Abstract
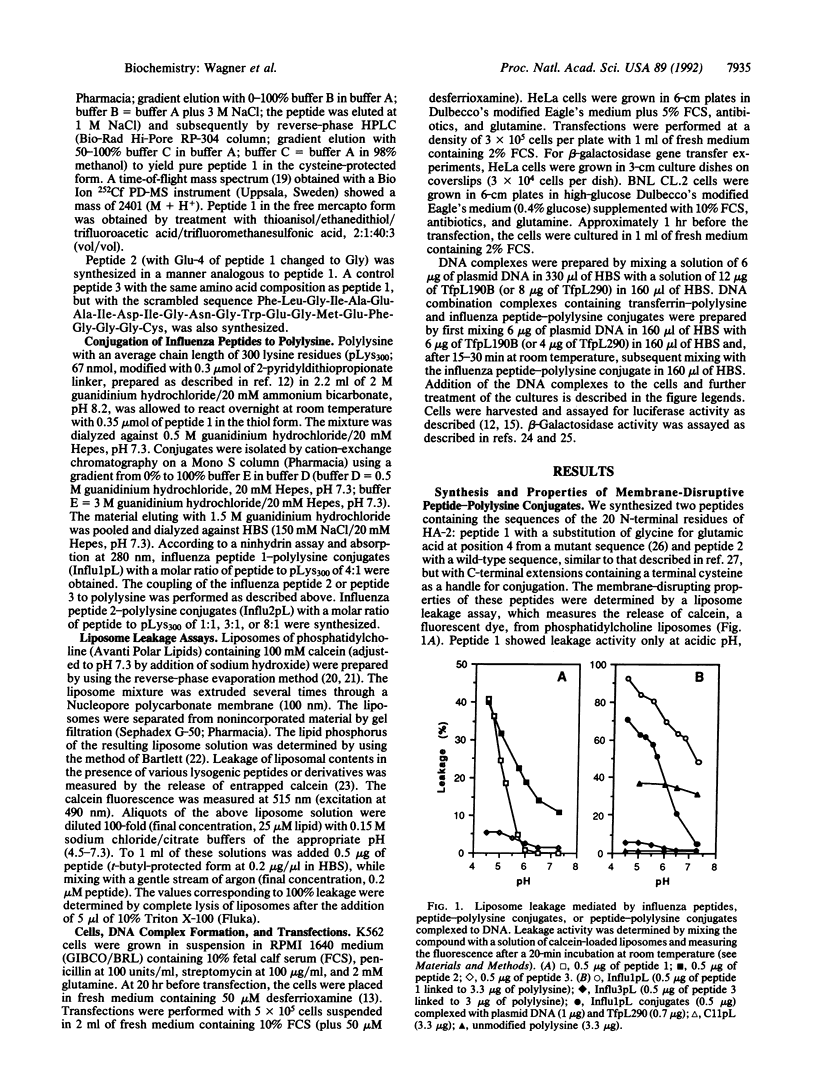
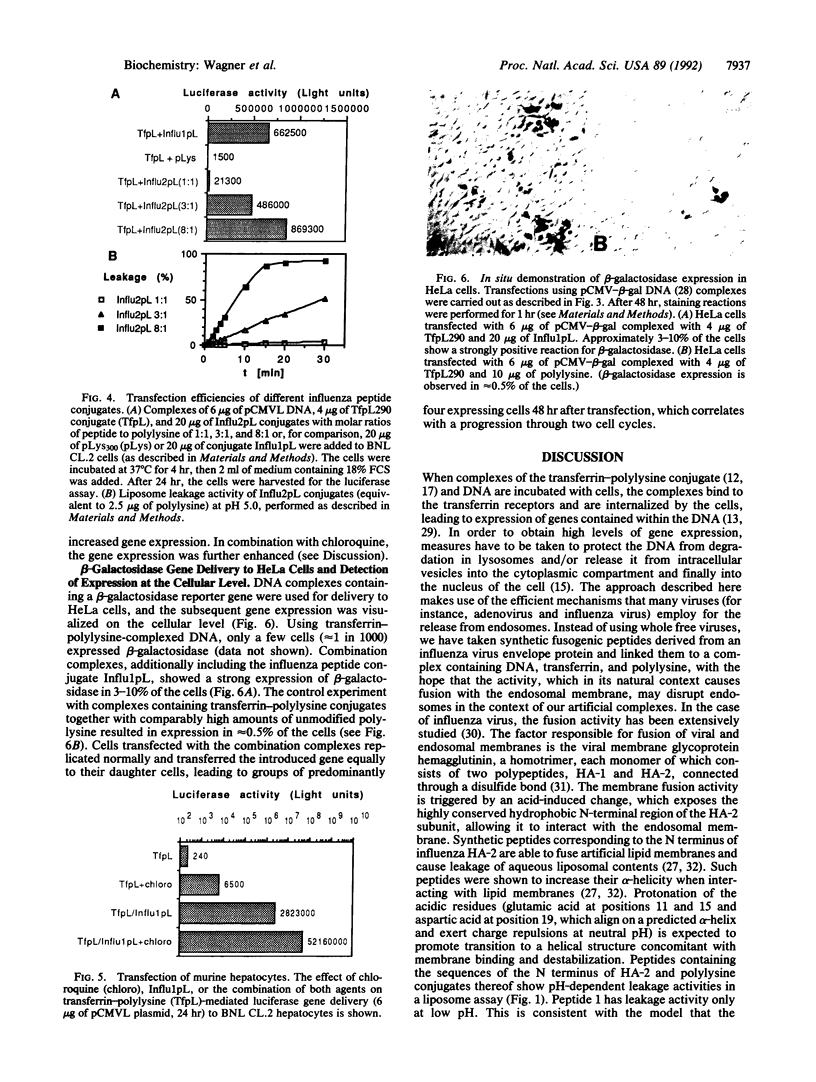
Complexes containing plasmid DNA, transferrin-polylysine conjugates, and polylysine-conjugated peptides derived from the N-terminal sequence of the influenza virus hemagglutinin subunit HA-2 have been used for the transfer of luciferase or beta-galactosidase marker genes to K562 cells, HeLa cells, and BNL CL.2 hepatocytes. These DNA complexes mimic the entry of viruses into cells, as they contain functions for (i) the packaging of the nucleic acid with polylysine, (ii) the attachment to the cell and receptor-mediated endocytosis with transferrin as a ligand, and (iii) the release from endosomes by using membrane-disrupting influenza peptides. The presence of these influenza peptide conjugates in the DNA complexes renders the complexes active in membrane disruption in a liposome leakage assay and results in a substantial augmentation of the transferrin-polylysine-mediated gene transfer.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Armentano D., Yu S. F., Kantoff P. W., von Ruden T., Anderson W. F., Gilboa E. Effect of internal viral sequences on the utility of retroviral vectors. J Virol. 1987 May;61(5):1647–1650. doi: 10.1128/jvi.61.5.1647-1650.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BARTLETT G. R. Phosphorus assay in column chromatography. J Biol Chem. 1959 Mar;234(3):466–468. [PubMed] [Google Scholar]
- Ballay A., Levrero M., Buendia M. A., Tiollais P., Perricaudet M. In vitro and in vivo synthesis of the hepatitis B virus surface antigen and of the receptor for polymerized human serum albumin from recombinant human adenoviruses. EMBO J. 1985 Dec 30;4(13B):3861–3865. doi: 10.1002/j.1460-2075.1985.tb04158.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berkner K. L. Development of adenovirus vectors for the expression of heterologous genes. Biotechniques. 1988 Jul-Aug;6(7):616–629. [PubMed] [Google Scholar]
- Bondeson J., Wijkander J., Sundler R. Proton-induced membrane fusion. Role of phospholipid composition and protein-mediated intermembrane contact. Biochim Biophys Acta. 1984 Oct 17;777(1):21–27. doi: 10.1016/0005-2736(84)90492-9. [DOI] [PubMed] [Google Scholar]
- Cain C. C., Sipe D. M., Murphy R. F. Regulation of endocytic pH by the Na+,K+-ATPase in living cells. Proc Natl Acad Sci U S A. 1989 Jan;86(2):544–548. doi: 10.1073/pnas.86.2.544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cotten M., Längle-Rouault F., Kirlappos H., Wagner E., Mechtler K., Zenke M., Beug H., Birnstiel M. L. Transferrin-polycation-mediated introduction of DNA into human leukemic cells: stimulation by agents that affect the survival of transfected DNA or modulate transferrin receptor levels. Proc Natl Acad Sci U S A. 1990 Jun;87(11):4033–4037. doi: 10.1073/pnas.87.11.4033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cotten M., Wagner E., Zatloukal K., Phillips S., Curiel D. T., Birnstiel M. L. High-efficiency receptor-mediated delivery of small and large (48 kilobase gene constructs using the endosome-disruption activity of defective or chemically inactivated adenovirus particles. Proc Natl Acad Sci U S A. 1992 Jul 1;89(13):6094–6098. doi: 10.1073/pnas.89.13.6094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curiel D. T., Agarwal S., Wagner E., Cotten M. Adenovirus enhancement of transferrin-polylysine-mediated gene delivery. Proc Natl Acad Sci U S A. 1991 Oct 1;88(19):8850–8854. doi: 10.1073/pnas.88.19.8850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falkner F. G., Moss B. Escherichia coli gpt gene provides dominant selection for vaccinia virus open reading frame expression vectors. J Virol. 1988 Jun;62(6):1849–1854. doi: 10.1128/jvi.62.6.1849-1854.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gething M. J., Doms R. W., York D., White J. Studies on the mechanism of membrane fusion: site-specific mutagenesis of the hemagglutinin of influenza virus. J Cell Biol. 1986 Jan;102(1):11–23. doi: 10.1083/jcb.102.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermonat P. L., Muzyczka N. Use of adeno-associated virus as a mammalian DNA cloning vector: transduction of neomycin resistance into mammalian tissue culture cells. Proc Natl Acad Sci U S A. 1984 Oct;81(20):6466–6470. doi: 10.1073/pnas.81.20.6466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huckett B., Ariatti M., Hawtrey A. O. Evidence for targeted gene transfer by receptor-mediated endocytosis. Stable expression following insulin-directed entry of NEO into HepG2 cells. Biochem Pharmacol. 1990 Jul 15;40(2):253–263. doi: 10.1016/0006-2952(90)90686-f. [DOI] [PubMed] [Google Scholar]
- Keller G., Paige C., Gilboa E., Wagner E. F. Expression of a foreign gene in myeloid and lymphoid cells derived from multipotent haematopoietic precursors. Nature. 1985 Nov 14;318(6042):149–154. doi: 10.1038/318149a0. [DOI] [PubMed] [Google Scholar]
- Lear J. D., DeGrado W. F. Membrane binding and conformational properties of peptides representing the NH2 terminus of influenza HA-2. J Biol Chem. 1987 May 15;262(14):6500–6505. [PubMed] [Google Scholar]
- Lim K., Chae C. B. A simple assay for DNA transfection by incubation of the cells in culture dishes with substrates for beta-galactosidase. Biotechniques. 1989 Jun;7(6):576–579. [PubMed] [Google Scholar]
- MacGregor G. R., Caskey C. T. Construction of plasmids that express E. coli beta-galactosidase in mammalian cells. Nucleic Acids Res. 1989 Mar 25;17(6):2365–2365. doi: 10.1093/nar/17.6.2365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piccini A., Perkus M. E., Paoletti E. Vaccinia virus as an expression vector. Methods Enzymol. 1987;153:545–563. doi: 10.1016/0076-6879(87)53077-4. [DOI] [PubMed] [Google Scholar]
- Samulski R. J., Chang L. S., Shenk T. Helper-free stocks of recombinant adeno-associated viruses: normal integration does not require viral gene expression. J Virol. 1989 Sep;63(9):3822–3828. doi: 10.1128/jvi.63.9.3822-3828.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Straubinger R. M., Papahadjopoulos D. Liposomes as carriers for intracellular delivery of nucleic acids. Methods Enzymol. 1983;101:512–527. doi: 10.1016/0076-6879(83)01035-6. [DOI] [PubMed] [Google Scholar]
- Szoka F., Jr, Papahadjopoulos D. Procedure for preparation of liposomes with large internal aqueous space and high capture by reverse-phase evaporation. Proc Natl Acad Sci U S A. 1978 Sep;75(9):4194–4198. doi: 10.1073/pnas.75.9.4194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner E., Cotten M., Foisner R., Birnstiel M. L. Transferrin-polycation-DNA complexes: the effect of polycations on the structure of the complex and DNA delivery to cells. Proc Natl Acad Sci U S A. 1991 May 15;88(10):4255–4259. doi: 10.1073/pnas.88.10.4255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner E., Cotten M., Mechtler K., Kirlappos H., Birnstiel M. L. DNA-binding transferrin conjugates as functional gene-delivery agents: synthesis by linkage of polylysine or ethidium homodimer to the transferrin carbohydrate moiety. Bioconjug Chem. 1991 Jul-Aug;2(4):226–231. doi: 10.1021/bc00010a006. [DOI] [PubMed] [Google Scholar]
- Wagner E., Zatloukal K., Cotten M., Kirlappos H., Mechtler K., Curiel D. T., Birnstiel M. L. Coupling of adenovirus to transferrin-polylysine/DNA complexes greatly enhances receptor-mediated gene delivery and expression of transfected genes. Proc Natl Acad Sci U S A. 1992 Jul 1;89(13):6099–6103. doi: 10.1073/pnas.89.13.6099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner E., Zenke M., Cotten M., Beug H., Birnstiel M. L. Transferrin-polycation conjugates as carriers for DNA uptake into cells. Proc Natl Acad Sci U S A. 1990 May;87(9):3410–3414. doi: 10.1073/pnas.87.9.3410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wharton S. A., Martin S. R., Ruigrok R. W., Skehel J. J., Wiley D. C. Membrane fusion by peptide analogues of influenza virus haemagglutinin. J Gen Virol. 1988 Aug;69(Pt 8):1847–1857. doi: 10.1099/0022-1317-69-8-1847. [DOI] [PubMed] [Google Scholar]
- Wiley D. C., Skehel J. J. The structure and function of the hemagglutinin membrane glycoprotein of influenza virus. Annu Rev Biochem. 1987;56:365–394. doi: 10.1146/annurev.bi.56.070187.002053. [DOI] [PubMed] [Google Scholar]
- Wiley D. C., Skehel J. J. The structure and function of the hemagglutinin membrane glycoprotein of influenza virus. Annu Rev Biochem. 1987;56:365–394. doi: 10.1146/annurev.bi.56.070187.002053. [DOI] [PubMed] [Google Scholar]
- Wiley D. C., Skehel J. J., Waterfield M. Evidence from studies with a cross-linking reagent that the haemagglutinin of influenza virus is a trimer. Virology. 1977 Jun 15;79(2):446–448. doi: 10.1016/0042-6822(77)90371-3. [DOI] [PubMed] [Google Scholar]
- Wu C. H., Wilson J. M., Wu G. Y. Targeting genes: delivery and persistent expression of a foreign gene driven by mammalian regulatory elements in vivo. J Biol Chem. 1989 Oct 15;264(29):16985–16987. [PubMed] [Google Scholar]
- Zenke M., Steinlein P., Wagner E., Cotten M., Beug H., Birnstiel M. L. Receptor-mediated endocytosis of transferrin-polycation conjugates: an efficient way to introduce DNA into hematopoietic cells. Proc Natl Acad Sci U S A. 1990 May;87(10):3655–3659. doi: 10.1073/pnas.87.10.3655. [DOI] [PMC free article] [PubMed] [Google Scholar]








