We report inhibition of Ca channels in horizontal cells by dopamine, a retinal neuromodulator regulated by light and the circadian clock. The work reveals that dopamine type 1 receptors mediate inhibition of Ca channels via a direct, voltage-dependent inhibition by Gβγ subunits. With Ca channels mediating horizontal cell neurotransmission, these results suggest that dopamine reduces inhibition of bipolar cells and photoreceptors, altering regulation of synaptic gain and lateral inhibition in visual processing in the retina.
Keywords: retinal horizontal cells, Ca2+ currents, Ca channels, G protein βγ-subunit, inhibition
Abstract
Horizontal cells form the first laterally interacting network of inhibitory interneurons in the retina. Dopamine released onto horizontal cells under photic and circadian control modulates horizontal cell function. Using isolated, identified horizontal cells from a connexin-57-iCre × ROSA26-tdTomato transgenic mouse line, we investigated dopaminergic modulation of calcium channel currents (ICa) with whole cell patch-clamp techniques. Dopamine (10 μM) blocked 27% of steady-state ICa, an action blunted to 9% in the presence of the L-type Ca channel blocker verapamil (50 μM). The dopamine type 1 receptor (D1R) agonist SKF38393 (20 μM) inhibited ICa by 24%. The D1R antagonist SCH23390 (20 μM) reduced dopamine and SKF38393 inhibition. Dopamine slowed ICa activation, blocking ICa by 38% early in a voltage step. Enhanced early inhibition of ICa was eliminated by applying voltage prepulses to +120 mV for 100 ms, increasing ICa by 31% and 11% for early and steady-state currents, respectively. Voltage-dependent facilitation of ICa and block of dopamine inhibition after preincubation with a Gβγ-blocking peptide suggested involvement of Gβγ proteins in the D1R-mediated modulation. When the G protein activator guanosine 5′-O-(3-thiotriphosphate) (GTPγS) was added intracellularly, ICa was smaller and showed the same slowed kinetics seen during D1R activation. With GTPγS in the pipette, additional block of ICa by dopamine was only 6%. Strong depolarizing voltage prepulses restored the GTPγS-reduced early ICa amplitude by 36% and steady-state ICa amplitude by 3%. These results suggest that dopaminergic inhibition of ICa via D1Rs is primarily mediated through the action of Gβγ proteins in horizontal cells.
NEW & NOTEWORTHY
We report inhibition of Ca channels in horizontal cells by dopamine, a retinal neuromodulator regulated by light and the circadian clock. The work reveals that dopamine type 1 receptors mediate inhibition of Ca channels via a direct, voltage-dependent inhibition by Gβγ subunits. With Ca channels mediating horizontal cell neurotransmission, these results suggest that dopamine reduces inhibition of bipolar cells and photoreceptors, altering regulation of synaptic gain and lateral inhibition in visual processing in the retina.
dopamine, a modulatory neurotransmitter in the retina whose synthesis and release is regulated by light and the retinal circadian clock, is released from inhibitory interneurons called dopaminergic amacrine cells (Doyle et al. 2002; Iuvone et al. 1978). Dopamine is well known for the numerous modulatory actions it has in the daily switch-overs from night vision, mediated by rods, to day vision, mediated by cones (Jackson et al. 2012; Witkovsky 2004). To accomplish many tasks, dopaminergic amacrine cells release dopamine onto multiple targets throughout the retina, including horizontal cells, in which it has been shown to modulate gap junction coupling, glutamate receptor activity, and spinule formation (Djamgoz et el. 1989; Iuvone et al. 2005; Knapp and Dowling 1987; Reitsamer et al. 2006; Ribelayga and Mangel 2003; Witkovsky 2004; Zhang et al. 2011).
A critical role for dopamine type 1 receptors (D1Rs) in maintaining the amplitude, dynamic range, and sensitivity of the b-wave of the electroretinogram (ERG) has been shown in D1R-knockout mice (Herrmann et al. 2011). This action was proposed to be due to D1Rs mediating increased release of GABA from horizontal cells to rod bipolar cell dendrites. Feedforward GABAergic transmission from horizontal cell to bipolar cells is considered likely in many reports investigating mammalian and nonmammalian vertebrate retinas (Herrmann et al. 2011; Thoreson and Mangel 2012; Yang and Wu 1991), based on the identification of GABA receptors in bipolar cell dendrites (Haverkamp et al. 2000; Vardi and Sterling 1994; Wässle et al. 1998) and responses of bipolar cell dendrites to GABA application (Hoon et al. 2015; Kaneda et al. 2000; Qian et al. 1997; Shields et al. 2000; Varela et al. 2005). Modulation of horizontal cell inhibition of bipolar cells by dopamine could have profound actions on retinal information processing.
Horizontal cell-mediated inhibition of photoreceptors has been shown in both mammalian and nonmammalian vertebrate retinas, in which depolarization of the horizontal cell membrane with injected current or activation of glutamate receptors leads to inhibition of voltage-gated Ca channels in photoreceptors (Babai and Thoreson 2009; Cadetti and Thoreson 2006; Davenport et al. 2008; Fahrenfort et al. 2009; Hirano et al. 2016; Hirasawa and Kaneko 2003; Liu et al. 2013; Thoreson et al. 2008; Vessey et al. 2005). Unlike the case in horizontal cells of most nonmammalian vertebrates studied, where GABA is released via reversed uptake, in mammalian horizontal cells this does not happen, as there are no GAT-type GABA uptake proteins to do this (Guo et al. 2009, 2010). Instead, mammalian horizontal cells express the proteins responsible for calcium-dependent SNARE protein-mediated vesicular release (reviewed in Hirano et al. 2016). Depolarization-induced inhibition of photoreceptors by horizontal cells depends on the voltage-dependent gating of the several Ca channel subtypes in rat horizontal cells (Liu et al. 2013). GABA release from horizontal cells has also been linked to inhibition of mammalian photoreceptors (Hirano et al. 2016; Kemmler et al. 2014; Liu et al. 2013). Should horizontal cell signaling to photoreceptors be modulated by dopamine, important changes in synaptic gain and lateral inhibition at the photoreceptor presynaptic terminal could occur.
Dopaminergic modulation of Ca channel-dependent signaling from horizontal cells to photoreceptors and bipolar cells has not been investigated in mammalian retina. Modulation of Ca channels in horizontal cells by dopamine would add to the many known actions of dopaminergic modulation of horizontal cell function. The goal of this study was to investigate the contribution of dopamine to the modulation of Ca channel currents in isolated horizontal cells, to add to our understanding of how dopamine may regulate horizontal cell inhibitory actions in the outer retina. The investigation tests a cellular mechanism potentially underlying the hypothesis that D1R activation could increase horizontal cell GABA release (Herrmann et al. 2011) by examining the actions of dopaminergic agents on the voltage-dependent gating of Ca channels of horizontal cells.
MATERIALS AND METHODS
This study used a connexin-57-iCre × ROSA26-tdTomato (Cx57-tdTomato) transgenic mouse line, in which only horizontal cells express tdTomato fluorescence, allowing positive identification of the isolated cells (Hoon et al. 2015). Experiments were performed in accordance with the guidelines for the welfare of experimental animals issued in the U.S. Public Health Service Policy on Humane Care and Use of Laboratory Animals (2002) and were reviewed and approved by the University of California-Los Angeles (UCLA) Animal Research Committee.
Patch-clamp recording from isolated horizontal cells.
Ca channel currents were measured in isolated Cx57-tdTomato mouse horizontal cells with standard whole cell patch clamp. Mice were deeply anesthetized with 1–3% isoflurane (IsoFlo, Abbott Laboratories, Abbott Park, IL) and decapitated, the eyes were enucleated, and the anterior portion of an eye including the lens was removed. The retina was removed and incubated in Hanks' balanced salt solution (HBSS; Hyclone, Logan, UT) containing 18 U/ml papain and 100 U/ml DNase I (Worthington Biochemical, Freehold, NJ) at 37°C for 40 min. Isolated cells were obtained by gentle trituration after digestion. The cells were kept in Dulbecco's modified Eagle's medium (DMEM; Life Technologies, Grand Island, NY) with 10% fetal bovine serum (FBS; Life Technologies) and penicillin-streptomycin (1×; Invitrogen) in a 5% CO2 incubator at 37°C. For fluorescent cell identification and initial patch-clamp recording, the solution was changed to a standard bathing solution containing (in mM) 138 NaCl, 3 KCl, 2 CaCl2, 1.25 NaH2PO4, 1 MgCl2, 10 glucose, and 10 HEPES, adjusted to pH 7.4 with NaOH, which was delivered via a gravity-driven fast flow system. To record whole cell Ca channel currents carried by Ba2+, isolated horizontal cells were bathed in a solution containing (in mM) 115 NaCl, 2.5 KCl, 5 CsCl, 10 BaCl2, 15 TEA Cl, 10 glucose, and 15 HEPES, adjusted to pH 7.4 with NaOH, while the pipette solution contained (in mM) 140 CsCl, 0.1 CaCl2, 1 EGTA, 10 HEPES, 3 Mg-ATP, 0.2 Li-GTP, and 8 phosphocreatine, at pH 7.2 with CsOH. TTX (100 nM) was added to the bath solution to block sodium currents in some experiments. Room-temperature (21–24°C) solutions were superfused via a gravity-driven system. Patch electrodes with 5- to 10-MΩ tip resistance were pulled from fire-polished borosilicate glass capillary tubes with a micropipette puller (Sutter Instrument, Novato, CA). The bath reference electrode consisted of an AgCl wire in a side chamber. Cell voltage was clamped with an Axopatch-200B amplifier (Molecular Devices, Sunnyvale, CA) using whole cell capacitance and series resistance compensation. The current signal was filtered at 2 kHz and digitized at 10 kHz with an Axon Digidata 1320A for storage on the hard disk of a computer running pCLAMP 8.2 acquisition software (Molecular Devices). All data are reported as means ± SE. Graphing and statistical analyses were performed with MATLAB 7.6 (MathWorks, Natick, MA). Student's t-test was used to determine whether significant differences existed between paired and unpaired data sets as appropriate, with other tests used as described in the text. P values of <0.05 were considered statistically significant.
Drugs and chemicals.
All chemicals and reagents, unless otherwise noted, were obtained from Sigma-Aldrich (St. Louis, MO). SKF38393 and ω-conotoxin GVIA were obtained from Tocris (Ellisville, MOA). SCH23390 and ω-agatoxin IVA were obtained from Ascent Scientific (Princeton, NJ). Drugs and reagents were either prepared in double-distilled water as stock solutions (frozen at −20°C) or prepared fresh. Spiperone was prepared as a stock solution in DMSO. Superfused drugs normally produced their full effects in ∼1 min, but in cases where no response was seen, a limit of 5 min was deemed sufficient to conclude an absence of action.
RESULTS
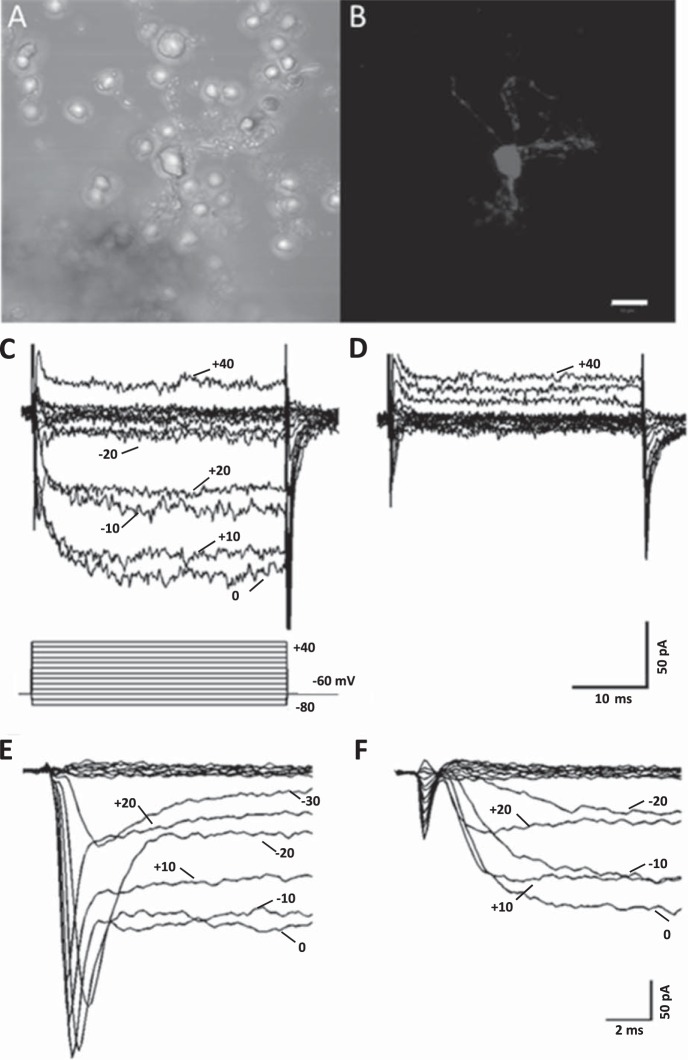
In this study, the dopamine sensitivity of Ca channel currents in isolated horizontal cells from Cx57-Tomato transgenic mouse retinas was explored. Figure 1, A and B, show an example of an enzymatically isolated horizontal cell in phase contrast and under fluorescence excitation light. Only horizontal cells express tdTomato fluorescence in this retina, so the identity of isolated cells could be readily established. Once isolated and identified, horizontal cells were whole cell patch-clamped in bathing and intracellular (pipette) solutions designed to isolate and enhance Ca channel currents. Inward Ca channel currents carried by Ba2+ were recorded with the voltage step paradigm shown in Fig. 1C. These currents were completely blocked during superfusion with 10 μM Cd2+, as shown in Fig. 1D. In a sample of six isolated horizontal cells, 10 μM Cd2+ blocked 90.0 ± 1.9% (P < 0.05) of the inward current at 0 mV. Fast transient inward currents were present in many but not all recordings, and their identity as voltage-gated Na channels, not T-type Ca channels, was confirmed by superfusion with TTX (100 nM), as shown in Fig. 1, E and F, in which complete block of the transient currents was seen. Previous reports of voltage-gated Na channels in horizontal cells from nonmammalian and mammalian vertebrates have been made (Malchow et al. 1990; Martin et al. 1996; Mojumder et al. 2007; Shingai and Christensen 1983, 1986; Ueda et al. 1992).
Fig. 1.
Ca channel currents in an isolated horizontal cell from a connexin-57-iCre × ROSA26-tdTomato (Cx57-tdTomato) mouse retina. A: phase-contrast image of an enzymatically treated, isolated horizontal cell. B: same field of view under green excitation light (560 nm) with red emission filter (610 nm), showing red tdTomato fluorescence from cell body and surrounding cellular processes. Scale bar for both images is 10 μm. C: inward Ca channel currents carried by Ba2+ recorded in response to the voltage step paradigm shown below traces. Cell was voltage-clamped at a holding potential of −60 mV, and steps lasting 40 ms were applied to potentials from −80 mV to 40 mV in 10-mV increments. D: same cell as in C shows that Ca channel currents were completely blocked during superfusion with 10 μM Cd2+. E: Na channel currents and Ca channel currents in response to voltage clamp steps from −80 to +20 mV, shown on an expanded timescale and from a different cell. F: same cell as in E showing 100 nM TTX blocking the Na channel currents.
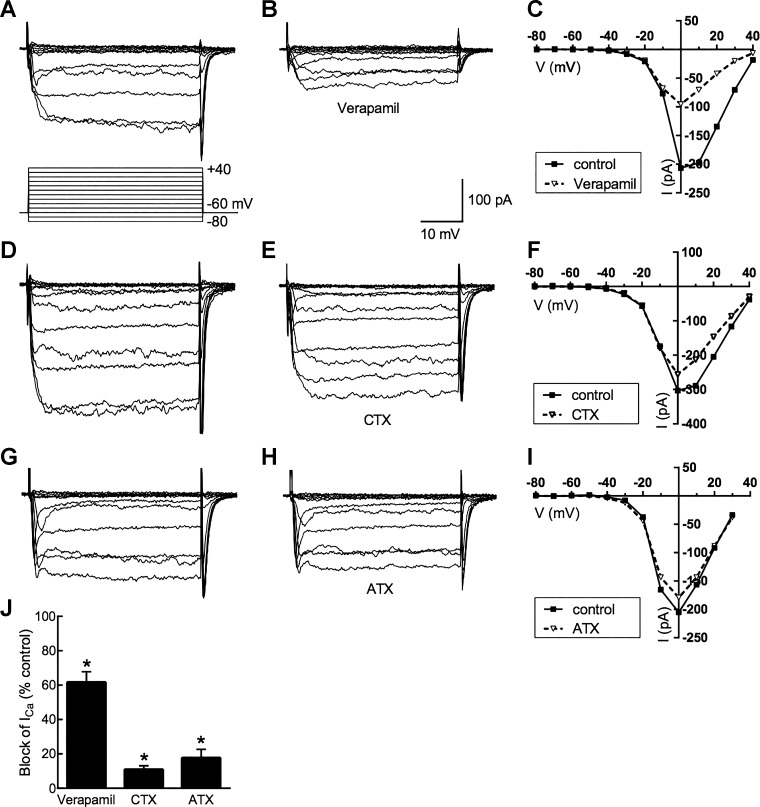
Figure 2 shows that the whole cell Ca channel current was carried predominantly in L-type Ca channels. The partial blocking effects of the L-type channel blocker verapamil (50 μM; 62% ± 6% reduction; n = 15, P < 0.05), the N-type channel blocker ω-conotoxin GVIA (1 μM; 9 ± 1% reduction; n = 6, P < 0.05), and the P/Q-type channel blocker ω-agatoxin IVA (200 nM; 18 ± 4% reduction; n = 3, P < 0.05) are similar to previous observations in mouse retina (Schubert et al. 2006) but indicate less N-type Ca channel activity in the isolated cell preparations used here. Peak Ca channel currents usually occurred at 0 mV, but this value was seen to occur between −10 and +20 mV in different cells.
Fig. 2.
Pharmacology of horizontal cell Ca channels. Horizontal cells were voltage-clamped at −60 mV and subsequently depolarized in steps lasting 40 ms to potentials between −80 mV and 40 mV. A–C: Ca channel currents before and during application of L-type Ca channel blocker verapamil (50 μM) and the current-voltage (I-V) relations in the steady state (averaged over the final 5 ms during each voltage step). D–F: Ca channel currents before and during application of N-type Ca channel blocker ω-conotoxin GVIA (CTX; 1 μM) and the steady-state I-V relations. G–I: Ca channel currents before and during application of P/Q-type Ca channel blocker ω-agatoxin IVA (ATX; 200 nM) and the steady-state I-V relations. J: pharmacology of horizontal cell peak Ca channel currents: % of peak control current that was blocked. *P < 0.05.
Dopamine modulates horizontal cell Ca channel currents.
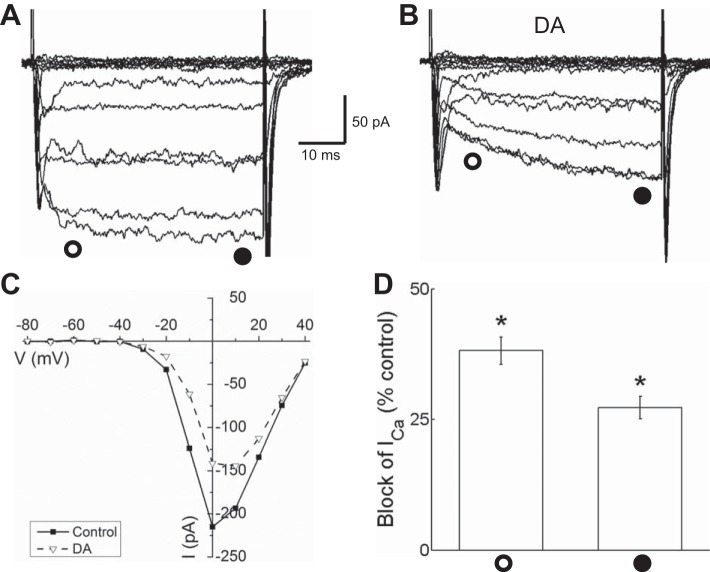
Dopamine application to isolated horizontal cells rapidly reduced Ca channel currents. Figure 3A shows control Ca channel currents recorded before dopamine application that were significantly inhibited during superfusion with 10 μM dopamine (shown in Fig. 3B). The inward current amplitude is reduced, and activation of the currents is slowed. In Fig. 3C, current-voltage (I-V) relations that were measured in control conditions and in the presence of dopamine show reduction of the peak inward current. Comparing the reduction by dopamine of the peak Ca channel current measured early in the voltage step (38.2% ± 2.6%; n = 6, P < 0.05) and the reduction near the end of the step (27.3% ± 2.2%; n = 6, P < 0.05) emphasizes that the dopamine-induced slowing of activation kinetics is produced by more inhibition in the first 10 ms of each step than after 40 ms of depolarization (Fig. 3D). The difference in early and steady-state current reduction was significant (P < 0.01).
Fig. 3.
Dopamine reduced Ca channel currents in isolated mouse horizontal cells identified by tdTomato fluorescence. A: Ca channel currents recorded under voltage clamp in response to the 40-ms voltage step paradigm shown in Fig. 1C before dopamine application. B: Ca channel currents recorded during superfusion with 10 μM dopamine (DA) show reduction of inward current amplitude and slowed activation. C: steady-state current-voltage (I-V) relations measured in control conditions and in the presence of DA near the end of each voltage step (time of measurement denoted by filled circle below the current traces) show reduction of the current between −30 and +30 mV. D: % of peak control current that was blocked by DA ∼10 ms after beginning of each voltage step (open circle) and at end of voltage steps (filled circle). *P < 0.05.
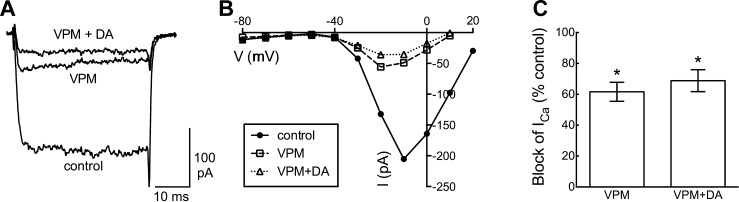
L-type Ca channels, which account for over half of the Ca channel current, are the only single subtype in horizontal cells that would account for the 38% block by dopamine. To test whether other subtypes are also modulated by dopamine, the amount by which dopamine suppressed Ca channel current in the presence of verapamil was measured. Figure 4A shows results from a cell having ∼200 pA of peak Ca channel current in control conditions (−10 mV), which in the presence of verapamil (50 μM) was reduced to ∼50 pA at peak and for which the addition of dopamine (10 μM) then suppressed an additional ∼20 pA. The I-V relations for this cell in the control, verapamil, and dopamine + verapamil conditions are shown in Fig. 4B. Figure 4C summarizes the results, showing that verapamil decreased Ca channel current by 61.7 ± 6.1% (P < 0.001, n = 15) and that addition of dopamine to the remaining current produced an additional decrease of 9.5 ± 7.3% (P < 0.001, n = 12). This indicates that while most of the current blocked by dopamine is carried in L-type channels, other Ca channel types appear to be inhibited as well.
Fig. 4.
Verapamil occludes the current reduced by dopamine. A: current traces recorded in sequence during voltage clamp step to −10 mV in control, verapamil (50 μM; VPM), and verapamil + dopamine (10 μM; VPM+DA). B: steady-state current-voltage relations show reduction of current by verapamil and subsequent modest further reduction by dopamine. C: % of peak control current that was blocked by verapamil (same data as in Fig. 2J) compared with block of current by verapamil and dopamine together. *P < 0.05.
Suppression of Ca channel currents in horizontal cells by dopamine is mediated by D1Rs.
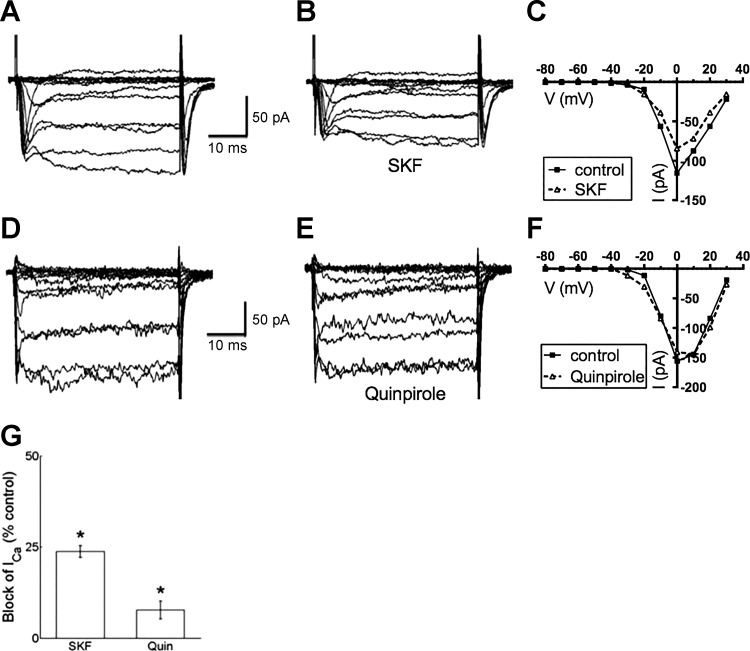
To test which type of dopamine receptor mediated this Ca channel current suppression, the D1R agonist SKF38393 was applied and found to reduce peak Ca channel currents in a kinetically similar manner, although more modestly, compared with dopamine (Fig. 5, A–C). SKF38393 significantly reduced early calcium current by 25.3 ± 2.0% and current during steady state by 19.5 ± 1.9%, and the early vs. late difference was significant (P < 0.001, n = 15).
Fig. 5.
Dopamine D1R agonist SKF38393 reduced Ca channel currents in isolated mouse horizontal cells, while dopamine D2R agonist quinpirole had small effect. A: Ca channel currents recorded in response to the voltage step paradigm shown in Fig. 1C before SKF38393 application. B: Ca channel currents recorded during treatment with 20 μM SKF38393 (SKF) show reduction of amplitude and slowed activation. C: steady-state I-V relations for recordings in A and B. D: Ca channel currents recorded before application of dopamine D2R agonist quinpirole. E: Ca channel currents recorded during treatment with 20 μM quinpirole show little reduction of amplitude and normal activation kinetics. F: steady-state I-V relations for recordings in D and E. G: % of peak control current that was blocked by SKF38393 (SKF) and by quinpirole (Quin). *P < 0.05.
In contrast, the dopamine type 2 receptor (D2R) agonist quinpirole had much less of an effect (Fig. 5, D–F). These results are summarized in Fig. 5G for cells tested with SKF38393 producing a mean reduction of 23.8 ± 1.7% (n = 9, P < 0.05) and quinpirole reducing the current by 7.7 ± 2.5% (n = 5, P < 0.05).
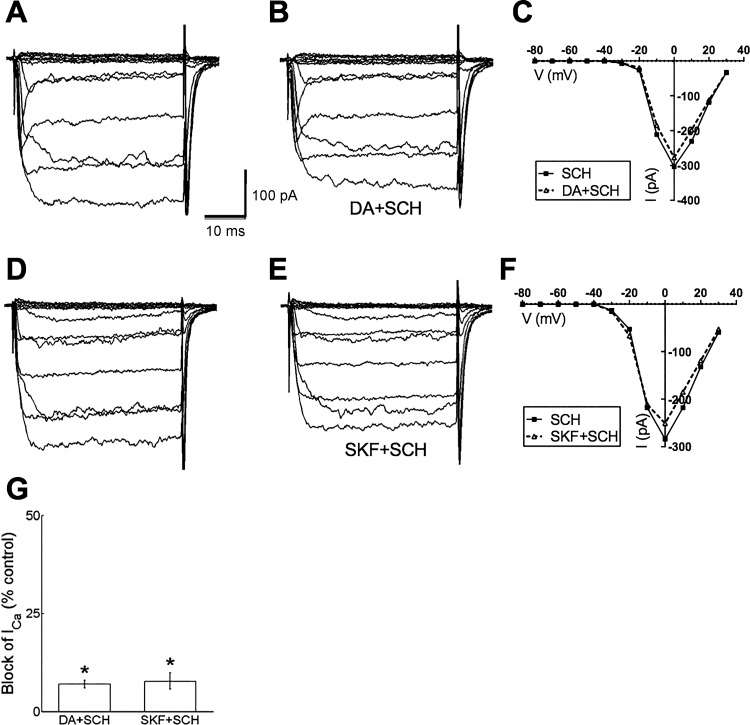
D1R antagonist blocks inhibition by dopamine.
To further determine the specificity of the role of the D1R, we tested whether the D1R antagonist SCH23390 interfered with the actions of dopamine. Figure 6, A–C, show that application of SCH23390 strongly blunted the inhibition of peak Ca channel currents normally caused by dopamine, reducing the inhibition to just 7.0 ± 1.0% (n = 6, P < 0.05). The involvement of the dopamine D1R was further scrutinized by determining whether the D1R antagonist SCH23390 interfered with the actions of the D1R agonist SKF38393. As shown in Fig. 6, D–F, SCH23390 limited the inhibitory actions of the D1R agonist SKF38393 to 7.8 ± 2.1% (n = 6, P < 0.05). Figure 6G summarizes the reduction of steady-state Ca channel current inhibition caused by dopamine and SKF38393 in the presence of SCH23390.
Fig. 6.
Dopamine D1R antagonist SCH23390 blocked Ca channel current reduction by dopamine and D1R agonist SKF38393. A: Ca channel currents recorded in the presence of 20 μM SCH23390 before dopamine application. B: Ca channel currents recorded in the same cell in the presence of 20 μM SCH23390 (SCH) and 10 μM dopamine (DA) show small reduction of amplitude. C: steady-state I-V relations for recordings in A and B. D: Ca channel currents recorded in the presence of 20 μM SCH23390 before SKF38393 application. E: Ca channel currents from the same cell in the presence of 20 μM SCH23390 and 20 μM SKF38393 show small reduction of amplitude. F: steady-state I-V relations for recordings in D and E. G: % of peak control current that was blocked by dopamine (DA) and SKF38393 (SKF) in the presence of SCH23390 (SCH). *P < 0.05.
In an additional test of specificity, spiperone, a D2R antagonist, failed to blunt the inhibitory actions of dopamine. In the presence of 20 μM spiperone, 10 μM dopamine inhibited peak Ca channel currents in horizontal cells by 30.8 ± 0.7% (n = 5, P < 0.05; data not shown). This suggests that the major action of dopamine on Ca channel currents in horizontal cells is mediated by D1Rs, not D2Rs.
Depolarization-induced facilitation of Ca channel currents inhibited by dopamine.
Previous studies of Ca channel inhibition by dopamine and other neurotransmitters have shown that the inhibition is sometimes mediated directly by the βγ-subunit of the GTP-binding protein (Gβγ). Activation of G protein-coupled receptors, such as the dopamine D1R, leads to separation of the Gα and Gβγ subunits of the G protein, which can exert actions on a number of cellular targets (Tedford and Zamponi 2006). For Gβγ subunits, one of these targets can be the voltage-gated Ca channel itself, with direct binding of the Gβγ subunit causing inhibition of channel gating, reducing Ca channel currents. We investigated a role for Gβγ subunits in the direct inhibition of Ca channels in horizontal cells with a test that involves applying strong depolarizing prepulses to the inhibited channels (Tedford and Zamponi 2006), which can produce a disinhibiting action called depolarization-dependent facilitation.
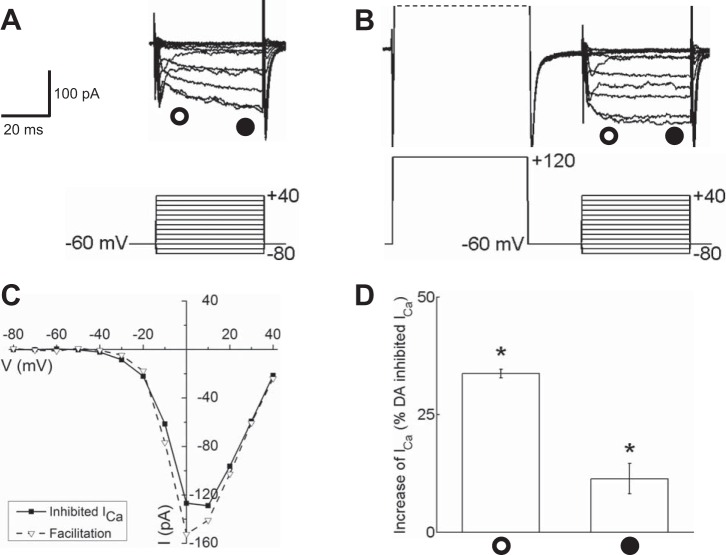
Ca channel currents that had been inhibited by dopamine in isolated mouse horizontal cells responded to +120-mV depolarizing prepulses with larger inward currents and faster activation kinetics (Fig. 7). Figure 7A shows Ca channel currents inhibited by dopamine in the characteristic manner (reduced amplitude and slowed kinetics of activation), which respond to the strong depolarizing prepulses with increased amplitudes and faster activation kinetics (Fig. 7, A and B). In the dopamine-inhibited state, the slowed activation kinetics are considered to reflect the unbinding of Gβγ subunits during the moderate depolarizations to voltages around zero. The much stronger depolarizing step to +120 mV is thought to produce rapid and near-complete dissociation of the inhibitory Gβγ subunits from the Ca channel, thus eliminating the slowed activation and producing larger, disinhibited currents. Figure 7C shows the I-V relations for the dopamine-inhibited and prepulse-disinhibited states, measured near the end of the 40-ms steps, and measurements of the peak currents summarized for all cells tested are shown in Fig. 7D, revealing that the measurement near the end of the voltage step produced an increase of 11.3 ± 2.2% in Ca channel current (n = 5, P < 0.05), while the increase in current in the first 10 ms was much greater at 33.7 ± 0.9% (n = 5, P < 0.05). The difference between the early and late current amplitude increases was significant (P < 0.0001). If the strong depolarizing voltage steps were able to “unblock” all of the dopamine-inhibited Ca channel current, which was ∼38% in the first 10 ms of the test voltage step, the recovery should have been closer to 50% of the remaining current, more than the increase seen here. The ∼34% recovery of early current observed might reflect the prepulse facilitation process operating at ∼68% efficiency. Were uncontaminated measurements possible at earlier times in the test steps, this apparent value might rise. The results are consistent with the inhibition of Ca channel currents by dopamine being mediated in large part by activation of Gβγ subunits.
Fig. 7.
Strong depolarizing prepulses restored Ca channel currents that had been inhibited by dopamine in isolated mouse horizontal cells. A: Ca channel currents evoked by the voltage step paradigm illustrated below the current traces are shown partially blocked after application of 10 μM dopamine. B: in the same dopamine-treated cell, Ca channel currents recorded after a depolarizing prepulse to +120 mV show current amplitudes and kinetics restored (facilitated) to the typical unblocked state. C: I-V relationships for Ca channel currents shown in A (inhibited ICa) and B (facilitation) recorded near the end of each voltage step (time of measurement denoted by the filled circle below the current traces in A and B). D: % increase of dopamine-reduced peak ICa induced by the prepulse to +120 mV ∼10 ms after the beginning of each voltage step (open circle) and at end of voltage steps (filled circle). *P < 0.05.
Gβγ proteins mediate Ca channel current suppression by DA.
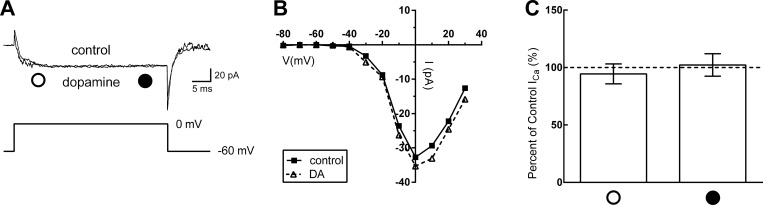
We note that prepulses to +120 mV did not significantly alter the Ca channel currents in horizontal cells that had not been treated with dopamine. Strong depolarizing prepulses had no significant effect on Ca channel current at 0 mV, tending to increase the current by 5.5 ± 1.7 pA (n = 7, P > 0.05; data not shown). This implies that, within the context of this model, there was little or no basal inhibition by Gβγ subunits under control conditions in our isolated horizontal cells. To further test the role that Gβγ plays in DA signaling, an inhibitor of Gβγ proteins was incubated with isolated horizontal cells prior to recording. Anti-βγ MPS-phosducin-like protein C terminus peptide is a small membrane-permeant peptide that interacts with Gβγ, preventing its dissociation from the G protein trimeric complex after receptor stimulation (Farrell et al. 2014; Morrey et al. 2008; Orr et al. 2002). Figure 8A shows a voltage-clamp recording from an isolated horizontal cell after pretreatment with anti-βγ peptide (1 μM, 15 min) in which there was no difference before or after DA superfusion. The I-V relation in Fig. 8B shows no difference as well. The data summary in Fig. 8C shows that in cells pretreated with the anti-βγ peptide dopamine had no significant effect on calcium current at either the early or late points of the voltage step. On average, dopamine decreased Ca channel current at the early point of the voltage step by 5.5 ± 5.9% (n = 7, P = 0.39) and increased Ca channel current near the end of the voltage step by 2.3 ± 6.7% (n = 7, P = 0.89).
Fig. 8.
Inhibition of Gβγ subunits by phosphoducin-like anti-βγ peptide occluded Ca channel current reduction by dopamine. A: inward Ca channel currents recorded at 0 mV in a cell pretreated in 1 μM anti-βγ. No reduction was found with 10 μM dopamine compared with control. Open and filled circles show early and late time points at which the data in C were obtained. B: steady-state I-V relations measured in control conditions and in the presence of 10 μM DA near the end of each voltage step from the same cell shown in A. C: comparison of peak current of anti-βγ pretreated mouse horizontal cells in 10 μM DA to control ∼10 ms into each voltage step (open circle) and near the end of each voltage step (filled circle).
We noted that cells pretreated with anti-βγ peptide had significantly smaller Ca channel currents at both early time points and the end of the voltage step compared with those without pretreatment. At the beginning of the voltage step, anti-βγ peptide-pretreated cells had Ca channel currents with an average amplitude of 43.3 ± 4.8 pA, while cells without this pretreatment had currents with an average amplitude of 104.0 ± 9.3 pA. This difference was significant (P < 0.00001, Welch 2-sample test; n = 7 and 18). Similarly, near the end of the voltage step, anti-βγ-pretreated cells had currents with an average amplitude of 44.0 ± 4.8 pA, while cells without this pretreatment had Ca channel currents with an average amplitude of 108.2 ± 9.4 pA. This difference was significant (P < 0.0001, Welch 2-sample test; n = 7 and 18).
G protein activator GTPγS occludes inhibition by dopamine.
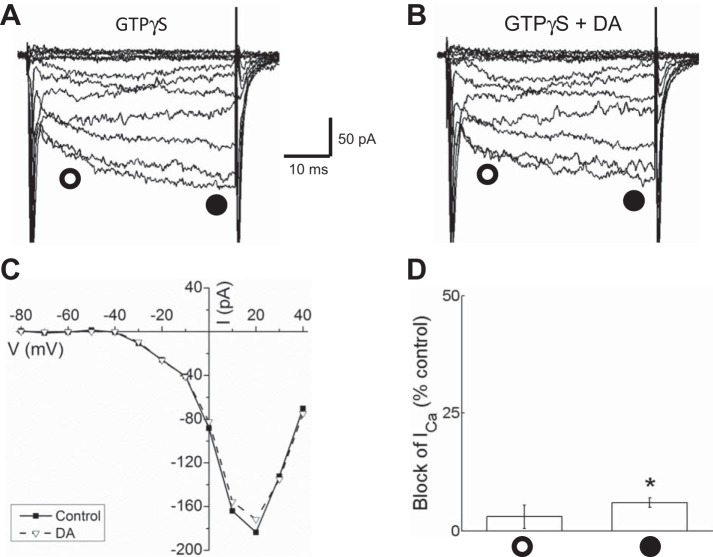
As a second test for a role of Gβγ subunits in the inhibition of Ca channel currents in horizontal cells, we used the GTP-binding protein activator guanosine 5′-O-(3-thiotriphosphate) (GTPγS) to determine whether dopamine would continue to inhibit the currents. Inclusion of GTPγS in the pipette occluded further inhibition of peak Ca channel currents by dopamine, as shown in Fig. 9, A and B. For the cell illustrated the I-V relations before and after dopamine application are nearly indistinguishable (Fig. 9C), and for the group of cells tested there was a reduction of only 3.3 ± 2.5% (n = 5, P < 0.05) at the early time point during the voltage step and a reduction of 6 ± 1% (n = 5, P < 0.05) near the end of the 40-ms step (Fig. 9D). These reductions are much smaller than the 38.2% reduction at the early time point and the 27.3% reduction at the end of the step, suggesting that GTPγS blocked further reduction of Ca channel current by dopamine, most likely by activating GTP-binding proteins and Gβγ subunits.
Fig. 9.
Activation of G proteins with GTPγS occluded inhibition of Ca channel currents by dopamine in isolated mouse horizontal cells. A: ensemble of traces recorded in the presence of GTPγS in the intracellular solution showing slowed activation kinetics. B: Ca channel currents in the same GTPγS-filled cell recorded after treatment with 10 μM dopamine showed modest inhibition. C: I-V relationships of Ca channel currents measured near the end of each voltage step (filled circles) from the same cell in A and B recorded from with GTPγS in the intracellular solution before (control) and after treatment with 10 μM dopamine (DA). D: % of peak control current that was blocked by dopamine in GTPγS-treated cells ∼10 ms after the beginning of each voltage step (open circle) and at end of voltage steps (filled circle). *P < 0.05.
Ca channel current inhibited by GTPγS is facilitated by depolarization.
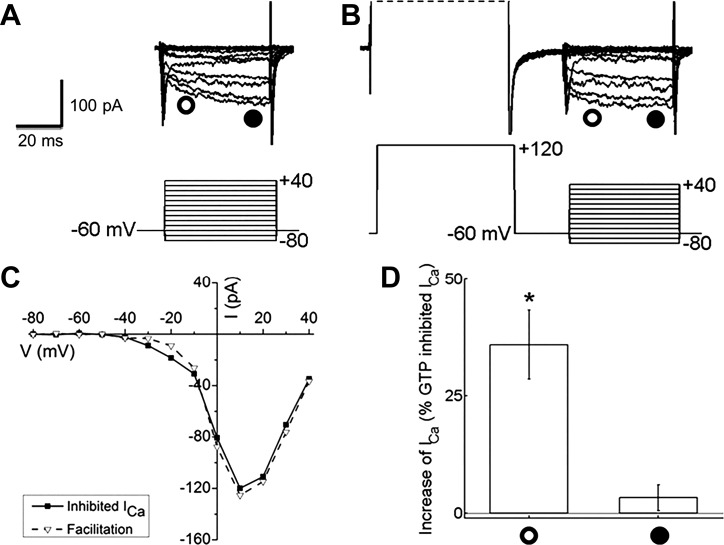
If Gβγ subunits released by dopamine are linked to inhibition of Ca channel current, then direct activation of these subunits with GTPγS in the recording pipette should produce inhibition that can be reversed with strong depolarizing prepulses. Figure 10A shows another example of horizontal cell Ca channel currents in the inhibited state, induced here as in Fig. 9 by inclusion of GTPγS in the pipette. In Fig. 10B depolarizing prepulses to +120 mV were applied before each step voltage, and the resulting Ca channel current was larger and showed restored, fast activation kinetics. The peak I-V curves, measured near the end of the 40-ms voltage steps, for these two conditions virtually overlap (Fig. 10C), but the early phase of the facilitation is large at 35.9 ± 7.4% (n = 5, P < 0.05) and much smaller near the end of the voltage steps (3.3 ± 2.8%; n = 5, P < 0.05; Fig. 10D). The difference between the early and late measurements is significant (P < 0.01). In other neurons, facilitation of this nature has been attributed to voltage-dependent unbinding of inhibitory Gβγ subunits.
Fig. 10.
Strong depolarizing prepulses restored Ca channel current inhibited by G protein activation with GTPγS in isolated mouse horizontal cells. A: Ca channel currents, evoked by the voltage step paradigm illustrated below the current traces, from a cell treated with GTPγS in the recording pipette. B: in the same cell, Ca channel currents in response to voltage steps but recorded after a depolarizing prepulse to +120 mV show current amplitudes and kinetics restored to unblocked state. C: I-V relationships for Ca channel currents shown in A (inhibited ICa) and B (facilitation) recorded near the end of each voltage step (time of measurement denoted by filled circle below current traces in A and B). D: % increases induced by the prepulse to +120 mV of ICa measured at the peak of the I-V relation. Open circle indicates the depolarization-induced increase measured ∼10 ms after the beginning of the voltage steps, and filled circle indicates the increase near the end of that voltage step. *P < 0.05.
DISCUSSION
The principal result presented here is that dopamine suppresses voltage-gated Ca channel activity in horizontal cells via activation of D1Rs. Since mammalian horizontal cells inhibit photoreceptors when they are depolarized to levels that activate these Ca channels (Hirano et al. 2016; Liu et al. 2013), this result implies that dopamine could alter horizontal cell inhibitory signaling.
An additional major finding is that, at the cellular level, much of the D1R-mediated inhibition of voltage-gated Ca channels in horizontal cells bears the hallmarks of a direct, voltage-dependent, inhibitory Gβγ subunit interaction with the Ca channels (Khan et al. 2013). However, while ruling out actions mediated by D2Rs in horizontal cells, the results presented do not preclude multiple D1R-mediated signaling pathways, as discussed below.
Impacts of dopaminergic modulation on horizontal cell inhibitory output.
In all species studied, light-induced dopamine release in the outer plexiform layer of the retina is believed to act at D1Rs in horizontal cells (Hampson et al. 1992; Witkovsky 2004). The best-studied action of dopamine is its reduction of gap junction coupling of horizontal cells, which has been shown through receptive field size measurements and dye coupling and with dual-electrode recordings of cocultured horizontal cells, where all observations are consistent with D1R cAMP-mediated mechanisms (He et al. 2000).
Dopaminergic modulation that increases the release of GABA from horizontal cells was hypothesized to be a plausible mechanism responsible for maintaining large b-wave amplitudes of the mouse ERG (Herrmann et al. 2011). While the GABAergic feedforward pathway from horizontal cells to bipolar cell dendrites has anatomical and physiological bases in both mammalian and nonmammalian species (Du and Yang 2000; Hare and Owen 1996; Haverkamp et al. 2000; Herrmann et al. 2011; Kaneda et al. 2000; Qian et al. 1997; Shields et al. 2000; Thoreson and Mangel 2012; Vardi and Sterling 1994; Varela et al. 2005; Wässle et al. 1998; Yang and Wu 1991), whether dopamine modulates this pathway has not been determined. However, the interpretation emerging from the present work suggests that dopamine would reduce GABA release since the action of dopamine is to reduce Ca channel activity in horizontal cells, upon which GABAergic inhibition depends (Hirano et al. 2016). Our investigation does not support the hypothesis of Herrmann et al. (2011). Other retinal pathways exist, likely acting at the bipolar cell axon terminals, which could provide D1R-mediated enhancement of GABA release onto bipolar cells. A D1R agonist suppresses the b-wave by acting on interneurons expressing voltage-gated Na channels, suggesting that the responsible GABAergic pathway is in the inner retina (Smith et al. 2015). GABAergic inhibitory amacrine cells synapse onto bipolar cell axon terminals (Chávez et al. 2006, 2010; Eggers and Lukasiewicz 2006; Hartveit 1999; Wässle et al. 1998). Serial inhibition between amacrine cells is a pathway known to suppress GABA input to some bipolar cells and, modulated by D1Rs, provides an alternate inner retinal mechanism accounting for the actions of GABA and dopamine in maintaining b-wave amplitude (Eggers and Lukasiewicz 2010, 2011; Smith et al. 2015).
Horizontal cell-mediated inhibition of both rod and cone photoreceptors has been documented in mammals and nonmammalian vertebrates, where depolarization of the horizontal cell membrane with injected current or activation of glutamate receptors leads to inhibition of voltage-gated, L-type Ca channels in photoreceptors (Babai and Thoreson 2009; Cadetti and Thoreson 2006; Davenport et al. 2008; Fahrenfort et al. 2009; Hirano et al. 2016; Hirasawa and Kaneko 2003; Liu et al. 2013; Thoreson et al. 2008; Vessey et al. 2005). This voltage dependence is integral to our understanding of the inhibitory signaling from horizontal cells to photoreceptors, with one report providing evidence for the involvement of voltage-dependent gating of N- and P/Q-type Ca channel subtypes in mammalian horizontal cells (Liu et al. 2013). Horizontal cell signaling to photoreceptors and bipolar cells could be altered if dopamine modulates the Ca channels responsible for sending inhibition. An earlier study showed that D1R activation had no effect on the inhibition of rod photoreceptor [Ca2+]i during horizontal cell depolarization (Liu et al. 2013), suggesting that this mechanism does not have a role in the modulation of rod photoreceptor synaptic signaling. This could be due to different Ca channel subtypes (N, P/Q) mediating the synaptic inhibition, rather than the L-type Ca channels implicated here as being the subtype predominantly modulated by D1Rs, or the fact that horizontal cell-to-rod interactions occur via the horizontal cell axon terminal, a structure separate from the isolated horizontal cell bodies from which we recorded in this work.
Possible direct action of G proteins in dopaminergic modulation.
GTP-binding protein coupled receptors (GPCRs), such as dopamine receptors, use multiple mechanisms for regulating calcium channels (Khan et al. 2013). In addition to the established mechanisms involving changes in phosphorylation state, which may be regulated through second messenger activation by GPCRs, many reports provide evidence that Gβγ subunits directly interact with their cellular effector systems, and these include voltage-gated Ca channels (Bourinet et al. 1994; Ikeda 1996; Zamponi et al. 1997). An established mechanism involves direct interaction of Gβγ subunits with the Ca channel, which produces voltage-dependent inhibition (Khan et al. 2013). This is seen as a reduction in the peak amplitude of whole cell Ca channel current together with a slowing of channel activation kinetics, and both inhibitory effects are relieved by strong depolarizing prepulses that reduce Gβγ binding, which restores channel kinetics. This action of Gβγ has been seen in L-, N-, P/Q-, and T-type Ca channels and is most thoroughly characterized in N-type Ca channels (Currie 2010).
Previous investigations have shown voltage-dependent and voltage-independent Gβγ interactions with N-type Ca channels that result in reduced Ca channel activation (Kisilevsky and Zamponi 2008; Tedford and Zamponi 2006). Our results indicate that voltage-dependent Gβγ interactions were largely responsible for the D1R-mediated reduction in Ca channel activation in horizontal cells, since most of the Ca channel current amplitude reduction and slowed activation kinetics in dopamine- and GTPγS-treated cells were reversed with depolarizing voltage steps to +120 mV. This prepulse paradigm has been shown to cause conformational changes in N-type calcium channels that favor dissociation of the Gβγ subunit from the channel protein, resulting in an increase in channel activation (Zamponi and Snutch 1998).
Neuronal L-type Ca channels composed of α1C-subunits (Cav1.2), which have been identified in mammalian horizontal cells (Liu et al. 2013; Schubert et al. 2006), have also been shown to be directly inhibited by Gβγ subunits and possess Gβγ binding sites in their NH2 and COOH termini (Ivanina et al. 2000). In striped bass retinal horizontal cells tested with dopamine, L-type Ca channels were found to undergo a decrease in activation while T-type Ca channel activation was increased (Pfeiffer-Linn and Lasater 1993). We found no evidence for T-type Ca channels in mouse horizontal cells.
Our results do not preclude additional D1R-mediated pathways, such as cAMP-dependent actions, direct Gβγ interactions with other PLC/DAG/PKC signaling pathways (Camps et al. 1992; Day et al. 2002; Park et al. 1993; Zamponi and Currie 2013), or even direct neurotransmitter receptor-channel interactions such as that in which dopamine modulates N-type Ca channels via a D1R that is directly coupled to the Ca channel, with no G protein involved (Kisilevsky et al. 2008).
It is clear that a large number of distinct GPCR-mediated mechanisms are available for the modulation of voltage-gated Ca channels. There are even inhibitory pathways by which dopamine, acting through a D1R, can inhibit Ca channels directly, independent of a GPCR (Kisilevsky et al. 2008). The inhibition by dopamine of Ca channels shown here appears to be mostly attributable to Gβγ subunits having direct actions on the Ca channels, a well-established voltage-dependent mechanism seen in many neurons. How this inhibitory modulation affects horizontal cell function in the retina is not yet fully established, but some of the numerous actions that dopamine has on these interneurons may be related to the direct Ca channel inhibition described here.
GRANTS
This work was supported by National Eye Institute Grant R01 EY-15573 (N. C. Brecha), the Plum Foundation (S. Barnes and N. C. Brecha), Canadian Institutes of Health Research-Nova Scotia Health Research Foundation Regional Partnership Program MOP-10968 (S. Barnes), Natural Sciences and Engineering Research Council of Canada (NSERC) Discovery Award 194640-2011 (S. Barnes), and a Veterans Administration Merit Review (N. C. Brecha). N. C. Brecha is a Veterans Administration Career Research Scientist.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
X.L., J.C.G., A.A.H., N.C.B., and S.B. conception and design of research; X.L. and J.C.G. performed experiments; X.L., J.C.G., and S.B. analyzed data; X.L., J.C.G., and S.B. interpreted results of experiments; X.L. and J.C.G. prepared figures; X.L., J.C.G., A.A.H., N.C.B., and S.B. approved final version of manuscript; J.C.G. and S.B. drafted manuscript; J.C.G., A.A.H., N.C.B., and S.B. edited and revised manuscript.
REFERENCES
- Babai N, Thoreson WB. Horizontal cell feedback regulates calcium currents and intracellular calcium levels in rod photoreceptors of salamander and mouse retina. J Physiol 587: 2353–2364, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourinet E, Charnet P, Tomlinson WJ, Stea A, Snutch TP, Nargeot J. Voltage-dependent facilitation of a neuronal alpha1C L-type calcium channel. EMBO J 13: 5032–5039, 1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cadetti L, Thoreson WB. Feedback effects of horizontal cell membrane potential on cone calcium currents studied with simultaneous recordings. J Neurophysiol 95: 1992–1995, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camps M, Carozzi A, Schnabel P, Scheer A, Parker PJ, Gierschik P. Isozyme-selective stimulation of phospholipase C-beta 2 by G protein beta gamma-subunits. Nature 360: 684–686, 1992. [DOI] [PubMed] [Google Scholar]
- Chávez AE, Grimes WN, Diamond JS. Mechanisms underlying lateral GABAergic feedback onto rod bipolar cells in rat retina. J Neurosci 30: 2330–2339, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chávez AE, Singer JH, Diamond JS. Fast neurotransmitter release triggered by Ca influx through AMPA-type glutamate receptors. Nature 443: 705–708, 2006. [DOI] [PubMed] [Google Scholar]
- Currie KP. G protein modulation of CaV2 voltage-gated calcium channels. Channels (Austin) 4: 497–509, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davenport CM, Detwiler PB, Dacey DM. Effects of pH buffering on horizontal and ganglion cell light responses in primate retina: evidence for the proton hypothesis of surround formation. J Neurosci 28: 456–64, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Day M, Olson PA, Platzer J, Striessnig J, Surmeier DJ. Stimulation of 5-HT2 receptors in prefrontal pyramidal neurons inhibits Cav1.2 L-type Ca2+ currents via a PLCbeta/IP3/calcineurin signaling cascade. J Neurophysiol 87: 2490–2504, 2002. [DOI] [PubMed] [Google Scholar]
- Djamgoz MB, Kirsch M, Wagner HJ. Haloperidol suppresses light-induced spinule formation and biphasic responses of horizontal cells in fish (roach) retina. Neurosci Lett 107: 200–204, 1989. [DOI] [PubMed] [Google Scholar]
- Doyle SE, McIvor WE, Menaker M. Circadian rhythmicity in dopamine content of mammalian retina: role of the photoreceptors. J Neurochem 83: 211–219, 2002. [DOI] [PubMed] [Google Scholar]
- Du JL, Yang XL. Subcellular localization and complements of GABAA and GABAC receptors on bullfrog retinal bipolar cells. J Neurophysiol 84: 666–676, 2000. [DOI] [PubMed] [Google Scholar]
- Eggers ED, Lukasiewicz PD. GABAA, GABAC and glycine receptor-mediated inhibition differentially affects light-evoked signalling from mouse retinal rod bipolar cells. J Physiol 572: 215–225, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eggers ED, Lukasiewicz PD. Interneuron circuits tune inhibition in retinal bipolar cells. J Neurophysiol 103: 25–37, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eggers ED, Lukasiewicz PD. Multiple pathways of inhibition shape bipolar cell responses in the retina. Vis Neurosci 28: 95–108, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fahrenfort I, Steijaert M, Sjoerdsma T, Vickers E, Ripps H, van Asselt J, Endeman D, Klooster J, Numan R, ten Eikelder H, von Gersdorff H, Kamermans M. Hemichannel-mediated and pH-based feedback from horizontal cells to cones in the vertebrate retina. PLoS One 4: e6090, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farrell SR, Rankin DR, Brecha NC, Barnes S. Somatostatin receptor subtype 4 modulates L-type calcium channels via Gβγ and PKC signaling in rat retinal ganglion cells. Channels (Austin) 8: 519–527, 2014. [Erratum. Channels (Austin) 9: February 2015, p. 56–57.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo C, Hirano AA, Stella SL Jr, Bitzer M, Brecha NC. Guinea pig horizontal cells express GABA, the GABA-synthesizing enzyme GAD 65, and the GABA vesicular transporter. J Comp Neurol 518: 1647–1669, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo C, Stella SL Jr, Hirano AA, Brecha NC. Plasmalemmal and vesicular gammaaminobutyric acid transporter expression in the developing mouse retina. J Comp Neurol 512: 6–26, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hampson EC, Vaney DI, Weiler R. Dopaminergic modulation of gap junction permeability between amacrine cells in mammalian retina. J Neurosci 12: 4911–4922, 1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hare WA, Owen WG. Receptive field of the retinal bipolar cell: a pharmacological study in the tiger salamander. J Neurophysiol 76: 2005–2019, 1996. [DOI] [PubMed] [Google Scholar]
- Hartveit E. Reciprocal synaptic interactions between rod bipolar cells and amacrine cells in the rat retina. J Neurophysiol 81: 2923–2936, 1999. [DOI] [PubMed] [Google Scholar]
- Haverkamp S, Grünert U, Wässle H. The cone pedicle, a complex synapse in the retina. Neuron 27: 85–95, 2000. [DOI] [PubMed] [Google Scholar]
- He S, Weiler R, Vaney DI. Endogenous dopaminergic regulation of horizontal cell coupling in the mammalian retina. J Comp Neurol 418: 33–40, 2000. [DOI] [PubMed] [Google Scholar]
- Herrmann R, Heflin SJ, Hammond T, Lee B, Wang J, Gainetdinov RR, Caron MG, Eggers ED, Frishman LJ, McCall MA, Arshavsky VY. Rod vision is controlled by dopamine-dependent sensitization of rod bipolar cells by GABA. Neuron 72: 101–110, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirano AA, Liu X, Boulter J, Grove JC, Pérez de Sevilla Müller L, Barnes S, Brecha NC. Targeted deletion of vesicular GABA transporter from retinal horizontal cells eliminates feedback modulation of photoreceptor calcium signaling. eNeuro 3: 1–13, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirasawa H, Kaneko A. pH changes in the invaginating synaptic cleft mediate feedback from horizontal cells to cone photoreceptors by modulating Ca2+ channels. J Gen Physiol 122: 657–671, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoon M, Sinha R, Okawa H, Suzuki SC, Hirano AA, Brecha N, Rieke F, Wong RO. Neurotransmission plays contrasting roles in the maturation of inhibitory synapses on axons and dendrites of retinal bipolar cells. Proc Natl Acad Sci USA 112: 12840–12845, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikeda SR. Voltage-dependent modulation of N-type calcium channels by G-protein beta gamma subunits. Nature 380: 255–258, 1996. [DOI] [PubMed] [Google Scholar]
- Iuvone PM, Galli CL, Garrison-Gund CK, Neff NH. Light stimulates tyrosine hydroxylase activity and dopamine synthesis in retinal amacrine neurons. Science 202: 901–902, 1978. [DOI] [PubMed] [Google Scholar]
- Iuvone PM, Tosini G, Pozdeyev N, Haque R, Klein DC, Chaurasia SS. Circadian clocks, clock networks, arylalkylamine N-acetyltransferase, and melatonin in the retina. Prog Retin Eye Res 24: 433–456, 2005. [DOI] [PubMed] [Google Scholar]
- Ivanina T, Blumenstein Y, Shistik E, Barzilai R, Dascal N. Modulation of L-type Ca2+ channels by gbeta gamma and calmodulin via interactions with N and C termini of alpha 1C. J Biol Chem 275: 39846–39854, 2000. [DOI] [PubMed] [Google Scholar]
- Jackson CR, Ruan GX, Aseem F, Abey J, Gamble K, Stanwood G, Palmiter RD, Iuvone PM, McMahon DG. Retinal dopamine mediates multiple dimensions of light-adapted vision. J Neurosci 32: 9359–9368, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaneda M, Andrásfalvy B, Kaneko A. Modulation by Zn2+ of GABA responses in bipolar cells of the mouse retina. Vis Neurosci 17: 273–281, 2000. [DOI] [PubMed] [Google Scholar]
- Kemmler R, Schultz K, Dedek K, Euler T, Schubert T. Differential regulation of cone calcium signals by different horizontal cell feedback mechanisms in the mouse retina. J Neurosci 34: 11826–11843, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan SM, Sleno R, Gora S, Zylbergold P, Laverdure JP, Labbé JC, Miller GJ, Hébert TE. The expanding roles of Gβγ subunits in G protein-coupled receptor signaling and drug action. Pharmacol Rev 65: 545–577, 2013. [DOI] [PubMed] [Google Scholar]
- Kisilevsky AE, Mulligan SJ, Altier C, Iftinca MC, Varela D, Tai C, Chen L, Hameed S, Hamid J, Macvicar BA, Zamponi GW. D1 receptors physically interact with N-type calcium channels to regulate channel distribution and dendritic calcium entry. Neuron 58: 557–570, 2008. [DOI] [PubMed] [Google Scholar]
- Kisilevsky AE, Zamponi GW. D2 dopamine receptors interact directly with N-type calcium channels and regulate channel surface expression levels. Channels (Austin) 2: 269–277, 2008. [DOI] [PubMed] [Google Scholar]
- Knapp AG, Dowling JE. Dopamine enhances excitatory amino acid-gated conductances in cultured retinal horizontal cells. Nature 325: 437–439, 1987. [DOI] [PubMed] [Google Scholar]
- Liu X, Hirano AA, Sun X, Brecha NC, Barnes S. Calcium channels in rat horizontal cells regulate feedback inhibition of photoreceptors through an unconventional GABA- and pH-sensitive mechanism. J Physiol 59: 3309–3324, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malchow RP, Qian HH, Ripps H, Dowling JE. Structural and functional properties of two types of horizontal cell in the skate retina. J Gen Physiol 95: 177–198, 1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin DK, Anderton PJ, Bursill JA, Le Dain AC, Millar TJ. Kainic acid blocks a TTX-sensitive sodium channel in retinal horizontal cells of the turtle (Pseudemys scripta elegans). Neuroreport 7: 2429–2433, 1996. [DOI] [PubMed] [Google Scholar]
- Mojumder DK, Frishman LJ, Otteson DC, Sherry DM. Voltage-gated sodium channel alpha-subunits Nav1.1, Nav12, and Nav16 in the distal mammalian retina. Mol Vis 13: 2163–2182, 2007. [PubMed] [Google Scholar]
- Morrey C, Estephan R, Abbott GW, Levi R. Cardioprotective effect of histamine H3-receptor activation: pivotal role of Gβγ-dependent inhibition of voltage-operated Ca2+ channels. J Pharmacol Exp Ther 326: 871–878, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orr AW, Pallero MA, Murphy-Ullrich JE. Thrombospondin stimulates focal adhesion disassembly through Gi- and phosphoinositide 3-kinase-dependent ERK activation. J Biol Chem 277: 20453–20460, 2002. [DOI] [PubMed] [Google Scholar]
- Park D, Jhon DY, Lee CW, Lee KH, Rhee SG. Activation of phospholipase C isozymes by G protein beta gamma subunits. J Biol Chem 268: 4573–4576, 1993. [PubMed] [Google Scholar]
- Pfeiffer-Linn C, Lasater EM. Dopamine modulates in a differential fashion T- and L-type calcium currents in bass retinal horizontal cells. J Gen Physiol 102: 277–294, 1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pflug R, Nelson R, Huber S, Reitsamer H. Modulation of horizontal cell function by dopaminergic ligands in mammalian retina. Vision Res 48: 1383–1390, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qian H, Li L, Chappell RL, Ripps H. GABA receptors of bipolar cells from the skate retina: actions of zinc on GABA-mediated membrane currents. J Neurophysiol 78: 2402–2412, 1997. [DOI] [PubMed] [Google Scholar]
- Reitsamer HA, Pflug R, Franz M, Huber S. Dopaminergic modulation of horizontal-cell-axon-terminal receptive field size in the mammalian retina. Vision Res 46: 467–474, 2006. [DOI] [PubMed] [Google Scholar]
- Ribelayga C, Mangel SC. Absence of circadian clock regulation of horizontal cell gap junctional coupling reveals two dopamine systems in the goldfish retina. J Comp Neurol 467: 243–253, 2003. [DOI] [PubMed] [Google Scholar]
- Schubert T, Weiler R, Feigenspan A. Intracellular calcium is regulated by different pathways in horizontal cells of the mouse retina. J Neurophysiol 96: 1278–1292, 2006. [DOI] [PubMed] [Google Scholar]
- Shields CR, Tran MN, Wong RO, Lukasiewicz PD. Distinct ionotropic GABA receptors mediate presynaptic and postsynaptic inhibition in retinal bipolar cells. J Neurosci 20: 2673–2682, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shingai R, Christensen BN. Sodium and calcium currents measured in isolated catfish horizontal cells under voltage clamp. Neuroscience 10: 893–897, 1983. [DOI] [PubMed] [Google Scholar]
- Shingai R, Christensen BN. Excitable properties and voltage-sensitive ion conductances of horizontal cells isolated from catfish (Ictalurus punctatus) retina. J Neurophysiol 56: 32–49, 1986. [DOI] [PubMed] [Google Scholar]
- Smith BJ, Côté PD, Tremblay F. Dopamine modulation of rod pathway signaling by suppression of GABAc feedback to rod-driven depolarizing bipolar cells. Eur J Neurosci 42: 2258–2270, 2015. [DOI] [PubMed] [Google Scholar]
- Tedford HW, Zamponi GW. Direct G protein modulation of Cav2 calcium channels. Pharmacol Rev 58: 837–862, 2006. [DOI] [PubMed] [Google Scholar]
- Thoreson WB, Babai N, Bartoletti TM. Feedback from horizontal cells to rod photoreceptors in vertebrate retina. J Neurosci 28: 5691–5695, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thoreson WB, Mangel SC. Lateral interactions in the outer retina. Prog Retin Eye Res 31: 407–441, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueda Y, Kaneko A, Kaneda M. Voltage-dependent ionic currents in solitary horizontal cells isolated from cat retina. J Neurophysiol 68: 1143–1150, 1992. [DOI] [PubMed] [Google Scholar]
- Vardi N, Sterling P. Subcellular localization of GABAA receptor on bipolar cells in macaque and human retina. Vision Res 34: 1235–1246, 1994. [DOI] [PubMed] [Google Scholar]
- Varela C, Blanco R, De la Villa P. Depolarizing effect of GABA in rod bipolar cells of the mouse retina. Vision Res 45: 2659–2667, 2005. [DOI] [PubMed] [Google Scholar]
- Vessey JP, Stratis AK, Daniels BA, Da Silva N, Jonz MG, Lalonde MR, Baldridge WH, Barnes S. Proton-mediated feedback inhibition of presynaptic calcium channels at the cone photoreceptor synapse. J Neurosci 25: 4108–4117, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wässle H, Koulen P, Brandstätter JH, Fletcher EL, Becker CM. Glycine and GABA receptors in the mammalian retina. Vision Res 38: 1411–11430, 1998. [DOI] [PubMed] [Google Scholar]
- Witkovsky P. Dopamine and retinal function. Doc Ophthalmol 108: 17–40, 2004. [DOI] [PubMed] [Google Scholar]
- Yang XL, Wu SM. Feedforward lateral inhibition in retinal bipolar cells: input-output relation of the horizontal cell-depolarizing bipolar cell synapse. Proc Natl Acad Sci USA 88: 3310–3313, 1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zamponi GW, Bourinet E, Nelson D, Nargeot J, Snutch TP. Crosstalk between G proteins and protein kinase C mediated by the calcium channel alpha1 subunit. Nature 385: 442–446, 1997. [DOI] [PubMed] [Google Scholar]
- Zamponi GW, Currie KP. Regulation of CaV2 calcium channels by G protein coupled receptors. Biochim Biophys Acta 1828: 1629–1643, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zamponi GW, Snutch TP. Decay of prepulse facilitation of N type calcium channels during G protein inhibition is consistent with binding of a single Gbeta subunit. Proc Natl Acad Sci USA 95: 4035–4039, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang AJ, Jacoby R, Wu SM. Light- and dopamine-regulated receptive field plasticity in primate horizontal cells. J Comp Neurol 519: 2125–2134, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]