Abstract
Viral hepatitis is a cause for major health care burden in India and is now equated as a threat comparable to the “big three” communicable diseases – HIV/AIDS, malaria and tuberculosis. Hepatitis A virus and Hepatitis E virus are predominantly enterically transmitted pathogens and are responsible to cause both sporadic infections and epidemics of acute viral hepatitis. Hepatitis B virus and Hepatitis C virus are predominantly spread via parenteral route and are notorious to cause chronic hepatitis which can lead to grave complications including cirrhosis of liver and hepatocellular carcinoma. Around 400 million people all over the world suffer from chronic hepatitis and the Asia-Pacific region constitutes the epicentre of this epidemic. The present article would aim to cover the basic virologic aspects of these viruses and highlight the present scenario of viral hepatitis in India.
Keywords: Viral hepatitis, Indian scenario, Health care burden
Introduction
Viral hepatitis is a cause for major health care burden in India and is now equated as a threat comparable to the “big three” communicable diseases – HIV/AIDS, malaria and tuberculosis.1 Hepatitis A virus (HAV) and Hepatitis E virus (HEV) are predominantly enterically transmitted pathogens and are responsible to cause both sporadic infections and epidemics of acute viral hepatitis (AVH). Hepatitis B virus (HBV) and Hepatitis C virus (HCV) are predominantly spread via parenteral route and are notorious to cause chronic hepatitis, which can lead to grave complications including cirrhosis of liver and hepatocellular carcinoma (HCC). Around 400 million people all over the world suffer from chronic hepatitis and the Asia-Pacific region constitutes the epicentre of this epidemic.1 The present article would aim to cover the basic virologic aspects of these viruses and highlight the present scenario of viral hepatitis in India.
HAV
HAV is a single-stranded RNA virus belonging to the family Picornaviridae. It is spread via the faecal–oral route and is closely associated with poor sanitary and bad hygienic conditions. HAV infection is common during childhood in developing countries like India and usually results in mild anicteric hepatitis. Majority of children (85%) below the age of 2 years and around 50% aged between 2 and 5 years have non-specific symptoms and are usually anicteric.2 However, HAV infection was reported to cause severe disease with increasing age of the patient and with the presence of underlying chronic liver disease.3, 4 The case fatality rate has been mentioned to be the highest in patients over the age of 50 years (1.8%) when compared to younger adults (0.3%).3
Scenario in India
HAV is responsible for several outbreaks of sporadic viral hepatitis in India. In a recent study by Rakesh et al. from Kerala, HAV was identified as the aetiology for an outbreak of AVH at Mylopore village of Kollam district. The authors noted a high attack rate in subjects aged 15–24 years (4.6%) when compared to subjects aged 5–14 years (3.1%). Pipe water contamination from a borewell was identified as a source of infection in this village.5 In an elegant study by Acharya et al., the seroprevalence of anti-HAV antibodies was assessed in 1424 school children aged between 4 and 18 years and 93.2% of children were found to have anti-HAV antibodies in their sera. The seroprevalence of anti-HAV was also assessed in 256 patients with underlying chronic liver disease and 97.6% tested positive indicating the high seroprevalence of HAV in India.6 In another study from Kottayam, HAV was deemed responsible for an outbreak of AVH in the medical college area and the authors emphasized on the need for a definite policy for control of viral hepatitis.7 In a study from Lucknow, 267 patients with AVH were evaluated and HAV was identified as the most common aetiology responsible in 26.96% patients followed HEV in 17.97%.8
However, in the recent times there has been a sero-epidemiological shift in HAV infection in India, with increasing incidence of infection being noted in the adult and adolescent population compared with children. Arankalle et al. in their study on 928 children aged between 18 months and 10 years attempted to estimate the age-related seroprevalence of HAV. Of the 348 children who tested positive for anti-HAV, 50.3% were in the age group of 6–10 years and 30.3% were in the 18 months to 6 years age group. It was also noted that the seropositivity of HAV was closely linked to the educational and socioeconomic status of the parents. Subjects who used a private toilet within the house were less often seropositive (33.1%) when compared to subjects who used an open field for excreta disposal (75%). This study clearly demonstrated the higher seroprevalence of HAV in older children and established a clear link between improved living standard and decreased seropositivity of HAV.9
Prevention of HAV infection
Improving the sanitary conditions and provision of safe, clean drinking water forms an imperative pillar in curtailing the spread of the HAV. Simple methods like maintaining proper hand hygiene is an effective method to curtail the virus. Vaccination forms a cornerstone in preventing HAV and both inactivated and live attenuated vaccines are available for use. The inactivated vaccine is derived from the HM 175/GBM strain whereas the live attenuated vaccine is derived from the H2 strain of the virus.
Vaccination in India
Indian Academy of Pediatrics recommends two doses for any of the licensed vaccines which have to be given 6 months apart to children aged 1 year or older. The dose recommended is 720 ELU for those aged <19 years and 1440 ELU for those above. Protective titres of antibodies are seen in almost 100% after the second dose of injection.2 Adverse reactions are minor and usually include local pain and swelling.
HEV
HEV is positive-stranded RNA virus belonging to the family hepeviridae. HEV has 4 genotypes of which genotypes 1 and 2 exclusively infect humans whereas genotypes 3 and 4 also infect several other mammalian species. HEV is primarily spread via the faecal–oral route and is an enterically transmitted pathogen like HAV. The incubation period of HEV infection is estimated to be around 2–9 weeks and during an epidemic of HEV, anicteric hepatitis is more common than icteric hepatitis and clinical hepatitis is seemingly more frequent in adults than in children aged <15 years.10 In addition to the classical routes described, HEV is also reported to spread by blood transfusion and via allograft.11, 12 HEV infection can also cause, albeit rarely, a chronic hepatitis which occurs when HEV replication persists for at least 6 months. Chronic HEV infection is classically described with HEV genotype 3 and can lead to cirrhosis in immunosuppressed patients and in patients undergoing a solid organ transplantation.13
Scenario in India
HEV is responsible for majority of the sporadic and epidemic cases of AVH in India.14, 15 The major epidemics caused by HEV in India are highlighted in Table 1.10 During an HEV epidemic, the secondary attack rate among the household contacts is estimated to be about 0.7–2% when compared to 50–75% for HAV.16 During an outbreak, it is observed that pregnant women have a higher likelihood to get infected (12–20%) with HEV and have a higher propensity to develop acute liver failure (ALF) (10–22%) when compared to non-pregnant females and males (1–2%).10 However, once ALF develops, it is noted that the pregnancy per se does not alter the mortality when compared to non-pregnant females and males.17
Table 1.
Major HEV epidemics in India.
| Location | Year | Number of infected subjects |
|---|---|---|
| Delhi | 1955 | 29,300 |
| Aurangabad | 1961 | 865 |
| Siliguri | 1966 | 4287 |
| Ahmedabad | 1975 | 2572 |
| Kanpur | 1990 | 79,091 |
| Nellore | 2008 | 23,915 |
In a study from Rajasthan, HEV infection among acute sporadic hepatitis was studied. 736 patients suspected to have viral hepatitis were evaluated and HEV was noted to be the predominant pathogen responsible in 49.7% of patients.18 Khuroo et al. studied the spectrum of HEV infection in India and noted that 92% (23/25) of patients with epidemic non-A, non-B hepatitis had acute HEV infection. Cholestasis was documented to be a predominant symptom in 25% and all patients recovered from the disease.19 HEV is a major aetiology for AVH in paediatric population and is reported to be responsible for over 70% of cases of acute hepatitis.20
HEV causes severe liver disease in individuals with underlying chronic liver disease, an entity recently termed as acute-on-chronic liver failure (ACLF). Several studies from India have depicted the prevalence of HEV-related ACLF as shown in Table 2.21 However, in a recent study by Shalimar et al. from New Delhi, HEV-related ACLF has been shown to have lower mortality when compared to ACLF due to other aetiologies (12.8% vs. 33–54%).22
Table 2.
HEV-related ACLF – data from India.
| Authors | Place of study | Year | Number (%) of cases of HEV related ACLF out of all ACLF |
|---|---|---|---|
| Duseja et al. | Chandigarh | 2010 | 4/102 (4%) |
| Garg et al. | Delhi | 2012 | 14/91 (15%) |
| Jagadisan et al. | Lucknow | 2012 | 23/36 (64%) |
| Jha et al. | Jaipur | 2013 | 5/52 (10%) |
| Shalimar et al. | Delhi | 2016 | 39/213 (18.3%) |
Prevention of HEV infection
Just like preventing HAV infection, improving the living standards and providing better sanitary conditions help in curtailing the spread of HEV in the community. Adequate sewage disposal and maintaining adequate personal hygiene are imperative to check the spread of the virus.
HEV vaccine
Different types of HEV vaccines are being developed resulting in recombinant protein vaccines, DNA vaccines or recombinant HEV virus like particles (rHEV-VLPs). Studies have evaluated the recombinant Hepatitis E virus open reading frame 2 (HEV ORF2) as the candidate vaccine and a study from Nepal including 2000 volunteers demonstrated an impressive vaccine efficacy rate of 95%. The dose of vaccine used was 20 μg given as a three-dose schedule at 0, 1 and 6 months. The only significant adverse effect was injection site pain.23 A recent study by Zhang et al. evaluated the long-term efficacy of HEV vaccine in 56,302 participants. The vaccine was administered as a three-dose schedule at 0, 1 and 6 months. The authors demonstrated a vaccine efficacy of 86.8% and 87% of those participants who received three doses of HEV vaccine maintained an adequate titre of antibodies against HEV for 4.5 years.24
HBV
HBV is a small DNA virus belonging to the family Hepadnaviridae. HBV is transmitted via permucosal or percutaneous exposure to infected body fluids or blood products and it replicates via an RNA intermediate that can integrate itself into the host genome. The spectrum of HBV infection varies from acute to chronic depending on the duration of persistence of HBV surface antigen (HBsAg) in the serum. Majority of patients with acute infection would remain asymptomatic and only 30% develop icteric hepatitis. The incidence of developing fulminant hepatic failure remains low (0.1–0.5%). When HBsAg persists in the serum for over 6 months, the patient has chronic HBV infection. The likelihood to go on to chronicity varies with age, with the risk being ≥90% in neonates and <5% in adults.25 Based on the prevalence of HBsAg, various geographic areas in the world are classified as having high (≥8%), intermediate (2–7%) and low (<2%) endemicity.26
Scenario in India
India falls into the category of intermediate endemicity for HBV and the common genotypes reported from India are A followed by D.27 In a population-based study conducted by Chowdhury et al., 7653 subjects were screened and 2.97% tested positive for HBsAg, of whom majority (90%) were Hepatitis B e antigen (HbeAg) negative and Hepatitis B e antibody (anti-HBe)-positive.28 A study by Lodha et al. from New Delhi depicted the prevalence of HBV infection in India to be 1–2%.29
A well designed meta-analysis by Batham et al. noted a high prevalence of HBV infection in the tribal population of India (15.9%) when compared to the non-tribal population (2.4%).30 However, in order to overcome the fallacy of publication bias which can distort the results drawn in systematic reviews, the authors used population-weights and estimated a prevalence of HBV infection to be 3.07% in the non-tribal population when compared to 11.85% in the tribal population.31 Few other studies have assessed the prevalence of HBV infection in the tribal population of India and have noted prevalence ranging from 4.4% in the Baiga tribal population of Madhya Pradesh32 to 37.8% in the Shompen tribe and 65% in the Jarawa tribe of Andaman and Nicobar islands.33, 34 Due to the bias of self-selection, screening blood donors usually underestimates the real prevalence of HBV in a community and most of the blood bank data from India depict a prevalence of HBV infection of about 0.2–4%.35, 36
The predominant mode of transmission of HBV in India is horizontal,37 although a recent study by Dwivedi et al. has shown 56.8% of pregnant women with HBV infection to be in the high replicative phase and having HBeAg positivity, suggesting vertical transmission to play a significant role in India as well. The prevalence of HBsAg positivity in antenatal women was noted to be 0.9% in this study.38
In order to reduce the complications associated with chronic Hepatitis B (CHB) infection, it is imperative to suppress viral replication before the development of cirrhosis and HCC. However, treatment is not recommended for patients with mild CHB infection in view of the low efficacy of existing therapies. Interferon-based therapy and oral treatment with drugs like entecavir and tenofovir disoproxil fumarate form the major cornerstone of therapy presently; however, newer drugs like besifovir and tenofovir alafenamide are expected to expand the therapeutic armamentarium against HBV in near future.39, 40
Prevention of HBV infection
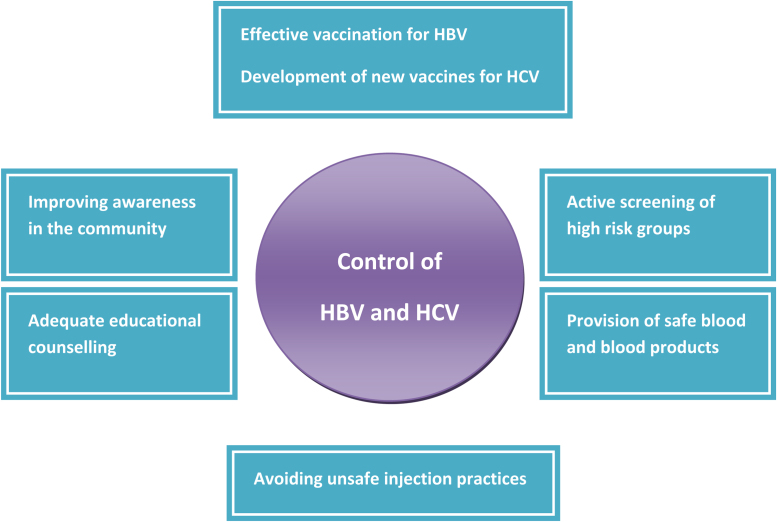
The preventive strategy of HBV infection should include vigilant screening of blood and blood products and routine testing of tissue and organ donors. A lot of emphasis needs to be laid on screening the high-risk group of patients which include those who receive blood transfusions (patients with thalassemia major), subjects actively involved in intravenous drug use (IVDU), tattooing, acupuncture and health care workers (HCWs). Harm reduction advice needs to be given to subjects with IVDU and adequate education regarding the usage of barrier contraceptives (e.g. condoms) and safe sexual practices need to be provided.26 The preventive strategies to control HBV and HCV infection are depicted in Fig. 1.
Fig. 1.
Preventive strategies to control HBV and HCV.
Vaccination in India
Vaccination forms an imperative pillar in the preventive strategy framed for HBV infection and studies in the regions of high endemicity have shown a steady decline in the incidence of HBV infection among children to <2% with the institution of effective vaccination protocol.41 Recombinant DNA-based vaccines are primarily used in India at a dosing schedule of 0, 1 and 6 months and is aimed to achieve a protective anti-HBs titre of >10 mIU/ml. A recent study from India has critically analyzed the immunologic benefits of HBV vaccination and noted that effective vaccination does not reduce the carrier rate of HBV and at 6 years of age, protective titres of anti-HBs antibody (>10 mIU/ml) were present in only 59% of those immunized which subsequently dropped to 13% at 11 years of age.42 These are disappointing results for an efficacious vaccine that results in the formation of a protective titre of antibodies in 95% of vaccinated subjects, suggesting that there is a urgent need to carefully scrutinize the HBV vaccination program in India.
HCV
HCV is a single-stranded RNA virus belonging to the family Flaviviridae. HCV causes both acute and chronic infection; however, unlike HBV, HCV has a higher propensity to lead onto chronic viraemia and 25% of these patients can develop chronic hepatitis.43 HCV has six major genotypes,1, 2, 3, 4, 5, 6 with genotype 1 being the most prevalent genotype globally (46%), followed by genotype 3 in 22% and genotypes 2 and 4 in 13% each.44
Scenario in India
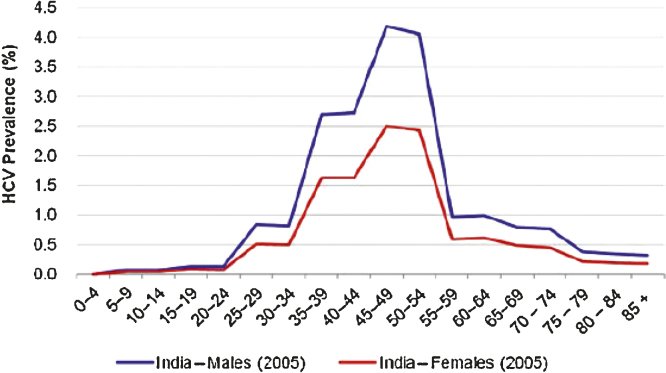
The estimated prevalence of HCV infection in India is about 1–1.9%45 although variations have been reported in literature across various geographical regions in India. The HCV prevalence in India across males and females has been depicted in Fig. 2.46 In an elegant population-based study done by Chowdhury et al. from West Bengal, 3579 individuals were preselected from 10,737 inhabitants off whom 83.1% agreed to participate in the study. The seroprevalence of HCV antibody positivity was noted in 0.87%. The prevalence showed an increasing trend from 0.31% in children aged below 10 years to 1.85% in adults aged 60 years or more with no difference in prevalence between males and females.47 In another study carried out by Singh et al. on 22,666 trainees of Indian Armed Forces, the seroprevalence of anti-HCV positivity was noted to be 0.44%. The possible explanation to this low prevalence was exclusion of those who may be at risk for HCV infection from recruitment as military trainees.48
Fig. 2.
Prevalence of HCV infection across males and females in India.46
In a study from New Delhi, 28,956 healthy blood donors were tested and the prevalence of anti-HCV was noted in 0.66%.49 The prevalence of HCV infection in high-risk group of patients like those receiving blood transfusions (patients with thalassemia major), subjects with IVDU, HCWs, subjects on hemodialysis (HD) is expected to be higher than in general population. In a study by Chakravarti et al. from New Delhi, 51 patients with anti-HCV positivity were enrolled and iatrogenic procedures were found to be responsible for transmission of HCV in 83.3% of patients with blood transfusion alone responsible in 67%.50 Jasuja et al. in their study on 119 patients receiving HD noted HCV infection in 27.7%. Longer duration of HD and getting HD at more than one centre are deemed important in acquiring HCV infection.51
Sood et al. in a study from Ludhiana in 2012 screened 5258 subjects and the seroprevalence of anti-HCV positivity was noted in 5.2%, with highest prevalence being noted in the age group of 41–60 years.52 However, a recent study by the same group in 2016 has demonstrated a falling seroprevalence of HCV to 3.29% (personal communication). In another study from the state of Haryana, 150,000 residents of Fatehabad district were screened and a prevalence of 1% was demonstrated. Prevalence of anti-HCV seropositivity has been shown to be higher in the tribal populations of India ranging from 2.02% in Lambada tribe in Andhra Pradesh to 14.4% in the Bharia tribe in Madhya Pradesh.53, 54
In published studies, genotype 3 is reported as the most common genotype in India, accounting for 54–80% of cases.55, 56 Studies from northern, western and eastern parts of the country have uniformly shown predominance of genotype 3; however, in southern India, both genotypes 1 and 3 HCV are found to be prevalent.57
There has been a paradigm shift in the management of chronic Hepatitis C in recent years with the availability of the new direct acting antiviral agents (DAAs). Sofosbuvir was the first DAA to make its way to the Indian market in March 2015 followed by Ledipasvir and Daclatasvir in December 2015. Excellent response rates have been noted with the use of these drugs; however, real world data from the Indian subcontinent are still awaited.
Prevention of HCV
Prevention of HCV infection shares a similar notion as mentioned for preventing HBV infection. Active screening of high-risk groups for HCV infection and meticulous educational training targeting not only the groups at risk but also the general population and HCWs can help curtail the spread of HCV. With the availability of newer DAAs, extremely effective treatment regimens are now available to treat HCV infection in India.
In India, it was shown that prevention of HCV decreased the overall prevalence but it did not impact the short-term liver-related mortality or development of HCC. Thus, a dual approach of decreasing the incidence of new cases and treatment of old cases would likely a play a vital role in bringing down the burden of the disease.58 Presently, no HCV vaccines are available for routine use; however, new vaccines like recombinant protein, peptide and vector-based vaccines have shown promise and have lately entered phase I/II clinical trials.59
Global fightback against viral hepatitis!
UN Member States have made a commitment to combat hepatitis under the goal 3.3 of the Sustainable Development Goals. This depicts a global effort to fight hepatitis; however, the only indicator for hepatitis that is being considered is “new hepatitis B infections per 100,000 people in a given year”. This indictor would not completely address the situation and tackle the burden associated with chronic Hepatitis C. WHO's Draft Global Health Sector Strategy on Viral Hepatitis gives the much needed roadmap and targets to combat hepatitis. It provides realistic targets and action plans to eliminate hepatitis by 2030.1
Conclusion
Viral hepatitis imposes a major healthcare burden in the India subcontinent. Maintaining adequate sanitary and hygienic conditions can help tackle the problem associated with enterically transmitted pathogens like HAV and HEV. HBV and HCV infection can cause chronic hepatitis, which can lead to ensuing complications like development of cirrhosis of liver and HCC. Following a multipronged approach of active screening, adequate treatment, universal vaccination against HBV and educational counselling can help decrease the burden of liver diseases associated with HBV and HCV infection in India.
Conflicts of interest
The authors have none to declare.
References
- 1.Locarnini S., Chen D.-S., Shibuya K. No more excuses: viral hepatitis can be eliminated. Lancet. 2016;387(10029):1703–1704. doi: 10.1016/S0140-6736(16)30295-1. [DOI] [PubMed] [Google Scholar]
- 2.Verma R., Khanna P. Hepatitis A vaccine should receive priority in National Immunization Schedule in India. Hum Vaccines Immunother. 2012;8(8):1132–1134. doi: 10.4161/hv.20475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Keeffe E.B. Hepatitis A and B superimposed on chronic liver disease: vaccine-preventable diseases. Trans Am Clin Climatol Assoc. 2006;117:227–238. [PMC free article] [PubMed] [Google Scholar]
- 4.Koff R.S. Hepatitis A. Lancet. 1998;351(9116):1643–1649. doi: 10.1016/S0140-6736(98)01304-X. [DOI] [PubMed] [Google Scholar]
- 5.Rakesh P.S., Sherin D., Sankar H., Shaji M., Subhagan S., Salila S. Investigating a community-wide outbreak of Hepatitis A in India. J Glob Infect Dis. 2014;6(2):59–64. doi: 10.4103/0974-777X.132040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Acharya S.K., Batra Y., Bhatkal B. Seroepidemiology of Hepatitis A virus infection among school children in Delhi and north Indian patients with chronic liver disease: implications for HAV vaccination. J Gastroenterol Hepatol. 2003;18(7):822–827. doi: 10.1046/j.1440-1746.2003.03051.x. [DOI] [PubMed] [Google Scholar]
- 7.Arankalle V.A., Sarada Devi K.L., Lole K.S., Shenoy K.T., Verma V., Haneephabi M. Molecular characterization of Hepatitis A virus from a large outbreak from Kerala, India. Indian J Med Res. 2006;123(6):760–769. [PubMed] [Google Scholar]
- 8.Jain P., Prakash S., Gupta S. Prevalence of Hepatitis A virus, Hepatitis B virus, Hepatitis C virus, Hepatitis D virus and Hepatitis E virus as causes of acute viral hepatitis in North India: a hospital based study. Indian J Med Microbiol. 2013;31(3):261–265. doi: 10.4103/0255-0857.115631. [DOI] [PubMed] [Google Scholar]
- 9.Arankalle V., Mitra M., Bhave S. Changing epidemiology of Hepatitis A virus in Indian children. Dovepress. 2014;4:7–13. [Google Scholar]
- 10.Acharya S.K., Madan K., Dattagupta S., Panda S.K. Viral hepatitis in India. Natl Med J India. 2006;19(4):203–217. [PubMed] [Google Scholar]
- 11.Hewitt P.E., Ijaz S., Brailsford S.R. Hepatitis E virus in blood components: a prevalence and transmission study in southeast England. Lancet. 2014;384(9956):1766–1773. doi: 10.1016/S0140-6736(14)61034-5. [DOI] [PubMed] [Google Scholar]
- 12.Hosseini Moghaddam S.M. Hepatitis E virus and renal transplantation. Hepat Mon. 2011;11(8):599–600. doi: 10.5812/kowsar.1735143X.770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kamar N., Izopet J., Dalton H.R. Chronic Hepatitis E virus infection and treatment. J Clin Exp Hepatol. 2013;3(2):134–140. doi: 10.1016/j.jceh.2013.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wong D.C., Purcell R.H., Sreenivasan M.A., Prasad S.R., Pavri K.M. Epidemic and endemic hepatitis in India: evidence for a non-A, non-B hepatitis virus aetiology. Lancet. 1980;2(8200):876–879. doi: 10.1016/s0140-6736(80)92045-0. [DOI] [PubMed] [Google Scholar]
- 15.Chauhan A., Dilawari J.B., Jameel S. Common aetiological agent for epidemic and sporadic non-A, non-B hepatitis. Lancet. 1992;339(8808):1509–1510. doi: 10.1016/0140-6736(92)91267-c. [DOI] [PubMed] [Google Scholar]
- 16.Aggarwal R., Jameel S. Hepatitis E. Hepatology. 2011;54(6):2218–2226. doi: 10.1002/hep.24674. [DOI] [PubMed] [Google Scholar]
- 17.Bhatia V., Singhal A., Panda S.K., Acharya S.K. A 20-year single-center experience with acute liver failure during pregnancy: is the prognosis really worse? Hepatology. 2008;48(5):1577–1585. doi: 10.1002/hep.22493. [DOI] [PubMed] [Google Scholar]
- 18.Chandra N.S., Sharma A., Rai R.R., Malhotra B. Contribution of Hepatitis E virus in acute sporadic hepatitis in north western India. Indian J Med Res. 2012;136(3):477–482. [PMC free article] [PubMed] [Google Scholar]
- 19.Khuroo M.S., Rustgi V.K., Dawson G.J. Spectrum of Hepatitis E virus infection in India. J Med Virol. 1994;43(3):281–286. doi: 10.1002/jmv.1890430316. [DOI] [PubMed] [Google Scholar]
- 20.Tomar B.S. Hepatitis E in India. Zhonghua Min Guo Xiao Er Ke Yi Xue Hui Za Zhi. 1998;39(3):150–156. [PubMed] [Google Scholar]
- 21.Kumar A., Saraswat V.A. Hepatitis E and acute-on-chronic liver failure. J Clin Exp Hepatol. 2013;3(3):225–230. doi: 10.1016/j.jceh.2013.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shalimar, Kumar D., Vadiraja P.K. Acute on chronic liver failure because of acute hepatic insults: etiologies, course, extrahepatic organ failure and predictors of mortality. J Gastroenterol Hepatol. 2016;31(4):856–864. doi: 10.1111/jgh.13213. [DOI] [PubMed] [Google Scholar]
- 23.Shrestha M.P., Scott R.M., Joshi D.M. Safety and efficacy of a recombinant Hepatitis E vaccine. N Engl J Med. 2007;356(9):895–903. doi: 10.1056/NEJMoa061847. [DOI] [PubMed] [Google Scholar]
- 24.Zhang J., Zhang X.-F., Huang S.-J. Long-term efficacy of a Hepatitis E vaccine. N Engl J Med. 2015;372(10):914–922. doi: 10.1056/NEJMoa1406011. [DOI] [PubMed] [Google Scholar]
- 25.Fattovich G., Bortolotti F., Donato F. Natural history of chronic Hepatitis B: special emphasis on disease progression and prognostic factors. J Hepatol. 2008;48(2):335–352. doi: 10.1016/j.jhep.2007.11.011. [DOI] [PubMed] [Google Scholar]
- 26.Puri P. Tackling the Hepatitis B disease burden in India. J Clin Exp Hepatol. 2014;4(4):312–319. doi: 10.1016/j.jceh.2014.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kumar A., Kumar S.I., Pandey R., Naik S., Aggarwal R. Hepatitis B virus genotype A is more often associated with severe liver disease in northern India than is genotype D. Indian J Gastroenterol. 2005;24(1):19–22. [PubMed] [Google Scholar]
- 28.Chowdhury A., Santra A., Chakravorty R. Community-based epidemiology of Hepatitis B virus infection in West Bengal, India: prevalence of Hepatitis B e antigen-negative infection and associated viral variants. J Gastroenterol Hepatol. 2005;20(11):1712–1720. doi: 10.1111/j.1440-1746.2005.04070.x. [DOI] [PubMed] [Google Scholar]
- 29.Lodha R., Jain Y., Anand K., Kabra S.K., Pandav C.S. Hepatitis B in India: a review of disease epidemiology. Indian Pediatr. 2001;38(4):349–371. [PubMed] [Google Scholar]
- 30.Batham A., Narula D., Toteja T., Sreenivas V., Puliyel J.M. Systematic review and meta-analysis of prevalence of Hepatitis B in India. Indian Pediatr. 2007;44(9):663–674. [PubMed] [Google Scholar]
- 31.Batham A., Gupta M.A., Rastogi P., Garg S., Sreenivas V., Puliyel J.M. Calculating prevalence of Hepatitis B in India: using population weights to look for publication bias in conventional meta-analysis. Indian J Pediatr. 2009;76(12):1247–1257. doi: 10.1007/s12098-009-0246-3. [DOI] [PubMed] [Google Scholar]
- 32.Reddy P.H., Tedder R.S. Hepatitis virus markers in the Baiga tribal population of Madhya Pradesh, India. Trans R Soc Trop Med Hyg. 1995;89(6):620. doi: 10.1016/0035-9203(95)90413-1. [DOI] [PubMed] [Google Scholar]
- 33.Murhekar M.V., Murhekar K.M., Sehgal S.C. Epidemiology of Hepatitis B virus infection among the tribes of Andaman and Nicobar Islands, India. Trans R Soc Trop Med Hyg. 2008;102(8):729–734. doi: 10.1016/j.trstmh.2008.04.044. [DOI] [PubMed] [Google Scholar]
- 34.Murhekar M.V., Murhekar K.M., Das D., Arankalle V.A., Sehgal S.C. Prevalence of Hepatitis B infection among the primitive tribes of Andaman & Nicobar Islands. Indian J Med Res. 2000;111:199–203. [PubMed] [Google Scholar]
- 35.Meena M., Jindal T., Hazarika A. Prevalence of Hepatitis B virus and Hepatitis C virus among blood donors at a tertiary care hospital in India: a five-year study. Transfusion. 2011;51(1):198–202. doi: 10.1111/j.1537-2995.2010.02801.x. [DOI] [PubMed] [Google Scholar]
- 36.Garg S., Mathur D.R., Garg D.K. Comparison of seropositivity of HIV, HBV, HCV and syphilis in replacement and voluntary blood donors in western India. Indian J Pathol Microbiol. 2001;44(4):409–412. [PubMed] [Google Scholar]
- 37.Gupta S., Gupta R., Joshi Y.K., Singh S. Role of horizontal transmission in Hepatitis B virus spread among household contacts in north India. Intervirology. 2008;51(1):7–13. doi: 10.1159/000118790. [DOI] [PubMed] [Google Scholar]
- 38.Dwivedi M., Misra S.P., Misra V. Seroprevalence of Hepatitis B infection during pregnancy and risk of perinatal transmission. Indian J Gastroenterol. 2011;30(2):66–71. doi: 10.1007/s12664-011-0083-y. [DOI] [PubMed] [Google Scholar]
- 39.Yuen M.F., Ahn S.H., Lee K.S. Two-year treatment outcome of chronic Hepatitis B infection treated with besifovir vs. entecavir: results from a multicentre study. J Hepatol. 2015;62(3):526–532. doi: 10.1016/j.jhep.2014.10.026. [DOI] [PubMed] [Google Scholar]
- 40.Sax P.E., Wohl D., Yin M.T. Tenofovir alafenamide versus tenofovir disoproxil fumarate, coformulated with elvitegravir, cobicistat, and emtricitabine, for initial treatment of HIV-1 infection: two randomised, double-blind, phase 3, non-inferiority trials. Lancet. 2015;385(9987):2606–2615. doi: 10.1016/S0140-6736(15)60616-X. [DOI] [PubMed] [Google Scholar]
- 41.Chen D.S. Toward elimination and eradication of Hepatitis B. J Gastroenterol Hepatol. 2010;25(1):19–25. doi: 10.1111/j.1440-1746.2009.06165.x. [DOI] [PubMed] [Google Scholar]
- 42.Kumar R., Puliyel J. Utility of Hepatitis B vaccination in India. Indian Pediatr. 2014;51(11):870–872. [PubMed] [Google Scholar]
- 43.Alter H.J., Seeff L.B. Recovery, persistence, and sequelae in Hepatitis C virus infection: a perspective on long-term outcome. Semin Liver Dis. 2000;20(1):17–35. doi: 10.1055/s-2000-9505. [DOI] [PubMed] [Google Scholar]
- 44.Gower E., Estes C., Blach S., Razavi-Shearer K., Razavi H. Global epidemiology and genotype distribution of the Hepatitis C virus infection. J Hepatol. 2014;61(1 suppl):S45–S57. doi: 10.1016/j.jhep.2014.07.027. [DOI] [PubMed] [Google Scholar]
- 45.Sievert W., Altraif I., Razavi H.A. A systematic review of Hepatitis C virus epidemiology in Asia, Australia and Egypt. Liver Int. 2011;31(suppl 2):61–80. doi: 10.1111/j.1478-3231.2011.02540.x. [DOI] [PubMed] [Google Scholar]
- 46.Saraswat V., Norris S., de Knegt R.J. Historical epidemiology of Hepatitis C virus (HCV) in select countries – volume 2. J Viral Hepat. 2015;22(suppl 1):6–25. doi: 10.1111/jvh.12350. [DOI] [PubMed] [Google Scholar]
- 47.Chowdhury A., Santra A., Chaudhuri S. Hepatitis C virus infection in the general population: a community-based study in West Bengal, India. Hepatology. 2003;37(4):802–809. doi: 10.1053/jhep.2003.50157. [DOI] [PubMed] [Google Scholar]
- 48.Singh M., Kotwal A., Gupta R.M., Adhya S., Chatterjee K., Jayaram J. Sero-epidemiological and behavioural survey of HIV, HBV and HCV amongst Indian armed forces trainees. Med J Armed Forces India. 2010;66(1):50–54. doi: 10.1016/S0377-1237(10)80093-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pahuja S., Sharma M., Baitha B., Jain M. Prevalence and trends of markers of Hepatitis C virus, Hepatitis B virus and human immunodeficiency virus in Delhi blood donors: a hospital based study. Jpn J Infect Dis. 2007;60(6):389–391. [PubMed] [Google Scholar]
- 50.Chakravarti A., Ashraf A., Malik S. A study of changing trends of prevalence and genotypic distribution of Hepatitis C virus among high risk groups in North India. Indian J Med Microbiol. 2013;31(4):354–359. doi: 10.4103/0255-0857.118877. [DOI] [PubMed] [Google Scholar]
- 51.Jasuja S., Gupta A.K., Choudhry R. Prevalence and associations of Hepatitis C viremia in hemodialysis patients at a tertiary care hospital. Indian J Nephrol. 2009;19(2):62–67. doi: 10.4103/0971-4065.53324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sood A., Sarin S.K., Midha V. Prevalence of Hepatitis C virus in a selected geographical area of northern India: a population based survey. Indian J Gastroenterol. 2012;31(5):232–236. doi: 10.1007/s12664-012-0251-8. [DOI] [PubMed] [Google Scholar]
- 53.Chandra M., Khaja M.N., Farees N. Prevalence, risk factors and genotype distribution of HCV and HBV infection in the tribal population: a community based study in south India. Trop Gastroenterol. 2003;24(4):193–195. [PubMed] [Google Scholar]
- 54.Puri P., Anand A.C., Saraswat V.A. Consensus statement of HCV task force of the Indian National Association for Study of the Liver (INASL). Part I. Status report of HCV infection in India. J Clin Exp Hepatol. 2014;4(2):106–116. doi: 10.1016/j.jceh.2014.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Verma V., Chakravarti A., Kar P. Genotypic characterization of Hepatitis C virus and its significance in patients with chronic liver disease from Northern India. Diagn Microbiol Infect Dis. 2008;61(4):408–414. doi: 10.1016/j.diagmicrobio.2008.03.011. [DOI] [PubMed] [Google Scholar]
- 56.Narahari S., Juwle A., Basak S., Saranath D. Prevalence and geographic distribution of Hepatitis C Virus genotypes in Indian patient cohort. Infect Genet Evol. 2009;9(4):643–645. doi: 10.1016/j.meegid.2009.04.001. [DOI] [PubMed] [Google Scholar]
- 57.Chandra M., Thippavuzzula R., Ramachandra Rao V.V. Genotyping of Hepatitis C virus (HCV) in infected patients from South India. Infect Genet Evol. 2007;7(6):724–730. doi: 10.1016/j.meegid.2007.03.001. [DOI] [PubMed] [Google Scholar]
- 58.Puri P., Anand A.C., Saraswat V.A. Disease burden of chronic Hepatitis C Virus (HCV) infection in India. Presented at: 65th Annual Meeting of the American Association for the Study of Liver Diseases; November 7–11, Boston, MA; 2014. [Google Scholar]
- 59.Naderi M., Gholipour N., Zolfaghari M.R., Moradi Binabaj M., Yegane Moghadam A., Motalleb G. Hepatitis C virus and vaccine development. Int J Mol Cell Med. 2014;3(4):207–215. [PMC free article] [PubMed] [Google Scholar]