Abstract
The response of the tympanic membrane (TM) to transient environmental sounds and the contributions of different parts of the TM to middle-ear sound transmission were investigated by measuring the TM response to global transients (acoustic clicks) and to local transients (mechanical impulses) applied to the umbo and various locations on the TM. A lightly-fixed human temporal bone was prepared by removing the ear canal, inner ear, and stapes, leaving the incus, malleus, and TM intact. Motion of nearly the entire TM was measured by a digital holography system with a high speed camera at a rate of 42 000 frames per second, giving a temporal resolution of <24 μs for the duration of the TM response. The entire TM responded nearly instantaneously to acoustic transient stimuli, though the peak displacement and decay time constant varied with location. With local mechanical transients, the TM responded first locally at the site of stimulation, and the response spread approximately symmetrically and circumferentially around the umbo and manubrium. Acoustic and mechanical transients provide distinct and complementary stimuli for the study of TM response. Spatial variations in decay and rate of spread of response imply local variations in TM stiffness, mass, and damping.
Keywords: Tympanic membrane, transient response, click response, mechanical impulse, impulse response, human
Graphical abstract

1. Introduction
Though most characterizations of tympanic membrane (TM) response to sound have used steady-state tonal stimuli, most environmental sounds are transient in nature. Studies of TM motion in response to steady-state stimuli include, among others, Tonndorf and Khanna (1972), Khanna and Tonndorf (1972), Decraemer et al. (1989), and Cheng et al. (2010, 2013). In animals, an early holographic study (Dancer et al., 1975) examined guinea pig TM motion amplitude in response to acoustic transients, and de La Rochefoucauld et al. (2010) examined the gerbil middle ear response to clicks. Dobrev (2014; Dobrev et al., 2014a) has demonstrated techniques to measure TM transient responses, but the complex response of the entire human TM to transients has not yet been studied in any detail.
Like most mammals, the human TM is attached to the tympanic ring along its edge and to the malleus manubrium at more central locations. Unlike in other mammals, the human TM is attached firmly to the manubrium at only two points: the umbo (extreme end of the manubrium) and the lateral process near the TM rim (e.g., Gea et al., 2010; De Greef et al., 2014). Between these two firm attachment locations is a thin epithelial fold, the plica mallearis, that attaches the TM to the manubrium.
There is an ongoing debate about how different parts of the TM contribute to sound transmission to the middle ear with low or high frequency stimuli. For example, some have argued that the motion of the TM distant from the umbo contribute little to motion of the umbo (and the coupled ossicular chain and cochlea) at high frequencies (Tonndorf and Khanna, 1970, 1972; Shaw, 1977, 1982; Shaw and Stinson, 1981), while others suggest that the radial fibers within the TM help couple distant TM motions to the TM at all frequencies (O'Connor et al., 2008). The debate includes questions of how the response characteristics and mechanical properties of the TM may vary over its surface; for instance, the TM thickness and presumably its mass varies among regions (Van der Jeught et al., 2013), and different parts of the TM may have different natural frequencies (Fay et al., 2006).
Transients provide a means to investigate these ideas by determining how different parts of the TM contribute to middle-ear input. Sound stimuli activate the entire TM nearly identically and simultaneously (Ravicz et al. 2014), and motion of the umbo in response to acoustic transients (“clicks”) represents a summing of the contributions of the entire surface of the TM to umbo motion, where the contribution of different TM locations to the total umbo motion may vary with location and frequency. In contrast, mechanical transients (“pokes”) that directly stimulate only a discrete small region of the TM allow the assessment of how individual small regions contribute to the motion of the umbo. Transient stimulation also provides a rich data set for modeling, including the determination of best frequencies and damping along the membrane surface. Since transients contain many frequencies, TM responses to transients are amenable to modal analysis.
In this paper we investigate the response of the TM in a human cadaveric temporal bone to global transient stimuli (acoustic clicks) and to local transient stimuli (mechanical impulses) applied to several different locations on the TM. We present TM transient responses measured using a newly-developed high-speed holographic system capable of measuring TM motion over most of its surface with a temporal resolution of less than 24 μs (Dobrev et al., 2014a). We use this technique to acquire preliminary results with both clicks and pokes in the same bone to examine three questions: (1) How do TM responses to global and local transient excitation differ? (2) Do the mechanical properties of the TM differ among locations and, if so, how? (3) Do different parts of the TM contribute differently to umbo motion?
2. Methods
2.1 Preparation of specimens
A de-identified human temporal bone was collected by the Massachusetts Eye and Ear Infirmary and fixed for three weeks according to the method of Thiel (2002; Stieger et al., 2012).1 The specimen was prepared by removing as much of the ear canal as possible, the inner ear, and the stapes to provide visual access to as much of the TM as possible. The TM, tympanic ring, and the malleus, incus, and their supporting ligaments were maintained (Fig. 1b, c). After preparation, the specimen was taken to the Center for Holographic Studies and Laser micro-mechaTronics at the Worcester Polytechnic Institute for holographic measurements. The lateral surface of the TM was painted with a zinc oxide (ZnO) solution to increase the reflectivity of the TM surface (Rosowski et al., 2009; Cheng et al., 2013). We have shown that neither the Thiel fixation nor the paint have a substantial effect on TM motion (Guignard et al., 2014; Cheng et al., 2013).
Fig. 1.
(color online) (a) Schematic of high-speed laser holography system. A laser beam is split into reference (dashed) and object (solid) illumination beams. The object illumination beam reflects off the TM of the temporal bone specimen and creates an interference pattern with the reference beam in the high-speed camera. The stapes and cochlea were removed from the specimen, but the malleus and incus and their ligaments were preserved. (b) Acoustic clicks were delivered by a speaker near the lateral TM and measured by a microphone at the tympanic ring. (c) Mechanical pokes were delivered to the medial side of the TM by a piezoelectric stack actuator with extension located at the umbo (not shown) or at nearby locations (e.g., ∼1.5 mm inferior to the umbo, shown here). (d) Schematic of TM showing poke locations.
2.2 Stimuli and acoustic responses
Acoustic impulses (“clicks”) generated by a 50-μs square voltage pulse through a power amplifier (2100; NAD Electronics, Pickering, ON, Canada) were delivered to the lateral side of the TM by a loudspeaker (SB29RDC-C-4; SB Acoustics, Denmark) located about 12 cm laterally to the TM, at approximately 30° off axis. The click had a peak sound pressure of 10 Pa as measured by a microphone (ER-7; Etymotic Research, Elk Grove Village, IL, USA) positioned at the posterior TM rim (Fig. 1b). Examples of the voltage pulse and resulting click waveform near the lateral TM surface are shown in Figure 2a. Note that the (rarefaction) click waveform measured near the TM begins approximately 441 μs after the voltage pulse, due to the propagation time from the speaker to the TM. Irregularities in the click waveform after 500 μs are likely due to reflections off nearby structures. Because the middle ear was open, sound could reach the medial TM surface also, but the acoustic baffle action of the preparation and its mounting and the additional sound propagation distance to the medial TM reduced the significance of medial sound pressures on early TM responses.
Fig. 2.
(color online) (a) Waveforms of the acoustic click measured near the lateral TM surface (in Pa, solid, right axis), mechanical poke (in nm, dashed, left axis), and electrical pulse to actuator (mV, dot-dashed, left axis; electrical pulse to speaker was similar). (b) Spectral magnitudes of the waveforms in panel a.
Mechanical displacement impulses (“pokes”) in response to the same 50-ms square voltage pulse were delivered by a small actuator comprising a piezoelectric stack (PI P-882.51, Karlsruhe, Germany; 3 × 2 × 18 mm long) with a thin extension rod made of stainless steel hypodermic tubing (22 ga: 0.7 mm outer diameter (o.d.) × 0.4 mm inner diameter (i.d.) × 10 mm long) anchored in a 3 × 2 × 0.8 mm thick aluminum plate glued to the end of the piezoelectric stack to produce a contact area 0.7 mm in diameter. Pokes were delivered to the medial side of the TM at the umbo or at three other locations (Fig. 1c, d). The mechanical stiffness of the piezoelectric stimulator is very high, so the displacement amplitude of the poke stimulus was effectively the same among poke locations. The acoustic response to the poke was recorded by the microphone as described above. An example of the poke waveform is also shown in Figure 2a. Spectra of these stimuli are shown in Fig. 2b. The spectral rolloff of the voltage stimulus at 20 kHz is consistent with the 50-μs voltage pulse width.
2.3 Measurement of TM motion
Motion of nearly the entire TM surface was measured by a high-speed digital holography system (Fig. 1a), which includes a continuous-wave (CW) 532 nm laser (SLIM-532, 50mW; Oxxius, O'Fallon, MO, USA), variable ratio beam splitter, an optical phase shifter, a high-speed camera (SA5 1000k; Photron, San Diego, CA, USA), and beam combining and imaging optics (Razavi et al., 2015a).
Digital holograms were recorded by splitting the laser into two beams, object and reference, by the use of a variable ratio beam splitter; see Figure 1a. A reference/object beam power ratio of about two was used. The reference beam illuminated the camera detector, whereas the object beam was directed and expanded to illuminate the specimen. The irradiance that reflected off the specimen was captured by a telecentric lens and combined with the reference beam. The interference pattern of the two beams was digitized by the camera. Displacements of the TM toward the camera are described as in the positive direction.
High-speed acquisition was achieved by a novel high-speed 2+N frame acquisition method (Razavi et al., 2015a) based on a hybrid spatio-temporal local correlation (LC) phase sampling approach (Dobrev et al. 2014a, b, c), that allows quantification of the TM transient deformations by utilizing two reference frames and N consecutive deformed frames recorded before and throughout the evolution of the event of interest. The high-speed camera capabilities allow for frame rates from 7 000 frames per second (fps) at full resolution (i.e., 106 pixels) to 106 fps at reduced resolutions (i.e. 1,000 pixels). For measurements of the TM transient motion presented in this paper, a trade-off was made by setting the camera at 384 × 384 pixels and at 42 000 fps to achieve both (a) adequate spatial resolution (∼22 μm per pixel) to resolve the complex deformation patterns of the TM and (b) adequate temporal resolution (<24 μs for 25 ms after the stimulus) to resolve the full hearing range bandwidth.
The amount of the TM we could measure was constrained by the field of view of the camera, which was limited by how completely we could expose the TM and by the focal length of the camera lens, all of which influenced the tradeoff between spatial and temporal resolution. Our choice of parameters provides sufficient field of view and spatial and temporal resolution for the measurements and analyses described here. The displacement resolution and noise floor are about 10 nm.
3. Results
The results describe here are preliminary in nature, as they were obtained from one temporal bone. As such, they serve to illustrate the nature of transient responses observed and how they might be analyzed and interpreted. Measurements of poke responses were repeated after the bone had been removed, moistened, and replaced, with results very similar to those shown here.
3.1 Response to impulsive acoustic stimuli – clicks
Figure 3a shows four maps of the displacement of the TM in response to an acoustic click. Each panel shows a map of the TM displacement at an instant in time after the click is produced by the speaker. Recall that the TM is a curved surface, so each panel shows the change in position of each visible point on the TM in response to the stimulus relative to the reference position (its position before the stimulus). The first panel (500 μs) shows the TM at rest, before the TM has begun to respond to the click.2 Subsequent panels show the TM displacement at later instants in time relative to the reference state. (See video “Payam transient Fig3.mp4” in Supplemental Materials.)
Fig. 3.
(a) TM displacement maps at four representative instants following an acoustic rarefaction click: 500, 571, 595, 643 μs. (b) Displacement waveforms at the umbo and three other TM locations (denoted 1, 2, 3, 4; see panel a) in response to an acoustic click. The response at each location is the mean over a 5 × 5 pixel array (110 × 110 μm) near the center of each numeral in panel (a). The vertical dot-dashed line shows the time of peak displacement response at the umbo (595 μs). Positive displacement is toward the camera (laterally).
The entire visible TM responded nearly instantaneously to acoustic transient stimuli. For the initial response (571 μs), the entire TM moved in phase, and the response peaked by about 595 μs. By 643 μs (about 95 μs after the beginning of the response), a region anterior to the manubrium moved with substantial amplitude and with opposite phase to another area 1–2 mm posterior to it. After another 100 μs or so (not shown), small areas of the TM moved with opposite phase. Because the middle ear was open, the click stimulus reached the medial side of the TM after a few hundred μs and could have influenced later TM motion, but we saw no later uniform TM displacement that would be evidence of this. The early in-phase motion and higher motion amplitudes away from the umbo are consistent with previous observations (Dobrev, 2014; Dobrev et al., 2014a, b).
To facilitate comparisons of TM responses between stimulus type and location, we evaluate the TM response in more detail at a few locations of interest (first panel of Fig. 3a): Location 1 (Loc. 1) at the umbo, Loc. 2 1.6 mm anterior to the umbo, Loc. 3 1.5 mm inferior, and Loc. 4 1.6 mm posterior and a little superior to the umbo. We compute the representative displacement at each of these locations as the mean of a 5 × 5 pixel array (∼110 × 110 μm) around the point about at the center of the numeral. The click-induced time waveforms of the TM displacement response at these locations are shown in Fig. 3b. The responses at all three locations on the TM (Locs. 2, 3, 4) began and peaked at about the same time (within the 24-μs time resolution of our measurements), while the peak umbo response occurred slightly later (vertical line). The peaks of the waveforms at Locs. 2, 3, and 4 appear to be split between two time samples, which suggests that those peaks occurred about one-half sample (∼12 μs) before the peak at the umbo. The amplitudes of these initial responses were smaller at the umbo than at other locations. At Loc. 4 (postero-superior to the umbo), the response 100–150 μs later was of opposite sign and larger amplitude than the initial response.
3.2 Response to impulsive mechanical stimuli – “pokes”
3.2.1 At the umbo
Figure 4a shows TM displacement maps of the response to a poke at the umbo. With this local stimulus, the TM responded first locally at the umbo (48 μs), and the response spread approximately symmetrically and circumferentially around the umbo and manubrium (later panels). The time waveforms of TM displacement (Fig. 4b) at Locations 1, 2, 3, 4 (as described in Fig. 3 above) show how the umbo response was maximal at 71 μs (vertical line) and decayed monotonically and smoothly over the next 1000 μs. The response peaked later at other locations anterior, inferior, or postero-superior to the umbo (see Fig. 4a for map); and at Loc. 4 (postero-superior), the second response peak was larger than the first. By 143 μs, anterior and posterior parts of the TM moved in opposite directions. As with acoustic clicks, the response was smaller at the umbo than at other locations. (See “Payam transient Fig4.mp4” in Supplemental Materials.)
Fig. 4.
(a) TM displacement maps at four consecutive instants following a mechanical poke at the umbo: 48, 71, 95, 143 μs. (b) Displacement waveforms at umbo and three other TM locations (denoted 1, 2, 3, 4; see panel a) in response to a mechanical poke at the umbo. The response at each location is the mean over a 5 × 5 pixel array as described for Fig. 3. The vertical dot-dashed line shows the time of peak displacement response at the umbo (71 μs).
3.2.2 At other locations
Figure 5 shows maps of TM displacement at three instants for identical pokes at the other stimulus locations: (a) Loc. 2 anterior, (b) Loc. 3 inferior, and (c) Loc. 4 superior-posterior to the umbo. With pokes at other locations, as with a poke at the umbo, the TM responded first locally at the site of stimulation, and the response spread approximately symmetrically and circumferentially around the umbo and manubrium. The first response at the poke location and the spread of the response are similar to previous observations (Dobrev, 2014). (See videos “Payam transient Fig5a.mp4”, “Payam transient Fig5b.mp4”, and “Payam transient Fig5c.mp4” in Supplemental Materials.)
Fig. 5.
TM displacement maps at various instants in response to a mechanical poke at (a) Loc. 2 anterior, (b) Loc. 3 inferior, and (c) Loc. 4 postero-superior to the umbo (see Fig. 4a).
4. Discussion
4.1 Comparison of responses to global and local impulsive stimuli
In this section, we examine the response of the TM to global (acoustic clicks) and local stimuli (mechanical pokes) at the four locations described and discussed in Sec. 3 above (1, 2, 3, 4) and at five additional locations for a more representative survey (2a, 2b, 3a, 3b, 4a). The locations are shown on a map of the TM in Figure 6a and in order from antero-superior to postero-superior in Fig. 6b. The following figures in this section and the next display response properties at the TM locations in this order.
Fig. 6.
(color online) (a) Outline of TM showing the 4 stimulus locations (1, 2, 3, 4) described in previous figures and 5 additional locations (2a, 2b, 3a, 3b, 4a) where TM displacement was evaluated. (b) Polar-coordinate representation of all 9 locations of interest. Horizontal axis: Azimuth from manubrium; vertical axis: radius from umbo.
4.1.1 Timing and amplitude of responses to clicks and umbo pokes
The timing and amplitude of the first peak of the TM displacement response at different locations differed between a click stimulus and a poke at the umbo, as seen by comparing Figures 3 and 4. Figure 7 explores these response differences in more detail.
Fig. 7.
(color online) (a) Time and (b) amplitudes of first response peak (filled symbols) and maximum response peak (open symbols), at each of the 9 locations where TM displacement was evaluated (over a 5 × 5 pixel array as described for Fig. 3), in order from antero-superior to postero-superior (as described in Fig. 6), for acoustic clicks (circles) and mechanical pokes at the umbo (squares). At locations with concentric symbols, the first response peak was the highest peak. For ease of comparison, click response times in (a) have been normalized to the umbo poke response time (71 μs), and amplitudes in (b) have been normalized by the response amplitude at the umbo.
The timing of the first response peak (closed symbols) and the maximum response (open symbols) is shown for clicks and umbo pokes in Fig. 7a at each of the nine locations described in Fig. 6 above. For convenience and ease of comparison, the peak umbo response time to a click is aligned with the peak umbo response time to an umbo poke (71 μs – see Fig. 4b).
Figure 7a highlights differences and similarities in the TM response to global (click) or local (poke) stimuli. (1) With click stimuli, the first response peak (filled circles) at most evaluation locations occurs at about the same time as the umbo (see Fig. 3). With umbo pokes (squares), the umbo responds first (see Fig. 4), and the response spreads radially and from more inferior to more superior locations: The locations closest to the umbo (2, 3, 4; see Fig. 6) respond next, and the locations most superior (2a, 4a) or further from the umbo (2b, 3a, 3b) respond later. (2) At locations inferior to the umbo (2, 3a, 3, 3b), the initial response peak to clicks and especially to pokes is also the maximum peak (open symbols), while at locations superior to the umbo (2a, 4a, 4b), the response builds over several hundred μs.
The variations in the amplitudes of the first TM response peaks and maximum peaks with location, shown in Fig. 7b, show considerable commonalities between clicks and umbo pokes. For both stimuli, the response (normalized by umbo response) was smallest at the umbo (see Figs. 3, 4) and generally larger at locations further from the umbo (2a, 2b, 3a, 3b).
Figure 7 also provides evidence of variations in mechanical coupling between different parts of the TM. The click and poke responses at the antero-superior and postero-superior locations (2a, 2b, 4, 4a) generally built up with time – the maximum displacement peak (open symbols) occurs later than the first peak – while at the other locations, the initial peak was the maximum. The buildup in response at these more superior locations suggests that motion of other parts of the TM contributed to the response at these locations, which in turn suggests that these locations were less tightly coupled to the umbo. The similarity in response characteristics among the other locations and the umbo suggests that these other locations have a tighter mechanical coupling to the umbo than the more superior locations.
4.1.2 Other aspects of click responses
As noted above and in Fig. 3, the peak umbo response to clicks occurs at 595 μs, which is slightly later than other parts of the TM and about 100 μs after the arrival of the click stimulus at the edge of the TM (476–500 μs; see Fig. 2a). The 100 μs delay between the click and the umbo displacement peak is of the same order of magnitude as the group delay observed previously between sound pressure near the umbo and umbo velocity (e.g., 68–92 μs by Ravicz et al., 2004), attributed to the TM (32 μs; O'Connor and Puria, 2008), or observed between ear-canal and cochlear sound pressures (83 μs; Nakajima et al., 2009).
4.2 Evidence of spatial variations in tympanic membrane mechanical properties
4.2.1 Decay time constants
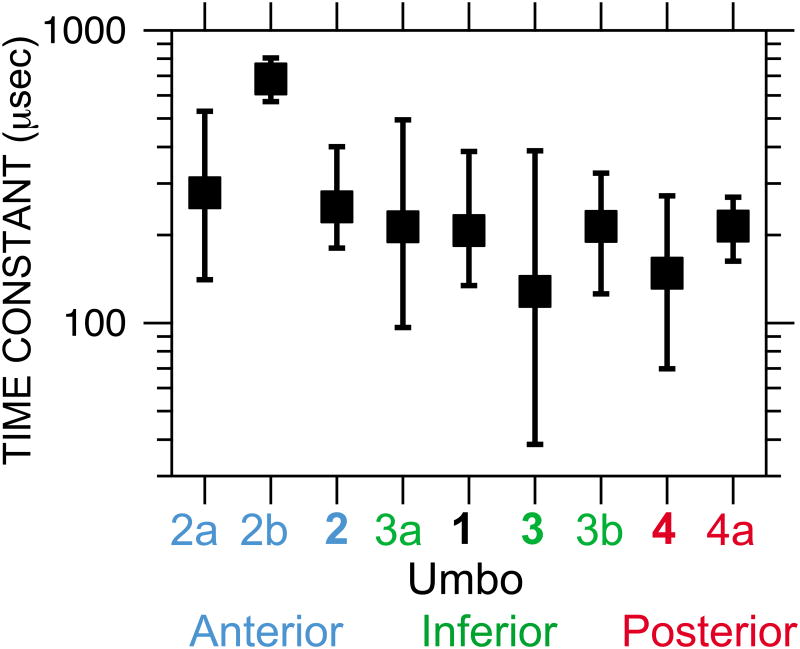
The damping of the TM response is described by the decay time constant, defined as the time by which the response decays to 1/e of its maximum. Decay time constants were computed from the TM displacement waveforms at each of the nine locations in response to mechanical pokes by fitting a straight line to the logarithm of the rectified waveform between the initial peak and the first minimum.3 The means and ranges of the time constants are shown for the nine locations in Figure 8. (Data at the poke location have been omitted, as the presence of the stimulator might affect the response decay.)
Fig. 8.
(color online) Map of means (squares) and ranges of decay time constants (bars) at each of the 9 evaluation locations (Fig. 6), in order from anterior to posterior. Time constants were computed from responses to mechanical pokes at the umbo and the other three TM locations. Note logarithmic vertical scale.
Though the range of decay time constants computed by this method was fairly large (about a factor of 20), the mean decay time constants computed from pokes at three or four locations were relatively uniform: Mean time constants generally varied between 150 and 250 μs (squares) except at antero-superior Loc. 2b (680 μs). The longer time constants observed at Locs. 2a and 2b suggest that TM mechanical damping may be lower in antero-superior parts of the TM than in other parts.
The values of (single) decay time constants we obtained by this fairly direct method are within the range of those (multiple) estimated from dynamic measurements on isolated TM samples (85, 5700 μs; Zhang and Gan, 2013) or from finite-element models fit to TM responses to tones (6.6, 40, 255, 2500 μs; De Greef et al., 2014) by less direct means. An advantage of our technique is that the effects of interactions between different TM regions excited by a global acoustic stimulus is minimized.
4.2.2 Response propagation velocities
Local wave propagation velocities along the TM surface were computed from poke response data at each of the nine evaluation locations. At each location, the propagation velocity was computed from the difference between the time of the first peak in the response at that location (Fig. 7) and the first response peak at the poke location, normalized by the linear distance between the evaluation location and the stimulus location from Fig. 6a. (For example, Fig. 6b shows linear distances between the evaluation locations and the umbo. Data at the poke locations for a poke at that location were omitted.) Figure 9 shows the propagation velocity at each evaluation location from a poke at each stimulus location (symbols). For example: (a) the open circle at Loc. 4 indicates that a poke at the umbo produced a response at Loc. 4, 1.3 mm from the umbo (Fig. 6b), whose initial peak was 24 μs after the peak at the umbo, which implies a propagation velocity of 56 m/s between the umbo and Loc. 4; and (b) the right-facing triangle at Loc. 2 indicates that the initial response peak at Loc. 2 from a poke at Loc. 4 (3.0 mm away) occurred 36 μs after the peak at Loc. 4, which implies a propagation velocity of 85 m/s between Loc. 4 and Loc. 2.4 The means of these velocities at each location, computed from pokes at each of the stimulus locations, are also shown in Fig. 9 by the short horizontal lines.
Fig. 9.
Propagation velocities at locations of interest in response to mechanical pokes at various stimulus locations (see Fig. 6): umbo (1; open circles), anterior (2; left triangles), inferior (3; inverted triangles), and postero-superior (4; right triangles). Mean velocities at each location are shown by horizontal bars. Propagation velocities at poke locations for pokes at those locations were omitted.
The propagation velocities computed by this method ranged from 18 m/s (at Loc. 4 for a poke at Loc. 3) to 170 m/s (at the umbo for a poke at Loc. 3), but 80% of the velocities were between 26 and 67 m/s with a mean of 43 m/s. Velocities were highest from the inferior and postero-superior poke locations (Locs. 3, 4) to the umbo and anterior Loc. 2. Pokes at Loc. 3 (inferior; inverted triangles) generally produced the highest velocities to the other TM locations (62 m/s average among the other 8 locations), and pokes at Loc. 2 (anterior; left triangles) generally produced the lowest velocities (average 33 m/s). These velocity estimates are similar to those estimated from clicks (24 m/s; Dobrev, 2014) and a factor of 5–10 higher than traveling-wave velocities estimated from displacements measured with steady-state acoustic stimuli (tones; 3–13 m/s; Cheng et al., 2013). The propagation velocity is determined by the local mechanical properties, the local mass and stiffness (e.g., Fletcher, 1992); and the stiffness may be influenced by the local TM curvature. The variations in propagation velocities to and from different locations suggest that TM mechanical properties can vary over the TM.
4.3. Contribution of different TM locations to umbo displacement
Mechanical stimuli applied locally can also reveal the contribution of different parts of the TM to umbo response. Umbo displacement waveforms in response to pokes at different TM locations are shown in Figure 10. The umbo response amplitude to a TM poke differed by poke location: The response was largest from a poke at the umbo, while a poke at Loc. 3 or Loc. 4 also produced a substantial response. In contrast, the response to a poke at anterior Loc. 2 was considerably smaller and barely distinguishable from noise.
Fig. 10.
(color online) Umbo displacement waveforms in response to a poke at the umbo or the three other TM stimulation locations.
The umbo displacements produced by pokes at these three TM poke locations were not well correlated with the response of those locations to a poke at the umbo, which can be seen in Table 1. An umbo poke produced the highest response at Loc. 4, and a poke at Loc. 4 produced the highest umbo response among the TM poke locations (except for the umbo itself). In contrast, an umbo poke produced large displacements at Locs. 2 and Loc. 3 that were larger than the umbo response, while a poke at Loc. 2 produced almost no umbo response. (The amplitude of the poke stimulus was effectively constant among poke locations, as mentioned in Sec. 2.2.) The large responses at Locs. 3 and 4 to an umbo poke and robust umbo response to pokes at Locs. 3 and 4 support the suggestion in Sec. 4.1.1 above that the inferior and posterior poke locations were fairly well coupled to the umbo. The low umbo response to a poke at Loc. 2 and the relatively long response latency between Loc. 2 and the umbo support the suggestion that the anterior poke location was less well coupled to the umbo.
Table 1.
Amplitudes and latencies of first response peaks at Locations 1, 2, 3, and 4 to an umbo poke compared to umbo first response peak amplitudes and latencies in response to pokes at Locations 1, 2, 3, and 4.
| Location | Displacement Peak at Loc. from Poke at Umbo | Displacement Peak at Umbo from Poke at Loc. | ||
|---|---|---|---|---|
| Displacement (nm) | Latency (μs) | Displacement (nm) | Latency (μs) | |
| 1 (umbo) | 90 | 0 | 90 | 0 |
| 2 (anterior) | 150 | 36 | <10 | 59 |
| 3 (inferior) | 180 | 24 | 30 | 12 |
| 4 (postero-superior) | 220 | 24 | 46 | 24 |
The results from pokes at only three TM locations provide intriguing insights into the mechanical coupling between different parts of the TM and the relative contributions of different parts of the TM to the umbo response in the case of acoustic stimulation. Further work in this area is likely to be fruitful and may contribute to a rethinking of the best location on the TM for ossicular prostheses to couple sound energy efficiently from the TM to the inner ear.
5. Conclusions
Acoustic and mechanical transients provide distinct and complementary stimuli for the study of TM dynamics.
Systematic spatial variations in response time and amplitude, decay, and rate of spread of response imply the existence of local variations in TM stiffness, mass, damping, and coupling between different TM regions.
Knowledge of how these TM mechanical properties vary over the TM can influence the design of TM and ossicular prostheses.
Supplementary Material
Fig. 3 Video of response to acoustic click: Payam transient Fig3.mp4
Fig. 4 Video of response to mechanical poke at umbo: Payam transient Fig4.mp4
Fig. 5a Video of response to mechanical poke at Loc. 2: Payam transient Fig5a.mp4
Fig. 5b Video of response to mechanical poke at Loc. 3: Payam transient Fig5b.mp4
Fig. 5c Video of response to mechanical poke at Loc. 4: Payam transient Fig5c.mp4
Research Highlights.
Tympanic membrane (TM) responses to impulsive stimuli were measured.
Stimuli: Acoustic clicks and mechanical “pokes” at the umbo and elsewhere on TM.
A high-speed holography system measured with 24-μsec, 22-μm × 10 nm resolution.
Response onset times and amplitudes and decay times varied with TM location.
These variations identify spatial variations in TM mechanical properties.
Acknowledgments
We thank Nima Maftoon for helpful discussions and the staffs of CHSLT at the Worcester Polytechnic Institute and the Eaton-Peabody Lab. Funded by NIDCD R01 DC008642, the Massachusetts Eye & Ear Infirmary, the Worcester Polytechnic Institute, and the Mittal Fund.
Footnotes
The specimen was the left ear from a 71-year-old male and was frozen for approximately 2 years before being thawed and fixed.
This 500-μs latency includes the acoustic propagation time from the speaker to the TM (see Fig. 2a).
The rectified waveforms generally showed several minima at different latencies from the initial peak (as in Dobrev, 2014, Fig. 13.10), which suggests that a full description of the TM viscoelastic behavior should include several time constants (e.g., De Greef et al., 2014). For simplicity, we examined only the decay to the first minimum.
It should be kept in mind that the approximately 12-μs timing resolution (24-μs frame interval + half-cycle adjustment for response peaks split between samples) places a distance-related limitation on the precision of velocity estimates: For instance, the 12-μs resolution limits the precision of the 56 m/s estimate of the velocity between the umbo and Loc. 4 1.3 mm away to about 30 m/s.
For submission to Hearing Research as part of the proceedings of the MEMRO 2015 meeting, Aalborg, Denmark, July 1-5, 2015 (Razavi et al., 2015b)
Supplemental materials: All available at <http://chslt.wpi.edu/MEMRO2015.html>.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Cheng JT, Aarnisalo AA, Harrington E, Hernandez-Montes MdeS, Furlong C, Merchant SN, Rosowski JJ. Motion of the surface of the human tympanic membrane measured with stroboscopic holography. Hear Res. 2010;263:66–77. doi: 10.1016/j.heares.2009.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng JT, Hamade M, Harrington E, Furlong C, Merchant SN, Rosowski JJ. Wave motion on the surface of the human tympanic membrane: holographic measurement and modeling analysis. J Acoust Soc Am. 2013;133:918–937. doi: 10.1121/1.4773263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dancer AL, Franke RB, Smigieleski P, Albe F, Fagot H. Holographic interferometry applied to the investigation of tympani-membrane displacements in guinea pig ears subjected to acoustic impulses. J Acoust Soc Am. 1975;58:223–228. doi: 10.1121/1.380649. [DOI] [PubMed] [Google Scholar]
- Decraemer WF, Khanna SM, Funnell WRJ. Interferometric measurement of the amplitude and phase of tympanic membrane vibrations in cat. Hear Res. 1989;38:1–18. doi: 10.1016/0378-5955(89)90123-8. [DOI] [PubMed] [Google Scholar]
- De Greef D, Aernouts J, Aerts J, Cheng JT, Horwitz R, Rosowski JJ, Dirckx JJJ. Viscoelastic properties of the human tympanic membrane studied with stroboscopic holography and finite element modeling. Hear Res. 2014;312:69–80. doi: 10.1016/j.heares.2014.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobrev IT. Ph D dissertation. Worcester Polytechnic Institute; 2014. Full-field vibrometry by high-speed digital holography for middle-ear mechanics; p. 245. [Google Scholar]
- Dobrev I, Furlong C, Cheng JT, Rosowski JJ. Full-field transient vibrometry of the human tympanic membrane by local phase correlation and high-speed holography. J Biomed Optics. 2014a;19(9):096001. doi: 10.1117/1.JBO.19.9.096001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobrev I, Furlong C, Cheng JT, Rosowski JJ. Optimization of a Lensless Digital Holographic Otoscope System for Transient Measurements of the Human Tympanic Membrane. Experimental Mechanics. 2014b doi: 10.1007/s11340-014-9945-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobrev I, Furlong C, Cheng JT, Harrington EJ, Rosowski JJ. Implementation and Evaluation of Single Frame Recording Techniques for Holographic Measurements of the Tympanic Membrane. In: Jin Helena, Sciammarella Cesar, Yoshida Sanichiro, Lamberti Luciano., editors. In-Vivo Advancement of Optical Methods in Experimental Mechanics. Vol. 3. Springer International Publishing; 2014c. pp. 85–92. 01/2014. [Google Scholar]
- Fay JP, Puria S, Steele CR. The discordant eardrum. Proc Nat Acad Sci. 2006;103:19743–19748. doi: 10.1073/pnas.0603898104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fletcher NH. Acoustic Systems in Biology. Oxford Univ. Press; New York: 1992. p. 333. [Google Scholar]
- Gea SLR, Decraemer WF, Funnell WRJ, Dirckx JJJ, Maier H. Tympanic membrane boundary deformations derived from static displacements observed with computerized tomography in human and gerbil. J Assoc Res Otolaryngol. 2010;11:1–17. doi: 10.1007/s10162-009-0192-9. Erratum in J Assoc Res Otolaryngol 11, 525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guignard J, Fullerton BC, Rosowski JJ, Cheng JT. Unpublished data 2014 [Google Scholar]
- Khanna SM, Tonndorf J. Tympanic-membrane vibrations in cats studied by time-averaged holography. J Acoust Soc Am. 1972;51:1904–1920. doi: 10.1121/1.1913050. [DOI] [PubMed] [Google Scholar]
- de La Rochefoucauld O, Kachroo P, Olson ES. Ossicular motion related to middle ear transmission delay in gerbil. Hear Res. 2010;270:158–172. doi: 10.1016/j.heares.2010.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakajima HH, Dong W, Olson ES, Merchant SN, Ravicz ME, Rosowski JJ. Differential intracochlear sound pressure measurements in normal human temporal bones. J Assoc Res Otolaryngol. 2009;10:23–36. doi: 10.1007/s10162-008-0150-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Connor KN, Puria S. Middle-ear circuit model parameters based on a population of human ears. J Acoust Soc Am. 2008;123:197–211. doi: 10.1121/1.2817358. [DOI] [PubMed] [Google Scholar]
- O'Connor KN, Tam M, Blevins NH, Puria S. Tympanic membrane collagen fibers: A key to high-frequency sound conduction. Laryngoscope. 2008;118:483–490. doi: 10.1097/MLG.0b013e31815b0d9f. [DOI] [PubMed] [Google Scholar]
- Ravicz ME, Rosowski JJ, Merchant SN. Mechanisms of hearing loss resulting from middle-ear fluid. Hear Res. 2004;195:103–130. doi: 10.1016/j.heares.2004.05.010. [DOI] [PubMed] [Google Scholar]
- Ravicz ME, Cheng JT, Rosowski JJ. Sound pressure distribution within the human ear canal and models: I. Forward stimulation. J Acoust Soc Am. 2014;136(6):3132–3146. doi: 10.1121/1.4898420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Razavi P, Dobrev I, Ravicz M, Cheng JT, Furlong C, Rosowski JJ. Transient response of the eardrum excited by localized mechanical forces. In: Tekalur Srinivasan Arjun, Zavattieri Pablo, Korach Chad S., editors. Mechanics of Biological Systems and Materials. Vol. 6. Springer International Publishing; 2015a. pp. 31–37. [Google Scholar]
- Razavi P, Ravicz ME, Dobrev I, Cheng JT, Furlong C, Rosowski JJ. Response of the human tympanic membrane to transient acoustic and mechanical stimuli. 7th International Symposium on Middle Ear Mechanics in Research and Otology; Aalborg, Denmark, A3-6. 2015b. [Google Scholar]
- Rosowski JJ, Cheng JT, Ravicz ME, Hulli N, Harrington EJ, Hernandez-Montes MS, Furlong C. Computer-assisted time-averaged holography of the motion of the surface of the tympanic membrane with sound stimuli of 0.4 to 25 kHz. Hear Res. 2009;253:83–96. doi: 10.1016/j.heares.2009.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw EAG. Eardrum representation in middle-ear acoustical networks. J Acoust Soc Am. 1977;62(Suppl 1):S12. [Google Scholar]
- Shaw EAG. The 1979 Rayleigh Medal Lecture: The elusive connection. In: Gatehouse RW, editor. Localization of Sound. Amphora; Groton, CT: 1982. [Google Scholar]
- Shaw EAG, Stinson MR. Network concepts and energy flow in the human middle ear. J Acoust Soc Am. 1981;69(Suppl 1):S43. [Google Scholar]
- Stieger C, Candreia C, Kompis M, Herrmann G, Pfiffner F, Widmer D, Arnold A. Laser Doppler vibrometric assessment of middle ear motion in Thiel-embalmed heads. Otol Neurotol. 2012;33:311–318. doi: 10.1097/MAO.0b013e3182487de0. [DOI] [PubMed] [Google Scholar]
- Thiel W. Ergänzung für die Konservierung ganzer Leichen nach W. Thiel. Ann Anat. 2002;184:267–269. doi: 10.1016/s0940-9602(02)80121-2. [DOI] [PubMed] [Google Scholar]
- Tonndorf J, Khanna SM. The role of the tympanic membrane vibrations in middle ear transmission. Ann Otol. 1970;79:743–753. doi: 10.1177/000348947007900407. [DOI] [PubMed] [Google Scholar]
- Tonndorf J, Khanna SM. Tympanic-membrane vibrations in human cadaver ears studied by time-averaged holography. J Acoust Soc Am. 1972;52:1221–1233. doi: 10.1121/1.1913236. [DOI] [PubMed] [Google Scholar]
- Van der Jeught S, Dirckx JJJ, Aerts JRM, Bradu A, Podoleanu AG, Buytaert JAN. Full-field thickness distribution of human tympanic membrane obtained with optical coherence tomography. J Assoc Res Otolaryngol. 2013;14:483–494. doi: 10.1007/s10162-013-0394-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Gan RZ. Dynamic properties of human tympanic membrane based on frequency-temperature superposition. Ann Biomed Eng. 2013;41:205–214. doi: 10.1007/s10439-012-0624-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. 3 Video of response to acoustic click: Payam transient Fig3.mp4
Fig. 4 Video of response to mechanical poke at umbo: Payam transient Fig4.mp4
Fig. 5a Video of response to mechanical poke at Loc. 2: Payam transient Fig5a.mp4
Fig. 5b Video of response to mechanical poke at Loc. 3: Payam transient Fig5b.mp4
Fig. 5c Video of response to mechanical poke at Loc. 4: Payam transient Fig5c.mp4