Abstract
Purpose
The purpose of this secondary analysis was to determine change in overall Health-related Quality of Life (HRQOL) based on patient data obtained from NRG Oncology RTOG 0537 as measured by the RTOG-modified University of Washington Head and Neck Symptom Score (RM-UWHNSS).
Methods
A multi-site prospective randomized clinical trial design stratified 137 patients with post-radiation therapy xerostomia according to prior pilocarpine (PC) treatment and time after radiation therapy and/or chemotherapy and randomized patients into two groups. Patients were assigned to acupuncture or PC. Twenty-four sessions of acupuncture-like transcutaneous nerve stimulation (ALTENS) were administered over 12 weeks, or oral PC (5 mg) three times daily over the same 12 weeks. The RM-UWHNSS was administered at baseline and at 4, 6, 9 and 15 months after the date of randomization.
Results
There were no between-arm differences in change scores on the RM-UWHNSS in the individual items, total score, or factor scores. For statistical modeling, race and time were significant for all outcomes (total and factor scores), while treatment arm was not significant. The ALTENS arm showed greater yet non-significant improvement on outcomes compared to the PC arm.
Conclusion
Although no significant treatment differences were seen in this trial, patients receiving ALTENS consistently had lower scores, indicating better function, as compared to those receiving PC. Radiation-induced xerostomia improved over time for all patients.
Keywords: Radiation-induced xerostomia (RIX), symptom management, head & neck cancer, acupressure-like transcutaneous nerve stimulation (ALTENS)
Introduction
Xerostomia is a common symptom among head and neck cancer patients undergoing external beam radiation. Xerostomia occurs in about 65% [1] of head and neck cancer patients who receive external beam radiation. This symptom can be distressing and cannot be reversed. Cholinergic agonists such as pilocarpine (PC) have minimal benefit and significant side effects [2]. Post-radiation xerostomia has been shown to reduce health related quality of life (HRQOL) [1].
One non-invasive therapy that has potential to improve post-radiation xerostomia is acupuncture-like transcutaneous nerve stimulation (ALTENS) [3]. ALTENS is a non-invasive alternative to needle acupuncture that provides low-intensity stimulation to acupuncture points. Eliminating the requirement for invasive needling allows ALTENS treatments to be administered with minimal training, requiring primarily the knowledge of the location of the active acupuncture points.
The Radiation Therapy Oncology Group (RTOG) conducted a Phase II study of ALTENS for radiation-induced xerostomia wherein patients reported improved saliva production and a reduction in xerostomia symptoms, there was no significant change in HRQOL when compared with baseline data [3].The RTOG then completed a randomized trial of ALTENS for radiation-induced xerostomia, NRG Oncology RTOG 0537, which showed that ALTENS did not increase whole salivary production over that seen with PC [4].
However, the sensation of xerostomia is a complex phenomenon, and patients may benefit from ALTENS beyond just whole salivary production. Therefore, this is a secondary analysis of the change in overall HRQOL, as measured by the RTOG-modified University of Washington Head and Neck Symptom Score (RM-UWHNSS) for the NRG Oncology RTOG 0527 patients. Specifically assessed was the effect of treatment on the total and subscale score across time while adjusting for patient and clinical factors.
Materials and Methods
Eligibility criteria
Inclusion criteria were:
age ≥18 years;
completion of radiation (intensity modulated, IMRT or standard conformal) with or without chemotherapy 3 months to 2 years before study entry;
no evidence of head/neck disease recurrence and patients who were disease free from other invasive malignancies for at least 3 years prior to study entry;
reported grade 1 or higher xerostomia (CTCAE v3.0) with a residual basal WSP ≥ 0.1 ml per minute;
0 to 2 Zubrod performance status;
if receiving PC or cevimeline, were required to discontinue these medications at least 2 weeks prior to randomization.
Exclusion criteria included unstable cardiac disease, pacemaker in-situ, chronic obstructive pulmonary disease, respiratory illness requiring hospitalization, acute bacterial or fungal infection requiring intravenous treatments and pregnancy.
The study was reviewed and approved by the Institutional Review Board at the participating institutions. Written informed consent was obtained from all patients prior to randomization.
Study design
This phase II–III randomized clinical trial (RCT) was conducted comparing ALTENS with oral PC [5,6]. The phase II portion was designed to determine feasibility of delivery of ALTENS at multiple sites and measured preliminary efficacy. The phase III portion stratified patients according to prior PC treatment and time after radiation therapy and/or chemotherapy (Figure 1). Zelen’s treatment allocation scheme was used to balance patient factors other than institutions [7]. Within each stratum, patients were randomized in a 1:1 ratio to either ALTENS or PC treatment.
Fig. 1.
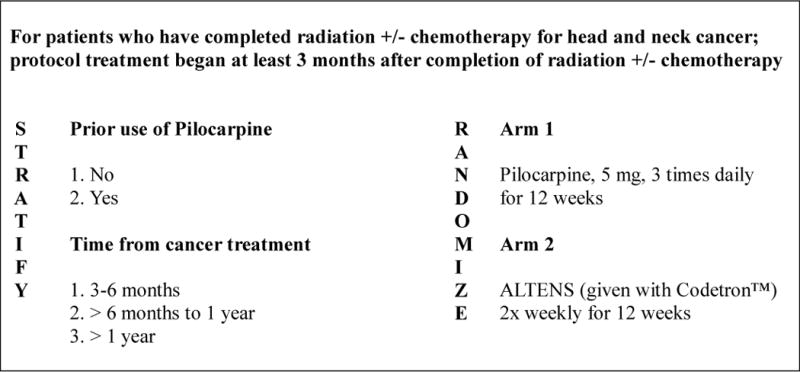
NRG Oncology/RTOG 0537 Study Schema
Instrument
Patient-reported HRQOL assessment was prospectively measured using the RM-UWHNSS (Appendix 1). It contains components of the original UWHNSS and additional questions assessing pain and mucous resulting in 15 total items. The UWHNSS is a self-administered, validated instrument designed for head and neck cancer patients with varying tumor sites and stages that has demonstrated responsiveness to clinical change [8]. The four major discriminant factors have been determined to be mucous amount and consistency, eating, pain, and activities. The RM-UWHNSS contains the employment question from UWHNSS version 1, all questions in the UWHNSS version 3 except for shoulder disability [9], and additional questions assessing mouth pain, throat pain, mucous amount, and mucous consistency. It has been used in previous RTOG clinical trials including RTOG 9901 and RTOG 0244. The item format for new items is modeled after the question stems for the original UWHNSS. Each question has five levels of functioning (Likert-type scale), ranging from no dysfunction to total dysfunction. For each question, patients are instructed to circle the statement that best describes their level of function during the past week. The RM-UWHNSS was administered at baseline and at 4, 6, 9 and 15 months after the date of randomization.
Intervention Arms
ALTENS Arm
ALTENS treatments were administered with a Codetron™ (model 902-C, EHM Rehabilitation Technologies Ltd., Ontario, Canada) trans-epidermal neural stimulator (TENS) unit and Karaya electrode pads. Bilateral acupuncture points: SP6, ST36, LI4 using negative electrodes and CV24 using the positive electrode were stimulated [3,5]. Sequences of 250 millisecond square pulses with a 4 Hz repetition rate were delivered. Each acupuncture point, except CV24, was stimulated for 10 seconds at a time. CV24, the site for the common electrode, was stimulated throughout the treatment session. Stimulation intensity (between level 3 to 6 on the machine) was adjusted to produce a deep strong aching sensation at each acupuncture point. Random switching among electrodes enabled by the Codetron™ embedded random circuit was employed to prevent brain habituation to stimulation [10].
ALTENS treatment was started within 14 days after study enrollment. All patients were scheduled for 24 ALTENS sessions (20 minutes each, two sessions per week), over 12 weeks. Two weeks without treatment was allowed and all outstanding sessions were administered in the remainder of the 12 week period, not to exceed three sessions per week. All treatments were delivered at RTOG participating academic and community-based institutions.
Staff administering the ALTENS received training at RTOG meetings. Slides of training materials and a training video were posted on the RTOG website. For each patient, photographs of electrode pad positions on the acupuncture points were sent electronically to the principal investigator for rapid approval before the third treatment session.
Pilocarpine (PC) Arm
PC is the most commonly used sialogogic agent approved by the FDA for RIX. Pilocarpine is a naturally occurring alkaloid that is a muscarinic-cholinergic agonist, and it causes stimulation of cholinergic receptors on the surface of the salivary exocrine glands, resulting in salivation [11].
For this study, the PC treatment started within 14 days of enrollment. Patients received 5 mg PC orally three times daily for 12 weeks and then stopped. There was no make-up for missed doses. Dose modification was permitted due to PC intolerance. Patients completed drug diaries and returned all medications for counting to determine treatment compliance.
Statistical considerations
Descriptive statistics were generated to characterize the study cohort. Patients who completed the 15-item RTOG-modified UWHNSS were compared to those who did not complete it. Respondents in the two arms were compared at each time point. Fisher’s exact test was used to compare categorical variables. Wilcoxon–Mann–Whitney test using the normal approximation and t-test were used to compare continuous variables depending on the normality of the data. RTOG-modified UWHNSS factor domain and total symptom scores were averaged using all items answered by the patient, similar to that of the validated tool. Specifically, for the total score, at most two missing items were permitted (B. Yueh, personal communication, 2012) while subscale scores required all items to be completed.
All item scores were transformed onto a scale from 20 to 100 with a score of 100 indicating poor HRQOL and a score of 20 indicating good HRQOL. Change scores were calculated by subtracting baseline scores from follow-up scores (follow-up – baseline). Thus a positive change score indicates a worsening HRQOL while a negative change score indicates an improvement. Change in individual question scores, factor domain scores, and total symptom score from baseline were evaluated at all follow-up time points (3, 6, 9, and 15 months). Graphs with 95% confidence intervals demonstrated the change in factor scores and total score over time for all patients. Following a previous analysis using the RTOG-modified UWHNSS, a 5 point difference in the mean change score was determined to be meaningful (Hoffman 2014) [12]. Potential floor and ceiling effects for deterioration status were evaluated using the 5 point difference.
A linear fixed-effect model, using maximum likelihood as the method of estimation with random intercepts and slopes, was constructed for the total symptom score and each factor score. Baseline score, time, and treatment arm were forced into the model as covariates. Time-by-treatment interaction, stratification factors and other baseline characteristics such as age (< 60 vs. ≥ 60 years old, Zubrod performance status (0 vs. 1, 2), gender (male vs. female), race (white vs. other), country (US vs. Canada), and prior chemotherapy (yes vs. no) were also considered for inclusion. Variables were retained in the model if ρ < 0.10.
To adjust for multiplicity while accounting for the correlated nature of the items and factors, alpha = 0.01 was used when testing the 15 items individually and the 4 factors. An alpha = 0.05 was used for all other tests, including the total score. All data were analyzed with SAS (v9.2 for Windows, SAS institute, Cary, NC) [13].
Results
To answer the Phase II portion of the study, the designed was feasible for delivery of ALTENS at multiple sites while measuring preliminary efficacy. Of these 146 eligible patients, 137 consented to participate in the HRQOL portion of the study and all of these patients completed the RM-UWHNSS at least once during the study (Figure 2). Pretreatment characteristics were similar between study arms (Table 1). Compliance for use of the tool was also relatively high with the lowest completion rate of 64.7% occurring at 15 months in the PC arm. Patients had similar baseline scores (Table 2).
Fig 2.

CONSORT Diagram
Table 1.
Pretreatment Characteristics of Patients who Consented to QOL
| Pilocarpine (n=68) |
ALTENS (n=69) |
P-value§ | |
|---|---|---|---|
| Age (years) | |||
| Median | 58.5 | 58 | 0.61† |
| Min – Max | 29 – 78 | 46 – 72 | |
| Q1 – Q3 | 52.5 – 63 | 53 – 65 | |
| Gender | |||
| Male | 59 (86.8%) | 59 (85.5%) | 0.99 |
| Female | 9 (13.2%) | 10 (14.5%) | |
| Race | |||
| American Indian or Alaska Native | 1 (1.5%) | 2 (2.9%) | 0.51 |
| Asian | 4 (5.9%) | 2 (2.9%) | |
| Black or African American | 5 (7.4%) | 2 (2.9%) | |
| White | 58 (85.3%) | 63 (91.3%) | |
| Ethnicity | |||
| Hispanic or Latino | 5 (7.4%) | 1 (1.4%) | 0.13 |
| Not Hispanic or Latino | 59 (86.8%) | 66 (95.7%) | |
| Unknown | 4 (5.9%) | 2 (2.9%) | |
| Zubrod Performance Status | |||
| 0 | 55 (80.9%) | 56 (81.2%) | 0.99 |
| 1 | 13 (19.1%) | 12 (17.4%) | |
| 2 | 0 (0.0%) | 1 (1.4%) | |
| Country of Residence | |||
| United States | 50 (73.5%) | 50 (72.5%) | 0.99 |
| Canada | 18 (26.5%) | 19 (27.5%) | |
| Prior Chemotherapy | |||
| No | 13 (19.1%) | 12 (17.4%) | 0.83 |
| Yes | 55 (80.9%) | 57 (82.6%) | |
| Time since RT +/− Chemotherapy* | |||
| 3–6 months ago | 18 (26.5%) | 18 (26.1%) | 0.99 |
| More than 6 months to 1 year ago | 26 (38.2%) | 27 (39.1%) | |
| 1–2 years ago | 24 (35.3%) | 24 (34.8%) | |
| Prior Use of Pilocarpine* | |||
| No | 58 (85.3%) | 58 (84.1%) | 0.99 |
| Yes | 10 (14.7%) | 11 (15.9%) |
Stratification factor;
P-value from fisher’s exact test
P-value from two-sided t-test assuming equal variances
Q1 = first quartile; Q3 = third quartile.
Table 2.
RTOG-Modified UWHNSS Baseline Score
| Pilocarpine | ALTENS | P-value§ | |
|---|---|---|---|
| Total Score | (n=65) | (n=60) | |
| Mean (Std. Dev.) | 47.9 (11.1) | 44.5 (10.1) | 0.23* |
| Median (Range) | 49.1 (25.5–72.7) | 43.6 (27.3–74.5) | |
| Mucus Factor Score | (n=65) | (n=63) | |
| Mean (Std. Dev.) | 54.6 (23.1) | 48.7 (24.7) | |
| Median (Range) | 60.0 (20.0–100.0) | 40.0 (20.0–100.0) | 0.22 |
| Eating Factor Score | (n=66) | (n=61) | |
| Mean (Std. Dev.) | 61.7 (14.7) | 59.3 (14.3) | |
| Median (Range) | 60.0 (30.0–90.0) | 55.0 (35.0–100.0) | 0.44 |
| Pain Factor Score | (n=66) | (n=64) | |
| Mean (Std. Dev.) | 30.4 (11.9) | 28.1 (10.9) | |
| Median (Range) | 26.7 (20.0–60.0) | 20.0 (20.0–60.0) | 0.32 |
| Activity Factor Score | (n=65) | (n=63) | |
| Mean (Std. Dev.) | 39.1 (14.3) | 37.1 (14.1) | |
| Median (Range) | 40.0 (20.0–70.0) | 40.0 (20.0–80.0) | 0.73 |
In answer to the phase III (primary aim) of this secondary analysis, there were no differences in change scores in the individual items, total score, or factor scores (results not shown) of the RM-UWHNSS. Due to the strong correlations between baseline and follow-up scores as well as the lack of any differences in baseline scores between treatment arms, baseline was included as part of the outcome variable in the longitudinal models rather than as a covariate.
For the statistical modeling of the total and subscale scores, race and time were significant for all outcomes (total and factors scores) while treatment arm was not significant (Table 3). Specifically, time had a negative effect, meaning that scores improved over time, as seen in Figure 3. White patients tended to have better scores than non-white patients for total score and all four factor scores. Patients with prior chemotherapy tended to demonstrate more dysfunction in terms of the total score and eating factor score than patients with no prior chemotherapy (estimate of 4.10, p=0.058 for total score; estimate of 6.03, p=0.034 for eating factor score). Patients with prior PC use had a better mucus factor score meaning less dysfunction (estimate = −8.95, p=0.023). Patients with a Zubrod of 1 or 2 tended to have a poorer activity score, or more dysfunction, than patients of Zubrod 0. There were minimal ceiling effects, but due to the large number of floor effects, deterioration was measured as decline vs. no decline since many patients were unable to improve. There were no significant differences in deterioration status for each item at any of the follow-up time points between the treatment arms (results not shown).
Table 3.
Fixed Effects Model
| Estimate (Std Error) | P-value* | |
|---|---|---|
| Total Score | ||
| Treatment (ALTENS vs. Pilocarpine) | −1.87 (1.66) | 0.260 |
| Time | −1.53 (0.23) | <0.001 |
| Prior Chemotherapy (Yes vs. No) | 4.10 (2.15) | 0.058 |
| Race (White vs. Other) | −10.04 (2.61) | <0.001 |
| Mucus Factor | ||
| Treatment (ALTENS vs. Pilocarpine) | −2.71 (2.89) | 0.349 |
| Time | −1.73 (0.64) | 0.008 |
| Race (White vs. Other) | −10.22 (4.59) | 0.027 |
| Prior Pilocarpine (Yes vs. No) | −8.95 (3.93) | 0.023 |
| Eating Factor | ||
| Treatment (ALTENS vs. Pilocarpine) | −2.33 (2.18) | 0.286 |
| Time | −2.61 (0.32) | <0.001 |
| Prior Chemotherapy (Yes vs. No) | 6.03 (2.83) | 0.034 |
| Race (White vs. Other) | −12.27 (3.45) | <0.001 |
| Pain Factor | ||
| Treatment (ALTENS vs. Pilocarpine) | −1.23 (1.68) | 0.464 |
| Time | −0.43 (0.21) | 0.049 |
| Race (White vs. Other) | −6.35 (2.65) | 0.017 |
| Activity Factor | ||
| Treatment (ALTENS vs. Pilocarpine) | −1.41 (2.09) | 0.845 |
| Time | −0.69 (0.28) | 0.017 |
| Race (White vs. Other) | −10.68 (3.39) | 0.002 |
| Zubrod (1,2 vs. 0) | 5.11 (2.73) | 0.062 |
P-value from t-test in comparison to the reference level
Bolded level is the Reference level.
Variables considered in model: age, Zubrod, gender, race, country, prior chemotherapy, time from end of prior therapy to registration, prior pilocarpine
Fig. 3.
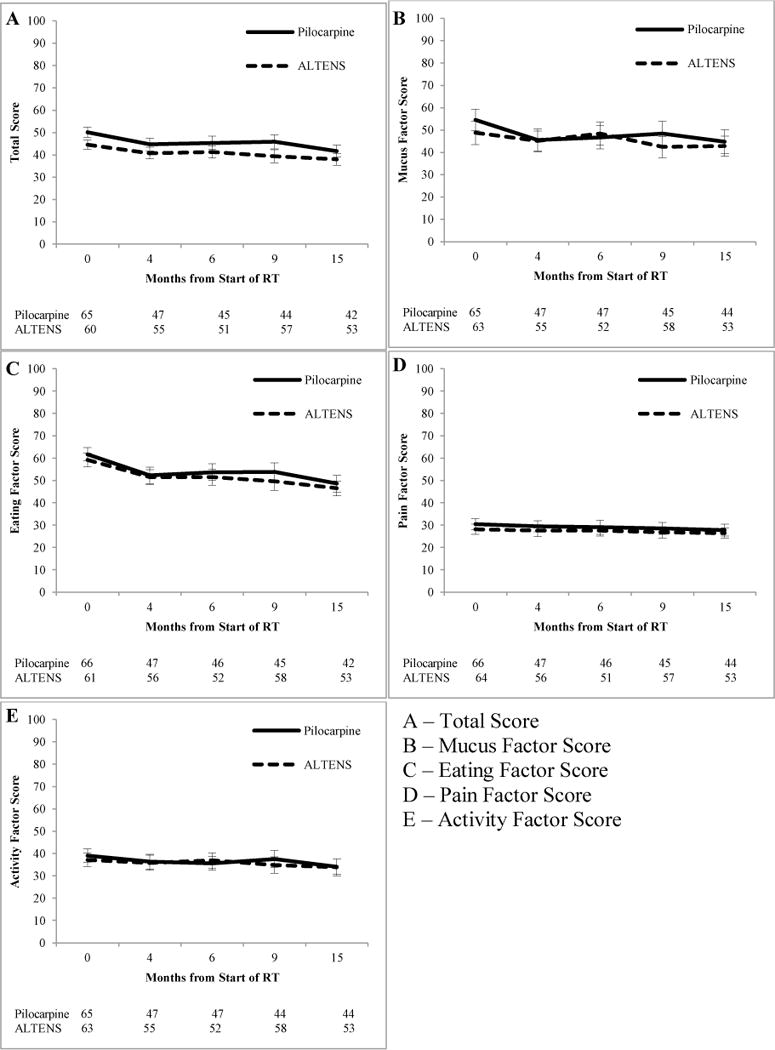
A Comparison of ALTENS vs. PC in Treating Radiation-Induced Xerostomia using the RM-UWHNSS
Discussion
Although no significant treatment differences were seen in this prospective phase III trial evaluating ALTENS vs. PC in treating radiation-induced xerostomia, patients receiving ALTENS consistently had lower scores on the RM-UWHNSS, indicating better function, as compared to those receiving PC (Figure 2). A similar trend was noted in the primary analysis of this trial using a different xerostomia measure, The University of Michigan Xerostomia-Related Quality of Life Scale (XeQOLS) (Wong, 2015). Patients in both arms had similar baseline scores, and no significant differences were found with respect to change from baseline or deterioration status for the total score and each factor score. RIX did improve over time for all patients. Finally, the consistent positive trends noted in the ALTENS arm may suggest ALTENS can enhance recovery of salivary function. Future research is warranted to examine this hypothesize.
This study is distinct from other studies addressing RIX because it was a large randomized trial that incorporated both the standard of care with PC and introduced acupressure-like ALTENS, plus it included formal patient-reported assessments [14–16]. It is of utmost importance to include patient-reported end points on symptom intervention trials [17].
One limitation was the lower than anticipated patient compliance. The total score of the RM-UWHNSS had only 61% statistical power to detect an effect size of 0.5 at 9 months. The number of patients who withdrew consent was 16 with 11 patients enrolled on the PC arm. A large contributing factor to missing data was the number of consent withdrawals, which were almost double in the PC arm. This was a substantial case of missing data, and contributed to the imbalance in evaluable patients between the arms. Patients appeared to be missing at random, but there may have been unaccounted differences between patients in the ALTENS and PC arms. Second, although the phase III portion of the protocol called for a sample size of 144 patients, only 103 were evaluated for the RTOG-modified UWHNSS end point; therefore, the lack of difference between ALTENS and PC may have been a result of insufficient sample size.
Despite the challenges of this study, ALTENS produced comparable HRQOL to PC. It is also important to note the non-invasive and non-medicating factors associated with ALTENS. Further, no side effects are noted with ALTENS. Anecdotally, study clinicians reported that more patients dropped out of the PC arm because they were looking for a non-medication intervention and preferred to not be inconvenienced by visits for standard of care medication and monitoring. When designing HRQOL studies, convenience and patient burden must be major considerations.
Given the considerable morbidity associated with RIX, efforts are still needed to more successfully intervene to prevent or diminish this incapacitating toxicity. While there are new initiatives on the horizon such as gland sparing RT, gene transfer and bone marrow cells, the discovery must go on for ways to improve salivary gland function [18,19]. Finally, symptom intervention trials must continue to include patient-reported outcomes since provider perceptions of RIX can differ from patient perception of symptom burden [20].
Acknowledgments
This project was supported by grants U10CA21661 (RTOG-Ops-Stat), U10CA180868 (NRG Oncology Operations), U10CA180822 (NRG Oncology SDMC), U10CA37422 (CCOP), UG1CA189867 (NCORP) from the National Cancer Institute (NCI).
Appendix 1–The Head and Neck Symptom Scale of the University of Washington Quality of Life Questionnaire (UWHNSS)

Footnotes
Conflict of Interest: Dr. Singh reports that his institution has received per-case reimbursement from RTOG. Dr. Yom reports a research grant from Genentech, Inc. and royalties from UpToDate, Inc.
Ethical approval: All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent: Informed consent was obtained from all individual participants included in the study.
Author Contributions
Conception and design: Drs. Wyatt, Pugh, Wong, Sagar, Yom, Berk
Data acquisition: Drs. Wong, Sagar, Singh, Koyfman, Nguyen-Tân, Yom, Cardinale, Sultanem Hodson, Krempl, Yeh, Berk, Ms. Lukaszczyk
Data analysis and interpretation: Drs. Wyatt, Pugh, Wong, Sagar, Yom, Berk
Drafting and revision of manuscript: Drs. Wyatt, Pugh, Wong, Sagar, Singh, Koyfman, Nguyen-Tân, Yom, Cardinale, Sultanem, Hodson, Krempl, Yeh, Berk, Ms. Lukaszczyk
Final approval of manuscript: Drs. Wyatt, Pugh, Wong, Sagar, Singh, Koyfman, Nguyen-Tân, Yom, Cardinale, Sultanem, Hodson, Krempl, Yeh, Berk, Ms. Lukaszczyk, Pugh, Wong, Sagar, Singh, Koyfman, Nguyen-Tân, Cardinale, Sultanem, Hodson, Krempl, Lukaszczyk, Yeh, Berk All authors agree to be accountable for all aspects of the work.
References
- 1.Dirix P, Nuytx S, Vander Poorten V, Delaere P, Van den Bogaert W. The influence of xerostomia after radiotherapy on quality of life: results of a questionnaire in head and neck cancer. Supportive Care in Cancer. 2008;16(2):171–179. doi: 10.1007/s00520-007-0300-5. [DOI] [PubMed] [Google Scholar]
- 2.Scarantino CLF, Swann RS, et al. Effect of pilocarpine during radiation therapy: Results of RTOG 97-09, a phase III randomized study in head and neck cancer patients. Journal of Support Oncology. 2006;4(5):252–258. [PubMed] [Google Scholar]
- 3.Wong R, Jones G, Sagar S, Babjak A, Whelan T. A Phase I-II study in the use of acupuncture-like transcutaneous nerve stimulation in the treatment of radiation-induced xerostomia in head-and-neck cancer patients treated with radical radiotherapy. International Journal of Radiation Oncology Biology Physics. 2003 Oct 1;57(2):472–480. doi: 10.1016/s0360-3016(03)00572-8. [DOI] [PubMed] [Google Scholar]
- 4.Wong R, Deshmukh S, Wyatt G, et al. Acupuncture-like Transcutaneous Electrical Nerve Stimulation versus Pilocarpine in Treating Early Radiation-Induced Xerostomia: Results of RTOG 0537 Phase 3 Study. International Journal of Radiation Oncology Biology Physics. 2015 doi: 10.1016/j.ijrobp.2015.01.050. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wong R, James J, Sagar S, et al. Phase 2 results from Radiation Therapy Oncology Group Study 0537: a phase 2/3 study comparing acupuncture-like transcutaneous electrical nerve stimulation versus pilocarpine in treating early radiation-induced xerostomia. Cancer. 2012 Sep 1;118(17):4244–4252. doi: 10.1002/cncr.27382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wong R, Deshmukh S, Wyatt G, et al. RTOG 0537 Phase 2/3 Study Comparing Acupuncture-Like Transcutaneous Electrical Nerve Stimulation (ALTENS) Versus Pilocarpine (PC) in Treating Early Radiation-Induced Xerostomia (RIX): Phase 3 Preliminary Analysis. International Journal of Radiation Oncology Biology Physics. 2013;87(2):S116. doi: 10.1016/j.ijrobp.2015.01.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zelen M. The randomization and stratification of patients to clinical trials. Journal of Chronic Disease. 1974;27(7–8):365–375. doi: 10.1016/0021-9681(74)90015-0. [DOI] [PubMed] [Google Scholar]
- 8.Hassan S, Weymuller E., Jr Assessment of quality of life in head and neck cancer patients. Head and Neck. 1993;15(6):485–496. doi: 10.1002/hed.2880150603. [DOI] [PubMed] [Google Scholar]
- 9.Weymuller E, Jr, Alsarraf R, Yueh B, Deleyiannis F, Coltera M. Analysis of the performance characteristics of the University of Washington Quality of Life instrument and its modification (UW-QOL-R) Archives of Otolaryngology: Head and Neck Surgery. 2001;127(5):489–493. doi: 10.1001/archotol.127.5.489. [DOI] [PubMed] [Google Scholar]
- 10.Pomeranz B, Niznik G. Codetron, a new electrotherapy device overcomes the habituation problems of conventional TENS devices. American Journal of Electromedicine. 1987;(First Quarter):22–26. [Google Scholar]
- 11.Dirix P, Nuyts S, Van den Bogaert W. Radiation-Induced Xerostomia in Patients with Head and Neck Cancer. Cancer. 2006;107:2525–2534. doi: 10.1002/cncr.22302. [DOI] [PubMed] [Google Scholar]
- 12.Hoffman K, Pugh S, James J, et al. The Impact of Concurrent Granulocyte Macrophage-Colony Stimulating Factor on Quality of Life in Head and Neck Cancer Patients: Results of the Randomized Placebo-Controlled Radiation Therapy Onocology Group 9901 Trial. Quality of Life Research. 2014;23:1841–1858. doi: 10.1007/s11136-014-0628-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.SAS Software . Version 9.2 of the SAS System for Windows. Copyright 2002–2003 SAS Institute Inc. 2008. [Google Scholar]
- 14.Kannan V, Bapsy P, Anantha N, Doval D, Vaithianathan H, Benumathy G. Efficacy and safety of granulcyte macrophage-colony stimulating factor (GM-CSF) on the frequency and severity of radiation mucositis in patients with head and neck carcinoma. International Journal of Radiation Oncology Biology Physics. 1997;37(5):1005–1010. doi: 10.1016/s0360-3016(97)00105-3. [DOI] [PubMed] [Google Scholar]
- 15.Chi K, Chen C, Chan W, Chow K, Chen S, Yen S. Effect of granulocyte-macrophage colony-stimulating factor on oral mucositis in head and neck cancer patients after cisplatin, fluorouracil, and leucovorin chemotherapy. Journal of Clinical Oncology. 1995;13(10):2620–2628. doi: 10.1200/JCO.1995.13.10.2620. [DOI] [PubMed] [Google Scholar]
- 16.Sprinzl G, Galvan O, de Vries A, Ulmer H, Gunkel A, Lukas P. Local application of granulocyte-macrophage colony stimulating factor (GM-CSF) for the treatment of oral mucositis. European Journal of Cancer. 2001;37(16):2003–2009. doi: 10.1016/s0959-8049(01)00170-8. [DOI] [PubMed] [Google Scholar]
- 17.Sloan JA, Berk L, Roscoe J, et al. Integrating patient-reported outcomes into cancer symptom management clinical trials supported by the National Cancer Institute-sponsored clinical trials networks. Journal of Clinical Oncology. 2007;25(32):5070–5077. doi: 10.1200/JCO.2007.12.7670. [DOI] [PubMed] [Google Scholar]
- 18.Delporte C, O’Connell B, Xinjun H, et al. Increased fluid secretion after adenoviral-mediated transfer of the aquaporin-1 cDNA to irradiated rat salivary glands. Proc Natl Acad Sci USA. 1997;94:3268–3278. doi: 10.1073/pnas.94.7.3268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lombaert I, Wierenga P, Kok T, et al. Mobilization of bone marrow stem cells by granulocyte colony-stimulating factor ameliorates radiation-induced damage to salivary glands. Clin Cancer Res. 2006;12:1804–1812. doi: 10.1158/1078-0432.CCR-05-2381. [DOI] [PubMed] [Google Scholar]
- 20.Sikorskii A, Wyatt G, Tamkus D, Victorson D, Rahbar M, Ahn S. Concordance between patient reports of cancer-related symptoms and medical records documentation. Journal of Pain and Symptom Management. 2012;44(3):362–372. doi: 10.1016/j.jpainsymman.2011.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]


