Abstract
Background
Enteric fevers contribute majorly to the burden of morbidity from infectious diseases in the developing world. Due to growing antibiotic resistance seen in their management, Salmonella and its various species are required to be periodically tested for sensitivity and resistance patterns, to guide the clinical management at the local level. This will also enable planning of antibiotic recycling wherever feasible.
Methods
A retrospective study of the results of blood culture isolates covering a period of 27 months was done at a tertiary care hospital. Blood samples were directly inoculated in Bactalert culture bottles and sub culture was done on Mac Conkey and Salmonella–Shigella Agar. Non-lactose fermenting colonies were processed for identification, antibiotic sensitivity and MIC value. Slide agglutination test using specific antisera was also done to confirm the serotype. Antimicrobial susceptibility was done in accordance with CLSI standards.
Results
8413 blood samples were processed and 1027 (12.20%) were assessed as ‘culture positive’. Salmonella were isolated in 46 samples of which 38 (83%) were Salmonella typhi and a single isolate was Salmonella paratyphi B.
S.typhi showed maximum sensitivity to imipenem (100%) (MIC <0.25 μg/ml) followed by ciprofloxacin (76.8%) (MIC >1 μg/ml) and nalidixic acid (50%) (MIC ≥ 32 μg/ml). S. paratyphi B showed 100% sensitivity to all the common antibiotics. Four samples (8%) were classified as multi drug resistant (MDR).
Conclusion
Our study has shown improved sensitivity to ceftriaxone and cotrimoxazole. A high degree of susceptibility to ampicillin among both S. typhi and S.paratyphi A is encouraging. However, low susceptibility to nalidixic acid and ciprofloxacin is a cause for concern. There is a need for further clinical studies to evaluate the response to chloramphenicol in MDR cases and to formulate uniform laboratory guidelines to test antibiotic sensitivity of S. typhi isolates.
Keywords: Enteric fever, Salmonella typhi, Antimicrobial resistance
Introduction
Enteric fever has been an important cause of human morbidity and mortality in developing nations like India. This acute systemic infection is caused mainly by Salmonella enteric serotype typhi and paratyphi A, rarely by Salmonellaparatyphi B and C.1 In the Delhi region, the incidence of enteric fever has been reported to be 9.8 cases per 1000 person years.2
The classical presentation of the disease includes malaise, fever, diffuse abdominal pain, occasional loose stool and constipation. In the past, chloramphenicol, ampicillin and cotrimoxazole had been the drugs of choice for management of enteric fevers. But slowly, strains resistant to all three first-line drugs have emerged. Further, continued use of ciprofloxacin for empirical treatment of typhoid has led to the emergence of ciprofloxacin resistant Salmonella. Emergence of multi drug resistant (MDR) Salmonella has become a cause of great concern to clinicians. All species of Salmonella are now developing multidrug resistance and complicating the management.3
Infection caused by Salmonella at tertiary care centres is not very common. It is difficult to isolate Salmonella in patients who are on potent antibiotic therapy. Since there are significant variations in the sensitivity patterns reported for Salmonellatyphi and S.Paratyphi A, it becomes clinically important to monitor the antibiotic sensitivity patterns to provide suitable guidance for treatment.
We analysed the antibiogram of isolates obtained from blood culture as recent studies from different regions have shown a shifting pattern of susceptibility to conventional drugs.
Material and methods
A retrospective study was done of the laboratory records and linked relevant data, maintained over a period from January 2012 to March 2014 in a tertiary care hospital. Blood samples were obtained from febrile patients of different clinical wards using aseptic means. Samples were directly inoculated in Bactalert culture bottles. Later on, sub culture was done on Mac Conkey and Salmonella–Shigella Agar and incubated at 37 °C for 18–24 h and in negative cases, subcultures were repeated for one week. Subsequently, non-lactose fermenting colonies were processed for identification, antibiotic sensitivity and MIC value with the help of Vitek2 (Biomerieux System). Slide agglutination test using specific antisera was also done to confirm the serotype. Antimicrobial susceptibility testing was also done in accordance with Clinical and Laboratory Standards Institute (CLSI). Antibiotics discs included in this study, by Kirby–Bauer's disc diffusion method, were ampicillin (10 μg), ceftriaxone (30 μg), nalidixic acid (30 μg), ciprofloxacin (5 μg), cotrimoxazole (25 μg) and imipenem (30 μg) (Hi-Media Laboratories). However Vitek2 gave results of nearly all relevant antibiotics. We did not check the drug resistance to chloramphenicol as the drug was not being procured for clinical use during the study period. Phase typing was not carried out and no correlation with Widal test was done.
Results
A total of 8413 blood samples were processed and 1027 (12.20%) were assessed as ‘culture positive’. Staphylococcus aureus was the commonest isolate followed by coagulase negative Staphylococcus, E.coli and other bacteria (Table 1). Salmonella were isolated in samples from 46 patients, provisionally diagnosed as pyrexia of unknown origin (PUO). Out of these 46 patients, 26 were children (<16 years); and the balance were adults. Of the Salmonella isolates, 38 were S. typhi (83%), seven were S. paratyphi A (15%) and a single isolate was S. paratyphi B. No S. paratyphi C was isolated during the study period.
Table 1.
Bacterial isolates (n = 1027).
| Bacterial isolates | Positive samples |
|---|---|
| Staphylococcus aureus | 340 (33.10%) |
| CONS | 229 (22.29%) |
| E. coli | 124 (12.07%) |
| Acinetobacter | 109 (10.61%) |
| Klebsiella | 79 (7.69%) |
| Pseudomonas | 66 (6.42%) |
| Salmonella | 46 (4.47%) |
| Proteus | 26 (2.53%) |
| Candida | 8 (0.77%) |
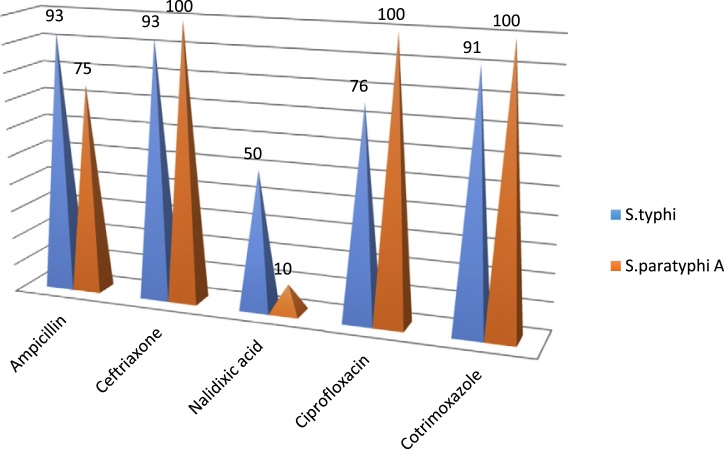
Salmonella typhi showed maximum sensitivity to imipenem (100%) (MIC < 0.25 μg/ml) followed by ciprofloxacin (76.8%) (MIC > 1.0 μg/ml) and nalidixic acid (50%) (MIC > 32 μg/ml). Salmonella paratyphi B showed 100% sensitivity to all the commonly used antibiotics. Four samples (8%) were noted to be having multidrug resistant (i.e. resistant to ampicillin, ceftriaxone, ciprofloxacin and cotrimoxazole). All the strains were resistant to nalidixic acid (MIC > 32 μg/ml) and to ciprofloxacin (MIC > 4 μg/ml). Antibiogram of common antibiotics for all the three strains are depicted in Table 2. Salmonella typhi A showed poorer sensitivity to ampicillin (75%) and nalidixic acid (10%) as compared to S. typhi. As we had only one isolate of S. paratyphi B, hence not much conclusion can be drawn from these results (Fig.)
Fig.
Sensitivity to antibiotics (Percentage of isolates).
.
Table 2.
Salmonella isolates sensitive to various antibiotics.
| Antibiotics | Present study (2012–14) | Lynch et al7 (2009) | Krishan et al8 (2009) | Nagshetty et al5 (2010) | Chaudhary et al9 (2013) |
|---|---|---|---|---|---|
| Ampicillin | 42/46 (91.30%) | 1744/2016 (86.50%) | 42/50 (84%) | 67/95 (70.53%) | 290/322 (90.68%) |
| Ceftriaxone | 43/46 (93.47%) | – | 50/50 (100%) | 89/95 (93.69%) | 322/322 (100%) |
| Nalidixic acid | 23/46 (50%) | 1258/2016 (62.40%) | – | 65/95 (68.42%) | 26/322 (8.07%) |
| Ciprofloxacin | 35/46 (76.80%) | 1282/2016 (63.59%) | 17/21 (81%) | 91/95 (95.78%) | 175/322 (54.34%) |
| Chloramphenicol | – | 1744/2016 (86.50%) | 29/50 (58%) | 68/95 (71.57%) | 322/322 (100%) |
| Cotrimoxazole | 42/46 (91.30%) | 1744/2016 (86.50%) | 44/50 (88%) | 78/95 (82.10%) | 306/322 (95.03%) |
| Imipenem | 46/46 (100%) | – | – | 95/95 (100%) | – |
Discussion
Enteric fever, though a major public health problem in India today, usually doesn't present itself as a febrile illness at tertiary care hospitals and is mostly managed at peripheral health care facilities. Most of the cases in our present study were transferred in for management from smaller hospitals located in and around the catchment areas. Not many cases of typhoid are reported in our clientele population as they are residing in garrisons with relatively better hygiene and sanitation conditions, safe water supply systems, and better immunization coverage as recommended in the Armed Forces immunization programme.
Our study has only 46 cases of typhoid fever (0.5%) out of 8413 blood samples processed during the study period, as compared to similar studies where the overall positivity varied between 5% and 9%.2, 4, 5
Drug resistance in typhoid fever is considered one of the important factors in the morbidity and mortality from the disease.6 Ampicillin, chloramphenicol and ciprofloxacin have been the main drugs used for treatment. There has been a wide variation in sensitivity to ampicillin (MIC < 2 μg/ml). In our study, sensitivity was observed to be around 91%. Half of the strains showed sensitivity to nalidixic acid (MIC > 32 μg/ml). This resistance is much higher as compared to a population based study in the USA where 38% isolated strains were resistant to nalidixic acid (nalidixic acid-resistant S. typhi [NARST]).7 Their analysis revealed that most of the patients, who had travelled to the Indian subcontinent, were infected with 85% MDRST and 94% NARST strains.7 It indicates that drug resistant strains are widely distributed in India.5, 8, 9 Nalidixic acid resistance may be an indicator of treatment failure to ciprofloxacin, hence both these drugs should be simultaneously tested for sensitivity. The antimicrobial susceptibility of Salmonella enterica serovars typhi and paratyphi A and B do vary in different populations (Table 2).
Our study has shown 93.47% sensitivity to ceftriaxone (MIC < 1.0 μg/ml) and 91.30% sensitivity to cotrimoxazole (MIC < 20.0 μg/ml) and is comparable with other studies.5, 8, 9 A high degree (>90%) of susceptibility to ampicillin (MIC < 2.0 μg/ml) and ceftriaxone (MIC < 1.0 μg/ml) among both S. typhi and S.paratyphi A is encouraging. However, low susceptibility to nalidixic (50%; MIC > 32 μg/ml) and ciprofloxacin (76.8%; MIC > 1.0 μg/ml) is cause for concern. Hence ciprofloxacin can no longer be considered to be the keystone for treatment. In a study in Orissa, prevalence of MDR Salmonella was found to be 12.75%.6 Nearly 8% of our isolates were multidrug resistant, however this was lower than other similar studies.5, 8
Conclusion
Typhoid fever remains a significant health burden, especially in low- and middle-income countries.10 Our study highlights the changing antibiotic sensitivity pattern of Salmonella and brings out the presence of MDR microorganisms in a tertiary care hospital. We report ampicillin and cotrimoxazole sensitive Salmonella strains, but rather higher resistance to nalidixic acid and ciprofloxacin. There is a need for further clinical studies to evaluate the response to chloramphenicol in such MDR cases. Our antimicrobial susceptibility data and MIC distribution too, suggests that ampicillin and cotrimoxazole may once again be useful for the treatment of enteric fever in the future.9 Third generation cephalosporins are valuable but their use should be restricted to complicated cases. It is necessary to do blood culture and antibiotic susceptibility testing on patients with suspected typhoid fever. This will help in preventing the spread of antibiotic –resistant S. typhi and development of MDR strains. There is also a need to have uniform laboratory testing guidelines for testing susceptibility to ciprofloxacin and azithromycin for S. typhi isolates.11
Conflicts of interest
The authors have none to declare.
References
- 1.Singla N., Bansal N., Gupta V., Chander J. Outbreak of Salmonella typhi enteric fever in sub-urban area of North India: a public health perspective. Asian Pac J Trop Med. 2013:167–168. doi: 10.1016/S1995-7645(13)60017-6. [DOI] [PubMed] [Google Scholar]
- 2.Sinha A., Sazawal S., Kumar R. Typhoid fever in children aged less than 5 years. Lancet. 1999;354:734–737. doi: 10.1016/S0140-6736(98)09001-1. [DOI] [PubMed] [Google Scholar]
- 3.Chandel D.S., Chaudhary R., Dhawan B., Pandey A., Dey A.B. Drug resistant Salmonella enterica serotype paratyphi A in India. Emerg Infect Dis. 2006;6:420–421. doi: 10.3201/eid0604.000420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bhattacharya S.S., Das U., Chaudhury B.K. Occurrence & antibiogram of Salmonella typhi & S. paratyphi a isolated from Rourkela, Orissa. Indian J Med Res. 2011;133:431–433. [PMC free article] [PubMed] [Google Scholar]
- 5.Nagshetty K., Channappa S.T., Gaddad S.M. Antimicrobial susceptibility of Salmonella typhi in India. J Infect Dev Ctries. 2010;4:70–73. doi: 10.3855/jidc.109. [DOI] [PubMed] [Google Scholar]
- 6.Chowta M.N., Chowta N.K. Study of clinical profile and antibiotic response in typhoid fever. Indian J Med Microbiol. 2005 Apr;23:125–127. doi: 10.4103/0255-0857.16054. [DOI] [PubMed] [Google Scholar]
- 7.Lynch M.F., Blanto E.M., Bulens S. Typhoid fever in the United States, 1990–2006. JAMA. 2009;302:859–865. doi: 10.1001/jama.2009.1229. [DOI] [PubMed] [Google Scholar]
- 8.Krishan P., Stalin M., Balasubramanian S. Changing trends in antimicrobial resistance of Salmonella enterica serovar typhi and Salmonella enterica serovar paratyphi A in Chennai. Indian J Pathol Microbiol. 2009;52:505–508. doi: 10.4103/0377-4929.56140. [DOI] [PubMed] [Google Scholar]
- 9.Choudhary A., Gopalkrishnan R., Nambi P.S., Ramasubramanian V., Ghafur K.A., Thirunarayan M.A. Antimicrobial susceptibility of Salmonella enterica serovars in a tertiary care hospital in southern India. Indian J Med Res. 2013;137:800–802. [PMC free article] [PubMed] [Google Scholar]
- 10.Buckle G.C., Fischer Walker C.L., Black R.E. Typhoid fever and paratyphoid fever: systematic review to estimate global morbidity and mortality for 2010. J Glob Health. 2012;2:010401. doi: 10.7189/jogh.02.010401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Srirangaraj S., Kali A., Charles M.V. A study of antibiogram of Salmonella enterica serovar typhi isolates from Pondicherry, India. Australas Med J. 2014 Apr 30;7:185–190. doi: 10.4066/AMJ.2014.2010. e Collection 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]