Abstract
A complete description of the swimming behavior of a bacterium requires measurement of the displacement and orientation of the cell body together with a description of the movement of the flagella. We rebuilt a tracking microscope so that we could visualize flagellar filaments of tracked cells by fluorescence. We studied Escherichia coli (cells of various lengths, including swarm cells), Bacillus subtilis (wild-type and a mutant with fewer flagella), and a motile Streptococcus (now Enterococcus). The run-and-tumble statistics were nearly the same regardless of cell shape, length, and flagellation; however, swarm cells rarely tumbled, and cells of Enterococcus tended to swim in loops when moving slowly. There were events in which filaments underwent polymorphic transformations but remained in bundles, leading to small deflections in direction of travel. Tumble speeds were ∼2/3 as large as run speeds, and the rates of change of swimming direction while running or tumbling were smaller when cells swam more rapidly. If a smaller fraction of filaments were involved in tumbles, the tumble intervals were shorter and the angles between runs were smaller.
Introduction
Early work with a tracking microscope (1) revealed the strategy used by peritrichously flagellated bacteria, such as Escherichia coli, to navigate the spatial gradients of chemical attractants. Cells alternately run (move steadily forward) or tumble (move erratically with little net displacement), changing directions. If a run carries a cell up the gradient, the next tumble is postponed. If a run carries a cell down the gradient, the next tumble occurs as it would in the absence of a stimulus. Thus, cells move up gradients in a random walk with a positive bias.
Flagella were visualized in dark-field microscopy by Macnab and Ornston (2), who found that flagellar filaments switched from normal (left-handed) to curly (right-handed) when cells tumbled. This work was hampered by the large amount of light scattered by cell bodies, which prevented the visualization of short polymorphic forms. This problem was eliminated when flagellar filaments were visualized in fluorescence (3). Cells run when pushed from behind by bundles of normal filaments spinning counterclockwise (CCW). Tumbles occur when the sense of rotation switches from CCW to clockwise (CW), but not all of the flagellar motors on a cell need to change direction at once: the reversal of one is sufficient to cause a cell to change course (3, 4). The filament changes from normal to semicoiled (right-handed with half the pitch) and comes out of the bundle. This maneuver reorients the cell body. Then the semicoiled filament relaxes to the curly form (right-handed with half the pitch and half the amplitude), aligning with the bundle and pushing the cell in the same direction as the filaments that have remained in the bundle. When the aberrant motor (or motors) switches back to CCW, the filaments relax to normal and rejoin the bundle. Cells of E. coli in swarms are relatively long and prefer to back up rather than tumble, by swimming back through the middle of the flagellar bundle (5).
Most modern methods of tracking are based on video imaging. These methods have been extended from two to three dimensions by out-of-focus image analysis (e.g., of fluorescent (6), dark-field (7), or phase-contrast (8) images) or by piezo-driven displacement of the microscope objective combined with a two-dimensional motorized stage (9). These schemes are an improvement on the tracking method used here, because more rapidly moving objects can be followed. Other strategies are to hold the bacterium in an optical trap in the presence of transverse flow (10) or to employ two optical traps, one near the front of the cell and the other near the back (11). Both of these schemes allow one to visualize fluorescently labeled flagella. The first scheme was employed in the discovery of the reverse, forward, and flick navigational strategy of Vibrio alginolyticus (12), and the second was used in a systematic analysis of E. coli tumbles (13). Finally, a growing body of work is employing holographic video microscopy (e.g., (14)).
We rebuilt a tracking microscope on an inverted platform that allowed for laser fluorescence excitation, working first with a dark-phase objective and later with a bright-phase objective of higher numerical aperture. We compared cells of E. coli grown under different conditions, including cells lengthened by treatment with cephalexin. We also tracked cells of two other peritrichously flagellated species, Bacillus subtilis and Enterococcus. However, we were not able to label the filaments of Enterococcus.
Materials and Methods
The tracker
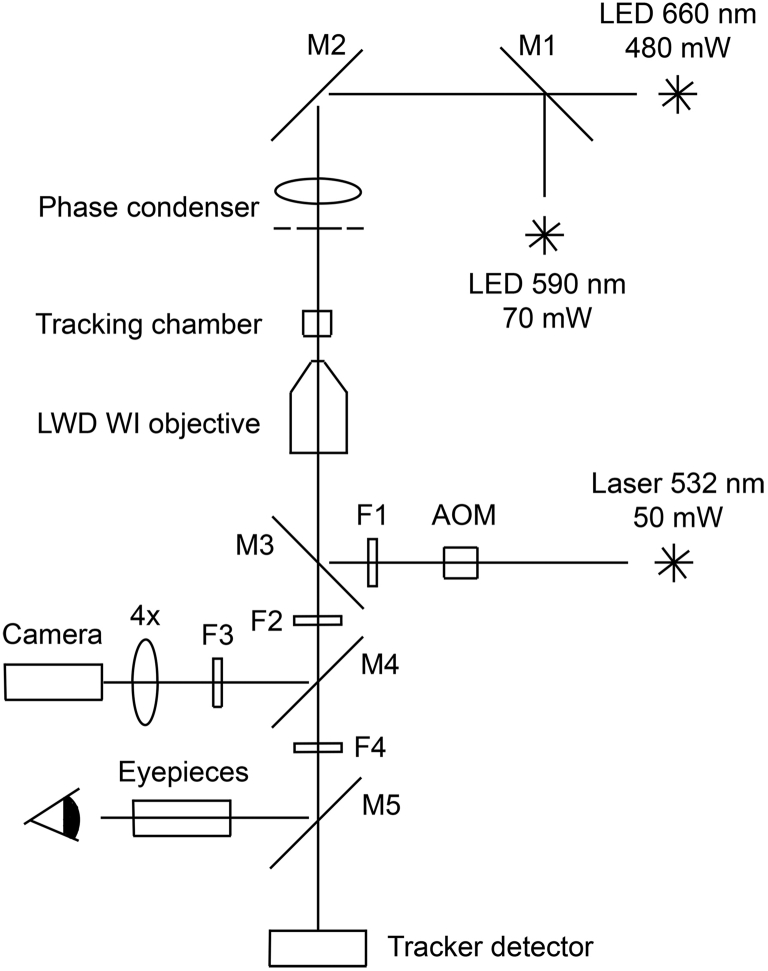
We rebuilt a tracking microscope (15, 16) by equipping a Diaphot 200 inverted microscope and later an Eclipse Ti-U inverted microscope (both Nikon, Melville, NY) with dark-phase and bright-phase optics, respectively; a fluorescence cube; and three light sources (one for tracking, one for visualizing cell bodies, and one (pulsed in synchrony with a CCD camera) for exciting fluorescently labeled flagellar filaments; see Fig. 1). A dichroic mirror (M4) relayed phase-contrast and fluorescence images to the CCD camera (KPC-650BH; KT&C, Fairfield, NJ) and a digital video recorder (GV-HD700/1; Sony, New York, NY), which recorded images at 30 fps. The tracking detector, equipped with three pairs of optical fibers (±X, ±Y, and ±Z), provided error signals to which a mechanical transducer responded by moving the tracking chamber (tracker box) in three dimensions. The box moved in such a way that the tracked cell remained fixed in the laboratory reference frame, so that the displacement of the box revealed the cell’s trajectory. A joystick (J50-NK30-FMB; ETI Systems, Carlsbad, CA) allowed the operator to change the position of the box to catch cells in the tracking spot. The tracker box, a hollow titanium cylinder (2.5 mm i.d. and 1.6 mm inside height) with upper and lower 5-mm-diameter glass windows (#72296-05; Electron Microscopy Sciences, Fort Washington, PA) was assembled, filled with ∼15 μL of cell suspension, and maintained during an experiment at the cells’ growth temperature. A damping solution of polydimethylsiloxane (500 cSt, n = 1.040; Clearco Products, Bensalem, PA) filled the space between the objective and the bottom of the box. A given cell preparation was tracked for as long as ∼2 h or until there was a noticeable decrease in the swimming speeds. Since the cells had been washed free of growth medium and their densities were small, most preparations remained well oxygenated. Tracking signals, comprising time (30 points/s), voltages representing the X, Y, Z positions, and a marker indicating when the laser was on, were processed with the use of a data-acquisition system (NI 6052E board using LabView, National Instruments, Austin, TX). The LabView data were analyzed with a custom MATLAB program (The MathWorks, Natick, MA) and the video data were analyzed with ImageJ (NIH, Bethesda, MD).
Figure 1.
Tracking microscope optical paths. Light from the 660 nm LED goes to the tracker detector, light from the 590 nm LED goes to the camera (phase illumination of the cell bodies), and light from stimulation by the 532 nm laser (Samba; Cobolt AB, Solna, Sweden) goes to the camera (fluorescence of flagellar filaments). The laser is attenuated in the standard way with a λ/2 plate to rotate the plane of polarization, followed by a polarizing cube beamsplitter. Fine adjustments during tracking are made by changing the AOM pulse width (0.2–1 ms). The output of the AOM is circularly polarized by a λ/4 plate at 45° to the plane of polarization of the laser beam. The dark-phase embodiment was on a Nikon Diaphot 200 stand (condenser LWD 0.52 with LWD ∞ Ph3 phase ring, objective 40× acromat, na 0.75, wd 1.6 mm (46-17-03; Zeiss, Peabody, MA)). The bright-phase embodiment was on a Nikon Eclipse Ti-U stand with an external phase-ring attachment (condenser CLWD 0.72 with a Ph3 ring from an Optiphot 1.25 phase condenser, objective Nikon CF175 Apo LWD 25×, na 1.1, wd 2.0 mm, with the external phase ring from a Nikon S-Ke BM10 objective). The objectives were used with a 500 cSt, n = 1.040 polydimethylsiloxane damping fluid rather than with water. The following filters were used: F1 ZET532/10x (Chroma, Bellows Falls, VT), F2 ET542lp (Chroma), F3 ET575/50m (Chroma), and F4 HQ620lp (Chroma). The following mirrors were used: M1 DMLP605R (Thorlabs, Newton, NJ), M2 DMLP900R (Thorlabs), M3 ZT532rdc (Chroma), M4 T620lpxr (Chroma), and M5 in a Nikon trinocular beamsplitter (15, 16). An AOM acousto-optical modulator (ATM-110A1 with E1101T730 driver; IntraAction, Bellwood, IL) moved the laser beam on or off a pinhole in synchrony with the video vertical sync pulse.
E. coli swimming cells
HCB1737 (17) is isogenic with strain AW405 (1), which is wild-type for chemotaxis except for a single cysteine substitution, S219C, in the flagellar filament protein, FliC. HCB1737 was cultured from frozen stocks (−80°C) either in 10 mL of Luria Bertani broth (LB; 10 g Bacto-tryptone, 5 g yeast extract, and 5 g NaCl per liter) or in swarm medium (SM; 10 g Bacto-peptone, 3 g beef extract, and 5 g NaCl per liter) in 125 mL Erlenmeyer flasks, and grown to saturation at 30°C with aeration by gyration at 125 rpm. A 1/100 dilution of the saturated LB culture was grown in 10 mL of tryptone broth (TB; 10 g Bacto-tryptone and 5 g NaCl per liter) for untreated cells or in 10 mL of SM for the swarm liquid cells in 125 mL Erlenmeyer flasks at 30°C, with gyration at 125 rpm for 4 h to a cell density of ∼4.1 × 108 cells/mL. For moderate-length cells, cephalexin was added after 2.5 h of incubation at a final concentration of 5 μg/mL, and incubation was continued for an additional 1.5 h. For very long cells, cephalexin at a final concentration of 50 μg/mL was used instead. Cultures were checked for motility using a phase-contrast microscope (Nikon Optiphot). Cells were washed free of growth medium by 10 min of centrifugation at 1200 × g, followed by gentle resuspension of the pellet and addition of 10 mL of motility buffer with Tween-20 (MBT; 0.01 M potassium phosphate, pH 7.0, 10−4 M EDTA, 0.067 M NaCl, 0.0001% Tween-20) four times. The cells were then labeled with Alexa Fluor 532 maleimide dye as described in (5) for ∼30 min. Once labeled, the cell aliquots were brought to 10 mL volumes with MBT and washed by centrifugation three times (as above), centrifuged a final time, and resuspended in 2 mL of tracking buffer (MBT with 0.4% (w/v) methylcellulose, 4000 cP (MBTM)). A further cell dilution of ∼1/100 was used for tracking. Methylcellulose suppresses Brownian motion, which smooths the tracks and makes it easier to follow the cells. At concentrations of <0.2%, cells speed up. At 0.4%, they tend to slow down, but not by much. The tracking statistics are largely unaffected. Cells were tracked at their growth temperature, 30°C.
E. coli swarm cells
HCB1737 swarm plates were prepared as described in (5), except that the inoculation was done with a 1 μL drop of saturated culture diluted to 10−5 with SM. Swarm cells were collected by repeated rinsing within 5 mm of the advancing edge of the swarm with 2 mL aliquots of MBT. They were then treated as described above by washing with centrifugation, labeling, and washing again to remove excess dye, with a final suspension in MBTM. Cells were tracked at their growth temperature, 30°C.
B. subtilis
B. subtilis strains DS9540 and DK2002 were a gift from Daniel Kearns (Indiana University Bloomington). DS9540 is wild-type, with a single mutation (hagT209C) in flagellin that permits labeling with maleimide dyes. DS9540 was further altered (motAB::tet, amyE::Physpank-motAB spec, swrA::kan, slrA::cat) and named DK2002. The phenotype of DK2002 is reduced in flagellar number, inducible motor proteins, and enrichment for single cells (i.e., unchained cells). Strains were streaked onto LB 2% agar plates and grown at room temperature (21°C). A scraping from the plate was used to inoculate 10 mL of LB and then grown overnight with aeration by gyration at 100 rpm and 21°C. The saturated culture was diluted in 10 mL of LB by 5 × 10−3, 10−3, and 10−4, and grown overnight. The next morning, the optical density (OD) was measured and a motile mid-log phase culture was chosen and labeled with fluorescent dye. For strain DK2002, 1 mM of isopropyl β-D-1-thiogalactopyranoside was added to induce motility 2.5 h before measurements of OD and labeling were performed. The labeling procedure was similar to that described above for E. coli. Cells were washed free of growth medium by 10 min of centrifugation at 1200 × g, followed by gentle resuspension of the pellet and addition of 10 mL of chemotaxis buffer (CB; 0.01 M potassium phosphate pH 7.0, 0.14 mM CaCl2, 0.3 mM (NH4)2SO4, 0.01 mM EDTA, 5 mM sodium lactate, and 0.5% glycerol) (18) four times. The cells were then labeled with Alexa Fluor 532 maleimide dye, as described in (5), for ∼30 min. Once labeled, the cell aliquots were brought to 10 mL volumes with CB and washed by centrifugation three times (as above). They were then centrifuged a final time and resuspended in 2 mL of CB with 0.4% (w/v) methylcellulose, 4000 cP. A further cell dilution of ∼1/100 was used for tracking. Labeled cells were tracked as described above for E. coli, but at their growth temperature, 21°C.
Streptococcus (now Enterococcus)
Streptococcus strain V4051 (19), which we identified by its 16S rRNA sequence as Enterococcus saccharolyticus, is motile in the presence of glucose. We grew it from frozen (−80°C) stocks in 10 mL of KTY-glucose medium (10 g Bacto tryptone, 5 g yeast extract, 8.7 g dibasic potassium phosphate, and 10 g glucose per liter) in 125 mL Erlenmeyer flasks to saturation at 34°C with aeration by gyration at 125 rpm. A 1/100 dilution of the saturated culture in 10 mL of fresh KTY-glucose medium was grown as above for 3.0 h to a cell density of ∼8 × 108 cells/mL. The cells were washed free of growth medium as described above for E. coli with a buffer containing glucose (0.1 M sodium phosphate pH 7.5, 0.2 M KCl, 10−4 M EDTA, and 0.01 M glucose) (SB) and a final resuspension of the pellet into the SB with 0.4% (w/v) methylcellulose 4000 cP, followed by a further ∼1/100 cell dilution for tracking. V4051 cells were not fluorescently labeled, because when they were labeled with a maleimide dye the filaments were not visible and the cell bodies had bright patches, and when they were labeled with a succinimidyl-ester dye the filaments were very dim, the cell bodies were overly bright, and the protocol interfered with motility. Unlabeled V4051 cells were tracked as described above for E. coli, but at their growth temperature, 34°C.
Data analysis
We recorded tracks first and visualized flagella later using brief exposures of laser illumination (Fig. 2), or we used prolonged laser illumination (Fig. 3) and did not include the track measurements in the data tables. All tabulated tracks contained more than 50 data points and were terminated when laser illumination began, because it can depress motility. The first data point with laser illumination was recorded in the data file and on the video, thus providing accurate synchronization of tracks to images.
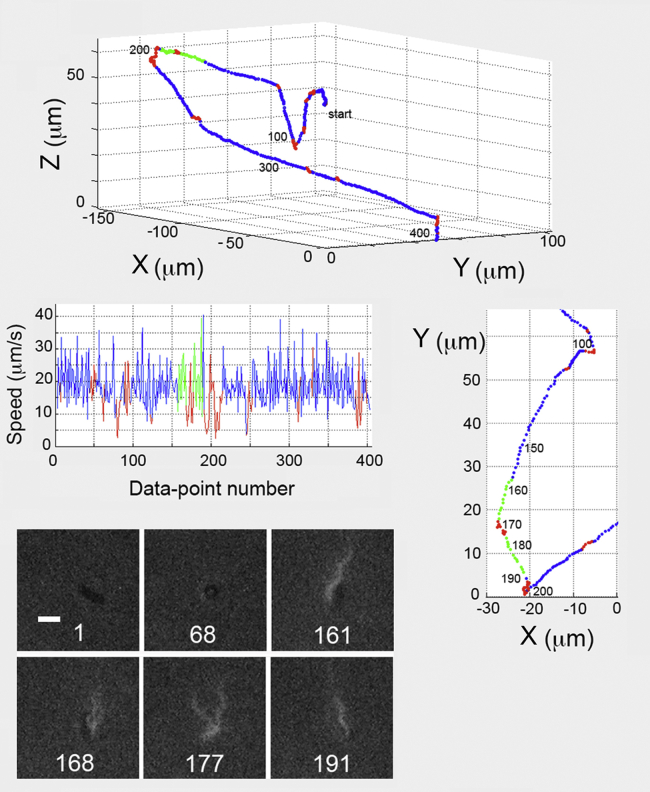
Figure 2.
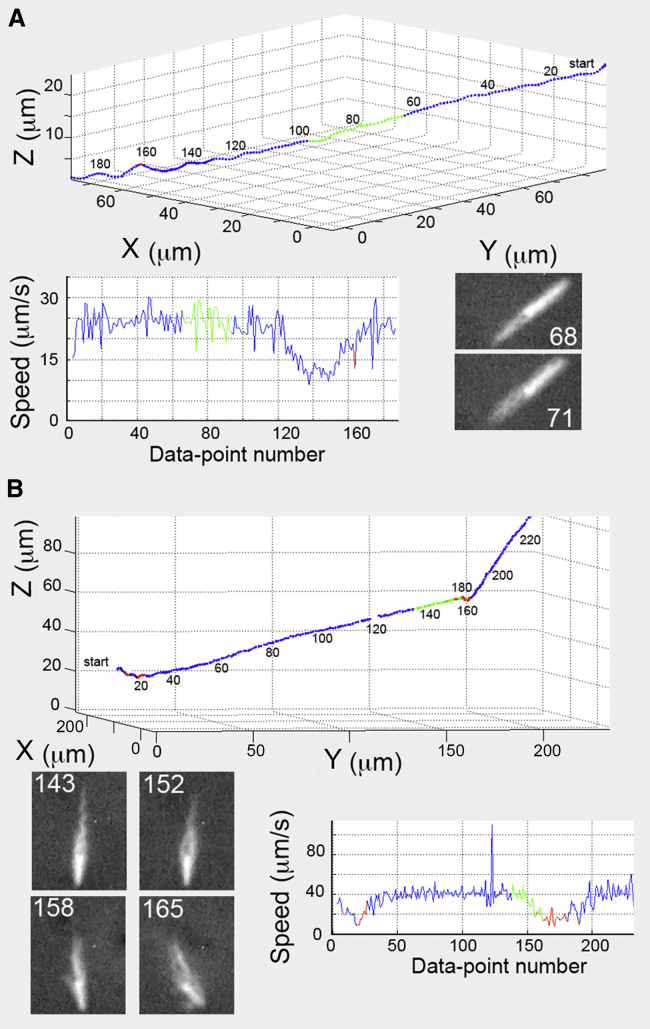
Track of a typical untreated E. coli cell (upper panel) with combined phase-contrast and fluorescence images (dark and light, respectively, lower-left panel). For cells of all kinds, runs are shown in blue, tumbles are in red, and runs with the laser on are in green. Each data point along the trajectory corresponds to one video frame (30/s). At the start, image 1 (lower-left panel), the phase-contrast image shows the cell moving parallel to the X-Y image plane. Cell-body measurements were made from this image. At image 68, the cell is seen end-on while moving along the Z axis at track point 68. The overview (right panel) shows the track in the X-Y plane from points 100–200, and includes laser illumination points 157–191. The cell was running with a tight bundle in image 161. A tumble occurred during points 168–177. In image 177, as the tumble ended, the bundle started to reform, a process that was completed at point 188 (not shown). A tightly formed bundle is evident in image 191. After this point, another tumble began. The scale bar here and in all other figures is 2 μm.
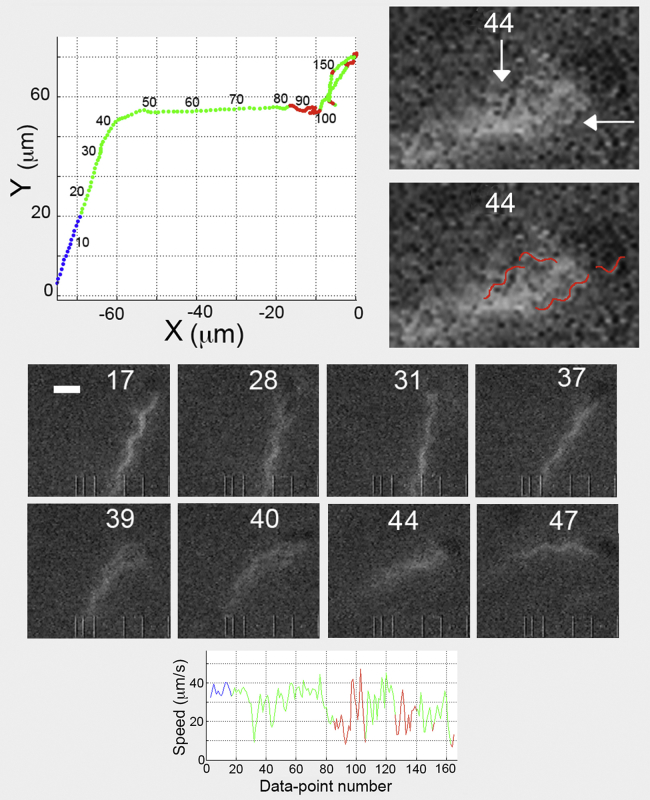
Figure 3.
Track of an untreated E. coli cell with an extended period of laser illumination moving normally in the X-Y plane (upper-left panel) until the first tumble. A comparison of the images (middle panel) with the data in the track suggests that although no filaments appeared outside the bundle and no tumbles were detected by our tracking criteria, motor reversals occurred: the cell altered course with a gradual change in the trajectory. An enlargement of image 44 (upper-right panel) shows curly filaments marked with white arrows (top) or highlighted in red (bottom).
The MATLAB program identified runs and tumbles according to algorithms specified earlier by Berg and Brown (20), with the exception that the first 10 points of each track were deleted to avoid noise as the cell settled into the tracking spot. Here, we recorded a data point in a track for each video frame, i.e., at a rate of 30/s, whereas previously specified data points (1, 20) were recorded at a rate of 12.6/s. As before, we calculated the instantaneous velocity at point J in the track using the second-order central difference equation , where is the position of the bacterium at point J, and T is the time interval between J and . The instantaneous speed at point J is the magnitude of . The angle by which the bacterium changes direction between J and is . When this angle is ≤35°/point for at least three points, the bacterium is in a run. When the angle is >35° for at least two points, the bacterium is in a tumble. Otherwise, if the angle is >35° for only a single point J, we find the angle between the vector sums and . If that angle is >35°, the bacterium has tumbled at point J. The run or tumble speed is the average of the instantaneous speeds at every point J contained in a single run or tumble. The angular speed while running or tumbling (the rate of change in swimming direction) is the average of the instantaneous rate of change between successive points at every point J contained in a single run or tumble. The run or tumble interval is the total time the bacterium spends in a single run or tumble, and it is calculated by subtracting the time point at the beginning of the run or tumble from the time point at the end of the run or tumble. The angle by which the bacterium changes direction between two runs is calculated from the sum of the last three velocity vectors of the previous run and the sum of the first three vectors of the following run, respectively. The angle by which the bacterium changes direction within a single run is calculated from the sum of the first three velocity vectors and the sum of the last three vectors of that run, respectively. The tumble frequency is defined as the number of tumbles divided by the time of the trajectory.
Long cells (E. coli cephalexin-treated and swarm cells, and B. subtilis DS9540) tended to wobble and thus exhibited helical trajectories. These tracks were smoothed into straighter lines either by using a moving average with a span between three and nine trajectory points or by projecting the vectors defined by successive trajectory points onto the central axis of each helical turn, to differentiate runs from tumbles. Tumbles were flagged from the smoothed data by using the standard 35° criterion. With the first method, smoothing changed the tumble shapes. With the second method, the trajectory points that occurred during a tumble were not modified.
The means and standard deviations (SDs) for a single cell were obtained by computing the means for each run or tumble separately, weighting the results for each run or tumble equally, and then computing the means and SDs of these means. The means and SDs for cell populations were computed by weighting the results for each cell equally.
Results
Motile cultures of E. coli and B. subtilis were fluorescently labeled with a sulfhydryl-specific (maleimide) dye and then tracked. Enterococcus was not labeled, because cells either failed to show fluorescent filaments after treatment with a maleimide dye or stopped swimming after treatment with a succinimidyl ester dye. Individual cells were imaged in phase contrast and, when labeled, in fluorescence (Fig. 1). We characterized the tracks of the cells by measuring run and tumble speeds (μm/s), run and tumble intervals (s), angular speeds while running and tumbling (°/point), the change in direction from run to run (°), and the change in direction during runs (°). For all cells and growth conditions, the data exhibited symmetric or skewed distributions, except for run and tumble intervals, which were exponentially distributed, with the shortest events being the most probable (see Figs. S9–S12 in the Supporting Material). A summary of the results for all cells is given in Table 1. Table S1 shows the subset of cells that ran and tumbled, and Table S2 shows the subset of cells that only ran. For comparison, tracking measurements from E. coli wild-type strain AW405 reported in 1972 (1) are shown in column 1 of Tables 1 and S1. In the 1972 study, the cells were grown in minimal medium with glycerol, and thus were not as fully energized. Also, the concentration of methylcellulose was smaller (0.18% as compared with the 0.4% used here), which changes the propulsion efficiency (21, 22). The E. coli strain tracked here, HCB1737, is derived from AW405 and shows similar behavior. B. subtilis is slightly larger and swims a bit faster, but behaves essentially the same as E. coli. The data for Enterococcus were similar to those obtained for E. coli and B. subtilis, but at low speeds the tracks were loopy.
Table 1.
Tracking Data for All Cells, Showing Means ± SD
| Parameter | E. coli AW405a | E. coli HCB1737 | E. coli HCB1737 Cephalexin | E. coli HCB1737 Swarm | B. subtilis DS9540 | B. subtilis DK2002 | Enterococcus V4051 |
|---|---|---|---|---|---|---|---|
| Number of cells | 35 | 137 | 155 | 274 | 117 | 139 | 64 |
| Number of cells that tumbled | 35 | 105 | 119 | 41 | 71 | 119 | 59 |
| Run speed (μm/s) | 14.2 ± 3.4 | 19.9 ± 5.6 | 23.0 ± 6.7 | 19.8 ± 6.9 | 23.3 ± 7.3 | 24.8 ± 8.2 | 20.3 ± 4.7 |
| Tumble speed (μm/s) | 14.3 ± 4.8 | 15.7 ± 5.3 | 16.7 ± 9.6 | 16.6 ± 9.3 | 15.8 ± 5.2 | 14.9 ± 3.9 | |
| Angular speed while running (°/point)b | 5.9 ± 3.2 | 10.8 ± 4.3 | 10.2 ± 4.1 | 3.9 ± 3.8 | 7.3 ± 5.0 | 11.5 ± 2.7 | 13.2 ± 5.2 |
| Angular speed while tumbling (°/point) | 23.5 ± 12.2 | 33.2 ± 7.5 | 30.4 ± 5.9 | 27.2 ± 7.0 | 27.6 ± 8.3 | 29.0 ± 5.0 | 28.0 ± 4.7 |
| Run interval (s) | 0.86 ± 1.18 | 2.9 ± 4.9 | 1.6 ± 2.9 | 7.1 ± 5.8 | 2.3 ± 2.0 | 0.91 ± 0.72 | 0.91 ± 1.4 |
| Tumble interval (s) | 0.14 ± 0.19 | 0.32 ± 0.18 | 0.28 ± 0.15 | 0.19 ± 0.12 | 0.17 ± 0.10 | 0.27 ± 0.14 | 0.33 ± 0.19 |
| Change in direction from run to run (°) | 68 ± 36 | 60.0 ± 29.2 | 64.7 ± 28.0 | 50.7 ± 32.8 | 51.8 ± 26.9 | 61.4 ± 25.3 | 68.0 ± 26.6 |
| Change in direction during runs (°) | 23 ± 23 | 17.4 ± 7.9 | 17.7 ± 10.3 | 23.8 ± 10.9 | 24.8 ± 13.7 | 23.5 ± 12.6 | 20.8 ± 10.5 |
| Tumble frequency (s−1) | ∼1 | 0.72 ± 0.60 | 1.0 ± 0.6 | 0.11 ± 0.34 | 0.50 ± 0.61 | 0.90 ± 0.62 | 1.2 ± 0.6 |
| Number of events (runs, tumbles) | 856, 754 | 898, 794 | 504, 232 | 397, 299 | 851, 749 | 505,467 | |
| Total tracking time (s) | 1094 | 828 | 2213 | 552 | 823 | 399 | |
| Body diameter (μm) | 0.73 ± 0.1 (89) | 1.0 ± 0.1 (103) | 1.1 ± 0.08 (24) | 1.2 ± 0.1 (68) | 1.2 ± 0.1 (117) | 1.3 ± 0.11 (90) | |
| Body length (μm) | 1.9 ± 0.5 (89) | 5.0 ± 1.9 (103) | 7.9 ± 4.8 (24) | 6.9 ± 2.3 (68) | 5.0 ± 1.7 (117) | 4.4 ± 2.1 (90) | |
| Number of filaments | 3.3 ± 0.9 (39) | 3.4 ±1.7 (40) | 3.9 ± 0.9 (7) | ∼26c | 5.8 ± 2.1 (22) |
E. coli was grown in three different ways: in T-broth (untreated), in T-broth containing cephalexin (cephalexin), and in a richer medium on an agar surface (swarm). Fig. 2 (upper panel) shows a track of a typical untreated cell, with a body length of 2.6 μm and bundle length of 4.3 μm, using intermittent laser illumination. The phase-contrast images show the cell body moving parallel (image 1) and then perpendicular (image 68) to the X-Y focal plane (the camera image plane). The cell was unaffected by the laser illumination: there was no decrement in cell speed (Fig. 2, speed) and the cell did not become tumbly. The track showed only a single tumble during the laser illumination (red section of the green track). The X-Y projection of the swimming path is shown in greater detail in the right panel of Fig. 2. The cell swam with a tight bundle before the tumble (image 161) and with a loosening bundle (image 168) just as the tumble began. During this tumble, the speed was 12.2 μm/s. The angular speed while tumbling was high at 61.6°/point, lasting over an interval of 0.53 s and resulting in a slight change in direction from run to run of 27.5°. Recorded images for the track of Fig. 2 are given in Movie S1.
Two other examples of tracks for untreated E. coli are shown in Figs. S1 and S2. The cell in Fig. S1 was only 1.5 μm long and had two remarkably short filaments (∼1 and 1.5 μm). However, all tracking measurements were within 1 SD of the population average. The run speed was slightly low and the change in direction during runs was somewhat high, with the runs having relatively high curvatures. The swimming speed did not diminish during tracking.
Filaments were visualized for 69 tumbles for 64 untreated cells (Table S3). We determined the fraction of filaments that were involved in a tumble by counting the number of filaments that caused the tumble and dividing by the total number of filaments on a cell, confirming that large angles between runs occurred when the fraction was large and smaller angles occurred when the fraction was small (Fig. S5). This was noted before for swimming cells (see Fig. 12 of (3)) and for swarm cells on a surface (see Fig. 8 of (5)). We also found that when the fraction was small, the tumble durations were shorter (Fig. S5).
We wanted to learn more about flagella and their function by using the laser for extended periods of illumination (Fig. 3). We looked for a correspondence between a greater run speed and bundle tightness, and between a lower run speed and bundle looseness over ∼75 cells. We did not find a strong correlation of this kind. As shown in Fig. 3, when we compared the bundle appearance with the speed, we found a tight bundle in image 17 and a speed of 35.6 μm/s; a loose bundle in image 28 and a lower speed of 24.6 μm/s; a tight bundle in images 31 and 37 with increased speeds of 33.5 μm/s and 32.2 μm/s, respectively; and a loose bundle in images 39 and 40 with speeds of 32.9 μm/s and 34.7 μm/s, respectively. What looked like a correlation in images 17, 28, 31, and 37 failed in images 39 and 40, where the loose bundle generated high speeds. In image 44, the bundle was not tight: two filaments were in a right-handed curly form (upper-right panels marked with arrows or highlighted in red), so transformations must have occurred. The speed was again high at 39.5 μm/s, and bundle reformation had begun. In image 47, the bundle appeared tight, with all filaments being in the normal helical form and all motors spinning CCW, with the speed 40.1 μm/s. The tracking data, however, showed the first tumble at track point 85 and the second tumble at track point 141, after which the cell speed decreased to an average of 22 μm/s for the remainder of the track, indicative of damage from the laser illumination. The speed fluctuations and small track deflections were not the result of loose or tight bundles, but instead were indicative of motor reversals and filament transformations occurring without filaments leaving the bundle. This is why tumbles involving a small number of filaments are not observed when the total number of filaments on a cell is large (≥5), as noted previously (see Fig. 12 D of (3)). Therefore, motor reversals can cause more subtle events than is usually imagined, including slight alterations of speed and trajectory (for the track of Fig. 3, see Movie S2).
We wanted to understand the behavior of long cells. E. coli cells elongate when treated with cephalexin or when grown on swarm plates. When long cells were studied in tracking buffer, tumbles that generated large changes in direction were observed, with the flagellar bundle switching from one end of the cell to the other, as seen on swarm plates (5). However, small changes in direction also occurred that were not seen on the swarm plates, with the flagellar bundle remaining at the same end of the cell. Curiously, the average track measurements of such elongated cells were indistinguishable from those of untreated cells (Table 1). When the cells were short, their flagellar filaments were usually longer than the cell body and could be visualized, so filament and bundle measurements could be made. When the cells were long, many flagellar filaments were obscured: sometimes the cell body did not roll fast enough to uncover them, and sometimes the region of laser illumination was too small. Therefore, filament and bundle measurements were rarely made. However, of 70 swarm cells, 52% had loosely bundled trailing filaments, 32% had well-formed trailing bundles, 16% had bundles along the sides of the cell body, and 13% had no bundles, only separate filaments. The longest cells were frequently (76%) bent or curved, and they wobbled and generated helical tracks: 25.2% of the cephalexin-treated cells and 83.6% of the swarm cells exhibited helical trajectories that required smoothing to determine the location of tumbles (for details regarding the methods used, see “Data analysis” above).
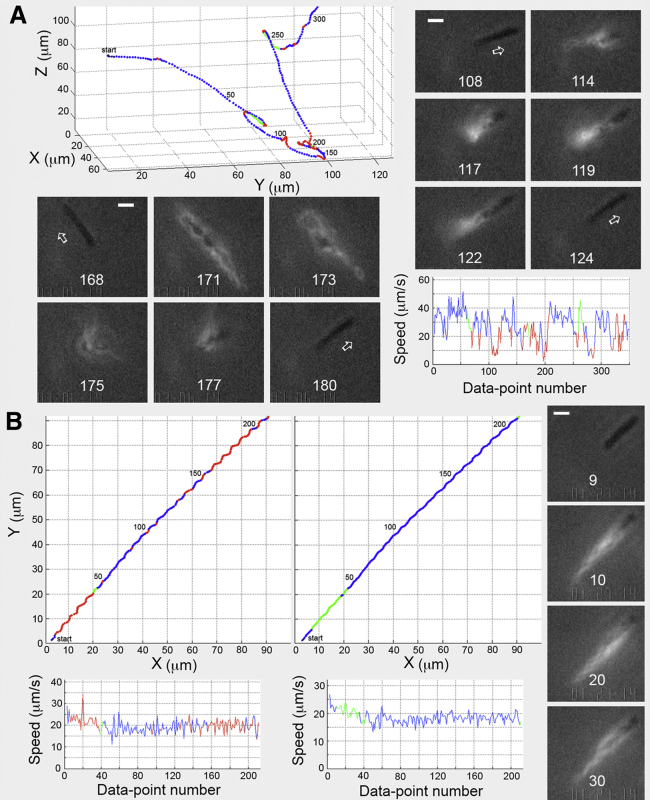
Fig. 4 A shows a cephalexin-treated cell with a 7.2-μm-long body and a 6.8-μm-long bundle that exhibits the typical diversity of tumbles. This cell appeared to be sensitive to laser illumination: the speed of the cell diminished slightly after laser illumination, and the cell reversed its direction of motion upon exposure to the laser (data points 69–73, where the average angular speed while tumbling, 38.6°/point, resulted in a larger-than-average net change in direction from run to run of 138° over the average tumble duration of 0.14 s; see Table 1). All of the cell’s filaments were active in the tumble, and the bundle reformed at what had been the leading end of the cell (images not shown). Another tumble (points 105–124) also appeared to involve all the filaments (images 108–124), with an angular speed while tumbling of 31.6°/point. However, the net change in direction between runs was small, at 30°, over a longer-than-average tumble interval of 0.63 s (compare Tables 1 and S1). When the bundle reformed, it remained at what had been the trailing end of the cell. Yet another tumble, data points 173–191 and images 171–177 (Fig. 4 A, lower-left panel), also appeared to involve all of the filaments, with the bundle remaining at the trailing end of the cell.
Figure 4.
Tracks of elongated E. coli cells. (A) A track of a cephalexin-treated cell (upper-left panel) shows both moderate and small changes in direction (tumbles) between runs. A small change in direction, 30.2°, occurred between runs with the bundle remaining at the same end of the cell body (images 108–124), and a larger change, 82.6°, occurred with the bundle also remaining at the same end of the cell (lower left, images 168–180). All of the cell’s filaments were involved in the tumbles, as evident in images 114–119 and 173–177. The speed graph shows that the tumble speed was significantly lower than the run speed. (B) Track of a typical swarm cell. The helical track in the left panel is the X-Y projection of the 3D trajectory obtained before smoothing. The track in the middle panel is the same data after smoothing with a running average of span 5. The video confirmed the cell was running. The corresponding speed data are shown below the tracks. Image 9 shows the cell before laser illumination, as it ran across the field of view. Only the filaments in the tracking area were illuminated by the laser light.
Fig. 4 B shows a swarm cell with a 5.7-μm-long body and a 6.8-μm-long trailing bundle. The tracking data were not used for behavioral analysis because the laser was turned on early in the track. The left panel shows the X-Y projection of the helical track before smoothing, with intermittent tumbles scored, even though the cell was running (confirmed by video). The right panel shows the track smoothed by a five-point running average. The cell did not tumble. The swimming speed (Fig. 4 B, below the tracks) diminished slightly after laser illumination. For the unsmoothed and smoothed tracks, the angular speed while running was 15.5 ± 5.1°/point and 8.2°/point, respectively, and the change in direction during runs was 18.5 ± 15.2° and 14.8°, respectively. The images show that the axis of the flagellar bundle was not in line with the long axis of the cell body, accounting for the helical swimming path. Recorded images of this track are provided in Movie S3; for the track of the much longer cell shown in Fig. S3, see Movie S4.
We found that the swarm-cell behavior was different from that of untreated or cephalexin-treated cells (Tables 1, S1, and S2): the run intervals were much longer and the tumble frequencies were much smaller. For control purposes, we also grew untreated and cephalexin-treated cells in liquid swarm medium, and found their behavior to be the same as when they were grown in the standard medium (Table S4). Thus, the richness of the swarm medium did not account for the differences in behavior. Since the swarm cells were, on average, only a few microns longer than the cephalexin-treated cells, we conclude that the differences in behavior were not simply due to differences in cell length, but presumably were due to the growth of swarm cells in a thin film of rich medium on an agar surface.
B. subtilis behaved much like E. coli (Tables 1, S1, and S2). However, the cells also were long. For DK2002, the slightly shorter and less well-flagellated mutant, only 4% of the tracks required smoothing. Most of these cells behaved similarly to untreated E. coli. For DS9540, the heavily flagellated and longer wild-type, 66% of the tracks required smoothing. These cells behaved similarly to both E. coli cephalexin and swarm cells: 61% ran and tumbled (Table S1) and 39% ran only (Table S2). Overall, their run intervals were long (Tables S1 and S2) and their tumble frequencies fell between those of cephalexin and swarm cells (Table 1). Fig. S4 shows strain DK2002 executing a typical track with intermittent laser illumination. This cell had a 3.0-μm-long body and a 9.4-μm-long flagellar bundle. The swimming speed did not diminish during tracking. All averages for this cell were within the SDs for the cell population.
Fig. 5 A shows a cell of wild-type strain DS9540, with a 10.9-μm-long body and a 9.8-μm-long flagellar bundle, that swam in a helical path like the swarm cells (Fig. 4 B). The track was smoothed by a five-point running average. The swimming speed diminished at track point 128 as the helical track radius increased, and recovered at point 168 as the helical track radius decreased. A brief tumble occurred at point 160. The angular speed while running was low due to smoothing. The images of the cell bodies appear in bright phase rather than in dark phase, as in Fig. 4. The bundle is tight in image 68 and loose in image 71.
Figure 5.
Tracks for B. subtilis DS9540. (A) A heavily flagellated cell executing a relatively straight track. The period of laser illumination (points 65–94) occurred during a run. Image 53 shows the cell body in bright phase, though slightly out of focus. At the start of laser illumination, the cell was running with a tight bundle (image 68). At 0.1 s later, the bundle began to loosen (image 71). In the video, the cell began to wobble and the track’s helicity increased (points 120–180). Although this cell did not tumble, the laser deenergized the cell and affected its motility. This is evident in the slowing speed from points 120–180. (B) Another heavily flagellated but shorter cell. This cell swam with a typical run-and-tumble trajectory. At trajectory point 120, a data point was dropped, which caused doubling of the speed (the peak in the speed graph). During laser illumination (track points 137–173), the cell initially ran with a tight bundle (image 143), but the bundle began to loosen (image 152). At track points 156–158, the cell tumbled, as evident in image 158, but individual filaments could not be resolved. Image 159 shows the cell body moving along a new trajectory, even though the filaments had not formed a tight bundle. This cell tumbled again from track points 161–172 and the cell slowed (speed trace 150–180), after which time the cell swam at the original speed.
Fig. 5 B shows a wild-type cell that ran and tumbled like untreated E. coli (Fig. 2). This cell had a 5.5-μm-long body and a 12.3-μm-long flagellar bundle. The swimming speed diminished during laser illumination. All averages for this cell were within the SDs for the cell population, with the exception that the angular speed while running was high at 16.3 ± 4.0°/point. The cell swam with a tight bundle in image 143, began to tumble in image 158, and was still tumbling in image 165 with the cell body and the bundle oriented at different angles. The resolution was not good enough to distinguish individual filaments.
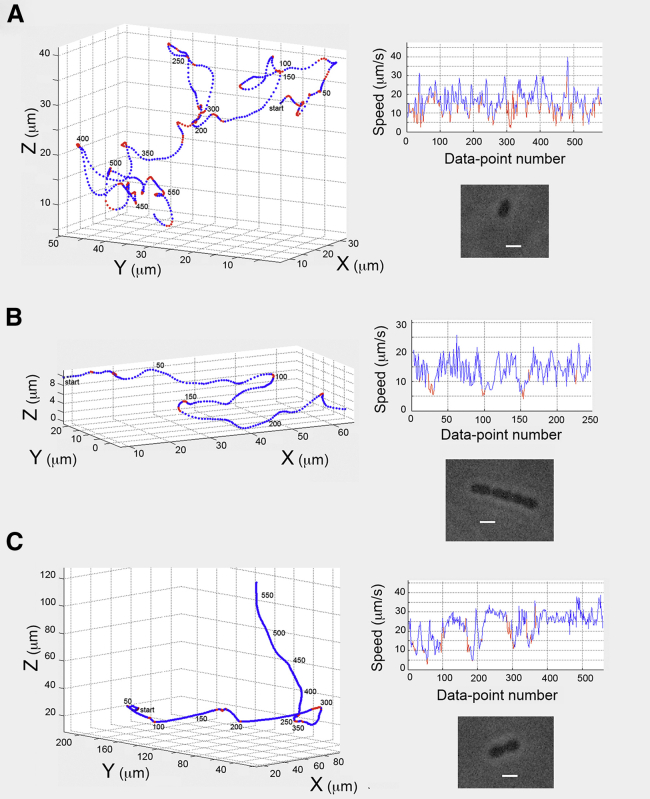
Enterococcus swam with several different styles: along loopy paths (41%), with helical paths (29%), with runs interrupted by tumbles (18%), and along paths that were combinations of the above (12%) (Fig. 6, A–C).
Figure 6.
Tracks for Enterococcus. (A) A typical loopy track, smoothed by a running average of span 5, showing runs and tumbles. (B) A helical track smoothed by a running average of span 5, showing runs and tumbles, including two reversals. (C) A run-and-tumble track smoothed using the vector sum method with a 0.3 radian tumble threshold.
Fig. 6 A shows a typical monococcus swimming with a loopy path. The cell body was 2.5 μm long. The track was smoothed using a running average with span 5.
Fig. 6 B shows a long cell comprising a chain of three diplococci that swam along a helical path. The composite cell body was 9.6 μm long. The track was smoothed using a running average with span 5.
Fig. 6 C shows a cell comprising two diplococci swimming along a typical run-and-tumble path. The composite cell body was 4.5 μm long. The track was smoothed using the vector sum method with a 0.3 radian tumble threshold.
The Enterococcus tracking measurements sorted by style are summarized in Table S5. There was a correspondence between swimming style and average run and tumble speeds (μm/s), with loopy and combined tracks having the lowest values. Run intervals were longest for cells that swam in a traditional run-and-tumble style and shortest for cells with loopy paths. Tumble intervals (s) and the angular speed while running and tumbling (°/point) were similar for all cells. although the angular speed while running was slightly lower for helical paths, where the data had been smoothed. Longer cells tended to swim along helical paths.
For all of the bacteria, we looked for correlations across the entire set of parameters averaged for each cell. The strongest correlation was between the tumble speed and run speed (μm/s; Fig. 7), due to the dependence of both speeds on motor speed. The mean angular speed while running (°/point) and the mean angular speed while tumbling (°/point) decreased as the mean run and tumble speeds increased, respectively (Fig. S6). Thus, the cells moved along straighter paths when they moved faster. The mean change in direction from run to run (°) was largest when the mean angular speed while tumbling was largest (°/point; Fig. S7), although the association was weak.
Figure 7.
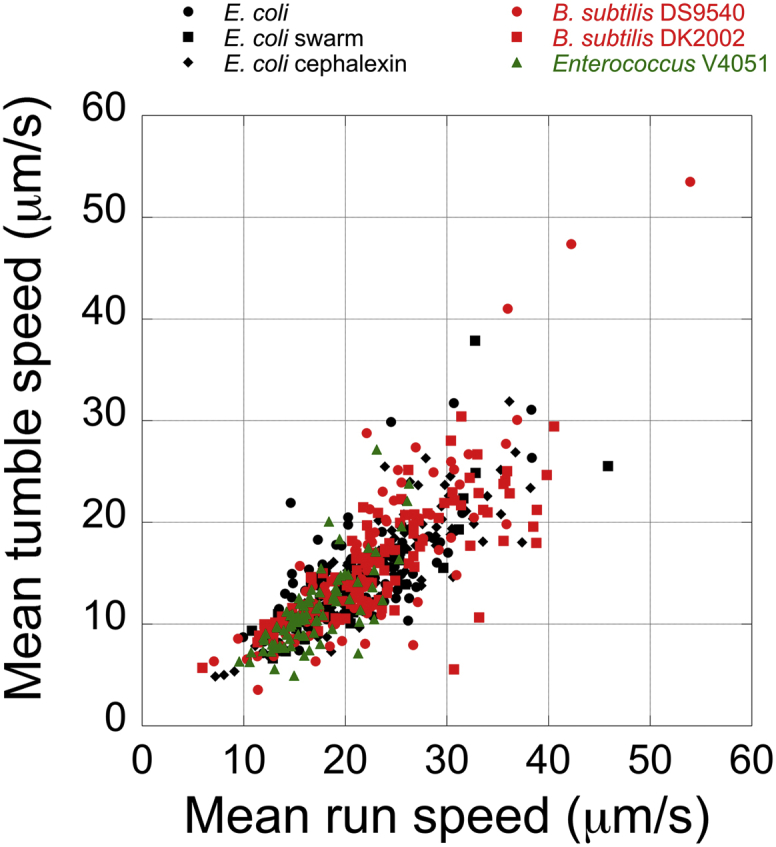
Correlation between mean tumble speeds and mean run speeds for cells of different types. Black dot, untreated E. coli; black squares, E. coli swarm cells; black diamonds, cephalexin-treated E. coli; red dots, B. subtilis DS9540 wild-type; red squares, B. subtilis DK2002; green squares, Enterococcus V4051.
We measured cell lengths and diameters, and when possible, the number and lengths of the filaments (Tables 1 and S3–S5). To our surprise, there were no strong correlations between the track measurements and the cell size or number of filaments. However, short tumble intervals (s) and low angular speed while tumbling (°/point) were favored by longer cells (Fig. S8). Comparatively few swarm cells tumbled, so the data were sparse. The increased lengths of cephalexin-treated and swarm cells did not lead to a large increase in filament number (Tables 1 and S3). The filaments of long, heavily flagellated cells were difficult to count because they tended to be obscured or intertwined, so the data were biased toward low filament numbers.
In general, measurements of the means for all the different bacteria were very similar, with the exception of the less tumbly behavior of the E. coli swarm cells and the heavily flagellated B. subtilis wild-type, so it seemed reasonable to consider all of the measurements as equally weighted independent events. Therefore, we plotted distributions and scatter plots for all runs and tumbles (n > 5000) for cells of all types. The angular speeds while running or tumbling were smaller when the run or tumble speeds were larger, with distinct decreasing rates (Fig. S13). Changes in direction from run to run were larger when angular speeds while tumbling were larger (Fig. S14).
Discussion
We visualized flagella while tracking cells of E. coli, B. subtilis, and a motile Enterococcus, varying the cell length and flagellar number in E. coli by treatment with cephalexin or by growth on swarm plates, and varying the flagellar number in B. subtilis by genetic construction. These species are all peritrichous, but the flagella of Enterococcus emerge around the waist of each coccus rather than at random points on the sides of the cell (see Fig. 1 of (19)). As expected, the cells moved in a run-and-tumble fashion (1). Data are shown in Table 1 for all cells, in Table S1 for cells that tumbled during the course of an experiment, and in Table S2 for cells that did not tumble.
A surprising result was that the run-and-tumble statistics were nearly the same regardless of species and cell length, with two exceptions: 1) swarm cells of E. coli rarely tumbled, and 2) wild-type cells of B. subtilis tumbled less than cells of a strain with fewer flagella (see Tables 1, S1, and S2). The failure of swarm cells to tumble was not due to cell length, since cephalexin-treated cells tumbled normally, or to effects of the swarm medium, since both wild-type and cephalexin-treated cells grown on swarm medium (in broth) swam as they did when grown on T-broth (compare Tables 1 and S4). The difference was due to growth in a thin film on agar. We suspect that wild-type cells of B. subtilis tumble less than constructs with fewer flagella because it is more difficult for right-handed filaments of the semicoiled or curly form to escape from bundles when the bundles contain large numbers of left-handed normal filaments. This effect is shown here in image 44 of Fig. 3 and has been noted previously (see Fig. 12 D of (3)).
Another intriguing result was that the run speeds were all about the same—the number of flagella did not matter. Cells of E. coli strain AW405 swam more slowly, but those cells were grown in a minimal medium containing glycerol rather than on T-broth, a mixture of amino acids (a casein hydrolysate). Wild-type B. subtilis did not swim faster than the mutant, even though wild-type cells had many more flagellar filaments, indeed, too many to count. Naively, one might expect a flagellar bundle with more filaments to generate more thrust, but this advantage might be dissipated by friction, a likelihood that was noted earlier for E. coli (4).
The longest cells were not able to tumble in the ordinary manner; instead, flagellar bundles tended to reform at one end of the cell or the other. The changes in direction from run to run tended to be small when the bundle reformed at the rear end of the cell where it had originally been, or large when the bundle reformed at the leading end of the cell. We looked to see whether the distribution of angles between runs became bimodal for cells >2 μm long, but this was not obvious. There were a number of intermediate angles, with a mean angle between runs of 59° ± 31°. Furthermore, for all E. coli the mean angle from run to run was 64° ± 42° (n = 1636), and for all cells of E. coli, B. subtilis, and Enterococcus the mean angle from run to run was 61° ± 42° (n = 4674). Both are nearly identical to the mean angle from run to run, 62° ± 26° (n = 1166), reported in 1972 (see Fig. 3 of (1)). The situation is different for swarm cells on an agar surface, where crowding suppresses small angular changes from run to run and cells prefer to back up (see Fig. 6 of (5)). For untreated E. coli, we looked at tumbles when filaments could be counted. We found that tumble times increased when the fraction of filaments out of the bundle increased, and confirmed that the angle between runs increased then as well (Fig. S5; see also Fig. 12 of (3) and Fig. 8 of (5)).
Tumble and run speeds were highly correlated (Fig. 7). The best linear fits for these correlations had slopes of 0.69 (R = 0.64), 0.68 (R = 0.85), 0.67 (R = 0.83), 0.76 (R = 0.83), 0.67 (R = 0.78), and 0.68 (R = 0.75) for E. coli untreated, cephalexin-treated, and swarm cells; B. subtilis wild-type and mutant cells; and Enterococcus cells, respectively. Thus, the cells moved ∼2/3 as fast when they tumbled as when they ran. As noted earlier (1), tumbling is an active process and is not determined by Brownian motion. The angular speed while running or tumbling decreased with speed (Figs. S6 and S13), suggesting that the cell body and the flagellar bundle are more closely aligned at higher speeds. The tumble interval and the angular speed while tumbling were affected by cell size (Fig. S8), with shorter cells tumbling for both large and small intervals of time and at large and small angular speeds, and longer cells tumbling more for shorter intervals of time at small angular speeds. For the angular speed while running, the data were complicated by high values when cells swam in helical paths and low values when those paths were smoothed. Helical paths occur when cells are bent or their flagellar bundles are off axis. If the image of the cell is longer than the span of the tracker detector, the tracker follows one edge of the cell, typically one end. As the cell is driven forward by the CCW rotation of one or more flagellar bundles (as viewed from behind the cell), the cell body rotates more slowly in a CW direction. If the cell is bent, its ends follow helical paths. The tracker does not see the flagellar bundles, since their fluorescence is blocked by filter F4 (Fig. 1)—it only sees the phase-contrast image of the cell body.
So far, we have discussed tumbles that we identified when the change in swimming direction from data point to data point was >35° (see “Data analysis” above for the criteria used to identify tumbles). However, there were events that were very subtle, where filaments changed their polymorphic form (from normal to semicoiled, followed immediately by curly) but did not come out of the bundle. Fig. 3 shows a cell in which a possible tumble seems obscured by filament transformations remaining within the bundle. The change in the track angle was too shallow to indicate a tumble, but a motor reversal was suggested by a change in the alignment of the bundle with the cell body and its direction of motion. Later in the track, the run speed increased with the appearance of a curly filament in the bundle. For this cell, had the tumble threshold been set at 27°, brief tumbles would have been flagged at the track inflections at points 30, 40, and 50. Therefore, the choice of a tumble threshold is somewhat arbitrary. However, the statistics do not change significantly from a choice in the range of 25–45°.
Author Contributions
L.T. and H.C.B. designed the research. H.C.B. built the apparatus. L.T., H.C.B., L.P., and M.N. performed the research. L.T. and M.N. analyzed the data. L.T. and H.C.B. wrote the manuscript.
Acknowledgments
We thank Dan Kearns for the gift of the B. subtilis strains, and Karen Fahrner for the rRNA sequencing that identified strain V4051 as Enterococcus.
This work was supported by a grant from the Physics of Living Systems program of the U.S. National Science Foundation and by the Rowland Institute at Harvard.
Editor: Dennis Bray.
Footnotes
Fourteen figures, five tables, and four movies are available at http://www.biophysj.org/biophysj/supplemental/S0006-3495(16)30478-7.
Supporting Material
References
- 1.Berg H.C., Brown D.A. Chemotaxis in Escherichia coli analysed by three-dimensional tracking. Nature. 1972;239:500–504. doi: 10.1038/239500a0. [DOI] [PubMed] [Google Scholar]
- 2.Macnab R.M., Ornston M.K. Normal-to-curly flagellar transitions and their role in bacterial tumbling. Stabilization of an alternative quaternary structure by mechanical force. J. Mol. Biol. 1977;112:1–30. doi: 10.1016/s0022-2836(77)80153-8. [DOI] [PubMed] [Google Scholar]
- 3.Turner L., Ryu W.S., Berg H.C. Real-time imaging of fluorescent flagellar filaments. J. Bacteriol. 2000;182:2793–2801. doi: 10.1128/jb.182.10.2793-2801.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Darnton N.C., Turner L., Berg H.C. On torque and tumbling in swimming Escherichia coli. J. Bacteriol. 2007;189:1756–1764. doi: 10.1128/JB.01501-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Turner L., Zhang R., Berg H.C. Visualization of flagella during bacterial swarming. J. Bacteriol. 2010;192:3259–3267. doi: 10.1128/JB.00083-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wu M., Roberts J.W., DeLisa M.P. Collective bacterial dynamics revealed using a three-dimensional population-scale defocused particle tracking technique. Appl. Environ. Microbiol. 2006;72:4987–4994. doi: 10.1128/AEM.00158-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Edwards M., Carlsen R.W., Zhuang J., Sitti M. Swimming characterization of Serratia marcescens for bio-hybrid micro-robotics. J. Micro-Bio. Robot. 2014;9:47–60. [Google Scholar]
- 8.Taute K.M., Gude S., Shimizu T.S. High-throughput 3D tracking of bacteria on a standard phase contrast microscope. Nat. Commun. 2015;6:8776. doi: 10.1038/ncomms9776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liu B., Gulino M., Breuer K.S. Helical motion of the cell body enhances Caulobacter crescentus motility. Proc. Natl. Acad. Sci. USA. 2014;111:11252–11256. doi: 10.1073/pnas.1407636111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chattopadhyay S., Wu X.L. The effect of long-range hydrodynamic interaction on the swimming of a single bacterium. Biophys. J. 2009;96:2023–2028. doi: 10.1016/j.bpj.2008.11.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Min T.L., Mears P.J., Chemla Y.R. High-resolution, long-term characterization of bacterial motility using optical tweezers. Nat. Methods. 2009;6:831–835. doi: 10.1038/nmeth.1380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Xie L., Altindal T., Wu X.L. From the cover: bacterial flagellum as a propeller and as a rudder for efficient chemotaxis. Proc. Natl. Acad. Sci. USA. 2011;108:2246–2251. doi: 10.1073/pnas.1011953108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mears P.J., Koirala S., Chemla Y.R. Escherichia coli swimming is robust against variations in flagellar number. eLife. 2014;3:e01916. doi: 10.7554/eLife.01916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cheong F.C., Wong C.C., Lim C.T. Rapid, high-throughput tracking of bacterial motility in 3D via phase-contrast holographic video microscopy. Biophys. J. 2015;108:1248–1256. doi: 10.1016/j.bpj.2015.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Berg H.C. How to track bacteria. Rev. Sci. Instrum. 1971;42:868–871. doi: 10.1063/1.1685246. [DOI] [PubMed] [Google Scholar]
- 16.Berg H.C. The tracking microscope. Adv. Opt. Elect. Microsc. 1978;7:1–15. [Google Scholar]
- 17.Turner L., Stern A.S., Berg H.C. Growth of flagellar filaments of Escherichia coli is independent of filament length. J. Bacteriol. 2012;194:2437–2442. doi: 10.1128/JB.06735-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ordal G.W., Goldman D.J. Chemotaxis away from uncouplers of oxidative phosphorylation in Bacillus subtilis. Science. 1975;189:802–805. doi: 10.1126/science.808854. [DOI] [PubMed] [Google Scholar]
- 19.Manson M.D., Tedesco P.M., Berg H.C. Energetics of flagellar rotation in bacteria. J. Mol. Biol. 1980;138:541–561. doi: 10.1016/s0022-2836(80)80017-9. [DOI] [PubMed] [Google Scholar]
- 20.Berg H.C., Brown D.A. Chemotaxis in Escherichia coli analyzed by three-dimensional tracking. Antibiot. Chemother. (1971) 1974;19:55–78. doi: 10.1159/000395424. [DOI] [PubMed] [Google Scholar]
- 21.Magariyama Y., Kudo S. A mathematical explanation of an increase in bacterial swimming speed with viscosity in linear-polymer solutions. Biophys. J. 2002;83:733–739. doi: 10.1016/S0006-3495(02)75204-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schneider W.R., Doetsch R.N. Effect of viscosity on bacterial motility. J. Bacteriol. 1974;117:696–701. doi: 10.1128/jb.117.2.696-701.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Guttenplan S.B., Shaw S., Kearns D.B. The cell biology of peritrichous flagella in Bacillus subtilis. Mol. Microbiol. 2013;87:211–229. doi: 10.1111/mmi.12103. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.