Abstract
Mammals develop in a physiologically hypoxic state and the oxygen tension of different tissues in the embryo is precisely controlled. Deviation from normal oxygenation, such as what occurs in placental insufficiency, can disrupt development. Several studies demonstrate that intrauterine hypoxia has a negative effect on kidney development. As nascent nephrons are forming from nephron progenitors in the nephrogenic zone, they are exposed to varying oxygen tension by virtue of the development of the renal vasculature. Thus, nephrogenesis may be linked to oxygen tension. However, the mechanism(s) by which this occurs remains unclear. This review will focus on what is known about the molecular mechanisms that are active in physiological and pathological hypoxia, and their effects on kidney development.
Keywords: nephrogenesis, nephron progenitors, hypoxia, placental insufficiency
Hypoxia and Mammalian Development
Most tissues reside normally in “physiological hypoxia,” with oxygen tension levels ranging from 1 to 9%, and oxygen levels below 1% being considered “pathological hypoxia” [1–4]. The mammalian embryo first begins development in a state of hypoxia, the placenta not developing until several days after fertilization [3, 4]. Portions of the developing embryo are vascularized and perfused at different rates; so physiological hypoxia varies throughout development. Tissue oxygen tension levels remain difficult to determine experimentally, so the relative changes in levels with vascularization remain undetermined.
The importance of appropriate oxygen tension during development is evidenced by culturing embryonic stem cell lines and early mouse embryos in low oxygen conditions to prevent spontaneous differentiation [5, 6]. For example, human embryonic stem cells have been used to show that the cellular main hypoxia responders, the transcription factors known as hypoxia inducible factors (HIFs), and low oxygen tension direct appropriate differentiation into neurons or glia [7]. Interestingly, the expansion both of mouse neural progenitors and post-natal cardiomyocytes is altered by both hypoxia and hyperoxia [8, 9]. Activation of the hypoxic stress pathway represses cardiomyocyte cell stress pathways to allow proliferation of the hypoxic fetal cardiomyocytes [10]. This review will discuss recent advances in understanding how changes in oxygen tension are critically important during kidney development.
Intrauterine Hypoxia
Although changes in relative oxygen tension levels are normal during development, exposure to additional hypoxia is known to negatively impact fetal development. Indeed, intrauterine hypoxia is a common environmental stressor as a result of maternal (smoking, environmental pollutants), placental (placental insufficiency) or fetal factors (anemia, cardiac defects). Of these factors, placental insufficiency is one of the main causes of intrauterine growth restriction (IUGR) [11–14]. Placental insufficiency increases a fetus’s risk for adverse health throughout life [11, 15, 16]. Depending on the severity of the hypoxic insult, affected fetuses will have varying degrees of injury, from alterations in metabolic, endocrine, and hematological systems [11], to overt tissue necrosis in the most severe settings. The placenta is responsible for delivering nutrients to the developing fetus; therefore an immature or abnormal placenta can restrict fetal growth. In response to decreased nutrient and oxygen availability, the fetus alters blood flow, preferentially supporting brain, heart, liver and adrenal growth, preventing proper development of other organs, such as the kidney [15, 16]. Impaired kidney development may reduce the number of nephrons that develop, increasing the risk for developing disease [17, 18]. Individuals affected by IUGR and decreased nephron number, have a higher risk for developing hypertension throughout life [19, 20]. Since IUGR is estimated to occur in 3–5% of pregnancies, this presents a life-long health risk to a significant portion of the population [21].
Several animal models of placental insufficiency have been developed to better understand the causes and impact of fetal hypoxia on mammalian development [22]. Placental insufficiency can be induced through gene deletions, such as eNOS, and placental-specific Igf2, to cause defects in placental maturation and vasculature [22–24]. A carefully designed Cited1 mouse study demonstrates that placental insufficiency results in renal medullary dysplasia [25]. Since placental Cited1 is only expressed from the maternally inherited X chromosome, the researchers compared female Cited1 heterozygous knockouts with paternally versus maternally inherited wild type X chromosomes, and found that only the paternally inherited null allele (null for Cited1 in the placenta and heterozygous for Cited1 in the embryo) caused placental defects. In these animals, placental insufficiency resulted in renal medullary dysplasia. However, most mouse genetic models are inefficient in producing affected embryos or are embryonic lethal, making them a poor choice to study the life-long risk associated with placental insufficiency. Furthermore, several of these models involve the knockout of genes that are expressed in the kidney, making it difficult to use these models to make observations about kidney development.
Uterine vessel ligation studies have also been used to model placental insufficiency, and its effects on development, in various animal species [22, 26]. However this method is technically challenging in mice due to their small size, so mouse models for placental insufficiency are typically produced using a hypoxia chamber. Pregnant dams are housed in an atmospherically controlled chamber, often using 12% O2, for several days to reduce oxygen delivery to embryos. The hypoxia chamber assay may also be performed with mice harboring a genetic mutation to investigate how the embryo’s hypoxia response is altered [27].
The Cellular Response to Hypoxia
Cells respond to hypoxic conditions mainly through the activation of hypoxia-inducible factors (HIFs). The HIFs are a family of transcription factors that bind to hypoxia-responsive elements (HREs) and mediate the transcription of many genes, including those involved in energy conservation and generation, angiogenesis, apoptosis, and cell growth and survival [28–34]. For example, HIF increases transcription of several genes involved in anaerobic glycolysis—such as hexokinase and phosphoglycerate kinase—which is the primary metabolic pathway of stem cells [35]. Furthermore, several microRNAs (miRNAs) have been shown to be hypoxia-responsive, aiding in the regulation of the cellular hypoxic response expression [36–38].
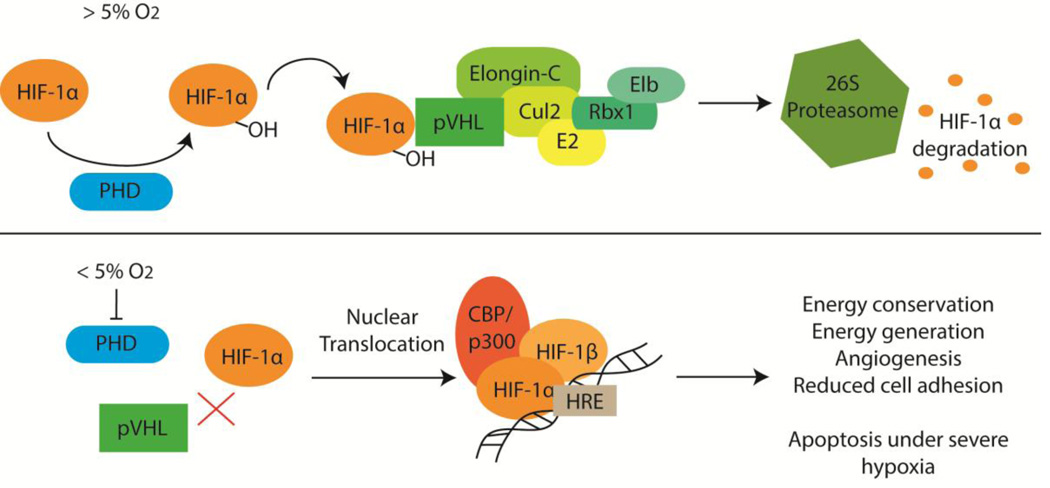
HIF is composed of an oxygen-sensitive α-subunit and a constitutively expressed β-subunit, known as HIF-β or arylhydrocarbon receptor nuclear translocator (ARNT). There are three known HIF α-subunits—HIF-1α, HIF-2α and HIF-3α—and two known HIF β-subunits—HIF-1β/ARNT and HIF-2β/ARNT2. As shown in Figure 1, when oxygen levels drop below 5%, prolyl-4-hydroxylases can no longer target HIF-α for degradation, resulting in the accumulation of HIF-α, which binds HIF-β. The HIF-αβ heterodimer binds the transcriptional co-activator CBP/p300 and then translocates to the nucleus, binding HREs to alter expression of the numerous HIF target genes. For a more detailed explanation of the HIF pathway, see these recent reviews [39–41].
Figure 1.
The hypoxia inducible factor 1 α-subunit (HIF-1α) is constitutively expressed. When the cellular oxygen tension is above 5%, HIF-1α is degraded. This is initiated when prolyl-4-hydroxylases (PHD) add hydroxyl (-OH) groups to HIF-1α. The von-Hippel Lindau protein (pVHL) binds hydroxylated HIF-1α and recruits other proteins to aid in the ubiquitinylation of HIF-1α, targeting it for degradation by the 26S proteasome. When the oxygen tension drops below 5%, PHDs can no longer hydroxylate HIF-1α, preventing pVHL from binding. HIF-1α accumulates in the cytosol and binds the HIF-1β/ARNT subunit. This heterodimeric transcription factor complex binds the CBP/p300 coactivator and this complex then translocates to the nucleus where it binds hypoxia-responsive elements (HRE) throughout the genome to up-regulate transcription of many genes involved in various processes.
HIF Function During Kidney Development
Metanephric kidney development begins with the outgrowth of the ureteric bud (UB) from the mesonephric duct into the adjacent metanephric mesenchyme (MM). The UB undergoes a series of branching events to form the collecting system of the kidney. Meanwhile, the MM condenses to form a “cap” of nephron progenitors around the ureteric tips. These progenitors possess the ability to self-renew during kidney development and are multipotent, giving rise to the multiple cell types required to form a functional nephron (from the glomerular epithelial cells to the distal tubule). The progenitors differentiate by undergoing a mesenchymal to epithelial transition followed by a series of proximal-distal patterning events to form the mature nephron. This process of nephrogenesis occurs in the relatively avascular nephrogenic zone of the developing kidney. Thus, it is believed that the MM is maintained in a hypoxic environment. However, measuring tissue oxygen tension in developing tissues remains technically challenging, and there is no clear data regarding the true oxygen tension of the nephrogenic zone or other parts of the developing kidney.
Given the relatively hypoxic niche in which nephron progenitors are likely to reside, this would predict a role for HIFs during nephrogenesis. Human and rat kidney HIF-1α co-localize with vascular endothelial growth factor (VEGF) in medullary collecting ducts and early glomeruli during kidney development, suggesting that HIF-1α plays a role in tubulogenesis and glomerulogenesis [42]. The same study demonstrated a distinct HIF-2α expression pattern—podocytes of more mature glomeruli, interstitial and peritubular cells—and co-localization with the endothelium-associated angiogenic factor endoglin [42]. Together, this suggests that HIF-2α is involved in renal vasculogenesis, likely in later stages of kidney development. Similar expression patterns were found in mice, with the nephrogenic zone of newborn kidney cortex and the medulla showing high HIF-1α and HIF-2α expression [43]. Genetic knockout of HIF-1α in mice leads to death by E9.5 from vascular and neural tube defects [44, 45]. HIF-2α knockout mice exhibit varying phenotypes—lethality from different causes at mid-gestational to post-gestational ages—based on the genetic background of the mouse. The mice that survive long enough for a metanephric kidney to develop had no nephrogenic or renal vasculature defects, suggesting that HIF-1α is the critical HIF during kidney development [41]. At present, there is no data regarding the potential developmental effects of a kidney-specific HIF-1α conditional knockout, although it is clear that HIF-1α is essential for normal vascular and organ development.
The expression of the HIF β-subunits during kidney development has also recently been investigated. HIF-2β was highly expressed in newborn mouse kidney, but expression decreased with age. In contrast, HIF-1β levels remained constant in the developing and mature kidney [46]. The authors suggest that high HIF-2β levels are needed in distal tubules during nephrogenesis, but later only needed in thick ascending limb. Metanephric development cannot be studied in global HIF-1β knockout mice, since the embryos do not survive past E10.5 from defects in both branchial arches and yolk sac angiogenesis [47]. To date, there are no kidney-specific HIF-β knockouts. Further studies of specific target genes for each subunit—and heterodimer pair—would prove useful in determining their roles during mammalian development.
Oxygen Tension and Ureteric Branching
HIFs have been shown to be important during development, but the role oxygen tension plays during kidney development has only recently been more thoroughly investigated. A 2005 study demonstrated that cultured E12 mouse kidney explants grown in 5% O2 had more extensive ureteric branching than those cultured in 20% O2 [48] (Table 1). Furthermore, E13 rat kidney explants cultured in 5% O2 for 96 hours had significantly more UB branching than kidneys cultured in 21% O2 [49]. In contrast, a 2015 study found that E12 and E13 mouse kidney explants cultured in 21% O2 had improved kidney growth and ureteric branching, while kidneys cultured in 5% and 1% O2 were significantly smaller and had fewer ureteric branches [50]. This disparity may be attributed to different culturing conditions, since the same mouse strains were used. The 2015 study cultured dissected E12 or E13 kidneys at 21% O2 for 24 hours and then exposed contralateral kidneys to different conditions for 48 hours, while the explants in the 2005 study were dissected and immediately cultured in different oxygen tensions for 48 hours. Together, this suggests that the timing of exposure to different oxygen tensions may be critical for proper kidney development.
Table 1.
Summary of studies evaluating the effect of hypoxia on kidney development.
| Hypoxia Model | Result | Citation |
|---|---|---|
|
|
[48] |
|
|
[49] |
|
|
[50] |
|
|
[55] |
|
|
[52] |
|
|
[61] |
VEGF= vascular endothelial growth factor
The observed oxygen-dependent effects on kidney development has prompted further research into the molecular signaling mediated by changes in oxygen tension. The previously described 2005 study showed that VEGF and FGF2 played a role in UB branching in both oxygen tension conditions [48]. The 2015 study furthered this, demonstrating that hypoxic HIF activation increases paracrine VEGFA signaling to inhibit nephrogenesis but enhance tubulogenesis [50]. The rat kidney explants mentioned earlier were also treated with the HIF-1α inhibitor digoxin, showing that HIF-1α regulates mRNA levels of Gdnf and its receptor while also regulating Fgf7 levels to play a role in regulating UB branching morphogenesis [49]. Several studies of pancreatic development—which occurs much like ureteric branching—reveal that the branching epithelium recruits blood vessels via VEGFA, which then signal to maintain trunk epithelium and inhibit acinar cell differentiation [51]. VEGFA and the vascular endothelium likely play similar roles in kidney development, but more studies are needed to more precisely define the molecular mechanism through which these hypoxia-responsive proteins are altering ureteric branching.
Oxygen Tension and Nephron Progenitors
As noted above, many stem cells are maintained in hypoxic niches and the same appears to hold true for nephron progenitors. Mouse kidney explant studies demonstrate that the nephrogenic zone of the developing kidney does not receive blood flow, and that the differentiation of nephron progenitors appears to be induced by vascular perfusion and the accompanying increase in oxygen tension [52]. However, how this process is regulated by hypoxia is poorly understood.
In other developing and adult organs, such as the intestine and liver, canonical developmental pathways are used for stem cell maintenance and differentiation. Similar mechanisms are found in the kidney with Wnt/β-catenin and Notch pathways regulating nephron progenitor expansion and differentiation [53, 54]. It is possible that these developmental pathways are hypoxia-responsive as part of their regulatory role in nephron progenitor maintenance and differentiation. In fact, ureteric Wnt/β-catenin signaling is suppressed during in utero hypoxia in mice [55] (Table 1). However, the specific hypoxia-responsive mechanism that results in altered Wnt signaling is unclear.
Looking at the canonical Notch pathway, Notch and HIF-1α interact under hypoxic conditions to inhibit myogenic and neuronal differentiation [56]. Nephron progenitor-specific Notch2 activation results in their depletion, ectopic Wnt4 expression, and premature tubule formation [57]. However, more studies are required to define the mechanism(s) by which hypoxia affects nephron progenitor biology.
Placental Insufficiency Effects on Nephrogenesis
Animal models of placental insufficiency demonstrate the various effects that in utero hypoxia can have on developing embryos. However, placental insufficiency also affects metabolite exchange, and separating pathological hypoxia from reduced nutrient availability is difficult. Adolescent guinea pigs that suffered from placental insufficiency had low birth weights, exhibited increased fibrosis in the heart and kidney, and decreased nephron number [58]. Further studies of how disease manifests in these guinea pigs will be useful in understanding similar cases in humans.
As mentioned earlier, reduced nephron number in humans can increase the risk for development of kidney disease. Several animal models show that placental insufficiency can reduce the number of nephrons that form. A rat model of placental insufficiency resulted in a nephron deficit and modest renal insufficiency [59]. Even though these rats did not develop hypertension, they may still be useful in determining the mechanism by which kidney development is altered by hypoxia. Similarly, IUGR rabbit fetuses from placental insufficiency pregnancies showed altered hypoxia-responsive gene expression and developed fewer nephrons [60]. Importantly, this study was able to show a direct link between placental insufficiency and nephrogenesis, although further studies are required to elucidate the mechanism of its effect on development.
Another rat study revealed that placental insufficiency reduced the kidney-to-body weight ratio and caused histological changes to the glomeruli—specifically, an enlarged Bowman space, flattened and decreased podocyte foot processes, and swelling and deformed mitochondria—and a wider interstitium in hypoxic fetuses [61]. The researchers demonstrated that the fetal kidneys had increased apoptosis and Beclin I signaling-mediated autophagy, but it remains unclear whether there were direct effects of hypoxia on nephron progenitors.
Mouse embryos exposed to severe acute early gestational hypoxia exhibited congenital anomalies of the kidney and urinary tract (commonly known as CAKUT) [55]. Interestingly, milder, prolonged hypoxia initiated a few days later in gestation resulted in a milder phenotype characterized by a smaller body size and reduced nephron number. These differences suggest that the degree of hypoxia is tightly controlled during nephrogenesis and that abnormalities in nephron development occur in a dose-dependent fashion.
Summary
Kidney development relies on physiological hypoxia for proper nephrogenesis, while pathological hypoxia inhibits the process and can result in a range of adverse effects. More research on the effect of fetal hypoxia on nephrogenesis needs to be conducted so that we can better understand how human health is affected in IUGR and placental insufficiency. While technically challenging, it will be important to define oxygen tension levels in developing tissues exposed physiological and pathological hypoxia, and link this information to the expression of hypoxia-responsive genes. Fetal hypoxia can restrict renal blood flow, causing the kidney to be pathologically hypoxic. While outside the scope of this review, the effects of hypoxia on the development of the renal vasculature also remains unclear.
HIFs are active during normal kidney development, and likely has increased activity during pathological hypoxia. The role that HIFs play should be further investigated by examining the gene expression profiles of nephron progenitors and nascent nephron structures (renal vesicle, comma-shaped body, s-shaped body, etc.) in both physiological and pathological hypoxia to identify the hypoxia-response signature of these lineages. This information will lead to a better mechanistic understanding of the pathologies resulting from intrauterine hypoxia and placental defects and deficiencies, and their consequent effects on human health.
Acknowledgments
Dr. Ho’s laboratory is supported by funding by an NIDDK R00DK087922, NIDDK R01DK103776, March of Dimes Basil O’Connor Starter Scholar Award and NIDDK Diabetic Complications Consortium grant DK076169. Dr. Ho’s laboratory has received research grant funding from Satellite Healthcare as part of the Norman S. Coplon Extramural Grant Program previously. Dr. Sims-Lucas’s laboratory is supported by an NIDDK K01DK096996.
Footnotes
Conflict of Interest The authors declare no conflict of interest.
References
- 1.Okazaki K, Maltepe E. Oxygen, epigenetics and stem cell fate. Regen Med. 2006;1:71–83. doi: 10.2217/17460751.1.1.71. [DOI] [PubMed] [Google Scholar]
- 2.Ivanovic Z. Hypoxia or in situ normoxia: The stem cell paradigm. J Cell Physiol. 2009;219:271–275. doi: 10.1002/jcp.21690. [DOI] [PubMed] [Google Scholar]
- 3.Mohyeldin A, Garzon-Muvdi T, Quinones-Hinojosa A. Oxygen in stem cell biology: a critical component of the stem cell niche. Cell Stem Cell. 2010;7:150–161. doi: 10.1016/j.stem.2010.07.007. [DOI] [PubMed] [Google Scholar]
- 4.Dunwoodie SL. The role of hypoxia in development of the Mammalian embryo. Dev Cell. 2009;17:755–773. doi: 10.1016/j.devcel.2009.11.008. [DOI] [PubMed] [Google Scholar]
- 5.Ezashi T, Das P, Roberts RM. Low O2 tensions and the prevention of differentiation of hES cells. Proc Natl Acad Sci U S A. 2005;102:4783–4788. doi: 10.1073/pnas.0501283102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rinaudo PF, Giritharan G, Talbi S, Dobson AT, Schultz RM. Effects of oxygen tension on gene expression in preimplantation mouse embryos. Fertil Steril. 2006;86:1252–1265. doi: 10.1016/j.fertnstert.2006.05.017. 1265 e1251-1236. [DOI] [PubMed] [Google Scholar]
- 7.Xie Y, Zhang J, Lin Y, Gaeta X, Meng X, Wisidagama DR, Cinkornpumin J, Koehler CM, Malone CS, Teitell MA, Lowry WE. Defining the role of oxygen tension in human neural progenitor fate. Stem Cell Reports. 2014;3:743–757. doi: 10.1016/j.stemcr.2014.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wagenfuhr L, Meyer AK, Braunschweig L, Marrone L, Storch A. Brain oxygen tension controls the expansion of outer subventricular zone-like basal progenitors in the developing mouse brain. Development. 2015;142:2904–2915. doi: 10.1242/dev.121939. [DOI] [PubMed] [Google Scholar]
- 9.Puente BN, Kimura W, Muralidhar SA, Moon J, Amatruda JF, Phelps KL, Grinsfelder D, Rothermel BA, Chen R, Garcia JA, Santos CX, Thet S, Mori E, Kinter MT, Rindler PM, Zacchigna S, Mukherjee S, Chen DJ, Mahmoud AI, Giacca M, Rabinovitch PS, Aroumougame A, Shah AM, Szweda LI, Sadek HA. The oxygen-rich postnatal environment induces cardiomyocyte cell-cycle arrest through DNA damage response. Cell. 2014;157:565–579. doi: 10.1016/j.cell.2014.03.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Guimaraes-Camboa N, Stowe J, Aneas I, Sakabe N, Cattaneo P, Henderson L, Kilberg MS, Johnson RS, Chen J, McCulloch AD, Nobrega MA, Evans SM, Zambon AC. HIF 1alpha Represses Cell Stress Pathways to Allow Proliferation of Hypoxic Fetal Cardiomyocytes. Dev Cell. 2015;33:507–521. doi: 10.1016/j.devcel.2015.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Eleftheriades M, Creatsas G, Nicolaides K. Fetal growth restriction and postnatal development. Ann NY Acad Sci. 2006;1092:319–330. doi: 10.1196/annals.1365.047. [DOI] [PubMed] [Google Scholar]
- 12.Henriksen T, Clausen T. The fetal origins hypothesis: placental insufficiency and inheritance versus maternal malnutrition in well-nourished populations. Acta Obstet Gynecol Scand. 2002;81:112–114. doi: 10.1034/j.1600-0412.2002.810204.x. [DOI] [PubMed] [Google Scholar]
- 13.Stallmach T, Hebisch G, Meier K, Dudenhausen JW, Vogel M. Rescue by birth: defective placental maturation and late fetal mortality. Obstet Gynecol. 2001;97:505–509. doi: 10.1016/s0029-7844(00)01208-4. [DOI] [PubMed] [Google Scholar]
- 14.Kingdom J, Huppertz B, Seaward G, Kaufmann P. Development of the placental villous tree and its consequences for fetal growth. Eur J Obstet Gynecol Reprod Biol. 2000;92:35–43. doi: 10.1016/s0301-2115(00)00423-1. [DOI] [PubMed] [Google Scholar]
- 15.Baschat AA. Fetal responses to placental insufficiency: an update. BJOG. 2004;111:1031–1041. doi: 10.1111/j.1471-0528.2004.00273.x. [DOI] [PubMed] [Google Scholar]
- 16.Cosmi E, Fanelli T, Visentin S, Trevisanuto D, Zanardo V. Consequences in infants that were intrauterine growth restricted. J Pregnancy. 2011;2011:364381. doi: 10.1155/2011/364381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bertram JF, Douglas-Denton RN, Diouf B, Hughson MD, Hoy WE. Human nephron number: implications for health and disease. Pediatr Nephrol. 2011;26:1529–1533. doi: 10.1007/s00467-011-1843-8. [DOI] [PubMed] [Google Scholar]
- 18.Cebrian C, Asai N, D'Agati V, Costantini F. The number of fetal nephron progenitor cells limits ureteric branching and adult nephron endowment. Cell Rep. 2014;7:127–137. doi: 10.1016/j.celrep.2014.02.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Paixao AD, Alexander BT. How the kidney is impacted by the perinatal maternal environment to develop hypertension. Biol Reprod. 2013;89:144. doi: 10.1095/biolreprod.113.111823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Luyckx VA, Brenner BM. Birth weight, malnutrition and kidney-associated outcomes--a global concern. Nat Rev Nephrol. 2015;11:135–149. doi: 10.1038/nrneph.2014.251. [DOI] [PubMed] [Google Scholar]
- 21.Parker SE, Werler MM. Epidemiology of ischemic placental disease: a focus on preterm gestations. Semin Perinatol. 2014;38:133–138. doi: 10.1053/j.semperi.2014.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Swanson AM, David AL. Animal models of fetal growth restriction: Considerations for translational medicine. Placenta. 2015;36:623–630. doi: 10.1016/j.placenta.2015.03.003. [DOI] [PubMed] [Google Scholar]
- 23.Constancia M, Hemberger M, Hughes J, Dean W, Ferguson-Smith A, Fundele R, Stewart F, Kelsey G, Fowden A, Sibley C, Reik W. Placental-specific IGF-II is a major modulator of placental and fetal growth. Nature. 2002;417:945–948. doi: 10.1038/nature00819. [DOI] [PubMed] [Google Scholar]
- 24.Dilworth MR, Kusinski LC, Baker BC, Renshall LJ, Baker PN, Greenwood SL, Wareing M, Sibley CP. Crossing mice deficient in eNOS with placental-specific Igf2 knockout mice: a new model of fetal growth restriction. Placenta. 2012;33:1052–1054. doi: 10.1016/j.placenta.2012.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sparrow DB, Boyle SC, Sams RS, Mazuruk B, Zhang L, Moeckel GW, Dunwoodie SL, de Caestecker MP. Placental insufficiency associated with loss of Cited 1 causes renal medullary dysplasia. J Am Soc Nephrol. 2009;20:777–786. doi: 10.1681/ASN.2008050547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Habli M, Jones H, Aronow B, Omar K, Crombleholme TM. Recapitulation of characteristics of human placental vascular insufficiency in a novel mouse model. Placenta. 2013;34:1150–1158. doi: 10.1016/j.placenta.2013.09.011. [DOI] [PubMed] [Google Scholar]
- 27.Larkin J, Chen B, Shi XH, Mishima T, Kokame K, Barak Y, Sadovsky Y. NDRG1 deficiency attenuates fetal growth and the intrauterine response to hypoxic injury. Endocrinology. 2014;155:1099–1106. doi: 10.1210/en.2013-1425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Elvidge GP, Glenny L, Appelhoff RJ, Ratcliffe PJ, Ragoussis J, Gleadle JM. Concordant regulation of gene expression by hypoxia and 2-oxoglutarate-dependent dioxygenase inhibition: the role of HIF-1alpha, HIF-2alpha, and other pathways. J Biol Chem. 2006;281:15215–15226. doi: 10.1074/jbc.M511408200. [DOI] [PubMed] [Google Scholar]
- 29.Greijer AE, van der Groep P, Kemming D, Shvarts A, Semenza GL, Meijer GA, van de Wiel MA, Belien JA, van Diest PJ, van der Wall E. Up-regulation of gene expression by hypoxia is mediated predominantly by hypoxia-inducible factor 1 (HIF-1) J Pathol. 2005;206:291–304. doi: 10.1002/path.1778. [DOI] [PubMed] [Google Scholar]
- 30.Hu CJ, Wang LY, Chodosh LA, Keith B, Simon MC. Differential roles of hypoxia-inducible factor 1 alpha (HIF-1 alpha) and HIF-2 alpha in hypoxic gene regulation. Mol Cell Biol. 2003;23:9361–9374. doi: 10.1128/MCB.23.24.9361-9374.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Manalo DJ, Rowan A, Lavoie T, Natarajan L, Kelly BD, Ye SQ, Garcia JG, Semenza GL. Transcriptional regulation of vascular endothelial cell responses to hypoxia by HIF-1. Blood. 2005;105:659–669. doi: 10.1182/blood-2004-07-2958. [DOI] [PubMed] [Google Scholar]
- 32.Wenger RH, Stiehl DP, Camenisch G. Integration of oxygen signaling at the consensus HRE. Sci STKE. 2005;2005:re12. doi: 10.1126/stke.3062005re12. [DOI] [PubMed] [Google Scholar]
- 33.Semenza GL. Regulation of mammalian O2 homeostasis by hypoxia-inducible factor 1. Annu Rev Cell Dev Biol. 1999;15:551–578. doi: 10.1146/annurev.cellbio.15.1.551. [DOI] [PubMed] [Google Scholar]
- 34.Kato H, Tamamizu-Kato S, Shibasaki F. Histone deacetylase 7 associates with hypoxia-inducible factor 1alpha and increases transcriptional activity. J Biol Chem. 2004;279:41966–41974. doi: 10.1074/jbc.M406320200. [DOI] [PubMed] [Google Scholar]
- 35.Vacanti NM, Metallo CM. Exploring metabolic pathways that contribute to the stem cell phenotype. Biochim Biophys Acta. 2013;1830:2361–2369. doi: 10.1016/j.bbagen.2012.08.007. [DOI] [PubMed] [Google Scholar]
- 36.Kulshreshtha R, Ferracin M, Wojcik SE, Garzon R, Alder H, Agosto-Perez FJ, Davuluri R, Liu CG, Croce CM, Negrini M, Calin GA, Ivan M. A microRNA signature of hypoxia. Mol Cell Biol. 2007;27:1859–1867. doi: 10.1128/MCB.01395-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nallamshetty S, Chan SY, Loscalzo J. Hypoxia: a master regulator of microRNA biogenesis and activity. Free Radic Biol Med. 2013;64:20–30. doi: 10.1016/j.freeradbiomed.2013.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pocock R. Invited review: decoding the microRNA response to hypoxia. Pflugers Arch. 2011;461:307–315. doi: 10.1007/s00424-010-0910-5. [DOI] [PubMed] [Google Scholar]
- 39.Majmundar AJ, Wong WJ, Simon MC. Hypoxia-inducible factors and the response to hypoxic stress. Mol Cell. 2010;40:294–309. doi: 10.1016/j.molcel.2010.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kaelin WG, Jr, Ratcliffe PJ. Oxygen sensing by metazoans: the central role of the HIF hydroxylase pathway. Mol Cell. 2008;30:393–402. doi: 10.1016/j.molcel.2008.04.009. [DOI] [PubMed] [Google Scholar]
- 41.Gunaratnam L, Bonventre JV. HIF in kidney disease and development. J Am Soc Nephrol. 2009;20:1877–1887. doi: 10.1681/ASN.2008070804. [DOI] [PubMed] [Google Scholar]
- 42.Bernhardt WM, Schmitt R, Rosenberger C, Munchenhagen PM, Grone HJ, Frei U, Warnecke C, Bachmann S, Wiesener MS, Willam C, Eckardt KU. Expression of hypoxia-inducible transcription factors in developing human and rat kidneys. Kidney Int. 2006;69:114–122. doi: 10.1038/sj.ki.5000062. [DOI] [PubMed] [Google Scholar]
- 43.Freeburg PB, Robert B, St John PL, Abrahamson DR. Podocyte expression of hypoxia-inducible factor (HIF)-1 and HIF-2 during glomerular development. J Am Soc Nephrol. 2003;14:927–938. doi: 10.1097/01.asn.0000059308.82322.4f. [DOI] [PubMed] [Google Scholar]
- 44.Iyer NV, Kotch LE, Agani F, Leung SW, Laughner E, Wenger RH, Gassmann M, Gearhart JD, Lawler AM, Yu AY, Semenza GL. Cellular and developmental control of O2 homeostasis by hypoxia-inducible factor 1a. Genes & Dev. 1998;12:149–162. doi: 10.1101/gad.12.2.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ryan HE, Lo J, Johnson RS. HIF-1 alpha is required for solid tumor formation and embryonic vascularization. EMBO J. 1998;17:3005–3015. doi: 10.1093/emboj/17.11.3005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Freeburg PB, Abrahamson DR. Divergent expression patterns for hypoxia-inducible factor-1beta and aryl hydrocarbon receptor nuclear transporter-2 in developing kidney. J Am Soc Nephrol. 2004;15:2569–2578. doi: 10.1097/01.ASN.0000141464.02967.29. [DOI] [PubMed] [Google Scholar]
- 47.Maltepe E, Schmidt JV, Baunoch D, Bradfield CA, Simon MC. Abnormal angiogenesis and responses to glucose and oxygen deprivation in mice lacking the protein ARNT. Nature. 1997;386:403–407. doi: 10.1038/386403a0. [DOI] [PubMed] [Google Scholar]
- 48.Akimoto T, Hammerman MR, Kusano E. Low ambient O2 enhances ureteric bud branching in vitro. Organogenesis. 2005;2:17–21. doi: 10.4161/org.2.1.1726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tsuji K, Kitamura S, Makino H. Hypoxia-inducible factor 1alpha regulates branching morphogenesis during kidney development. Biochem Biophys Res Commun. 2014;447:108–114. doi: 10.1016/j.bbrc.2014.03.111. [DOI] [PubMed] [Google Scholar]
- 50.Schley G, Scholz H, Kraus A, Hackenbeck T, Klanke B, Willam C, Wiesener MS, Heinze E, Burzlaff N, Eckardt KU, Buchholz B. Hypoxia inhibits nephrogenesis through paracrine Vegfa despite the ability to enhance tubulogenesis. Kidney Int. 2015;88:1283–1292. doi: 10.1038/ki.2015.214. [DOI] [PubMed] [Google Scholar]
- 51.Cleaver O, Dor Y. Vascular instruction of pancreas development. Development. 2012;139:2833–2843. doi: 10.1242/dev.065953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rymer C, Paredes J, Halt K, Schaefer C, Wiersch J, Zhang G, Potoka D, Vainio S, Gittes GK, Bates CM, Sims-Lucas S. Renal blood flow and oxygenation drive nephron progenitor differentiation. Am J Physiol Renal Physiol. 2014;307:F337–F345. doi: 10.1152/ajprenal.00208.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Boyle SC, Kim M, Valerius MT, McMahon AP, Kopan R. Notch pathway activation can replace the requirement for Wnt4 and Wnt9b in mesenchymal-to-epithelial transition of nephron stem cells. Development. 2011;138:4245–4254. doi: 10.1242/dev.070433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Karner CM, Das A, Ma Z, Self M, Chen C, Lum L, Oliver G, Carroll TJ. Canonical Wnt9b signaling balances progenitor cell expansion and differentiation during kidney development. Development. 2011;138:1247–1257. doi: 10.1242/dev.057646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wilkinson LJ, Neal CS, Singh RR, Sparrow DB, Kurniawan ND, Ju A, Grieve SM, Dunwoodie SL, Moritz KM, Little MH. Renal developmental defects resulting from in utero hypoxia are associated with suppression of ureteric beta-catenin signaling. Kidney Int. 2015;87:975–983. doi: 10.1038/ki.2014.394. [DOI] [PubMed] [Google Scholar]
- 56.Gustafsson MV, Zheng X, Pereira T, Gradin K, Jin S, Lundkvist J, Ruas JL, Poellinger L, Lendahl U, Bondesson M. Hypoxia requires notch signaling to maintain the undifferentiated cell state. Dev Cell. 2005;9:617–628. doi: 10.1016/j.devcel.2005.09.010. [DOI] [PubMed] [Google Scholar]
- 57.Fujimura S, Jiang Q, Kobayashi C, Nishinakamura R. Notch2 activation in the embryonic kidney depletes nephron progenitors. J Am Soc Nephrol. 2010;21:803–810. doi: 10.1681/ASN.2009040353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Briscoe TA, Rehn AE, Dieni S, Duncan JR, Wlodek ME, Owens JA, Rees SM. Cardiovascular and renal disease in the adolescent guinea pig after chronic placental insufficiency. Am J Obstet Gynecol. 2004;191:847–855. doi: 10.1016/j.ajog.2004.01.050. [DOI] [PubMed] [Google Scholar]
- 59.Moritz KM, Mazzuca MQ, Siebel AL, Mibus A, Arena D, Tare M, Owens JA, Wlodek ME. Uteroplacental insufficiency causes a nephron deficit, modest renal insufficiency but no hypertension with ageing in female rats. J Physiol. 2009;587:2635–2646. doi: 10.1113/jphysiol.2009.170407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Figueroa H, Lozano M, Suazo C, Eixarch E, Illanes SE, Carreno JE, Villanueva S, Hernandez-Andrade E, Gratacos E, Irarrazabal CE. Intrauterine growth restriction modifies the normal gene expression in kidney from rabbit fetuses. Early Hum Dev. 2012;88:899–904. doi: 10.1016/j.earlhumdev.2012.07.010. [DOI] [PubMed] [Google Scholar]
- 61.Xia S, Lv J, Gao Q, Li L, Chen N, Wei X, Xiao J, Chen J, Tao J, Sun M, Mao C, Zhang L, Xu Z. Prenatal exposure to hypoxia induced Beclin 1 signaling-mediated renal autophagy and altered renal development in rat fetuses. Reprod Sci. 2015;22:156–164. doi: 10.1177/1933719114536474. [DOI] [PMC free article] [PubMed] [Google Scholar]