Abstract
Cell motility is required for diverse biological processes including development, homing of immune cells, wound healing, and cancer cell invasion. Motile neutrophils exhibit a polarized morphology characterized by the formation of leading edge pseudopods and a highly contractile cell rear known as the uropod. Although it is known that perturbing uropod formation impairs neutrophil migration, the role of the uropod in cell polarization and motility remains incompletely understood. Here we discuss cell intrinsic mechanisms that regulate neutrophil polarization and motility with a focus on the uropod, and examine how relationships among regulatory mechanisms change when cells change their direction of migration.
Introduction
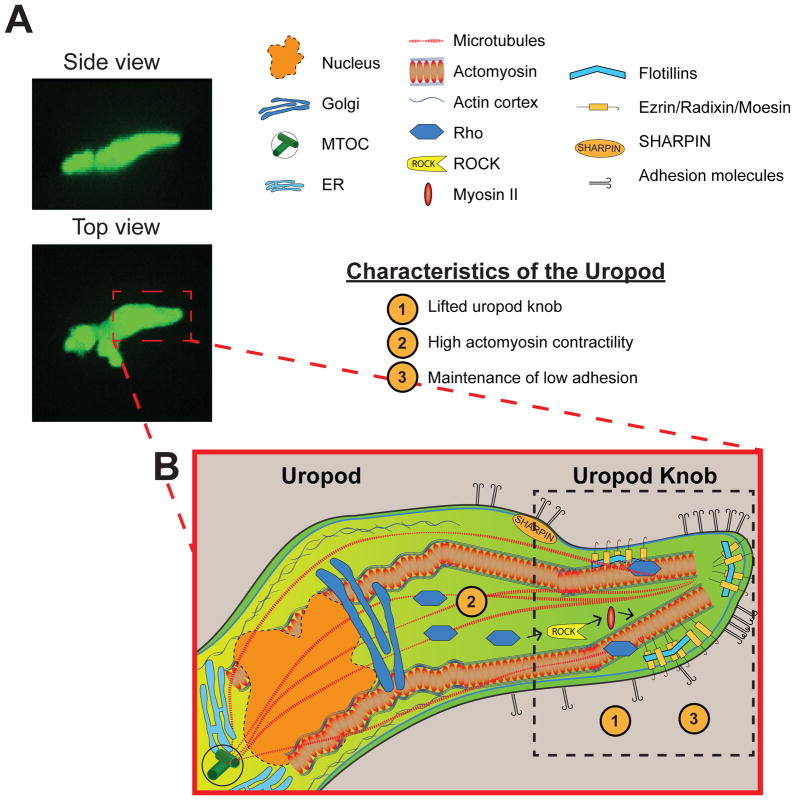
Eukaryotic cell motility is observed in many cell types throughout biology, ranging from single celled organisms such as Dictyostelium discoideum and other amoeba, to cells in multicellular eukaryotes including fibroblasts, germ cells, and immune cells. Historic designations have characterized two major forms of eukaryotic cell motility: amoeboid and mesenchymal migration. Amoeboid migration has been defined by low adhesion, independence from proteolytic degradation of the extracellular matrix (ECM), and a rounded cell morphology with a highly contractile rear uropod (Lammermann and Sixt, 2009). Amoeboid migration is classically used to define the migration of Dictyostelium or primary cells of the innate immune response, including neutrophils, that display highly rapid migration (Friedl and Wolf, 2010). Fibroblasts and other mesenchymal cells, on the other hand, generally migrate in an adhesion and proteolysis dependent manner, and typically exhibit the more elongated cell tail found in fibroblasts without the formation of a rearward uropod (Carragher et al., 2006, Sahai and Marshall, 2003, Sanz-Moreno et al., 2008). Here, we discuss neutrophil polarization and migration with a focus on the mechanisms that regulate neutrophil polarization, particularly the role of the uropod. For the purposes of this review we define the uropod as the rearward part of the cell that includes a distinctive trailing protrusion or knob-like structure, referred to as the “uropod knob” (Figure 1).
Figure 1.
Schematic of Neutrophil Uropod. (A) Side and top view of a neutrophil in a zebrafish larva shows polarized morphology of a migrating neutrophil. (B) Organization and makeup of the uropod. Molecular components of the uropod including Rho, ROCK, and myosin II maintain contractility and generate force at the rear. Other signaling proteins, such as adhesion molecules, flotillins, ERM proteins, and microtubules, are localized to the uropod and contribute to neutrophil polarization and migration. The neutrophil uropod functions by providing high contractility and breaking of adhesions at the rear.
Neutrophils are key mediators of host defense responses and an effective immune response depends on their ability to rapidly migrate through tissues to reach sites of infection and tissue damage. As the first wave of immune cells to arrive during an immune response, neutrophils in particular must be able to efficiently move to sites of inflammation (Nourshargh et al., 2010). A defining characteristic of neutrophil motility, as compared to mesenchymal migration, is the generation of a cell intrinsic polarization that generates a persistent and defined leading edge pseudopod with an opposing rear uropod (Figure 1A). This self-organizing neutrophil polarization does not require an exogenous gradient of chemoattractant since it occurs even in uniform concentrations of chemoattractant. It has been previously suggested that some cells break symmetry and become polarized in the absence of a gradient of chemoattractant by first forming the cell rear through actomyosin activity (Cramer, 2010). This polarization is stable, as neutrophils rarely reverse polarity when changing directions in vitro or within tissues, even in response to changing external cues. Neutrophils instead make U-turns to change directions while maintaining a polarized front and rear (Gerisch and Keller, 1981). How neutrophils maintain this polarity, and in particular the role of the uropod, still remains an enigma. We will discuss the communication between leading and trailing edge signals that mediate the maintenance of polarization, and how neutrophil polarization changes as neutrophils switch directions or, more rarely, when they reverse their polarity. We will also discuss the unique features of signaling at the uropod and of neutrophil force asymmetry.
Front and rear signaling and the maintenance of polarity
Efficient neutrophil motility requires close coordination between processes at the leading and trailing edge of the cell. Although the presence of a distinct leading edge pseudopod and a rear uropod are key features of neutrophil polarized migration, it is not entirely clear what occurs first and which is the “leader” during neutrophil migration. Moreover, there remain gaps in understanding how the leading and trailing edges of neutrophils are coordinated to allow for persistent neutrophil polarization even in the absence of chemokine gradients. This regulation likely involves both positive and negative feedback mechanisms that govern the highly efficient migration of neutrophils. There has been substantial interest in the idea that the self-organizing polarity of neutrophils is mediated by positive feedback loops at the leading edge and signaling that is inhibitory to protrusion at the rear of the cell. Some models have been proposed to explain this regulation, including Local Excitation and Global Inhibition (LEGI), that have been reviewed elsewhere (Devreotes and Horwitz, 2015). In this section we discuss studies that focus on how Rho GTPase signaling is coordinated between the front and rear of motile neutrophils.
The leading edge
Pseudopods are the most common leading edge protrusion during neutrophil migration. In neutrophils migrating in vivo, actin stability is polarized, with dynamic F-actin concentrated in leading edge pseudopods and stable F-actin concentrated at the rear, where there is high actomyosin contractility (Yoo et al., 2010). During chemotactic migration, Rac activation at the leading edge induces actin polymerization and pseudopod formation (Charest and Firtel, 2007, Lam and Huttenlocher, 2013). This actin polymerization drives protrusions at the leading edge of neutrophils and contributes to forward migration even when the uropod is compromised (Houk et al., 2012, Lammermann et al., 2008). Following the recognition of chemoattractants, effectors such as PIP3, a product of PI3K, are concentrated at the leading edge of migrating neutrophils and act through positive feedback with Rac to polarize dynamic actin at the leading edge and induce protrusions and motility (Hirsch et al., 2000, Sasaki et al., 2000, Yoo et al., 2010) (Figure 2A). Localized Rac activation using photoactivation at the leading edge is sufficient to drive neutrophil migration in vivo (Yoo et al., 2010). Although Rac at the leading edge is both essential and sufficient for pseudopod formation, substantial evidence implicates Cdc42 as a key player in steering cells during chemotaxis. In recent work, locally excitable networks of leading edge Cdc42 signaling have been implicated in steering cells during chemotaxis, while Rac mediates the more robust generation of actin dynamics at the cell front (Yang et al., 2016).
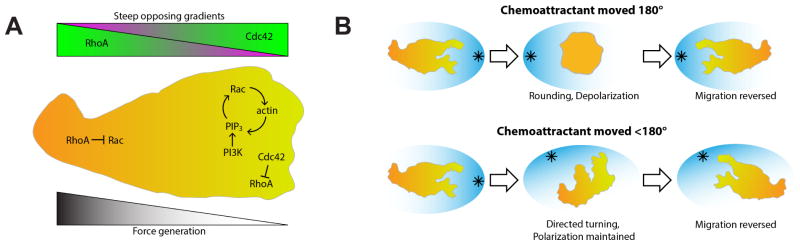
Figure 2.
Neutrophil front and rear signaling influence each other and may contribute to neutrophil turning. (A) Neutrophil polarization is initially established by steep opposing gradients of RhoA and Cdc42 signaling. A positive feedback loop involving PIP3, Rac, and actin operates at the leading edge of the cell where Cdc42 activity inhibits RhoA. At the uropod of the cell RhoA inhibits Rac, stabilizing neutrophil polarity. These signaling patterns contribute to the asymmetric generation of force at the trailing edge of the cell. (B) Neutrophils respond to changes in the direction of chemoattractant gradient in two ways. In response to a 180° change, neutrophils oft en become round and then reverse their polarity (top). In response to smaller changes in the gradient and in most in vivo situations, neutrophils instead make a U-turn by forming a new pseudopod on the side facing the new gradient (bottom).
Rho GTPase signaling and front-rear polarity
Rho GTPase activity is commonly thought to be restricted to one pole of migrating neutrophils with Cdc42 and Rac in the pseudopod and RhoA toward the uropod (Meili and Firtel, 2003). The combined activity of these GTPases controls actin dynamics throughout the cell. In response to chemoattractant, a steep gradient of Cdc42 at the leading edge is formed at the same time as an opposing gradient of RhoA signaling forms toward the cell’s rear (Yang et al., 2016) (Figure 2A), suggesting that front and rear polarity are formed simultaneously. Cdc42 inhibits Rho activity at the leading edge, and likely contributes to the maintenance of polarized RhoA signaling at the uropod. Many models have suggested distinct signaling pathways that lead to the segregation of “frontness” and “backness” signaling nodes within the neutrophil, but the interactions between signaling molecules localized to the leading and trailing edges that maintain neutrophil polarity even under uniform concentrations of attractant remain unclear (Meili and Firtel, 2003, Xu et al., 2003).
Many signaling molecules that localize to the uropod help drive neutrophil polarization and chemotaxis at least in part through RhoA and myosin II activity (Pestonjamasp et al., 2006, Van Keymeulen et al., 2006, Yang et al., 2016). While PIP3 accumulation at the leading edge helps to drive cell protrusion, kinases that generate another phosphinostitide, PI(4,5)P2, localize to the rear of the cell and drive rear retraction during neutrophil migration (Lacalle et al., 2007, Lokuta et al., 2007). Phosphatidylinositol phosphate kinase type Iγ (PIPKIγ661) localizes to the uropod of neutrophils and is important for detachment of the trailing edge during directed migration (Lokuta et al., 2007). Key RhoA regulators including guanine nucleotide exchange factors or GEFs also localize to the uropod. One RhoA GEF, PDZRhoGEF (PRG), localizes to the rear of polarized cells and acts in a positive feedback loop with RhoA, ROCK, and myosin II to stabilize neutrophil polarity and restrict contractility to the rear of the cell (Wong et al., 2007). This localization is dependent on myosin II and locally activates RhoA to create a stable uropod and allow for contraction and de-adhesion, two major components of uropod function (Wong et al., 2007). In addition to RhoA and myosin II signaling that limits membrane protrusion at the rear, another recent study suggests that the ERM protein moesin (discussed below) plays an essential role in neutrophil polarization by inhibiting Rac signaling and protrusion at the cell rear (Liu et al., 2015a) (Figure 2A).
In addition to signaling in the uropod, both positive and negative signaling at the leading edge helps to promote polarized neutrophil migration. The interaction of PIP3 and actin is part of a positive feedback loop that stabilizes PIP3 accumulation at the leading edge and requires PI3K, Rac and Cdc42, and interacting proteins such as Hem-1, a member of the WAVE complex that regulates actin polymerization (Srinivasan et al., 2003, Weiner et al., 2002, Weiner et al., 2006). The establishment of polarized actin polymerization is not solely mediated by PI3K and Rho GTPase activity at the leading edge, as other proteins that play an important role at the leading edge, such as Homer3, a Gαi2- binding protein, have also been identified (Wu et al., 2015). Rac and Cdc42 play divergent roles in regulating polarity following stimulation. Rac activity can regulate the accumulation of PIP3 at leading edge of polarized cells and the development of a polarized morphology in neutrophils (Srinivasan et al., 2003). Cdc42 activity is not required for PIP3 accumulation at the leading edge of cells, but rather stabilizes a single leading edge in the direction of a gradient (Srinivasan et al., 2003, Yang et al., 2016). In addition to their roles in establishing and maintaining the neutrophil pseudopod, Rac and Cdc42 regulate polarized RhoA activity in the uropod. Rac is necessary for both the formation of a leading edge and for tail retraction during chemotaxis (Gardiner et al., 2002). Cells deficient in Rac have impaired detachment of the cell rear during migration due to a decrease in RhoA activity and inhibited myosin light chain phosphorylation (Pestonjamasp et al., 2006). Cdc42 also mediates the effect of PIP3 on RhoA activation and Rho-dependent actomyosin contraction leading to stable polarity and the formation of a single pseudopod (Van Keymeulen et al., 2006). In addition to its effect on RhoA downstream of PIP3 accumulation, Cdc42 also mediates myosin contraction at the trailing edge by controlling the redistribution of WASp, which controls clustering of integrin CD11b in the neutrophil uropod (described in more detail below) (Kumar et al., 2012, Szczur et al., 2009). This redistribution of CD11b by WASp through Cdc42 is important for myosin contraction through ROCK and the phosphorylation of myosin light chain leading to a stable uropod (Kumar et al., 2012, Szczur et al., 2009). It is important to note that it is not just positive signaling that functions at the leading edge but also negative signaling. For example, the activation of myosin phosphatase at the leading edge helps to confine moesin’s inhibitory activity to the uropod (Liu et al., 2015a).
Cell intrinsic polarity and neutrophil reverse migration
Neutrophils rapidly polarize and migrate to sites of tissue damage by responding to complex gradients of chemoattractants. Neutrophils change their direction in response to the movement of a chemoattractant gradient in two ways: by maintaining their polarity and making a U-turn or by reversing their polarity and moving in the opposite direction (Albrecht and Petty, 1998, Gerisch and Keller, 1981, Ramsey, 1972, Zigmond et al., 1981) (Figure 2B). It is particularly intriguing that neutrophils generally maintain polarity and turn even when changing directions in response to new or competing attractants rather than reversing their polarity. Maintenance of neutrophil polarity is also observed during neutrophil migration in vivo in response to tissue damage in zebrafish larvae. In zebrafish, neutrophils are rapidly recruited to a wound and subsequently the majority of neutrophils reverse their migration away from the wound as they leave the site of tissue damage (Mathias, 2006). Although some neutrophils will pause at the wound and reverse polarity, many neutrophils maintain polarity with a sustained uropod and make a U-turn away from the wound (unpublished observations). How neutrophils maintain polarity remains unclear and likely involves the coordinated positive and negative feedback mechanisms between the leading and trailing edges of the cell discussed above. It has also been shown that fast moving cells, such as neutrophils, are able to sustain polarity longer using a positive feedback loop between actin flow and the asymmetric distribution of signaling components (Maiuri et al., 2015).
To probe the role of polarity in neutrophil directional motility, many groups have challenged neutrophils with point sources of chemoattractant at different angles from the direction of polarized migration. The most extreme case occurs when the chemoattractant is moved from directly in front of a polarized migrating neutrophil to directly behind the cell’s uropod. In response to a change in the location of the chemoattractant gradient, some neutrophils will temporarily lose polarity and become rounded. Following this rounding, the neutrophils may reverse polarity by producing a new pseudopod in the location of the previous uropod and create a uropod in the location of the former pseudopod (Albrecht and Petty, 1998, Dehghani Zadeh et al., 2003, Gerisch and Keller, 1981, Ramsey, 1972) (Figure 2B, top). This response usually occurs when the chemotactic gradient has moved 180°, from directly in front of a polarized cell to directly behind the cell (Figure 2) (Dehghani Zadeh et al., 2003, Gerisch and Keller, 1981, Ramsey, 1972); however, this response was also seen when neutrophils were exposed to increasingly lower uniform fields of chemoattractant,(Albrecht and Petty, 1998). In response to smaller movements in the chemoattractant gradient and occasionally in response to the gradient moving 180°, neutrophils will change their direction of movement by making a U-turn. This involves a series of smaller turns in which neutrophils form a new pseudopod at the side of the leading edge while maintaining their uropod (Gerisch and Keller, 1981, Hamza et al., 2014, Zigmond et al., 1981) (Figure 2B, bottom). This mechanism for reversing neutrophil motility has been observed in a variety of platforms including experiments in which a micropipette producing a gradient of chemoattractant is moved, as in the experiments discussed above. U-turn behavior has also been seen in vitro in microfluidic devices used to study 1D neutrophil migration to a source of fMLP (Hamza et al., 2014). We have also seen the turning of neutrophils in our in vivo system during the reverse migration of neutrophils from a wound in zebrafish (Mathias et al., 2006, Tauzin et al., 2014).
Several mechanisms have been proposed to explain the ability of neutrophils to maintain polarity and make a U-turn in response to a change in the gradient. The feedback loops discussed above involving GTPases and phosphoinositides, responsible for stabilizing polarity at the leading and trailing edges of the cell, likely have a role in maintaining this polarity (Xu et al., 2003). Furthermore, Rac activation in the uropod of migrating neutrophils fails to induce protrusions, indicating the uropod is resistant to leading-edge signaling pathways (Yoo et al., 2010). Moreover, it has been suggested that membrane tension generated by leading edge protrusion plays a role in maintaining polarity by acting as a long-range inhibitor of actin assembly and Rac activation in the uropod (Houk et al., 2012). A recent study in fact suggests that membrane tension works in conjunction with a mechano-sensory feedback loop involving phospholipase D2 (PLD2) and mTORC2 to control actin protrusions and maintain polarity (Diz-Munoz et al., 2016). In some cases, subsets of neutrophils experiencing the same change in the gradient will respond in both ways, with some cells reversing their polarity while other cells make large U-turns (Dehghani Zadeh et al., 2003, Gerisch and Keller, 1981). This might be partially explained by a model developed by Prentice-Mott et al. that describes the changes in intracellular signaling gradients in a model of directional memory that is driven at least in part by the rear of the cell via moesin and ROCK (Prentice-Mott et al., 2016).
Signaling and the neutrophil uropod
The classic uropod is characterized by high actomyosin contractility and low adhesion (Figure 1). Indeed, the high contractility generated in the uropod of neutrophils are likely important for efficient neutrophil migration by both providing a rearward squeezing that promotes forward protrusion and by inducing sufficient tension to release adhesive contacts of the uropod from the underlying substratum. Key signaling components that regulate contractility at the uropod, as discussed above, include the rearward concentration of myosin II, actomyosin bundles, and active Rho (Heasman et al., 2010, Sanchez-Madrid and Serrador, 2009). Other uropod components, discussed in more detail below, include signaling molecules such as PIPKIs (Lacalle et al., 2007, Lokuta et al., 2007) and Proline serine threonine phosphatase interacting protein 1 (PSTPIP1) (Cooper et al., 2008), cytoskeletal proteins of the ezrin/radixin/moesin family (Serrador et al., 1997, Serrador et al., 2002, Yonemura et al., 1998), flotillins (Ludwig et al., 2010) and specific adhesion receptors such as P-selectin glycoprotein ligand-1 (PSGL-1), CD44, ICAMs, LFA-1, and integrins (for a full review of uropod components see Sánchez-Madrid and Serrador, 2009) (Figure 1B). Microtubules also orient toward the uropod in neutrophils (Eddy et al., 2002) and can be stabilized by CD11b integrin in the uropod (Kumar et al., 2012). Below we discuss the function of some of the key uropod components.
Myosin II
Myosin II helps maintain uropod polarity (Smith et al., 2007, Uchida et al., 2003) and inhibition of RhoA, Rho-associated protein kinase (ROCK), or myosin II activity leads to impaired uropod formation, rear release, and reduced migration. The impaired retraction caused by myosin II inhibitors is not seen on poorly adhesive substrates supporting the idea that one important role myosin II plays in the rear of the cell is to break adhesions with the underlying matrix (Eddy et al., 2000). This myosin II contraction requires myosin II to be activated by the Ca2+- dependent myosin light chain kinase (MLCK) (Eddy et al., 2000). The integrin CD11b, also known as αM integrin, plays a role in maintaining neutrophil polarity by modulating myosin light chain mediated contractility (Kumar et al., 2012). During polarization, CD11b redistributes to the uropod in a manner dependent on Cdc42. At the uropod, it suppresses protrusions by modulating the MLC pathway in a ROCK dependent process and by stabilizing polarity (Szczur et al., 2009). In addition to playing a role in breaking cellular adhesions at the uropod, myosin II activity affects migration speed and directionality (Cavnar et al., 2011, Eddy et al., 2000, Pestonjamasp et al., 2006, Yoshinaga-Ohara et al., 2002). Defects in neutrophil motility caused by inhibition of ROCK or myosin II are, therefore, at least in part, caused by defects in uropod retraction and induce an elongated morphology (Eddy et al., 2000), further supporting a central role for uropod signaling in the maintenance of neutrophil migration. The absence of polarized myosin II has also been shown to lead to chemotaxis defects in neutrophils downstream of the mammalian target of rapamycin complex 2 (mTORC2). Depletion of mTORC2 reduces both actin polymerization at the leading edge and myosin II regulation at the rear resulting in impaired rear retraction in a RhoA/cAMP-dependent pathway (Liu et al., 2010).
Adhesion and neutrophil migration
Uropod detachment is required for efficient neutrophil motility. However, engagement of adhesion is needed for cell stopping (Saez de Guinoa et al., 2013) as well as extravasation (Hyun et al., 2012). The adhesion state of the uropod must therefore be tightly regulated to maintain low adhesion at the cell rear, facilitating neutrophil migration. Several adhesion molecules are localized to the uropod, including PSGL-1, LFA-1 (integrin αLβ2), and intercellular adhesion molecules (ICAMs). This localization is often used as a marker for uropod identification (Sanchez-Madrid and Serrador, 2009), and is thought to be important for neutrophil adhesion during transmigration across the endothelium. Neutrophils and other immune cells like lymphocytes also rely on uropod adhesion when they recruit and activate each other. Uropod adhesion thus also plays a role in mediating cell-cell interactions (del Pozo et al., 1997, Saez de Guinoa et al., 2013).
Several pathways regulate adhesion at the uropod during motility. Many adhesion receptors at the uropod have ligands that are expressed only in specific contexts or cell types. For example, uropod attachment and elongation is a crucial step prior to extravasation in leukocytes, and is dependent upon ICAM1 engagement on endothelial cells by LFA-1 on leukocytes (Hyun et al., 2012). During migration, however, leukocytes expressing LFA-1 maintain high affinity LFA-1, mediated by integrin inside-out signaling, at the mid-cell and leading edge, but a low affinity LFA-1 at the uropod (Smith et al., 2005). These findings suggest that integrin engagement is important for adhesion and extravasation, but a low affinity form of LFA-1 at the rear of the cell is necessary for efficient migration. Indeed, it was shown that an adaptor protein, SHARPIN, directly interacts with LFA-1 at the uropod of motile T cells, maintaining inactive LFA-1 and inhibiting the expression of LFA-1 activation epitopes (Pouwels et al., 2013). Alteration of SHARPIN leads to defects in proper LFA-1 control and impaired uropod detachment (Morin et al., 2008, Pouwels et al., 2013, Smith et al., 2003).
Other mechanisms also regulate the adhesion state of the neutrophil uropod to facilitate detachment and motility. For example, junctional adhesion molecule A (JAM-A) regulates integrin internalization and recycling and is found in intracellular vesicles co-localized with β1 and β2 integrins. This internalization is crucial for proper neutrophil migration by reducing integrin surface expression at the rear, as neutrophils deficient in JAM-A have impaired uropod retraction, likely due to a defect in integrin internalization (Cera et al., 2009).
There is substantial evidence to support the idea that amoeboid motility is different in 2 and 3 dimensional spaces (2D and 3D). In fact, neutrophil migration can occur in three dimensional spaces, like interstitial tissues in vivo, in the absence of integrin-mediated adhesion (Lammermann et al., 2008). This is in contrast to neutrophil adhesion and migration along endothelial surfaces (a type of 2D in vivo migration), where integrin-mediated adhesion is generally required. It has also been shown that neutrophils can migrate in 3D without talin, a key regulator of integrin activation. Studies have shown that in vivo talin is not required for neutrophil motility (Lammermann et al., 2013); however in 3D in vitro gels, neutrophil directed migration to a gradient of chemoattractant was impaired in talin-deficient neutrophil-like cells (Yamahashi et al., 2015). Interestingly, in a more recent study, Toyjanova et al. showed that spatial confinement of neutrophils is sufficient to cause a switch to integrin-independent motility (Toyjanova et al., 2015), It has also been shown that in similar confined environments mesenchymal cancer cells are capable of adopting a fast moving amoeboid-like mode of migration (Liu et al., 2015b). This finding might provide insight into the mesenchymal to amoeboid transition seen in some migrating cancer cells (Friedl and Wolf, 2003), and raises interesting questions about the role of a uropod-like rear in the more rapid migration of cancer cells.
ERM proteins
Chemoattractant induced cell signaling promotes rapid polarization of key components to the uropod, including RhoA regulatory proteins and the ezrin/radixin/moesin (ERM) family of proteins. For example, the adaptor protein HS1-associated protein X-1 (Hax1) and integrin associated protein filamin A are both necessary for RhoA activation, uropod formation, and efficient neutrophil migration (Cavnar et al., 2011, Sun et al., 2013). An early step in uropod formation is the localization of ERM family proteins in the knob-like uropod structure (Martinelli et al., 2013, Sanchez-Madrid and Serrador, 2009). The ERM family of proteins links the actin cytoskeleton to membrane receptors and transduces signals involved in cytoskeletal remodeling and cell motility (Ivetic and Ridley, 2004, Yoshinaga-Ohara et al., 2002). ERM family proteins are also known to act both upstream and downstream of Rho family GTPases in a positive feedback loop (Ivetic and Ridley, 2004). Myosin II activity is essential for the localization of ERM proteins to the uropod, where ERM proteins in turn regulate Rho activity providing a positive feedback loop that sustains uropod formation. ERM proteins are necessary for efficient migration, and interact with flotillins to form lipid raft clusters at the uropod (Chen et al., 2013, Serrador et al., 1997, Tomas et al., 2002). Accordingly, uropod formation and myosin II activity are impaired in neutrophils from flotillin 1 knockout mice (Ludwig et al., 2010). Constitutively active ERM proteins are sufficient to trigger uropod formation, likely, at least in part, through feedback signaling with RhoA and ROCK. Accordingly, the uropod does not form when dominant negative ERM proteins are expressed in T cells (Martinelli et al., 2013). The similarities between T cell and neutrophil uropods suggest ERM proteins may play a similar role in neutrophil polarization. In neutrophils, ERM proteins bind to ICAM-3 and PSGL-1 in the uropod following stimulation (Alonso-Lebrero et al., 2000). It is also known that ERM proteins interact with F-actin and that this interaction is dependent on RhoA (Matsui et al., 1998, Nakamura et al., 1999). It is, therefore, likely that the ERM proteins link signaling from adhesion receptors to the cytoskeleton, in a Rho-dependent manner, leading to efficient polarization of the actin network. As discussed above, a recent study highlights a key role for moesin in limiting membrane protrusions at the uropod, helping maintain neutrophil polarization during motility (Liu et al., 2015a). Thus, the localization and regulation of uropod-associated proteins, like ERM proteins, represents a key feature of the rapid motility of neutrophils and other amoeboid cells.
Microtubules
In contrast to mesenchymal cells where microtubules orient toward the leading edge, microtubules orient toward the uropod in neutrophils and can be stabilized by CD11b integrin in the uropod (Eddy et al., 2002, Kumar et al., 2012). This microtubule organization is maintained during neutrophil motility and does not depend on adhesion (Eddy et al., 2002). Interestingly, disruption of the microtubule network with a chemical inhibitor induces neutrophil polarization and random migration both in vitro (Niggli, 2003) and in vivo (Yoo et al., 2010) but reduces chemotaxis to the chemoattractant f-Met-Leu-Phe (Xu et al., 2005) supporting an essential role for the microtubule network in directed migration. Microtubules interact with the actin cytoskeleton to regulate polarized migration. Inhibition of F-actin prevents the reorientation of microtubules following polarization and disruption of microtubules leads to reduced levels of F-actin in the pseudopods of migrating neutrophils (Eddy et al., 2002, Xu et al., 2005). The establishment of polarity and migration in neutrophils following microtubule disruption is likely due to the activation of Rho- and actomyosin-dependent contractility in the neutrophil uropod (Niggli, 2003, Xu et al., 2005). We postulate that this increase in Rho activation is due to the activation of Rho GEF-H1, a microtubule regulated GEF. Interestingly, the rapid motility appears to be driven largely by rearward contractility, and occurs in the absence of PIP3 concentration at the leading edge, which is required for the generation of directed pseudopods (Xu et al., 2005, Yoo et al., 2010). The lack of PIP3 would also explain the reduced chemotaxis seen in these cells (Xu et al., 2005). Thus, microtubule disassembly alters polarized signaling likely because of effects on specific targets that regulate the actin cytoskeleton at the front and rear of migrating neutrophils.
Neutrophil polarization: force centers at the neutrophil uropod
In contrast to mesenchymal cells, the uropod is the primary site for force generation in motile neutrophils. Mesenchymal cells generate traction stresses against their underlying substratum through integrin-ligand interactions that connect intracellular cytoskeletal networks to the extracellular matrix. Traction forces were originally measured in mesenchymal cells such as fibroblasts and endothelial cells (Lo et al., 2000, Reinhart-King et al., 2005). These traction forces were found to be relatively large, on the order of kilopascals, and located along the leading edge of migrating cells. In addition, macrophages, another type of leukocyte, can migrate in a mesenchymal mode (Van Goethem et al., 2010) and have traction forces at the leading edge of the cell (Hind et al., 2015). These findings led to the forward towing explanation of migration. This model suggests that cells possess transient towing units under their leading edge that adhere to the substratum and transmit strong traction forces, pulling the cell forward (Munevar et al., 2001). In contrast to this mode of migration, which generally applies to two dimensional mesenchymal migration, neutrophils migrate using a significantly different pattern of traction asymmetry.
The first study on traction force generation by neutrophils found that migrating neutrophils consistently generate traction stresses in the rear of the cell relative to the direction of migration (Smith et al., 2007). This pattern of traction stresses was observed with neutrophils in a uniform field of the neutrophil chemoattractant f-Met-Leu-Phe (fMLP) during chemokinesis and in response to a point source of fMLP during chemotaxis, although rearward traction forces were significantly higher in chemotaxing neutrophils. Furthermore, it was determined that force generation in the uropod preceded a turn during neutrophil migration, indicating that the location of the force center is partially responsible for influencing the direction of neutrophil migration (Smith et al., 2007). This pattern of uropod force generation was subsequently confirmed by a number of groups looking at force generation in primary neutrophils and neutrophil-like cell lines (Bastounis et al., 2014, Jannat et al., 2011, Oakes et al., 2009). Interestingly, this same force generation pattern was also found in migrating Dictyostelium (Bastounis et al., 2014, Lombardi et al., 2007). The forces generated by neutrophils were found to be significantly smaller than those generated by mesenchymal cells (Reinhart-King et al., 2005, Smith et al., 2007), in agreement with the theory that force generation is inversely correlated with migration speed (Beningo et al., 2001). The orientation and magnitude of traction stresses in neutrophils suggests that neutrophils migrate using a fundamentally different mode than mesenchymal cells. Rather than pulling themselves forward as the forward towing model describes, neutrophils use a rearward squeezing mode of migration in which the cell uses strong forces at the rear to push the contents of the cell forward, much like squeezing toothpaste out of a tube, and simultaneously detaches the uropod from the underlying substratum (Smith et al., 2007). Similar to neutrophil migration in two dimensions, neutrophils under confinement display traction forces at the rear of the cell and the magnitude of traction stresses is higher on stiffer substrates. While blockade of both β1 and β2 integrins did not cause a change in cell speed or force generation, it did cause a change in the pattern of force distribution, indicating that while not necessary for migration, integrins are important for the organization of forces in confined spaces (Toyjanova et al., 2015).
Many of the studies on force generation have found that the magnitude of force generation and the involvement of specific signaling molecules are dependent on the stiffness of the underlying matrix. It has been shown that neutrophils spread more, migrate more slowly but persistently, and generate larger and more organized forces on stiff matrices compared to soft matrices (Jannat et al., 2011, Jannat et al., 2010, Oakes et al., 2009). The correlation between chemotactic prowess and force asymmetry and magnitude indicates that force generation in neutrophils is critical for efficient motility (Jannat et al., 2010). It has been suggested that PI3K might also play a role in sensing changes in matrix stiffness during neutrophil chemotaxis. Oakes et al. found that while neutrophils spread more and had a lower velocity on stiffer gels than softer gels, neutrophils treated with a PI3K inhibitor spread and migrated equally well on both stiff and soft matrices. This result suggests that PI3K plays a mechanosensing role during neutrophil migration (Oakes et al., 2009). PI3K has been shown to regulate neutrophil motility by regulating the antereoposterior polarization of F-actin dynamics both in vitro and in vivo (Martin et al., 2015, Wang et al., 2002, Yoo et al., 2010) and PIP3, a product of PI3K, has been shown to mediate RhoA activity at the uropod of migrating neutrophils (Van Keymeulen et al., 2006). It is, therefore, possible that in addition to sensing matrix stiffness, PI3K plays an indirect role in force generation by mediating RhoA activity and myosin II contraction at the rear of the cell. In support of this hypothesis, it has been shown that PI3K activity is important for force generation in other immune cells (Hind et al., 2015). In neutrophils, reports have shown that myosin II contraction through the RhoA kinase ROCK is partially responsible for force generation (Jannat et al., 2011). Neutrophils incubated with the Y27632 ROCK inhibitor showed significantly reduced force generation and lost their asymmetric pattern of force generation. These cells had limited motility, further indicating that the asymmetrically distributed forces at the uropod are necessary for efficient neutrophil migration (Jannat et al., 2011). However, neutrophils are capable of migrating in the absence of a functional force-generating uropod. For example, the leading edge can remain migratory following cleavage from the cell body (Houk et al., 2012) and neutrophils are able to migrate following the loss of their uropods during incomplete extravasation (Hyun et al., 2012). Furthermore, leukocytes in 3D environments do not require contraction through myosin II for forward migration of the cells’ leading edge; instead, myosin II is necessary for deforming the nucleus to allow for migration through the 3D matrix (Lammermann et al., 2008). These results indicate that a second force generating mechanism, such as actin polymerization, at the leading edge of the cell also contributes to neutrophil migration; however, the findings also illustrate the importance of a contractile uropod for optimal neutrophil migration. The localization of traction forces to the neutrophil uropod highlights the importance of the uropod in driving neutrophil migration. Furthermore, it has also been shown that the force center moves prior to a neutrophil turn suggesting the uropod force center is partially responsible for the direction of migration during a turn (Smith et al., 2007). Thus, the correlation between force magnitude and chemotactic index and the observation that the force center moves prior to a turn indicates that the uropod can also play a role in the directionality of migration.
Conclusions
Neutrophil migration is crucial for a proper immune response. This migration requires the coordination of many signaling pathways at the front and rear of the cell that lead to neutrophil polarization and motility. There has been substantial focus on how the leading edge regulates polarized neutrophil migration. We have discussed how the neutrophil uropod has emerged as a crucial structure for proper neutrophil motility that can also be the driving center for neutrophil polarization and migration. This is supported by several lines of evidence. Inhibition of uropod formation significantly impairs neutrophil migration. It has also been shown recently that Rho signaling polarizes at the rear at the same time as Cdc42 activity localizes to the front, indicating the rear and front of the cell are formed simultaneously. Furthermore, the maintenance of polarity during neutrophil turning and reverse migration suggests that the uropod is a stable structure and that turning may be a more efficient method than polarity reversal for changing direction in highly motile cells. It is also possible that the uropod drives the turning behavior as turns can be predicted by localized force changes in the uropod. In addition to its role in neutrophil motility, the neutrophil uropod has also been implicated in cellular communication through adhesion interactions (Cera et al., 2009) and secreting trails of chemokine to direct the recruitment of other immune cells (Lim et al., 2015). The uropod remains an enigma in neutrophil migration and more needs to be done to understand how it drives intrinsic polarity and motility of amoeboid cells. It is interesting to speculate that the uropod may be a defining feature of the amoeboid motility of other cells such as cancer cells where a uropod-like structure has been seen and could represent a novel therapeutic target.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- ALBRECHT E, PETTY HR. Cellular memory: neutrophil orientation reverses during temporally decreasing chemoattractant concentrations. Proc Natl Acad Sci U S A. 1998;95:5039–44. doi: 10.1073/pnas.95.9.5039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ALONSO-LEBRERO JL, SERRADOR JM, DOMINGUEZ-JIMENEZ C, BARREIRO O, LUQUE A, DEL POZO MA, SNAPP K, KANSAS G, SCHWARTZ-ALBIEZ R, FURTHMAYR H, LOZANO F, SANCHEZ-MADRID F. Polarization and interaction of adhesion molecules P-selectin glycoprotein ligand 1 and intercellular adhesion molecule 3 with moesin and ezrin in myeloid cells. Blood. 2000;95:2413–9. [PubMed] [Google Scholar]
- BASTOUNIS E, MEILI R, ALVAREZ-GONZALEZ B, FRANCOIS J, DEL ALAMO JC, FIRTEL RA, LASHERAS JC. Both contractile axial and lateral traction force dynamics drive amoeboid cell motility. J Cell Biol. 2014;204:1045–61. doi: 10.1083/jcb.201307106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BENINGO KA, DEMBO M, KAVERINA I, SMALL JV, WANG YL. Nascent focal adhesions are responsible for the generation of strong propulsive forces in migrating fibroblasts. J Cell Biol. 2001;153:881–8. doi: 10.1083/jcb.153.4.881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CARRAGHER NO, WALKER SM, SCOTT CARRAGHER LA, HARRIS F, SAWYER TK, BRUNTON VG, OZANNE BW, FRAME MC. Calpain 2 and Src dependence distinguishes mesenchymal and amoeboid modes of tumour cell invasion: a link to integrin function. Oncogene. 2006;25:5726–40. doi: 10.1038/sj.onc.1209582. [DOI] [PubMed] [Google Scholar]
- CAVNAR PJ, BERTHIER E, BEEBE DJ, HUTTENLOCHER A. Hax1 regulates neutrophil adhesion and motility through RhoA. J Cell Biol. 2011;193:465–73. doi: 10.1083/jcb.201010143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CERA MR, FABBRI M, MOLENDINI C, CORADA M, ORSENIGO F, REHBERG M, REICHEL CA, KROMBACH F, PARDI R, DEJANA E. JAM-A promotes neutrophil chemotaxis by controlling integrin internalization and recycling. J Cell Sci. 2009;122:268–77. doi: 10.1242/jcs.037127. [DOI] [PubMed] [Google Scholar]
- CHAREST PG, FIRTEL RA. Big roles for small GTPases in the control of directed cell movement. Biochem J. 2007;401:377–90. doi: 10.1042/BJ20061432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHEN EJ, SHAFFER MH, WILLIAMSON EK, HUANG Y, BURKHARDT JK. Ezrin and moesin are required for efficient T cell adhesion and homing to lymphoid organs. PLoS One. 2013;8:e52368. doi: 10.1371/journal.pone.0052368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COOPER KM, BENNIN DA, HUTTENLOCHER A. The PCH family member proline-serinethreonine phosphatase-interacting protein 1 targets to the leukocyte uropod and regulates directed cell migration. Mol Biol Cell. 2008;19:3180–91. doi: 10.1091/mbc.E08-02-0225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CRAMER LP. Forming the cell rear first: breaking cell symmetry to trigger directed cell migration. Nat Cell Biol. 2010;12:628–32. doi: 10.1038/ncb0710-628. [DOI] [PubMed] [Google Scholar]
- DEHGHANI ZADEH A, SEVEAU S, HALBWACHS-MECARELLI L, KELLER HU. Chemotactically-induced redistribution of CD43 as related to polarity and locomotion of human polymorphonuclear leucocytes. Biol Cell. 2003;95:265–73. doi: 10.1016/s0248-4900(03)00053-4. [DOI] [PubMed] [Google Scholar]
- DEL POZO MA, CABANAS C, MONTOYA MC, AGER A, SANCHEZ-MATEOS P, SANCHEZ-MADRID F. ICAMs redistributed by chemokines to cellular uropods as a mechanism for recruitment of T lymphocytes. J Cell Biol. 1997;137:493–508. doi: 10.1083/jcb.137.2.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DEVREOTES P, HORWITZ AR. Signaling networks that regulate cell migration. Cold Spring Harb Perspect Biol. 2015;7:a005959. doi: 10.1101/cshperspect.a005959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DIZ-MUNOZ A, THURLEY K, CHINTAMEN S, ALTSCHULER SJ, WU LF, FLETCHER DA, WEINER OD. Membrane Tension Acts Through PLD2 and mTORC2 to Limit Actin Network Assembly During Neutrophil Migration. PLoS Biol. 2016;14:e1002474. doi: 10.1371/journal.pbio.1002474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EDDY RJ, PIERINI LM, MATSUMURA F, MAXFIELD FR. Ca2+-dependent myosin II activation is required for uropod retraction during neutrophil migration. J Cell Sci. 2000;113(Pt 7):1287–98. doi: 10.1242/jcs.113.7.1287. [DOI] [PubMed] [Google Scholar]
- EDDY RJ, PIERINI LM, MAXFIELD FR. Microtubule asymmetry during neutrophil polarization and migration. Mol Biol Cell. 2002;13:4470–83. doi: 10.1091/mbc.E02-04-0241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FRIEDL P, WOLF K. Tumour-cell invasion and migration: diversity and escape mechanisms. Nat Rev Cancer. 2003;3:362–74. doi: 10.1038/nrc1075. [DOI] [PubMed] [Google Scholar]
- FRIEDL P, WOLF K. Plasticity of cell migration: a multiscale tuning model. J Cell Biol. 2010;188:11–9. doi: 10.1083/jcb.200909003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GARDINER EM, PESTONJAMASP KN, BOHL BP, CHAMBERLAIN C, HAHN KM, BOKOCH GM. Spatial and temporal analysis of Rac activation during live neutrophil chemotaxis. Curr Biol. 2002;12:2029–34. doi: 10.1016/s0960-9822(02)01334-9. [DOI] [PubMed] [Google Scholar]
- GERISCH G, KELLER HU. Chemotactic reorientation of granulocytes stimulated with micropipettes containing fMet-Leu-Phe. J Cell Sci. 1981;52:1–10. doi: 10.1242/jcs.52.1.1. [DOI] [PubMed] [Google Scholar]
- HAMZA B, WONG E, PATEL S, CHO H, MARTEL J, IRIMIA D. Retrotaxis of human neutrophils during mechanical confinement inside microfluidic channels. Integr Biol (Camb) 2014;6:175–83. doi: 10.1039/c3ib40175h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HEASMAN SJ, CARLIN LM, COX S, NG T, RIDLEY AJ. Coordinated RhoA signaling at the leading edge and uropod is required for T cell transendothelial migration. J Cell Biol. 2010;190:553–63. doi: 10.1083/jcb.201002067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HIND LE, DEMBO M, HAMMER DA. Macrophage motility is driven by frontal-towing with a force magnitude dependent on substrate stiffness. Integr Biol (Camb) 2015;7:447–53. doi: 10.1039/c4ib00260a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HIRSCH E, KATANAEV VL, GARLANDA C, AZZOLINO O, PIROLA L, SILENGO L, SOZZANI S, MANTOVANI A, ALTRUDA F, WYMANN MP. Central role for G protein-coupled phosphoinositide 3-kinase gamma in inflammation. Science. 2000;287:1049–53. doi: 10.1126/science.287.5455.1049. [DOI] [PubMed] [Google Scholar]
- HOUK AR, JILKINE A, MEJEAN CO, BOLTYANSKIY R, DUFRESNE ER, ANGENENT SB, ALTSCHULER SJ, WU LF, WEINER OD. Membrane tension maintains cell polarity by confining signals to the leading edge during neutrophil migration. Cell. 2012;148:175–88. doi: 10.1016/j.cell.2011.10.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HYUN YM, SUMAGIN R, SARANGI PP, LOMAKINA E, OVERSTREET MG, BAKER CM, FOWELL DJ, WAUGH RE, SARELIUS IH, KIM M. Uropod elongation is a common final step in leukocyte extravasation through inflamed vessels. J Exp Med. 2012;209:1349–62. doi: 10.1084/jem.20111426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- IVETIC A, RIDLEY AJ. Ezrin/radixin/moesin proteins and Rho GTPase signalling in leucocytes. Immunology. 2004;112:165–76. doi: 10.1111/j.1365-2567.2004.01882.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JANNAT RA, DEMBO M, HAMMER DA. Traction forces of neutrophils migrating on compliant substrates. Biophys J. 2011;101:575–84. doi: 10.1016/j.bpj.2011.05.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JANNAT RA, ROBBINS GP, RICART BG, DEMBO M, HAMMER DA. Neutrophil adhesion and chemotaxis depend on substrate mechanics. J Phys Condens Matter. 2010;22:194117. doi: 10.1088/0953-8984/22/19/194117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KUMAR S, XU J, PERKINS C, GUO F, SNAPPER S, FINKELMAN FD, ZHENG Y, FILIPPI MD. Cdc42 regulates neutrophil migration via crosstalk between WASp, CD11b, and microtubules. Blood. 2012;120:3563–74. doi: 10.1182/blood-2012-04-426981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LACALLE RA, PEREGIL RM, ALBAR JP, MERINO E, MARTINEZ AC, MERIDA I, MANES S. Type I phosphatidylinositol 4-phosphate 5-kinase controls neutrophil polarity and directional movement. J Cell Biol. 2007;179:1539–53. doi: 10.1083/jcb.200705044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LAM PY, HUTTENLOCHER A. Interstitial leukocyte migration in vivo. Curr Opin Cell Biol. 2013;25:650–8. doi: 10.1016/j.ceb.2013.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LAMMERMANN T, AFONSO PV, ANGERMANN BR, WANG JM, KASTENMULLER W, PARENT CA, GERMAIN RN. Neutrophil swarms require LTB4 and integrins at sites of cell death in vivo. Nature. 2013;498:371–5. doi: 10.1038/nature12175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LAMMERMANN T, BADER BL, MONKLEY SJ, WORBS T, WEDLICH-SOLDNER R, HIRSCH K, KELLER M, FORSTER R, CRITCHLEY DR, FASSLER R, SIXT M. Rapid leukocyte migration by integrin-independent flowing and squeezing. Nature. 2008;453:51–5. doi: 10.1038/nature06887. [DOI] [PubMed] [Google Scholar]
- LAMMERMANN T, SIXT M. Mechanical modes of ‘amoeboid’ cell migration. Curr Opin Cell Biol. 2009;21:636–44. doi: 10.1016/j.ceb.2009.05.003. [DOI] [PubMed] [Google Scholar]
- LIM K, HYUN YM, LAMBERT-EMO K, CAPECE T, BAE S, MILLER R, TOPHAM DJ, KIM M. Neutrophil trails guide influenza–specific CD8(+) T cells in the airways. Science. 2015;349:aaa4352. doi: 10.1126/science.aaa4352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LIU L, DAS S, LOSERT W, PARENT CA. mTORC2 regulates neutrophil chemotaxis in a cAMP- and RhoA-dependent fashion. Dev Cell. 2010;19:845–57. doi: 10.1016/j.devcel.2010.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LIU X, YANG T, SUZUKI K, TSUKITA S, ISHII M, ZHOU S, WANG G, CAO L, QIAN F, TAYLOR S, OH MJ, LEVITAN I, YE RD, CARNEGIE GK, ZHAO Y, MALIK AB, XU J. Moesin and myosin phosphatase confine neutrophil orientation in a chemotactic gradient. J Exp Med. 2015a;212:267–80. doi: 10.1084/jem.20140508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LIU YJ, LE BERRE M, LAUTENSCHLAEGER F, MAIURI P, CALLAN-JONES A, HEUZE M, TAKAKI T, VOITURIEZ R, PIEL M. Confinement and low adhesion induce fast amoeboid migration of slow mesenchymal cells. Cell. 2015b;160:659–72. doi: 10.1016/j.cell.2015.01.007. [DOI] [PubMed] [Google Scholar]
- LO CM, WANG HB, DEMBO M, WANG YL. Cell movement is guided by the rigidity of the substrate. Biophys J. 2000;79:144–52. doi: 10.1016/S0006-3495(00)76279-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOKUTA MA, SENETAR MA, BENNIN DA, NUZZI PA, CHAN KT, OTT VL, HUTTENLOCHER A. Type Igamma PIP kinase is a novel uropod component that regulates rear retraction during neutrophil chemotaxis. Mol Biol Cell. 2007;18:5069–80. doi: 10.1091/mbc.E07-05-0428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOMBARDI ML, KNECHT DA, DEMBO M, LEE J. Traction force microscopy in Dictyostelium reveals distinct roles for myosin II motor and actin-crosslinking activity in polarized cell movement. J Cell Sci. 2007;120:1624–34. doi: 10.1242/jcs.002527. [DOI] [PubMed] [Google Scholar]
- LUDWIG A, OTTO GP, RIENTO K, HAMS E, FALLON PG, NICHOLS BJ. Flotillin microdomains interact with the cortical cytoskeleton to control uropod formation and neutrophil recruitment. J Cell Biol. 2010;191:771–81. doi: 10.1083/jcb.201005140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MAIURI P, RUPPRECHT JF, WIESER S, RUPRECHT V, BENICHOU O, CARPI N, COPPEY M, DE BECO S, GOV N, HEISENBERG CP, LAGE CRESPO C, LAUTENSCHLAEGER F, LE BERRE M, LENNON-DUMENIL AM, RAAB M, THIAM HR, PIEL M, SIXT M, VOITURIEZ R. Actin flows mediate a universal coupling between cell speed and cell persistence. Cell. 2015;161:374–86. doi: 10.1016/j.cell.2015.01.056. [DOI] [PubMed] [Google Scholar]
- MARTIN KJ, MUESSEL MJ, PULLAR CE, WILLARS GB, WARDLAW AJ. The role of phosphoinositide 3-kinases in neutrophil migration in 3D collagen gels. PLoS One. 2015;10:e0116250. doi: 10.1371/journal.pone.0116250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MARTINELLI S, CHEN EJ, CLARKE F, LYCK R, AFFENTRANGER S, BURKHARDT JK, NIGGLI V. Ezrin/Radixin/Moesin proteins and flotillins cooperate to promote uropod formation in T cells. Front Immunol. 2013;4:84. doi: 10.3389/fimmu.2013.00084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MATHIAS JR, PERRIN BJ, LIU TX, KANKI J, LOOK AT, HUTTENLOCHER A. Resolution of inflammation by retrograde chemotaxis of neutrophils in transgenic zebrafish. J Leukoc Biol. 2006;80:1281–8. doi: 10.1189/jlb.0506346. [DOI] [PubMed] [Google Scholar]
- MATSUI T, MAEDA M, DOI Y, YONEMURA S, AMANO M, KAIBUCHI K, TSUKITA S, TSUKITA S. Rho-kinase phosphorylates COOH-terminal threonines of ezrin/radixin/moesin (ERM) proteins and regulates their head-to-tail association. J Cell Biol. 1998;140:647–57. doi: 10.1083/jcb.140.3.647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MEILI R, FIRTEL RA. Two poles and a compass. Cell. 2003;114:153–6. doi: 10.1016/s0092-8674(03)00553-1. [DOI] [PubMed] [Google Scholar]
- MORIN NA, OAKES PW, HYUN YM, LEE D, CHIN YE, KING MR, SPRINGER TA, SHIMAOKA M, TANG JX, REICHNER JS, KIM M. Nonmuscle myosin heavy chain IIA mediates integrin LFA-1 de-adhesion during T lymphocyte migration. J Exp Med. 2008;205:195–205. doi: 10.1084/jem.20071543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MUNEVAR S, WANG Y, DEMBO M. Traction force microscopy of migrating normal and H-ras transformed 3T3 fibroblasts. Biophys J. 2001;80:1744–57. doi: 10.1016/s0006-3495(01)76145-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NAKAMURA F, HUANG L, PESTONJAMASP K, LUNA EJ, FURTHMAYR H. Regulation of F-actin binding to platelet moesin in vitro by both phosphorylation of threonine 558 and polyphosphatidylinositides. Mol Biol Cell. 1999;10:2669–85. doi: 10.1091/mbc.10.8.2669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NIGGLI V. Microtubule-disruption-induced and chemotactic-peptide-induced migration of human neutrophils: implications for differential sets of signalling pathways. J Cell Sci. 2003;116:813–22. doi: 10.1242/jcs.00306. [DOI] [PubMed] [Google Scholar]
- NOURSHARGH S, HORDIJK PL, SIXT M. Breaching multiple barriers: leukocyte motility through venular walls and the interstitium. Nat Rev Mol Cell Biol. 2010;11:366–78. doi: 10.1038/nrm2889. [DOI] [PubMed] [Google Scholar]
- OAKES PW, PATEL DC, MORIN NA, ZITTERBART DP, FABRY B, REICHNER JS, TANG JX. Neutrophil morphology and migration are affected by substrate elasticity. Blood. 2009;114:1387–95. doi: 10.1182/blood-2008-11-191445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PESTONJAMASP KN, FORSTER C, SUN C, GARDINER EM, BOHL B, WEINER O, BOKOCH GM, GLOGAUER M. Rac1 links leading edge and uropod events through Rho and myosin activation during chemotaxis. Blood. 2006;108:2814–20. doi: 10.1182/blood-2006-01-010363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- POUWELS J, DE FRANCESCHI N, RANTAKARI P, AUVINEN K, KARIKOSKI M, MATTILA E, POTTER C, SUNDBERG JP, HOGG N, GAHMBERG CG, SALMI M, IVASKA J. SHARPIN regulates uropod detachment in migrating lymphocytes. Cell Rep. 2013;5:619–28. doi: 10.1016/j.celrep.2013.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PRENTICE-MOTT HV, MEROZ Y, CARLSON A, LEVINE MA, DAVIDSON MW, IRIMIA D, CHARRAS GT, MAHADEVAN L, SHAH JV. Directional memory arises from long-lived cytoskeletal asymmetries in polarized chemotactic cells. Proc Natl Acad Sci U S A. 2016;113:1267–72. doi: 10.1073/pnas.1513289113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RAMSEY WS. Analysis of individual leucocyte behavior during chemotaxis. Exp Cell Res. 1972;70:129–39. doi: 10.1016/0014-4827(72)90190-5. [DOI] [PubMed] [Google Scholar]
- REINHART-KING CA, DEMBO M, HAMMER DA. The dynamics and mechanics of endothelial cell spreading. Biophys J. 2005;89:676–89. doi: 10.1529/biophysj.104.054320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SAEZ DE GUINOA J, BARRIO L, CARRASCO YR. Vinculin arrests motile B cells by stabilizing integrin clustering at the immune synapse. J Immunol. 2013;191:2742–51. doi: 10.4049/jimmunol.1300684. [DOI] [PubMed] [Google Scholar]
- SAHAI E, MARSHALL CJ. Differing modes of tumour cell invasion have distinct requirements for Rho/ROCK signalling and extracellular proteolysis. Nat Cell Biol. 2003;5:711–9. doi: 10.1038/ncb1019. [DOI] [PubMed] [Google Scholar]
- SANCHEZ-MADRID F, SERRADOR JM. Bringing up the rear: defining the roles of the uropod. Nat Rev Mol Cell Biol. 2009;10:353–9. doi: 10.1038/nrm2680. [DOI] [PubMed] [Google Scholar]
- SANZ-MORENO V, GADEA G, AHN J, PATERSON H, MARRA P, PINNER S, SAHAI E, MARSHALL CJ. Rac activation and inactivation control plasticity of tumor cell movement. Cell. 2008;135:510–23. doi: 10.1016/j.cell.2008.09.043. [DOI] [PubMed] [Google Scholar]
- SASAKI T, IRIE-SASAKI J, JONES RG, OLIVEIRA-DOS-SANTOS AJ, STANFORD WL, BOLON B, WAKEHAM A, ITIE A, BOUCHARD D, KOZIERADZKI I, JOZA N, MAK TW, OHASHI PS, SUZUKI A, PENNINGER JM. Function of PI3Kgamma in thymocyte development, T cell activation, and neutrophil migration. Science. 2000;287:1040–6. doi: 10.1126/science.287.5455.1040. [DOI] [PubMed] [Google Scholar]
- SERRADOR JM, ALONSO-LEBRERO JL, DEL POZO MA, FURTHMAYR H, SCHWARTZ-ALBIEZ R, CALVO J, LOZANO F, SANCHEZ-MADRID F. Moesin interacts with the cytoplasmic region of intercellular adhesion molecule-3 and is redistributed to the uropod of T lymphocytes during cell polarization. J Cell Biol. 1997;138:1409–23. doi: 10.1083/jcb.138.6.1409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SERRADOR JM, VICENTE-MANZANARES M, CALVO J, BARREIRO O, MONTOYA MC, SCHWARTZALBIEZ R, FURTHMAYR H, LOZANO F, SANCHEZ-MADRID F. A novel serine-rich motif in the intercellular adhesion molecule 3 is critical for its ezrin/radixin/moesin-directed subcellular targeting. J Biol Chem. 2002;277:10400–9. doi: 10.1074/jbc.M110694200. [DOI] [PubMed] [Google Scholar]
- SMITH A, BRACKE M, LEITINGER B, PORTER JC, HOGG N. LFA-1-induced T cell migration on ICAM-1 involves regulation of MLCK-mediated attachment and ROCK-dependent detachment. J Cell Sci. 2003;116:3123–33. doi: 10.1242/jcs.00606. [DOI] [PubMed] [Google Scholar]
- SMITH LA, ARANDA-ESPINOZA H, HAUN JB, DEMBO M, HAMMER DA. Neutrophil traction stresses are concentrated in the uropod during migration. Biophys J. 2007;92:L58–60. doi: 10.1529/biophysj.106.102822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SRINIVASAN S, WANG F, GLAVAS S, OTT A, HOFMANN F, AKTORIES K, KALMAN D, BOURNE HR. Rac and Cdc42 play distinct roles in regulating PI(3,4,5)P3 and polarity during neutrophil chemotaxis. J Cell Biol. 2003;160:375–85. doi: 10.1083/jcb.200208179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SUN C, FORSTER C, NAKAMURA F, GLOGAUER M. Filamin-A regulates neutrophil uropod retraction through RhoA during chemotaxis. PLoS One. 2013;8:e79009. doi: 10.1371/journal.pone.0079009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SZCZUR K, ZHENG Y, FILIPPI MD. The small Rho GTPase Cdc42 regulates neutrophil polarity via CD11b integrin signaling. Blood. 2009;114:4527–37. doi: 10.1182/blood-2008-12-195164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TAUZIN S, STARNES TW, BECKER FB, LAM PY, HUTTENLOCHER A. Redox and Src family kinase signaling control leukocyte wound attraction and neutrophil reverse migration. J Cell Biol. 2014;207:589–98. doi: 10.1083/jcb.201408090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TOMAS EM, CHAU TA, MADRENAS J. Clustering of a lipid-raft associated pool of ERM proteins at the immunological synapse upon T cell receptor or CD28 ligation. Immunol Lett. 2002;83:143–7. doi: 10.1016/s0165-2478(02)00075-5. [DOI] [PubMed] [Google Scholar]
- TOYJANOVA J, FLORES-CORTEZ E, REICHNER JS, FRANCK C. Matrix confinement plays a pivotal role in regulating neutrophil-generated tractions, speed, and integrin utilization. J Biol Chem. 2015;290:3752–63. doi: 10.1074/jbc.M114.619643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- UCHIDA KS, KITANISHI-YUMURA T, YUMURA S. Myosin II contributes to the posterior contraction and the anterior extension during the retraction phase in migrating Dictyostelium cells. J Cell Sci. 2003;116:51–60. doi: 10.1242/jcs.00195. [DOI] [PubMed] [Google Scholar]
- VAN GOETHEM E, POINCLOUX R, GAUFFRE F, MARIDONNEAU-PARINI I, LE CABEC V. Matrix architecture dictates three-dimensional migration modes of human macrophages: differential involvement of proteases and podosome-like structures. J Immunol. 2010;184:1049–61. doi: 10.4049/jimmunol.0902223. [DOI] [PubMed] [Google Scholar]
- VAN KEYMEULEN A, WONG K, KNIGHT ZA, GOVAERTS C, HAHN KM, SHOKAT KM, BOURNE HR. To stabilize neutrophil polarity, PIP3 and Cdc42 augment RhoA activity at the back as well as signals at the front. J Cell Biol. 2006;174:437–45. doi: 10.1083/jcb.200604113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WANG F, HERZMARK P, WEINER OD, SRINIVASAN S, SERVANT G, BOURNE HR. Lipid products of PI(3)Ks maintain persistent cell polarity and directed motility in neutrophils. Nat Cell Biol. 2002;4:513–8. doi: 10.1038/ncb810. [DOI] [PubMed] [Google Scholar]
- WEINER OD, NEILSEN PO, PRESTWICH GD, KIRSCHNER MW, CANTLEY LC, BOURNE HR. A PtdInsP(3)- and Rho GTPase-mediated positive feedback loop regulates neutrophil polarity. Nat Cell Biol. 2002;4:509–13. doi: 10.1038/ncb811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WEINER OD, RENTEL MC, OTT A, BROWN GE, JEDRYCHOWSKI M, YAFFE MB, GYGI SP, CANTLEY LC, BOURNE HR, KIRSCHNER MW. Hem-1 complexes are essential for Rac activation, actin polymerization, and myosin regulation during neutrophil chemotaxis. PLoS Biol. 2006;4:e38. doi: 10.1371/journal.pbio.0040038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WONG K, VAN KEYMEULEN A, BOURNE HR. PDZRhoGEF and myosin II localize RhoA activity to the back of polarizing neutrophil-like cells. J Cell Biol. 2007;179:1141–8. doi: 10.1083/jcb.200706167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WU J, PIPATHSOUK A, KEIZER-GUNNINK A, FUSETTI F, ALKEMA W, LIU S, ALTSCHULER S, WU L, KORTHOLT A, WEINER OD. Homer3 regulates the establishment of neutrophil polarity. Mol Biol Cell. 2015;26:1629–39. doi: 10.1091/mbc.E14-07-1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- XU J, WANG F, VAN KEYMEULEN A, HERZMARK P, STRAIGHT A, KELLY K, TAKUWA Y, SUGIMOTO N, MITCHISON T, BOURNE HR. Divergent signals and cytoskeletal assemblies regulate self-organizing polarity in neutrophils. Cell. 2003;114:201–14. doi: 10.1016/s0092-8674(03)00555-5. [DOI] [PubMed] [Google Scholar]
- XU J, WANG F, VAN KEYMEULEN A, RENTEL M, BOURNE HR. Neutrophil microtubules suppress polarity and enhance directional migration. Proc Natl Acad Sci U S A. 2005;102:6884–9. doi: 10.1073/pnas.0502106102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- YAMAHASHI Y, CAVNAR PJ, HIND LE, BERTHIER E, BENNIN DA, BEEBE D, HUTTENLOCHER A. Integrin associated proteins differentially regulate neutrophil polarity and directed migration in 2D and 3D. Biomed Microdevices. 2015;17:100. doi: 10.1007/s10544-015-9998-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- YANG HW, COLLINS SR, MEYER T. Locally excitable Cdc42 signals steer cells during chemotaxis. Nat Cell Biol. 2016;18:191–201. doi: 10.1038/ncb3292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- YONEMURA S, HIRAO M, DOI Y, TAKAHASHI N, KONDO T, TSUKITA S, TSUKITA S. Ezrin/radixin/moesin (ERM) proteins bind to a positively charged amino acid cluster in the juxta-membrane cytoplasmic domain of CD44, CD43, and ICAM-2. J Cell Biol. 1998;140:885–95. doi: 10.1083/jcb.140.4.885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- YOO SK, DENG Q, CAVNAR PJ, WU YI, HAHN KM, HUTTENLOCHER A. Differential regulation of protrusion and polarity by PI3K during neutrophil motility in live zebrafish. Dev Cell. 2010;18:226–36. doi: 10.1016/j.devcel.2009.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- YOSHINAGA-OHARA N, TAKAHASHI A, UCHIYAMA T, SASADA M. Spatiotemporal regulation of moesin phosphorylation and rear release by Rho and serine/threonine phosphatase during neutrophil migration. Exp Cell Res. 2002;278:112–22. doi: 10.1006/excr.2002.5571. [DOI] [PubMed] [Google Scholar]
- ZIGMOND SH, LEVITSKY HI, KREEL BJ. Cell polarity: an examination of its behavioral expression and its consequences for polymorphonuclear leukocyte chemotaxis. J Cell Biol. 1981;89:585–92. doi: 10.1083/jcb.89.3.585. [DOI] [PMC free article] [PubMed] [Google Scholar]