Abstract
Background
Infectious enteritis is a commonly identified risk factor for irritable bowel syndrome (IBS). The incidence of Clostridium difficile infection (CDI) is on the rise. However, there is limited information on post-infectious IBS (PI-IBS) development following CDI and the host- and infection-related risk factors are not known.
Aim
Our aim was to determine the incidence and risk factors for PI-IBS following CDI.
Methods
684 cases of CDI identified from Sep 2012–Nov 2013 were surveyed. Participants completed the Rome III IBS questionnaire and details on the CDI episode. Predictive modelling was done using logistic regression to evaluate risk factors for PI-IBS development.
Results
315 CDI cases responded (46% response rate) and 205 were at-risk (no pre-CDI IBS) for PI-IBS development. 52/205 (25%) met the Rome III criteria for IBS ≥6 months following CDI. IBS-mixed was most common followed by IBS-diarrhea. In comparison to those without subsequent PI-IBS, greater percentage of PI-IBS patients had CDI symptoms >7 days, nausea, vomiting, abdominal pain during CDI, anxiety, and a higher BMI. Using logistic regression, CDI symptoms >7 days {Odds ratio (OR):2.96, p=0.01}, current anxiety (OR:1.33, p<0.0001) and a higher BMI (OR:1.08, p=0.004) were independently associated with PI-IBS development; blood in the stool during CDI was protective (OR: 0.44, p=0.06).
Conclusions
In this cohort study, new-onset IBS is common after CDI. Longer CDI duration, current anxiety and higher BMI are associated with the diagnosis of C. difficile PI-IBS. This chronic sequela should be considered during active management and follow up of patients with CDI.
Keywords: gastrointestinal infections, functional gastrointestinal disorders
INTRODUCTION
Irritable bowel syndrome (IBS) is one of the most prevalent gastrointestinal disorders, affecting 15% of the U.S. population.1 The role of infectious enteritis in development of IBS was first described in the early 1960s,2 and since then, there has been growing epidemiological evidence supporting the association between gastrointestinal infections and subsequent development of IBS.3–5 Different microorganism types have been associated with post-infectious IBS (PI-IBS) including bacteria,6–7 viruses,8 and protozoa.9 It has been shown that 4–36% of individuals with infectious enteritis develop PI-IBS,4 and symptoms persist for ≥10 years in some cases.10
The incidence of Clostridium difficile infection (CDI) has risen over the last decade and it has become a significant source of mortality, morbidity and a major health care burden.11–12 The main risk factors for CDI are advanced age (>65 years), antibiotic treatment and hospitalization.13–14 However, CDI is now being increasingly recognized as a cause of diarrhea in the community, and community acquired CDI accounts for 20–40% of all CDI cases.15 These patients are younger and often lack the above mentioned risk factors for CDI.16–18 The median risk for at least one recurrence following an index episode of CDI is 22%,19 and patients who experience a first recurrence are at a higher risk for subsequent recurrences.20 There is some evidence that patients get diagnosed with “recurrent CDI” based on clinical suspicion alone,21 with some being re-treated without laborotary confirmation. C. difficile PI-IBS can theoretically mimic symptoms of a recurrence, resulting in unnecessary antibiotic treatment. Systemic antibiotic use in turn can increase the recurrent CDI.22
The risk of PI-IBS following CDI has been studied but the data available is scarce.23–24 Two small studies have shown that 4–12% of individuals may have PI-IBS symptoms following CDI. Another recent retrospective cohort study among military personnel showed an incidence of IBS of 5–9/100,000 person years following CDI.25 None of these studies have examined host- or infection-related risk factors for development of PI-IBS following CDI.
Our aim was to determine the incidence and risk factors for new-onset PI-IBS development among laboratory-confirmed cases of CDI from a large community-based cohort who did not have IBS prior to the infection. We hypothesized that CDI is associated with PI-IBS development and that specific host- and infection-related factors predict development of PI-IBS following an episode of CDI.
METHODS
Patients (>18 years) with positive C. difficile PCR from September 2012–November 2013 were identified using the microbiology laboratory database at Mayo Clinic. Clinical notes and laboratory results were reviewed to confirm CDI diagnoses. A diagnosis of CDI required the presence of diarrhea (three or more watery stools per day, not on laxatives or with an alternative explanation for diarrhea) in addition to the positive stool PCR. A total of 684 CDI cases identified over this time frame were eligible to be contacted. A standardized questionnaire was mailed to these participants ≥6 months following the CDI (supplementary material). Another mailing was made one month later for the passive non-responders. For non-responders to two postal mailing attempts, telephone calls were made to ensure they had received the survey and to allow completion of the survey over the phone, if needed. A maximum 3-month period was allowed for survey completion by each participant (6–9 months from the CDI episode). The survey consisted of a brief demographic questionnaire, Rome III diagnostic criteria for IBS (for current and pre-CDI gastrointestinal symptoms), IBS symptom severity score (IBS-SSS), and the hospital anxiety and depression scale (HADS). IBS phenotype was defined using the Rome III criteria as well. The Mayo Clinic Survey Research Center conducted the survey. Electronic medical records were reviewed to rule out alternate diagnoses that may cause symptoms mimicking IBS. Additionally, patients with recurrent episodes of CDI after the index episode were identified and excluded. The electronic medical records were also reviewed to determine diagnosis of anxiety and depression prior to the infection using ICD codes and clinician documentation. The study was approved by the Mayo Clinic Institutional Review Board.
Statistical Analysis
Means and standard errors are reported for continuous variables, while frequencies and percentages are reported for categorical variables. Univariate logistic regression models were used to measure the association between potential risk factors and the outcome of new-onset PI-IBS 6 months or more following the CDI. Based on the number of patients with C. difficile PI-IBS (n=52) and the 10 events per variable rule-of-thumb, a logistic model having no more than about 5 variables was used to prevent over fitting. Based on prior research, age at CDI and gender were forced into the multivariable model regardless of statistical significance and all other variables were candidates for inclusion into the final model. Forward and stepwise variable selection using a type I error of 0.10 was utilized. In addition, best subsets selection using the score statistic was also used with a maximum of 7 variables included. All techniques supported the same multivariable model which is the one presented. A complete case analysis was used for all models.
RESULTS
Survey Response Rate
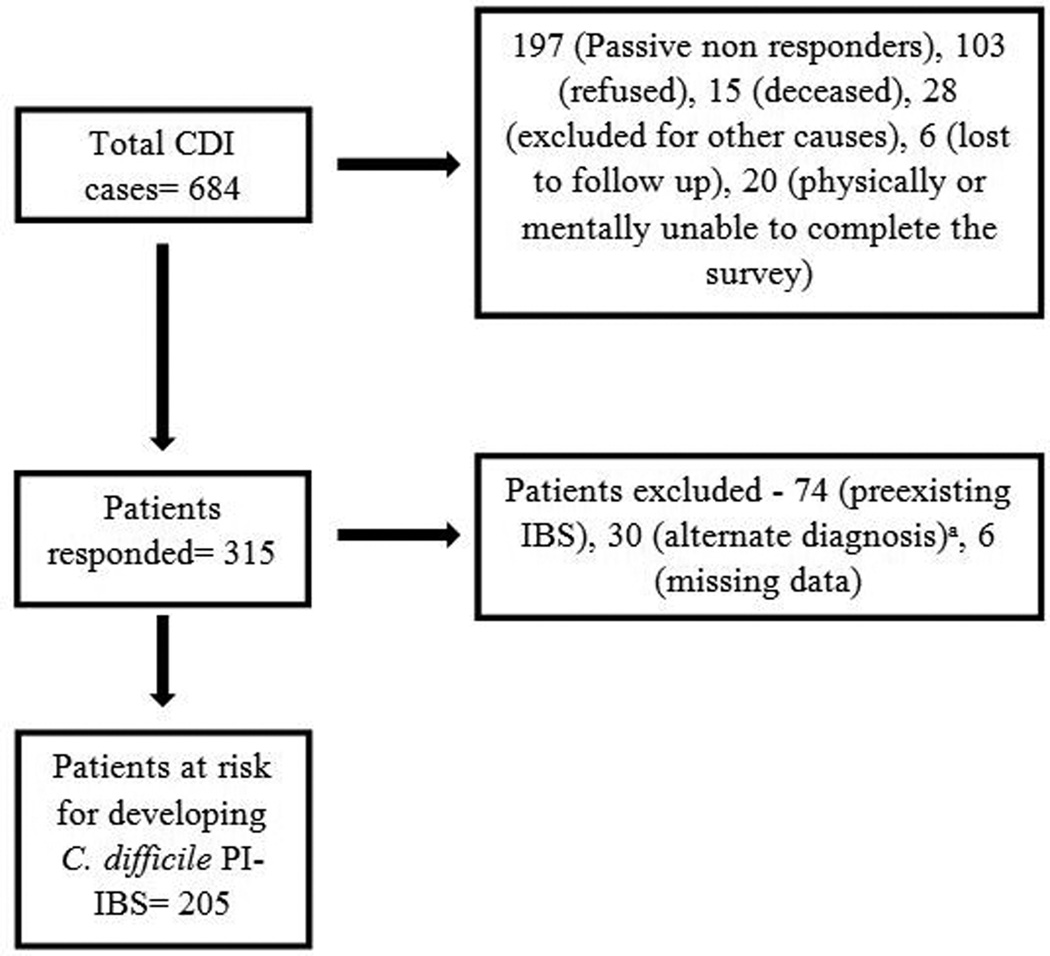
The overall response rate was 46.1% (315/684). One hundred and three patients actively refused to participate in the study, while 197 patients did not respond (passive non-responders). Fifteen patients were deceased, 20 patients were physically or mentally unable to participate in the study. Twenty eight patients did not participate due to other exclusions. Six patients returned partially completed questionnaire and were lost to follow up for subsequent contact.
Study Population
Of the 315 responders, 74 were excluded for meeting Rome III criteria for IBS prior to the CDI episode based on Rome III survey responses for pre-CDI IBS or documentation in the electronic medical record. Thirty were excluded due to alternate diagnoses, such as inflammatory bowel disease, microscopic colitis or celiac disease. Six were excluded due to missing data. Thus, 205 of the 315 responders were defined as at-risk for C. difficile PI-IBS development (Figure 1). Responders (without prior IBS) were more likely to be older (mean 57.6 years vs 45.8 years) but had similar gender distribution (43.4% vs 39.1%) and BMI (mean 27.3 kg/m2 vs 27.8 kg/m2) as non-responders.
Figure 1. Study population flow for determination of C. difficile PI-IBS cases and controls.
CDI, C. difficile infection; IBD, inflammatory bowel disease; IBS- irritable bowel syndrome, PI-IBS, post-infectious IBS
a. IBD, microscopic colitis, celiac disease
Incidence of PI-IBS following CDI
Of the 205 at-risk patients, 57 met Rome III IBS criteria for ongoing gastrointestinal symptoms ≥6 months following the CDI episode. Five of these were considered not to have IBS since they had at least one additional episode of CDI following the index episode. Overall, 52/205 (25.4%) patients were defined as having PI-IBS following the CDI episode. Of these, 27 (52%) were IBS-M (IBS-mixed), 21 (40%) were IBS-D (IBS-diarrhea predominant), 2 (4%) were IBS-C (IBS-constipation predominant), and 2 (4%) were IBS-U (IBS-undifferentiated). The mean (SEM) IBS symptom severity score (IBS-SSS) was 238 (14) on a scale of 0–500 with 500 being the most severe symptoms. Of the 52 PI-IBS patients, 44 were tested for recurrence and were found to be negative for C. difficile.
Demographic Characteristics
The mean (SEM) age was similar for the C. difficile PI-IBS and non PI-IBS groups [56 (2.4) years vs 58 (1.5) years]. The distribution of gender was also similar, with 38% males in the C. difficile PI-IBS cohort and 45% males in the non PI-IBS group. Patients with C. difficile PI-IBS had a higher mean BMI compared to the non PI-IBS group (29 kg/m2 vs 27 kg/m2, p= 0.06). Patients with C. difficile PI-IBS had a higher mean (SEM) HADS anxiety score of 9.8 (0.4) as compared to 8.1 (0.2) (p <0.0001) for individuals without PI-IBS. The HADS depression score was not different among the two groups. The prevalence of anxiety prior to infection was similar in the PI-IBS (13.5%) and non PI-IBS group (6.5%). Additionally, the prevalence of depression prior to infection was similar in the PI-IBS (7.7%) and non PI-IBS group (11.8%). Distribution summaries of these and other demographic variables among C. difficile PI-IBS and non PI-IBS cohorts are shown in Table 1.
Table 1.
Distribution of demographic, infection-related and psychological variables among C. difficile IBS patients and controls
| Variable |
C. difficile PI-IBS (N=52) |
No PI-IBS (N=153) |
No. of Missing values |
Univariate PI- IBS OR (95% CI) |
P value |
|---|---|---|---|---|---|
| Demographic and psychological | |||||
| Age, mean (SEM) | 55.7 (2.4) | 58.3 (1.5) | 0 | 0.99 (0.98– 1.01) | 0.39 |
| Male gender, n (%) | 20 (38.5%) | 69 (45.1%) | 0 | 0.76 (0.40–1.45) | 0.40 |
| Current Smoker, n (%) | 8 (15.7%) | 13 (8.6%) | 3 | 1.97 (0.77–5.08) | 0.16 |
| Caucasian, n (%) | 51 (98.1%) | 142 (93.4%) | 1 | 3.59 (0.45–28.71) | 0.23 |
| BMI, mean (SEM) | 28.8 (1.2) | 26.8 (0.5) | 1 | 1.04 (0.998–1.094) | 0.06 |
| HADS anxiety score, mean (SEM) |
9.8 (0.4) | 8.1 (0.2) | 2 | 1.27 (1.13– 1.43) | <0.000 1 |
| HADS depression score, mean (SEM) |
8.5 (0.2) | 8.5 (0.1) | 3 | 1.03 (0.83– 1.29) | 0.78 |
| Pre-CDI anxiety diagnosis, n (%) |
7 (13.5%) | 10 (6.5%) | 0 | 2.22 (0.80–6.18) | 0.12 |
| Pre-CDI depression diagnosis, n (%) |
4 (7.7%) | 18 (11.8%) | 0 | 0.62 (0.20–1.94) | 0.41 |
| CDI episode-related | |||||
| Nausea, n (%) | 33 (68.8%) | 60 (42.9%) | 17 | 2.93 (1.46–5.88) | 0.002 |
| Vomiting, n (%) | 15 (31.9%) | 22 (16.2%) | 22 | 2.43 (1.13–5.22) | 0.02 |
| Diarrhea, n (%) | 49 (94.2%) | 138 (91.4%) | 2 | 1.54(0.42–5.62) | 0.51 |
| Abdominal pain, n (%) | 43 (86.0%) | 95 (67.9%) | 15 | 2.91 (1.21–6.97) | 0.02 |
| Fever, n (%) | 13 (26%) | 52 (38.5%) | 20 | 0.56 (0.27– 1.15) | 0.12 |
| Blood in stool, n (%) | 11 (22.4%) | 46 (32.9%) | 16 | 0.59 (0.28– 1.26) | 0.17 |
| Duration of CDI symptoms >7 days, n (%) |
41 (78.8%) | 90 (60.4%) | 4 | 2.44 (1.16– 5.13) | 0.02 |
| Hospitalized during CDI, n (%) |
31 (59.6%) | 88 (57.9%) | 1 | 1.07 (0.57– 2.04) | 0.83 |
Clinical Characteristics during CDI Episode
A higher proportion of patients in the C. difficile PI-IBS cohort reported a >7 day duration of CDI symptoms as compared to the control group (79% vs 61%, p= 0.02). During the CDI episode, a higher percentage of patients who later developed PI-IBS reported nausea (69% vs 43%, p= 0.002), vomiting (32% vs 16%, p= 0.02) and abdominal pain (86% vs 68%, p= 0.02). Distribution summaries of these and other infection-related variables among C. difficile PI-IBS and non PI-IBS cohorts are shown in Table 1 along with univariate odds ratios.
Logistic Regression Model for Variables Associated with C. difficile PI-IBS
In a multivariable model, CDI symptom duration >7 days was independently associated with an increased risk of PI-IBS diagnosis with an OR (95% CI) of 3.0 (1.24 – 7.10). The current anxiety score from the HADS and BMI were associated with an increased risk of PI-IBS diagnosis following the CDI episode with ORs (95% CI) of 1.33 (1.16 – 1.53) and 1.08 (1.02 – 1.14), respectively. Reporting of blood in stool was associated with lower odds of C. difficile PI-IBS diagnosis with an OR of 0.44 (0.19– 1.02). Variables included in the multivariable logistic regression model are listed in Table 2.
Table 2.
Multivariable model for new-onset IBS following C. difficile infection (N=183)
| Variable |
C. difficile PI-IBS OR (95% CI) |
P value |
|---|---|---|
| Male gender | 0.62 (0.28–1.34) | 0.22 |
| Age at CDI | 1.00 (0.98– 1.02) | 0.97 |
| BMI | 1.08 (1.02– 1.14) | 0.004 |
| HADS anxiety score | 1.33 (1.16– 1.53) | <0.0001 |
| Blood in stool during CDI | 0.44 (0.19– 1.02) | 0.06 |
| Duration of CDI >7 days | 2.96 (1.24– 7.10) | 0.01 |
DISCUSSION
In this study of 205 CDI patients without prior IBS, we demonstrate that a quarter of patients report symptoms consistent with IBS ≥6 months following their CDI episode. This is significant considering the progressively increasing burden of CDI. Previous studies have shown a wide range of PI-IBS incidence, from 4–36% following gastrointestinal infections, however, most of the studies report a 10–15% incidence.4,26 The incidence of C. difficile PI-IBS in our study is also higher than the previously reported incidence. One of these studies showed a incidence of 4% but that study only had 3 months of follow-up and a sample size of 23 patients, of which one reported C. difficile PI-IBS.23 The CDI diagnosis in this study was made using both culture and by detection of toxins by rapid immunoassay. Another small study of 94 CDI patients showed an incidence of 12% and over 50% were lost to follow-up, leaving 41 CDI patients available for analysis.24 None of the controls (non-infected) in this study developed IBS over the duration of follow-up.
Several studies have looked at risk factors for PI-IBS development following gastrointestinal infections but this is the first to determine risk factors associated with PI-IBS development following CDI in those without prior IBS. Severity of initial illness has been proposed as risk factor for PI-IBS following bacterial enteritis,27–29 but no evidence is available for patients following CDI. We showed that patients with prolonged symptom duration (i.e., >7 days) have an increased likelihood of developing PI-IBS following CDI. We also found that patients reporting nausea, vomiting and abdominal pain had higher odds of PI-IBS development following CDI on univariate analysis. On multivariable analysis, however, only duration of CDI symptoms (>7 days) was found to be significantly associated. Blood in the stool, which can be perceived as a marker for severe disease was not associated with C. difficile PI-IBS diagnosis on univariate analysis and was found to be protective against PI-IBS on multivariable analysis. Previous studies have found mixed results as pertains to association between bloody diarrhea and PI-IBS development.30–31 Our patient population was specifically asked about “blood in the stool” and not bloody diarrhea in the survey which could have led to inaccurate responses or this could be due to recall bias.
Previous studies have shown that psychological factors such as anxiety and depression at the time of enteritis may increase the risk of PI-IBS.32–34 We found that current anxiety was associated with a diagnosis of PI-IBS following CDI. We did not find any association between depression and PI-IBS. It is possible that PI-IBS is making individuals more anxious since anxiety and depression prior to infection were not associated with PI-IBS development.
Age has been proposed as a risk factor with individuals less than 60 years at a higher risk for PI-IBS,4,27 however, we did not find any significant correlation with age. This could be due to over representation of the elderly in our responder population. Additionally, there have been other studies which found no effect of age in increasing the risk of PI-IBS.35–36 There is conflicting data regarding gender as a risk factor for PI-IBS. We didn’t find an association between gender and PI-IBS in contrast to previous studies.30,37–38 However female patients have a higher incidence of psychological disorders, including anxiety and depression,4 and in two studies, the association of gender was not observed after multivariate analysis despite being significant in univariate analysis, thereby supporting the above argument.33,39 A relationship between obesity and IBS has been studied in the past.40 We found patients with a higher BMI to be at an increased risk for C. difficile PI-IBS, although there is much to be explored in this area in order to understand the mechanism involved in obesity increasing the risk of IBS. Changes in gut microbiota have been proposed as one mechanism and have been observed in obese patients,41 and IBS,42 separately. Interestingly, fecal microbiota diversity was found to be reduced in PI-IBS which inversely correlated with lamina propria lymphocytes and psychological scores suggesting a possible link between dysbiosis, inflammation and stress in PI-IBS.43
Our study has limitations. It was a cross-sectional study with patients contacted via a survey which makes it prone to recall and survey response bias. Additionally, a control group without CDI derived from the same population would have been helpful in defining risk-ratios. A number of epidemiological studies in PI-IBS7,29 including ours are prone to recall bias for pre-infection IBS symptoms. The CDI diagnosis was made on the basis of a positive PCR and presence of ≥3 watery stools/day and it is possible that some of the patients had a false positive diagnosis. We demonstrate that 25% of CDI patients without prior IBS develop PI-IBS at least 6 months after CDI which is higher than the median PI-IBS incidence following infection from other pathogens. This makes it important to consider the possibility of PI-IBS when patients with a history of CDI present with ongoing gastrointestinal symptoms. Such patients may be inappropriately re-treated for CDI. Ongoing anxiety and higher BMI independently increase the odds of PI-IBS diagnosis following CDI. Additionally, longer duration of CDI symptoms is also moderately associated. Considering the significant incidence of C. diificile PI-IBS, retreatment for recurrence should only be offered after laboratory confirmation of the diagnosis. The risk factors for C. difficile PI-IBS can also be potentially modified to help reduce the incidence of PI-IBS following CDI. A potential implication is that a more prompt diagnosis and treatment for CDI does not only have implications for treating the acute infection but also on the delayed sequela of PI-IBS. A prospective cohort study with assessment of prior IBS symptoms at the time of infection and with a control group will be needed to define the incidence and the risk factors for PI-IBS with a clearer perspective during the course of the disease and help determine the sequelae. Additionally, mechanistic studies to determine the pathophysiology of C. difficile PI-IBS will be helpful in understanding this entity and probably IBS in general. These include the role of gut microbiota and ascertaining changes over a longer period among CDI patients who resolve their symptoms and those that go on to develop PI-IBS.
Supplementary Material
Acknowledgments
The authors thank Ms. Lori Anderson and Ms. Kristy Zodrow for administrative assistance.
Statement of Interests: This study was funded in parts by NIH K23 (DK103911), Small Grant Program Award from Mayo Clinic Center for Clinical and Translational Science (NIH UL1TR000135), and American Gastroenterological Association Rome Foundation Functional Gastroenterology and Motility Disorders Pilot Research Award to Madhusudan Grover.
Abbreviations
- CDI
Clostridium difficile infection
- PI-IBS
post-infectious irritable bowel syndrome
Footnotes
Authorship Statement:
Guarantor of the Article: Madhusudan Grover, M.B.B.S.
Author Contributions:
A. Wadhwa: study concept and design; acquisition of data; analysis and interpretation of data; drafting of the manuscript; critical revision of the manuscript for important intellectual content
MF. AlNahhas: study concept and design; acquisition of data; analysis and interpretation of data; drafting of the manuscript; critical revision of the manuscript for important intellectual content
R. Dierkhising: analysis and interpretation of data; drafting of the manuscript; critical revision of the manuscript for important intellectual content; statistical analysis
R. Patel: analysis and interpretation of data; critical revision of the manuscript for important intellectual content; administrative, technical, or material support
P. Kashyap: analysis and interpretation of data; critical revision of the manuscript for important intellectual content
D. Pardi: study design; analysis and interpretation of data; critical revision of the manuscript for important intellectual content
S. Khanna: study design; acquisition of data; analysis and interpretation of data; critical revision of the manuscript for important intellectual content
M. Grover: study concept and design; acquisition of data; analysis and interpretation of data; drafting of the manuscript; critical revision of the manuscript for important intellectual content; statistical analysis; obtained funding; administrative, technical, or material support; study supervision
All authors approved the final version of this manuscript.
Disclosures: None
REFERENCES
- 1.American Gastroenterological Association medical position statement: irritable bowel syndrome. Gastroenterology. 2002;123:2105–2107. doi: 10.1053/gast.2002.37095b. [DOI] [PubMed] [Google Scholar]
- 2.Chaudhary NA, Truelove SC. The irritable colon syndrome. A study of the clinical features, predisposing causes, and prognosis in 130 cases. Q J Med. 1962;31:307–322. [PubMed] [Google Scholar]
- 3.Barbara G, Cremon C, Pallotti F, De Giorgio R, Stanghellini V, Corinaldesi R. Postinfectious irritable bowel syndrome. J Pediatr Gastroenterol Nutr. 2009;48(Suppl 2):S95–S97. doi: 10.1097/MPG.0b013e3181a15e2e. [DOI] [PubMed] [Google Scholar]
- 4.Spiller R, Garsed K. Postinfectious irritable bowel syndrome. Gastroenterology. 2009;136:1979–1988. doi: 10.1053/j.gastro.2009.02.074. [DOI] [PubMed] [Google Scholar]
- 5.Schwille-Kiuntke J, Mazurak N, Enck P. Systematic review with meta-analysis: post-infectious irritable bowel syndrome after travellers' diarrhoea. Aliment Pharmacol Ther. 2015;41:1029–1037. doi: 10.1111/apt.13199. [DOI] [PubMed] [Google Scholar]
- 6.Cremon C, Stanghellini V, Pallotti F, Fogacci E, Bellacosa L, Morselli-Labate AM, et al. Salmonella gastroenteritis during childhood is a risk factor for irritable bowel syndrome in adulthood. Gastroenterology. 2014;147:69–77. doi: 10.1053/j.gastro.2014.03.013. [DOI] [PubMed] [Google Scholar]
- 7.Schwille-Kiuntke J, Enck P, Zendler C, Krieg M, Polster AV, Klosterhalfen S, et al. Postinfectious irritable bowel syndrome: follow-up of a patient cohort of confirmed cases of bacterial infection with Salmonella or Campylobacter. Neurogastroenterol Motil. 2011;23:e479–e488. doi: 10.1111/j.1365-2982.2011.01779.x. [DOI] [PubMed] [Google Scholar]
- 8.Zanini B, Ricci C, Bandera F, Caselani F, Magni A, Laronga AM, et al. Incidence of post-infectious irritable bowel syndrome and functional intestinal disorders following a water-borne viral gastroenteritis outbreak. Amer J Gastroenterol. 2012;107:891–899. doi: 10.1038/ajg.2012.102. [DOI] [PubMed] [Google Scholar]
- 9.Hanevik K, Dizdar V, Langeland N, Hausken T. Development of functional gastrointestinal disorders after Giardia lamblia infection. BMC Gastroenterol. 2009;9:27. doi: 10.1186/1471-230X-9-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schwille-Kiuntke J, Mazurak N, Enck P. Systematic review with meta-analysis: post-infectious irritable bowel syndrome after travellers' diarrhoea. Aliment Pharmacol Ther. 2015 Jun;41(11):1029–1037. doi: 10.1111/apt.13199. [DOI] [PubMed] [Google Scholar]
- 11.Dubberke ER, Olsen MA. Burden of Clostridium difficile on the healthcare system. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2012;55(Suppl 2):S88–S92. doi: 10.1093/cid/cis335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lessa FC, Winston LG, McDonald LC. Burden of Clostridium difficile infection in the United States. N Engl J Med. 2015;372:2369–2370. doi: 10.1056/NEJMc1505190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Freeman J, Wilcox MH. Antibiotics and Clostridium difficile. Microbes Infect. 1999;1:377–384. doi: 10.1016/s1286-4579(99)80054-9. [DOI] [PubMed] [Google Scholar]
- 14.Pepin J, Saheb N, Coulombe MA, Alary ME, Corriveau MP, Authier S, et al. Emergence of fluoroquinolones as the predominant risk factor for Clostridium difficile-associated diarrhea: a cohort study during an epidemic in Quebec. Clin Infect Dis. 2005;41:1254–1260. doi: 10.1086/496986. [DOI] [PubMed] [Google Scholar]
- 15.Gupta A, Khanna S. Community-acquired Clostridium difficile infection: an increasing public health threat. Infect Drug Resist. 2014;7:63–72. doi: 10.2147/IDR.S46780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Khanna S, Pardi DS. The growing incidence and severity of Clostridium difficile infection in inpatient and outpatient settings. Expert Rev Gastroenterol Hepatol. 2010;4:409–416. doi: 10.1586/egh.10.48. [DOI] [PubMed] [Google Scholar]
- 17.Khanna S, Pardi DS, Aronson SL, Kammer PP, Orenstein R, St Sauver JL, et al. The epidemiology of community-acquired Clostridium difficile infection: a population-based study. Am J Gastroenterol. 2012;107:89–95. doi: 10.1038/ajg.2011.398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bauer MP, Goorhuis A, Koster T, Numan-Ruberg SC, Hagen EC, Debast SB, et al. Community-onset Clostridium difficile-associated diarrhoea not associated with antibiotic usage--two case reports with review of the changing epidemiology of Clostridium difficile-associated diarrhoea. Neth J Med. 2008;66:207–211. [PubMed] [Google Scholar]
- 19.Abou Chakra CN, Pepin J, Sirard S, Valiquette L. Risk factors for recurrence, complications and mortality in Clostridium difficile infection: a systematic review. PLoS One. 2014;9:e98400. doi: 10.1371/journal.pone.0098400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fekety R, McFarland LV, Surawicz CM, Greenberg RN, Elmer GW, Mulligan ME. Recurrent Clostridium difficile diarrhea: characteristics of and risk factors for patients enrolled in a prospective, randomized, double-blinded trial. Clin Infect Dis. 1997;24:324–333. doi: 10.1093/clinids/24.3.324. [DOI] [PubMed] [Google Scholar]
- 21.Sheitoyan-Pesant C, Abou Chakra CN, Pepin J, Marcil-Heguy A, Nault V, Valiquette L. Clinical and healthcare burden of multiple recurrences of Clostridium difficile infection. Clin Infect Dis. 2016;62:574–580. doi: 10.1093/cid/civ958. [DOI] [PubMed] [Google Scholar]
- 22.Shivashankar R, Khanna S, Kammer PP, Scott Harmsen W, Zinsmeister AR, Baddour LM, Pardi DS. Clinical predictors of recurrent Clostridium difficile infection in out-patients. Aliment Pharmacol Ther. 2014;40:518–522. doi: 10.1111/apt.12864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Piche T, Vanbiervliet G, Pipau FG, Dainese R, Hebuterne X, Rampal P, et al. Low risk of irritable bowel syndrome after Clostridium difficile infection. Can J Gastroenterol. 2007;21:727–731. doi: 10.1155/2007/262478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sethi S, Garey KW, Arora V, Ghantoji S, Rowan P, Smolensky M, et al. Increased rate of irritable bowel syndrome and functional gastrointestinal disorders after Clostridium difficile infection. J Hosp Infect. 2011;77:172–173. doi: 10.1016/j.jhin.2010.09.024. [DOI] [PubMed] [Google Scholar]
- 25.Gutierrez RL, Riddle MS, Porter CK. Increased risk of functional gastrointestinal sequelae after Clostridium difficile infection among active duty United States military personnel (1998–2010) Gastroenterology. 2015;149:1408–1414. doi: 10.1053/j.gastro.2015.07.059. [DOI] [PubMed] [Google Scholar]
- 26.Grover M. Role of gut pathogens in development of irritable bowel syndrome. Indian J Med Res. 2014;139:11–18. [PMC free article] [PubMed] [Google Scholar]
- 27.Neal KR, Hebden J, Spiller R. Prevalence of gastrointestinal symptoms six months after bacterial gastroenteritis and risk factors for development of the irritable bowel syndrome: postal survey of patients. Bmj. 1997;314:779–782. doi: 10.1136/bmj.314.7083.779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang LH, Fang XC, Pan GZ. Bacillary dysentery as a causative factor of irritable bowel syndrome and its pathogenesis. Gut. 2004;53:1096–1101. doi: 10.1136/gut.2003.021154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Marshall JK, Thabane M, Garg AX, Clark WF, Salvadori M, Collins SM. Incidence and epidemiology of irritable bowel syndrome after a large waterborne outbreak of bacterial dysentery. Gastroenterology. 2006;131:445–450. doi: 10.1053/j.gastro.2006.05.053. quiz 660. [DOI] [PubMed] [Google Scholar]
- 30.Thabane M, Simunovic M, Akhtar-Danesh N, Garg AX, Clark WF, Collins SM, et al. An outbreak of acute bacterial gastroenteritis is associated with an increased incidence of irritable bowel syndrome in children. Amer J Gastroenterol. 2010;105:933–939. doi: 10.1038/ajg.2010.74. [DOI] [PubMed] [Google Scholar]
- 31.Kowalcyk BK, Smeets HM, Succop PA, De Wit NJ, Havelaar AH. Relative risk of irritable bowel syndrome following acute gastroenteritis and associated risk factors. Epidemiol Infect. 2014;142:1259–1268. doi: 10.1017/S0950268813001891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ruigomez A, Garcia Rodriguez LA, Panes J. Risk of irritable bowel syndrome after an episode of bacterial gastroenteritis in general practice: influence of comorbidities. Clin Gastroenterol Hepatol. 2007;5:465–469. doi: 10.1016/j.cgh.2007.02.008. [DOI] [PubMed] [Google Scholar]
- 33.Gwee KA, Leong YL, Graham C, McKendrick MW, Collins SM, Walters SJ, et al. The role of psychological and biological factors in postinfective gut dysfunction. Gut. 1999;44:400–406. doi: 10.1136/gut.44.3.400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gwee KA, Graham JC, McKendrick MW, Collins SM, Marshall JS, Walters SJ, et al. Psychometric scores and persistence of irritable bowel after infectious diarrhoea. Lancet. 1996;347:150–153. doi: 10.1016/s0140-6736(96)90341-4. [DOI] [PubMed] [Google Scholar]
- 35.Mearin F, Perez-Oliveras M, Perello A, Vinyet J, Ibanez A, Coderch J, et al. Dyspepsia and irritable bowel syndrome after a Salmonella gastroenteritis outbreak: one-year follow-up cohort study. Gastroenterology. 2005;129:98–104. doi: 10.1053/j.gastro.2005.04.012. [DOI] [PubMed] [Google Scholar]
- 36.Moss-Morris R, Spence M. To "lump" or to "split" the functional somatic syndromes: can infectious and emotional risk factors differentiate between the onset of chronic fatigue syndrome and irritable bowel syndrome? Psychosom Med. 2006;68:463–469. doi: 10.1097/01.psy.0000221384.07521.05. [DOI] [PubMed] [Google Scholar]
- 37.Wensaas K-A, Langeland N, Hanevik K, Morch K, Eide GE, Rortveit G. Irritable bowel syndrome and chronic fatigue 3 years after acute giardiasis: historic cohort study. Gut. 2012;61:214–219. doi: 10.1136/gutjnl-2011-300220. [DOI] [PubMed] [Google Scholar]
- 38.Halvorson HA, Schlett CD, Riddle MS. Postinfectious irritable bowel syndrome--a meta-analysis. Am J Gastroenterol. 2006;101:1894–1899. doi: 10.1111/j.1572-0241.2006.00654.x. quiz 942. [DOI] [PubMed] [Google Scholar]
- 39.Dunlop SP, Jenkins D, Neal KR, Spiller RC. Relative importance of enterochromaffin cell hyperplasia, anxiety, and depression in postinfectious IBS. Gastroenterology. 2003;125:1651–1659. doi: 10.1053/j.gastro.2003.09.028. [DOI] [PubMed] [Google Scholar]
- 40.Pickett-Blakely O. Obesity and irritable bowel syndrome: a comprehensive review. Gastroenterol Hepatol. 2014;10:411–416. [PMC free article] [PubMed] [Google Scholar]
- 41.Ley RE, Backhed F, Turnbaugh P, Lozupone CA, Knight RD, Gordon JI. Obesity alters gut microbial ecology. Proc Natl Acad Sci U S A. 2005;102:11070–11075. doi: 10.1073/pnas.0504978102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bennet SM, Ohman L, Simren M. Gut microbiota as potential orchestrators of irritable bowel syndrome. Gut Liver. 2015;9:318–331. doi: 10.5009/gnl14344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sundin J, Rangel I, Fuentes S, Heikamp-de Jong I, Hultgren-Hornquist E, deVos WM, Brummer RJ. Altered faecal and mucosal microbial composition in post-infectous irritable bowel syndrome patients correlates with mucosal lymphocyte phenotypes and psychological distress. Aliment Pharamcol Ther. 2015;41:342–351. doi: 10.1111/apt.13055. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.