Lymphopenia accompanies some autoimmune diseases.1 Several studies,2,3 but not others,4,5 have suggested that lymphopenia occurs in treatment-naive multiple sclerosis (MS), so the issue remains unresolved. This is important since lymphopenia may identify an immunologically distinct subset of MS. Also, lymphopenia may emerge as a risk factor for serious viral infections of the brain during dimethyl fumarate treatment.2 We therefore embarked on a retrospective controlled study of pretreatment lymphopenia in relapsing MS.
Methods.
Data were collected retrospectively during an institutionally approved service evaluation of blood test monitoring of patients with relapsing MS in a regional MS service in Southampton, UK, over a 2-year period (2012–2014). McDonald criteria were used for MS diagnosis. The following data were collected: age, sex, comorbidities, type of relapsing MS, date of first MS symptom onset, number of functional systems affected, pretreatment and posttreatment lymphocyte counts and their dates, relapse date and severity (3 categories6), and date of treatment initiation. To calculate a relapsing disease severity index, mean relapse severity was multiplied by relapse rate. Control lymphocyte data were derived from preoperative blood counts of age- and sex-matched individuals undergoing septoplasty in the same hospital for structural reasons, excluding neoplastic and infective operative indications. Lymphopenia was classified according to CTCAE (Common Terminology Criteria for Adverse Events) version 4. Statistical analysis was conducted in SPSS version 22 (IBM Corp., Armonk, NY). Null hypotheses were rejected at p < 0.05.
Results.
Seven hundred sixty-four patients were identified with blood test data (table). Baseline and posttreatment blood tests were available in 466 and 247 patients, respectively. Average blood test frequency was 4 per year. Lymphocyte counts were relatively stable with time, with a coefficient of variation of 7.5%.
Table.
Characteristics of the study populations
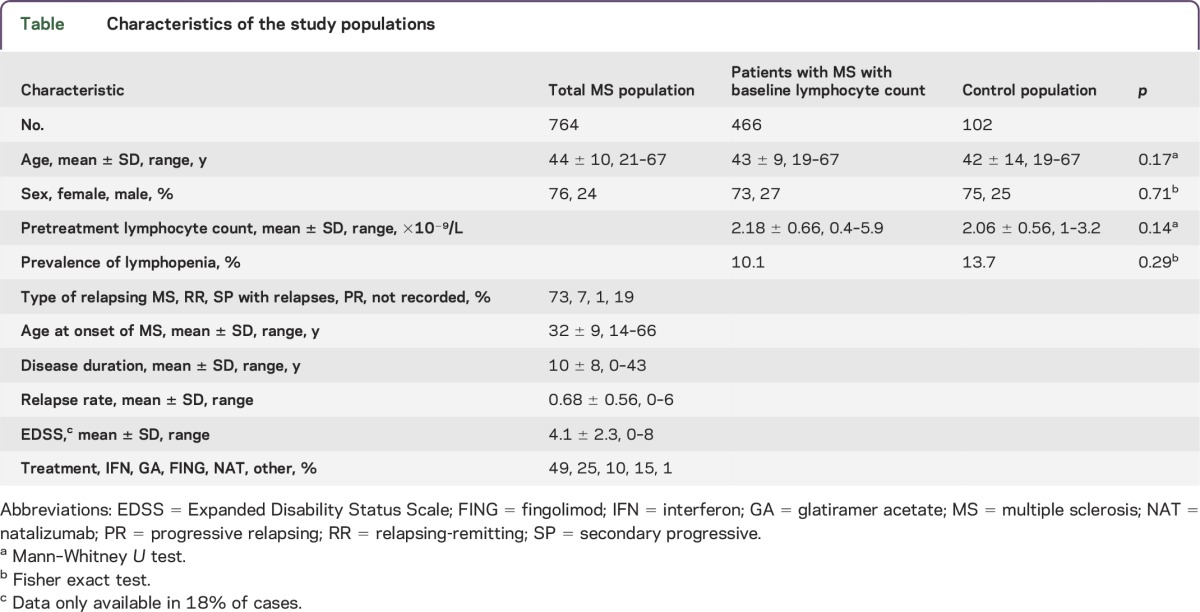
The mean lymphocyte count in treatment-naive patients with MS was 2.18 × 109/L with an SD of 0.66 × 109. Lymphopenia was present in 10% (48 patients; 46 grade I, one grade II, one grade III). A detailed retrospective review of the medical records of all patients with lymphopenia was undertaken to look for recognized causes of lymphopenia.1 In only 3 cases steroids were administered in the month before lymphopenia; one case with borderline baseline lymphocytes developed a grade III lymphopenia 2 days after steroids, while blood tests before steroids were unavailable in 2 cases with a grade I lymphopenia.
There was no association between pretreatment lymphocyte count and any patient characteristic (age, sex, MS type, autoimmune comorbidities, age at onset of first MS symptom, disease duration, time since last relapse, number of functional systems affected, relapse rate, last relapse severity, and severity index) or month or season (using correlation coefficients, group comparison tests, and linear or logistic regression). In the UK, during the study period, patients with higher disease activity (≥2 disabling relapses in 1 year) received natalizumab, and treatment options for lower disease activity included interferon beta, glatiramer acetate, and fingolimod. This binary treatment destination was used as a marker of disease activity. In a multivariate logistic regression, pretreatment lymphocyte count or lymphopenia did not predict natalizumab use, while younger age and higher relapsing disease severity index did (odds ratios of 0.9 and 25, respectively, p < 0.0001).
We compared the lymphopenia observed in the pretreatment MS population with an age- and sex-matched control hospital population undergoing cosmetic septoplasty who had preoperative blood tests on the same hematology analyzer. There was no statistical difference in mean lymphocyte count or prevalence of lymphopenia (table).
After treatment with interferon beta or glatiramer acetate, the prevalence of sustained lymphopenia increased to 28% (26% grade I, 2% grade II). Lymphocytes decreased after starting interferon beta (mean decrease of 0.3 × 109/L, p < 0.0001, paired t test), but not glatiramer acetate. Multiple logistic regression identified low pretreatment lymphocyte count as the only predictor of posttreatment lymphopenia (odds ratio of 0.1, p = 0.001).
Fingolimod caused a lymphopenia in all patients (range: 0.3–1.4 × 109/L; median decrease of 1.5 × 109/L compared to baseline, p < 10−6, Wilcoxon); using multiple linear regression, pretreatment lymphocyte count was the only predictor of posttreatment count (R2 = 0.22, B = 0.1, p = 0.003). Natalizumab decreased lymphocyte count (median decrease of 1.3 × 109/L compared to baseline, p < 10−14, Wilcoxon; lymphocytosis >4 × 109/L in 23% vs 0%).
Discussion.
Since the lymphocyte reference range covers 95% of values in a healthy population, lymphopenia is expected in 2.5%.1 In our treatment-naive relapsing MS population, we found lymphopenia in 10%. However, this was not different from a well-matched healthy control population. Moreover, lymphopenia was not associated with relapsing activity. Hence, the lymphopenia in patients with MS is unlikely to be related to autoimmunity. A more likely explanation is stress-induced lymphopenia in both cohorts, through cortisol or Epstein-Barr activation.7
We found that pretreatment lymphopenia predicts posttreatment lymphopenia; this is useful since it identifies at-risk patients needing frequent monitoring. Because of this study's retrospective nature, lymphocyte subsets were not available, and these are important.4,5 Further work is needed to determine whether lymphocyte subsets during lymphopenia differ in patients with MS vs controls.
Footnotes
Author contributions: Conceived and designed study: I. Galea. Performed the data collection: Z. Lim, E. Elwood, H. Naveed, I. Galea. Analyzed the data: Z. Lim, I. Galea. Wrote the manuscript: Z. Lim, E. Elwood, I. Galea.
Study funding: This study was supported by funding from the University of Southampton.
Disclosure: Z.W. Lim, E. Elwood, and H. Naveed report no disclosures. I. Galea received travel funding from Teva-UK, received research support from Evgen, Merck Serono, Spire, Engineering and Physical Sciences Research Council UK, Medical Research Council UK, Wessex Medical Research, NIHR Clinical Research Network Wessex, Royal College of Surgeons of Edinburgh, University of Southampton, MS Society, Association of British Neurologists, Smile4Wessex. Go to Neurology.org/nn for full disclosure forms. The Article Processing Charge was paid by The University of Southampton.
References
- 1.Brass D, McKay P, Scott F. Investigating an incidental finding of lymphopenia. BMJ 2014;348:g1721. [DOI] [PubMed] [Google Scholar]
- 2.Longbrake EE, Naismith RT, Parks BJ, Wu GF, Cross AH. Dimethyl fumarate-associated lymphopenia: risk factors and clinical significance. Mult Scler J Exp Transl Clin 2015;1. pii: 2055217315596994. Epub 2015 Jul 31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rieckmann P, O'Connor P, Francis GS, Wetherill G, Alteri E. Haematological effects of interferon-beta-1a (Rebif) therapy in multiple sclerosis. Drug Saf 2004;27:745–756. [DOI] [PubMed] [Google Scholar]
- 4.Habib J, Deng J, Lava N, Tyor W, Galipeau J. Blood B cell and regulatory subset content in multiple sclerosis patients. J Mult Scler 2015;2. pii: 1000139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Puissant-Lubrano B, Viala F, Winterton P, Abbal M, Clanet M, Blancher A. Thymic output and peripheral T lymphocyte subsets in relapsing–remitting multiple sclerosis patients treated or not by IFN-beta. J Neuroimmunol 2008;193:188–194. [DOI] [PubMed] [Google Scholar]
- 6.NHS England. Clinical commissioning policy: disease modifying therapies for patients with multiple sclerosis [online]. Available at: http://www.england.nhs.uk/wp-content/uploads/2013/10/d04-p-b.pdf. Accessed June 28, 2016.
- 7.Coskun O, Sener K, Kilic S, et al. . Stress-related Epstein-Barr virus reactivation. Clin Exp Med 2010;10:15–20. [DOI] [PubMed] [Google Scholar]


