Abstract
Background
Comparing surgical outcomes of management of posterior polar cataract, a congenital cataract, which is difficult to manage surgically and has been associated with poor surgical outcomes.
Methods
46 eyes of 38 patients with posterior polar cataract underwent phacoemulsification and PCIOL implantation.
Results
In a prospective analytical study, 46 eyes of 38 patients with posterior polar cataracts underwent surgery at a zonal hospital of the armed forces. The posterior polar cataract incidence was 1.23 per 1000 with confidence interval (CI) of 0.0012. Of the 46 eyes operated, 6 had a posterior capsular rupture (PCR) (13.04%). The PCR incidence in normal cataracts is reported at approx. 1.1%, whereas, various studies have reported incidence of 6–36% in posterior polar cataracts. 41 eyes achieved a visual acuity of 6/12 or better (89.13%) and 39 eyes of 6/9 or better (84.78%). 4 patients had amblyopia (8.6%), Two patients developed macular edema (4.34%). Mean follow-up was 7 months (range 3–11 months). There was no case of nucleus drop or retinal detachment.
Conclusion
Posterior polar cataracts are a surgical challenge. With controlled surgery, well defined techniques, a good surgical outcome can be achieved with reduced incidence of PCR.
Keywords: Posterior polar, Posterior capsular rent, Congenital
Introduction
Posterior polar cataract is a disorder, generally present at birth, which is a dominantly inherited disorder with variable expressivity; however, it can be sporadic and generally manifests at a later age. Dominantly inherited cataracts tend to be bilateral, whereas sporadic ones are generally unilateral. There is a positive family history in 40–55% and it is bilateral in 65–80%.1 There is no sex predilection. The reported incidence is 3–5 in 1000.2 Recently, evidence has linked it to a mutation on PITX3, chromosome 10 and 16q22.3 It is a cataract, which is situated on the posterior pole of the lens associated with remnants of the tunica vasculosa lentis, an embryologic hyaloid structure that fails to regress.
Duke-Elder has classified it into stationary and progressive forms. The stationary form is a well-circumscribed circular opacity, localized on the central posterior capsule. Progression begins in any decade. In the progressive type, whitish opacification takes place in the posterior cortex in the form of radiating rider opacity. It has feathery and scalloped edges, but they do not involve the nucleus. Both stationary and progressive posterior polar cataracts may become symptomatic.
Singh classified posterior polar cataract into:
-
Type 1
The posterior polar opacity is associated with posterior subcapsular cataract.
-
Type 2
Sharply defined round or oval opacity with ringed appearance like an onion with or without grayish spots at the edge.
-
Type 3
Sharply defined round or oval white opacity with dense white spots at the edge often associated with thin or absent PC. These dense white spots are a diagnostic sign (Daljit Singh sign) of posterior capsule leakage and extreme fragility.
-
Type 4Combination of the above 3 types with nuclear sclerosis. Schroeder on the other hand graded posterior polar cataract in his pediatric patients according to its effect on pupillary obstruction in the red reflex testing as follows:
-
Grade 1A small opacity without any effect on the optical quality of the clear part of the lens.
-
Grade 2A two-thirds obstruction without other effect.
-
Grade 3The disk-like opacity in the posterior capsule is surrounded by an area of further optical distortion. Only the dilated pupil shows a clear red reflex surrounding this zone.
-
Grade 4The opacity is totally occlusive; no sufficient red reflex is obtained by dilation of the pupil. It thus interferes with central vision, especially in bright light, when the pupil is constricted.
-
Grade 1
The posterior polar cataract is tightly adherent to the underlying posterior capsule and often associated with a pre-existing posterior capsular dehiscence or a very thin capsule under the plaque (Fig. 1). Because of its predisposition to posterior capsular dehiscence during surgery, it represents a challenge to the surgeon.4, 5 Various studies have reported different rates of capsular dehiscence during surgery, varying from 7.1% to as high as 36%.2, 6 A number of surgical techniques and approaches have been defined for improving surgical outcomes and reducing the rate of complications during surgery.7, 8, 9, 10 The timing of surgery is also of great importance as the risks of early surgery at a younger age have to be balanced with the difficulty of performing the surgery on a harder, more mature, advanced cataract.
Fig. 1.

Posterior polar cataract.
Most studies quoted above have examined a number of cases ranging from 25 to 36. In this study, we have performed surgery on 46 eyes of 38 patients and tried to describe a surgical approach, which included manual IA with a Simcoe cannula, which will reduce the complication rate and improve the surgical outcome in these patients.
Materials and methods
In a prospective analytical study of posterior polar cataract, 46 eyes of 38 patients with posterior polar cataract underwent phacoemulsification with posterior chamber intra ocular lens (PCIOL) implantation, over a three-year period, at a zonal hospital of the army. All the patients underwent surgery with a similar technique over a period of three years. The surgical factors of interest were the rate of posterior capsular rupture (PCR), nucleus drop and postoperatively, and the best corrected visual acuity.
All patients were counselled preoperatively and the risks involved were explained to the patient. The possibility of pre-existing amblyopia6 was also explained to the patients. All cases with associated problems such as glaucoma, pterygium, uveitis and nuclear sclerosis greater than grade III were excluded from the study.
Surgery – All patients underwent phacoemulsification surgery under peribulbar anesthesia on a Stellaris phaco machine, a venturi pump based machine. In contrast to routine cases, the following parameters were used during surgery, vacuum – 150–200 mmHg, bottle height – 50–70 cm and power varied from 20 to 30 and vacuum 100 mmHg for epinucleus removal.
A 2.8 mm corneal entry was made at 10 o’clock position and side port at 1 o’clock. The central curvilinear capsulorhexis (CCC) was kept slightly smaller at 5 mm (Fig. 2), to support a sulcus implanted intra ocular lens (IOL) if required. However, in denser cataracts, the CCC was kept larger than 5 mm to prevent rupture during hydro procedures. No hydrodissection was done; only gentle hydrodelineation was done using two or three waves of BSS fluid (Fig. 3). A golden ring indicated successful delineation. No rotation of the nucleus was done and an attempt was made to ensure partial prolapse of the nucleus by hydrodelineation.
Fig. 2.
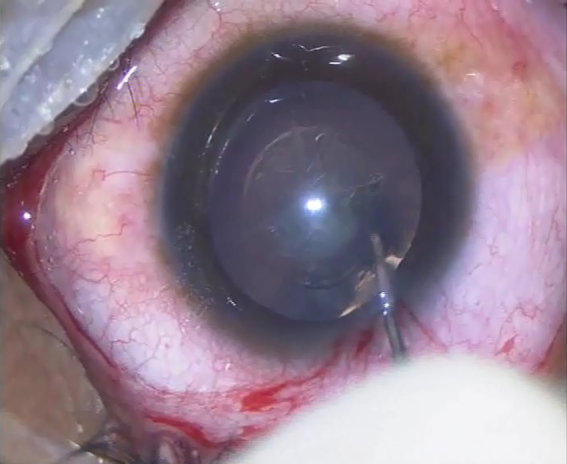
Slightly small central curvilinear capsulorhexis of 5 mm radius.
Fig. 3.

Nucleus hydrodelineation with golden ring, no hydrodissection done.
Less dense nuclei were emulsified in the bag as a whole and slow controlled chopping was done if the nucleus was grade 2 or grade 2+. To prevent capsular rupture during the phaco hand-piece removal, the Sinskey hook was removed first and viscoelastic was injected gradually through the side port as the phaco handpiece is being removed. Cortical aspiration was done using a Simcoe cannula (Fig. 4), which is gentler but slightly increases the surgical time. Further surgical steps were assessed based on the condition of the posterior capsule. In general an effort was made to implant an in-the-bag foldable PCIOL. Here, the appearance of a Pseudo Hole (Fig. 5), a common appearance in Post polar cataracts must be kept in mind. Posterior capsular polishing was totally avoided in all cases. After IOL implantation, anterior capsule polishing was done in all cases to reduce chances of capsular contraction because of the smaller capsulorhexis.
Fig. 4.

Irrigation aspiration of cortical matter being done with Simcoe cannula.
Fig. 5.

Appearance of a Pseudo Hole, giving a false appearance of a posterior capsular rent (PCR).
All patients were placed on antibiotic steroid drops and Betablockers for the first week and subsequently on a tapering dose of antibiotic + steroids for six weeks. Patients with co-morbidities such as diabetes and patients with a compromised posterior capsule were given Bromfenac eye drops in addition for a period of four weeks. All patients were given spectacle prescriptions at the end of six weeks. Patients with compromised posterior capsules were followed up monthly for an additional period of three months to look for raised IOP, cystoid macular edema and retinal breaks.
Results
A total of 46 eyes of 38 patients underwent surgery for Posterior polar cataract during the period from July 2010 to July 2013 at a zonal hospital of the armed forces. Out of the 38 patients, 20 were male and 18 were female. The ages ranged from 31 to 52 years. During this period, a total of 4046 cataract surgeries were performed; out of which 46 were posterior polar cataracts. The total OPD attendance during this period was 68,956 patients, of which 37,262 were new patients.
All the patients, who underwent surgery were implanted with hydrophobic IOLS. Of the 46 eyes operated, 6 had a PCR (13.04%), two of which were patients with bilateral posterior polar cataracts. No patient with bilateral posterior cataract had a PCR in both eyes. No vitreous loss was present except in one case, which required anterior vitrectomy and IOL was implanted in the sulcus. All patients who had a PCR were >40 years of age and had an associated nuclear sclerosis of Gd II/III and a large posterior plaque.
41 eyes achieved a visual acuity of 6/12 or better (89.13%) and 39 eyes of 6/9 or better (84.78%). All patients with bilateral cataract had a final visual acuity of better than 6/12, except two eyes, which had a visual acuity of less than 6/12. 4 patients had amblyopia (8.6%), one of which was a patient with bilateral posterior polar cataract, which explained the visual acuity below 6/12. Mean follow-up was 7 months (range 3–11 months). Two patients developed macular edema (4.34%), none of which were patients with both eyes operated. There was no case of nucleus drop or retinal detachment, both of which are complications reported after surgery for posterior polar cataracts.
Discussion
Posterior polar cataracts are a known surgical challenge for any ophthalmic surgeon. The complications involved are well known and documented.11, 12, 13 The approach to such patients has varied over the years with surgeons even advising patients not to get operated and prescribing them dilating drops to get over the problem of reduced vision in bright light.
With the improvement in equipment, especially phacoemulsification machines, the concept has gradually changed from non-interventional to one of a planned intervention to achieve the best possible outcome with adequate pre-op counseling of the patient.
In this study, we have compiled one of the largest case studies in relation to posterior polar cataracts.1 A number of techniques have been outlined over the years to achieve the best surgical outcome. Based on our case series, we have listed out the best possible approach to a case of posterior polar cataract to achieve a good surgical outcome. Our surgical approach adopted the use of Simcoe cannula for irrigation aspiration (IA),2 which we feel, is one of the major factor contributing to a lower incidence of PCR. The PCR incidence in normal cataracts is reported at approx. 1.1%, whereas, various studies have reported incidence of 6–36% in posterior polar cataracts6, 2, 14 (Table 1). Our case series reported a PCR rate of 13.04%, which is in the reported range of 6–36% worldwide. The maximum risk of PCR is during IA and during changes in the anterior chamber pressure. We also adopted the technique of injecting viscoelastic through the side port during phaco hand piece removal to reduce chances of sudden changes in chamber pressure.5 Both these steps would contribute greatly to reducing the PCR rate in these cases. The disadvantage of manual IA is a slightly increased surgical time, which is a small concession when compared to the improved outcomes.
Table 1.
Comparison of visual outcome and complication rates with other studies.
| Parameters | This study | Lee and Lee | Das et al. | Vasavada and Singh | Osher et al. | Kumar et al. | Hayashi et al. | Nagappa et al. |
|---|---|---|---|---|---|---|---|---|
| Number of eyes | 46 eyes of 38 patients | 36 eyes of 31 patients | 81 eyes of 59 patients | 25 eyes | 31 eyes of 22 patients | 58 eyes of 51 patients | 28 eyes | 17 eyes |
| Mean preop VA | 6/18 (6/24–6/12) | – | 22% 20/30 or better | – | – | – | 20/133 | – |
| Post op VA | 89.13% 6/12 or better 84.78% 6/9 or better | 94% 20/40 or better | 93% 20/30 or better | 72% 20/30, Post Yag, 96% 20/30 or better | 96.7%, 20/40 or better | 94.8% 20/40 or better | 20/22 | 20/28 |
| Mean follow-up | 7 months (3–11 months) | – | 9 months (7–20) | 13.72 | 7.2 (1 week to 32 months) | 15.4 (12–40) | – | – |
| PCR rate | 13.04%(06) | 11.1% | 31% | 36% | 26% | 15.51% | 7.1% | 6% |
| Dropped nucleus | Nil | Nil | 02 | Nil | Nil | Nil | 01 | Nil |
Other authors have described various chopping techniques to reduce complications,5, 2, 8 but since we only evaluated Grade II nuclei, we did a direct chop in all cases and we felt it was not affecting the outcome if a good hydrodelineation10 was done in all cases.
The prudent approach for any surgeon would be to modify his existing technique; however, the basic tenets of being gentle, using low flow parameters, no hydrodissection, no nucleus rotation, reduced bottle height, aspiration using Simcoe cannula5 and a stable anterior chamber cannot be overemphasized in achieving a good surgical outcome. Posterior polar cataracts remain a surgical dilemma and challenge; however, the right steps and approach would greatly reduce the complications associated with them.
Conflicts of interest
The authors have none to declare.
References
- 1.Kalantan H. Posterior polar cataract: a review. Saudi J Ophthalmol. 2012;26:41–49. doi: 10.1016/j.sjopt.2011.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lee M.W., Lee Y.C. Phacoemulsification of posterior polar cataracts—a surgical challenge. Br J Ophthalmol. 2003;87:1426–1427. doi: 10.1136/bjo.87.11.1426-a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Addison P.K., Berry V., Ionides A.C., Francis P.J., Bhattacharya S.S., Moore A.T. Posterior polar cataract is the predominant consequence of a recurrent mutation in the PITX3 gene. Br J Ophthalmol. 2005;89(February (2)):138–141. doi: 10.1136/bjo.2004.053413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Osher R.H., Yu B.C., Koch D.D. Posterior polar cataracts: a predisposition to intraoperative posterior capsular rupture. J Cataract Refract Surg. 1990;16:157–162. doi: 10.1016/s0886-3350(13)80724-9. [DOI] [PubMed] [Google Scholar]
- 5.Vasavada A.R., Singh R. Phacoemulsification with posterior polar cataract. J Cataract Refract Surg. 1999;25:238–245. doi: 10.1016/s0886-3350(99)80133-3. [DOI] [PubMed] [Google Scholar]
- 6.Hayashi K., Hayashi H., Nakao F. Outcomes of surgery for posterior polar cataract. J Cataract Refract Surg. 2003;29:45–49. doi: 10.1016/s0886-3350(02)01692-9. [DOI] [PubMed] [Google Scholar]
- 7.Fine I.H., Packer M., Hoffman R.S. Management of posterior polar cataract. J Cataract Refract Surg. 2003;29:16–19. doi: 10.1016/s0886-3350(02)01616-4. [DOI] [PubMed] [Google Scholar]
- 8.Allen D., Wood C. Minimizing risk to the capsule during surgery for posterior polar cataract. J Cataract Refract Surg. 2002;28:742–744. doi: 10.1016/s0886-3350(02)01244-0. [DOI] [PubMed] [Google Scholar]
- 9.Vasavada A.R., Raj S.M. Inside-out delineation. J Cataract Refract Surg. 2004;30:1167–1169. doi: 10.1016/j.jcrs.2003.10.034. [DOI] [PubMed] [Google Scholar]
- 10.Anis A.Y. Understanding hydrodelineation: the term and the procedure. Doc Ophthalmol. 1994;87:123–137. doi: 10.1007/BF01204789. [DOI] [PubMed] [Google Scholar]
- 11.Kumar S., Ram J., Sukhija J., Severia S. Phacoemulsification in posterior polar cataract: does size of lens opacity affect surgical outcome? Clin Experiment Ophthalmol. 2010;38(9):857–861. doi: 10.1111/j.1442-9071.2010.02354.x. [DOI] [PubMed] [Google Scholar]
- 12.Vajpayee R.B., Sinha R., Singhvi A., Sharma N., Titiyal J.S., Tandon R. ‘Layer by layer’ phacoemulsification in posterior polar cataract with pre-existing posterior capsular rent. Eye (Lond) 2008;22(8):1008–1010. doi: 10.1038/sj.eye.6702795. [DOI] [PubMed] [Google Scholar]
- 13.Taskapili M., Gulkilik G., Kocabora M.S., Ozsutcu M. Phacoemulsification with viscodissection in posterior polar cataract: minimizing risk of posterior capsule tear. Ann Ophthalmol (Skokie) 2007;39(2):145–149. doi: 10.1007/s12009-007-0004-y. [DOI] [PubMed] [Google Scholar]
- 14.Vasavada A.R., Vasavada V.A., Raj S.M. Approaches to a posterior polar cataract. Saudi J Ophthalmol. 2012;26:51–54. doi: 10.1016/j.sjopt.2011.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]


