Abstract
Rho kinase (ROCK) has been implicated in physiological and pathophysiological processes, including regulation of vascular function. ROCK signaling is thought to be a critical contributor to cardiovascular disease, including hypertension and effects of angiotensin II (Ang II). Two isoforms of ROCK (1 and 2) have been identified, and are expressed in vascular cells. In this study, we examined the importance of ROCK2 in relation to vessel function using several models and a novel inhibitor of ROCK2. First, incubation of carotid arteries with the direct RhoA activator CN-03 or Ang II impaired endothelium-dependent relaxation by approximately 40-50% (P<0.05) without altering endothelium-independent relaxation. Both CN-03- and Ang II-induced endothelial dysfunction was prevented by Y-27632 (an inhibitor of both ROCK isoforms) or the selective ROCK2 inhibitor SLX-2119. In contrast, SLX-2119 had little effect on contraction of carotid arteries to receptor-mediated agonists (serotonin, phenylephrine, vasopressin or U46619). Second, in basilar arteries, SLX-2119 inhibited constriction to Ang II by approximately 90% without significantly affecting responses to serotonin or KCl. Third, in isolated pressurized brain parenchymal arterioles, SLX-2119 inhibited myogenic tone in a concentration-dependent manner (eg, 1 μmol/L SLX-2119 dilated by 79±4%). Lastly, SLX-2119 dilated small pial arterioles in vivo, an effect that was augmented by inhibition of NO synthase. These findings suggest that ROCK2 has major, but heterogeneous, effects on function of endothelium and vascular muscle. The data support the concept that aberrant ROCK2 signaling may be a key contributor to select aspects of large and small vessel disease including Ang II-induced endothelial dysfunction.
Keywords: angiotensin II, cerebral circulation, nitric oxide, Rho kinase, myogenic tone, parenchymal arterioles, endothelium
Introduction
The Rho-associated coiled-coil kinase, Rho kinase (ROCK), has emerged as a key regulator of vascular function in health and disease. Based largely on studies using pharmacological inhibitors that do not distinguish between isoforms, ROCK has been implicated in various diseases including hypertension1, 2, diabetes3 and stroke4, 5.
Two widely expressed isoforms of ROCK (ROCK1 and ROCK2) have been identified. For example, ROCK1 and ROCK2 are expressed at the mRNA and protein level in the vasculature, including in cerebral arteries6, 7. The small Rho GTPase, RhoA, is an important activator of ROCK8. Activation of RhoA involves the conversion of GDP-bound RhoA to GTP-bound RhoA by guanine nucleotide exchange factors. The relative abundance of activated RhoA can also be modulated by GTPase-activating proteins and guanine nucleotide dissociation inhibitors8. Once active, RhoA translocates to the cell membrane to activate ROCK.
ROCK has diverse effects depending on the cell type. In vascular muscle, ROCK serves as an important regulator of contraction8. Activation of ROCK inhibits myosin light chain phosphatase, thus preventing dephosphorylation of myosin and a maintenance or increase in contractile force. This effect on contractile force is not dependent on increases in intracellular calcium. Although ROCK has also been shown to function in endothelium, much less is known about the role of ROCK enzymes in regulating endothelial function during health and disease. One effect of ROCK is the down regulation of nitric oxide (NO) production via inhibitory effects on endothelial NO synthase (eNOS)9.
The brain is one of the end-organs most affected by hypertension. Deleterious effects of hypertension include impaired vasodilation, augmented vasoconstriction, and blood-brain barrier dysfunction10. Hypertension is a major risk factor for large and small vessel disease and stroke11-13. ROCK has emerged as a key contributor to regulation of vessel function, influencing vascular tone normally and in disease6, 7, 14-16. ROCK activity is associated with increased cardiovascular morbidity and mortality17. Basic and clinical evidence have implicated angiotensin II (Ang II) in hypertension and vascular disease18, 19, with other studies showing ROCK is activated by Ang II1, 20-22.
Little is known regarding the role of specific ROCK isoforms in the regulation of vascular function. In the present study, we examined the hypothesis that ROCK2 plays an important role in intact vessels under physiological and pathological conditions. Because of its key role in hypertension and vessel disease, we used a model of Ang II-induced vascular dysfunction for portions of the study. As part of our approach, we tested effects of a new, selective ROCK2 inhibitor, SLX-2119 (also known as KD025)4, 23 on regulation of vascular tone in carotid arteries and resistance vessels in brain. Our findings suggest that ROCK2 plays a key role in endothelial dysfunction produced in response to direct RhoA activation or Ang II. We also found that constriction of cerebral arteries to Ang II and myogenic tone in small parenchymal arterioles is ROCK2-dependent. Overall, these findings support the concept that ROCK2 is a key ROCK isoform in relation to regulation of carotid and cerebrovascular function.
Materials and Methods
Experimental animals
The protocols used were approved by the University of Iowa Animal Care and Use Committee. We studied male C57Bl6/J mice. All mice were fed standard chow and water ad libitum and studied at 4-6 months of age. Care of mice met the standards set forth by the National Institute of Health for the care and use of experimental animals. Details regarding the experimental procedures are presented in the on-line only Data Supplement. Briefly, four different segments of the vasculature were used to examine the role of ROCK in various aspects of vascular function. We took advantage of specific features in each vessel type in order to address specific questions (endothelial function in carotid arteries, myogenic tone in brain parenchymal arterioles, etc).
Statistical analysis
All data are expressed as mean±SE. Data were evaluated using one- or two-way analysis of variance (ANOVA) followed by Tukey post hoc test (for one-way ANOVA), as appropriate. Statistical significance was accepted at P<0.05.
Results
Direct activation of RhoA impairs endothelial function in carotid arteries in a ROCK2-dependent manner
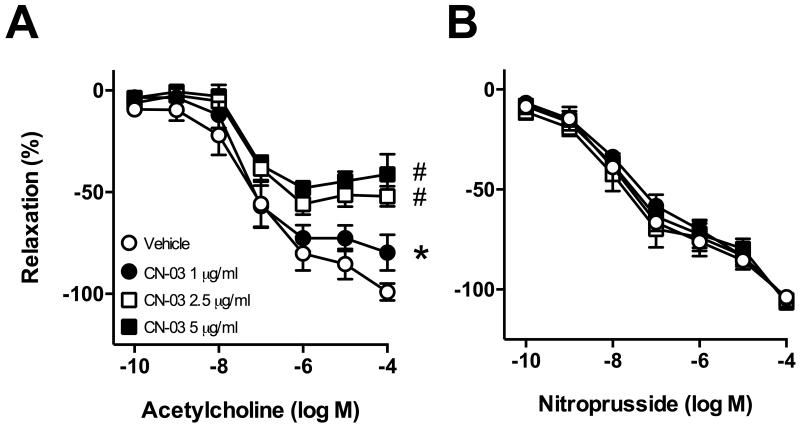
Incubation of arteries with the direct RhoA activator elicited concentration-dependent effects. At 1 μg/ml, CN-03 moderately impaired relaxation to acetylcholine (Figure 1A). In contrast, incubation with CN-03 at 2.5 or 5 μg/ml greatly impaired endothelium-dependent relaxation to acetylcholine (Figure 1A). Endothelium-independent relaxation to nitroprusside was not affected by CN-03 at any concentration (Figure 2B) suggesting effects were selective for endothelial cells. In most groups, contraction of carotid arteries to U46619 was not significantly affected by incubation with CN-03 (data not shown). As we obtained a similar degree of endothelial dysfunction with 2.5 or 5 μg/ml CN-03, we used the 2.5 μg/ml concentration in subsequent experiments.
Figure 1.
Responses of carotid arteries to (A) acetylcholine and (B) nitroprusside following 22 hr incubation with CN-03 (1, 2.5 or 5 μg/mL) or vehicle. (A and B) n=4 for all groups, *P<0.05 for 1 μg/mL curve vs vehicle curve; #P<0.001 for 2.5 μg/mL and 5 μg/mL CN-03 curves vs. vehicle curve.
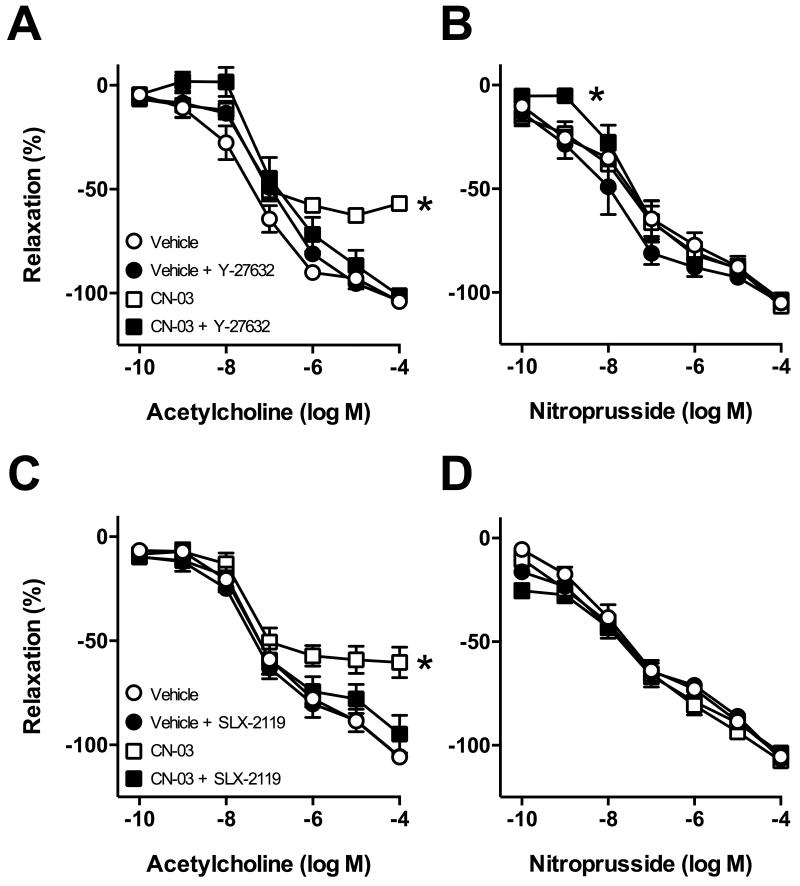
Figure 2.
Responses of carotid arteries to acetylcholine (left panels) and nitroprusside (right panels) following incubation with CN-03 (2.5 μg/mL) or vehicle in the absence or presence of the ROCK inhibitor Y-27632 (A and B) or ROCK2 inhibitor SLX-2119 (C and D). (A and B) n=4 for all groups; (A) *P<0.05 for CN-03 alone curve vs. all other groups; (B) *P<0.05 CN-03 + Y-27632 curve vs. vehicle curve. (C and D) n=6 for all groups, P<0.001 for CN-03 alone curve vs all other groups.
Impaired endothelium-dependent relaxation induced by CN-03 was reversed by Y-27632 (3 μmol/L)(Figure 2A). We next sought to determine which ROCK isoform was being activated by CN-03. The ROCK2 inhibitor SLX-2119 (1 μmol/L) also significantly improved endothelium-dependent responses following CN-03 treatment (Figure 2C). In contrast, treatment with Y-27632 or SLX-2119 did not affect responses in vehicle treated groups (Figure 2A and C). In the majority of groups, CN-03 alone or in combination with Y-27632 or SLX-2119 had no effect on relaxation to nitroprusside (Figure 2B and D). We did observe a small shift in the response to submaximal concentrations of nitroprusside with CN-03 + Y-27632 versus vehicle only (Figure 2B). In this group, contraction of carotid arteries to U46619 was somewhat augmented following incubation with 2.5 μg/ml CN-03 (Figure S1A).
Ang II impairs endothelial function in carotid arteries in a ROCK2-dependent manner
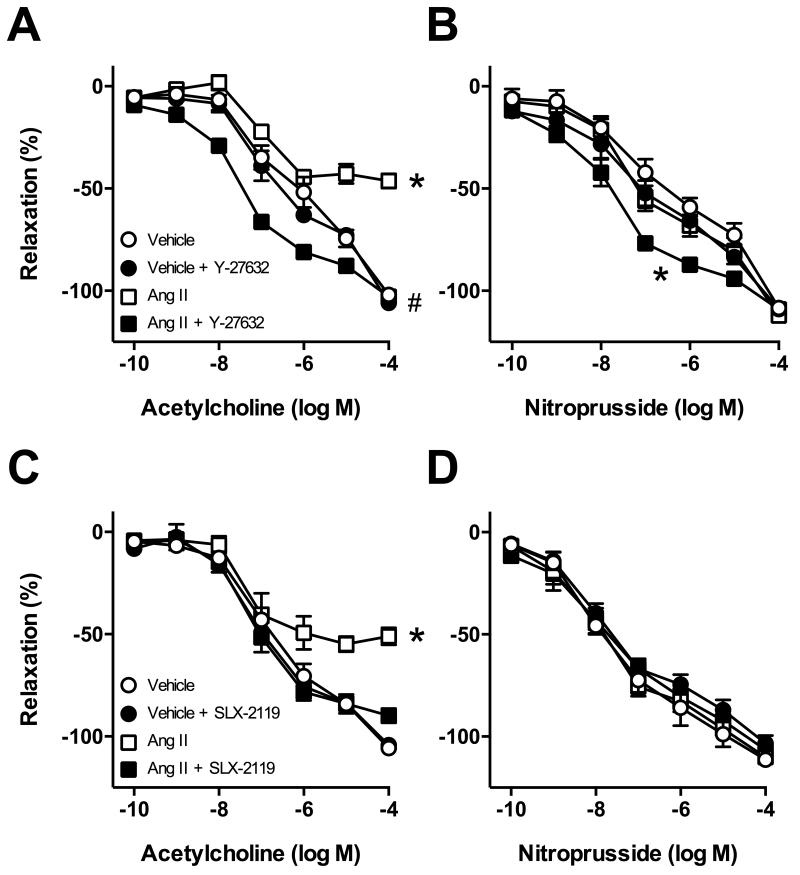
Consistent with previous studies24, Ang II (10 nmol/L) impaired endothelium-dependent relaxation of carotid arteries by ~50% (Figure 3A). Endothelial function was restored to normal by treatment with Y-27632 (Figure 3A). We observed a leftward shift in the concentration-response curve to acetylcholine compared with the vehicle groups with Ang II + Y-27632 treatment (Figure 3A). In addition, endothelial function was restored by SLX-2119 treatment (Figure 3C). In most groups, relaxation to nitroprusside was not significantly affected by incubation with Ang II or treatment with Y-27632 or SLX-2119 (Figure 3B and D). However, treatment with Ang II + Y-27632 resulted in a small leftward shift in the concentration-response curve to nitroprusside (Figure 3B). Contraction of carotid arteries to U46619 was not significantly affected by incubation with Ang II (Figure S1B).
Figure 3.
Responses of carotid arteries to (A) acetylcholine and (B) nitroprusside following incubation with Ang II (10 nmol/L) or vehicle in the absence or presence of the ROCK inhibitor Y-27632 (A and B) or the ROCK2 inhibitor SLX-2119 (C and D). n=6 for all groups. (A) *P<0.001 for Ang II curve vs. all other groups, (A) #P<0.001 for Ang II + Y-27632 curve vs. all other groups. (B) *P<0.001 for Ang II +Y-27632 curve vs. all other groups.
Because SLX-2119 improved endothelial function after treatment with Ang II, we tested if the increased vasorelaxation to acetylcholine was mediated by NO using L-NNA in an additional group. We found that the increased response to acetylcholine produced by SLX-2119 (after treatment with Ang II) was prevented by L-NNA (Figure S2A). Relaxation of arteries to nitroprusside was similar in these groups (Figure S2B).
Contraction of carotid arteries to several receptor-mediated agonists is independent of ROCK2
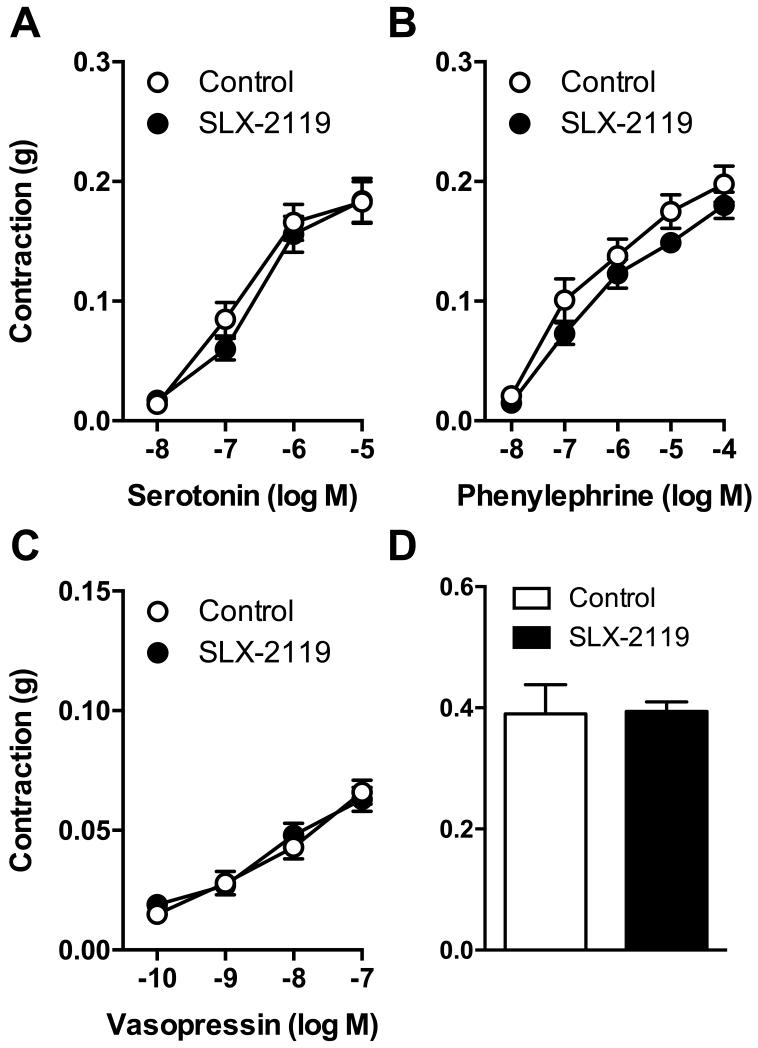
We next tested effects of ROCK2 inhibition on contractile responses to several agonists, including two that are inhibited by Y-27632 (serotonin and phenylephrine) in carotid arteries25. Contraction of freshly isolated carotid arteries (i.e. no incubation with CN-03 or Ang II) to serotonin (Figure 4A), phenylephrine (Figure 4B), vasopressin (Figure 4C) or U46619 (Figure 4D) was not significantly affected by SLX-2119 (1 μmol/L).
Figure 4.
Contraction of carotid arteries to (A) serotonin, (B) phenylephrine, (C) vasopressin, and (D) U46619 in the absence and presence of SLX-2119 (1 μmol/L). n=6 for all groups.
ROCK2 mediates constriction to select agents in basilar arteries
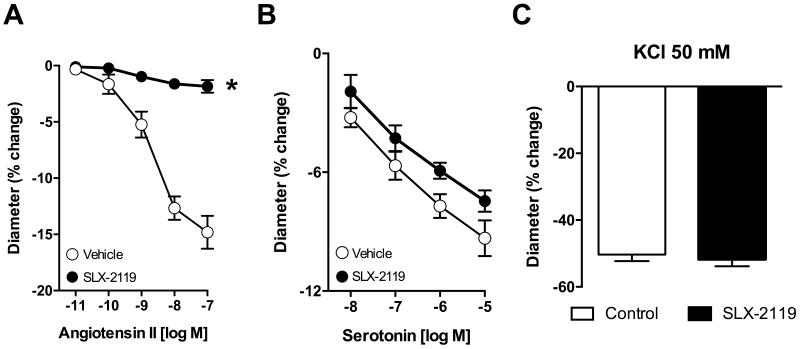
We previously found that Y-27632 essentially abolished constriction of basilar arteries to Ang II20. SLX-2119 did not affect baseline diameter of basilar arteries (162±4 and 161±4 μm in vehicle and SLX-2119 treated, respectively; n=10). However, constriction to Ang II in basilar arteries was reduced by about 90% by SLX-2119 (1 μmol/L)(Figure 5A). In contrast, vasoconstriction to serotonin (Figure 5B) or KCl (50 mmol/L; 5C) were not significantly affected by SLX-2119.
Figure 5.
Constriction of basilar arteries to (A) angiotensin II, (B) serotonin or (C) KCl in the absence and presence of SLX-2119. (A) n=6, *P<0.001 for SLX-2119 vs. vehicle, (B) n=4, (C) n=4-6.
Myogenic tone is dependent on ROCK2 in parenchymal arterioles
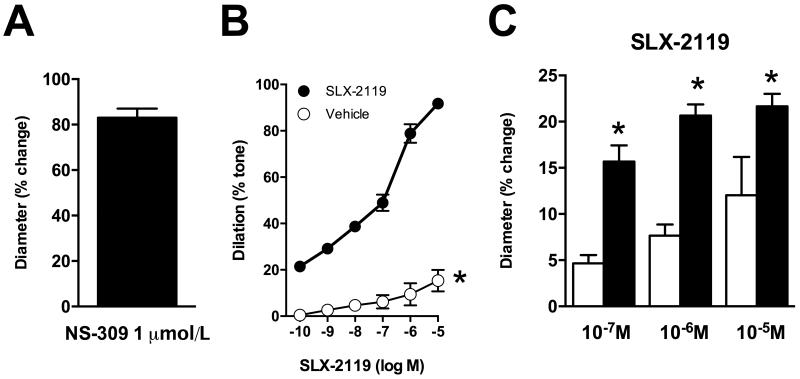
Generation of myogenic tone by cerebral arteries and arterioles is a major element of mechanisms that underlie autoregulation of cerebral blood flow. Parenchymal arterioles develop substantial myogenic tone26-28 and are key contributors to overall cerebral vascular resistance29. In the current study, parenchymal arterioles generated spontaneous tone once pressurized (37.1±3.0% of maximum diameter; n=10 arterioles) to a baseline diameter of 17±2 μm (active diameter). Subsequent application of NS-309 caused marked dilation of parenchymal arterioles (Figure 6A)(n=8 arterioles). The observation that NS-309, an activator of small- and intermediate-conductance Ca2+ activated K+ channels, produced robust vasodilation suggested endothelial function was intact in these arterioles. The cumulative addition of SLX-2119 (0.1 nmol/L - 10 μmol/L) dilated parenchymal arterioles (Figure 6B). At 1 μmol/L, a concentration where SLX-2119 is highly selective for ROCK2 vs ROCK14, SLX-2119 dilated parenchymal arterioles by 78.9±4.0%. In contrast, addition of vehicle (DMSO) had minimal effect on myogenic tone (Figure 6B). In a separate group of vessels studied under the same conditions, Y-27632 dilated parenchymal arterioles to a comparable degree as SLX-2119 with a maximum response of 89.8±2.3% (10 μmol/L; n=6). Diameter of parenchymal arterioles under passive, calcium-free conditions, was 26±3 μm.
Figure 6.
(A) Dilation of parenchymal arterioles to NS-309. (B) Effect of SLX-2119 or vehicle (DMSO) on myogenic tone in brain parenchymal arterioles. (C) Responses of pial arterioles in vivo to SLX-2119 in the absence and presence of L-NNA (100 μmol/L). (A) n=8 parenchymal arterioles. (B) n=5 parenchymal arterioles for both groups, *P<0.001 for SLX-2119 vs. vehicle. (C) n=3, *P<0.05 for SLX-2119 + L-NNA vs. SLX-2119 alone.
ROCK2 influences resting diameter of pial arterioles in vivo
In pial arterioles in vivo, SLX-2119 produced moderate dilation (Figure 6C). Local treatment with the NOS inhibitor L-NNA did not alter baseline diameter (34±3 μm before L-NNA vs. 34±3 μm after 30 min L-NNA incubation), but the magnitude of the response to SLX-2119 was significantly augmented (Figure 6C).
Discussion
In the present study, we obtained evidence supporting the concept that ROCK2 plays a key role in regulating the function of both large arteries as well as the smallest arterioles. We made several novel findings. First, direct activation of RhoA using CN-03 selectively impairs endothelial function in carotid arteries via a ROCK2-dependent mechanism. Second, Ang II-induced endothelial dysfunction in carotid arteries is also dependent on ROCK2. The ROCK2 inhibitor restored the NO component of this response. Third, contraction of carotid arteries to phenylephrine, serotonin, vasopressin and U46619 are largely independent of ROCK2. Fourth, constriction of basilar arteries to Ang II, but not serotonin or KCl, requires normal activity of ROCK2. Fifth, myogenic tone in brain parenchymal arterioles is highly dependent on ROCK2. Lastly, the influence of ROCK2 on arteriolar microvascular tone in vivo is modulated by NO. These novel findings suggest that ROCK2 is a major regulator of vascular tone in muscular arteries as well as resistance vessels in brain.
Experimental approaches to study ROCK
The majority of findings dealing with the impact of ROCK in the vasculature are based on studies that used pharmacological inhibitors. In relation to selectivity, two of the most commonly used inhibitors of ROCK (Y-27632 and fasudil) can also inhibit other enzymes, with fasudil being the least selective of the two30, 31. Of importance for the present study, Y-27632 and fasudil have similar inhibitory constants (Ki) for ROCK1 and ROCK24. In contrast, SLX-2119 is highly selective for ROCK2 compared with ROCK1 (Ki: >10,000 nmol/L for ROCK1 vs 41 nmol/L for ROCK2)4.
In contrast to pharmacological inhibitors, only a small number of studies have utilized genetic manipulation of ROCK to study its functional effects. The lack of available genetic models has contributed to our limited understanding of the relative contribution of specific ROCK isoforms in vascular disease. More than 90% of mice fully deficient in either ROCK isoform (Rock1−/− and Rock2−/−) die in utero or early in the postnatal period9. Therefore, studies using such mice need to be interpreted with caution as surviving animals may have compensatory effects of the remaining ROCK isoform.9 For such reasons, a few studies have used heterozygous Rock1+/− and Rock2+/− mice. Using such mice for example, diabetes-induced endothelial dysfunction in aorta was primarily mediated by ROCK1, with a smaller role for ROCK23. Neointima formation following carotid artery ligation was reduced in Rock1+/− mice32, 33. In cell culture studies, gene silencing techniques suggested that ROCK1 contributes to stiffening of endothelial cells and increased vascular permeability34. Furthermore, in aortic vascular muscle in culture, siRNA against ROCK2, but not ROCK1 reduced contraction to the phospholipid lysophosphatidic acid35. Effects of other vasoconstrictor stimuli were not tested. While such efforts have begun to define roles for different ROCK isoforms, the limited data to date in intact blood vessels makes it difficult to predict which isoform would be of more important in regulating function in endothelium versus vascular muscle.
An important gap in our understanding is that previous work has not provided insight into the impact of specific ROCK isoforms in resistance vessels. In brain, both arteries and arterioles are resistance vessels. For example, surface arteries and arterioles contribute approximately 50% of total vascular resistance18, 29. Parenchymal arterioles are the major resistance vessel within the brain itself 18, 29. Thus, dysfunction at any of these levels can have a significant impact on local vascular resistance and thus blood flow. In some cases, such vascular changes have long-term consequences for neurological function10.
Influence of ROCK on endothelial function
The function of ROCK in the vasculature has been studied most widely in relation to its role in vascular muscle, where it regulates contraction. Increases in calcium sensitivity is achieved via the ROCK mediated inhibition of myosin light chain phosphatase (MLCP). Inhibition of MLCP activity decreases the dephosphorylation of myosin light chain. Thus, even in the absence of any further rise in intracellular calcium, contractile force can be maintained or increased, due to the lack of phosphatase activity by MLCP.
Much less is known regarding the function of ROCK in endothelium. One area that has received some attention relates to how ROCK affects eNOS. Such effects may occur via several mechanisms. ROCK can impair eNOS activity via a reduction in the phosphorylation of a key residue, serine 117736. In addition, ROCK can directly phosphorylate eNOS at threonine 495, which inhibits eNOS activity36, 37. The balance between phosphorylation at serine 1177 and threonine 495 determines the activity of eNOS. Other studies suggest ROCK inhibition increases eNOS expression via increased eNOS mRNA stability5, 38. Either alone or in combination, these effects of ROCK on eNOS expression and activity would be predicted to substantially alter NO bioavailability.
In the present study, we used two agents to induce endothelial dysfunction, CN-03 and Ang II. While beneficial effects of ROCK inhibition on vascular function are known, it was unclear whether direct activation of this pathway is sufficient to cause endothelial dysfunction. The direct RhoA activator, CN-03, mimics the effect of cytotoxic necrotizing factor-1 (CNF-1) from Escherichia coli, de-amidating the glutamine at position 63 to glutamate39. CN-03 has been used mostly in cell culture, so data on effects in intact vascular tissue are limited. We6 and others40 found that CN-03 increases myogenic tone in cerebral arteries and arterioles in a ROCK-dependent manner.
We initially tested whether direct activation of RhoA, and therefore ROCK, with CN-03 was sufficient to impair endothelial function. CN-03 produced concentration-dependent impairment of endothelium-dependent relaxation, but did not inhibit endothelium-independent relaxation. Impaired relaxation to acetylcholine following incubation with CN-03 was reversed by both Y-27632 and the ROCK2 inhibitor SLX-2119. The finding that both inhibitors restored endothelium-dependent responses suggests that ROCK2 is the isoform that is primarily responsible for the dysfunction. These findings show for the first time that treatment of arteries with CN-03 is a novel approach to induce endothelial dysfunction specifically via activation of ROCK.
We next tested whether ROCK2 also mediated Ang II-induced endothelial dysfunction. As previously reported24, incubation with Ang II induced endothelial dysfunction in wild-type mice. Endothelial dysfunction in this model, which examines direct effects of Ang II on the vessel wall, is dependent on interacting immune- and oxidative stress-dependent mechanisms18. The novel finding of the present study is that ROCK2 plays an essential role in Ang II-induced endothelial dysfunction. There are several ways such interactions might occur including activation of RhoA or ROCK by reactive oxygen species.
We observed a leftward shift in the concentration response curves to acetylcholine and nitroprusside in the group treated with Ang II and Y-27632. The physiological relevance of this finding is unclear, as Y-27632 did not have similar effects in vessels not treated with Ang II or treated with CN-03. One potential explanation is that as Y-27632 would also inhibit ROCK1, its use may unmask an influence of ROCK1 in modulating endothelial function. This possibility would need further investigation.
The finding that a ROCK2 inhibitor improves endothelium-dependent (NO-mediated) relaxation in arteries following CN-03 or Ang II treatment suggests a key role for this isoform in endothelium. These findings are consistent with previous studies where fasudil improved endothelial function in an Ang II-dependent model of hypertension1. Like SLX-2119 in the current study, fasudil restored the NO-component of the response in that study1, consistent with the concept that increased ROCK activity impairs endothelial function via effects on production of NO1. This negative interaction between ROCK2 and NO during vascular disease is consistent with the finding that ROCK activity correlates positively with the incidence of cerebrovascular events17.
Role of ROCK in receptor-mediated vasoconstriction
Although previous studies revealed that contraction of carotid arteries to serotonin and phenylephrine are inhibited by Y-2763225, inhibition of ROCK2 did not significantly impair contraction of the same arteries to phenylephrine, serotonin, vasopressin or U46619. Such findings suggest that ROCK1 may be the more important as a mediator of agonist-induced contraction in large muscular arteries. Consistent with this concept, contraction to phenylephrine is reduced in aorta from Rock1+/− mice whereas it is not significantly altered in Rock2+/− mice3. Collectively, such findings suggest a heterogeneous contribution of ROCK isoforms to various agonist-induced vasoconstrictor responses.
Ang II constricts many vessels, including cerebral arteries via activation of AT1 receptors20. Previous work has shown that Ang II constricts basilar arteries via activation of ROCK, as the response was abolished by Y-2763220. In the present study, we extend those findings by examining effects of ROCK2 inhibition on vasoconstriction to Ang II. Our results suggest that vasoconstrictor effects of Ang II in cerebral arteries are predominantly ROCK2-dependent. Consistent with findings in the carotid artery, SLX-2119 did not have a significant effect on constriction of the basilar artery to serotonin.
Regulation of myogenic tone by ROCK
Myogenic tone of vascular muscle in resistance vessels is a key contributor to the autoregulatory response. Autoregulation is the collective process by which blood flow is kept relatively constant over a range of perfusion pressures41. Numerous molecules and pathways have been suggested to contribute to myogenic tone and myogenic responses (for review see42). However, the precise molecular details by which cerebral and systemic arteries and arterioles generate myogenic tone remains unsettled. We6 and others16, 43 have provided evidence that ROCK is a key determinant of myogenic tone in middle and posterior cerebral arteries. Furthermore, ROCK inhibitors with no isoform selectivity (Y-27632 and H-1152) cause marked dilation of parenchymal arterioles during health and disease (present work and previous studies26, 40, 44), highlighting the importance of ROCK in regulating myogenic tone at multiple levels within the cerebral vasculature. In the current study, we extend those findings by obtaining evidence that ROCK2 specifically is a major regulator of myogenic tone in brain parenchymal arterioles. Parenchymal arterioles connect larger pial arteries and arterioles to the underlying capillary network. As parenchymal arterioles are immediately upstream of capillaries, they are a key regulators of local cerebral blood flow45. These arterioles have been described as a bottleneck in delivering nutrients to the brain parenchyma and as such, disruption of parenchymal arteriolar function has dramatic effects on downstream perfusion46. Although emerging evidence indicates that this segment of the vasculature is particularly affected by small vessel disease11, 12, there is still relatively little known regarding regulation of function in this important portion of the vasculature. Our findings suggest that myogenic tone in parenchymal arterioles is almost completely dependent on activity of ROCK2.
Lastly, we examined the influence of ROCK2 on pial microvascular diameter in vivo. Previous work using Y-27632 suggested that ROCK influences arteriolar tone under normal conditions15. The findings of the present study suggest that ROCK2 is the isoform that mediates this effect. Consistent with previous work with Y-2763215, we found that the NOS inhibitor L-NNA augmented vasodilator responses to SLX-2119. It is unlikely this effect of L-NNA is non-specific as we and others have found previously that vasodilation to papaverine, low concentrations of potassium, adenosine, and norepinephrine are not augmented by L-NNA (or L-NAME)47-50. Overall, these new findings suggest that in cerebral arterioles from normal animals, basal NO inhibits the influence of ROCK2 on vessel tone. Such an effect may serve as an important protective mechanism to limit the generation of excessive levels of arteriolar tone.
Links between Ang II and ROCK2
The present findings have implications for hypertension and vascular disease. Ang II has been widely implicated in various forms of hypertension and the renin-angiotensin system is a major therapeutic target19. Two of the key vascular effects of Ang II are endothelial dysfunction and vasoconstriction, both with significant physiological consequences. Endothelial dysfunction plays a fundamental role in the onset and progression of vascular disease18. In addition to hypertension, Ang II promotes endothelial abnormalities and atherosclerosis in the presence of other cardiovascular risk factors including diabetes and aging18. Considering this broad impact, the finding that ROCK2 inhibition restored endothelial function in a model of Ang II-induced vascular disease suggests that ROCK2 may be an important therapeutic target.
Vasoconstrictor effects of Ang II have several potential consequences. Increases in vascular tone can contribute to global or local changes in vascular resistance as well as producing hypoperfusion, which in brain, may contribute to the cognitive effects of hypertension. Lastly, sustained increases in vascular tone may be involved in initiating structural changes in the vasculature known as inward remodeling. The finding that SLX-2119 inhibited the majority of constriction of cerebral arteries to Ang II is further evidence that ROCK2 may be involved in more than one component of the disease process, implying that targeting ROCK2 may have broad therapeutic potential.
In summary, the present study suggests that ROCK2 contributes to several aspects of vascular function under normal conditions and in two models of vascular disease. Activation of RhoA, and as a consequence ROCK, is sufficient to cause profound endothelial dysfunction. We identify a novel, highly targeted method to impair endothelial function using CN-03. Our data suggest that ROCK2 contributes to both CN-03 and Ang II-induced endothelial dysfunction. In addition, key roles for ROCK2 as a mediator of vasoconstriction to Ang II and myogenic tone in small parenchymal arterioles were revealed. In contrast, ROCK2 appears to play a much smaller role in vasoconstriction to several receptor-mediated agonists.
Perspective
ROCK has been implicated in cardiovascular disease in both animal models and humans. Thus, therapeutic targeting of ROCK may have great potential. While beneficial effects of ROCK inhibition are well known, treatment of human subjects have been limited to the use of fasudil in subarachnoid hemorrhage51, although clinical trails with other inhibitors and diseases are ongoing. The recent development of more selective inhibitors like SLX-2119, in combination with better insight into the impact of specific ROCK isoforms at the cellular and molecular level, should facilitate this translational effort. SLX-2119 is safe, well tolerated, and orally active in humans and mice4, 23, making it or related approaches that target ROCK2 a good candidate for therapeutic use. The current studies highlight the potential benefit of ROCK2 inhibition in models of disease as well new insight into the functional importance of ROCK2 in resistance vessels. Further studies in this area may facilitate approaches that could be used to prevent, delay, and possibly reverse key elements in the progression of large and/or small vessel disease.
Supplementary Material
Novelty and Significance.
1. What Is New?
Our findings suggest that ROCK2 plays a key role in endothelial dysfunction produced in response to angiotensin II (Ang II) or direct activation of RhoA.
Constriction of cerebral arteries to Ang II and myogenic tone in small parenchymal arterioles was ROCK2-dependent. In contrast, contraction of carotid arteries to several receptor-mediated agonists was largely ROCK2-independent.
These findings support the concept that ROCK2 is a key ROCK isoform in relation to regulation of carotid and cerebrovascular function. These are the first functional data related to the impact of ROCK2 in resistance vessels.
2. What Is Relevant?
Little is known regarding the role of specific ROCK isoforms in the regulation of vascular function.
Considering the importance of Rho kinase in vascular cells, these findings have implications for mechanisms that regulate brain perfusion, endothelial function, and the pathogenesis of large and small vessel disease.
3. Summary
This study provides new insight into the impact of ROCK2 on several aspects of vascular function under normal conditions and in two models of vascular disease. Activation of ROCK2 was sufficient to cause profound endothelial dysfunction. We describe a new approach to study endothelial function using CN-03, obtaining evidence that ROCK2 contributes to both CN-03 and Ang II-induced endothelial dysfunction. In addition, key roles for ROCK2 in vasoconstrictor responses to Ang II and myogenic tone in small parenchymal arterioles were revealed. The studies highlight the potential benefit of ROCK2 inhibition in models of disease as well new insight into the functional importance of ROCK2 in resistance vessels.
Acknowledgements
The authors would like to thank Fabrice Dabertrand, PhD for technical assistance with initiating studies of parenchymal arterioles. The authors would also like to acknowledge Cynthia Lynch for technical assistance.
Sources of Funding
This work was supported by research grants from the National Institute of Health (HL-62984, HL-113863, NS-096465), the Department of Veteran’s Affair’s (BX001399), the Fondation Leducq (a Transatlantic Network of Excellence), and the National Health and Medical Research Council of Australia (1053786).
Footnotes
Disclosures
None.
References
- 1.Higashi M, Shimokawa H, Hattori T, Hiroki J, Mukai Y, Morikawa K, Ichiki T, Takahashi S, Takeshita A. Long-term inhibition of Rho-kinase suppresses angiotensin II-induced cardiovascular hypertrophy in rats in vivo: Effect on endothelial NAD(P)H oxidase system. Circ Res. 2003;93:767–775. doi: 10.1161/01.RES.0000096650.91688.28. [DOI] [PubMed] [Google Scholar]
- 2.Mukai Y, Shimokawa H, Matoba T, Kandabashi T, Satoh S, Hiroki J, Kaibuchi K, Takeshita A. Involvement of Rho-kinase in hypertensive vascular disease -a novel therapeutic target in hypertension. FASEB J. 2001;15:1062–1064. doi: 10.1096/fj.00-0735fje. [DOI] [PubMed] [Google Scholar]
- 3.Yao L, Chandra S, Toque HA, Bhatta A, Rojas M, Caldwell RB, Caldwell RW. Prevention of diabetes-induced arginase activation and vascular dysfunction by Rho kinase (ROCK) knockout. Cardiovasc Res. 2013;97:509–519. doi: 10.1093/cvr/cvs371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lee JH, Zheng Y, von Bornstadt D, et al. Selective ROCK2 inhibition in focal cerebral ischemia. Ann Clin Transl Neurol. 2014;1:2–14. doi: 10.1002/acn3.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rikitake Y, Kim H-H, Huang Z, Seto M, Yano K, Asano T, Moskowitz MA, Liao JK. Inhibition of Rho kinase (ROCK) leads to increased cerebral blood flow and stroke protection. Stroke. 2005;36:2251–2257. doi: 10.1161/01.STR.0000181077.84981.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.De Silva TM, Ketsawatsomkron P, Pelham C, Sigmund CD, Faraci FM. Genetic interference with peroxisome proliferator-activated receptor γ in smooth muscle enhances myogenic tone in the cerebrovasculature via a Rho kinase-dependent mechanism. Hypertension. 2015;65:345–351. doi: 10.1161/HYPERTENSIONAHA.114.04541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.De Silva TM, Modrick ML, Ketsawatsomkron P, Lynch C, Chu Y, Pelham CJ, Sigmund CD, Faraci FM. Role of peroxisome proliferator-activated receptor-γ in vascular muscle in the cerebral circulation. Hypertension. 2014;64:1088–1093. doi: 10.1161/HYPERTENSIONAHA.114.03935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Somlyo AP, Somlyo AV. Ca2+ sensitivity of smooth muscle and nonmuscle myosin II: Modulated by G proteins, kinases, and myosin phosphatase. Physiol Rev. 2003;83:1325–1358. doi: 10.1152/physrev.00023.2003. [DOI] [PubMed] [Google Scholar]
- 9.Loirand G. Rho kinases in health and disease: From basic science to translational research. Pharmacol Rev. 2015;67:1074–1095. doi: 10.1124/pr.115.010595. [DOI] [PubMed] [Google Scholar]
- 10.De Silva TM, Faraci FM. Microvascular dysfunction and cognitive impairment. Cell Mol Neurobiol. 2016;36:241–258. doi: 10.1007/s10571-015-0308-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Joutel A, Faraci FM. Cerebral small vessel disease: Insights and opportunities from mouse models of collagen IV-related small vessel disease and cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy. Stroke. 2014;45:1215–1221. doi: 10.1161/STROKEAHA.113.002878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wardlaw JM, Smith C, Dichgans M. Mechanisms of sporadic cerebral small vessel disease: Insights from neuroimaging. Lancet Neurol. 2013;12:483–497. doi: 10.1016/S1474-4422(13)70060-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lawes CM, Bennett DA, Feigin VL, Rodgers A. Blood pressure and stroke: An overview of published reviews. Stroke. 2004;35:1024–1033. [PubMed] [Google Scholar]
- 14.Chrissobolis S, Sobey CG. Evidence that Rho-kinase activity contributes to cerebral vascular tone in vivo and is enhanced during chronic hypertension: Comparison with protein kinase C. Circ Res. 2001;88:774–779. doi: 10.1161/hh0801.090441. [DOI] [PubMed] [Google Scholar]
- 15.Didion SP, Lynch CM, Baumbach GL, Faraci FM. Impaired endothelium-dependent responses and enhanced influence of Rho-kinase in cerebral arterioles in type II diabetes. Stroke. 2005;36:342–347. doi: 10.1161/01.STR.0000152952.42730.92. [DOI] [PubMed] [Google Scholar]
- 16.Gokina NI, Park KM, McElroy-Yaggy K, Osol G. Effects of Rho kinase inhibition on cerebral artery myogenic tone and reactivity. J Appl Physiol. 2005;98:1940–1948. doi: 10.1152/japplphysiol.01104.2004. [DOI] [PubMed] [Google Scholar]
- 17.Kajikawa M, Noma K, Maruhashi T, Mikami S, Iwamoto Y, Iwamoto A, Matsumoto T, Hidaka T, Kihara Y, Chayama K, Nakashima A, Goto C, Liao JK, Higashi Y. Rho-associated kinase activity is a predictor of cardiovascular outcomes. Hypertension. 2013;63:856–864. doi: 10.1161/HYPERTENSIONAHA.113.02296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Faraci FM. Protecting against vascular disease in brain. Am J Physiol. 2011;300:H1566–1582. doi: 10.1152/ajpheart.01310.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Te Riet L, van Esch JH, Roks AJ, van den Meiracker AH, Danser AH. Hypertension: Renin-angiotensin-aldosterone system alterations. Circ Res. 2015;116:960–975. doi: 10.1161/CIRCRESAHA.116.303587. [DOI] [PubMed] [Google Scholar]
- 20.Faraci FM, Lamping KG, Modrick ML, Ryan MJ, Sigmund CD, Didion SP. Cerebral vascular effects of angiotensin II: New insights from genetic models. J Cereb Blood Flow Metabol. 2006;26:449–455. doi: 10.1038/sj.jcbfm.9600204. [DOI] [PubMed] [Google Scholar]
- 21.Sagara Y, Hirooka Y, Nozoe M, Ito K, Kimura Y, Sunagawa K. Pressor response induced by central angiotensin II is mediated by activation of Rho/Rho-kinase pathway via AT1 receptors. J Hypertens. 2007;25:399–406. doi: 10.1097/HJH.0b013e328010b87f. [DOI] [PubMed] [Google Scholar]
- 22.Matrougui K, Tanko LB, Loufrani L, Gorny D, Levy BI, Tedgui A, Henrion D. Involvement of Rho-kinase and the actin filament network in angiotensin II-induced contraction and extracellular signal-regulated kinase activity in intact rat mesenteric resistance arteries. Arterioscler Thromb Vasc Biol. 2001;21:1288–1293. doi: 10.1161/hq0801.093653. [DOI] [PubMed] [Google Scholar]
- 23.Zanin-Zhorov A, Weiss JM, Nyuydzefe MS, et al. Selective oral ROCK2 inhibitor down-regulates IL-21 and IL-17 secretion in human T cells via STAT3-dependent mechanism. Proc Natl Acad Sci. 2014;111:16814–16819. doi: 10.1073/pnas.1414189111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Didion SP, Kinzenbaw DA, Faraci FM. Critical role for CuZn-superoxide dismutase in preventing angiotensin II-induced endothelial dysfunction. Hypertension. 2005;46:1147–1153. doi: 10.1161/01.HYP.0000187532.80697.15. [DOI] [PubMed] [Google Scholar]
- 25.Didion SP, Lynch CM, Faraci FM. Cerebral vascular dysfunction in TallyHo mice: A new model of type II diabetes. Am J Physiol. 2007;292:H1579–1583. doi: 10.1152/ajpheart.00939.2006. [DOI] [PubMed] [Google Scholar]
- 26.Cipolla MJ, Chan SL, Sweet J, Tavares MJ, Gokina N, Brayden JE. Postischemic reperfusion causes smooth muscle calcium sensitization and vasoconstriction of parenchymal arterioles. Stroke. 2014;45:2425–2430. doi: 10.1161/STROKEAHA.114.005888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dabertrand F, Kroigaard C, Bonev AD, Cognat E, Dalsgaard T, Domenga-Denier V, Hill-Eubanks DC, Brayden JE, Joutel A, Nelson MT. Potassium channelopathy-like defect underlies early-stage cerebrovascular dysfunction in a genetic model of small vessel disease. Proc Natl Acad Sci. 2015;112:E796–805. doi: 10.1073/pnas.1420765112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dabertrand F, Nelson MT, Brayden JE. Acidosis dilates brain parenchymal arterioles by conversion of calcium waves to sparks to activate BK channels. Circ Res. 2012;110:285–294. doi: 10.1161/CIRCRESAHA.111.258145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Faraci F, Heistad D. Regulation of large cerebral arteries and cerebral microvascular pressure. Circ Res. 1990;66:8–17. doi: 10.1161/01.res.66.1.8. [DOI] [PubMed] [Google Scholar]
- 30.Breitenlechner C, Gaßel M, Hidaka H, Kinzel V, Huber R, Engh RA, Bossemeyer D. Protein kinase A in complex with Rho-kinase inhibitors Y-27632, fasudil, and H-1152P. Structure. 2003;11:1595–1607. doi: 10.1016/j.str.2003.11.002. [DOI] [PubMed] [Google Scholar]
- 31.Davies SP, Reddy H, Caivano M, Cohen P. Specificity and mechanism of action of some commonly used protein kinase inhibitors. Biochem J. 2000;351:95–105. doi: 10.1042/0264-6021:3510095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rikitake Y, Oyama N, Wang CY, Noma K, Satoh M, Kim HH, Liao JK. Decreased perivascular fibrosis but not cardiac hypertrophy in ROCK1+/− haploinsufficient mice. Circulation. 2005;112:2959–2965. doi: 10.1161/CIRCULATIONAHA.105.584623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Noma K, Rikitake Y, Oyama N, Yan G, Alcaide P, Liu PY, Wang H, Ahl D, Sawada N, Okamoto R, Hiroi Y, Shimizu K, Luscinskas FW, Sun J, Liao JK. ROCK1 mediates leukocyte recruitment and neointima formation following vascular injury. J Clin Invest. 2008;118:1632–1644. doi: 10.1172/JCI29226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Huynh J, Nishimura N, Rana K, Peloquin JM, Califano JP, Montague CR, King MR, Schaffer CB, Reinhart-King CA. Age-related intimal stiffening enhances endothelial permeability and leukocyte transmigration. Sci Transl Med. 2011;3:112ra122. doi: 10.1126/scitranslmed.3002761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wang Y, Zheng XR, Riddick N, Bryden M, Baur W, Zhang X, Surks HK. ROCK isoform regulation of myosin phosphatase and contractility in vascular smooth muscle cells. Circ Res. 2009;104:531–540. doi: 10.1161/CIRCRESAHA.108.188524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Faraco G, Moraga A, Moore J, Anrather J, Pickel VM, Iadecola C. Circulating endothelin-1 alters critical mechanisms regulating cerebral microcirculation. Hypertension. 2013;62:759–766. doi: 10.1161/HYPERTENSIONAHA.113.01761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sugimoto M, Nakayama M, Goto TM, Amano M, Komori K, Kaibuchi K. Rho-kinase phosphorylates eNOS at threonine 495 in endothelial cells. Biochem Biophys Res Commun. 2007;361:462–467. doi: 10.1016/j.bbrc.2007.07.030. [DOI] [PubMed] [Google Scholar]
- 38.Laufs U, Liao JK. Post-transcriptional regulation of endothelial nitric oxide synthase mRNA stability by Rho GTPase. J Biol Chem. 1998;273:24266–24271. doi: 10.1074/jbc.273.37.24266. [DOI] [PubMed] [Google Scholar]
- 39.Schmidt G, Sehr P, Wilm M, Selzer J, Mann M, Aktories K. Gln 63 of Rho is deamidated by Escherichia coli cytotoxic necrotizing factor-1. Nature. 1997;387:725–729. doi: 10.1038/42735. [DOI] [PubMed] [Google Scholar]
- 40.Li Y, Brayden JE. Rho kinase activity governs arteriolar myogenic depolarization. J Cereb Blood Flow Metabol. 2015 doi: 10.1177/0271678X15621069. 0271678X15621069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cipolla MJ. The cerebral circulation. In: Granger DN, Granger J, editors. Integrated Systems Physiology: From Molecule to Function. Morgan & Claypool Life Sciences; San Rafael: 2009. pp. 1–59. [PubMed] [Google Scholar]
- 42.Hill-Eubanks DC, Gonzales AL, Sonkusare SK, Nelson MT. Vascular TRP channels: Performing under pressure and going with the flow. Physiology. 2014;29:343–360. doi: 10.1152/physiol.00009.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Johnson RP, El-Yazbi AF, Takeya K, Walsh EJ, Walsh MP, Cole WC. Ca2+ sensitization via phosphorylation of myosin phosphatase targeting subunit at threonine-855 by Rho kinase contributes to the arterial myogenic response. J Physiol. 2009;587:2537–2553. doi: 10.1113/jphysiol.2008.168252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ahnstedt H, Sweet J, Cruden P, Bishop N, Cipolla MJ. Effects of early post-ischemic reperfusion and tPA on cerebrovascular function and nitrosative stress in female rats. Transl Stroke Res. 2016;7:228–238. doi: 10.1007/s12975-016-0468-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hill RA, Tong L, Yuan P, Murikinati S, Gupta S, Grutzendler J. Regional blood flow in the normal and ischemic brain is controlled by arteriolar smooth muscle cell contractility and not by capillary pericytes. Neuron. 2015;87:95–110. doi: 10.1016/j.neuron.2015.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nishimura N, Schaffer CB, Friedman B, Lyden PD, Kleinfeld D. Penetrating arterioles are a bottleneck in the perfusion of neocortex. Proc Natl Acad Sci. 2007;104:365–370. doi: 10.1073/pnas.0609551104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sobey CG, Faraci FM. Effect of nitric oxide and potassium channel agonists and inhibitors on basilar artery diameter. Am J Physiol. 1997;272:H256–H262. doi: 10.1152/ajpheart.1997.272.1.H256. [DOI] [PubMed] [Google Scholar]
- 48.Chrissobolis S, Ziogas J, Chu Y, Faraci FM, Sobey CG. Role of inwardly rectifying K+ channels in K+-induced cerebral vasodilatation in vivo. Am J Physiol. 2000;279:H2704–2712. doi: 10.1152/ajpheart.2000.279.6.H2704. [DOI] [PubMed] [Google Scholar]
- 49.Kitazono T, Faraci FM, Heistad DD. Effect of norepinephrine on rat basilar artery in vivo. Am J Physiol. 1993;264:H178–182. doi: 10.1152/ajpheart.1993.264.1.H178. [DOI] [PubMed] [Google Scholar]
- 50.Yang G, Iadecola C. Activation of cerebellar climbing fibers increases cerebellar blood flow: Role of glutamate receptors, nitric oxide, and cGMP. Stroke. 1998;29:499–507. doi: 10.1161/01.str.29.2.499. [DOI] [PubMed] [Google Scholar]
- 51.Shibuya M, Hirai S, Seto M, Satoh S, Ohtomo E. Effects of fasudil in acute ischemic stroke: Results of a prospective placebo-controlled double-blind trial. J Neurol Sci. 2005;238:31–39. doi: 10.1016/j.jns.2005.06.003. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.