Abstract
Genetic testing for BRCA genes, associated with hereditary breast-ovarian cancer risk, is an accepted cancer control strategy. BRCA genetic testing has both medical and psychosocial implications for individuals seeking testing and their family members. However, promoting open and adaptive communication about cancer risk in the family is challenging for parents of minor children. Using prospective data collected from mothers undergoing BRCA genetic testing and their untested co-parents (N = 102 parenting dyads), we examined how maternal and co-parent characteristics independently and conjointly influenced the overall quality of parent–child communication with minor children. Statistical associations were tested in accordance with the Actor–Partner Interdependence Model. Significant Actor effects were observed among mothers, such that open parent–child communication prior to genetic testing was positively associated with open communication 6 months following receipt of genetic test results; and among co-parents, more open parent–child communication at baseline and greater perceived quality of the parenting relationship were associated with more open parent–child communication at follow-up. Partner effects were also observed: co-parents’ baseline communication and confidence in their ability to communicate with their minor children about genetic testing was positively associated with open maternal parent– child communication at follow-up. These results demonstrate that for families facing the prospect of cancer genetic testing, perceptions and behaviors of both members of child-rearing couples have important implications for the overall quality of communication with their minor children, including communication about cancer risk.
Keywords: BRCA genetic testing, Family, communication, Cancer, Dyadic data analysis
Introduction
Over the past two decades, genetic counseling and testing for mutations in the BRCA1 and BRCA2 (BRCA) and other genes has become a widely available and accepted component of hereditary cancer risk assessment and management [1–3]. Women who are carriers of a pathogenic BRCA mutation face heightened risks for breast (BRCA1: 44–78 % risk; BRCA2: 31–56 % risk) and ovarian (BRCA1: 18–54 % risk; BRCA2: 2.4–19 % risk) cancers [4]. If a woman learns her BRCA mutation status, she can gain valuable information to guide decisions about risk management and prevention strategies including prophylactic surgery, enhanced screening, and chemoprevention. This form of genetic testing also has important psychosocial implications, as individuals undergoing genetic testing have been observed to experience heightened anxiety, depression, and worry about cancer [5–7]. Additionally, because BRCA mutations are inherited in an autosomal dominant manner, women undergoing genetic testing will acquire risk information relevant to their family members. For instance, a BRCA mutation carrier’s first-degree relatives (i.e., biological parents, siblings, and children) each have a 50 % likelihood of possessing the same cancer-predisposing gene. Although not at genetic risk themselves, spouses can also experience concerns and distress regarding the future health of a mutation carrier and their at-risk children [8]. Thus, the BRCA genetic testing process affects the health and well-being of not only individuals seeking testing, but also their untested family members [9–11].
Family communication plays a central role in cancer genetic testing, allowing for the sharing of health risk information, the receipt and provision of social support, and the promotion of adaptive coping efforts [12]. In this context, patterns of communication that are open and expressive can be beneficial for the entire family. For example, the gathering and dissemination of family cancer experiences and genetic testing results across generations can help shape family members’ understanding of their cancer risks and prevention options [13, 14]. Prior research has also demonstrated that open family communication is an important factor for adaptive child functioning when a parent has been diagnosed with cancer [15]. Furthermore, children in families affected with a genetic condition experience worries and concerns about their health and the health of their parents [16]: greater open communication is associated with higher levels of children’s emotional and psychological resiliency, as well as with adaptive responses to their own inherited disease risks [17]. More broadly, communication within families about hereditary and genetic disease risk has also focused on cystic fibrosis [18], hemophilia [19], Huntington’s disease [20], inherited retinal disease [21], and heart disease [22]. As such, parents can benefit from open communication with their parenting partners and children. Individuals undergoing hereditary cancer risk testing have reported lower levels of cancer-specific distress and worry when they have open communication with their partner and with their children [23].
Although there are substantial benefits to open communication about hereditary cancer risk in the family, achieving or maintaining such communication patterns may be challenging given the diverse medical and psychosocial implications of genetic testing. Indeed, prior work has demonstrated that among hereditary breast-ovarian cancer families, those who sought genetic testing subsequently reported poorer outcomes related to family expressiveness and cohesion than did those who refused testing [24]. Similarly, men and women having BRCA genetic testing reported a significant decline in their perceived family cohesion and family emotional bonding following testing [25]. Parents of minor children who seek genetic testing may be particularly vulnerable to communication- related challenges, as they must navigate decisions about whether, when, and how to share aspects of their genetic testing experience with their children and adult relatives. Although many parents undergoing genetic testing ultimately disclose risk information to their children [26–28], others do not. All parents struggle with balancing a respect for keeping children informed about health risks in the family with a desire to protect them from potentially distressing information that they may not be cognitively or emotionally prepared to receive or able to medically act upon [29]. Members of parenting dyads (e.g., mothers and their co-parents) can also differ in their motivations for communicating about genetic testing with their children [30], which contributes to family communication discord and difficulty.
It appears that genetic testing strains families’ normal communication processes, impacting not only disclosure of genetic test results, but also the overall quality of communication. In the case of parents and their children, identifying factors that promote or inhibit open parent– child communication about familial cancer is essential because it permits anticipation, guidance, and preparation for cancer prevention. Past work suggests that parental communication about BRCA is influenced by aspects of the parenting relationship and by parental coping behaviors: mothers are more likely to discuss BRCA with their co-parents when they perceive a stronger parenting alliance (i.e., commitment and cooperation regarding childrearing) [31, 32]. Additionally, parents are more likely to have open conversations about their own BRCA genetic test results with their minor children when employing more active coping strategies such as seeking social support and expressing their emotions [26]. Whether or not these factors are associated with the overall quality of parent–child communication among families undergoing BRCA genetic testing remains to be seen. Although such processes occur in the context of a dynamic and complex family system [12, 33], prior studies have not examined independent effects of an individual parent’s perceptions and behaviors on communication with her/his children, or effects that are interdependent upon the co-parent.
The present study sought to address these gaps in the literature by examining how characteristics of both members of a parenting dyad influence the quality of their communication with children in the context of hereditary breast-ovarian cancer risk. Specifically, we examined how both parents’ perceptions, including the perceived quality of their parenting relationship, stress, and coping appraisals regarding communicating about maternal BRCA genetic test results with children, independently and conjointly influence parent–child communication. Consistent with past research and the Actor–Partner Interdependence Model (APIM) framework [34, 35], which identifies the dyadic influences of both actor (i.e., own) and partner (i.e., co-parent/parenting partner) effects on a given outcome, we hypothesized that greater parenting relationship quality would be associated with more open parent–child communication (both Actor and Partner effects). We also hypothesized that lower perceived stress regarding communicating with children about BRCA and greater confidence in the ability to cope with communicating about BRCA results to children would be associated with more open parent–child communication (both Actor and Partner effects).
Materials and methods
Setting
Data for this analysis originated from a prospective study of family communication of BRCA genetic test results. Descriptions of the overall study methods have been published previously [28, 36, 37]. Briefly, participants were mothers who underwent genetic counseling and testing for BRCA mutations and their untested parenting partners (i.e., co-parents). In order to participate in the study, women had to: (1) be at least 18 years of age; (2) obtain BRCA testing by providing a DNA sample; and (3) be mother to at least one child between 8 and 21 years of age. The lower age was selected based on research suggesting that children begin to learn of their mothers’ genetic breast-ovarian cancer risk at this age [26, 27]. The upper age was selected because young people may be considered for genetic testing beginning at age 18, although medical interventions for cancer risk reduction are not recommended until age 25 [2]. Co-parents completed study interviews but did not undergo genetic testing themselves. Mothers and co-parents were enrolled at three cancer centers in the United States, all of which have well-established clinical hereditary breast/ovarian cancer risk and prevention research programs.
Procedures
Study procedures were approved by the Institutional Review Boards at each participating clinical site. Mothers were recruited for participation by a cancer genetic counselor at the conclusion of their pre-test genetic counseling appointment and after providing a DNA sample for BRCA analysis. For mothers with a parenting partner, they were informed that their co-parent had an opportunity to participate in the study. Written informed consent was obtained from all participants. Mothers provided detailed information about their children, including children’s age, gender, birth order, and birth relationship (e.g., biological, step-child). For mothers with more than one child, a computer algorithm was used to randomly select a target child within the study age range for reference on assessments. This process was developed to reduce selection bias that may occur when parents are asked to choose a target child [38]. Mothers and their co-parents completed telephone interviews at baseline and 1 month following their posttest counseling session (when a genetic counselor informed them of the mother’s BRCA genetic test results), and again 6 months after posttest counseling. A modest incentive of a $5 gift card was provided upon completion of each study assessment. Descriptions of the parent study sample of mothers and co-parents completing baseline and follow-up interviews, including comparisons of sample characteristics across study data collection points, are available elsewhere [28, 39].
Participants
In total, 121 mother and co-parent dyads were enrolled in the study and completed a baseline assessment. The sample for this analysis included the 102 dyads (84 % of the total) where both members of the dyad completed telephone interviews at baseline and 6 months follow-up. Compared with dyads that were not included in analyses because one or more members of the couple did not complete a follow-up (n = 19), those who were included (n = 102) were more likely to be non-Hispanic white (as reported by the mother: 87 versus 63 %, p = 0.009). There were no other statistically significant differences based on demographic or clinical characteristics. In total, 98 % (n = 100) of co-parents were male and 89 % (n = 91) were biological parents of the target child.
Measures
Demographic and clinical information
Demographic information was obtained in the baseline interview for mothers and co-parents (age, gender, race, education, household income, marital status), and their target child (age, gender). Maternal clinical information collected included proband status (i.e., the first affected family member to seek BRCA genetic counseling/testing), and family history of breast/ovarian cancer. Mothers’ genetic test results (true negative, negative uninformative [i.e., no mutation identified in a member of a kindred without a known mutation], positive) were abstracted from their clinical records.
Parenting alliance
Parenting relationship quality was assessed using a reliable and valid self-report scale, the Parenting Alliance Measure [40, 41]. The PAM is a 20-item measure of the strength of the child-rearing relationship between two parents (e.g., “When there is a problem with our child, we work out a good solution together.”). Responses are based on a five point Likert-type scale (1 = Strongly disagree, 5 = Strongly agree) and summed to create a total score with a range from 20 to 100. Higher scores indicate stronger and more positive parenting alliance. Internal consistency of the PAM among mothers and co-parents in the sample was high (Cronbach α’s > 0.90).
Cognitive appraisals of parent–child communication
According to the Transactional Model of Stress and Coping, cognitive appraisal is a two-part process during which mental impressions are formed about the stress associated with a specific event (i.e., primary appraisals) and one’s confidence in the ability to cope with the event (i.e., secondary appraisals) [42]. At baseline, we assessed primary and secondary appraisals among mothers and co-parents with items developed to capture perceived stress and confidence related to communicating with their child about maternal BRCA genetic test results and the consequences of this communication. These items were developed and validated in prior studies of women’s cognitive appraisals of the BRCA genetic counseling and testing process and adapted for use in genetic family communication research [43, 44].
Since items were administered during baseline interviews prior to receipt of the mother’s genetic test results, participants responded to separate sets of items assessing primary and secondary appraisals based on the possibility of receiving a positive or negative test result. In total, mothers and co-parents responded to 12 items assessing cognitive appraisals: primary (three items) and secondary (three items) appraisals in the event of a positive test result, and corresponding items in the event of a negative test result. Each set of items was introduced by statements such as: “Let’s assume your (your child’s mother’s) test result is negative, meaning you (she) do not (does not) carry a known mutation in BRCA.” Primary appraisal items captured how stressful it would be to talk with their child(ren) about the test result, deal with the impact of the test result for themselves, and deal with the impact of the test result for their child(ren). For secondary appraisals, a similar set of items assessed how confident parents were in talking with their child(ren) about the test results, dealing with the impact for themselves, and dealing with the impact for their child(ren). Responses to all items were based on a four point Likert-type scale (1 = Not at all, 4 = Very). Scores for each set of items were summed to create a continuous variable with higher values indicating greater stress (primary appraisals, range 3–12) and greater confidence in communicating test results to offspring (secondary appraisals, range 3–12). For analyses, we created variables for primary and secondary appraisals using scores derived from the sets of items that were specific to the mother’s actual test result only (positive or negative/negative uninformative). Internal consistency reliability was high (Cronbach α’s > 0.80), except for the set of items assessing co-parents’ primary appraisals in the event of a negative test result where internal consistency was lower (Cronbach’s α = 0.67). This was due to one item with a modest item-total correlation (r = 0.38).
Parent–child communication
The 20-item Parent–Adolescent Communication (PAC) scale was administered at baseline and follow-up to capture the quality of the parent–child communication relationship [45]. The PAC is a valid and reliable measure of openness (e.g., “I find it easy to discuss problems with my child.”) and problems (e.g., “I don’t think I can tell my child how I really feel about some things.”) in parent–child communication. Items are based on a five point Likert-type response and summed to create a total score (range 20–100). Higher values indicate more open, less problematic parent–child communication. The PAC had good reliability among mothers and co-parents at both time points (all Cronbach α’s > 0.75).
Statistical analysis
Statistical analysis involved several steps. First, descriptive statistics were used to examine distributional properties of all variables and to characterize the sample. Bivariate tests (i.e., t tests, Pearson’s r correlation) were used to examine differences between mothers and co-parents on predictor and outcome variables and correlations of predictor and outcome variables among participant classes. Descriptive statistics and bivariate tests were conducted using SAS 9.3 (SAS Institute, Cary, NC). Structural equation modeling was conducted using Mplus 7.1 (Muthen & Muthen, Los Angeles, CA) to examine the interdependent relationships of mothers’ and co-parents’ parent–child communication at follow-up using the APIM. This allows for examination of relationships among interdependent dyads by modeling both “Actor” (i.e., own) and “Partner” effects (i.e., co-parent). The APIM estimates Actor effects controlling for Partner effects and vice versa, taking into account the correlated nature of observations within dyads [34, 35].
Parenting dyad members were distinguishable in the dataset as either mother/actor or co-parent/partner. Following the APIM approach for distinguishable dyads, a just-identified (i.e., saturated) model with zero degrees of freedom was created first based on the hypothesized relationships [34, 35]. In order to obtain initial model fit estimates and to provide a comparison for subsequent models, a zero correlation constraint was applied for two baseline predictor variables given the results of bivariate analyses and findings from the saturated model [46]. Additional constraints were imposed to test whether Actor and Partner effects were equal by using the Wald χ2 statistics. P values >0.05 from the Wald test were considered to indicate that parameters of interest could be constrained to be equal without adversely affecting model fit [34, 35].
The following criteria were used to evaluate model fit: the model χ2 test (p > 0.05), a Comparative Fit Index (CFI) > 0.96, and a Root Mean Square Error Approximation (RMSEA) < 0.05 [47]. We included demographic and clinical characteristics as covariates in the model, such as the target child’s gender and mother’s proband status, which have been identified as influential factors in parental communication of BRCA test results to children [26].
Results
Sample characteristics
Participant characteristics (N = 102 parenting dyads) are shown in Table 1. Mothers and co-parents were mostly non-Hispanic white (87.2 %), had attained a college education or higher (≥75 %), and were married or living as married (>90 %). Compared with co-parents, mothers reported a more open and less problematic parent–child communication relationship at baseline (t = 4.02, p < 0.001) and 6 months (t = 2.51, p = 0.014). Compared to mothers, co-parents were significantly older (t = 4.81, p < 0.001).
Table 1.
Sample characteristics (n = 102)
| Mothers | Co-parents | |
|---|---|---|
| Demographics | ||
| Age, years (M [SD])a | 46.0 (6.0) | 48.1 (6.8) |
| Race | ||
| Non-Hispanic white | 87.2 % | 87.2 % |
| Non-white | 12.8 % | 12.8 % |
| Education | ||
| <College education | 23.5 % | 19.6 % |
| ≥College education | 76.5 % | 80.4 % |
| Marital status | ||
| Married/living as married | 91.2 % | 91.7 % |
| Unmarried | 8.8 | 8.3 % |
| Maternal clinical characteristics | ||
| Proband status | ||
| Yes | 79.4 % | – |
| No | 20.6 % | – |
| Family history of breast/ovarian cancer | ||
| Yes | 45.1 % | – |
| No | 54.9 % | – |
| BRCA test resultsb | ||
| True negative | 14.7 % | – |
| Uninformative | 76.5 % | – |
| Positive | 8.8 % | – |
| Child demographics | ||
| Age (M [SD]) | 12.5 (3.3) | 12.5 (3.3) |
| Gender | ||
| Female | 56.9 % | 56.9 % |
| Male | 43.1 % | 43.1 % |
| Baseline cognitive appraisals (M [SD]) | ||
| Primary (stress) | 4.2 (2.2) | 4.2 (2.0) |
| Secondary (coping confidence) | 10.4 (2.1) | 10.3 (2.1) |
| Baseline parenting alliance (PAM, M [SD]) | 90.3 (9.9) | 90.0 (9.3) |
| Parent–child communication (PAC, M [SD]) | ||
| Baseline* | 81.9 (10.4) | 77.2 (9.4) |
| 6 months* | 81.4 (10.5) | 78.5 (10.5) |
PAM Parenting alliance measure, PAC Parent–Adolescent Communication scale
Mothers and co-parents differed significantly at p < 0.05
For subsequent analyses, “true negative” and “uninformative” results were combined into a single category
Bivariate analyses
Bivariate correlations among parent–child communication, parenting alliance, and cognitive appraisal variables are shown in Table 2. Among mothers, more open parent–child communication at follow-up was associated with a stronger mother-reported parenting alliance (r = 0.35, p < 0.001), less perceived stress of communicating with children about BRCA test results (i.e., reduced primary appraisals, r = −0.19, p = 0.059), greater confidence in communicating with minor offspring about genetic test results (i.e., elevated secondary appraisals, r = 0.20, p = 0.049), and more open parent–child communication at baseline (r = 0.80, p < 0.001) (Table 2). Maternal parent–child communication at follow-up was also associated with co-parent-reported variables including more open parent–child communication at follow-up (r = 0.38, p < 0.001), stronger parenting alliance at baseline (r = 0.29, p = 0.003), and more open parent–child communication at baseline (r = 0.37, p < 0.001). Co-parents’ parent–child communication at follow-up was associated with several mother-reported variables including stronger parenting alliance at baseline (r = 0.30, p = 0.003) and reduced primary appraisals (r = −0.18, p = 0.076). Co-parents’ open parent–child communication at follow-up was also associated with their own and stronger parenting alliance (r = 0.54, p < 0.001), greater confidence in communicating with their children about genetic test results (i.e., secondary appraisals, r = 0.24, p = 0.015) and more open parent–child communication at baseline (r = 0.77, p < 0.001).
Table 2.
Bivariate correlations between mothers’ and co-parents’ parent–child communication and predictor variables
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | |
|---|---|---|---|---|---|---|---|---|---|---|
| Mothers | ||||||||||
| 1. Six month parent–child communication | 1.0 | |||||||||
| 2. Parenting alliance | .35*** | 1.0 | ||||||||
| 3. Primary appraisals | −.19* | .03 | 1.0 | |||||||
| 4. Secondary appraisals | .20** | .02 | −.55*** | 1.0 | ||||||
| 5. Baseline parent–child communication | .80*** | .31** | −.21** | −.15 | 1.0 | |||||
| Co-parents | ||||||||||
| 6. Six month parent–child communication | .38** | .30** | −.18* | −.001 | .07 | 1.0 | ||||
| 7. Parenting alliance | .29** | .53*** | −.14 | −.07 | −.08 | .54*** | 1.0 | |||
| 8. Primary appraisals | −.12 | .13 | .62*** | −.25** | −.06 | −.10 | −.09 | 1.0 | ||
| 9. Secondary appraisals | .15 | .004 | −.41*** | .17 | −.05 | .24** | .17* | −.50*** | 1.0 | |
| 10. Baseline parent–child communication | .37*** | .21** | −.22** | .03 | .21** | .77*** | .50*** | −.15 | .21** | 1.0 |
p < 0.01;
p < 0.05;
p < 0.10
The results of bivariate analyses conducted between the demographic and clinical characteristics shown in Table 1 and mothers’ and co-parents’ parent–child communication at follow-up revealed that none were significantly associated with these outcomes of interest (data not shown).
Actor–partner interdependence models
To obtain an initial model fit estimate from the just-identified model, the correlation between maternal parenting alliance at baseline and co-parents’ baseline secondary appraisals was constrained to equal zero. This constraint was selected because the correlation between these two variables was close to zero and not statistically significant in bivariate analyses (r = 0.004, p = 0.97; see Table 2). This initial model was a good fit for the data (Model χ2 [17] = 19.31, p = 0.31, CFI = 0.989, RMSEA = 0.037 [90 % CI 0.0, 0.10]).
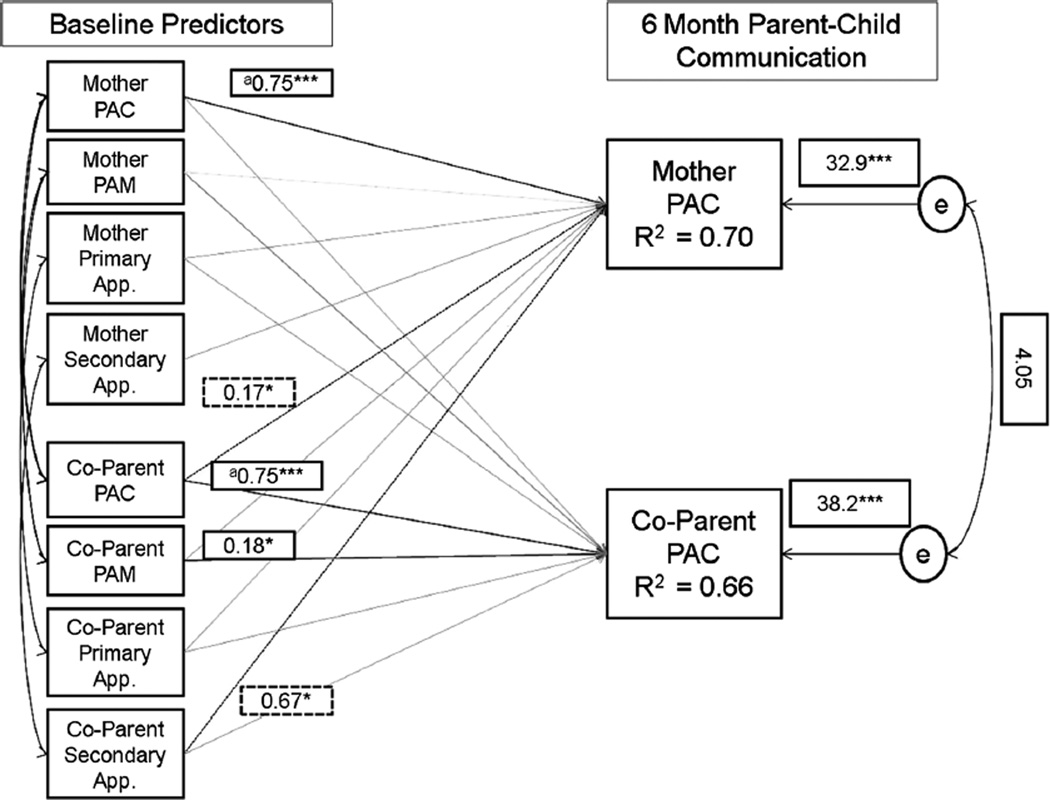
The final model fit the data well (Model χ2 [18] = 19.37, p = 0.37; CFI = 0.993; RMSEA = 0.027 [90 % CI 0.0, 0.09]) and is shown in Fig. 1. An equality constraint (χ2 [1] = 0.06, p = 0.81) revealed that both maternal and co-parent parent–child communication at baseline equally influenced their own parent–child communication at follow-up (B’s = 0.75, p < 0.001). Co-parents’ parent–child communication at baseline was also associated with maternal parent–child communication at follow-up (B = 0.17, p = 0.017), but a similar effect was not observed for the association between maternal parent–child communication and co-parent parent–child communication. In addition, greater confidence in co-parents’ ability to cope with maternal genetic test results at baseline (i.e., secondary appraisal) was associated with a more open parent–child communication relationship at follow-up for mothers (B = 0.67, p = 0.036). These findings lend support to the discovery of both actor and partner APIM effects in the data for mothers, but not for co-parents. In the final model, a stronger parenting alliance at baseline among co-parents was also associated with more open and less problematic parent–child communication at follow-up (B = 0.17, p = 0.036). Overall, the model explained 70 and 66 % of the variance in follow-up parent–child communication among mothers and co-parents, respectively.
Fig. 1.
Final actor partner interdependence model of mothers’ and co-parents’ parent–child communication. Note Model χ2 [18] = 19.37, p = 0.37. Comparative Fit Index = 0.993. Root mean square error approximation = 0.027 (90 % CI 0.0, 0.09). Raw (unstandardized) parameter estimates are displayed. Shaded lines represent non-statistically significant parameters (estimates are not displayed). Solid lines are actor effects, dashed lines are partner effects. aCoefficient paths constrained to be equal based on Wald χ2 test. Covariates included in the model were maternal proband status and child gender. For p values: * <0.05; ** <0.01; *** <0.001
Discussion
Results of the present study confirmed that for families facing the challenge of genetic testing for hereditary breast-ovarian cancer risk, the perceptions and behaviors of both parenting partners have implications for the ways in which they communicate with their minor children. Some of the observed effects were independent in nature. For example, Actor effects were observed such that BRCA-tested mothers’ and co-parents’ patterns of more open and less problematic parent–child communication prior to genetic testing were positively associated with their respective communication patterns 6 months after genetic testing. As hypothesized, co-parents with a stronger parenting alliance reported better communication with their children. However, a similar association was not found among mothers. It is possible that shared childrearing goals with the individual undergoing genetic testing is more important for the parenting partner not directly involved in genetic testing. A stronger parenting alliance may also assist co-parents in understanding maternal preferences and expectations about family communication and, in turn, communicate more freely and openly with their children.
Given the complexity of the family system, we had also predicted that one parent’s characteristics would directly influence the communication experiences of the other parent. Such effects were only observed for co-parents, as their parent–child communication and communication coping appraisals at baseline were associated with maternal parent–child communication 6 months after the receipt of BRCA test results. This suggests that for mothers undergoing genetic testing, their parenting partners play an important role in promoting healthy mother–child communication. By establishing a pattern of open communication with their children and expressing confidence in their own ability to cope with discussing BRCA test results with children, co-parents may implicitly or explicitly mitigate maternal concerns and buffer against stressors that could lead to poor mother–child communication outcomes. This notion is consistent with prior research in predictive genetic testing for hereditary cancer risk, which found that people who experience less support from their relationship partners tended to have more adverse effects on their interactions with their children [48].
Although we hypothesized that there would be both Actor and Partner effects of parents’ primary appraisals of the stress associated with communicating about BRCA testing to children on subsequent parent–child communications, no such effects were observed. However, it is important to note that the participants in this sample generally reported low levels of stress (Actor primary appraisals [M, SD] = 4.2, 2.2; Partners’ primary appraisals [M, SD] = 4.2, 2.0; range 3–12), which may be in part attributable to the fact that most mothers received negative/negative uninformative test results. Thus, it is not known how these cognitive appraisals may independently or conjointly affect the quality of parent–child communication for those who perceive such conversations as more distressing.
Strengths, limitations, and future directions
Through the use of a prospective design and the inclusion of both members of a parenting dyad, this study provides valuable insights into how the characteristics of both parents can influence the overall quality of communication with their minor children when mothers are undergoing cancer genetic counseling and testing. However, it is not known to what extent these results may be generalizable to the broader population of families utilizing other forms of genetic testing. The study sample consisted of parents who were predominantly non-Hispanic white, well-educated, and married, and included mothers with primarily negative/negative uninformative genetic test results. Furthermore, study participants reported generally low primary appraisals of the stress associated with communicating BRCA test results to their children, and high levels of parenting alliance. The effects of parents’ perceptions of their parenting relationship and their stress and coping appraisals on the quality of their parent–child communication may be different in families who are more diverse, in contexts where the gender of the parent and parenting partner are opposite from those studied herein (e.g., fathers undergoing genetic testing with untested mothers) or the same (e.g., same-sex couples), in families who are subjected to greater psychosocial stress due to the receipt of pathogenic results, and/or who report poorer premorbid relationship functioning. Finally, this study relied on parents’ self-reported assessments and the primary measures (PAM, PAC) did not assess parenting and communication styles specifically related to genetic information per se. Nevertheless, both measures have been used in previous studies of family functioning in health, cancer, and disease aggregation contexts [49–53]. Future studies that incorporate data derived from direct observation, genetic family communication, and the perspectives of minor children themselves may improve our understanding of how parental factors contribute to a healthy family communication environment.
Additional research is necessary to more fully understand which factors promote open parent–child communication in diverse familial and genetic testing contexts, including those contexts in which parents and families are likely to be most vulnerable (e.g., following the receipt of a pathogenic test result, those with poor parenting relationship quality, those coping with a current cancer diagnosis) and to identify factors that are amenable to intervention efforts. The results demonstrated that co-parents’ communication patterns and cognitive appraisals had important implications for the ways in which BRCA-tested mothers communicated with their children, and that their parenting relationship influenced their communication practices. Data suggested that co-parents played critical roles in shaping and maintaining open parent–child communication environments when families were subjected to the stressor of genetic testing for cancer risk. Future interventions must aim to incorporate and address the needs of parents as a means of promoting adaptive family communication and adjustment.
Conclusions
Genetic testing for hereditary breast-ovarian cancer risk affects not only the individual seeking testing, but family members as well. When the individual seeking testing is a parent of a young child, the challenges associated with genetic testing may be particularly pronounced and have far-reaching effects on family functioning and cancer preventive behaviors, including cancer risk communication. The present study demonstrated that the characteristics of both members of a parenting dyad, their existing patterns of communication, quality of the parenting relationship, and confidence in the ability to cope with communicating genetic test results to their children, all influenced the overall quality of parent–child communication that occurred in this context. Open family communication had benefits for all kindred members, and promoting such adaptive patterns of behavior among those seeking cancer genetic testing and their families requires consideration of multiple perspectives of agents in the family system.
Acknowledgments
This work was supported by National Institutes of Health Grants R01HG02686 and K18HG006754 to Kenneth P. Tercyak. Jada G. Hamilton was supported by National Institutes of Health Grant P30CA08748. We would like to thank Judy Garber, Andrea Patenaude, Beth Peshkin, and Katherine Schneider for their contributions to this research.
Footnotes
Compliance with ethical standards
Ethical approval All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent Informed consent was obtained from all individual participants included in the study.
Conflict of interest The authors declare that they have no conflict of interest.
References
- 1.Moyer VA. Risk assessment, genetic counseling, and genetic testing for BRCA-related cancer in women: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2014;160:271–281. doi: 10.7326/M13-2747. [DOI] [PubMed] [Google Scholar]
- 2.National Comprehensive Cancer Network. NCCN clinical practice guidelines in oncology (NCCN guidelines): genetic/familial high-risk assessment: breast and ovarian. [Accessed 10 Sept 2015];Version 2.2015. 2015 http://www.nccn.org/professionals/physician_gls/pdf/genetics_screening.pdf. [Google Scholar]
- 3.Robson ME, Storm CD, Weitzel J, Wollins DS, Offit K. American Society of Clinical Oncology policy statement update: genetic and genomic testing for cancer susceptibility. J Clin Oncol. 2010;28:893–901. doi: 10.1200/JCO.2009.27.0660. [DOI] [PubMed] [Google Scholar]
- 4.Antoniou A, Pharoah PD, Narod S, et al. Average risks of breast and ovarian cancer associated with BRCA1 or BRCA2 mutations detected in case series unselected for family history: a combined analysis of 22 studies. Am J Hum Genet. 2003;72:1117–1130. doi: 10.1086/375033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Butow PN, Lobb EA, Meiser B, Barratt A, Tucker KM. Psychological outcomes and risk perception after genetic testing and counselling in breast cancer: a systematic review. Med J Aust. 2003;178:77–81. doi: 10.5694/j.1326-5377.2003.tb05069.x. [DOI] [PubMed] [Google Scholar]
- 6.Cameron LD, Muller C. Psychosocial aspects of genetic testing. Curr Opin Psychiatry. 2009;22:218–223. doi: 10.1097/YCO.0b013e3283252d80. [DOI] [PubMed] [Google Scholar]
- 7.Hamilton JG, Lobel M, Moyer A. Emotional distress following genetic testing for hereditary breast and ovarian cancer: a meta-analytic review. Health Psychol. 2009;28:510–518. doi: 10.1037/a0014778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Metcalfe KA, Liede A, Trinkaus M, Hanna D, Narod SA. Evaluation of the needs of spouses of female carriers of mutations in BRCA1 and BRCA2. Clin Genet. 2002;62:464–469. doi: 10.1034/j.1399-0004.2002.620607.x. [DOI] [PubMed] [Google Scholar]
- 9.DeMarco TA, McKinnon WC. Life after BRCA1/2 testing: family communication and support issues. Breast Dis. 2006;27:127–136. doi: 10.3233/bd-2007-27108. [DOI] [PubMed] [Google Scholar]
- 10.Patenaude AF. Genetic testing for cancer: psychological approaches for helping patients and families. Washington: American Psychological Association; 2005. [Google Scholar]
- 11.Tercyak KP. Handbook of genomics and the family: psychosocial context for children and adolescents. New York: Springer; 2010. [Google Scholar]
- 12.Peterson SK. The role of the family in genetic testing: theoretical perspectives, current knowledge, and future directions. Health Educ Behav. 2005;32:627–639. doi: 10.1177/1090198105278751. [DOI] [PubMed] [Google Scholar]
- 13.Koehly LM, Peters JA, Kenen R, et al. Characteristics of health information gatherers, disseminators, and blockers within families at risk of hereditary cancer: implications for family health communication interventions. Am J Public Health. 2009;99:2203–2209. doi: 10.2105/AJPH.2008.154096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Palmquist AE, Koehly LM, Peterson SK, Shegog M, Vernon SW, Gritz ER. “The cancer bond”: exploring the formation of cancer risk perception in families with Lynch syndrome. J Genet Couns. 2010;19:473–486. doi: 10.1007/s10897-010-9299-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Visser A, Huizinga GA, van der Graaf WT, Hoekstra HJ, Hoekstra-Weebers JE. The impact of parental cancer on children and the family: a review of the literature. Cancer Treat Rev. 2004;30:683–694. doi: 10.1016/j.ctrv.2004.06.001. [DOI] [PubMed] [Google Scholar]
- 16.Tercyak KP, Peshkin BN, Streisand R, Lerman C. Psychological issues among children of hereditary breast cancer gene (BRCA1/2) testing participants. Psychooncology. 2001;10:336–346. doi: 10.1002/pon.531. [DOI] [PubMed] [Google Scholar]
- 17.Metcalfe A, Coad J, Plumridge GM, Gill P, Farndon P. Family communication between children and their parents about inherited genetic conditions: a meta-synthesis of the research. Eur J Hum Genet. 2008;16:1193–1200. doi: 10.1038/ejhg.2008.84. [DOI] [PubMed] [Google Scholar]
- 18.Ormond KE, Mills PL, Lester LA, Ross LF. Effect of family history on disclosure patterns of cystic fibrosis carrier status. Am J Med Genet C Semin Med Genet. 2003;119C:70–77. doi: 10.1002/ajmg.c.10008. [DOI] [PubMed] [Google Scholar]
- 19.Sorenson JR, Jennings-Grant T, Newman J. Communication about carrier testing within hemophilia A families. Am J Med Genet C Semin Med Genet. 2003;119C:3–10. doi: 10.1002/ajmg.c.10001. [DOI] [PubMed] [Google Scholar]
- 20.Forrest K, Simpson SA, Wilson BJ, et al. To tell or not to tell: barriers and facilitators in family communication about genetic risk. Clin Genet. 2003;64:317–326. doi: 10.1034/j.1399-0004.2003.00142.x. [DOI] [PubMed] [Google Scholar]
- 21.McKibbin M, Ahmed M, Allsop MJ, et al. Current understanding of genetics and genetic testing and information needs and preferences of adults with inherited retinal disease. Eur J Hum Genet. 2014;22:1058–1062. doi: 10.1038/ejhg.2013.296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cohen LL, Stolerman M, Walsh C, Wasserman D, Dolan SM. Challenges of genetic testing in adolescents with cardiac arrhythmia syndromes. J Med Ethics. 2012;38:163–167. doi: 10.1136/medethics-2011-100087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.van Oostrom I, Meijers-Heijboer H, Duivenvoorden HJ, et al. Family system characteristics and psychological adjustment to cancer susceptibility genetic testing: a prospective study. Clin Genet. 2007;71:35–42. doi: 10.1111/j.1399-0004.2007.00731.x. [DOI] [PubMed] [Google Scholar]
- 24.McInerney-Leo A, Biesecker BB, Hadley DW, et al. BRCA1/2 testing in hereditary breast and ovarian cancer families II: impact on relationships. Am J Med Genet A. 2005;133A:165–169. doi: 10.1002/ajmg.a.30566. [DOI] [PubMed] [Google Scholar]
- 25.Stroup AM, Smith KR. Familial effects of BRCA1 genetic mutation testing: changes in perceived family functioning. Cancer Epidemiol Biomarkers Prev. 2007;16:135–141. doi: 10.1158/1055-9965.EPI-06-0178. [DOI] [PubMed] [Google Scholar]
- 26.Tercyak KP, Hughes C, Main D, et al. Parental communication of BRCA1/2 genetic test results to children. Patient Educ Couns. 2001;42:213–224. doi: 10.1016/s0738-3991(00)00122-1. [DOI] [PubMed] [Google Scholar]
- 27.Tercyak KP, Peshkin BN, DeMarco TA, Brogan BM, Lerman C. Parent–child factors and their effect on communicating BRCA1/2 test results to children. Patient Educ Couns. 2002;47:145–153. doi: 10.1016/s0738-3991(01)00192-6. [DOI] [PubMed] [Google Scholar]
- 28.Tercyak KP, Mays D, DeMarco TA, et al. Decisional outcomes of maternal disclosure of BRCA1/2 genetic test results to children. Cancer Epidemiol Biomarkers Prev. 2013;22:1260–1266. doi: 10.1158/1055-9965.EPI-13-0198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Farkas Patenaude A, DeMarco TA, Peshkin BN, et al. Talking to children about maternal BRCA1/2 genetic test results: a qualitative study of parental perceptions and advice. J Genet Couns. 2013;22:303–314. doi: 10.1007/s10897-012-9549-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sharff ME, DeMarco TA, Mays D, et al. Parenting through genetic uncertainty: themes in the disclosure of breast cancer risk information to children. Genet Test Mol Biomarkers. 2012;16:376–382. doi: 10.1089/gtmb.2011.0154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.DeMarco TA, Peshkin BN, Valdimarsdottir HB, Patenaude AF, Schneider KA, Tercyak KP. Role of parenting relationship quality in communicating about maternal BRCA1/2 genetic test results with children. J Genet Couns. 2008;17:283–287. doi: 10.1007/s10897-007-9147-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Weissman S, Cohen RS. The parenting alliance and adolescence. Adolesc Psychiatry. 1985;12:24–45. [PubMed] [Google Scholar]
- 33.Rolland JS, Williams JK. Toward a biopsychosocial model for 21st-century genetics. Fam Process. 2005;44:3–24. doi: 10.1111/j.1545-5300.2005.00039.x. [DOI] [PubMed] [Google Scholar]
- 34.Kenny DA, Kashy DA, Cook WL. Dyadic data analysis. New York: Guilford Press; 2006. [Google Scholar]
- 35.Kenny DA, Ledermann T. Detecting, measuring, and testing dyadic patterns in the actor–partner interdependence model. J Fam Psychol. 2010;24:359–366. doi: 10.1037/a0019651. [DOI] [PubMed] [Google Scholar]
- 36.DeMarco TA, Nusbaum RH, Peshkin BN, et al. Prevalence and correlates of mothers and fathers attending pretest cancer genetic counseling together. Patient Educ Couns. 2010;78:29–33. doi: 10.1016/j.pec.2009.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tercyak KP, Peshkin BN, DeMarco TA, et al. Information needs of mothers regarding communicating BRCA1/2 cancer genetic test results to their children. Genet Test. 2007;11:249–255. doi: 10.1089/gte.2006.0534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Peshkin BN, DeMarco TA, Garber JE, et al. Brief assessment of parents’ attitudes toward testing minor children for hereditary breast/ovarian cancer genes: development and validation of the Pediatric BRCA1/2 Testing Attitudes Scale (P-TAS) J Pediatr Psychol. 2009;34:627–638. doi: 10.1093/jpepsy/jsn033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mays D, DeMarco TA, Luta G, et al. Distress and the parenting dynamic among BRCA1/2 tested mothers and their partners. Health Psychol. 2014;33:765–773. doi: 10.1037/a0033418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Abidin RR, Konold TR. Parenting alliance measure™ (PAM™): professional manual. Odessa: Psychological Assessment Resources; 1999. [Google Scholar]
- 41.Konold TR, Abidin RR. Parenting alliance: a multifactor perspective. Assessment. 2001;8:47–65. doi: 10.1177/107319110100800105. [DOI] [PubMed] [Google Scholar]
- 42.Lazarus RS, Folkman S. Stress, appraisal, and coping. New York: Springer; 1984. [Google Scholar]
- 43.Halbert CH, Schwartz MD, Wenzel L, et al. Predictors of cognitive appraisals following genetic testing for BRCA1 and BRCA2 mutations. J Behav Med. 2004;27:373–392. doi: 10.1023/b:jobm.0000042411.56032.42. [DOI] [PubMed] [Google Scholar]
- 44.O’Neill SC, Rini C, Goldsmith RE, Valdimarsdottir H, Cohen LH, Schwartz MD. Distress among women receiving uninformative BRCA1/2 results: 12-month outcomes. Psychooncology. 2009;18:1088–1096. doi: 10.1002/pon.1467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Barnes HL, Olson DH. Parent–Adolescent Communication Scale. In: Olson DH, McCubbin HI, Barnes HL, editors. Family inventories: inventories used in a national survey of families across the family life-cycle. St. Paul: Family Social Science, University of Minnesota; 1982. pp. 33–48. [Google Scholar]
- 46.Hasson-Ohayon I, Goldzweig G, Braun M, Galinsky D. Women with advanced breast cancer and their spouses: diversity of support and psychological distress. Psychooncology. 2010;19:1195–1204. doi: 10.1002/pon.1678. [DOI] [PubMed] [Google Scholar]
- 47.Hu L, Bentler PM. Cutoff criteria for fit indexes in covariance structure analysis: conventional criteria versus new alternatives. Struct Equ Model. 1999;6:1–55. [Google Scholar]
- 48.van Oostrom I, Meijers-Heijboer H, Duivenvoorden HJ, et al. A prospective study of the impact of genetic susceptibility testing for BRCA1/2 or HNPCC on family relationships. Psychooncology. 2007;16:320–328. doi: 10.1002/pon.1062. [DOI] [PubMed] [Google Scholar]
- 49.Cho OH, Yoo YS, Hwang KH. Comparison of parent–child communication patterns and parental role satisfaction among mothers with and without breast cancer. Appl Nurs Res. 2015;28:163–168. doi: 10.1016/j.apnr.2014.09.005. [DOI] [PubMed] [Google Scholar]
- 50.Mazzeschi C, Pazzagli C, Laghezza L, De GG, Reboldi G, De FP. Parental alliance and family functioning in pediatric obesity from both parents’ perspectives. J Dev Behav Pediatr. 2013;34:583–588. doi: 10.1097/DBP.0b013e3182a50a89. [DOI] [PubMed] [Google Scholar]
- 51.Phillips-Salimi CR, Robb SL, Monahan PO, Dossey A, Haase JE. Perceptions of communication, family adaptability and cohesion: a comparison of adolescents newly diagnosed with cancer and their parents. Int J Adolesc Med Health. 2014;26:19–26. doi: 10.1515/ijamh-2012-0105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Robertson HA, Kutcher SP, Bird D, Grasswick L. Impact of early onset bipolar disorder on family functioning: adolescents’ perceptions of family dynamics, communication, and problems. J Affect Disord. 2001;66:25–37. doi: 10.1016/s0165-0327(00)00281-0. [DOI] [PubMed] [Google Scholar]
- 53.Sales JM, Milhausen RR, Wingood GM, Diclemente RJ, Salazar LF, Crosby RA. Validation of a Parent–Adolescent Communication Scale for use in STD/HIV prevention interventions. Health Educ Behav. 2008;35:332–345. doi: 10.1177/1090198106293524. [DOI] [PubMed] [Google Scholar]