Abstract
Background
The European Randomized study of Screening for Prostate Cancer (ERSPC) has shown a 21% reduction in prostate cancer (PC) mortality and a 1·6-fold increase in PC incidence with PSA-based screening (at 13 years of follow-up). We evaluated PC incidence by risk category at diagnosis across the arms in order to assess the potential impact on PC mortality.
Design, setting, and participants
Information on arm, centre, T and M stage, Gleason score, serum PSA at diagnosis, age at randomization, follow-up time and vital status were extracted from the ERSPC database. Four risk categories at diagnosis were defined: 1-low, 2-intermediate, 3-high, 4-metastatic disease. PSA (<=100 / >100) was used as the indicator of metastasis.
Outcome measurements and statistical analysis
Incidence rate ratios (RR) for screening versus control arm by risk category at diagnosis and follow-up time were calculated using Poisson regression analysis for 7 centres. Follow-up was truncated at 13 years. Missing data were imputed using chained equations. The analyses were carried out on an intention-to-treat basis.
Results and limitations
7,408 PC cases were diagnosed in the screening and 6,107 in the control arm. Proportion of missing stage, Gleason or PSA was comparable in the two arms (8% versus 10%), but differed between centres. The RRs were elevated in the screening arm for low (2·14, 95% CI: 2·03-2·25) and intermediate risk categories at-diagnosis (1·24, 95% CI: 1·16-1·34), equal to unity for the high risk category at-diagnosis (1·00, 95%CI: 0·89-1·13) and reduced for metastatic disease at diagnosis (0·60, 95% CI: 0·52-0·70). The RR of metastatic disease had a similar temporal pattern as mortality, shifted forward an average of almost 3 years, though the mortality reduction was smaller.
Conclusions
The results confirm a reduction of metastatic disease at diagnosis in the screening arm, preceding mortality reduction by almost three years.
Patient summary
These findings indicate that the decrease of metastatic disease at diagnosis is the major determinant of the PC mortality reduction in the ERSPC trial.
Funding
Each centre had its own funding responsibility.
Introduction
An update to the European Randomized study of Screening for Prostate Cancer (ERSPC) has recently confirmed the reduction of prostate cancer (PC) mortality within the ERSPC screening arm invited to prostate-specific antigen (PSA) testing.1 The validity of the result has been questioned2,3 based mainly on the differences in treatment between the arms, ignoring the difference in disease characteristics at presentation.4 Previous evaluations of treatment and different methods of mortality analysis,5-8 however, have shown that differences in treatment are unlikely to explain the impact of screening on mortality. Nevertheless, detailed analysis of key prognostic features of PC across the study arms is worthwhile to better understand how the mortality difference has emerged.
In principle,9 assuming that the cancer staging system is consistent over the compared groups, the requirements of an effective screening programme are:
-
a)
an ability to produce a stage migration (‘down-staging’) in the screening arm;
-
b)
a stage migration accompanied by a decrease in the incidence of advanced cancers;
-
c)
a decrease in advanced stage incidence occurring before a mortality reduction in the screening arm.
Meeting these requirements would not mean that early diagnosis had been the cause (or the only cause) of any observed reduction in mortality; yet, if these requirements were not met, it would be unlikely that screening could explain any observed mortality reduction.
A reduction in the risk of diagnosis of metastatic disease in the screening arm of four ERSPC centres was shown in a previous work.10 That paper addressed the occurrence of metastatic disease both at diagnosis and in the post-treatment follow-up.
In the current study, we present up-dated results on the stage distribution by arm in 7 ERSPC centres. We evaluate whether the above mentioned requirements were met and explore the association of the decrease of advanced cancer incidence rates (if any) with the reduction in PC mortality.
Materials and Methods
The ERSPC is a multi-centre, randomized screening trial comparing an intervention arm of men to whom regular PSA screening is offered with a control arm to which such screening is not offered. The trial started in Belgium, the Netherlands, Finland, Sweden, Spain, Italy, Switzerland and France during the period 1993-1998.11 The study methodology has been described in detail previously.12 Data were obtained by linkage to local cancer registries for PC incidence and national registries for overall mortality. PC deaths were ascertained by local, independent cause-of-death committees.13 Data on trial arm, centre, T and M stage,14 Gleason score, serum PSA at diagnosis, age at randomization, follow-up time (until December 31, 2010) and vital status were extracted from the trial database.
We selected men aged 55 to 69 years (the core age group), excluding those who died, were diagnosed with PC or emigrated between randomization and screening invitation (N=145). The median follow-up for the two French centres, Herault and Tarn, was 6 to 7 years and, therefore, too short for their data to be included in this analysis.
The risk classification adopted in the last ERSPC analysis1 was used. Four risk categories at diagnosis were defined using information available at diagnosis: low risk (category 1) as clinical stage T1-2 and Gleason score <= 6; intermediate risk (2) as T1-2 and Gleason score = 7 or T3 and Gleason score <= 7; high risk (3) as T1-3 and Gleason score = 8-10 or T4 and any Gleason score; and the metastatic disease category (4) as M1 or PSA >100 ng/mL. Data on disease progression and metastasis status during the entire period of post diagnosis follow-up have already been published for those centres for which information was available.10 In the present analysis only information on risk category at diagnosis, available for all centres, is considered.
The accuracy of risk category definition was evaluated by a sensitivity analysis, restricting the evaluation of metastasis to M1 cases only.
We imputed missing data for the variables used in defining the risk categories at diagnosis using multiple imputations by the technique of chained equations (MICE).15 Briefly, the MICE uses the distribution of observed data to identify a set of plausible values for the missing data, by incorporating a random component to reflect the uncertainty of the estimate. Several data-sets are generated and the same analysis is conducted on each data-set with the aim of obtaining a set of parameter estimates that are combined in a single overall estimate (computed as the average of the estimates in each simulated dataset) with relative standard errors (computed including the between and within imputation variance) and confidence intervals.16
For the incomplete variables (T and M stage, Gleason score, and PSA level), we derived imputation models that included the complete variables - arm, centre, vital status and follow-up time, age at randomization and interactions between each variable and trial arm. The interactions between each variable and trial arm were included in order to incorporate potential differences among centres in the mechanism causing the missing data. Ten final data-sets were generated after the algorithm converged. In the literature on multiple imputation, between 3 and 5 imputed data-sets is considered adequate.17 The estimates were combined using Rubin’s rules.18 We conducted a sensitivity analysis by imputing directly the risk categories at diagnosis. We used version 12 of the STATA software, StataCorp, College Station, TX, USA. PC incidence rate ratios (RR) by centre and risk categories at diagnosis were calculated for the screening versus control arms using Poisson regression analysis. Person-years were calculated as time from randomization to PC diagnosis, death or end of follow-up, whichever came first. We adjusted all the analyses for the randomisation ratio 1:1·5 screening versus control arm in Finland. Primary analyses were carried out on an intention-to-treat (intention-to-screen) basis, i.e. men were included in the analysis in the arm they were randomly assigned to, regardless of compliance. Moreover, to provide a sensitivity analysis, we split the cumulative occurrence within the screening arm according to screening status defining subjects who underwent at least the first screening, after first invitation, test as attendees.
Complete case analysis (before missing data imputation) and all-case analysis (after data imputation) are presented.
Results
In the core age group, 162,338 men were randomized. The median age at randomization was 60·2 years.
With data truncated at 13·0 years of follow-up 7,408 and 6,107 PC cases were diagnosed in the screening and control arms, respectively. Overall, the distribution of missing values was rather similar in the two arms (8% in the screening and 10% in the control arm), but in some centres (Belgium, Italy, and Spain) data were missing for between 38% and 77% of cases and there was an imbalance between the arms (of at least 10 percentage points) (Table 1).
Table 1.
Numbers of randomized subjects and prostate cancer cases (total and by risk category) by centre.
| SCREENING ARM (Rand. age: 55-69 y.) | CONTROL ARM (Rand. age: 55-69 y.) | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Risk category | Risk category | |||||||||||||
| Centre | Rando- mized men |
PC cases | 1 (Low) |
2 (Intermediate) |
3 (High) |
4 (Metastatic) |
Missing | Rando- mized men |
PC cases | 1 (Low) |
2 (Intermediate) |
3 (High) |
4 (Metastatic) |
Missing |
| Netherlands | 17,443 | 2180 (100%) | 1447 (66%) | 472 (22%) | 126 (6%) | 39 (2%) | 96 (4%) | 17,390 | 1070 (100%) | 481 (45%) | 290 (27%) | 143 (13%) | 125 (12%) | 31 (3%) |
| Belgium | 4,307 | 417 (100%) | 85 (20%) | 28 (7%) | 10 (2%) | 14 (3%) | 280 (67%) | 4,255 | 321 (100%) | 25 (8%) | 5 (2%) | 8 (2%) | 11 (3%) | 272 (85%) |
| Sweden | 5,901 | 738 (100%) | 525 (71%) | 139 (19%) | 27 (4%) | 32 (4%) | 15 (2%) | 5,951 | 469 (100%) | 208 (44%) | 132 (28%) | 35 (7%) | 59 (13%) | 35 (7%) |
| Finland | 31,970 | 3018 (100%) | 1822 (60%) | 761 (25%) | 269 (9%) | 149 (5%) | 17 (1%) | 48,409 | 3609 (100%) | 1619 (45%) | 1165 (32%) | 419 (12%) | 361 (10%) | 45 (1%) |
| Italy | 7266 | 396 (100%) | 142 (36%) | 77 (19%) | 42 (11%) | 10 (3%) | 125 (32%) | 7,251 | 289 (100%) | 41 (14%) | 32 (11%) | 27 (9%) | 12 (4%) | 177 (61%) |
| Spain | 1,056 | 87 (100%) | 40 (46%) | 15 (17%) | 4 (5%) | 2 (2%) | 26 (30%) | 1,141 | 52 (100%) | 9 (17%) | 9 (17%) | 3 (6%) | 3 (6%) | 28 (54%) |
| Switzerland | 4,948 | 572 (100%) | 381 (67%) | 133 (23%) | 41 (7%) | 6 (1%) | 11 (2%) | 4,955 | 297 (100%) | 160 (54%) | 78 (26%) | 32 (11%) | 15 (5%) | 12 (4%) |
| Herault | 28,793 | 1196 (100%) | 433 (36%) | 269 (22%) | 40 (3%) | 51 (4%) | 403 (34%) | 28,869 | 1094 (100%) | 377 (34%) | 241 (22%) | 33 (3%) | 47 (4%) | 396 (36%) |
| Tarn | 10,879 | 559 (100%) | 248 (44%) | 192 (34%) | 74 (13%) | 21 (4%) | 24 (4%) | 10,470 | 506 (100%) | 207 (41%) | 193 (38%) | 71 (14%) | 14 (3%) | 21 (4%) |
| Total | 112,563 | 9163 (100%) | 5126 (56%) | 2086 (23%) | 633 (7%) | 324 (4%) | 997 (11%) | 128,691 | 7707 (100%) | 3127 (41%) | 2145 (28%) | 771 (10%) | 647 (8%) | 1017 (13%) |
|
Total (ex. Fr*) |
72,891 | 7408 (100%) | 4442 (60%) | 1625 (22%) | 519 (7%) | 252 (3%) | 570 (8%) | 89,352 | 6107 (100%) | 2543 (42%) | 1711 (28%) | 667 (11%) | 586 (10%) | 600 (10%) |
Herault and Tarn (French centres) excluded.
Imputation did not materially affect the results (Table 2) and therefore the results based on imputed data are shown henceforth.
Table 2.
Cumulative incidence rate ratio (RR) by risk category for the original data and after data imputation
| Original data | After data imputation | |||||||
|---|---|---|---|---|---|---|---|---|
| Risk Category | Screening | Control | RR (95%CI) | Screening | Control | RR (95%CI) | ||
| 1 LOW Risk | 4,442 | 60% | 2,543 | 42% | 2·29 (2·18 ; 2·42) | 65% | 47% | 2·14 (2·03 ; 2·25) |
| 2 INTERMEDIATE Risk | 1625 | 22% | 1711 | 28% | 1·27 (1·18 ; 1·37) | 24% | 30% | 1·24 (1·16 ; 1·34) |
| 3 HIGH Risk | 519 | 7% | 667 | 11% | 1·02 (0·90 ; 1·15) | 8% | 12% | 1·00 (0·89 ; 1·13) |
| 4 M1 And/Or Psa 100+ Risk | 252 | 3% | 586 | 10% | 0·56 (0·48 ; 0·65) | 4% | 11% | 0·60 (0·52 ; 0·70) |
| 5 Missing Values | 570 | 8% | 600 | 10% | 1·01 (0·90 ; 1·13) | - | - | |
| Total | 7,408 | 100% | 6,107 | 100% | 1·56 (1·50 ; 1·62) | 100% | 100% | 1·56 (1·51 ; 1·62) |
The RRs (screening versus control arm) for the low and intermediate risk categories at diagnosis were significantly elevated (RR=2·14, 95% CI: 2·03-2·25 and 1·24 95% CI: 1·16-1·34, respectively) and for the high risk category it was unity (RR=1·00, 95% CI: 0·89-1·13). The RR was significantly reduced by 40% for the metastatic disease category (RR=0·60, 95% CI: 0·52-0·70).
The reduction was even greater when restricting the evaluation to M1 cases only (RR=0·50 (0·43 ; 0·57)).
The absolute excess in the low risk category was larger than the deficit of advanced disease: the incidence rate difference per 1000 randomized men (IRD) for the low risk category was 34·26 (95% CI: 31·99; 36·52). The IRD for the intermediate, high and metastatic risk categories at diagnosis were 4·26 (95% CI: 2·74; 5·79), 0·47 (95% CI: −1·09; 0·74) and −3·14 (95% CI: −3·93; −2·35), respectively.
The RRs (screening versus control arm) for the low and intermediate risk categories dropped strongly and consistently with follow-up. The high RRs in the first 3 years were associated with the prevalence round and overdiagnosis. There was a sharp decrease of RR in the high risk category within the first six years, and a further reduction thereafter as the risk converged with the control arm. A slight decrease of RR was evident until the 9th year for the metastatic disease category but afterwards it remained constant (Table 3).
Table 3.
Cumulative incidence rate ratios (RR) by risk category and periods from randomization after data imputation. Incidence Rate difference (IRD) per 1000 randomized men.
| Time since randomization (years) | ||||||
|---|---|---|---|---|---|---|
| 0-3 years | 0-6 years | 0-9 years | 0-11 years | 0-13 years | 0-13 years | |
|
|
||||||
| Risk category | RR (95%CI) | RR (95%CI) | RR (95%CI) | RR (95%CI) | RR (95%CI) | IRD (95%CI) |
| 1 LOW risk | 3·82 (3·37 ; 4·33) | 3·09 (2·86 ; 3·34) | 2·56 (2·4 ; 2·72) | 2·22 (2·10 ; 2·34) | 2·14 (2·03 ; 2·25) | 34·26 (31·99 ; 36·52) |
|
| ||||||
| 2 INTERMEDIATE risk | 3·15 (2·57 ; 3·87) | 2·02 (1·79 ; 2·28) | 1·55 (1·42 ; 1·69) | 1·35 (1·25 ; 1·47) | 1·24 (1·16 ; 1·34) | 4·26 (2·74 ; 5·79) |
|
| ||||||
| 3 HIGH risk | 1·74 (1·3 ; 2·34) | 1·36 (1·12 ; 1·65) | 1·19 (1·02 ; 1·38) | 1·07 (0·93 ; 1·22) | 1·00 (0·89 ; 1·13) | 0·47 (−1·09 ; 0·74) |
|
| ||||||
| 4 M1 and/or psa 100+ | 0·94 (0·70 ; 1·27) | 0·74 (0·60 ; 0·9) | 0·63 (0·53 ; 0·74) | 0·61 (0·52 ; 0·71) | 0·60 (0·52 ; 0·70) | −3·14 (−3·93 ; −2·35) |
|
| ||||||
| Total | 3·07 (2·83 ; 3·34) | 2·36 (2·23 ; 2·49) | 1·91 (1·83 ; 1·99) | 1·66 (1·60 ; 1·72) | 1·56 (1·51 ; 1·62) | 3·44 (3·16 ; 3·72) |
A sharper contrast was seen between the arms for the entire 13 year period when only the attendees were used to calculate the RRs - 2·45 (95% CI: 2·32; 2·58) for low risk, 1·32 (95%CI: 1·23 ; 1·43) for intermediate risk , 1·00 (95% CI: 0·88; 1·14) for high risk PC, and 0·45 (95%CI: 0·38; 0·53) for metastatic disease at diagnosis.
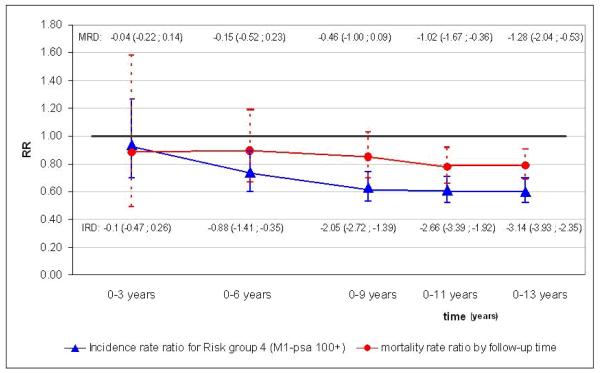
The reduction in cumulative (0-13 years) RRs (screening versus control arm) for metastatic disease at diagnosis (RR = 0·60) is clear and evident within the first six years of follow-up, while in mortality the reduction is smaller (RR=0·79) and emerges roughly three years later. (Figure 1).
Figure 1.
Cumulative incidence rate ratios for risk category 4 (after data imputation) and prostate cancer mortality rate ratio by time since randomization, 95% confidence interval. IRD = Incidence Rate difference per 1000 randomized men, MRD = Mortality Rate difference per 1000 randomized men.
Discussion
In the present study we have examined the incidence rates of PC by risk category at diagnosis between the two arms of the ERSPC trial.
Overall, the net effect in the screening arm was a marked increase of the cumulative incidence of low risk PC, along with a significant reduction of cumulative incidence of metastatic disease at diagnosis. A slight increase in the intermediate risk category and no difference in the high risk category (with an early excess disappearing by year 10) were observed. When interpreting these results, we must keep in mind that early detection resulting in a stage migration is expected to increase early disease with corresponding decreases in advanced disease.
In this study, the reduction found for metastatic disease at diagnosis was not reflected as an excess in the high risk category, but increased risk was found only in the intermediate and, above all, the low risk categories. The absolute excess in the low risk category was substantially larger than the deficit of advanced disease: the difference reflects the excess of incidence in the screening arm which was reported in the aforementioned update to the ERSPC trial along with the conclusion that one PC death is averted for 27 excess cases detected in 13 years of follow-up.1
In order to avoid potential biases due to the imbalance of the proportions of missing data in the two arms and among centres, we used multiple imputation. As the results did not change considerably with imputation, results with imputed data are shown because they are more comprehensive and precise.
The RRs for the screening versus control arms decreased with follow-up time both overall and for each risk category at-diagnosis, though not in a similar fashion. Cumulative incidence for low-risk PC was 3 times higher in the screening arm than in the control arm during the first 3 years of follow-up, and then fell off steadily to an incidence twice as high in the screening arm in the overall period of 13 years. The RRs for intermediate and high risk categories also decreased over time. In contrast, a decrease in the cumulative incidence for the metastatic disease category was not evident in the first 3 years of follow-up (RR=0·94) but thereafter it decreased until the 9th year (RR=0·63) and then remained stable.
The pattern described above was reinforced for those who were attendees. For example, the RR (screening versus control arm) for metastatic disease at diagnosis was 0·45. Obviously, a comparison of attendees with the entire control arm could be biased because of a difference in underlying risk due to selection bias. Nevertheless, such a comparison confirms that PSA-based early diagnosis determined a decrease of metastatic disease at diagnosis.
The current result is consistent with an earlier analysis10 in showing a reduction in metastatic disease at diagnosis, although the magnitude of effect is slightly smaller in the current analysis with data from a larger number of centres and longer follow-up and data imputation. In the previous paper, a relative reduction of 50% of metastatic disease at diagnosis was reported (RR=0·50, 95%C I 0·40; 0·62).
In the present paper, we had no information on disease progression after diagnosis. It is not sufficient for screening to increase detection of early disease, but it should affect the course of the disease so that deaths are averted or postponed by early treatment. This requires that there is no compensatory increase in disease progression after diagnosis. In the earlier paper, no differences were reported in metastatic disease emerging during the follow-up period (after diagnosis) although a slight, but statistically non-significant, increase was seen in the post-diagnosis incidence of metastasis in the screening arm (RR=1·16, 95% CI 0·9-1·47).10 The lack of further reduction in development of metastatic disease after diagnosis is not surprising given the higher incidence of low and intermediate risk PC in the screening arm and the lack of any difference in high risk PC between the two arms. Hence, a similar occurrence of metastatic disease after diagnosis seems plausible, likely indicating that early diagnosis and treatment could not stop disease progression in all cases. The lack of reduction in incidence of metastasis after diagnosis is in agreement with the smaller reduction of mortality (RR=0·79) than in incidence of metastatic PC (RR=0·60).
Limitations
Two potential limitations regarding the risk categories at diagnosis adopted in the present analysis should be considered.
First, we use a rather crude classification, based only on Gleason and clinical stage, including PSA > 100 ng / mL just as a criterion for metastasis. The choice was driven by a need to use the classification utilized in the last ERSPC analyses.1,19 Moreover PSA data for the control arm are incomplete and therefore unusable for finer groups’ definition. The accuracy of such a definition was evaluated by a sensitivity analysis and when restricting the evaluation to M1 cases only the reduction was even higher. The lower value is probably associated with the higher completeness of PSA information in the screening arm. The assumption of consistency in the cancer staging system over the compared groups is plausible in a randomized trial because a similar distribution by stages in the two arms would be expected in the absence of screening. We used the staging assigned by population-based cancer registries. Further information on staging and treatment were recovered from medical records in a non differential way for all cases in both arms.
Second, consistency in cancer staging presumably changed over time. Modifications are due to trends in pathology reporting but we have assumed that this phenomenon doesn’t affect the comparability between arms because there is no reason to suspect it would be differential by study assignment.
Conclusion
The present results confirm a stage migration in the screening arm with a 40% reduction in metastatic disease at diagnosis, which preceded a mortality reduction by almost three years.
These results strongly suggest that a decrease of metastatic disease at diagnosis is a major determinant of the reduction of PC mortality in the ERSPC trial, although we cannot exclude additional contributions from other factors.
References
- 1.Schröder FH, Hugosson J, Roobol MJ, et al. Screening and prostate cancer mortality: results of the European Randomised Study of Screening for Prostate Cancer (ERSPC) at 13 years of follow-up. Lancet. 2014 doi: 10.1016/S0140-6736(14)60525-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Miller AB. New Data on Prostate-Cancer Mortality after PSA Screening. N Engl J Med. 2012;366:1047–8. doi: 10.1056/NEJMe1200185. [DOI] [PubMed] [Google Scholar]
- 3.Haines IE, Gabor Miklos GL. Prostate-specific antigen screening trials and prostate cancer deaths: the androgen deprivation connection. J Natl Cancer Inst. 2013;105:1534–9. doi: 10.1093/jnci/djt248. [DOI] [PubMed] [Google Scholar]
- 4.Carlsson S, Roobol MJ, Schröder FH, Hugosson J, Auvinen A. RE: Prostate-specific antigen screening trials and prostate cancer deaths: the androgen deprivation connection. J Natl Cancer Inst. 2014;106 doi: 10.1093/jnci/dju079. [DOI] [PubMed] [Google Scholar]
- 5.Wolters T, Roobol MJ, Steyerberg EW, et al. The effect of study arm on prostate cancer treatment in the large screening trial ERSPC. Int J Cancer. 2010;126:2387–93. doi: 10.1002/ijc.24870. [DOI] [PubMed] [Google Scholar]
- 6.van Leeuwen PJ, Kranse R, Hakulinen T, et al. Impacts of a population based prostate cancer screening programme on excess all-mortality rates in men with prostate cancer: a randomized controlled trial. 2013 J Med Screen. 2013;20:33–8. doi: 10.1177/0969141313476632. [DOI] [PubMed] [Google Scholar]
- 7.Kranse R, van Leeuwen PJ, Hakulinen T, et al. Excess all-cause mortality in the evaluation of a screening trial to account for selective participation. J Med Screen. 2013;20:39–45. doi: 10.1177/0969141312474443. [DOI] [PubMed] [Google Scholar]
- 8.Zappa M, Puliti D, Hugosson J, et al. A Different Method of Evaluation of the ERSPC Trial Confirms That Prostate-specific Antigen Testing Has a Significant Impact on Prostate Cancer Mortality. Eur Urol. 2014;66:401–3. doi: 10.1016/j.eururo.2013.12.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Autier P, Héry C, Haukka J, Boniol M, Byrnes G. Advanced breast cancer and breast cancer mortality in randomized controlled trials on mammography screening. J Clin Oncol. 2009;27:5919–23. doi: 10.1200/JCO.2009.22.7041. [DOI] [PubMed] [Google Scholar]
- 10.Schröder FH, Hugosson J, Carlsson S, et al. Screening for prostate cancer decreases the risk of developing metastatic disease: findings from the European Randomized Study of Screening for Prostate Cancer (ERSPC) Eur Urol. 2012;62:745–52. doi: 10.1016/j.eururo.2012.05.068. [DOI] [PubMed] [Google Scholar]
- 11.Schröder FH, Denis LJ, Roobol MJ, et al. The story of the European Randomized Study of Screening for Prostate Cancer. BJU Int. 2003;92(Suppl 2):1–13. doi: 10.1111/j.1464-410x.2003.4698x.x. [DOI] [PubMed] [Google Scholar]
- 12.Schröder FH, Hugosson J, Roobol MJ, et al. Screening and prostate-cancer mortality in a randomized European study. N Engl J Med. 2009;360:1320–8. doi: 10.1056/NEJMoa0810084. [DOI] [PubMed] [Google Scholar]
- 13.De Koning HJ, Blom J, Merkelbach JW, et al. Determining the cause of death in randomized screening trial(s) for prostate cancer. BJU Int. 2003;92:71–78. doi: 10.1111/j.1465-5101.2003.04402.x. [DOI] [PubMed] [Google Scholar]
- 14.The AJCC Cancer Staging Atlas. 6nd Edition
- 15.Van Buuren S, Oudshoorn CGM. Multivariate Imputation by Chained Equations: MICE V1.0 User’s manual. Leiden; TNO Preventie en Gezondheid: 2000. TNO Report PG/VGZ/00.038. [Google Scholar]
- 16.Rubin DB. Inference and missing data. Biometrika. 1976;63:581–592. [Google Scholar]
- 17.White IR, Royston P, Wood AM. Multiple imputation using chained equations: Issues and guidance for practice. Stat Med. 2001;30:377–99. doi: 10.1002/sim.4067. [DOI] [PubMed] [Google Scholar]
- 18.Rubin DB. Multiple Imputation for Nonresponse in Surveys. Wiley; New York: 1987. [Google Scholar]
- 19.Schröder FH, Hugosson J, Roobol MJ, et al. Prostate-cancer mortality at 11 years of follow-up. N Engl J Med. 2012;366:981–90. doi: 10.1056/NEJMoa1113135. [DOI] [PMC free article] [PubMed] [Google Scholar]