Endocannabinoids are important signaling molecules that depress both excitatory synapses leading to decreases in neural circuit output and inhibitory synapses leading to increases in circuit output due to disinhibition. In this study, difference in Cl− gradients were found to strongly influence whether endocannabinoids ultimately decrease or increase circuit output. These findings may explain the pro- and antinociceptive effects of endocannabinoids. The role of Cl− gradient may also be relevant to other modulatory transmitters as well.
Keywords: endocannabinoids, TRPV, Cl− homeostasis, neuromodulation, Hirudo
Abstract
Endocannabinoids can elicit persistent depression of excitatory and inhibitory synapses, reducing or enhancing (disinhibiting) neural circuit output, respectively. In this study, we examined whether differences in Cl− gradients can regulate which synapses undergo endocannabinoid-mediated synaptic depression vs. disinhibition using the well-characterized central nervous system (CNS) of the medicinal leech, Hirudo verbana. Exogenous application of endocannabinoids or capsaicin elicits potentiation of pressure (P) cell synapses and depression of both polymodal (Npoly) and mechanical (Nmech) nociceptive synapses. In P synapses, blocking Cl− export prevented endocannabinoid-mediated potentiation, consistent with a disinhibition process that has been indicated by previous experiments. In Nmech neurons, which are depolarized by GABA due to an elevated Cl− equilibrium potentials (ECl), endocannabinoid-mediated depression was prevented by blocking Cl− import, indicating that this decrease in synaptic signaling was due to depression of excitatory GABAergic input (disexcitation). Npoly neurons are also depolarized by GABA, but endocannabinoids elicit depression in these synapses directly and were only weakly affected by disruption of Cl− import. Consequently, the primary role of elevated ECl may be to protect Npoly synapses from disinhibition. All forms of endocannabinoid-mediated plasticity required activation of transient potential receptor vanilloid (TRPV) channels. Endocannabinoid/TRPV-dependent synaptic plasticity could also be elicited by distinct patterns of afferent stimulation with low-frequency stimulation (LFS) eliciting endocannabinoid-mediated depression of Npoly synapses and high-frequency stimulus (HFS) eliciting endocannabinoid-mediated potentiation of P synapses and depression of Nmech synapses. These findings demonstrate a critical role of differences in Cl− gradients between neurons in determining the sign, potentiation vs. depression, of synaptic modulation under normal physiological conditions.
NEW & NOTEWORTHY
Endocannabinoids are important signaling molecules that depress both excitatory synapses leading to decreases in neural circuit output and inhibitory synapses leading to increases in circuit output due to disinhibition. In this study, difference in Cl− gradients were found to strongly influence whether endocannabinoids ultimately decrease or increase circuit output. These findings may explain the pro- and antinociceptive effects of endocannabinoids. The role of Cl− gradient may also be relevant to other modulatory transmitters as well.
many modulatory neurotransmitters, such as endocannabinoids, opioids, and adenosine, depress both excitatory (glutamatergic) and inhibitory (GABAergic/glycinergic) central synapses (Freund et al. 2003; Morairty et al. 2004; Lau and Vaughan 2014). Consequently, these transmitters are capable of either decreasing neural circuit output by depressing excitatory synapses or increasing circuit output by depressing inhibitory synapses (i.e., disinhibition). This raises a question; how do neural circuits regulate which synaptic pathways undergo depression vs. disinhibition? This has been a major concern in understanding how endocannabinoids modulate pain circuits given that these transmitters have been observed to produce both depression (Morisset and Urban 2001; Liang et al. 2004) and disinhibition (Pernia-Andrade et al. 2009) of nociceptive synaptic input.
It has been hypothesized that differences in Cl− gradients may act to regulate whether or not synapses are sensitive to disinhibition, potentially explaining how endocannabinoids can have both pro- and antinociceptive effects (Christie and Mallet 2009). Most neurons have low levels of intracellular Cl− that are maintained by the K+-Cl− cotransporter 2 (KCC2) that exports Cl− so that activation of ionotropic GABA receptors leads to Cl− influx and hyperpolarization. However, neurons can also have a high intracellular Cl− due to the influence of the Na+-K+-Cl− cotransporter 1 (NKCC1) that imports Cl− so that GABA receptor activation elicits Cl− efflux and depolarization (Kaila et al. 2014). While KCC2-expressing neurons would be sensitive to neuromodulator-induced disinhibition, NKCC1-expressing neurons would be presumably “protected” from such disinhibition.
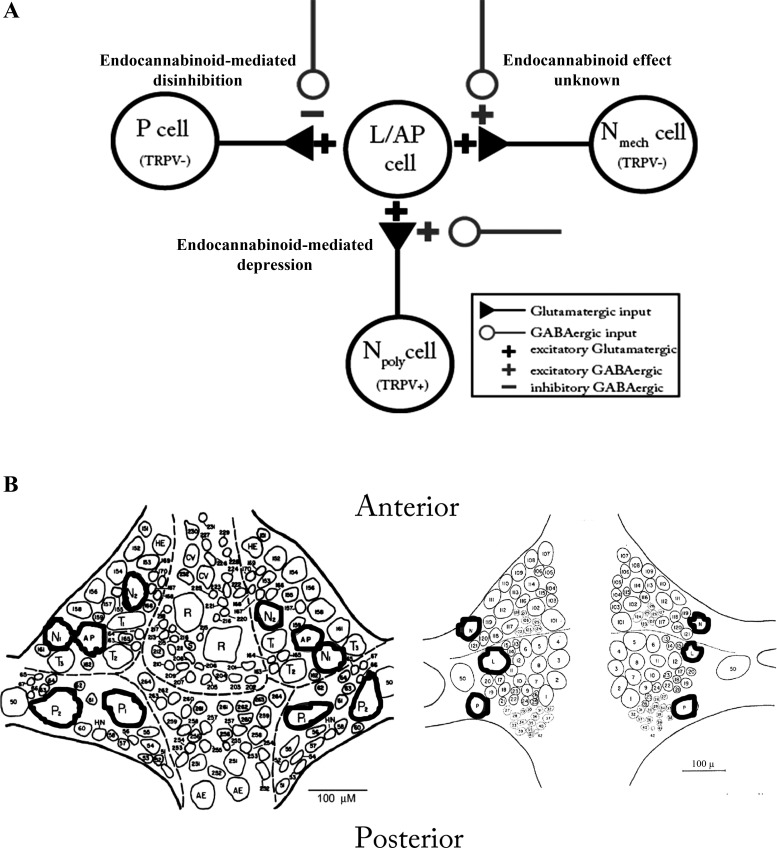
Testing the role of differences in Cl− gradients is impossible without prior knowledge of which neurons have normal vs. elevated intracellular Cl− and whether their synapses undergo endocannabinoid-mediated modulation. Knowing such details, a priori, is possible in the well-characterized central nervous system (CNS) of the medicinal leech, Hirudo verbana (Fig. 1). In Hirudo, nonnociceptive pressure-sensitive afferents (P cell) are inhibited by GABA while the polymodal nociceptive (Npoly) and mechano-only nociceptive (Nmech) afferents are excited by GABA (Sargent et al. 1977; Wang et al. 2015). These opposing effects of GABA are due to differences in the Cl− equilibrium potentials (ECl), which are regulated by KCC2- and NKCC1-like transporters in the P and N cells, respectively (Wang et al. 2015). Pharmacological inhibition of KCC2 in Hirudo produced a depolarizing shift in the ECl in the P cells, as well as reduced GABA-induced hyperpolarization and increased/disinhibited synaptic transmission. Inhibition of NKCC1 in the N cells, however, produced a hyperpolarizing shift in the ECl that was accompanied by a reduction in GABA-induced depolarization and a decrease in synaptic transmission.
Fig. 1.
A: diagram of Hirudo sensorimotor synapses including the putative GABAergic input they receive. The pressure (P), polymodal (Npoly), and mechanical (Nmech) sensory neurons all have excitatory, glutamatergic input onto shared postsynaptic targets (filled triangles) (Wessel et al. 1999; Yuan and Burrell, 2010; Higgins et al. 2013). In this study, the synapses used were the P- or Npoly-to-longitudinal motor neuron (L) and the Nmech-to-anterior pagoda neuron (AP). Tonic, GABAergic inputs (open circles) have an inhibitory effect on P cell synapses but have an excitatory effect on both Npoly- and Nmech-cell synapses (Wang et al. 2015). B: locations of identified neurons in the Hirudo central nervous system (CNS) used in these experiments (modified from Muller et al. 1981). Both the ventral (left) and dorsal (right) aspects of a single mid-body ganglion are shown. Neurons used in this study have been outlined; N1 = Npoly and N2 = Nmech.
Both the P and Npoly cells make monosynaptic, glutamatergic input onto a shared postsynaptic target, the longitudinal (L) motor neuron (Fig. 1A) (Baylor and Nicholls 1969; Yuan and Burrell 2010). Furthermore, both synapses contribute to the neural circuit that mediates whole body shortening, a defensive withdrawal reflex (Shaw and Kristan 1995). Finally, both synapses are modulated by endocannabinoids, depressing N-cell synapses and potentiating P-cell synapses for at least 1 h (Yuan and Burrell 2010, 2012; Higgins et al. 2013). Since it is known which synapses are modulated by endocannabinoids and which of these synapses are hyperpolarized vs. depolarized by GABA, it is possible to examine the potential role of Cl− homeostasis on endocannabinoid-mediated plasticity in Hirudo.
The Hirudo CNS possesses the two main endocannabinoids found in the mammalian nervous system, 2-arachydonoyl glycerol (2-AG) and anandamide (AEA) (Matias et al. 2001). Like other protostomal invertebrates, Hirudo lacks orthologues of the cannabinoid receptors CB1 and CB2 (Elphick 2012). Instead, modulation by endocannabinoids in Hirudo is mediated by a transient potential receptor vanilloid (TRPV)-like receptor (Summers et al. 2014) that mediates both endocannabinoid-dependent long-term depression (eCB-LTD) in Npoly synapses (Yuan and Burrell 2010, 2013b) and endocannabinoid-induced disinhibition of P synapses (Higgins et al. 2013). Npoly cells possess TRPV channels that are believed to be activated by 2-AG, resulting in eCB-LTD (Yuan and Burrell 2010). P cells lack TRPV channels and it is thought that 2-AG acts on unknown GABAergic neurons that are TRPV positive and undergo eCB-LTD resulting in disinhibition of the P-cell synapses (Higgins et al. 2013; Summers et al. 2014). It is well-established that endocannabinoids can activate mammalian TRPV1 channels (De Petrocellis et al. 2001; Qin et al. 2008; Zygmunt et al. 2013) and that endocannabinoid activation of TRPV1 can elicit LTD of excitatory and inhibitory synapses, the latter resulting in disinhibition of neural circuits (Gibson et al. 2008; Maione et al. 2009; Chavez et al. 2010; Puente et al. 2011; Kim et al. 2012; Brown et al. 2013). The cellular features of eCB-LTD in Hirudo exhibit considerable conservation with at least the presynaptic forms of TRPV-mediated LTD in mammalian synapses (Gibson et al. 2008; Jensen and Edwards 2012) so the effects of Cl− homeostasis on endocannabinoid-mediated plasticity in Hirudo are relevant to function in the mammalian CNS. In this study, differences in Cl− gradients were found to play a critical role in determining how endocannabinoids modulate synapses from three distinct cell types of neurons (P, Npoly, and Nmech).
MATERIALS AND METHODS
Animal preparation.
Hirudo verbana (3 g) were obtained from commercial suppliers (Leeches USA, Westbury, NY and Niagara Leeches, Cheyenne, WY) and maintained in artificial pond water (0.52 g/l H2O Instant Ocean, Aquarium Systems) on a 12-h light-dark cycle at 15°C. Individual ganglia were dissected and pinned in a recording chamber with constant perfusion of normal Hirudo saline (110 mM NaCl, 5 mM NaOH, 4 mM KCl, 1.8 mM CaCl2, 1 mM MgCl2, and 10 mM HEPES, pH 7.4) at ∼1.5 ml/min. Individual neurons were identified based on their position within the ganglion, size, and electrophysiological properties. Each ganglion contains two bilateral pairs of nociceptive (N) and pressure-sensitive cells (P) and three pairs of light touch-sensitive neurons (T) (Fig. 1B). The N cells are further divided into a lateral N pair that are polymodal nociceptors (Npoly) and a medial pair that are mechanical nociceptors (Nmech) (Nicholls and Baylor 1968; Blackshaw et al. 1982; Pastor et al. 1996). L-motor neuron identification was confirmed by recording from the electrically coupled contralateral L-motor neuron and observing synchronous activity (Stuart 1970). The L cells are located laterally on the dorsal side of the ganglion and the afferent cells are located on the ventral surface (Fig. 1B). However, it is possible to record from both the L and the Npoly or P cells from the dorsal side of the ganglion given the lateral positions of the cell bodies from both these primary afferents (Fig. 1B). It is not possible to record from the Nmech cell when the ganglion is dorsal side up because this neuron is located medially on the ventral side of the ganglion. Therefore, synaptic recordings were made from the Nmech and AP neurons, which are both located on the ventral surface of the ganglion (Fig. 1B). Where they have been compared, the properties of N- or P-to-AP synapses appear to be identical to other synaptic connections made by these sensory cells (Gaudry and Kristan 2009; Higgins et al. 2013).
Drug application.
Drugs used for each experiment were kept as frozen aliquot solutions and then diluted to their final concentration in normal saline just before each respective experiment. 2-Arachidonoylglycerol (2-AG), anandamide (AEA), capsaicin (CAP), SB 366791 (SB), tetrahydrolipstatin (THL, also known as Orlistat), bumetanide (BUM), and VU 0240551 (VU) stocks were made in dimethyl sulfoxide (DMSO). 2-AG, AEA, and SB were obtained from Tocris (Ellisville, MO), while THL, BUM, VU, CAP, and DMSO were obtained from Sigma-Aldrich (St. Louis, MO).
Electrophysiology.
Techniques used in this study have been described in detail previously (Yuan and Burrell 2013b). Briefly, current clamp (bridge balanced) intracellular recordings were carried out using sharp glass microelectrodes (tip resistance: 20–35 MΩ) made from borosilicate capillary tubing (1.0-mm OD, 0.75-mm ID; FHC, Bowdoinham, ME) using a horizontal puller (Sutter Instruments P-97; Novato, CA). Microelectrodes were filled with 3 M potassium acetate (KAc), which produced no obvious effects on synaptic inhibition in Hirudo neurons (Burgin and Szczupak 2003; Ivanov and Calabrese 2006). Manual micropositioners (Model 1480; Siskiyou, Grants Pass, OR) were used to impale individual neurons during experiments. Current pulses were delivered to electrodes using a multichannel programmable stimulator (STG 1004; Multi-Channel Systems; Reutlingen, Germany) and the signal was recorded using a bridge amplifier (BA-1S; NPI, Tamm, Germany) and digitally converted for analysis (Axoscope; Molecular Devices, Sunnyvale, CA).
Pretest recordings of the excitatory postsynaptic potentials (EPSPs) were conducted and then the intracellular electrodes were withdrawn. The ganglion was then treated with 2-AG (100 μM), AEA (100 μM), or capsaicin (1 μM) for 15 min, followed by a 60-min consolidation/washout period. The pre- and postsynaptic cells were then repenetrated and the posttest recordings of EPSPs were taken. Prolonged recordings with these sharp microelectrodes were not carried out because this results in rundown of EPSP amplitudes, likely due to damage to the recorded cell. For experiments using SB 366791, the pretest recording were conducted after bath application of SB 366791 for 15 min and then coapplication of SB 366791 and 2-AG, AEA, or capsaicin, and after a 60-min consolidation, posttest recordings of EPSPs were taken. For experiments using bumetanide (25 μM) or VU 0240551 (100 μM), the pretest recordings were conducted after bath application of either bumetanide or VU 0240551 for 15 min and then coapplication of bumetanide or VU 0240551 and 2-AG, AEA, or capsaicin, and after a 60-min consolidation, posttest recordings of EPSPs were taken (bumetanide and VU 0240551 were applied throughout the whole experiments). For experiments using THL (10 μM), pretest recordings of EPSPs were performed, then bath application of THL 15 min and then coapplication of THL and 2-AG, AEA, or capsaicin, and after a 60-min consolidation, posttest recordings of EPSPs were taken. All treatment groups were tested in parallel with a vehicle control group (0.01% DMSO).
For experiments testing activity-elicited synaptic modulation, low-frequency stimulation (LFS) of T cell was conducted by a well-established protocol in which the presynaptic cell was stimulated 900 times at 1 Hz. High-frequency stimulation (HFS) was delivered to the Npoly neuron that consisted of 20 trains delivered at a rate of 0.1 Hz with each train having 10 suprathreshold stimulus pulses at 25 Hz. For both LFS and HFS experiments, EPSPs and recordings were made before (pretest) and 60 min following either LFS or HFS.
To ensure that changes in synaptic transmission were not the result of changes in postsynaptic input resistance (IR) due to either experimental treatment or repenetration of the sharp electrode, IR was monitored during the pre- and posttest recordings by measuring the membrane potential change during a 500-ms, 1-nA negative current pulse. Only stable recordings (<10% change in IR) were included in the final data analysis. During the pre- and posttests, EPSPs were elicited at 0.1 Hz and the peak amplitude was calculated from the average of 5–10 EPSPs recordings.
Statistics.
EPSP amplitude measurements of the pre- and posttest recordings were normalized and presented as means ± SE. Statistical analyses using a one-way ANOVA were performed to determine main effects with Student-Newman-Keuls post hoc tests to confirm the ANOVA results. The analyses were carried out with Sigmaplot. All significance was determined at α level of at least P ≤ 0.05.
RESULTS
Effects of endocannabinoids on three different types of glutamatergic synapses.
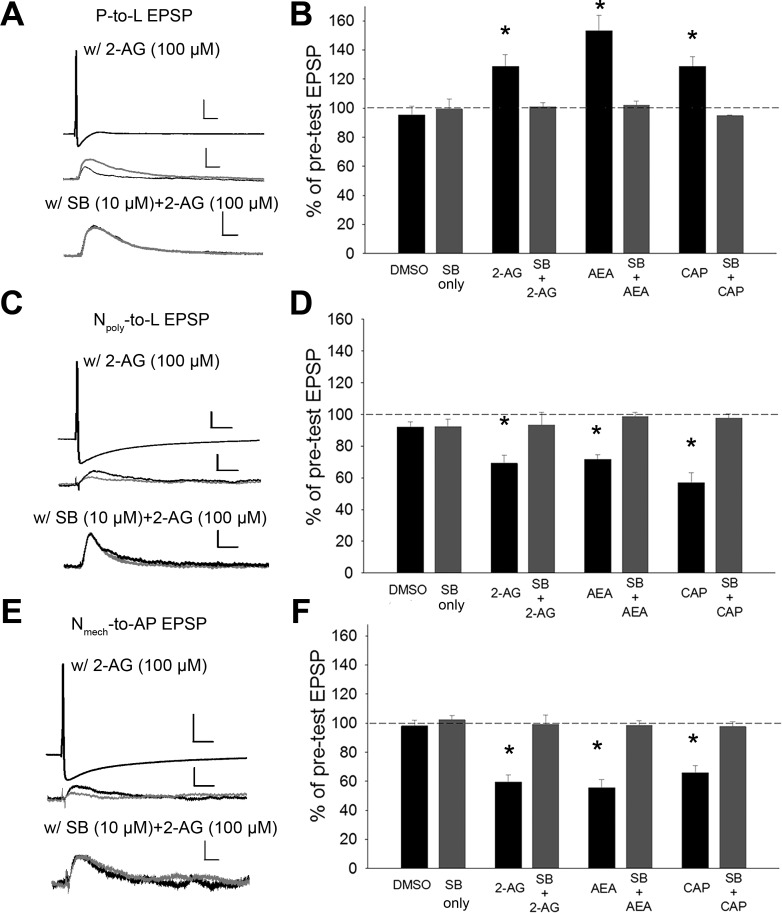
First, the effects of the endocannabinoids 2-AG and anandamide (AEA) and the TRPV1 agonist capsaicin (CAP) were tested on the P, Npoly, and Nmech synapses. Recent studies have shown that 2-AG activates TRPV1 channels (Qin et al. 2008; Zygmunt et al. 2013). Consistent with previous findings (Yuan and Burrell 2010; Higgins et al. 2013), bath application of 2-AG (100 μM for 15 min) elicited persistent changes in both synapses when recorded 1 h after treatment; potentiation of the P-to-L synapses and depression of the Npoly-to-L synapses [P cell synapses, Fig. 2, A and B; one-way ANOVA, F(7,39) = 11.47; P < 0.001; Student-Newman-Keuls post hoc test of 2-AG vs. DMSO, P < 0.01; Npoly synapses, Fig. 2, C and D, one-way ANOVA, F(7,39) = 11.13; P < 0.001; post hoc 2-AG vs. DMSO, P < 0.01]. In ganglia that were cotreated with 2-AG plus the TRPV1 channel antagonist SB 366791 (SB; 10 μM), no potentiation was observed in the P-cell synapses and no depression was observed in the Npoly synapses, again consistent with previous studies (Higgins et al. 2013; Yuan and Burrell 2013b) (P synapses, Fig. 2B, 2-AG vs. SB + 2-AG, P < 0.05; Npoly synapses, Fig. 2D, 2-AG vs. SB + 2-AG, P < 0.01).
Fig. 2.
Endocannabinoid-mediated synaptic plasticity in P, Npoly, and Nmech synapses. A, C, and E, top: example of changes in excitatory postsynaptic potential (EPSP) amplitude elicited by either the P (A), Npoly, (C) or Nmech neuron (E; scale bar = 20.0 mV/50 ms), following or 2-arachydonoyl glycerol (2-AG) treatment (middle; scales bars = 2.0 mV/50 ms) or 2-AG + SB 366791 (SB) (bottom; scale bars = 2.0 mV/50 ms for P and Npoly and 1.5 mV/50 ms for Nmech). Black and gray traces are pre- and posttest EPSPs (averaged from 5–10 recordings), respectively. B, D, and F: mean ± SE EPSP (shown as %pretest EPSP amplitude) following vehicle, endocannabinoid, or capsaicin (CAP) treatment with or without SB. 2-AG, anandamide (AEA), and CAP elicited potentiation in P synapses (B) but depressed Npoly (D) and Nmech (F) synapses. For all 3 synapse types, SB blocked synaptic changes elicited by 2-AG, AEA, and CAP and no change in EPSP amplitude was observed in the vehicle control (0.01% DMSO) or SB only groups. *Statistically significant differences based on one-way ANOVA with Student-Newman-Keuls post hoc test (see results); n = 5 (different animals) for all treatment and control groups.
The effects of AEA, which is a well-known endocannabinoid that activates TRPV channels (De Petrocellis et al. 2001), have not been tested in Hirudo. AEA (100 μM) produced the same effects as 2-AG; potentiating P synapses and depressing Npoly synapses (P synapses, Fig. 2B; AEA vs. DMSO, P < 0.001; Npoly synapses, Fig. 2D, AEA vs. DMSO, P < 0.005). The TRPV1 antagonist SB 366791 (10 μM) blocked AEA-mediated potentiation of P synapses and depression of Npoly synapses (P synapses, Fig. 2B, SB only vs. SB + AEA, P > 0.05; Npoly synapses, Fig. 2D, SB only vs. SB + AEA, P > 0.05).
As an additional test of TRPV involvement, the effects of the TRPV1 activator CAP (1 μM) were tested. The effects of capsaicin were identical to those of 2-AG and AEA, potentiation of P-cell synapses and depression of Npoly-cell synapses (P synapses, Fig. 2B, DMSO vs. CAP, P < 0.05; Npoly synapses, Fig. 2D, DMSO vs. CAP, P < 0.001). SB 366791 (10 μM) blocked capsaicin-induced modulation of P and Npoly synapses (P synapses, Fig. 2B, SB only vs. SB + CAP, P > 0.05; Npoly synapses, Fig. 2D, SB only vs. SB + CAP, P > 0.05). Together these findings support the idea that endocannabinoid/TRPV-mediated signaling elicits opposing forms of plasticity in these two synapse types in Hirudo.
The effects of endocannabinoids were also examined on synapses made by the previously untested mechanical N cell (Nmech), which, like the P cell, lacks TRPV channels (Summers et al. 2014). Surprisingly, 2-AG, AEA, and capsaicin all elicited persistent depression of the Nmech synapses when measured 1 h after treatment [Fig. 2, E and F; one-way ANOVA, F(7,39) = 19.76; P < 0.001; post hoc 2-AG vs. DMSO control, P < 0.001; AEA vs. DMSO control, P < 0.001; CAP vs. DMSO control, P < 0.001]. 2-AG-, AEA-, and capsaicin-mediated LTD was blocked by SB366791 [Fig. 2F, SB only vs. 2-AG + SB, P > 0.5; SB only vs. AEA + SB, P > 0.05; SB only vs. CAP + SB, P > 0.05], suggesting involvement of the Hirudo TRPV-like channel. How TRPV-activating ligands could affect the Nmech synapse is addressed in subsequent experiments. For all of these synaptic studies, no changes in the postsynaptic input resistance (IR) were observed during the P-to-L, Npoly-to-L and Nmech-to-AP synapses.
Cl− gradients and endocannabinoid modulation.
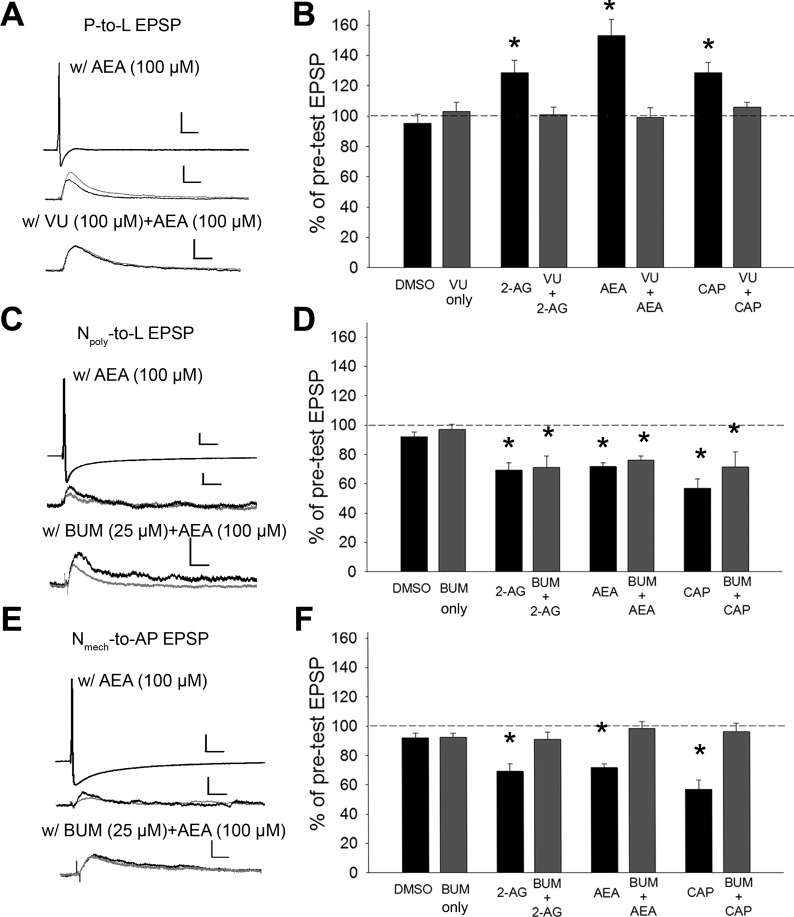
The role of differences in Cl− gradients in regulating the “sign” of endocannabinoid-mediated plasticity was examined. 2-AG-induced potentiation of P synapses requires GABAergic signaling indicating that endocannabinoids depress GABAergic (likely tonic) input and thereby disinhibiting the synapse (Higgins et al. 2013; Wang et al. 2015). Therefore, the effects of the KCC2 inhibitor on endocannabinoid-mediated potentiation/disinhibition were examined. VU 0240551 at 100 μM produces approximately a −20-mV positive shift of ECl in P cells, decreasing the hyperpolarizing effects of GABA and acutely increasing synaptic transmission by this afferent (Wang et al. 2015). Pretreatment with VU 0240551 followed by application of endocannabinoids or capsaicin (15 min) blocked 2-AG, AEA, and capsaicin-induced potentiation while pretreatment with VU 0240551 by itself had no effect [Fig. 3, A and B; one-way ANOVA, F(7,39) = 9.02; P < 0.001; post hoc VU vs. VU + 2-AG, P > 0.05; VU vs. VU + AEA, P > 0.05; VU vs. VU + CAP, P > 0.05]. These results indicate that disrupting Cl− export interfered with endocannabinoid-mediated disinhibition of the P-cell synapses.
Fig. 3.
Manipulating Cl− homeostasis alters endocannabinoid-mediated synaptic plasticity. A, C, and E, top: example of changes in EPSP amplitude elicited by either the P (A), Nmech (C), or Npoly neuron (E; scale bar = 20.0 mV/50 ms), following AEA (middle; scale bar = 2 mV/50 ms for P and 1 mV/50 ms for Npoly and Nmech), AEA + VU 0240551 (VU) (A), or AEA + bumetanide (BUM) (C and E) (bottom; scale bar = 2.0 mV/50 ms for P and Npoly and 1.0 mV/50 ms for Nmech). Black and gray traces are pre- and posttest EPSPs, respectively. B, D, and F: mean ± SE EPSP (%pretest amplitude) following vehicle, endocannabinoid, or CAP treatment with or without VU or BUM. B: in P synapses, 2-AG-, AEA-, and CAP-elicited potentiation was blocked by VU. D: BUM did not affect endocannabinoid- or CAP-mediated depression in Npoly synapses. F: in Nmech synapses, endocannabinoids- and CAP-mediated depression was blocked by BUM. For all three synapse types, no change in EPSP amplitude was observed in the vehicle control (0.01% DMSO) or in the VU- or BUM-only groups. *Statistically significant differences based on one-way ANOVA with Student-Newman-Keuls post hoc test (see results); n = 5 (different animals) for all treatment and control groups.
Next, the effects of disrupting Cl− import on eCB-LTD in the Npoly synapses were examined. As already stated, GABA has an excitatory effect on the Npoly cell and its synapse (Wang et al. 2015). If endocannabinoid is depressing GABAergic input sufficiently to disinhibit P-cell synapses, then it is possible that depression of this excitatory GABAergic input will “disexcite” Npoly synapses. Pretreatment with the NKCC1 inhibitor bumetanide has been shown to decrease the ECl in the Npoly cell and this was accompanied by a decrease in Npoly synaptic transmission, presumably by decreasing tonic depolarization by GABA (Wang et al. 2015). In the present experiments, 25 μM bumetanide were used, which can decrease GABA's depolarizing effect on Npoly. Pretreatment with bumetanide did not prevent 2-AG-, AEA-, or capsaicin-mediated LTD of Npoly synapses [Fig. 3, C and D; one-way ANOVA F7,39 = 4.89; P < 0.001; post hoc BUM vs. BUM + 2-AG, P < 0.05; BUM vs. BUM + AEA, P < 0.05; BUM vs. BUM + CAP, P < 0.05]. These results indicate that even though bumetanide decreases depolarizing GABAergic input, this does not contribute to eCB-LTD of Npoly synapses and that the observed depression is due entirely to the direct activation of the TRPV-like channel in the presynaptic Npoly neuron (Yuan and Burrell 2010, 2012, 2013a,b). However, the depolarized ECl may “protect” these synapses from the disinhibition that would normally be observed in neurons that receive GABAergic input and had low intracellular Cl− levels.
Finally, the effects of bumetanide were tested on endocannabinoid-mediated depression of the Nmech synapses. Recall that Nmech cells also have an elevated/depolarized ECl, like Npoly (Wang et al. 2015) and Nmech synapses undergo depression when treated with 2-AG, AEA, or capsaicin (Fig. 2, E and F) even though they lack TRPV channels (Summers et al. 2014). Bumetanide treatment (25 μM) completely blocked 2-AG-, AEA-, and capsaicin-mediated LTD of Nmech synapses [Fig. 3, E and F; one-way ANOVA, F7,39 = 11.19; P < 0.001; post hoc BUM vs. BUM + 2-AG, P > 0.05; BUM vs. BUM + AEA, P > 0.05; BUM vs. BUM + CAP, P > 0.05]. These results suggest that endocannabinoid-mediated modulation of the Nmech synapses utilizes the same mechanisms observed in P synapses but with the opposite effect. That is, endocannabinoids depress tonic GABAergic input to the Nmech, but since ECl is depolarized relative to resting potential in these afferents, this results in a decrease in excitatory modulation of the Nmech synapses or “disexcitation.” This represents a new and entirely novel mechanism of endocannabinoid modulation of excitatory synapses. Again, no statistically significant changes in postsynaptic input resistance (IR) were observed during the P-to-L, Npoly-to-L, and Nmech-to-AP synaptic recordings.
Patterns of activity that elicit endocannabinoid/TRPV-mediated synaptic modulation.
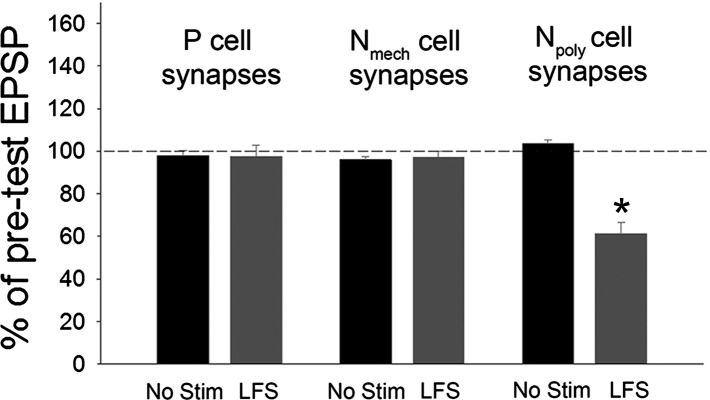
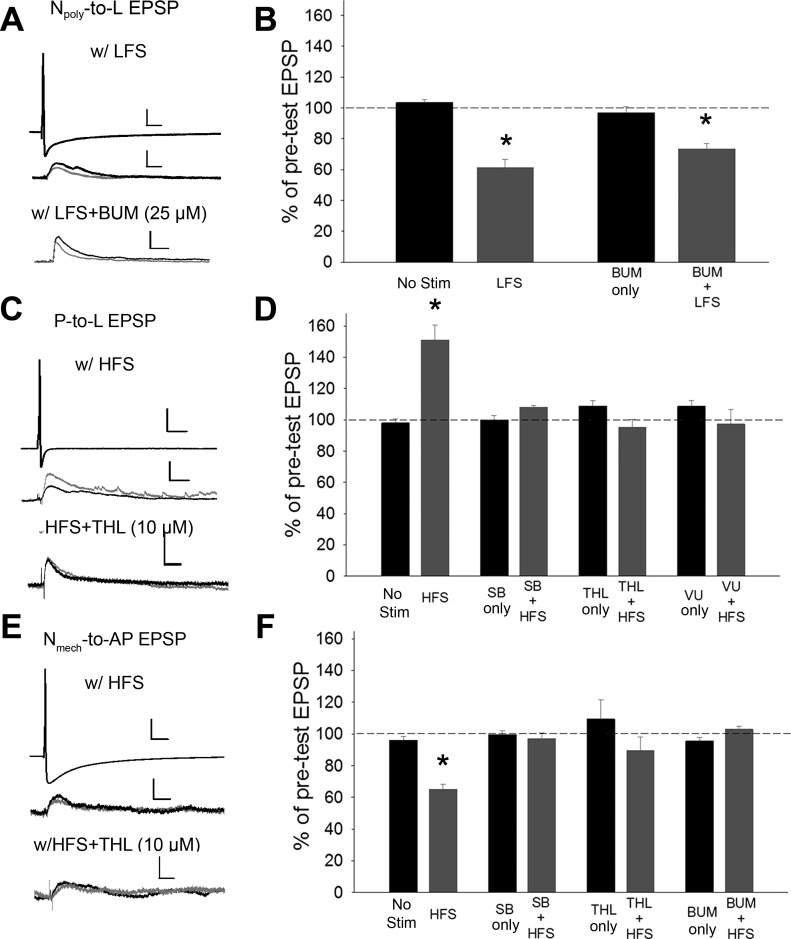
Given that exogenous activation of endocannabinoids elicits three distinct mechanisms of synaptic plasticity, two of which were directly influenced by differences in Cl− gradients, what patterns of circuit activity could elicit these forms of synaptic change? Previously, low-frequency stimulation (LFS; 1 Hz for 900 s) of the touch-sensitive afferents (T cells) was found to elicit endocannabinoid/TRPV-dependent depression of Npoly synapses (Yuan and Burrell 2010, 2013b) and this LTD was blocked by SB 366791 or by an inhibitor of the DAG lipase responsible for 2-AG synthesis, tetrahydrolipstatin (THL) (Lee et al. 1995). These experiments were repeated and, consistent with previous results, T-cell LFS heterosynaptically depressed Npoly synapses. However, T-cell LFS did not affect P or Nmech synapses (Fig. 4, t-test, Npoly synapses, P < 0.01; P and Nmech synapses, P > 0.05). LFS-induced depression of Npoly synapses was still observed following inhibition of Cl− import by bumetanide (25 μM) [Fig. 5, A and B; one-way ANOVA, F(3,19) = 27.74; P < 0.001; post hoc LFS vs. no stim control, P < 0.001; BUM only vs. BUM + LFS, P < 0.001]. However, the level of LFS-induced depression in bumetanide-treated synapses was significantly less than that observed in the LFS-only group (Fig. 4, A and B: LFS vs. BUM + LFS, P < 0.05). This result suggests that in addition to the direct activation of TRPV channels on Npoly neurons, T cell LFS elicits a decrease in an excitatory input that requires a Cl− conductance (presumably a GABAergic input) that contributes to eCB-LTD of the Npoly synapse, albeit to a small degree.
Fig. 4.
Effects of T cell with low-frequency stimulation (LFS) on synaptic transmission by the 3 synapses in this study. While LFS did depressed the Npoly synapses as previously observed (Yuan and Burrell 2010), no change was observed in either the P or Nmech synapses. No effect was observed in saline control (no stim) among 3 synapses. Data are presented as the mean ± SE percentage of the pretest EPSP (*P < 0.01, t-test); n = 5 (different animals) for all treatment and control groups.
Fig. 5.
Endocannabinoid-mediated synaptic modulation elicited by either LFS or high-frequency stimulation (HFS) and the role of Cl− homeostasis. A, C, and E, top: example of changes in EPSP amplitude elicited by either the Npoly (A), P (C). or Nmech neuron (E; scale bars = 20.0 mV/50 ms), following LFS (A) or HFS (C and E; scale bars = 2.0 mV/50 ms for Npoly and P and 1.0 mV/50 ms for Nmech), LFS + BUM (A) or HFS + tetrahydrolipstatin (THL) (C, E;) (bottom; scale bars = 2.0 mV/50 ms). Black and gray traces are pre- and posttest EPSPs, respectively. B, D, and F: mean ± SE EPSP (%pretest amplitude) following LFS or HFS with or without SB, THL, VU, or BUM. B: in Npoly synapses, LFS elicited synaptic depression. This depression was not blocked by BUM. D: in P synapses, HFS elicited synaptic potentiation and this potentiation was blocked by SB, THL, and VU. (F) In Nmech synapses, HFS elicited synaptic depression and this depression was blocked by SB, THL, and BUM. For all 3 synapse types no change in EPSP amplitude was observed in the saline (no stim) or in the SB-, THL-, VU-, or BUM-only groups. *Statistically significant differences based on one-way ANOVA with Student-Newman-Keuls post hoc test (see results); n = 5 (different animals) for all treatment and control groups.
If T-cell LFS does not affect the P or Nmech synapses, then what patterns of activity are effective in inducing endocannabinoid-mediated modulation of these pathways? Given that noxious stimuli produce sensitization of defensive withdrawal reflexes elicited by P-cell activity (Lockery and Kristan 1991; Ehrlich et al. 1992; Modney et al. 1997; Burrell et al. 2001; Burrell and Sahley 2005), the possibility that high-frequency stimulation (HFS) of the Npoly cell could elicit potentiation of P synapses was examined. HFS (20 trains at 0.1 Hz with 10 pulses at 25 Hz per train) of Npoly significantly potentiated P synapses when retested 1 h following later [Fig. 5, C and D; one-way ANOVA, F(7,39) = 12.87; P < 0.001; post hoc HFS vs. no stim control, P < 0.001]. Furthermore, this potentiation was blocked by either SB 366791 (10 μM; Fig. 5D, SB only vs. SB + HFS, P > 0.05) or THL (10 μM; Fig. 5D, THL only vs. THL + HFS, P > 0.05), indicating that increased signaling was 2-AG and TRPV dependent. When the Cl− export inhibitor VU 0240551 (100 μM) was applied during HFS stimulation, HFS-induced potentiation was blocked (Fig. 5D, VU only vs. VU + HFS, P > 0.05), indicating that normal Cl− export was required for this activity-dependent form of endocannabinoid-mediated plasticity, identical to what was observed during exogenous application of endocannabinoids.
HFS of the Npoly neuron depressed the Nmech synapses [Fig. 5, E and F; one-way ANOVA, F(7,39) = 5.29; post hoc HFS vs. no stim control, P < 0.001]. This LTD was also blocked by SB 366791 (Fig. 5F, SB only vs. SB + HFS, P > 0.5) or THL (THL only vs. THL + HFS, P > 0.05), indicating a requirement for 2-AG synthesis and TRPV activation. HFS-elicited depression of Nmech synapses was blocked by bumetanide treatment, implying that normal Cl− import was also required for this activity-dependent form of endocannabinoid-mediated plasticity (Fig. 5F, BUM only vs. BUM + HFS, P > 0.1). Again, identical to the results observed during exogenous application of endocannabinoids. No statistically significant changes in postsynaptic IR were observed during the P-to-L, Npoly-to-L, or Nmech-AP synapse recordings.
DISCUSSION
This study provides the first experimental confirmation of the hypothesis that differences in Cl− gradients can control whether or not synaptic circuits are sensitive to endocannabinoid-mediated disinhibition (Christie and Mallet 2009). In addition, the role of Cl− gradient control as a contributor to synaptic plasticity has been extended by the discovery of a completely novel form of neuromodulation observed in the Nmech synapses in which endocannabinoid-mediated depression is blocked by inhibition of Cl− import. These findings may explain how endocannabinoids in the spinal cord can have both pro- and antinociceptive effects (Morisset and Urban 2001; Liang et al. 2004; Hohmann et al. 2005; Christie and Mallet 2009; Pernia-Andrade et al. 2009; Kato et al. 2012).
The pattern of observed endocannabinoid-mediated synaptic modulation was not simply a product of exogenous drug application but could also be produced following distinct patterns of afferent activity. In P synapses, HFS of Npoly elicited potentiation that was blocked by inhibition of 2-AG synthesis or TRPV activation. These effects were observed even though P cells lack TRPV channels (Pastor et al. 1996; Summers et al. 2014). It is proposed that endocannabinoids depress tonic GABAergic input to the P cell presynaptic terminals, disinhibiting those synapses so that spike-evoked neurotransmitter release is now enhanced (Fig. 6A). P synapses are thought to receive tonic inhibitory GABAergic input (Wang et al. 2015) and endocannabinoid-mediated potentiation of P synapses requires functioning GABAA receptors (Higgins et al. 2013). In this study, inhibition of Cl− export by the KCC2 inhibitor VU 0240551 also blocked endocannabinoid-mediated potentiation by disrupting the Cl− gradient, confirming that the role of GABA is based on its activation of ligand-gated Cl− channels and not metabotropic GABA receptors.
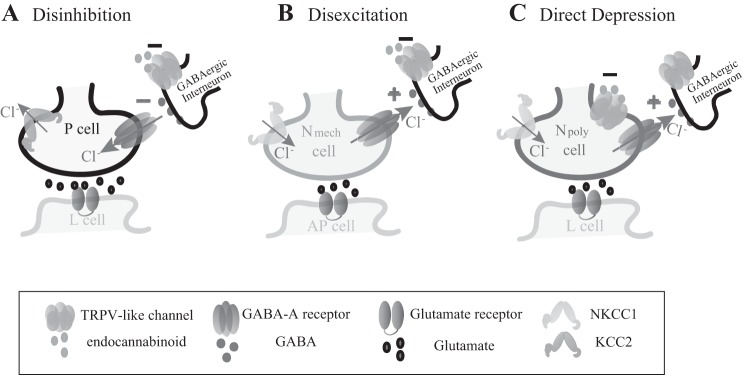
Fig. 6.
Diagram illustrating the three mechanisms of which endocannabinoids modulated synaptic transmission. All sensory neurons receive tonic GABAergic input from an unknown interneuron that has an inhibitory effect on P synapses and an excitatory on Npoly and Nmech synapses based on (Wang et al. 2015). The Npoly cell is of transient potential receptor vanilloid (TRPV) positive, while the P and Nmech neurons are TRPV negative (Pastor et al. 1996; Summers et al. 2014). A: endocannabinoids depress inhibitory GABAergic input to the P cell, increasing P glutamatergic transmission via a disinhibition mechanism that is dependent on activity of a KCC2-like Cl− exporter. B: endocannabinoids depress exhibitory GABAergic input to the Nmech cell, decreasing Nmech glutamatergic transmission via a disexcitation mechanism that is dependent on activity of a NKCC1-like Cl− importer. C: endocannabinoids depress the Npoly synapses directly via activation of their TRPV channels (Yuan and Burrell, 2010, 2012, 2013a,b). Although endocannabinoids presumably depress excitatory GABAergic input to the Npoly neurons, this did not appear to affect Npoly synapses.
Endocannabinoid-mediated modulation of Nmech synapses utilizes a mechanism that is identical to the P synapses except that the sign of the synaptic change is reversed due to Nmech having elevated levels of intracellular Cl−. In Nmech neurons, which also lack TRPV receptors, LTD can be induced by exogenous application of endocannabinoids (or capsaicin) and by HFS of the Npoly neuron that is 2-AG and TRPV dependent. Inhibition of Cl− import by the NKCC1 blocker bumetanide prevented endocannabinoid/TRPV-mediated depression elicited by exogenous drug application or HFS. From these results it is inferred that endocannabinoids depress a tonic excitatory/depolarizing input that requires Cl− efflux in the Nmech cell (Wang et al. 2015) and that a decrease in this excitatory input leads to depression of the Nmech synapse (Fig. 6B). It is thought that this excitatory, Cl−-based current represents a GABAergic input, but this has not been directly tested yet. Although the identities of at least two GABAergic neurons are known (Cline 1983), it is not known whether these cells are the source of the tonic GABAergic input. Future experiments will probe the GABA signaling component of this modulatory process.
2-AG- and capsaicin-elicited LTD of Npoly cell synapses has already been shown (Yuan and Burrell 2010, 2012, 2013a), but the capacity of AEA to also produce depression is a new discovery and is relevant given the presence of both 2-AG and AEA in the Hirudo CNS (Matias et al. 2001). That T-cell LFS can elicit LTD in the Npoly synapse that is 2-AG and TRPV dependent has also been previously reported (Yuan and Burrell 2010, 2013b). Npoly cells possess a TRPV-like receptor (Summers et al. 2014) so the effects of endocannabinoids modulation are due to the direct activation of this TRPV channel (Fig. 6C). As in Nmech neurons, Npoly cells are excited by GABA due to the high intracellular Cl− and bumetanide treatment alone can reduce this response to GABA and acutely disexcite Npoly synapses (Wang et al. 2015). Although manipulation of the Cl− gradient by bumetanide did not alter depression following exogenous application of 2-AG, AEA, or capsaicin, bumetanide treatment did cause a small but significant decrease in eCB-LTD elicited by T-cell LFS. Therefore, eCB-LTD in Npoly synapses is primarily due to direct activation of TRPV channels on the presynaptic, Npoly cell, with the indirect, disexcitation mechanism making a relatively small contribution. However, the depolarized ECl may have an additional role in that it may “protect” the Npoly and from endocannabinoid-mediated disinhibition that would occur in neurons that receive GABAergic input and possess the low intracellular Cl− levels that are more commonly observed in adult cells.
It is interesting that different forms of endocannabinoid-mediated modulation were elicited by distinct patterns of afferent activity. T-cell LFS elicited endocannabinoid-mediated plasticity only in Npoly synapses, while Npoly HFS produced endocannabinoid-mediated changes in the P and Nmech synapses. HFS does elicit homosynaptic LTP in Npoly, but this is mediated by NMDA receptors and not endocannabinoid/TRPV signaling (unpublished data). This suggests that the CNS has distinct endocannabinoid synthesizing sites that are selectively activated by distinct patterns of afferent stimulation. Previous studies in which the DAG lipase inhibitor THL was injected into either the presynaptic Npoly or postsynaptic L motor neuron have shown that the latter is the site of 2-AG synthesis activated by LFS (Yuan and Burrell 2013a,b), although this has not been confirmed with nonpharmaceutical approaches. The site of endocannabinoid synthesis activated by HFS is not known. These findings are similar to endocannabinoid modulation of striatal synapses that also exhibit long-lasting depression or disinhibition depending on the pattern of afferent activity (Adermark and Lovinger 2009).
These results suggest the presence of distinct endocannabinoid “modules”; combinations of excitatory and inhibitory circuits along with endocannabinoid-releasing neurons, which are accessed by different patterns of activity and have distinct functional roles. For example, eCB-LTD in Npoly synapses is elicited by T-cell LFS and this translates into a depression of defensive withdrawal behaviors elicited by Npoly (Yuan and Burrell 2013b). This strongly resembles gate control of pain in which stimulation of nonnociceptive (Aβ) afferents can diminish nociceptive signaling (Melzack and Wall 1965). Repetitive Aβ stimulation in mammals is known to induce LTD in nociceptive synapses (Wallin et al. 2003; Sdrulla et al. 2015) that is similar to the depression of the Hirudo Npoly synapses. The endocannabinoid-mediated depression observed here may represent a previously unrecognized form of neuroplasticity contributing to gate control.
HFS of Npoly, on the other hand, elicited endocannabinoid-mediated potentiation/disinhibition of P synapses and is remarkably similar to TRPV-mediated disinhibition that contributes to sensitization of nonnociceptive signaling (allodynia) in mammals (Kim et al. 2012). This endocannabinoid-mediated potentiation may represent a heretofore unrecognized form of heterosynaptic facilitation that contributes to central sensitization of primary somatosensory synapses and provides a potential activity-dependent mechanism by which disinhibition allows nonnociceptive afferents to become recruited into the nociceptive circuitry (Torsney and MacDermott 2006; Lu et al. 2013).
HFS also resulted in endocannabinoid-mediated depression of Nmech synapses, which would seem to oppose central sensitization. What this would imply is that a noxious stimulus that produces high-frequency activity in a nociceptive afferent (such as Npoly) can elicit depression in other nociceptive pathways (Nmech in this case). This has been observed before and referred to as diffuse nociceptive inhibitory control (DNIC) or conditioned inhibition of pain (Ernst et al. 1986; Yarnitsky 2010). The physiological mechanisms that mediate DNIC are poorly understood although descending modulatory input has been implicated. Based on the findings in the present study, it is proposed that endocannabinoid-mediated disexcitation of nociceptive synapses may represent an additional form of neuromodulation contributing to DNIC. Obviously, further studies are required to support or refute this hypothesis.
Although studied in the context of somatosensory afferents, the cellular features of endocannabinoid-mediated synaptic modulation observed here, the role of differences in Cl− gradients and the patterns of afferent activity that initiate specific endocannabinoid modules have broad applicability to circuits throughout the CNS. Endocannabinoid-mediated modulation via TRPV channels is observed in a wide range of neural circuits including those contributing to cognition, stress, addiction, fight-or-flight behaviors, and, of course, nociception (see review by Edwards 2014), although these findings would also be relevant for neuromodulation mediated by CB1 or CB2 receptors. There are also an increasing number of examples in which variability of Cl− gradients between adult neurons is observed in both vertebrates and invertebrates (Duchen 1986; Chavas and Marty 2003; Ruiz et al. 2003; Norekian and Malyshev 2005; Cheung et al. 2006; Szabadics et al. 2006; Gilbert et al. 2007; Pfeiffer et al. 2009; Pugh and Jahr 2011; Haam et al. 2012). These include examples in which neurons with different Cl− gradients are found within the same or overlapping neural circuits (Gilbert et al. 2007; Haam et al. 2012) and neurons in which different cellular compartments in neurons exhibit different Cl− gradients (Khirug et al. 2008).
The findings in this study represent a new and important mechanism for both the regulation of neuromodulatory processes and the organization of these processes at the microcircuit level. The role of differences in Cl− gradient observed here is unlikely to be limited to only endocannabinoid-based modulation but also impacts other modulatory transmitters known to depress both glutamatergic and GABAergic/glycinergic synapses, e.g., opioids (Lau and Vaughan 2014), glutamate (Zak et al. 2015), or adenosine (Morairty et al. 2004). Consequently, the integration of Cl− gradients into adult neuromodulatory processes represents a new avenue to study neural and behavioral plasticity throughout the CNS.
GRANTS
This work was funded by National Science Foundation IOS-1051734 (to B. D. Burrell) and the University of South Dakota Division of Basic Biomedical Sciences.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Y.W. performed experiments; Y.W. analyzed data; Y.W. and B.D.B. interpreted results of experiments; Y.W. prepared figures; Y.W. drafted manuscript; B.D.B. conception and design of research; B.D.B. edited and revised manuscript; B.D.B. approved final version of manuscript.
REFERENCES
- Adermark L, Lovinger DM. Frequency-dependent inversion of net striatal output by endocannabinoid-dependent plasticity at different synaptic inputs. J Neurosci 29: 1375–1380, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baylor DA, Nicholls JG. Chemical and electrical synaptic connexions between cutaneous mechanoreceptor neurones in the central nervous system of the leech. J Physiol 203: 591–609, 1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackshaw SE, Nicholls JG, Parnas I. Physiological responses, receptive fields and terminal arborizations of nociceptive cells in the leech. J Physiol 326: 251–260, 1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown TE, Chirila AM, Schrank BR, Kauer JA. Loss of interneuron LTD and attenuated pyramidal cell LTP in Trpv1 and Trpv3 KO mice. Hippocampus 23: 662–671, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgin AM, Szczupak L. Network interactions among sensory neurons in the leech. J Comp Physiol A Neuroethol Sens Neural Behav Physiol 189: 59–67, 2003. [DOI] [PubMed] [Google Scholar]
- Burrell BD, Sahley CL. Serotonin mediates learning-induced potentiation of excitability. J Neurophysiol 94: 4002–4010, 2005. [DOI] [PubMed] [Google Scholar]
- Burrell BD, Sahley CL, Muller KJ. Non-associative learning and serotonin induce similar bi-directional changes in excitability of a neuron critical for learning in the medicinal leech. J Neurosci 21: 1401–1412, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chavas J, Marty A. Coexistence of excitatory and inhibitory GABA synapses in the cerebellar interneuron network. J Neurosci 23: 2019–2031, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chavez AE, Chiu CQ, Castillo PE. TRPV1 activation by endogenous anandamide triggers postsynaptic long-term depression in dentate gyrus. Nat Neurosci 13: 1511–1518, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung U, Moghaddasi M, Hall HL, Smith JJ, Buck LT, Woodin MA. Excitatory actions of GABA mediate severe-hypoxia-induced depression of neuronal activity in the pond snail (Lymnaea stagnalis). J Exp Biol 209: 4429–4435, 2006. [DOI] [PubMed] [Google Scholar]
- Christie MJ, Mallet C. Endocannabinoids can open the pain gate. Sci Signal 2: pe57, 2009. [DOI] [PubMed] [Google Scholar]
- Cline HT. 3H-GABA uptake selectively labels identifiable neurons in the leech central nervous system. J Comp Neurol 215: 351–358, 1983. [DOI] [PubMed] [Google Scholar]
- De Petrocellis L, Harrison S, Bisogno T, Tognetto M, Brandi I, Smith GD, Creminon C, Davis JB, Geppetti P, Di Marzo V. The vanilloid receptor (VR1)-mediated effects of anandamide are potently enhanced by the cAMP-dependent protein kinase. J Neurochem 77: 1660–1663, 2001. [DOI] [PubMed] [Google Scholar]
- Duchen MR. Excitation of mouse motoneurones by GABA-mediated primary afferent depolarization. Brain Res 379: 182–187, 1986. [DOI] [PubMed] [Google Scholar]
- Edwards JG. TRPV1 in the central nervous system: synaptic plasticity, function and pharmacological implications. In; Capsaicin as a Therapeutic Molecule, edited by Abdel-Salam OM. Basel, Switzerland: 2014, p. 77–104. [DOI] [PubMed] [Google Scholar]
- Ehrlich JS, Boulis NM, Karrer T, Sahley CL. Differential effects of serotonin depletion on sensitization and dishabituation in the leech, Hirudo medicinalis. J Neurobiol 23: 270–279, 1992. [DOI] [PubMed] [Google Scholar]
- Elphick MR. The evolution and comparative neurobiology of endocannabinoid signalling. Philos Trans R Soc Lond B Biol Sci 367: 3201–3215, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ernst M, Lee MH, Dworkin B, Zaretsky HH. Pain perception decrement produced through repeated stimulation. Pain 26: 221–231, 1986. [DOI] [PubMed] [Google Scholar]
- Freund TF, Katona I, Piomelli D. Role of endogenous cannabinoids in synaptic signaling. Physiol Rev 83: 1017–1066, 2003. [DOI] [PubMed] [Google Scholar]
- Gaudry Q Jr, Kristan WB. Behavioral choice by presynaptic inhibition of tactile sensory terminals. Nat Neurosci 12: 1450–1457, 2009. [DOI] [PubMed] [Google Scholar]
- Gibson HE, Edwards JG, Page RS, Van Hook MJ, Kauer JA. TRPV1 channels mediate long-term depression at synapses on hippocampal interneurons. Neuron 57: 746–759, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert D, Franjic-Wurtz C, Funk K, Gensch T, Frings S, Mohrlen F. Differential maturation of chloride homeostasis in primary afferent neurons of the somatosensory system. Int J Dev Neurosci 25: 479–489, 2007. [DOI] [PubMed] [Google Scholar]
- Haam J, Popescu IR, Morton LA, Halmos KC, Teruyama R, Ueta Y, Tasker JG. GABA is excitatory in adult vasopressinergic neuroendocrine cells. J Neurosci 32: 572–582, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins A, Yuan S, Wang Y, Burrell B. Differential modulation of nociceptive vs. non-nociceptive synapses by endocannabinoids. Mol Pain 9: 26, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hohmann AG, Suplita RL, Bolton NM, Neely MH, Fegley D, Mangieri R, Krey JF, Walker JM, Holmes PV, Crystal JD, Duranti A, Tontini A, Mor M, Tarzia G, Piomelli D. An endocannabinoid mechanism for stress-induced analgesia. Nature 435: 1108–1112, 2005. [DOI] [PubMed] [Google Scholar]
- Ivanov AL, Calabrese RL. Spike-mediated and graded inhibitory synaptic transmission between leech interneurons: evidence for shared release sites. J Neurophysiol 96: 235–251, 2006. [DOI] [PubMed] [Google Scholar]
- Jensen T, Edwards JG. Calcineurin is required for TRPV1-induced long-term depression of hippocampal interneurons. Neurosci Lett 510: 82–87, 2012. [DOI] [PubMed] [Google Scholar]
- Kaila K, Price TJ, Payne JA, Puskarjov M, Voipio J. Cation-chloride cotransporters in neuronal development, plasticity and disease. Nat Rev Neurosci 15: 637–654, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato A, Punnakkal P, Pernia-Andrade AJ, von Schoultz C, Sharopov S, Nyilas R, Katona I, Zeilhofer HU. Endocannabinoid-dependent plasticity at spinal nociceptor synapses. J Physiol 590: 4717–4733, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khirug S, Yamada J, Afzalov R, Voipio J, Khiroug L, Kaila K. GABAergic depolarization of the axon initial segment in cortical principal neurons is caused by the Na-K-2Cl cotransporter NKCC1. J Neurosci 28: 4635–4639, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim YH, Back SK, Davies AJ, Jeong H, Jo HJ, Chung G, Na HS, Bae YC, Kim SJ, Kim JS, Jung SJ, Oh SB. TRPV1 in GABAergic interneurons mediates neuropathic mechanical allodynia and disinhibition of the nociceptive circuitry in the spinal cord. Neuron 74: 640–647, 2012. [DOI] [PubMed] [Google Scholar]
- Lau BK, Vaughan CW. Descending modulation of pain: the GABA disinhibition hypothesis of analgesia. Curr Opin Neurobiol 29: 159–164, 2014. [DOI] [PubMed] [Google Scholar]
- Lee MW, Kraemer FB, Severson DL. Characterization of a partially purified diacylglycerol lipase from bovine aorta. Biochim Biophys Acta 1254: 311–318, 1995. [DOI] [PubMed] [Google Scholar]
- Liang YC, Huang CC, Hsu KS, Takahashi T. Cannabinoid-induced presynaptic inhibition at the primary afferent trigeminal synapse of juvenile rat brainstem slices. J Physiol 555: 85–96, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lockery Sr Jr, Kristan WB. Two forms of sensitization of the local bending reflex of the medicinal leech. J Comp Physiol A 168: 165–177, 1991. [DOI] [PubMed] [Google Scholar]
- Lu Y, Dong H, Gao Y, Gong Y, Ren Y, Gu N, Zhou S, Xia N, Sun YY, Ji R, Xiong L. A feed-forward spinal cord glycinergic neural circuit gates mechanical allodynia. J Clin Invest 123: 4050–4062, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maione S, Cristino L, Migliozzi AL, Georgiou AL, Starowicz K, Salt TE, Di Marzo V. TRPV1 channels control synaptic plasticity in the developing superior colliculus. J Physiol 587: 2521–2535, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matias I, Bisogno T, Melck D, Vandenbulcke F, Verger-Bocquet M, De Petrocellis L, Sergheraert C, Breton C, Di Marzo V, Salzet M. Evidence for an endocannabinoid system in the central nervous system of the leech Hirudo medicinalis. Brain Res Mol Brain Res 87: 145–159, 2001. [DOI] [PubMed] [Google Scholar]
- Melzack R, Wall PD. Pain mechanisms: a new theory. Science 150: 971–979, 1965. [DOI] [PubMed] [Google Scholar]
- Modney BK, Sahley CL, Muller KJ. Regeneration of a central synapse restores nonassociative learning. J Neurosci 17: 6478–6482, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morairty S, Rainnie D, McCarley R, Greene R. Disinhibition of ventrolateral preoptic area sleep-active neurons by adenosine: a new mechanism for sleep promotion. Neuroscience 123: 451–457, 2004. [DOI] [PubMed] [Google Scholar]
- Morisset V, Urban L. Cannabinoid-induced presynaptic inhibition of glutamatergic EPSCs in substantia gelatinosa neurons of the rat spinal cord. J Neurophysiol 86: 40–48, 2001. [DOI] [PubMed] [Google Scholar]
- Muller KJ, Nicholls JG, Stent GS. Neurobiology of the Leech. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press, 1981. [Google Scholar]
- Nicholls JG, Baylor DA. Specific modalities and receptive fields of sensory neurons in CNS of the leech. J Neurophysiol 31: 740–756, 1968. [DOI] [PubMed] [Google Scholar]
- Norekian TP, Malyshev AY. Coordinated excitatory effect of GABAergic interneurons on three feeding motor programs in the mollusk Clione limacina. J Neurophysiol 93: 305–315, 2005. [DOI] [PubMed] [Google Scholar]
- Pastor J, Soria B, Belmonte C. Properties of the nociceptive neurons of the leech segmental ganglion. J Neurophysiol 75: 2268–2279, 1996. [DOI] [PubMed] [Google Scholar]
- Pernia-Andrade AJ, Kato A, Witschi R, Nyilas R, Katona I, Freund TF, Watanabe M, Filitz J, Koppert W, Schuttler J, Ji G, Neugebauer V, Marsicano G, Lutz B, Vanegas H, Zeilhofer HU. Spinal endocannabinoids and CB1 receptors mediate C-fiber-induced heterosynaptic pain sensitization. Science 325: 760–764, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfeiffer K, Panek I, Hoger U, French AS, Torkkeli PH. Random stimulation of spider mechanosensory neurons reveals long-lasting excitation by GABA and muscimol. J Neurophysiol 101: 54–66, 2009. [DOI] [PubMed] [Google Scholar]
- Puente N, Cui Y, Lassalle O, Lafourcade M, Georges F, Venance L, Grandes P, Manzoni OJ. Polymodal activation of the endocannabinoid system in the extended amygdala. Nat Neurosci 14: 1542–1547, 2011. [DOI] [PubMed] [Google Scholar]
- Pugh JR, Jahr CE. Axonal GABAA receptors increase cerebellar granule cell excitability and synaptic activity. J Neurosci 31: 565–574, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin N, Neeper MP, Liu Y, Hutchinson TL, Lubin ML, Flores CM. TRPV2 is activated by cannabidiol and mediates cgrp release in cultured rat dorsal root ganglion neurons. J Neurosci 28: 6231–6238, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruiz A, Fabian-Fine R, Scott R, Walker MC, Rusakov DA, Kullmann DM. GABAA receptors at hippocampal mossy fibers. Neuron 39: 961–973, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sargent PB, Yau KW, Nicholls JG. Extrasynaptic receptors on cell bodies of neurons in central nervous system of the leech. J Neurophysiol 40: 446–452, 1977. [DOI] [PubMed] [Google Scholar]
- Sdrulla AD, Xu Q, He SQ, Tiwari V, Yang F, Zhang C, Shu B, Shechter R, Raja SN, Wang Y, Dong X, Guan Y. Electrical stimulation of low-threshold afferent fibers induces a prolonged synaptic depression in lamina II dorsal horn neurons to high-threshold afferent inputs in mice. Pain 156: 1008–1017, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw BK Jr, Kristan WB. The whole-body shortening reflex of the medicinal leech: motor pattern, sensory basis, and interneuronal pathways. J Comp Physiol A 177: 667–681, 1995. [DOI] [PubMed] [Google Scholar]
- Stuart AE. Physiological and morphological properties of motoneurones in the central nervous system of the leech. J Physiol 209: 627–646, 1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Summers T, Holec S, Burrell BD. Physiological and behavioral evidence of a capsaicin-sensitive TRPV-like channel in the medicinal leech. J Exp Biol 217: 4167–4173, 2014. [DOI] [PubMed] [Google Scholar]
- Szabadics J, Varga C, Molnar G, Olah S, Barzo P, Tamas G. Excitatory effect of GABAergic axo-axonic cells in cortical microcircuits. Science 311: 233–235, 2006. [DOI] [PubMed] [Google Scholar]
- Torsney C, MacDermott AB. Disinhibition opens the gate to pathological pain signaling in superficial neurokinin 1 receptor-expressing neurons in rat spinal cord. J Neurosci 26: 1833–1843, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallin J, Fiska A, Tjolsen A, Linderoth B, Hole K. Spinal cord stimulation inhibits long-term potentiation of spinal wide dynamic range neurons. Brain Res 973: 39–43, 2003. [DOI] [PubMed] [Google Scholar]
- Wang Y, Summers T, Peterson W, Miiller E, Burrell BD. Differential effects of GABA in modulating nociceptive vs. non-nociceptive synapses. Neuroscience 298: 397–409, 2015. [DOI] [PubMed] [Google Scholar]
- Wessel R, Kristan WB Jr, Kleinfeld D. Supralinear summation of synaptic inputs by an invertebrate neuron: dendritic gain is mediated by an “inward rectifier” K(+) current. J Neurosci 19: 5875–5888, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yarnitsky D. Conditioned pain modulation (the diffuse noxious inhibitory control-like effect): its relevance for acute and chronic pain states. Curr Opin Anaesthesiol 23: 611–615, 2010. [DOI] [PubMed] [Google Scholar]
- Yuan S, Burrell BD. Endocannabinoid-dependent LTD in a nociceptive synapse requires activation of a presynaptic TRPV-like receptor. J Neurophysiol 104: 2766–2777, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan S, Burrell BD. Long-term depression of nociceptive synapses by non-nociceptive afferent activity: role of endocannabinoids, Ca(2)+, and calcineurin. Brain Res 1460: 1–11, 2012. [DOI] [PubMed] [Google Scholar]
- Yuan S, Burrell BD. Endocannabinoid-dependent long-term depression in a nociceptive synapse requires coordinated presynaptic and postsynaptic transcription and translation. J Neurosci 33: 4349–4358, 2013a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan S, Burrell BD. Nonnociceptive afferent activity depresses nocifensive behavior and nociceptive synapses via an endocannabinoid-dependent mechanism. J Neurophysiol 110, 2607–2616, 2013b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zak JD, Whitesell JD, Schoppa NE. Metabotropic glutamate receptors promote disinhibition of olfactory bulb glomeruli that scales with input strength. J Neurophysiol 113: 1907–1920, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zygmunt PM, Ermund A, Movahed P, Andersson DA, Simonsen C, Jonsson BA, Blomgren A, Birnir B, Bevan S, Eschalier A, Mallet C, Gomis A, Hogestatt ED. Monoacylglycerols activate TRPV1–a link between phospholipase C and TRPV1. PLoS One 8: e81618, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]