This is the first investigation of the relationship between short-latency afferent inhibition (SAI) and the sensory afferent volley and the first to examine the relationship between SAI and somatosensory evoked potentials (SEPs). The data indicate that 1) SAI increases with the recruitment of sensory fibers and 2) its stimulus-response profile is correlated with SEPs. These novel data provide practical guidelines and also contribute to our understanding of SAI mechanisms.
Keywords: TMS, short-latency afferent inhibition, somatosensory evoked potentials, sensory nerve action potential, recruitment curve, afferent volley
Abstract
Short-latency afferent inhibition (SAI) is characterized by the suppression of the transcranial magnetic stimulation motor evoked potential (MEP) by the cortical arrival of a somatosensory afferent volley. It remains unknown whether the magnitude of SAI reflects changes in the sensory afferent volley, similar to that observed for somatosensory evoked potentials (SEPs). The present study investigated stimulus-response relationships between sensory nerve action potentials (SNAPs), SAI, and SEPs and their interrelatedness. Experiment 1 (n = 23, age 23 ± 1.5 yr) investigated the stimulus-response profile for SEPs and SAI in the flexor carpi radialis muscle after stimulation of the mixed median nerve at the wrist using ∼25%, 50%, 75%, and 100% of the maximum SNAP and at 1.2× and 2.4× motor threshold (the latter equated to 100% of the maximum SNAP). Experiment 2 (n = 20, age 23.1 ± 2 yr) probed SEPs and SAI stimulus-response relationships after stimulation of the cutaneous digital nerve at ∼25%, 50%, 75%, and 100% of the maximum SNAP recorded at the elbow. Results indicate that, for both nerve types, SAI magnitude is dependent on the volume of the sensory afferent volley and ceases to increase once all afferent fibers within the nerve are recruited. Furthermore, for both nerve types, the magnitudes of SAI and SEPs are related such that an increase in excitation within somatosensory cortex is associated with an increase in the magnitude of afferent-induced MEP inhibition.
NEW & NOTEWORTHY
This is the first investigation of the relationship between short-latency afferent inhibition (SAI) and the sensory afferent volley and the first to examine the relationship between SAI and somatosensory evoked potentials (SEPs). The data indicate that 1) SAI increases with the recruitment of sensory fibers and 2) its stimulus-response profile is correlated with SEPs. These novel data provide practical guidelines and also contribute to our understanding of SAI mechanisms.
in an influential study, Tokimura et al. (2000) demonstrated that peripheral nerve stimulation reduces the amplitude of transcranial magnetic stimulation (TMS) motor evoked potentials (MEPs) when the two inputs are timed to nearly coincide within the motor cortex, a circuit called short-latency afferent inhibition (SAI). SAI is elicited by delivering a TMS pulse ∼2–8 ms after the arrival of the afferent volley in somatosensory cortex (SI) [i.e., corresponding to the N20 somatosensory evoked potential (SEP)]. SAI appears to originate within the sensorimotor cortices since it is not observed with transcranial electrical stimulation and there is a concomitant suppression of late indirect waves (Di Lazzaro et al. 2012; Tokimura et al. 2000) with no impact on spinal excitability as measured by F waves (Chen et al. 1999; Classen et al. 2000; Tamburin et al. 2001; Tokimura et al. 2000). SAI is found in both homo- and heterotopic muscles after stimulation of the mixed median nerve (MN) at the wrist (Fischer and Orth 2011). In contrast, SAI evoked by digital nerve (DN) stimulation demonstrates a somatotopic distribution such that SAI is greater (i.e., more inhibition) and occurs at an earlier latency in muscles that are in closer proximity to the stimulating electrode (Classen et al. 2000). SAI is modulated during specific phases of movement (Asmussen et al. 2013, 2014; Voller et al. 2006), indicating its use in studying motor control. SAI is reduced and/or abolished in Alzheimer's disease (Di Lazzaro 2004) and Parkinson's disease (Sailer et al. 2003, 2007), particularly in those presenting with mild cognitive impairment (Yarnall et al. 2013) or dementia (Celebi et al. 2012), although opposite effects are also observed (Di Lazzaro et al. 2004; Nardone et al. 2005). Pharmacological studies demonstrate that SAI is abolished in the presence of lorazepam and scopolamine (Di Lazzaro et al. 2000, 2005) and is increased with diazepam (Di Lazzaro et al. 2005), indicating the influence of different GABAA subunits and acetylcholine in the genesis and/or maintenance of this circuit.
SI is able to encode the intensity of afferent input (Lin et al. 2003). In humans, SEPs recorded over SI demonstrate an increasing stimulus-response relationship with the mixed median sensory nerve action potential (SNAPs). The amplitudes of MN SEPs are maximal at ∼50% of the maximum SNAP (i.e., SNAPmax), with further increases in SNAP attributed to contributions from antidromic efferent fibers (Gandevia and Burke 1984; Lesser et al. 1979). It is known that SAI increases as the conditioning stimulus intensity increases from sensory to motor threshold (Fischer and Orth 2011); however, its magnitude as a function of the sensory afferent volley has not been studied. We reasoned that if the magnitude of SAI depends on the volume of sensory afferent fibers then SAI should cease to increase once all sensory fibers are recruited. Therefore, SAI should increase to ∼50% SNAPmax for the mixed MN and 100% SNAPmax for a purely sensory nerve, analogous to changes in the SEP. Furthermore, by delivering MN stimulation as pairs of pulses the SEP responses to the first (i.e., SEP 1) and second (i.e., SEP 2) stimuli are recorded, the latter of which is typically suppressed (Gatica Tossi et al. 2013; Höffken et al. 2010, 2013). The relationship between the sensory afferent volley and SEP 2 also remains unknown.
The present study examined the stimulus-response relationship between SAI and SEPs for the mixed MN (Fischer and Orth 2011; Gandevia and Burke 1984) and purely cutaneous DN (Taylor et al. 1992). The data indicate that for each nerve type SEPs and SAI follow a similar trend as a function of the sensory afferent volley and that both measures are maximal when the sensory fibers are fully recruited. These data indicate that the magnitude of SAI is determined by the volume of the sensory afferent volley.
METHODS
Participants
Thirty-three self-reported right-handed individuals participated in one of two experiments with ten of those participating in both experiments [experiment 1: n = 23 (10 men, 13 women), mean age: 23 ± 1.5 yr; experiment 2: n = 20 (7 men, 13 women), age 23.1 ± 2 yr]. The study conformed to the Declaration of Helsinki and was approved by the McMaster Research Ethics Board. All individuals provided written consent prior to participation.
Experiment 1. Median Nerve Evoked SNAPs, SEPs, and SAI
SNAPs were recorded by placing surface electrodes over the MN at the elbow just proximal to the medial epicondyle. EMG signals were amplified (1,000×), band-pass filtered (high pass of 20 Hz and low pass of 2,500 Hz), collected, and stored (CED 1401, Signal v5). The MN, a mixed nerve containing both sensory and motor fibers, was stimulated with a bar electrode at the wrist slightly medial and proximal to the styloid process of the radius and just lateral to the tendons of the wrist with the cathode proximal. A constant-current stimulator (Digitimer DS7AH) provided square wave pulses with a duration of 0.5 ms. Initially, SNAP amplitude (peak to peak) was assessed at 5 mA and averaged over 50 epochs. The current was then increased by 1 mA, and SNAPs were again collected and averaged over 50 epochs. This procedure was repeated until the amplitude of the SNAP ceased to increase over three consecutive blocks of 50 epochs. Five MN stimulation intensities were subsequently tested and defined as the stimulator output that elicited ∼25%, 50%, and 75% of SNAPmax and 1.2× and 2.4× motor threshold (MT) based on a visible muscle twitch in the abductor pollicis brevis (APB).
SEPs were acquired at electrode position C3′ and referenced to Fz (International 10-20 System) with the ground electrode over the clavicle. Impedance was <5 kΩ at each scalp electrode. For each of the five intensities, 500 pairs of MN stimuli were delivered at a rate of 1 Hz and separated by an interstimulus interval (ISI) of 30 ms (Allison 1962; Höffken et al. 2007; McLaughlin and Kelly 1993) to allow the investigation of the first (SEP 1) and second (SEP 2) SEPs. SEP signals were amplified (10,000), band-pass filtered (high pass of 2 Hz and low pass of 2,500 Hz), and collected (CED 1401, Signal v5). SEPs were obtained from the time-locked average of ∼500 epochs. SEP 1 was determined as the peak-to-peak amplitude of the first N20-P25 potential. Intracortical inhibition within SI was determined by the amplitude of SEP 2, which is suppressed at ISIs of 30 ms (Allison 1962; Höffken et al. 2007; McLaughlin and Kelly 1993).
SAI was recorded with Ag-AgCl surface electrodes (9 mm) placed over the flexor carpi radialis (FCR) muscle of the right forearm with the active electrode placed ∼3 cm distal and 2 cm lateral to the medial epicondyle, the reference placed over the tendons of the wrist, and the ground electrode placed over the medial styloid process of the wrist. TMS was performed by delivering monophasic pulses with a 50-mm-inner diameter figure-of-eight branding coil connected to a Magstim 2002 stimulator (Magstim). The motor hot spot for FCR was determined as the location providing the most reproducible MEP in the relaxed FCR muscle. The coil was positioned ∼45° to the midsagittal line to induce a current in a posterolateral-to-anteromedial direction over the left-hemisphere motor cortex. Brainsight Neuronavigation 2 (Rogue Research) was used to target and track the TMS coil position and orientation corresponding to the location of the motor hot spot. To elicit SAI, the TMS intensity was set to evoke MEPs of ∼1 mV in the relaxed FCR. The ISI between the peripheral nerve stimulus and TMS pulse was derived from the latency of the N20 component from the SEP obtained in each participant, and an additional 2 ms was added to elicit greatest inhibition (Tokimura et al. 2000). For each of the five intensities tested, 15 conditioned MEPs (i.e., MEPnerve-TMS) and 15 unconditioned MEPs (i.e., MEPTMS) were randomly presented with a 5-s intertrial interval. SAI was expressed as a ratio of the amplitude of the conditioned to unconditioned MEPs
Experiment timeline.
Participants experienced five testing blocks that acquired SNAPs, SEPs, and SAI, each of which corresponded to a single SNAPmax intensity. The intensity order was pseudorandomized across participants with a Williams square design.
Experiment 2. Digital Nerve Evoked SNAPs, SEPs, and SAI
SNAPs and SEPs were recorded as described in experiment 1. To stimulate the DN, ring electrodes were placed over proximal and middle phalanges of the index finger of the right hand. A constant-current stimulator (Digitimer DS7AH) provided square wave pulses with a duration of 0.5 ms. The sensory threshold (ST) was obtained at this location and defined as the minimum stimulator intensity resulting in sensation in the vicinity of the ring electrodes. The DN was stimulated at four intensities each corresponding to ∼25%, 50%, 75%, and 100% of the SNAPmax in separate blocks. DN SEPs are typically smaller than those obtained by mixed nerve stimulation; therefore DN SEPs were averaged over 1,000 epochs and the stimuli were delivered at 3 Hz to reduce the overall time required to acquire the data. DN SEPs were quantified by the peak-to-peak amplitude of the N20-P25 potential. SAI was probed in two muscles: the first dorsal interosseous (FDI), for which the DN innervates the overlying skin, and FCR, which is not directly innervated by the DN. SAI is regularly obtained in FDI after DN stimulation (Asmussen et al. 2013), while it is less often reported in FCR. To elicit SAI, the TMS pulse was timed to the individual N20 latency plus 2 ms and the resulting MEP was normalized as above.
Experiment timeline.
Five testing blocks were performed. The first four included the collection of SEPs with the order of intensity pseudorandomized according to a Williams square design. SAI was acquired in the final block whereby MEPnerve-TMS (n = 15 per intensity) was presented with the same randomization as the SEP collection Williams square design and MEPTMS was acquired in blocks of five trials between each MEPnerve-TMS collection presented (i.e., n = 20, 4 blocks × 5 trials each).
Data Analyses
The actual SNAP amplitudes were computed and subsequently compared against neighboring intensities via two-tailed paired t-tests. SNAPs that were not significantly different from the neighboring SNAP amplitude were averaged to create a single intensity. One-way repeated-measures ANOVA with factor Intensity (4 levels) was performed for SEP 1, SEP 2, and SAI. For SAI in experiment 2, a two-way repeated-measures ANOVA [within-subject factors Intensity (4 levels) and Muscle (2 levels: FDI and FCR)] was performed. Post hoc Tukey's honestly significant difference tested for differences in the case of significant F-statistics. For experiment 1, we tested the hypothesis that SAI should increase with an increase in stimulation intensity to approximately MT (47% SNAPmax) (Dinse et al. 2013; Fischer and Orth 2011) using a one-tailed paired t-test (∼25% vs. ∼50% SNAPmax). Pearson's correlation analyses were performed between SEP 1 and SAI to assess the relationship between the excitation of SI and the inhibition in the MEP. Furthermore, the amount of change in SNAP, SAI, and SEP measures were assessed between each level of SNAPmax and were qualitatively assessed for similarities. If the assumptions of sphericity were not met, Greenhouse-Geisser corrections were used. The normality of the distribution was tested via Kolmogorov and Smirnov tests, and if normality was not met the data were subsequently log transformed before ANOVA. All normality tests and ANOVAs were performed with SPSS version 23 (IBM Technologies), and significance was set at P < 0.05. Post hoc testing was performed with SAS (SAS Institute).
RESULTS
Experiment 1. Median Nerve Evoked SNAPs, SEPs, and SAI
One participant was not able to complete the highest intensity because of discomfort, and one participant had SEP responses >2 SD away from the mean at all intensities. Data from the remaining 21 individuals (8 men, 13 women; mean age: 23 ± 1.54 yr) were included in the subsequent analyses. The MN SAI data were not normally distributed (P = 0.027), and the data were log transformed and subsequently passed normality (P = 0.2). Therefore, for MN SAI the ANOVA was performed on the logarithmic transformed data.
MN stimulation was delivered to obtain SNAPs of approximately 25%, 50%, and 75% SNAPmax and at 1.2× and 2.4× MT. After collection of the SNAPs all intensities were expressed as a percentage of SNAPmax. The intensity of 2.4× MT yielded SNAPs that reached 100% SNAPmax. Therefore, 2.4× MT was renamed as 100% SNAPmax. The amplitudes of all other stimulating intensities were subsequently normalized to this value and are expressed in Table 1. These values are referred to here. SNAPs were not significantly different between 44% SNAPmax and 1.2× MT (49% SNAPmax) (P = 0.106), and data for these two intensities were averaged for each individual prior to subsequent analyses. Following this average, all adjacent intervals of %SNAPmax were statistically different (20% vs. 47%, 47% vs. 71%, 71% vs. 100%, P < 0.001).
Table 1.
Measurements of afferent volley
| 25% SNAP | 50% SNAP | 1.2 × MT | 75% SNAP | 100% SNAP | 2.4 × MT | |
|---|---|---|---|---|---|---|
| MN, μV | 8.53 | 10.95 | 11.54 | 14.09 | 23.09 | |
| SE | 3.13 | 3.81 | 3.14 | 4.61 | 6.28 | |
| 11.25 | ||||||
| MN, %Max | 20 | 44 | 49 | 71 | 100 | |
| SE | 10.2 | 15.3 | 23.6 | 19.7 | 0.00 | |
| 47% | ||||||
| MN, mA | 9.16 | 11.82 | 12.64 | 15.05 | 24.88 | |
| SE | 3.77 | 4.40 | 4.23 | 5.27 | 8.48 | |
| DN, μV | 0.50 | 1.29 | 1.97 | 2.4 | ||
| SE | 0.08 | 0.13 | 0.19 | 0.25 | ||
| DN, %Max | 21 | 55 | 84 | 100 | ||
| SE | 10.7 | 10.5 | 14.3 | 0.00 | ||
| DN, mA | 3.85 | 5.40 | 7.30 | 11.43 | ||
| SE | 1.57 | 1.56 | 2.37 | 3.76 | ||
For measurements of median nerve (MN) sensory nerve action potential (SNAP), headings indicate intended values of stimulation intensity, with the actual group-averaged intensity collected represented below as actual amplitude (μV), in %SNAPmax (%Max), and in current (mA) with standard error (SE). Actual values obtained included 44% and 49% SNAPmax (at 1.2× MT), and these were not statistically different. Data from these intensities were averaged to create a single intensity at 47%. For measurements of digital nerve (DN) SNAP, headings indicate intended values of stimulation intensity, with the actual group-averaged intensity collected represented below as actual amplitude (μV), in %SNAPmax (%Max), and in current (mA) with standard error (SE).
Table 2 displays the group-averaged peak-to-peak N20-P25 amplitudes of both SEP 1 and SEP 2 as a function of the percentage of SNAPmax. For SEP 1, one-way ANOVA revealed a significant effect of Intensity [F(2.07,41.48) = 16.41; P < 0.001] whereby amplitudes were greater at 47%, 71%, and 100% vs. 20% SNAPmax (Tukey's, P = 0.05). Statistically, SEPs did not increase beyond the amplitude obtained at 47% SNAPmax. No effect was observed for SEP 2 [Intensity: F(3,60) = 1.118; P = 0.349], which had an amplitude of ∼2 μV at all intensities, suggesting a potential ceiling effect for activation within SI after a nerve stimulus 30 ms earlier. Figure 1A plots an example SNAP data set recorded from one participant after MN stimulation. Figure 1B plots an example data set of traces showing SEP 1 and SEP 2 from one individual at each percentage of SNAPmax.
Table 2.
Group-averaged data
| 20% SNAP | 21% SNAP | 47% SNAP | 55% SNAP | 71% SNAP | 84% SNAP | 100% SNAP | |
|---|---|---|---|---|---|---|---|
| MN SEP 1, μV | 4.78 | 5.82* | 6.33* | 6.19* | |||
| SE | 0.48 | 0.57 | 0.53 | 0.56 | |||
| MN SEP 2, μV | 2.03 | 2.22 | 2.29 | 2.37 | |||
| SE | 0.26 | 0.33 | 0.36 | 0.36 | |||
| MN SAI, % | 0.81 | 0.66* | 0.74 | 0.76 | |||
| SE | 0.08 | 0.04 | 0.07 | 0.07 | |||
| DN SEP, μV | 1.17 | 1.82* | 2.21* | 2.53*† | |||
| SE | 0.13 | 0.13 | 0.21 | 0.24 | |||
| DN SAI (FCR), % | 0.95 | 0.80* | 0.76* | 0.67* | |||
| SE | 0.05 | 0.04 | 0.05 | 0.05 | |||
| DN SAI (FDI), % | 0.86 | 0.55* | 0.67* | 0.59* | |||
| SE | 0.11 | 0.04 | 0.08 | 0.06 |
Values are group-averaged values for somatosensory evoked potential (SEP) and short-latency afferent inhibition (SAI) with standard error (SE) for both the median (MN) and digital (DN) nerves at each percentage of the actual maximum sensory nerve action potential (SNAP). MN SAI was evoked in the flexor carpi radialis (FCR), while DN SAI was performed in both the FCR and the first dorsal interosseous (FDI). For MN SEP 1, 47%, 71%, and 100% SNAPmax are larger than 20% SNAPmax (*). For MN SAI, 47% is larger than 20% SNAPmax (*). For DN SEP, 55%, 84%, and 100% are larger than 21% SNAPmax (*) and 100% is larger than 55% SNAPmax (†). For DN SAI in both FDI and FCR a main effect of intensity shows greater SAI at 100%, 84%, and 55% compared with 21% SNAPmax (*).
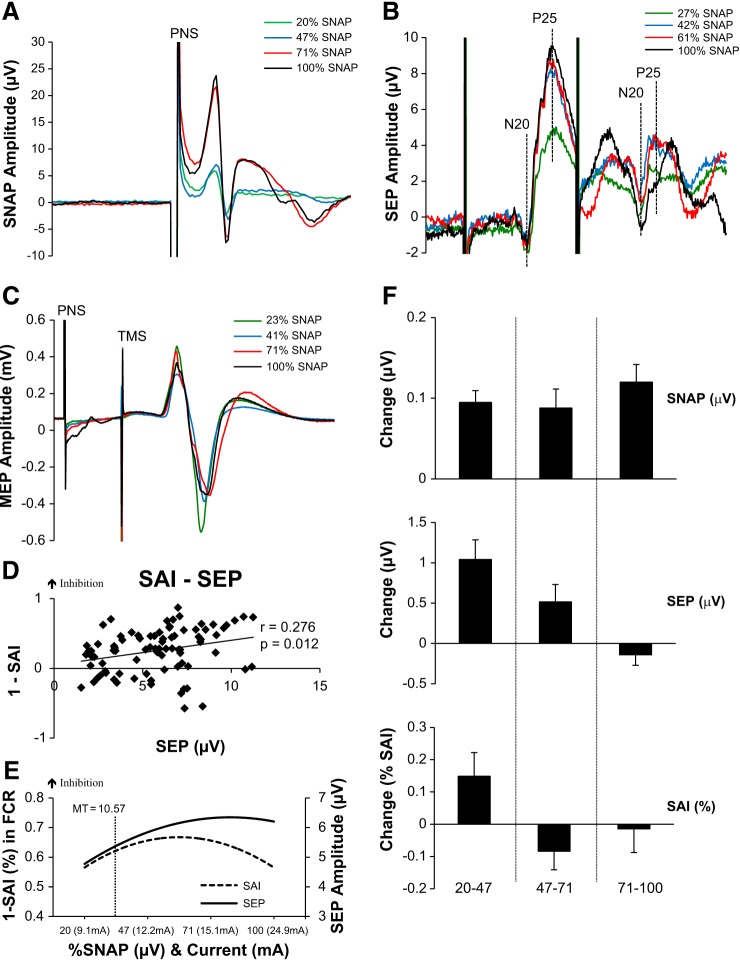
Fig. 1.
Median nerve stimulation. A: average SNAPs recorded from 1 individual at each intensity of median nerve stimulation. Timing of peripheral nerve stimulation (PNS) shown. B: 1ndividual SEP traces depicting N20-P25 amplitude of SEP 1 and SEP 2. Actual percentages of SNAPmax for this participant are shown in the key. C: individual SAI traces with actual %SNAPmax for this participant. D: correlations between SAI and SEP 1 reveal a significant positive correlation; as SEP increases SAI increases. E: second-order polynomial trend lines of the recruitment curves for each measurement are shown to represent how each measure responds to the increases in SNAP. Represented on the graph is the group-averaged motor threshold (MT) and its location on the recruitment curves. As indicated, MT lies slightly below the saturation point and may not allow increases in these measures to occur. Additionally, visually all 3 measures show the saturation occurring at ∼50% of the SNAPmax. F: the group-averaged % change (±SE) in between each block of stimulation intensity for the SNAP, SEP, and SAI. SNAP increases in each increment as depicted by the positive % change, although SEP and SAI reverse and actually show a decrease indicating that they are no longer increasing as the sensory afferent volley increases.
Table 2 displays the group-averaged SAI at each percentage of SNAPmax. One-way ANOVA revealed no effect of Intensity [F(3,60) = 0.469; P = 0.705]. However, in support of the hypothesis, SAI increases from 20% to 47% SNAPmax (P = 0.024, 1-tailed, paired t-test), where 47% is the maximum depth of SAI observed. Therefore, similar to SEP 1, SAI increased to ∼50% of SNAPmax. Figure 1C illustrates an example SAI data set from one individual at each percentage of SNAPmax.
Correlation analyses between the magnitude of SEPs and SAI are shown in Fig. 1D. SEP 1 and SAI are positively correlated (r = 0.276, P = 0.012), indicating that increases in SEP 1 are associated with increases in SAI (i.e., greater inhibition). Additional information is obtained from the second-order polynomial trend lines (Fig. 1E) that show that increases in SEPs are concomitant with increases in SAI (i.e., increased inhibition) and both measures cease to increase further at ∼50% SNAPmax. Figure 1E also plots the motor threshold for a twitch in APB (i.e., MT) to demonstrate that this intensity, frequently used in research, falls between 20% and 47% SNAPmax. For each of the three increments of SNAP (20–47%, 47–71%, 71–100%) the magnitude and direction of corresponding changes in SEPs and SAI are plotted in Fig. 1F. This plot reaffirms that the increase in SNAP is not always met with increases in SEPs or SAI, as both measures cease to increase despite the increase in the amplitude of SNAP. Collectively, experiment 1 data indicate that the MN evoked SAI and SEPs follow a similar recruitment pattern and also cease to increase beyond ∼50% of SNAPmax.
Experiment 2. DN Stimulation
For DN stimulation, 20 right-handed individuals were studied (7 men, 13 women; age 23.1 ± 2 yr) and included in all subsequent analyses. The data for DN SAI were not normally distributed (FCR, P = 0.003; FDI, P = 0.001), and two outliers originating from the same participant were identified and subsequently removed. After removal of the outliers the data were normally distributed (P = 0.20). The actual group-averaged DN nerve intensities delivered, based on percentage of SNAPmax, are shown in Table 1 and are referred to here.
Group-averaged peak-to-peak N20-P25 SEPs are shown in Table 2. One-way ANOVA revealed a significant effect of Intensity [F(1.469,27.907) = 29.682, P < 0.001] such that SEP 1 was greater at 55%, 84%, and 100% vs. 21% and greater at 100% vs. 55% SNAPmax (P < 0.05, Tukey's). For SAI (Table 2), two-way ANOVA revealed a significant main effect of Intensity [F(3,54) = 7.25, P < 0.001] and a trend toward a significant effect of Muscle [F(1,18) = 4.39, P = 0.051] without their interaction [F(3,54) = 1.13 P = 0.34]. For the factor Intensity, SAI is greater at all intensities compared with 21% SNAPmax (P < 0.05, Tukey's).
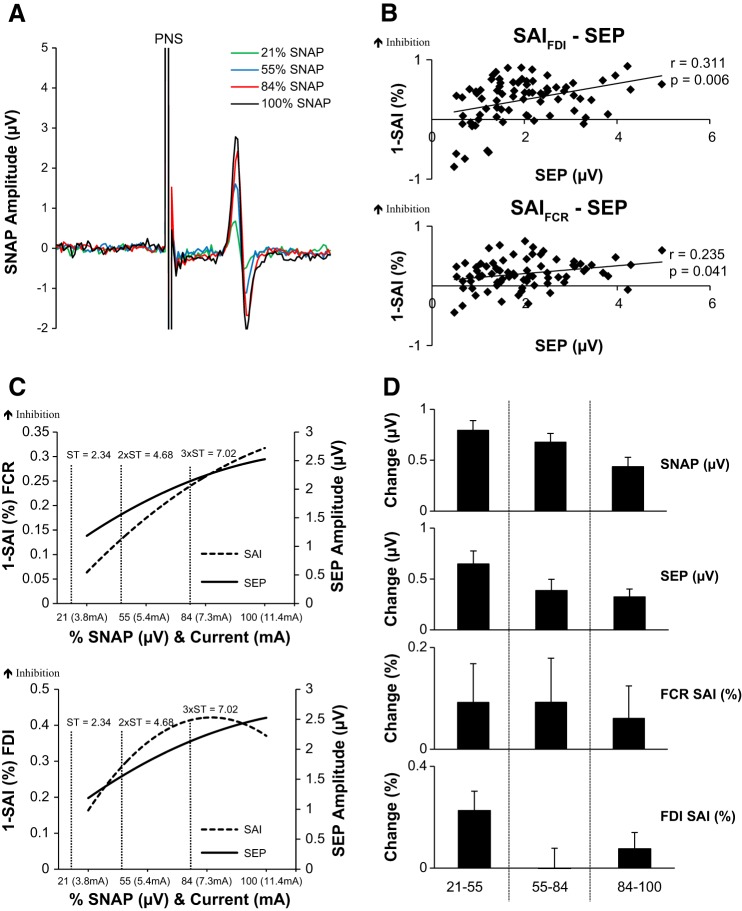
Figure 2A plots an example SNAP data set recorded from one participant after DN stimulation. Figure 2B plots the positive correlations between SEPs and SAI for FCR (Fig. 2B, bottom) and FDI (Fig. 2B, top). Corresponding trend lines are plotted in Figure 2C. Unlike the MN evoked responses, SEPs and SAI continue to increase until SNAPmax. For comparison, we have plotted the group-averaged sensory thresholds (STs) commonly used in research (ST, 2× ST, and 3× ST), all of which fall below 100% SNAPmax. Finally, Fig. 2D plots the change in SEPs and SAI within each SNAP increment and reveals that both measures increase until the maximum SNAP is achieved, in contrast to that observed after stimulation of the mixed MN. In summary, the DN data reveal that SEPs and SAI continue to increase with the SNAP until SNAPmax is reached.
Fig. 2.
Digital nerve stimulation. A: average SNAPs recorded from 1 individual at each intensity of digital nerve stimulation. B, top: correlations between SAI evoked in FDI and SEP 1 reveal a significant positive correlation showing that as SEPs increase the depth of SAI increases. Bottom: correlations between SAI evoked in FCR and SEP 1 reveal a significant positive correlation showing that as SEPs increase the depth of SAI increases. C, top: second-order polynomial trend lines of the recruitment curves for each measurement in FCR are plotted to represent how each measure responds to the increases in SNAP. For comparison, group-averaged sensory thresholds (ST, 2× ST, and 3× ST) are shown, all of which are below SNAPmax. Bottom: second-order polynomial trend lines for SAI in FDI and SEPs. D: the group-averaged % change (±SE) in each increment of stimulation intensity for the SNAP, SEP, and SAI. As shown, SNAP, SEP, and SAIFCR and SAIFDI increase, as depicted by the positive % change, to the highest intensity increment.
DISCUSSION
The present study examined the stimulus-response relationship between SAI and the sensory afferent volley and between SAI and SEPs, the latter of which is known to reliably reflect the afferent volley (Gandevia and Burke 1984; Lesser et al. 1979). Several novel observations were revealed. First, the depth of SAI is indeed linked to the sensory afferent volley; increases in SAI are associated with increases in the sensory afferent volley. This relationship persists for the mixed MN, where the afferent fibers are likely fully recruited by ∼50% SNAPmax, and also for the purely cutaneous DN, where afferent fibers contribute until the maximum SNAP is obtained. Second, measures of SAI and SEPs are closely related—as SEPs increase, SAI increases, and both measures behave similarly regardless of nerve stimulated and muscle tested.
The amplitude of the N20-P25 SEP generated after MN stimulation increased up to ∼50% SNAPmax (i.e., 1.2× MT) and plateaued beyond this point, similar to previous reports (Gandevia and Burke 1984; Lesser et al. 1979). An alternative explanation is that above 50% SNAPmax subcortical structures including the dorsal column nuclei, ventro-posterior lateral/anterior nuclei of the thalamus, and thalamic reticular nucleus may provide a gating mechanism to reduce the afferent information arriving at the cortex (Guillery et al. 1998). The present findings, however, suggest that the SEP plateau reflects the point at which all sensory fibers within the MN have been recruited (i.e., ∼50% SNAPmax), since the cutaneous DN SEPs increase until the maximum SNAP is reached. Therefore, our findings support the suggestion by Gandevia and Burke (1984) that MN evoked SNAPs are contributed by antidromic motor fibers beyond ∼50% SNAPmax. The stimulus response profile of SAI follows closely with that of SEP 1. Both measures increased up to 50% SNAPmax with MN stimulation, and both increased to 100% SNAPmax with DN stimulation. It is also interesting to note that MN SAI follows a U-shaped function and is decreased slightly beyond ∼71% SNAPmax. Although we did not assess the percept of pain in our study, SAI is reduced immediately after painful stimuli (Burns et al. 2016), and this may have contributed to our observation.
The pathway by which the afferent volley projects to M1 to produce SAI remains unclear—does the afferent volley proceed to M1 via a direct thalamo-cortical projection (Di Lazzaro et al. 2012) or via a relay through SI? Although our data cannot exclude a direct thalamo-cortical route for the SAI circuit, our results support a relay through SI since SEPs are significantly correlated with SAI after both MN and DN stimulation, suggesting that the activation of SI is closely linked to the inhibition present in M1. One mechanism to explain such cortico-cortical conditioning from SI involves pyramidal cells that, within SI, have long-range horizontal monosynaptic excitatory projections to neurons within M1 (Petrof et al. 2015) and are able to drive the output of cells in the upper layers of M1 but have little influence on the cells in the lower layers (Petrof et al. 2015; Sherman 2012). SAI is associated with a reduction in the amplitude of the indirect (I) waves, specifically the late I-3 wave but not the earlier I waves (Tokimura et al. 2000), and the GABAergic inhibitory neurons are involved in the suppression of late and not early I waves (Di Lazzaro et al. 2012). Furthermore, the interval we tested (i.e., N20 + 2 ms) yields stronger inhibition than that observed at longer intervals (i.e., N20 + 6 ms or + 8 ms), the latter of which appear to be mediated by the cerebellum (Dubbioso et al. 2015). We suggest that long-range horizontal projections from SI pyramidal cells may excite these GABAergic inhibitory cells, thereby increasing the inhibition on the late I waves resulting in SAI. Increasing the excitation of SI pyramidal cells with a larger afferent volley (and greater SEP amplitude) may potentiate the GABAergic inhibitory cells and increase SAI. In further support for the role of SI in SAI generation, TMS plasticity protocols targeting SI but not M1 modulate SAI (Kojima et al. 2015; Tsang et al. 2014, 2015).
The mechanism of SEP 2 suppression relative to SEP 1 is thought to proceed by GABAergic neurotransmission (Cruikshank et al. 2007; Gatica Tossi et al. 2013) whereby activated pyramidal cells excite inhibitory parvalbumin-expressing interneurons (Neske et al. 2015), likely double bouquet cells (Whitsel et al. 1999) that, via recurrent inhibition, act to inhibit the pyramidal cells themselves (Neske et al. 2015; Whitsel et al. 1999). Therefore, the response to the second nerve stimulus is suppressed relative to the first. One important observation, however, is that the amplitude of SEP 2 is not altered by changes in the sensory afferent volley. Therefore, the magnitude of recurrent inhibition is largely unchanged by increases in intensity. We note that SEP 2 is not only insensitive to changes in the afferent volley but also appears to not exceed ∼2 μV, suggesting that there is a ceiling effect to which cells can be activated immediately after activation.
Practical Implications of the Research
The data presented have important implications for basic and clinical neuroscience research. First, we have identified that the stimulus-response relationship of the SAI and SEP recruitment curve is intensity specific. For example, studies that aim to alter SAI magnitude (Quartarone et al. 2006; Tsang et al. 2014, 2015) would benefit from an intensity between 25% and 50% SNAPmax to allow SAI to increase or decrease accordingly. We note that MT for a twitch in APB is observed at approximately the same current needed to elicit ∼50% SNAPmax. Therefore, stimulation intensities set above MT are likely to lead to saturation in SAI and may limit opportunities to observe increases in SAI magnitude. Additionally, we note that the current for detecting ST is below the current to elicit 25% SNAPmax. Therefore a stimulation intensity delivered at ST may be insufficient to observe decreases in SEPs or SAI. However, stimulation intensity delivered at 2× ST provides the opportunity to observe both increases and decreases in SEPs and SAI. Furthermore, we have included the corresponding current intensity needed to achieve each SNAP. Although absolute values of current reflect impedance and hardware specifics, these data indicate that amperages associated with STs and MTs closely reflect those reported in the literature (Quartarone et al. 2006; Tsang et al. 2014, 2015).
Revealing the relationship between the cortical representation of the somatosensory afferent volley and the magnitude of M1 output may have implications for motor control. Recent research has shown the importance of SAI in both allowing movement and preventing unwanted actions (Asmussen et al. 2013, 2014; Voller et al. 2006). In individuals with Parkinson's disease, reduced SAI is associated with slower gait speed (Rochester et al. 2012) but is unrelated to freezing of gait symptoms (Picillo et al. 2015). The findings of the present study may help guide the methodologies of future studies examining the role of SAI in motor control.
Conclusions
Our data show that SAI and SEPs have similar recruitment profiles. These data support the hypothesis that the sensory afferent volley is a key determinant in the magnitude of SI cortical excitation (SEPs) and inhibition within M1 (SAI) and that neural activity within SI is a significant predictor of changes in SAI. Understanding the relationship between the afferent volley and measures of sensorimotor function is essential for designing effective research studies, and understanding their interrelatedness provides new insight into the neural mechanisms that mediate the SAI circuit.
GRANTS
This study was supported by a Natural Sciences and Engineering Research Council (NSERC) operating grant to A. J. Nelson.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
A.Z.B., M.J.A., and A.J.N. conception and design of research; A.Z.B. and M.J.A. performed experiments; A.Z.B. and A.J.N. analyzed data; A.Z.B., M.J.A., and A.J.N. interpreted results of experiments; A.Z.B. and A.J.N. prepared figures; A.Z.B., M.J.A., and A.J.N. drafted manuscript; A.Z.B., M.J.A., and A.J.N. edited and revised manuscript; A.Z.B., M.J.A., and A.J.N. approved final version of manuscript.
REFERENCES
- Allison T. Recovery functions of somatosensory evoked responses in man. Electroencephalogr Clin Neurophysiol 14: 331–343, 1962. [DOI] [PubMed] [Google Scholar]
- Asmussen MJ, Jacobs MF, Lee KG, Zapallow CM, Nelson AJ. Short-latency afferent inhibition modulation during finger movement. PLoS One 8: e60496, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asmussen MJ, Zapallow CM, Jacobs MF, Lee KG, Tsang P, Nelson AJ. Modulation of short-latency afferent inhibition depends on digit and task-relevance. PLoS One 9: e104807, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burns E, Chipchase LS, Schabrun SM. Reduced short- and long-latency afferent inhibition following acute muscle pain: a potential role in the recovery of motor output. Pain Med (February 13, 2016). doi: 10.1093/pm/pnv104. [DOI] [PubMed] [Google Scholar]
- Celebi O, Temuçin CM, Elibol B, Saka E. Short latency afferent inhibition in Parkinson's disease patients with dementia. Mov Disord 27: 1052–1055, 2012. [DOI] [PubMed] [Google Scholar]
- Chen R, Corwell B, Hallett M. Modulation of motor cortex excitability by median nerve and digit stimulation. Exp Brain Res 129: 77–86, 1999. [DOI] [PubMed] [Google Scholar]
- Classen J, Steinfelder B, Liepert J, Stefan K, Celnik P, Cohen LG, Hess A, Kunesch E, Chen R, Benecke R, Hallett M. Cutaneomotor integration in humans is somatotopically organized at various levels of the nervous system and is task dependent. Exp Brain Res 130: 48–59, 2000. [DOI] [PubMed] [Google Scholar]
- Cruikshank SJ, Lewis TJ, Connors BW. Synaptic basis for intense thalamocortical activation of feedforward inhibitory cells in neocortex. Nat Neurosci 10: 462–468, 2007. [DOI] [PubMed] [Google Scholar]
- Di Lazzaro V. Motor cortex hyperexcitability to transcranial magnetic stimulation in Alzheimer's disease. J Neurol Neurosurg Psychiatry 75: 555–559, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Lazzaro V, Oliviero A, Pilato F, Saturno E, Dileone M, Bentivoglio AR, Tonali PA. Normal or enhanced short-latency afferent inhibition in Parkinson's disease? Brain 127: E8–E9, 2004. [DOI] [PubMed] [Google Scholar]
- Di Lazzaro V, Oliviero A, Profice P, Pennisi MA, Di Giovanni S, Zito G, Tonali P, Rothwell JC. Muscarinic receptor blockade has differential effects on the excitability of intracortical circuits in the human motor cortex. Exp Brain Res 135: 455–461, 2000. [DOI] [PubMed] [Google Scholar]
- Di Lazzaro V, Pilato F, Dileone M, Tonali PA, Ziemann U. Dissociated effects of diazepam and lorazepam on short-latency afferent inhibition. J Physiol 569: 315–323, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Lazzaro V, Profice P, Ranieri F, Capone F, Dileone M, Oliviero A, Pilato F. I-wave origin and modulation. Brain Stimul 5: 512–525, 2012. [DOI] [PubMed] [Google Scholar]
- Dubbioso R, Pellegrino G, Antenora A, De Michele G, Filla A, Santoro L, Manganelli F. The effect of cerebellar degeneration on human sensori-motor plasticity. Brain Stimul 8: 1144–1150, 2015. [DOI] [PubMed] [Google Scholar]
- Fischer M, Orth M. Short-latency sensory afferent inhibition: conditioning stimulus intensity, recording site, and effects of 1 Hz repetitive TMS. Brain Stimul 4: 202–209, 2011. [DOI] [PubMed] [Google Scholar]
- Gandevia SC, Burke D. Saturation in human somatosensory pathways. Exp Brain Res 54: 582–585, 1984. [DOI] [PubMed] [Google Scholar]
- Gatica Tossi MA, Lillemeier AS, Dinse HR. Influence of stimulation intensity on paired-pulse suppression of human median nerve somatosensory evoked potentials. Neuroreport 24: 451–456, 2013. [DOI] [PubMed] [Google Scholar]
- Guillery RW, Feig SL, Lozsádi DA. Paying attention to the thalamic reticular nucleus. Trends Neurosci 21: 28–32, 1998. [DOI] [PubMed] [Google Scholar]
- Höffken O, Lenz M, Tegenthoff M, Schwenkreis P. Multichannel SEP-recording after paired median nerve stimulation suggests origin of paired-pulse inhibition rostral of the brainstem. Neurosci Lett 468: 308–311, 2010. [DOI] [PubMed] [Google Scholar]
- Höffken O, Tannwitz J, Lenz M, Sczesny-Kaiser M, Tegenthoff M, Schwenkreis P. Influence of parameter settings on paired-pulse-suppression in somatosensory evoked potentials: a systematic analysis. Clin Neurophysiol 124: 574–580, 2013. [DOI] [PubMed] [Google Scholar]
- Höffken O, Veit M, Knossalla F, Lissek S, Bliem B, Ragert P, Dinse HR, Tegenthoff M. Sustained increase of somatosensory cortex excitability by tactile coactivation studied by paired median nerve stimulation in humans correlates with perceptual gain. J Physiol 584: 463–471, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kojima S, Onishi H, Miyaguchi S, Kotan S, Sugawara K, Kirimoto H, Tamaki H. Effects of cathodal transcranial direct current stimulation to primary somatosensory cortex on short-latency afferent inhibition. Neuroreport 26: 634–637, 2015. [DOI] [PubMed] [Google Scholar]
- Lesser RP, Koehle R, Lueders H. Effect of stimulus intensity on short latency somatosensory evoked potentials. Electroencephalogr Clin Neurophysiol 47: 377–382, 1979. [DOI] [PubMed] [Google Scholar]
- Lin YY, Shih YH, Chen JT, Hsieh JC, Yeh TC, Liao KK, Kao CD, Lin KP, Wu ZA, Ho LT. Differential effects of stimulus intensity on peripheral and neuromagnetic cortical responses to median nerve stimulation. Neuroimage 20: 909–917, 2003. [DOI] [PubMed] [Google Scholar]
- McLaughlin DF, Kelly EF. Evoked potentials as indices of adaptation in the somatosensory system in humans: a review and prospectus. Brain Res Brain Res Rev 18: 151–206, 1993. [DOI] [PubMed] [Google Scholar]
- Nardone R, Florio I, Lochner P, Tezzon F. Cholinergic cortical circuits in Parkinson's disease and in progressive supranuclear palsy: a transcranial magnetic stimulation study. Exp Brain Res 163: 128–131, 2005. [DOI] [PubMed] [Google Scholar]
- Neske GT, Patrick SL, Connors BW. Contributions of diverse excitatory and inhibitory neurons to recurrent network activity in cerebral cortex. J Neurosci 35: 1089–1105, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrof I, Viaene AN, Sherman SM. Properties of the primary somatosensory cortex projection to the primary motor cortex in the mouse. J Neurophysiol 113: 2400–2407, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Picillo M, Dubbioso R, Iodice R, Iavarone A, Pisciotta C, Spina E, Santoro L, Barone P, Amboni M, Manganelli F. Short-latency afferent inhibition in patients with Parkinson's disease and freezing of gait. J Neural Transm 122: 1533–1540, 2015. [DOI] [PubMed] [Google Scholar]
- Quartarone A, Rizzo V, Bagnato S, Morgante F, Sant'Angelo A, Girlanda P, Siebner HR. Rapid-rate paired associative stimulation of the median nerve and motor cortex can produce long-lasting changes in motor cortical excitability in humans. J Physiol 575: 657–670, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rochester L, Yarnall AJ, Baker MR, David RV, Lord S, Galna B, Burn DJ. Cholinergic dysfunction contributes to gait disturbance in early Parkinson's disease. Brain 135: 2779–2788, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sailer A, Cunic DI, Paradiso GO, Gunraj CA, Wagle-Shukla A, Moro E, Lozano AM, Lang AE, Chen R. Subthalamic nucleus stimulation modulates afferent inhibition in Parkinson disease. Neurology 68: 356–363, 2007. [DOI] [PubMed] [Google Scholar]
- Sailer A, Molnar GF, Paradiso G, Gunraj CA, Lang AE, Chen R. Short and long latency afferent inhibition in Parkinson's disease. Brain 126: 1883–1894, 2003. [DOI] [PubMed] [Google Scholar]
- Sherman SM. Thalamocortical interactions. Curr Opin Neurobiol 22: 575–579, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamburin S, Manganotti P, Zanette G, Fiaschi A. Cutaneomotor integration in human hand motor areas: somatotopic effect and interaction of afferents. Exp Brain Res 141: 232–241, 2001. [DOI] [PubMed] [Google Scholar]
- Taylor JL, Burke D, Heywood J. Physiological evidence for a slow K+ conductance in human cutaneous afferents. J Physiol 453: 575–589, 1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tokimura H, Di Lazzaro V, Tokimura Y, Oliviero A, Profice P, Insola A, Mazzone P, Tonali P, Rothwell JC. Short latency inhibition of human hand motor cortex by somatosensory input from the hand. J Physiol 523: 503–513, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsang P, Bailey AZ, Nelson AJ. Rapid-rate paired associative stimulation over the primary somatosensory cortex. PLoS One 10: e0120731, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsang P, Jacobs MF, Lee KG, Asmussen MJ, Zapallow CM, Nelson AJ. Continuous theta-burst stimulation over primary somatosensory cortex modulates short-latency afferent inhibition. Clin Neurophysiol 125: 2253–2259, 2014. [DOI] [PubMed] [Google Scholar]
- Voller B, St Clair Gibson A, Dambrosia J, Pirio Richardson S, Lomarev M, Dang N, Hallett M. Short-latency afferent inhibition during selective finger movement. Exp Brain Res 169: 226–231, 2006. [DOI] [PubMed] [Google Scholar]
- Whitsel BL, Favorov O, Delemos KA, Lee C, Tommerdahl M, Essick GK, Nakhle B. SI neuron response variability is stimulus tuned and NMDA receptor dependent. J Neurophysiol 81: 2988–3006, 1999. [DOI] [PubMed] [Google Scholar]
- Yarnall AJ, Rochester L, Baker MR, David R, Khoo TK, Duncan GW, Galna B, Burn DJ. Short latency afferent inhibition: a biomarker for mild cognitive impairment in Parkinson's disease? Mov Disord 28: 1285–1288, 2013. [DOI] [PubMed] [Google Scholar]