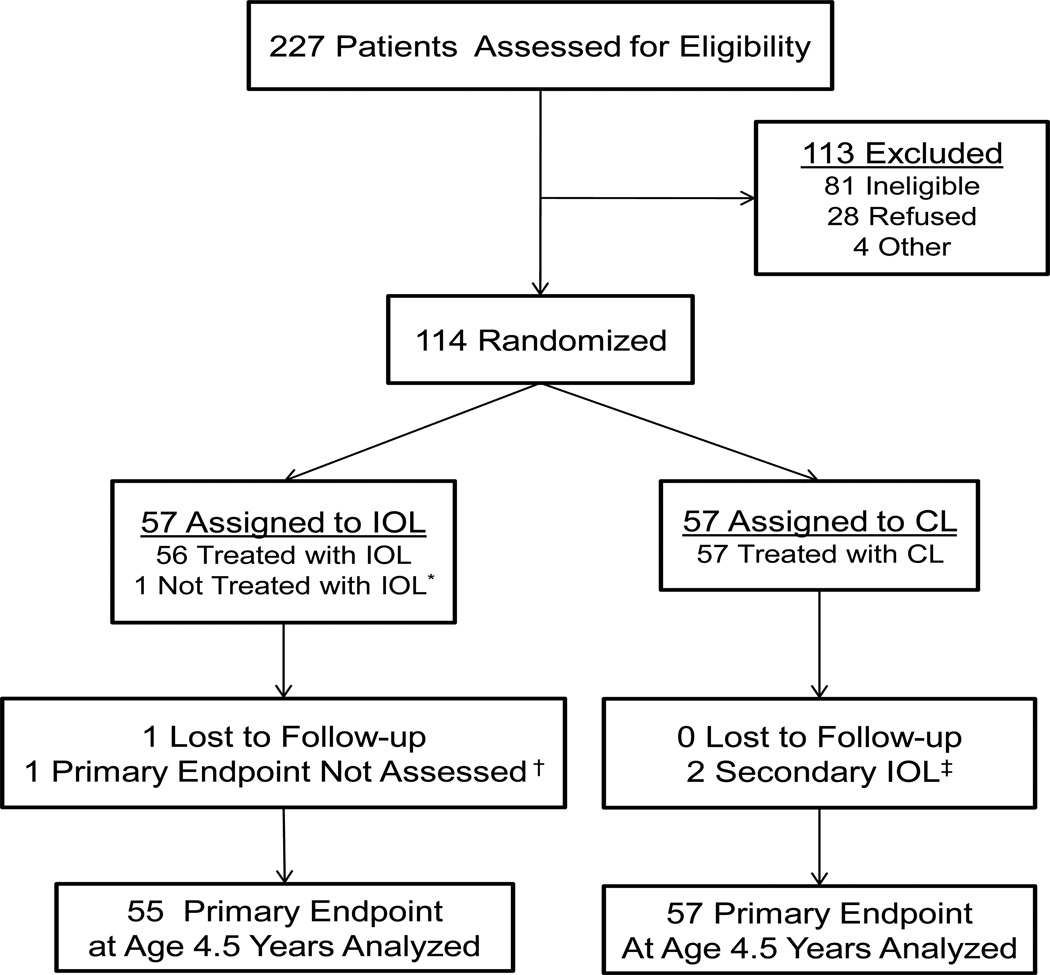
The IATS enrolled 114 children; 57 randomized to each treatment group (Figure 1). At age 4½ optotype visual acuity was assessed in 112. The current analyses exclude an additional three children: two who had adverse events that limited visual potential, and another who had Stickler’s Syndrome which resulted in his having better visual acuity in his treated, than fellow, eye.
Figure 1.
CONSORT Diagram for the Infant Aphakia Treatment Study
Adherence data were available from at least three interviews before their first birthday for 92 children, and 102 had data from at least five interviews between 12 and 48 months of age. Eighty-six children had at least three interviews in the first year and at least five between twelve and 48 months of age. The demographic and clinical characteristics were similar in children randomized to receive an IOL and those left aphakic (16), and similar in children included in these analyses as compared to the entire sample, with the exception that children with a later age at surgery were less likely to have at least three adherence assessments in the first year (table 1), because of the more limited time between surgery and the child’s first birthday.
Table 1.
Characteristics of Analyzed Sample
| All Participants |
Participants with ≥3 adherence assessments in 1st year of life |
Participants with ≥5 adherence assessments between 12 and 48 months of age |
Participants with ≥3 adherence assessments in 1st year of life and ≥5 adherence assessments between 12 and 48 months of age |
||
|---|---|---|---|---|---|
| N | 109 | 94 | 103 | 87 | |
| Female | 59 (54.1%) | 50 (53.2%) | 57 (55.3%) | 48 (55.2%) | |
| Private Insurance | 68 (62.4%) | 58 (61.7%) | 65 (63.1%) | 55 (63.2%) | |
| White Race | 92 (84.4%) | 80 (85.1%) | 88 (85.4%) | 75 (86.2%) | |
| Randomized to IOL | 54 (54.1%) | 47 (50.0%) | 53 (51.5%) | 45 (51.7%) | |
| <49 Days at Surgery | 47 (43.1%) | 48 (51.1%) | 45 (43.7%) | 44 (50.6%) | |
| Visual Acuity worse than 95% Prediction Limits5 at 12 months |
51 (46.8%) | 44 (46.8%) | 46 (44.7%) | 39 (44.8%) | |
| Additional Surgeries in 1st year of life |
38 (34.9%) | 22 (23.4%) | 37 (35.9%) | 33 (37.9%) | |
| Adverse Events in 1st year of life |
23 (21.1%) | 22 (23.4%) | 22 (21.4%) | 20 (23.0%) | |
| Number of Adherence interviews in 1st year |
3 | 32 (34.0%) | 29 (33.3%) | ||
| 4 | 61 (64.9%) | 58 (66.7%) | |||
| 5 | 1 (1.1%) | 0 (0.0%) | |||
| Number of Adherence interviews between 12 and 48 months of age |
5 | 2 (1.9%) | 2 (2.3%) | ||
| 6 | 3 (2.9%) | 1 (1.1%) | |||
| 7 | 7 (1.9%) | 2 (2.3%) | |||
| 8 | 6 (5.8%) | 6 (6.9%) | |||
| 9 | 7 (6.8%) | 5 (5.7%) | |||
| 10 | 17 (16.5%) | 14 (16.1%) | |||
| 11 | 31 (30.1%) | 28 (32.2%) | |||
| 12 | 35 (34.0%) | 29 (33.3%) | |||
<0.42 cycles per degree
On average, caregivers reported patching their children 3.73 ± 1.47 (Range: 0.06–7.11) hours per day in the first year of life and 3.43 ± 2.04 (range: 0–9.27) hours per day between twelve and forty-eight months of age. No differences were identified in reported patching for children randomized to receive an IOL and those left aphakic (average difference in 1st year: −0.29 hours per day 95% CI = −0.90,0.33; average difference between 12 and 48 months of age −0.40 95% CI = −1.20,0.40).
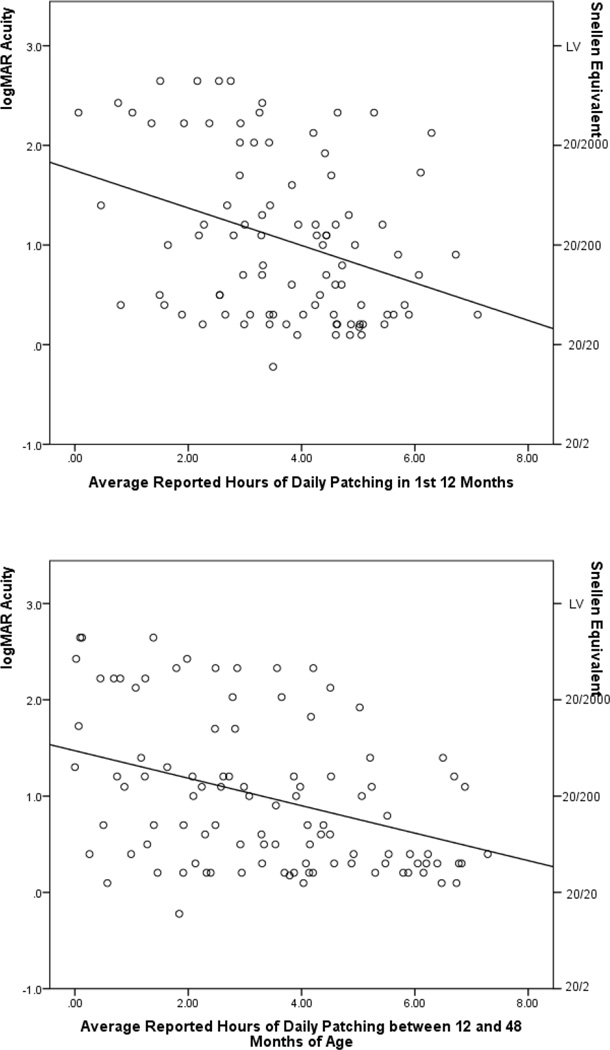
Reported adherence to patching was associated with optotype visual acuity at 4½ years of age in the first and subsequent three years (rpearson = −0.32 95% CI = −0.49, −0.13; −0.36 95% CI = −0.52, −0.18, respectively) (Figure 2) and the correlation was similar for children left aphakic (rpearson = −0.36 95% CI =−0.59, −0.08 in the first year; −0.29 95% CI = −0.53, −0.01 between 12 and 48 months of age) and for those receiving an IOL (rpearson = −0.27 95% CI = −0.52, 0.02 in the first year; −0.41 95% CI= −0.62, −0.15 between 12 and 48 months of age). These plots visually demonstrate two important features: the relationship between increased patching and visual acuity, and the variability in the relationship.
Figure 2.
Scatterplots showing the relationship between optotype visual acuity at 4 ½ years of age and reported average hours of daily patching in (a) the 1st year of life and (b) between 24 and 48 months of life
Findings from regression analyses support the existence of an association between adherence and visual acuity and suggest that the association is not confounded by sociodemographic factors (Table 2). Further, adherence between 12 and 48 months of age was significantly associated with visual acuity even after adjusting for both adherence in the 1st year of life and poor grating acuity at 12 months of age. However, together, average hours of patching in infancy and between 12 and 48 months of age collectively accounted for less than 15% (14.3%) of the observed variation in visual acuity.
Table 2.
Linear regression of the relationship between the average number of hours of patching per day on optotype visual acuity at 4 ½ years of life in the Infant Aphakia Treatment Study
| Model | Reported Average Hours of Patching per Day in 1st year of life |
Reported Average Hours of Patching per Day between 12 and 48 Months of Age |
||
|---|---|---|---|---|
| β6 (95% CI) | p | β (95% CI) | p | |
| Unadjusted | −0.077 (−0.125,−0.03) | 0.002 | −0.059 (−0.093,−0.026) | 0.001 |
| Adjusting for Race7, Gender, Age at Surgery, Insurance Type, Any Adverse Events in 1st year, Any Additional Surgeries in 1st year |
−0.075 (−0.12,−0.030) | 0.002 | −0.055 (−0.089,−0.022) | 0.002 |
| Adjusting for above plus adherence in 1st year of life |
−0.051 (−0.084,−−0.018) | 0.003 | ||
| Adjusting for above plus visual acuity at 12 months8 |
−0.040 (−0.075,−0.005) | 0.024 | ||
Beta represents the expected change in transformed visual acuity associated with a 1 hour increase in reported average hours of daily patching.
White vs. Other; Male vs. Female; <48 days versus 49 to 269 days; Private Insurance vs. other pay.
Better than 4.2 cycles per degree versus worse than 4.2 cycles per degree
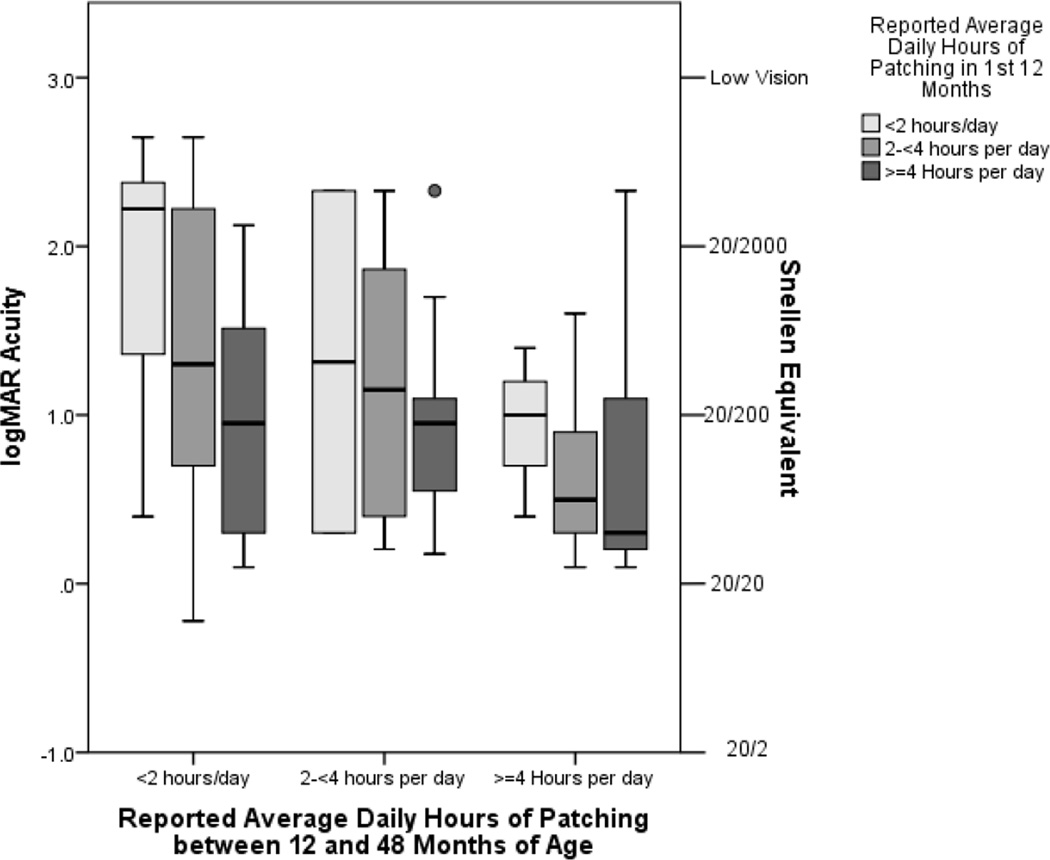
On average, children who were patched at least 4 hours per day throughout the first four years of life had the best visual acuities and those patched fewer than two hours per day had the worst (Figure 3). However, there was a wide range of visual acuity outcomes even among children who patched similar amounts further supporting the conclusion that there is considerable inter-individual variability in the relationship between patching and visual outcomes in these children.
Figure 3.
Boxplot showing the relationship between reported hours of patching in the first year of life and between 12 and 48 months of life and optotype visual acuity at 4 ½ years of age in the Infant Aphakia Treatment Study.
The boxes represent the interquartile range (i.e., 25th percentile to 75th percentile) of visual acuity associated with each combination of patching in the 1st year of life and patching between 12 and 48 months of age, with the whiskers representing the full range of vision excluding any outliers which are shown as circles. The center line indicates the median visual acuity.
Discussion
Our analyses suggest that implanting an IOL at the time of cataract extraction does not affect adherence to prescribed occlusion therapy and that the earlier observation of a lack of difference in visual acuity between aphakic and pseudophakic children (16) is unlikely attributable to an effect of treatment on adherence to patching. Further, our data support a hypothesized relationship between patching and visual acuity in infants treated for unilateral congenital cataract. These findings are consistent with most previous assessments of the importance of occlusion therapy to vision rehabilitation in these children (7, 21, 22). Further, patching in the first year of life and thereafter both contributed to visual acuity.
However, we also found substantial variability in the relationship between patching and visual acuity. The number of hours patched in the first year of life, and in the subsequent three years, each accounted for about 10% of the observed variance in optotype acuity at age 4½ years and together they accounted for less than 15%. Thus, there is considerable variation in visual outcome given a specific amount of patching. This finding is similar to that of Stewart et al. in older children with other types of amblyopia in whom adherence to occlusion therapy was monitored using Occlusion Dose Monitors (23). Although they found a monotonic relationship between total dose of occlusion and visual outcomes, there was substantial variability.
There are a number of study limitations. First, although assessing the relationship between adherence to patching and visual acuity was included in the initial study design, it was not powered for the current question. Additionally, the number of children in certain subgroups was relatively small. For example, only three children were patched fewer than 2 hours per day in the first year of life and an average of at least four hours per day in the subsequent three years. Thus, there may have been inadequate power to identify small differences in adherence between treatment groups or in the impact of adherence on grating visual acuity.
Additionally, although the IATS is a randomized controlled trial, there may be residual confounding by factors that are associated with both visual acuity and adherence. We attempted to account for this by controlling for sociodemographic factors, age at surgery, adverse outcomes and surgery. However, it is possible that there are other confounders about which we are unaware.
The use of clinical trial data also limits the generalizability of these results. The participating surgeons were highly skilled, and the participants provided with patches, contact lenses and spectacles. The quarterly adherence phone calls may also modify the amount of patching that was achieved. Thus, the observed association between patching and visual outcomes in other settings may be different from what we observed.
Preliminary analyses also suggest that caregivers who completed fewer adherence interviews reported somewhat less patching than caregivers who completed more interviews. However, this difference is not statistically significant and given that adherence data are available on more than 80% of IATS participants, we do not believe that these differences explain our findings.
Finally, we believe that the association between adherence and visual acuity is bidirectional. Children with the best vision may be easiest to patch and therefore be patched more than children with poorer vision. For example, half (52.1%) of the children with grating acuity of better than 4.2 cycles per degree at 12 months of age were patched approximately the same amount (± 1 hour per day) before and after their first birthdays; only 14 (29.2%) were patched much less (≤1 hour per day) after their first birthday than they had been patched earlier. In contrast, only one-third (n=13) of the children with poor vision at 12 months of age were patched the same amount (± 1 hour per day) their first birthday; while 17 (43.6%) of these children were patched substantially less than before (≤1 hour per day). This would have the effect of over-estimating the association between patching and visual acuity. Even so, we believe that the fact that visual acuity is associated with patching before 12 months of age, and the fact that visual acuity is associated with adherence to patching between 12 and 48 months of age in models which account for both visual acuity at 12 months of age and patching prior to that point, supports our conclusion that patching throughout the preschool years contributes to visual outcome.
In summary, these data suggest that adherence to occlusion therapy throughout the first four years of life contributes to visual acuity in children treated for unilateral congenital cataract although there is considerable individual variation. These data might be used to support caregivers’ continuing efforts to patch and can provide them with encouragement about the efficacy of patching, even if they have previously been unable to fully adhere to the prescribed patching regimen.
Supplementary Material
Key Points.
Question
Although patching has been a mainstay in treating children with a unilateral congenital cataract, its efficacy has not been rigorously assessed in children with deprivation amblyopia
Findings
In a randomized clinical trial of treatment for unilateral congenital cataract, we found that whether or not an intra-ocular lens was implanted at the time of cataract extraction did not affect caregivers’ reported adherence to prescribed patching. However, a greater number of reported hours of patching throughout the first four years of life was associated with better visual acuity.
Meaning
Our results confirm that occlusion throughout the preschool years is associated with improved visual acuity in infants treated for unilateral congenital cataract.
Acknowledgments
Trial Registration: NCT00212134
The design, data collection and conduct of the Infant Aphakia Treatment Study were supported by the National Eye Institute through grants: EY0113272, EY025553 and EY013287. Carolyn Drews-Botsch and George Cotsonis conducted all analyses at the Rollins School of Public Health at Emory University. They had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. Drs. Drews-Botsch, Celano, Hartmann and Lambert participated in development of the study protocol and data collection. Drs. Drews-Botsch, Celano, Hartmann and Lambert and Mr. Cotsonis reviewed and approved the manuscript, and participated in the decision to submit the manuscript for publication.
References
- 1.Beller R, Hoyt CS, Marg E, Odom JV. Good visual function after neonatal surgery for congenital monocular cataracts. American journal of ophthalmology. 1981;91(5):559–565. doi: 10.1016/0002-9394(81)90053-2. [DOI] [PubMed] [Google Scholar]
- 2.Birch EE, Stager DR. Prevalence of good visual acuity following surgery for congenital unilateral cataract. Archives of ophthalmology. 1988;106(1):40–43. doi: 10.1001/archopht.1988.01060130046025. [DOI] [PubMed] [Google Scholar]
- 3.Birch EE, Stager DR, Wright WW. Grating acuity development after early surgery for congenital unilateral cataract. Archives of ophthalmology. 1986;104(12):1783–1787. doi: 10.1001/archopht.1986.01050240057040. [DOI] [PubMed] [Google Scholar]
- 4.Lewis TL, Maurer D. Multiple sensitive periods in human visual development: evidence from visually deprived children. Developmental psychobiology. 2005;46(3):163–183. doi: 10.1002/dev.20055. [DOI] [PubMed] [Google Scholar]
- 5.Pratt-Johnson J, Tillson G. Visual results after removal of congenital cataracts before the age of 1 year. Canadian journal of ophthalmology Journal canadien d'ophtalmologie. 1981;16(1):19–21. [PubMed] [Google Scholar]
- 6.Chak M, Rahi JS. The health-related quality of life of children with congenital cataract: findings of the British Congenital Cataract Study. British journal of ophthalmology. 2007;91(7):922–926. doi: 10.1136/bjo.2006.109603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lambert SR, Plager DA, Lynn MJ, Wilson ME. Visual outcome following the reduction or cessation of patching therapy after early unilateral cataract surgery. Archives of ophthalmology. 2008;126(8):1071–1074. doi: 10.1001/archopht.126.8.1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Al-Zuhaibi S, Al-Harthi I, Cooymans P, Al-Busaidi A, Al-Farsi Y, Ganesh A. Compliance of amblyopic patients with occlusion therapy: A pilot study. Oman journal of ophthalmology. 2009;2(2):67. doi: 10.4103/0974-620X.53035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gunton K. Advances in amblyopia: what have we learned from PEDIG trials? Pediatrics. 2013;131:540–547. doi: 10.1542/peds.2012-1622. [DOI] [PubMed] [Google Scholar]
- 10.Antonio-Santos A, Vedula SS, Hatt SR, Powell C. Occlusion for stimulus deprivation amblyopia. The Cochrane Library. 2014 doi: 10.1002/14651858.CD005136.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lambert SR, Lynn MJ, Hartmann EE, et al. Comparison of contact lens and intraocular lens correction of monocular aphakia during infancy: a randomized clinical trial of HOTV optotype acuity at age 4.5 years and clinical findings at age 5 years. JAMA ophthalmology. 2014;132(6):676–682. doi: 10.1001/jamaophthalmol.2014.531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Drews-Botsch CD, Hartmann EE, Celano M Group IATS. Predictors of adherence to occlusion therapy 3 months after cataract extraction in the Infant Aphakia Treatment Study. Journal of American Association for Pediatric Ophthalmology and Strabismus. 2012;16(2):150–155. doi: 10.1016/j.jaapos.2011.12.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Drews-Botsch CD, Celano M, Kruger S, Hartmann EE. Adherence to occlusion therapy in the first six months of follow-up and visual acuity among participants in the Infant Aphakia Treatment Study (IATS) Investigative ophthalmology & visual science. 2012;53(7):3368. doi: 10.1167/iovs.11-8457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dobson V, Quinn G, Siatkowski R, et al. Agreement between grating acuity at age 1 year and Snellen acuity at age 5.5 years in the preterm child. Cryotherapy for Retinopathy of Prematurity Cooperative Group. Investigative ophthalmology & visual science. 1999;40(2):496–503. [PubMed] [Google Scholar]
- 15.Group IATS. A randomized clinical trial comparing contact lens to intraocular lens correction of monocular aphakia during infancy: grating acuity and adverse events at age 1 year. Archives of ophthalmology. 2010;128(7):810. doi: 10.1001/archophthalmol.2010.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Group IATS. The infant aphakia treatment study: design and clinical measures at enrollment. Archives of ophthalmology. 2010;128(1):21. doi: 10.1001/archophthalmol.2009.350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wallace M, Stewart C, Moseley M, Stephens D, Fielder A. Concordance with occlusion therapy for childhood amblyopia. Investigative ophthalmology & visual science. 2013;54(15):4979-. doi: 10.1167/iovs.13-11861. [DOI] [PubMed] [Google Scholar]
- 18.Nucci P, Alfarano R, Piantanida A, Brancato R. Compliance in antiamblyopia occlusion therapy. Acta ophthalmologica. 1992;70(1):128–131. doi: 10.1111/j.1755-3768.1992.tb02104.x. [DOI] [PubMed] [Google Scholar]
- 19.Holmes JM, Beck RW, Repka MX, et al. The amblyopia treatment study visual acuity testing protocol. Archives Of Ophthalmology (Chicago, Ill: 1960) 2001;119(9):1345–1353. doi: 10.1001/archopht.119.9.1345. [DOI] [PubMed] [Google Scholar]
- 20.Moke PS, Turpin AH, Beck RW, et al. Computerized method of visual acuity testing: adaptation of the amblyopia treatment study visual acuity testing protocol. American journal of ophthalmology. 2001;132(6):903–909. doi: 10.1016/s0002-9394(01)01256-9. [DOI] [PubMed] [Google Scholar]
- 21.Mayer D, Moore B, Robb R. Assessment of vision and amblyopia by preferential looking tests after early surgery for unilateral congenital cataracts. Journal of pediatric ophthalmology and strabismus. 1988;26(2):61–68. doi: 10.3928/0191-3913-19890301-05. [DOI] [PubMed] [Google Scholar]
- 22.Mayer D, Beiser A, Warner A, Pratt E, Raye K, Lang J. Monocular acuity norms for the Teller Acuity Cards between ages one month and four years. Investigative ophthalmology & visual science. 1995;36(3):671–685. [PubMed] [Google Scholar]
- 23.Lloyd IC, Dowler JG, Kriss A, et al. Modulation of amblyopia therapy following early surgery for unilateral congenital cataracts. The British journal of ophthalmology. 1995 Sep;79(9):802–806. doi: 10.1136/bjo.79.9.802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Uemura Y, Katsumi O. Form-vision deprivation amblyopia and strabismic amblyopia. Graefe's archive for clinical and experimental ophthalmology = Albrecht von Graefes Archiv fur klinische und experimentelle Ophthalmologie. 1988;226(2):193–196. doi: 10.1007/BF02173317. [DOI] [PubMed] [Google Scholar]
- 25.Stewart CE, Moseley MJ, Stephens DA, et al. Treatment dose-response in amblyopia therapy: The Monitored Occlusion Treatment of Amblyopia Study (MOTAS) Invest Opthalmol Vis Sci. 2004;45(9):3048–3054. doi: 10.1167/iovs.04-0250. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.