Abstract
Saturated and unsaturated phospholipids (PLs) can segregate into lateral domains. The preference of cholesterol for saturated acyl chains over monounsaturated, and especially polyunsaturated ones, may also affect lateral segregation. Here we have studied how cholesterol influenced the lateral segregation of saturated and unsaturated PLs, for which cholesterol had a varying degree of affinity. The fluorescence lifetime of trans-parinaric acid reported the formation of ordered domains (gel or liquid-ordered (lo)) in bilayers composed of different unsaturated phosphatidylcholines, and dipalmitoyl-phosphatidylcholine or n-palmitoyl-sphingomyelin, in the presence or absence of cholesterol. We observed that cholesterol facilitated lateral segregations and the degree of facilitation correlated with the relative affinity of cholesterol for the different PLs in the bilayers. Differential scanning calorimetry and 2H nuclear magnetic resonance showed that cholesterol increased the thermostability of both the gel and lo-domains. Increased number of double bonds in the unsaturated PL increased the order in the lo-domains, likely by enriching the ordered domains in saturated lipids and cholesterol. This supported the conclusions from the trans-parinaric acid experiments, and offers insight into how cholesterol facilitated lateral segregation. In conclusion, the relative affinity of cholesterol for different PLs appears to be an important determinant for the formation of ordered domains. Our data suggests that knowledge of the affinity of cholesterol for the different PLs in a bilayer allows prediction of the degree to which the sterol promotes lo-domain formation.
Introduction
An increasing amount of evidence suggests that the lateral structures of biological membranes are essential for many biological processes (reviewed in Lingwood et al. (1)). The majority of our knowledge of the mechanisms driving the lateral structuring in lipid bilayers comes from studies of relatively simple model systems. Ternary lipid bilayers composed of low gel to liquid-crystalline (liquid-disordered, ld) melting temperature (Tm) phospholipids (PLs), high Tm PLs, and cholesterol have been extensively studied (2, 3, 4). Such ternary systems have been especially informative as they show fluid-fluid phase coexistence, mimicking a likely scenario in biological membranes (5).
Cholesterol has an important role in the lateral structuring of membranes, as it together with PLs forms a lo-phase, where the lipids have a high acyl chain order but where the lateral diffusion is much faster than in the semicrystalline gel phase (6, 7). Cholesterol preferentially interacts with saturated PLs, and prefers sphingomyelins (SMs) over other PLs (8). It is not fully understood how cholesterol’s preferential interactions with different PLs affect the formation, thermostability, and size of cholesterol-rich domains.
PLs with polyunsaturated acyl chains are of special interest because they have many proposed biological functions (9). PLs in several membranes (e.g., synaptic termini in neurons, sperm, and retinal rod outer membrane segments) are highly enriched in the polyunsaturated docosahexaenoic acid (22:6) (10). Other membranes have much lower concentration of polyunsaturated PLs, but the levels can be dramatically enriched from exogenous sources (10). PLs can have unsaturation that are similar in both chains (called “symmetric” PLs) or only in the sn-2 chain (called “hybrid” PLs), the latter commonly found in biological systems (11, 12). Polyunsaturated PLs have very dynamic acyl chains, as shown by both nuclear magnetic resonance (NMR) and molecular dynamics simulations (9, 13). Due to their highly disordered nature, these PLs interact very poorly with cholesterol (14), and it has been proposed that polyunsaturated PLs could promote lateral segregation in biological membranes (15).
In this project our aim was to determine if and how cholesterol can facilitate the lateral segregation of lipids through its different interactions with saturated and unsaturated PLs. Our approach to gain insight into the role of cholesterol was to compare gel-domain formation in binary PL mixtures (data from Kullberg et al. (16)), and the formation of lo-domains in the same PL bilayers including cholesterol. The onset of ordered domain formation (gel or lo) was detected by measuring the fluorescence lifetime of trans-parinaric acid (tPA), which is very sensitive to the acyl chain order in the lipid bilayer (17). We included several unsaturated phosphatidylcholines (PC), which interacted differently with cholesterol (18), to examine how they affected cholesterol’s influence on the formation of ordered domains in the bilayers. We also compared n-palmitoyl-sphingomyelin (PSM) with dipalmitoyl-phosphatidylcholine (DPPC), because these are known to have different affinities for cholesterol (19). We investigated the nature of the domains formed in the different ternary bilayers using differential scanning calorimetry (DSC) and 2H NMR, to obtain additional insight into cholesterol’s influence on lateral segregation. The affinity of cholesterol for different PL bilayers was analyzed through sterol partitioning experiments. Our results showed that cholesterol facilitated lateral segregation, and the extent of segregation appeared to correlate closely with cholesterol’s relative affinity for PLs in the bilayer systems.
Materials and Methods
Materials
The lipids 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphocholine (POPC), 1-palmitoyl-2-linoleoyl-sn-glycero-3-phosphocholine (PLPC), 1-palmitoyl-2-arachidonoyl-sn-glycero-3-phosphocholine (PAPC), 1-palmitoyl-2-docosahexaenoyl-sn-glycero-3-phosphocholine (PDPC), 1,2 dioleoyl-sn-glycero-3-phosphocholine (DOPC), 1,2-dilinoleoyl-sn-glycero-3-phosphocholine (DLPC), 1,2-diarachidonoyl-sn-glycero-3-phosphocholine (DAPC), 1,2-didocosahexaenoyl-sn-glycero-3-phosphocholine (DDPC), 24:1-SM, and DPPC were bought from Avanti Polar Lipids (Alabaster, AL). N-Palmitoyl-D-erythro-sphingosylphosphorylcholine (PSM) was purified from chicken egg SM (ESM; Avanti Polar Lipids) as described in Kullberg et al. (16). N-Palmitoyl-(9′9′-d2)-SM (P(d2)SM) and 1-palmitoyl-2-palmitoyl-(9′9′-d2)-sn-glycero-3-phosphocholine (DP(d2)PC) were prepared by coupling palmitic-(9′9′-d2) acid (Cambridge Isotope Laboratories, Tewksbury, MA) with sphingosyl phosphorylcholine or 1-palmitoyl-2-OH-lyso PC, respectively (20). All PLs were dissolved in argon-purged methanol and stored at −20°C. Stock solutions and samples containing unsaturated lipids were saturated with argon and contained 0.5 mol % butylated hydroxytoluene as radical scavenger. The samples and stocks were kept shielded from light and regularly checked for oxidation with electron spray mass spectrometry, and analytical reverse-phase high-performance liquid chromatography (HPLC). PL concentration was determined by an inorganic phosphate assay (21). Cholesterol was purchased from Sigma-Aldrich (St, Louis, MO) and dissolved in hexane/isopropanol (3:2 by volume) and stored at −20°C. Cholesterol concentration was determined by a surface barostat as described in Jungner et al. (22).
TopFluor cholesterol (TF-cholesterol) was bought from Avanti Polar Lipids. 1-Palmitoyl-2-propionyl-DPH-sn-glycero-3-phosphocholine (DPH-PC) was prepared from 1-palmitoyl-2-OH-lyso-PC and propionyl-DPH (Setareh Biotech, Eugene, OR) as described previously in Mason et al. (20). Cholestatrienol (CTL) was prepared as described in Fischer et al. (23). tPA was synthesized from the methyl ester of α-linolenic acid as described by Kuklev and Smith (24). tPA was purified by crystallization from hexane at −80°C to yield 99% pure all trans parinaric acid. tPA identity was verified by electron spray mass spectrometry, analytical HPLC absorbance, and emission spectra. Fluorophores were stored at −87°C and contained 0.5 mol % butylated hydroxytoluene to prevent oxidation. Fluorophore concentrations were determined from absorbance based on molar extinction coefficients of 92,000 cm−1 M−1 at 300 nm in MeOH for tPA, 88,000 cm−1 M−1 at 350 nm in MeOH for DPH-PC, 90,000 cm−1 M−1 at 497 nm in MeOH for TF-cholesterol (Molecular Probes, Eugene, OR), and 11,250 cm−1 M−1 at 300 nm in MeOH for CTL (17). Water (MqH20) was purified by reverse osmosis followed by UF Plus treatment (to yield 18.2MΩcm resistivity; Millipore, Billerica, MA). Organic solvents were of HPLC purity and all other lab chemicals were of highest purity available.
Vesicle preparation
Samples for analysis were made by mixing lipids in organic solvents with or without a fluorescent probe. The samples were extensively dried under a nitrogen flow at 40°C. The dried lipid film was hydrated with prewarmed argon-purged MqH20 for 30 min at a temperature over Tm of the saturated lipid. Samples were vortexed and sonicated 5 min at 65°C in a model No. M3 Bath Sonicator (FinnSonic Oy, Lahti, Finland) to generate multilamellar vesicles (MLVs). For the partitioning experiments, large unilamellar vesicles (LUVs) were made by extruding the hydrated lipids 11 times through a 200-nm pore size polycarbonate filter (Whatman International, Maidstone, UK). The samples were argon-purged after sonication/extrusion and cooled to ambient temperature before measurements.
Time-resolved fluorescence of tPA
MLVs were prepared to a lipid concentration of 0.1 mM, including 1 mol % tPA, as described above. The time-resolved fluorescence experiments were performed using a FluoTime 100 spectrofluorimeter, with a PicoHarp 300E time-correlated single photon counting module (PicoQuant, Berlin, Germany). A 297-nm LED laser source (PLS300; PicoQuant) was used for excitation of tPA and emission was collected through a long-pass filter with a cut-off at 395 nm. The fluorescent decays were analyzed using the software FluoFit Pro (PicoQuant). Data were fitted to contain two or three lifetime components, whichever gave best unbiased residual plots and χ-squared closest to 1. The average lifetime was calculated as described in Lakowicz (25).
Partitioning experiments
Equilibrium partitioning of TF-cholesterol was studied with a Förster resonance energy transfer (FRET) approach, using donor and acceptor LUVs. The donor LUVs contained POPC and 0.5 mol % DPH-PC (FRET donor) and 0.5 mol % TF-cholesterol (FRET acceptor). The acceptor LUVs contained POPC with an increasing amount of DPPC or PSM (up to 100%). Donor and acceptor LUVs were mixed in a 1:1 molar ratio to a final lipid concentration of 0.08 mM. After 24 h incubation at 50°C, the fluorescence lifetime of DPH-PC was measured on a FluoTime 200 spectrometer (PicoQuant) at 50°C. DPH-PC was excited by a 378 nm PDL 800-D pulsed diode laser and emission was recorded at 430 nm. The data was analyzed using the software FluoFit Pro (PicoQuant). The FRET efficiency (E) was calculated according to (25):
| (1) |
where (τ0) is the fluorescence lifetime of DPH-PC in the absence of TF-cholesterol and (τi) is the lifetime of DPH-PC in the absence of acceptor LUVs. The fraction of TF-cholesterol (XD) in the donor LUVs was calculated from the FRET efficiency using:
| (2) |
where EINI is the FRET efficiency without transfer (with donor LUVs only) and Ei is the FRET efficiency in the particular mixture of donor and acceptor LUVs. When the molar fractions of TF-cholesterol in donor and acceptor LUVs are known, the partitioning coefficient (KR) can be calculated according to Eq. 3:
| (3) |
where XA and XD are the molar fraction sterols in the acceptor and donor LUVs and CA and CD are the concentration of acceptor and donor LUVs.
Partitioning of CTL between LUVs and methyl-β-cyclodextrin (Sigma-Aldrich) was determined as described previously in Nyström et al. (26). From the data, a molar fraction partitioning coefficient (Kx) was calculated.
DSC measurements
MLVs for DSC were prepared to a 1:1 molar ratio of unsaturated and saturated lipids with 0, 4, and 6 mol % cholesterol, and a final lipid concentration of 1.5 mM. All samples were stored at 4°C and degassed for 10 min using a ThermoVac instrument (MicroCal, Northampton, MA) before being loaded into the VP-DSC instrument (MicroCal). Samples were scanned from 0 to 60°C at the rate of 1°C/min. The software ORIGIN (OriginLab, Northampton, MA) was used for data analysis.
2H NMR experiments
Samples were made by mixing P(d2)SM or DP(d2)PC, unsaturated lipid (POPC, PLPC, PAPC, or DOPC), and cholesterol from organic solvents in the ratio 40:40:20, after which the solvent was evaporated under a constant stream of nitrogen at 40°C. The lipids were redissolved in 0.5 mL chloroform and were dried again under nitrogen flow at 40°C, after which they were kept in vacuum for 20 h. MLVs were prepared by hydrating the dried lipid films with deuterium-depleted water at 65°C, followed by vigorously vortexing. Each suspension was freeze-thawed 10 times followed by lyophilization, rehydration with deuterium-depleted water until 50% hydrated (w/w), and freeze-thawing 10 times. Each sample was transferred to a 5-mm glass tube (Wilmad, Vineland, NJ) and sealed using epoxy glue.
A 300-MHz CMX spectrometer (Chemagnetics, Agilent, Palo Alto, CA) with a 5-mm 2H static probe (Otsuka Electronics, Osaka, Japan) and a quadrupole echo sequence were used to record the 2H NMR spectra. The 90° pulse width was 2 μs, the interpulse delay 30 μs, and the repetition rate 0.5 s. The sweep width was 200 kHz and the number of scans ∼50 000. A usual 8-step phase cycle of the CYCLOPS type was adopted with an alternating ±90° phase shift for the second pulse. The data was analyzed with the software TopSpin 3.1 (Bruker, Berlin, Germany).
Results
Effect of cholesterol on lateral segregation in bilayers containing PSM and unsaturated PCs
To understand how cholesterol influenced the lateral segregation of unsaturated and saturated PLs, we determined how ordered domains formed in cholesterol-free and cholesterol-containing bilayers. This was done by performing time-resolved fluorescence measurements of tPA in lipid bilayers with systematically varied lipid compositions. As the excited state lifetime of tPA is very sensitive to the acyl chain order in the bilayer, the formation of ordered domains is effectively detected (17, 27). The onset of gel domain formation (for cholesterol-free bilayers) in the binary systems composed of POPC, PLPC, PAPC, PDPC, DOPC, or DLPC and PSM is available in a recent report from our group (16). The role of cholesterol in lateral segregation was determined by measuring the fluorescence lifetimes of tPA (1 mol %) in ternary bilayers with cholesterol, an unsaturated PL (POPC, PLPC, PAPC, PDPC, DOPC, or DLPC), and increasing concentrations of PSM (0–50 mol %). The cholesterol concentration was kept constant at 20 mol % while the saturated/unsaturated PL ratio was varied. The cholesterol concentration was chosen to be 20 mol %, because most published studies indicate that at 20 mol % only lo-domains are formed at low concentrations of saturated lipids (5, 28).
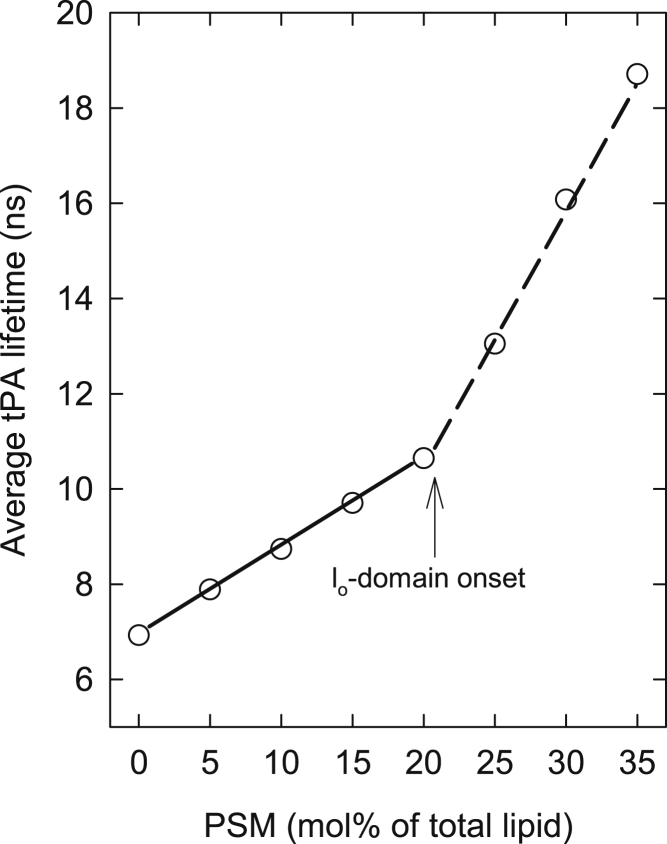
Representative fluorescence decays from the measurements are shown in Fig. S1 in the Supporting Material. The calculated average fluorescence lifetimes of tPA were plotted as a function of PSM content (representative data shown in Fig. 1 and in Fig. S2), and the onset of the lo-domain formation was determined.
Figure 1.
The average fluorescence lifetime of tPA in POPC bilayers with a constant 20 mol % cholesterol, as a function of PSM content: Representative data from one set of data recorded at 23°C is shown. The onset of ordered domain formation was determined as the point at which the linear lifetime function changed slope (as denoted in the figure).The same approach was used to analyze the data from the cholesterol-free bilayers (16). In cases of uncertainty of the slope change, more repeats with 2.5 mol % intervals were done.
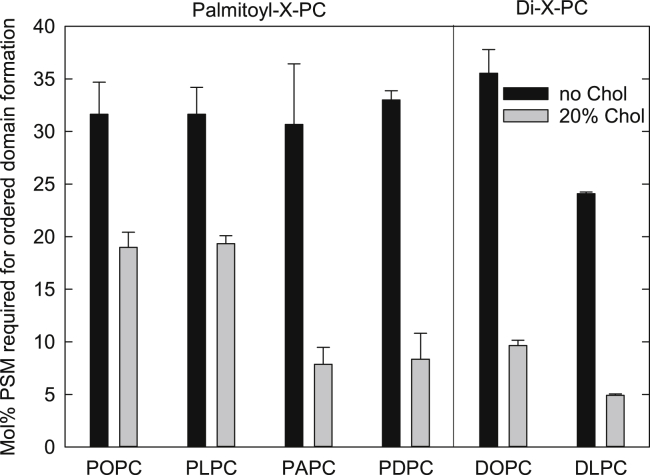
With the hybrid PLs (POPC, PLPC, PAPC, and PDPC), the number of double bonds did not affect the onset of gel-domain formation (Kullberg et al. (16), and Fig. 2). However, in bilayers containing 20 mol % cholesterol, much less PSM was required to induce lateral segregation than in the cholesterol-free bilayers, i.e., cholesterol facilitated lateral segregation. The number of double bonds in the unsaturated lipid also affected the amount of PSM needed to form lo-domains. In POPC with one double and PLPC with two double bonds, ∼19 mol % PSM was required to form lo-domains while only ∼8 mol % PSM was needed in PAPC and PDPC with four and six double bonds, respectively. However, also note that these acyl chains were longer than the chains in POPC and PLPC. In the bilayers with symmetric PLs (DOPC and DLPC), cholesterol also promoted lateral segregation. However, increasing the number of double bonds in symmetric PLs also affected the formation of gel domains in cholesterol-free bilayers, in contrast to the bilayers containing hybrid PLs (16).
Figure 2.
Amount of PSM required to form ordered domains in bilayers of different unsaturated PLs with or without cholesterol: The onset of gel-domain formation was taken from data in Kullberg et al. (16), and the onset of lo-domain formation was determined in bilayers with a constant cholesterol content of 20 mol % as described in Fig. 1. All experiments were performed at 23°C.
In conclusion, the tPA experiments indicate that cholesterol can facilitate lateral segregation of saturated and unsaturated PLs, and that the degree to which this occurs depends on the number of double bonds in the latter (and possibly chain length difference could have a minor impact). The driving force behind lateral segregation could be cholesterol’s aversion for unsaturated PLs (18). Indeed, this seems to be the case, as cholesterol could induce lateral segregation in bilayers composed of two unsaturated lipids (DDPC and 24:1 SM), when cholesterol’s aversion for one lipid was much larger than for the other lipid (Fig. S3).
Influence of cholesterol’s affinity for the saturated lipid on lateral segregation
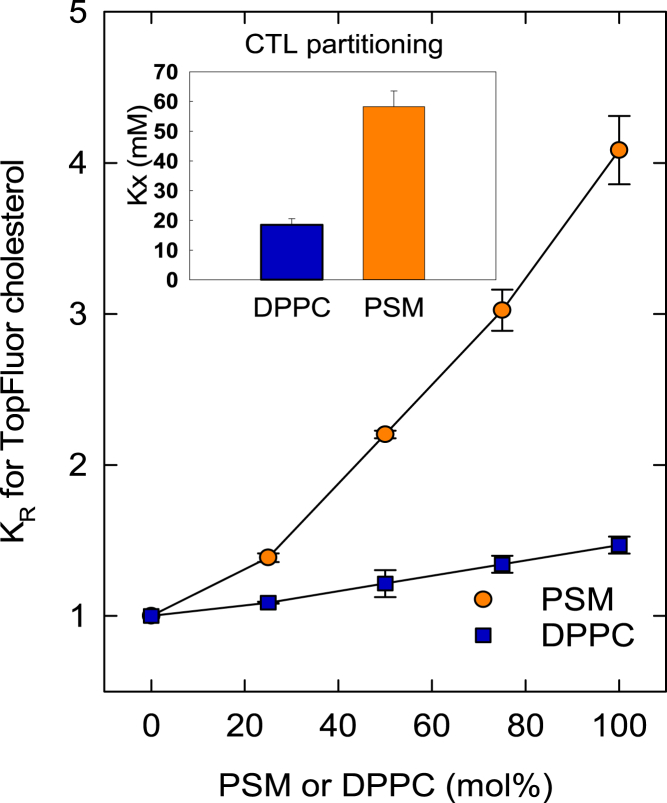
It is well documented that cholesterol has a high affinity for SM bilayers (19, 29). By replacing SM with a saturated PC, for which cholesterol has a lower affinity (30), we could determine the impact of cholesterol’s affinity for different PLs on cholesterol facilitated lateral segregation. We chose DPPC, which has similar thermotropic phase behavior to PSM (16). First, we quantified the affinity of cholesterol for fluid PSM and DPPC bilayers by performing partitioning experiments at 50°C. For such experiments, we have extensively used the cholesterol analog CTL, which has very similar properties to cholesterol (26, 31). The drawback with CTL is its low fluorescent intensity at higher temperatures, especially in unsaturated bilayers. Hence, CTL partitioning experiments could only be made with pure DPPC and PSM bilayers (Fig. 3). To allow comparison of sterol interactions with unsaturated and saturated the more temperature stable cholesterol analog, TF-cholesterol was used ((32); see Fig. S4 for structures). The equilibrium partitioning of TF-cholesterol between donor LUVs and acceptor LUVs was determined. Donor LUVs contained POPC with 0.5 mol % TF-cholesterol and 0.5 mol % DPH-PC and acceptor LUVs contained POPC and increasing concentration of PSM or DPPC (up to 100%). The relative partitioning coefficient (KR) with POPC acceptor LUVs (Fig. 3) was close to 1 (normalized to 1 to simplify interpretation). Addition of PSM to POPC bilayers increased KR up to ∼4 when the acceptor LUVs contained pure PSM. In the literature, KR values between 4.5 and 12 have been reported for cholesterol (33, 34). Accordingly, the relative affinity of TF-cholesterol for PSM over POPC may be slightly lower than that of cholesterol, but as the affinity of sterols for PL bilayers is affected by the sterol mol fraction in the bilayers and presence of lo-domains (3, 35), it may be misleading to compare KR values obtained with different cholesterol mol fractions in the bilayers.
Figure 3.
Equilibrium partitioning of TF-cholesterol between donor and acceptor LUVs: The donor LUVs were POPC prepared with 0.5 mol % TF-cholesterol and 0.5 mol % 16:0 DPH-PC. In the acceptor LUVs, the lipid composition was POPC with an increasing amount of PSM or DPPC (up to 100% PSM or DPPC). The relative partitioning coefficients (KR) were calculated from FRET efficiency. (Inset) Molar fraction partitioning coefficients (Kx) for CTL partitioning between methyl-β-cyclodextrin and DPPC or PSM LUVs; all measurements were made at 50°C to ensure fluid membranes. To see this figure in color, go online.
Inclusion of DPPC in POPC bilayers increased the partitioning coefficient very modestly, with a KR of ∼1.5 with 100% DPPC in the acceptor LUVs. TF-cholesterol partitioning data showed that the sterol had a 2.7 higher affinity for PSM than DPPC, which is in good agreement with the results obtained in CTL partitioning experiments (Fig. 3).
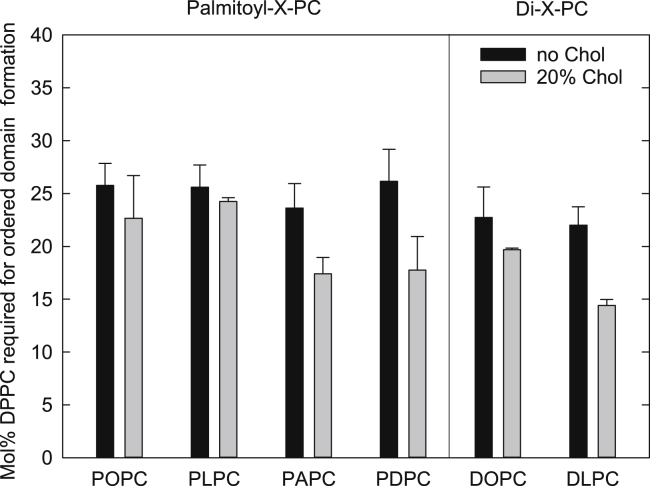
Next, the same tPA-fluorescence-lifetime based assay used in PSM systems (Figs. 1 and 2) was used to detect the onset of lo-domain formation in bilayers with a constant of 20 mol % cholesterol, an unsaturated PL (POPC, PLPC, PAPC, PDPC, DOPC, or DLPC), and an increasing amount of DPPC (0–50 mol %). Fig. 4 shows the effect of cholesterol on the onset of lo-domain formation, as compared to the onset of gel domain formation in unsaturated PCs (gel domain data taken from Kullberg et al. (16)).
Figure 4.
Amount of DPPC required to form ordered domains in bilayers of different unsaturated PCs without or with cholesterol: The onset of gel-domain formation was taken from Kullberg et al. (16), and the onset of lo-domain formation was determined in bilayers with a constant cholesterol content of 20 mol % as described in Fig. 1. All experiments were performed at 23°C.
Addition of 20 mol % cholesterol had no significant effect on the lateral segregation in bilayers composed of DPPC and POPC or PLPC as compared to binary systems. In PAPC and PDPC bilayers, the lo-domains formed at slightly lower DPPC concentration than in cholesterol-free bilayers (∼17 vs. ∼23 mol % DPPC). In bilayers composed of symmetrical unsaturated lipids (DOPC and DLPC) and DPPC, the presence of cholesterol modestly promoted lateral segregation as compared to cholesterol-free bilayers. There was a correlation between the number of double bonds in the unsaturated PLs and cholesterol’s facilitation of ordered domain formation in DPPC bilayers. However, the effect of cholesterol was not as pronounced in DPPC bilayers as it was in PSM bilayers (compared in Fig. S5). The results indicated that it was not only cholesterol’s aversion for unsaturated lipids but also the relative affinity of cholesterol for the two PLs (unsaturated and saturated) that had an impact on the degree to which cholesterol promoted lateral segregation.
Effect of low cholesterol concentrations on gel domain thermostability
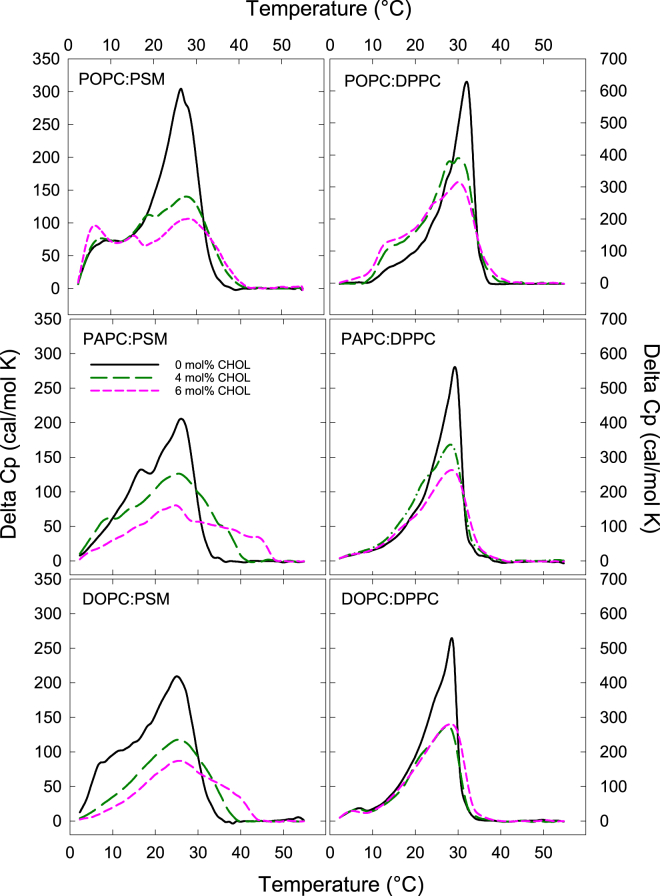
To gain more insight into cholesterol promoted lateral segregation in lipid bilayers, we studied how inclusion of small amounts of cholesterol affected the thermotropic behavior of binary PL bilayers. The bilayers were prepared from unsaturated lipids (POPC, PAPC, or DOPC), PSM or DPPC, and cholesterol. The PL molar ratio was 1:1, and cholesterol was present at 0, 4, and 6 mol %. The thermograms from all observed systems were complex, and contained several overlapping transitions (Fig. 5). As expected, the addition of 4 and 6 mol % cholesterol to the binary systems lowered the enthalpy and cooperativity of the gel domain melting in all samples (36). Interestingly, cholesterol addition significantly increased the thermostability of the gel domains in PSM-based systems, suggesting the formation of PSM-cholesterol-enriched domains. The increased thermostability was apparent in the POPC sample, but was much more pronounced in the DOPC and the PAPC samples. In the DPPC-based systems, the addition of cholesterol did not markedly affect the gel domain thermostability, irrespective of the degree of unsaturation in the unsaturated lipid.
Figure 5.
The effect of cholesterol on the thermostability of PSM or DPPC containing gel domains by DSC: Bilayers contained a 1:1 concentration of a saturated lipid (PSM or DPPC) and an unsaturated lipid (POPC, PAPC, or DOPC) and 0, 4, and 6 mol % cholesterol. Note that the scale of the y axis is different for PSM and DPPC. To see this figure in color, go online.
As a whole, the DSC results suggested that the ordered domains were increasingly enriched in SM and cholesterol when double bonds were increased in the unsaturated PLs. Although these domains most likely were gel domains at all cholesterol concentrations studied, the observed effects cholesterol are probably relevant for how cholesterol promote lateral segregation.
2H NMR show that the order in both disordered and ordered domains depended on the number of double bonds in the unsaturated lipid
Additional information about the properties of the ordered and disordered domains in the different systems was obtained using 2H NMR. 2H NMR can be used to detect lipid order of deuterated lipids in both the ld-domain and the lo-domains, and hence gives insight into the domain compositions (37). Detecting lipid order for both ld- and lo-domains has been done with several perdeuterated PLs by identifying two sets of Pake doublets from the terminal methyl group (38, 39). We used site-specific deuterated P(d2)SM or DP(d2)PC, allowing us to measure acyl chain order in the middle of the acyl chains where the acyl chains to a higher degree are ordered by cholesterol (40).
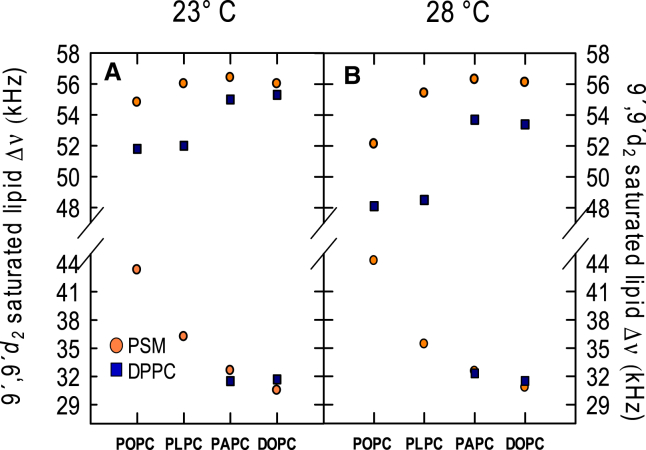
The samples contained an unsaturated lipid (POPC, PAPC, or DOPC), a saturated lipid (P(d2)SM or DP(d2)PC), and cholesterol mixed in the molar ratio 40:40:20. In the PSM samples, two sets of Pake doublets were observed in all spectra in all the samples (Fig. S6). The quadrupole splitting (Δν) describes the acyl chain order for the deuterated lipid. The Δν of the outer peaks denoted to PSM in the lo-domains was smallest in the POPC system, and Δν in the outer doublet increased modestly but significantly when the number of double bonds was increased in the unsaturated lipid at both 23 and 28°C (Fig. 6). In the ld-domains, the effect of increased degree of unsaturation was the opposite. Increasing the temperature from 23 to 28°C affected the order of the lo-domains in POPC- and PLPC-containing bilayers, but not in PAPC- and DOPC-based systems, suggesting higher thermostability in the latter, in line with the DSC results (Fig. 5). In the samples with deuterated PSM and POPC, two sets of Pake doublets were observed also at 28°C (Fig. S6), but the smaller difference in Δν between ld- and lo-domains suggested that a slight temperature increase would lead to only one set of Pake doublets. This is in good agreement with earlier reports with perdeuterated PSM in similar sample compositions, where two sets of Pake doublets were detected at 25°C but not 30°C (38).
Figure 6.
The quadrupole splittings of site-specific deuterated saturated lipid in both ld- and lo-domains at 23 and 28°C: Acyl chain order was deduced from the quadrupole splitting (Δν) from each peak doublets. MLVs were prepared to 50 wt % H2O containing an unsaturated lipid (POPC, PLPC, PAPC, or DOPC), a saturated lipid (P(d2)SM or DP(d2)PC), and cholesterol mixed in the ratio 40:40:20. To see this figure in color, go online.
In the DPPC-containing bilayers, two set of Pake doublets were only observed in the spectra from the samples containing the more unsaturated lipids (PAPC and DOPC). In these bilayers the order of deuterated DPPC in the lo-domains was lower especially at 28°C than the deuterated PSM, although the Δν in the ld-domains was similar. Earlier reports with DOPC/deuterated DPPC/cholesterol have shown the 40:20:20 composition have lo- and ld-phase coexistence, in agreement with our observations (39). Overall, there was a smaller difference between order in lo- and ld-domains in the DPPC-containing bilayers than observed with PSM, as indicated by the difference in Δν between the two domains, and by the single set of Pake doublet observed in the spectra in the POPC and PLPC samples. This agrees with cholesterol having less promotion of ordered domain formation in DPPC bilayers, and a lower cholesterol affinity for DPPC than PSM bilayers (Fig. 3).
Discussion
Cholesterol is an essential lipid component of mammalian cell membranes. It is well known that cholesterol together with PLs forms a lo-phase, and has an important role in the shaping of the lateral structure of membranes (6, 41). In this project our aim was to obtain detailed information about how cholesterol can promote lateral segregation of lipids, and therefore we compared how ordered domains (gel or lo) formed in binary PL bilayers with or without cholesterol. The ordered domains were detected using the fluorophore tPA that we previously have shown is highly sensitive to the presence of gel and lo-domains (3, 42). The mol fractions of saturated lipids needed to form lo-domains at 20 mol % cholesterol reported by tPA fluorescence are usually slightly lower than those reported by other methods (e.g., fluorescence microscopy or NMR). For example, microscopy data indicates that ∼20 mol % SM is needed to form lo-domains in DOPC/SM/cholesterol bilayers compared to ∼10 mol % reported by tPA (2, 43). 2H NMR indicates that ∼27 mol % DPPC is needed to form lo-domains in DOPC/DPPC/CHOL bilayers while tPA reports ∼20 mol %. Likely the lower fractions reported by tPA derives from the probes’ high sensitivity for ordered domains as well as the high resolution of single probe fluorescence methods allowing detection of small and dynamic structures.
Influence of cholesterol on the lateral segregation of saturated and unsaturated PLs
In a previous study it was observed that in binary bilayers composed of unsaturated hybrid PL and different saturated lipids, the number of double bonds in the unsaturated chains of hybrid PLs did not markedly affect the solubility of the saturated lipids in the ld-phase (16). However, in the presence of cholesterol, lo-domains segregated from the ld-phase more efficiently when the hybrid PLs were more unsaturated (Figs. 2 and 4). In addition, less PSM was needed to form lo-domains than was needed to form gel domains. A similar promotion of lateral segregation by cholesterol has previously been observed (44). It is also known that an increasing number of double bonds in the unsaturated lipid, in similar ternary bilayers to those used in our study, promote lateral segregation (45). However, in recently determined phase diagrams for POPC/bovine brain SM (bbSM)/cholesterol, DOPC/bbSM/cholesterol (28), and 1-stearoyl-2-docosahexaenoyl-PC/bbSM/cholesterol (46), the PL unsaturation does not appear to show clear effects on the formation of lo-domains. This could be due to the varying acyl chain composition in bbSM, as it has been shown that both PSM and ESM are stronger inducers of lo-domains and create larger domains than bbSM (28, 47).
In our study, cholesterol promoted lo-domain formation more strongly in bilayers with more double bonds in the unsaturated lipid. Even if PDPC and PAPC have more carbons in their acyl chains compared to POPC and PLPC, the increased number of double bonds should result in more gauche conformers, thereby reducing the effective chain length. We therefore believe that the number of double bonds was more important, compared to the number of carbons in the acyl chains of PAPC and PDPC, as determinants of promoted lateral segregation. It was also most likely the number of double bonds and not the chain length that lowered the preference of cholesterol for such bilayers, as determined by sterol partitioning studies (18, 33).
An increased amount of double bonds in PLs has been reported to lower the cholesterol solubility in the bilayer (48). Cholesterol solubility is the lowest in bilayers composed of PLs with several double bonds in both acyl chains. The 20 mol % cholesterol used in this study should not be close to the solubility limit in any of the phospholipid systems. Hence, the degree of solubility should not be directly influencing the onset of lo-domain formation. However, as the affinity of cholesterol for PL bilayers also is the lowest when the bilayer is composed of PLs with several double bonds in both acyl chains (33), and there is likely a correlation between the sterol solubility in PL bilayers and the sterol affinity for the bilayers.
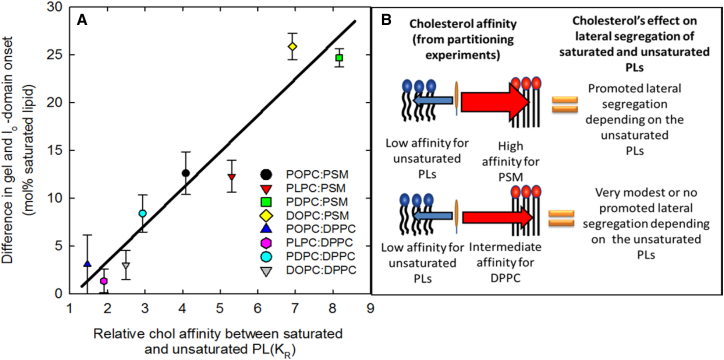
Although we observed similar cholesterol promoting effects on the lateral segregation in DPPC-based bilayers (Fig. 4), still the effects was markedly smaller than in PSM-containing bilayers (compared in Fig. S5). The more pronounced promotion of lateral segregation by cholesterol in SM bilayers is in agreement with observations made in fluorescence quenching experiments (44), and we assigned it to the lower affinity of cholesterol for DPPC bilayers as compared to PSM bilayers (Fig. 3). As cholesterol addition to DDPC/24:1-SM bilayers also promoted lateral segregation (Fig. S3 C), it seemed that the degree to which cholesterol promoted lateral domain formation depended on the relative affinity of cholesterol for the two PLs (unsaturated and saturated) in the ternary mixture. To test this, we plotted cholesterol’s relative affinity for the saturated and unsaturated lipids against the difference between lo- and gel-domain onset (Fig. 7 A). This resulted in a rather linear dependence between promotion of ordered domain formation and the relative affinity. We also included a scheme describing the cholesterol promoted lateral segregation in the studied systems (Fig. 7 B). The scheme shows that while cholesterol’s affinity for the unsaturated PLs remains the same in the PSM and DPPC bilayers, the effect on promoted lateral segregation is smaller when cholesterol’s affinity to the saturated lipid is lower, as seen in the DPPC bilayers. We suggest that this relative difference between cholesterol’s affinity for unsaturated and saturated PLs determines how cholesterol influences lateral segregation.
Figure 7.
Cholesterol’s relative affinity for unsaturated and saturated lipids correlates with the promoted lateral segregation measured with tPA-lifetimes (A). Cholesterol’s affinity for some of the unsaturated PLs was taken from Williams et al. (18). Model of how cholesterol’s influence on lateral segregation is dependent on cholesterol relative affinity between the saturated and the unsaturated lipid (B). To see this figure in color, go online.
Insight into how cholesterol facilitates lateral segregation in lipid bilayers
As discussed above, cholesterol facilitated lo-domain formation more efficiently in PSM-containing bilayers than in DPPC bilayers, an effect that we associated with the higher affinity that cholesterol has for SM compared to PC (Fig. 3 and Lönnfors et al. (30)). Support for this model (Fig. 7 B) and more insight into the mechanism through which cholesterol facilitated lateral segregation was obtained from DSC and 2H NMR experiments.
In DSC experiments, the effect of low cholesterol concentrations (4 or 6 mol %) on the thermotropic domain behavior of binary PL bilayers was studied (Fig. 5). In the PSM-containing bilayers, the addition of cholesterol led to the appearance of new peaks in the thermograms at higher temperatures. This resembles the scenario observed in binary PSM/cholesterol systems, i.e., formation of more thermostable cholesterol-enriched domains (49). In the ternary system, the shift in melting temperature was larger when the unsaturated lipid had more double bonds, and in the PAPC/PSM/cholesterol bilayers the melting was close to that observed in binary PSM/cholesterol bilayers (49). This suggests that, upon cholesterol addition, the ordered domains became more enriched in PSM.
In DPPC-containing bilayers, the addition of cholesterol clearly lowered the enthalpy of the transitions in the recorded thermograms, but no new melting peaks at higher temperatures appeared in any of the investigated systems (Fig. 5). Similarly, It has been shown that low cholesterol concentrations do not result in domains with increased thermostability in binary DPPC/cholesterol bilayers (50). This may be due to differences in how cholesterol interacts with DPPC as compared to PSM. Interestingly, a low concentration of cholesterol seemed to have a larger effect on the less thermostable peak (probably POPC rich gel-domains) in the DPPC samples compared to the PSM samples (Fig. 5). This could be related to the rather similar affinity of cholesterol for DPPC and POPC (Fig. 3). Hence, the reason that no increase in thermostability of ordered domains was observed upon cholesterol addition to DPPC containing bilayers, could also be due to less cholesterol interactions with DPPC than with PSM. However, published data suggests that cholesterol partitions similarly between ld-and gel domains in ternary POPC-based systems, irrespective of whether the saturated lipid is a PC or a SM (4, 28).
Although our 2H NMR data only had two temperature points (23 and 28°C), the data suggested a higher thermostability of the lo-domains when the number of double bonds was increased in the unsaturated PLs (Fig. 6). This agrees with the DSC data (Fig. 5) and is supported by microscopy experiments (51). The difference in thermostability between PC and SM lo-domains (Fig. 6) that was observed in our study was supported in the literature by microscopy (2), fluorescence spectroscopy (52), and 2H NMR experiments (40).
The 2H NMR data (Fig. 6) showed that the order in lo-domains was higher in PSM-containing bilayers compared to DPPC-containing bilayers with all unsaturated lipids used. This fits well with phase diagrams and simulations of similar systems, which indicate that SM lo-domains are more enriched in saturated lipid and cholesterol than PC lo-domains (4, 28, 53). The 2H NMR data also showed that the lo-domains were more ordered, and the ld-domains less ordered, when more double bonds were present in the unsaturated lipids, suggesting a clearer separation of ordered and disordered domains with more double bonds in the unsaturated lipids. The Δν from deuterium-labeled PSM in bilayers with PAPC and DOPC approached those observed in binary PSM/cholesterol (1:1) bilayers (54). A larger difference in acyl chain order between the lo- and ld-domains could give rise to larger domain sizes (55), as has been observed in a comparison of PDPC/ESM/Chol and POPC/ESM/Chol bilayers (56). It is likely that a higher degree of order in the lo domains also allows the lo-domains to form at lower concentrations of the saturated lipid.
In bilayers composed of POPC or PLPC with DPPC and Chol, only a single set of Pake doublet was detected (Fig. S6), likely due to small domains and/or fast exchange of lipids between the ld- and lo-domains. This further emphasizes the difference in lo-domain formation capability of PC and SM, as PSM spectra showed two set of Pake doublets with all unsaturated lipids, and supports the results in published comparisons of PC and SM in ternary systems (2, 57).
The difference between cholesterol’s affinity for saturated SM and PC could be due in part to different hydrogen-bonding abilities (58) and different headgroup mobility of the PLs (59). Cholesterol also affects SM acyl chain order more than it affects saturated PC acyl chain order (30). Different organization of the lo-domains in PC and SM bilayers could also play a role, because cholesterol could be at different depth in PC and SM lo-phases (40). SM’s domain-forming properties, and domain thermostability, are more affected by the presence of polyunsaturated PLs in cholesterol-containing bilayers than a saturated PC. This could be one reason why biological membranes have saturated SMs instead of saturated PCs.
In the absence of laterally segregated domains, it is believed that lipids have a nonrandom distribution in the fluid phase (60). We speculate that certain lipids have a higher tendency to cluster together, which at a critical composition can induce lateral segregation. From sterol partitioning experiments, we know that cholesterol has different affinities for different PLs in the fluid phase (Fig. 3; Williams et al. (18) and Lönnfors et al. (30)). Cholesterol’s different affinity for different PLs could enhance clustering of certain lipids, and alter the lipid composition in the clusters. In turn, the changed composition may increase the stability of the clusters, thereby promoting lateral segregation, as seen especially in the bilayers containing PSM and polyunsaturated PLs. An enrichment in cholesterol and saturated lipid in the clusters, as suggested by the NMR and DSC data, would also increase the acyl chain order difference between ld- and lo-domains, which may facilitate lateral segregation as it is likely to increase the line tension between ld- and lo-domains.
Conclusions
In this project we compared how the addition of cholesterol to binary mixtures of an unsaturated and a saturated PL affected the lateral segregation in the bilayers. From the results it was clear that both the number of double bonds in the unsaturated lipid and the structural features of the saturated lipid affect how cholesterol facilitated lateral segregation. By correlating the affinity of cholesterol for the different lipids with the influence on lateral segregation, we could conclude that the relative affinity of cholesterol for an unsaturated and a saturated lipid, in the ternary lipid bilayers, determined the degree to which cholesterol facilitated lateral segregation. The DSC and 2H NMR results suggested that cholesterol-promoted lateral segregation was linked to the enrichment of the lo-domains in saturated PLs and cholesterol. Based on our results, the amount of polyunsaturated PLs in a biological membrane may determine whether sphingolipids and cholesterol form lateral domains. Our data suggests that knowledge of the affinity of cholesterol for the different PLs in a bilayer allows prediction of the degree to which the sterol promotes lo-domain formation and the physical properties of the lo-domains.
Author Contributions
T.K.M.N., O.E., M.M., and J.P.S. designed research; O.E., V.H., T.Y., T.K.M.N., and H.T. performed research; all authors analyzed the data; and O.E. and T.K.M.N. wrote the article, with contributions from all authors.
Acknowledgments
Financial support was generously provided by the Academy of Finland (to J.P.S.), the Sigrid Juselius Foundation (to J.P.S.), and the ISB doctoral network at Åbo Akademi (to O.E.). M.M. is grateful to the ERATO “Lipid Active Structure Project” from the Japan Science and Technology Agency (to J.P.S.) and to Grant-In-Aids for Scientific Research (A) (No. 25242073) from MEXT, Japan. T.Y. was partly supported by the “International Joint Research Promotion Program” at Osaka University.
Editor: Klaus Gawrisch.
Footnotes
Supporting Materials and Methods and six figures are available at http://www.biophysj.org/biophysj/supplemental/S0006-3495(16)30513-6.
Supporting Material
References
- 1.Lingwood D., Kaiser H.J., Simons K. Lipid rafts as functional heterogeneity in cell membranes. Biochem. Soc. Trans. 2009;37:955–960. doi: 10.1042/BST0370955. [DOI] [PubMed] [Google Scholar]
- 2.Veatch S.L., Keller S.L. Seeing spots: complex phase behavior in simple membranes. Biochim. Biophys. Acta. 2005;1746:172–185. doi: 10.1016/j.bbamcr.2005.06.010. [DOI] [PubMed] [Google Scholar]
- 3.Nyholm T.K., Lindroos D., Slotte J.P. Construction of a DOPC/PSM/cholesterol phase diagram based on the fluorescence properties of trans-parinaric acid. Langmuir. 2011;27:8339–8350. doi: 10.1021/la201427w. [DOI] [PubMed] [Google Scholar]
- 4.Konyakhina T.M., Wu J., Feigenson G.W. Phase diagram of a 4-component lipid mixture: DSPC/DOPC/POPC/Chol. Biochim. Biophys. Acta. 2013;1828:2204–2214. doi: 10.1016/j.bbamem.2013.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Marsh D. Cholesterol-induced fluid membrane domains: a compendium of lipid-raft ternary phase diagrams. Biochim. Biophys. Acta. 2009;1788:2114–2123. doi: 10.1016/j.bbamem.2009.08.004. [DOI] [PubMed] [Google Scholar]
- 6.Ipsen J.H., Karlström G., Zuckermann M.J. Phase equilibria in the phosphatidylcholine-cholesterol system. Biochim. Biophys. Acta. 1987;905:162–172. doi: 10.1016/0005-2736(87)90020-4. [DOI] [PubMed] [Google Scholar]
- 7.Quinn P.J., Wolf C. The liquid-ordered phase in membranes. Biochim. Biophys. Acta. 2009;1788:33–46. doi: 10.1016/j.bbamem.2008.08.005. [DOI] [PubMed] [Google Scholar]
- 8.van Dijck P.W.M. Negatively charged phospholipids and their position in the cholesterol affinity sequence. Biochim. Biophys. Acta. 1979;555:89–101. doi: 10.1016/0005-2736(79)90074-9. [DOI] [PubMed] [Google Scholar]
- 9.Gawrisch K., Soubias O. Structure and dynamics of polyunsaturated hydrocarbon chains in lipid bilayers-significance for GPCR function. Chem. Phys. Lipids. 2008;153:64–75. doi: 10.1016/j.chemphyslip.2008.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stillwell W., Wassall S.R. Docosahexaenoic acid: membrane properties of a unique fatty acid. Chem. Phys. Lipids. 2003;126:1–27. doi: 10.1016/s0009-3084(03)00101-4. [DOI] [PubMed] [Google Scholar]
- 11.Heberle F.A., Doktorova M., Feigenson G.W. Hybrid and nonhybrid lipids exert common effects on membrane raft size and morphology. J. Am. Chem. Soc. 2013;135:14932–14935. doi: 10.1021/ja407624c. [DOI] [PubMed] [Google Scholar]
- 12.van Meer G., Voelker D.R., Feigenson G.W. Membrane lipids: where they are and how they behave. Nat. Rev. Mol. Cell Biol. 2008;9:112–124. doi: 10.1038/nrm2330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Feller S.E. Acyl chain conformations in phospholipid bilayers: a comparative study of docosahexaenoic acid and saturated fatty acids. Chem. Phys. Lipids. 2008;153:76–80. doi: 10.1016/j.chemphyslip.2008.02.013. [DOI] [PubMed] [Google Scholar]
- 14.Shaikh S.R., Cherezov V., Wassall S.R. Molecular organization of cholesterol in unsaturated phosphatidylethanolamines: x-ray diffraction and solid state 2H NMR reveal differences with phosphatidylcholines. J. Am. Chem. Soc. 2006;128:5375–5383. doi: 10.1021/ja057949b. [DOI] [PubMed] [Google Scholar]
- 15.Wassall S.R., Stillwell W. Polyunsaturated fatty acid-cholesterol interactions: domain formation in membranes. Biochim. Biophys. Acta. 2009;1788:24–32. doi: 10.1016/j.bbamem.2008.10.011. [DOI] [PubMed] [Google Scholar]
- 16.Kullberg A., Ekholm O.O., Slotte J.P. Miscibility of sphingomyelins and phosphatidylcholines in unsaturated phosphatidylcholine bilayers. Biophys. J. 2015;109:1907–1916. doi: 10.1016/j.bpj.2015.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sklar L.A., Hudson B.S., Simoni R.D. Conjugated polyene fatty acids as fluorescent probes: synthetic phospholipid membrane studies. Biochemistry. 1977;16:819–828. doi: 10.1021/bi00624a002. [DOI] [PubMed] [Google Scholar]
- 18.Williams J.A., Wassall C.D., Wassall S.R. An electron paramagnetic resonance method for measuring the affinity of a spin-labeled analog of cholesterol for phospholipids. J. Membr. Biol. 2013;246:689–696. doi: 10.1007/s00232-013-9586-z. [DOI] [PubMed] [Google Scholar]
- 19.Halling K.K., Ramstedt B., Nyholm T.K. Cholesterol interactions with fluid-phase phospholipids: effect on the lateral organization of the bilayer. Biophys. J. 2008;95:3861–3871. doi: 10.1529/biophysj.108.133744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mason J.T., Broccoli A.V., Huang C. A method for the synthesis of isomerically pure saturated mixed-chain phosphatidylcholines. Anal. Biochem. 1981;113:96–101. doi: 10.1016/0003-2697(81)90049-x. [DOI] [PubMed] [Google Scholar]
- 21.Rouser G., Fleischer S., Yamamoto A. Two-dimensional thin layer chromatographic separation of polar lipids and determination of phospholipids by phosphorus analysis of spots. Lipids. 1970;5:494–496. doi: 10.1007/BF02531316. [DOI] [PubMed] [Google Scholar]
- 22.Jungner M., Ohvo H., Slotte J.P. Interfacial regulation of bacterial sphingomyelinase activity. Biochim. Biophys. Acta. 1997;1344:230–240. doi: 10.1016/s0005-2760(96)00147-6. [DOI] [PubMed] [Google Scholar]
- 23.Fischer R.T., Stephenson F.A., Schroeder F. δ5,7,9(11)-Cholestatrien-3 β-ol: a fluorescent cholesterol analogue. Chem. Phys. Lipids. 1984;36:1–14. doi: 10.1016/0009-3084(84)90086-0. [DOI] [PubMed] [Google Scholar]
- 24.Kuklev D.V., Smith W.L. Synthesis of four isomers of parinaric acid. Chem. Phys. Lipids. 2004;131:215–222. doi: 10.1016/j.chemphyslip.2004.06.001. [DOI] [PubMed] [Google Scholar]
- 25.Lakowicz J.R. Kluwer Academic/Plenum Publishers; New York: 1999. Principles of Fluorescence Spectroscopy. [Google Scholar]
- 26.Nyström J.H., Lönnfors M., Nyholm T.K. Transmembrane peptides influence the affinity of sterols for phospholipid bilayers. Biophys. J. 2010;99:526–533. doi: 10.1016/j.bpj.2010.04.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Björkqvist Y.J., Nybond S., Ramstedt B. N-palmitoyl-sulfatide participates in lateral domain formation in complex lipid bilayers. Biochim. Biophys. Acta. 2008;1778:954–962. doi: 10.1016/j.bbamem.2007.12.016. [DOI] [PubMed] [Google Scholar]
- 28.Petruzielo R.S., Heberle F.A., Feigenson G.W. Phase behavior and domain size in sphingomyelin-containing lipid bilayers. Biochim. Biophys. Acta. 2013;1828:1302–1313. doi: 10.1016/j.bbamem.2013.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ramstedt B., Slotte J.P. Membrane properties of sphingomyelins. FEBS Lett. 2002;531:33–37. doi: 10.1016/s0014-5793(02)03406-3. [DOI] [PubMed] [Google Scholar]
- 30.Lönnfors M., Doux J.P., Slotte J.P. Sterols have higher affinity for sphingomyelin than for phosphatidylcholine bilayers even at equal acyl-chain order. Biophys. J. 2011;100:2633–2641. doi: 10.1016/j.bpj.2011.03.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Scheidt H.A., Muller P., Huster D. The potential of fluorescent and spin-labeled steroid analogs to mimic natural cholesterol. J. Biol. Chem. 2003;278:45563–45569. doi: 10.1074/jbc.M303567200. [DOI] [PubMed] [Google Scholar]
- 32.Milles S., Meyer T., Müller P. Organization of fluorescent cholesterol analogs in lipid bilayers—lessons from cyclodextrin extraction. Biochim. Biophys. Acta. 2013;1828:1822–1828. doi: 10.1016/j.bbamem.2013.04.002. [DOI] [PubMed] [Google Scholar]
- 33.Niu S.L., Litman B.J. Determination of membrane cholesterol partition coefficient using a lipid vesicle-cyclodextrin binary system: effect of phospholipid acyl chain unsaturation and headgroup composition. Biophys. J. 2002;83:3408–3415. doi: 10.1016/S0006-3495(02)75340-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tsamaloukas A., Szadkowska H., Heerklotz H. Thermodynamic comparison of the interactions of cholesterol with unsaturated phospholipid and sphingomyelins. Biophys. J. 2006;90:4479–4487. doi: 10.1529/biophysj.105.080127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tsamaloukas A., Szadkowska H., Heerklotz H. Interactions of cholesterol with lipid membranes and cyclodextrin characterized by calorimetry. Biophys. J. 2005;89:1109–1119. doi: 10.1529/biophysj.105.061846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Estep T.N., Mountcastle D.B., Thompson T.E. Thermal behavior of synthetic sphingomyelin-cholesterol dispersions. Biochemistry. 1979;18:2112–2117. doi: 10.1021/bi00577a042. [DOI] [PubMed] [Google Scholar]
- 37.Yasuda T., Tsuchikawa H., Matsumori N. Deuterium NMR of raft model membranes reveals domain-specific order profiles and compositional distribution. Biophys. J. 2015;108:2502–2506. doi: 10.1016/j.bpj.2015.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bartels T., Lankalapalli R.S., Brown M.F. Raftlike mixtures of sphingomyelin and cholesterol investigated by solid-state 2H NMR spectroscopy. J. Am. Chem. Soc. 2008;130:14521–14532. doi: 10.1021/ja801789t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Davis J.H., Clair J.J., Juhasz J. Phase equilibria in DOPC/DPPC-d62/cholesterol mixtures. Biophys. J. 2009;96:521–539. doi: 10.1016/j.bpj.2008.09.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yasuda T., Kinoshita M., Matsumori N. Detailed comparison of deuterium quadrupole profiles between sphingomyelin and phosphatidylcholine bilayers. Biophys. J. 2014;106:631–638. doi: 10.1016/j.bpj.2013.12.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Darke A., Finer E.G., Phillips M.C. Nuclear magnetic resonance study of lecithin-cholesterol interactions. J. Mol. Biol. 1972;63:265–279. doi: 10.1016/0022-2836(72)90374-9. [DOI] [PubMed] [Google Scholar]
- 42.Engberg O., Nurmi H., Slotte J.P. Effects of cholesterol and saturated sphingolipids on acyl chain order in 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphocholine bilayers—a comparative study with phase-selective fluorophores. Langmuir. 2015;31:4255–4263. doi: 10.1021/acs.langmuir.5b00403. [DOI] [PubMed] [Google Scholar]
- 43.Bezlyepkina N., Gracià R.S., Dimova R. Phase diagram and tie-line determination for the ternary mixture DOPC/eSM/cholesterol. Biophys. J. 2013;104:1456–1464. doi: 10.1016/j.bpj.2013.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ahmed S.N., Brown D.A., London E. On the origin of sphingolipid/cholesterol-rich detergent-insoluble cell membranes: physiological concentrations of cholesterol and sphingolipid induce formation of a detergent-insoluble, liquid-ordered lipid phase in model membranes. Biochemistry. 1997;36:10944–10953. doi: 10.1021/bi971167g. [DOI] [PubMed] [Google Scholar]
- 45.Filippov A., Orädd G., Lindblom G. Domain formation in model membranes studied by pulsed-field gradient-NMR: the role of lipid polyunsaturation. Biophys. J. 2007;93:3182–3190. doi: 10.1529/biophysj.107.111534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Konyakhina T.M., Feigenson G.W. Phase diagram of a polyunsaturated lipid mixture: brain sphingomyelin/1-stearoyl-2-docosahexaenoyl-sn-glycero-3-phosphocholine/cholesterol. Biochim. Biophys. Acta. 2016;1858:153–161. doi: 10.1016/j.bbamem.2015.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lindblom G., Orädd G., Filippov A. Lipid lateral diffusion in bilayers with phosphatidylcholine, sphingomyelin and cholesterol. An NMR study of dynamics and lateral phase separation. Chem. Phys. Lipids. 2006;141:179–184. doi: 10.1016/j.chemphyslip.2006.02.011. [DOI] [PubMed] [Google Scholar]
- 48.Brzustowicz M.R., Cherezov V., Wassall S.R. Molecular organization of cholesterol in polyunsaturated membranes: microdomain formation. Biophys. J. 2002;82:285–298. doi: 10.1016/S0006-3495(02)75394-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Nyholm T.K.M., Nylund M., Slotte J.P. A calorimetric study of binary mixtures of dihydrosphingomyelin and sterols, sphingomyelin, or phosphatidylcholine. Biophys. J. 2003;84:3138–3146. doi: 10.1016/s0006-3495(03)70038-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.McMullen T.P., Lewis R.N., McElhaney R.N. Differential scanning calorimetric study of the effect of cholesterol on the thermotropic phase behavior of a homologous series of linear saturated phosphatidylcholines. Biochemistry. 1993;32:516–522. doi: 10.1021/bi00053a016. [DOI] [PubMed] [Google Scholar]
- 51.Staneva G., Seigneuret M., Angelova M.I. Detergents induce raft-like domains budding and fission from giant unilamellar heterogeneous vesicles: a direct microscopy observation. Chem. Phys. Lipids. 2005;136:55–66. doi: 10.1016/j.chemphyslip.2005.03.007. [DOI] [PubMed] [Google Scholar]
- 52.Yasuda T., Matsumori N., Murata M. Formation of gel-like nanodomains in cholesterol-containing sphingomyelin or phosphatidylcholine binary membrane as examined by fluorescence lifetimes and 2H NMR spectra. Langmuir. 2015;31:13783–13792. doi: 10.1021/acs.langmuir.5b03566. [DOI] [PubMed] [Google Scholar]
- 53.Sodt A.J., Pastor R.W., Lyman E. Hexagonal substructure and hydrogen bonding in liquid-ordered phases containing palmitoyl sphingomyelin. Biophys. J. 2015;109:948–955. doi: 10.1016/j.bpj.2015.07.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Engberg O., Yasuda T., Slotte J.P. Lipid interactions and organization in complex bilayer membranes. Biophys. J. 2016;110:1563–1573. doi: 10.1016/j.bpj.2015.12.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Heberle F.A., Petruzielo R.S., Katsaras J. Bilayer thickness mismatch controls domain size in model membranes. J. Am. Chem. Soc. 2013;135:6853–6859. doi: 10.1021/ja3113615. [DOI] [PubMed] [Google Scholar]
- 56.Georgieva R., Chachaty C., Staneva G. Docosahexaenoic acid promotes micron scale liquid-ordered domains. A comparison study of docosahexaenoic versus oleic acid containing phosphatidylcholine in raft-like mixtures. Biochim. Biophys. Acta. 2015;1848:1424–1435. doi: 10.1016/j.bbamem.2015.02.027. [DOI] [PubMed] [Google Scholar]
- 57.van Duyl B.Y., Ganchev D., Killian J.A. Sphingomyelin is much more effective than saturated phosphatidylcholine in excluding unsaturated phosphatidylcholine from domains formed with cholesterol. FEBS Lett. 2003;547:101–106. doi: 10.1016/s0014-5793(03)00678-1. [DOI] [PubMed] [Google Scholar]
- 58.Slotte J.P. The importance of hydrogen bonding in sphingomyelin’s membrane interactions with co-lipids. Biochim. Biophys. Acta. 2016;1858:304–310. doi: 10.1016/j.bbamem.2015.12.008. [DOI] [PubMed] [Google Scholar]
- 59.Siminovitch D.J., Jeffrey K.R. Orientational order in the choline headgroup of sphingomyelin: a 14N-NMR study. Biochim. Biophys. Acta. 1981;645:270–278. doi: 10.1016/0005-2736(81)90198-x. [DOI] [PubMed] [Google Scholar]
- 60.Ackerman D.G., Feigenson G.W. Lipid bilayers: clusters, domains and phases. Essays Biochem. 2015;57:33–42. doi: 10.1042/bse0570033. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.