Summary
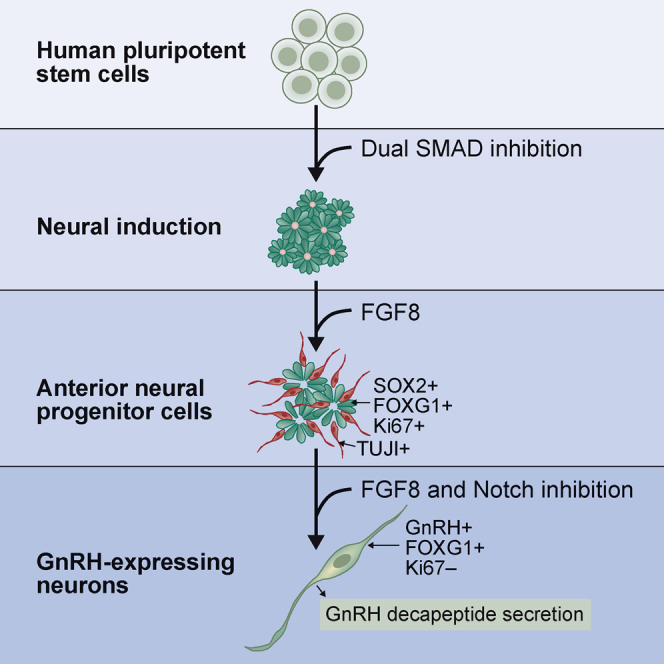
Gonadotropin-releasing hormone (GnRH) neurons regulate human puberty and reproduction. Modeling their development and function in vitro would be of interest for both basic research and clinical translation. Here, we report a three-step protocol to differentiate human pluripotent stem cells (hPSCs) into GnRH-secreting neurons. Firstly, hPSCs were differentiated to FOXG1, EMX2, and PAX6 expressing anterior neural progenitor cells (NPCs) by dual SMAD inhibition. Secondly, NPCs were treated for 10 days with FGF8, which is a key ligand implicated in GnRH neuron ontogeny, and finally, the cells were matured with Notch inhibitor to bipolar TUJ1-positive neurons that robustly expressed GNRH1 and secreted GnRH decapeptide into the culture medium. The protocol was reproducible both in human embryonic stem cells and induced pluripotent stem cells, and thus provides a translational tool for investigating the mechanisms of human puberty and its disorders.
Keywords: human pluripotent stem cells, induced pluripotent stem cells, human embryonic stem cells, gonadotropin-releasing hormone, GnRH, puberty, hypogonadotropic hypogonadism, Kallmann syndrome
Graphical Abstract
Highlights
-
•
GnRH neurons regulate puberty and reproduction
-
•
We generated GnRH-expressing and secreting neurons from hPSCs
-
•
These neurons can be used to study diseases affecting the reproductive system
Raivio and colleagues have developed a protocol for differentiating GnRH-expressing neurons from hPSCs. The cells were first differentiated into NPCs and then with combined FGF8 stimulation and Notch inhibition to GnRH-secreting neural cells. This protocol will provide an important platform for investigating the development and function of the human hypothalamic-pituitary-gonadal axis.
Introduction
The onset of puberty is regulated by a small population of hypothalamic gonadotropin-releasing hormone (GnRH) neurons, which secrete GnRH decapeptide to the hypophyseal portal system. Unlike other neuroendocrine cell types that reside in the hypothalamus, GnRH neurons are born outside the CNS in the frontonasal area. The olfactory pit, which is formed by the olfactory placodes (OP) and surrounding mesenchyme, provides a niche for GnRH neuron specification. Prenatally, GnRH neurons migrate from the frontonasal mesenchyme along with olfactory axons to the forebrain and into the hypothalamus, where their final maturation occurs (Wray, 2010). The early events that lead to GnRH neuron specification and the origin of their progenitors in the olfactory pit are currently poorly understood. Many existing data support OP as the source of GnRH neurons (Kim et al., 1999, Schwanzel-Fukuda and Pfaff, 1989, Wray et al., 1989a, Wray et al., 1989b), whereas others have proposed that at least some GnRH neurons arise from multiple sources, such as the neural crest, and adenohypophyseal or CNS progenitor cells (Forni et al., 2011, Markakis et al., 2004, Salvi et al., 2009, Whitlock et al., 2003).
GnRH neuron specification occurs under explicit spatiotemporal conditions, involving fibroblast growth factor 8 (FGF8) signaling and bone morphogenetic protein/transforming growth factor β (BMP/TGF-β) pathway inhibition (Forni et al., 2013, Kawauchi et al., 2005, Rawson et al., 2010). The indispensable role of FGF8 signaling through fibroblast growth factor receptor 1 (FGFR1) in this process has been established in various animal models (Chung and Tsai, 2010, Sabado et al., 2012). These findings are mirrored by reports in humans: mutations in FGF8 and FGFR1 are found in patients with congenital hypogonadotropic hypogonadism, a rare genetic disease that causes GnRH deficiency (Dode et al., 2003, Falardeau et al., 2008).
Human pluripotent stem cells (hPSCs), including embryonic stem cells (hESCs) and induced pluripotent stem cells (hiPSCs), allow in vitro differentiation of specialized cell types, including neurons (Chambers et al., 2009, Davis et al., 2012, Hay et al., 2008). Here we report a protocol for the generation of GnRH-expressing neurons from hPSCs.
Results
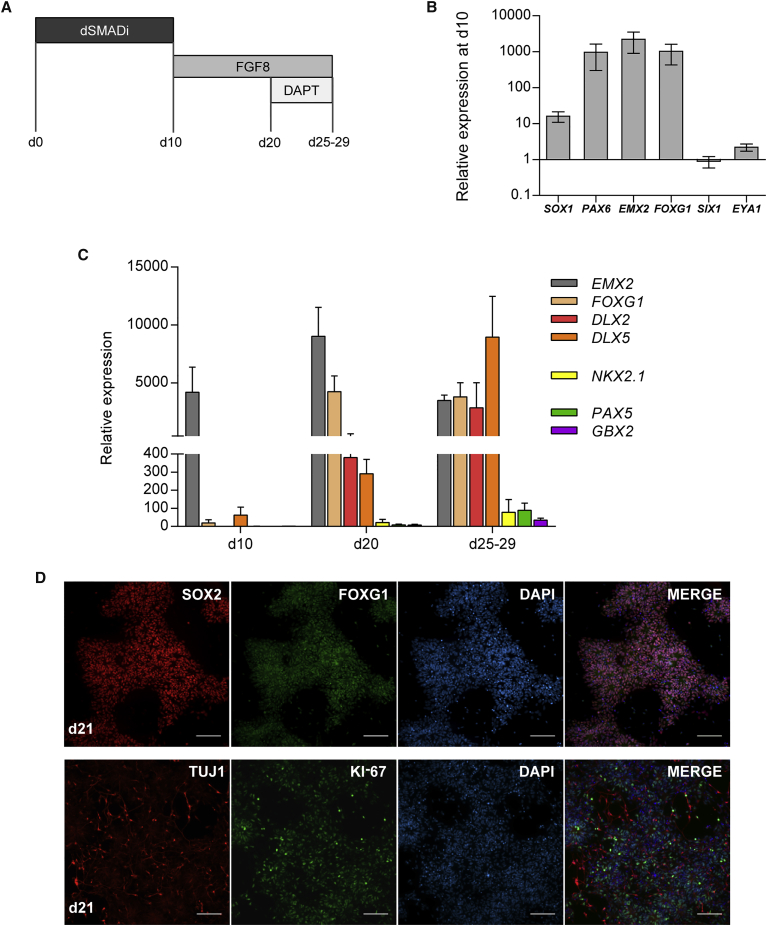
A schematic of the protocol is presented in Figure 1A. In the first step, we employed dual SMAD inhibition on hPSCs by blocking BMP and TGF-β/activin signaling pathways with dorsomorphin (DM) and SB431542 (SB), respectively, for 10 days to produce neural progenitor cells (NPCs) (Chambers et al., 2009). This was followed by 10-day treatment with FGF8, a key ligand in GnRH neuron development, and 4–8 days of treatment with both FGF8 and Notch inhibitor DAPT (N-[N-(3,5-difluorophenacetyl)-L-alanyl]-S-phenylglycine t-butyl ester), to induce terminal maturation of the neurons (Borghese et al., 2010). We used hiPSC line HEL11.4 (Mikkola et al., 2013), and hESC line H9 (Thomson et al., 1998; WiCell) for differentiation experiments, and the main results were repeated in hiPSC line HEL24.3 (Trokovic et al., 2015). Both hiPSC lines have been established from healthy donor fibroblasts.
Figure 1.
Differentiation Protocol Schematic, and Expression of Anterior Neural Progenitor Markers after Neural Induction
(A) Schematic representation of the protocol. For the first 10 days the cells were treated with dual SMAD inhibition (dSMADi) using dorsomorphin and SB431542. FGF8 was added at d11, and Notch inhibitor DAPT was added at d21.
(B) Real-time qPCR results at d10 showing an increased expression of pan-neural marker SOX1 and forebrain- and olfactory placode-associated genes EMX2 and FOXG1. Preplacodal markers SIX1 and EYA1 remained low. Expression levels are relative to d0 hPSCs (HEL11.4 and H9 representative experiments, n = 6, mean ± SEM).
(C) Relative expression of anterior neural progenitor (EMX2, FOXG1, DLX2, and DLX5), ventral forebrain (NKX2.1), and caudal CNS markers (PAX5 and GBX2) in FGF8-treated cells confirming anterior neural identity. HEL11.4-derived cells, n = 3 (mean + SEM). Corresponding data from H9-derived cells are shown in Figure S1B.
(D) Immunocytochemistry at d21 revealed an abundant expression of SOX2, FOXG1, and Ki-67 within neural rosettes. TUJ1 was found mainly outside the neural rosette structures. HEL11.4-derived cells. Scale bars, 100 μm.
Dual SMAD Inhibition Followed by FGF8 Treatment Produces Highly Proliferating Neural Progenitor Cells that Retain Anterior Identity
Ten days of dual SMAD inhibition induced early pan-neural marker SOX1 and neural progenitor markers PAX6, EMX2, and FOXG1 (Figure 1B), which are expressed in the developing forebrain and the OP (Duggan et al., 2008, Forni et al., 2011, Simeone et al., 1992, Zhang et al., 2010). In contrast, the expression of preplacodal genes EYA1 and SIX1 (Ikeda et al., 2007, Schlosser et al., 2008) remained low (Figure 1B). Immunocytochemical analyses confirmed the expression of neural progenitor markers including PAX6, FOXG1, and SOX2, whereas preplacodal marker SIX1 was undetectable (Figure S1A). The expression levels of ventral forebrain marker NKX2.1 and caudal markers PAX5 and GBX2 (Kirkeby et al., 2012, Maroof et al., 2013) were low (Figures 1C and S1B). These results indicate that dual SMAD inhibition with DM and SB for 10 days efficiently induces anteriorly patterned NPCs.
During the subsequent 10 days, the anteriorly primed NPCs were treated with FGF8. At day 20 (d20), FGF8-treated cells had further increased the expression of EMX2 and FOXG1, as well as DLX2 and DLX5, which are also anteriorly expressed; in the frontonasal area, the OP, and the anterior basal forebrain (Merlo et al., 2007, Simeone et al., 1994) (Figures 1C and S1B). A high expression of these anterior markers was sustained throughout the protocol, whereas the expressions of NKX2.1, PAX5, and GBX2 remained low (Figures 1C and S1B). Between d10 and d20, the cells formed neural rosettes that abundantly expressed both SOX2 and FOXG1 (Figure 1D). Overall, an average of 93% (±0.9%, n = 5) of cells were FOXG1 positive at d21. FGF8-treated cells expanded more rapidly than the controls (Figure S1C). Proliferation occurred predominantly in the rosette structures, as judged by abundant Ki-67 expression (Figure 1D). Neural-specific TUJ1-positive cells, which were consistently negative for Ki-67, were located at the periphery and around the neural rosettes, indicating neuronal maturation and cell-cycle exit (Figure 1D).
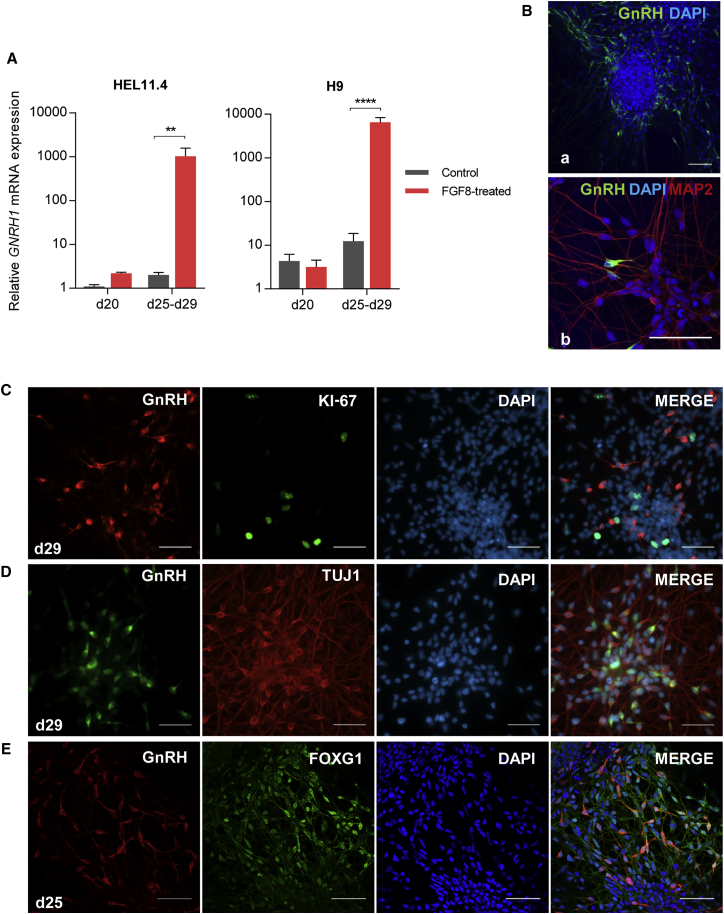
FGF8 and Subsequent Notch Inhibition Induces GnRH Expression
To induce the terminal differentiation of NPCs, we inhibited Notch signaling with DAPT from d21 onward. After 4–8 days, the GNRH1 mRNA level was highly increased in cells supplemented with FGF8, but not in the control cells (Figures 2A and S2A). Immunofluorescence showed presence of GnRH-positive cells mostly in the peripheral regions of condensed cell clusters (Figure 2Ba). The GnRH-positive cells were bipolar and expressed neural marker MAP2 (Figure 2Bb). Concurrently, the number of proliferating (Ki-67 positive) cells was low, and, importantly, no GnRH and Ki-67 double-positive cells were observed (Figure 2C). This indicates that the GnRH-positive cells had exited the cell cycle. The neural identity of the GnRH-expressing cells was further confirmed by double staining with TUJ1 (Figures 2D and S2B). A proportion of the GnRH-positive cells also expressed FOXG1 (Figure 2E). On average, 15.1% (±1.6%, n = 4) of the HEL11.4-derived cells and 12.2% (±4.2%, n = 3) of the H9-derived cells were GnRH positive.
Figure 2.
GnRH-Expressing Cells Emerge after Treatment with FGF8 and DAPT
(A) Relative expression of GNRH1 at the end of the protocol in HEL11.4-derived cells (n = 4), and H9-derived cells (n = 6) treated with or without FGF8 (mean + SEM). ∗∗p < 0.01, ∗∗∗∗p < 0.0001 (paired ratio t test).
(B–E) Immunocytochemistry in HEL11.4-derived cells at the end of the protocol. (B) GnRH-positive neurons appeared mostly in the periphery of condensed node-like structures. These cells appeared to be interconnected by long cellular projections (a), which stained positive for MAP2 (b). (C) The GnRH-expressing cells were not proliferating, as indicated by the absence of colocalization with Ki-67 staining. (D) The GnRH-positive cells also expressed neuron-specific marker TUJ1. See also Figure S2B. (E) At the end of the protocol, the majority of the cells expressed FOXG1. Some of the GnRH-positive cells were also FOXG1 positive. All scale bars represent 50 μm.
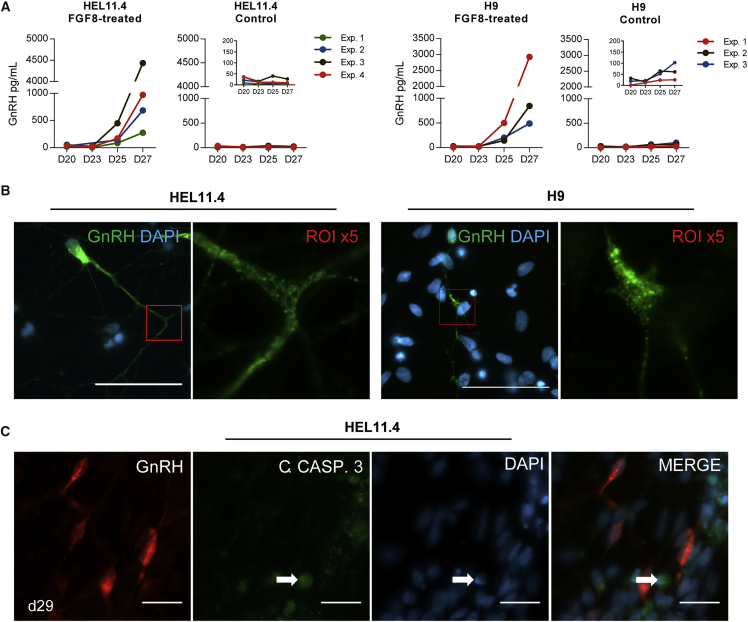
Secretion of GnRH Decapeptide
Human GnRH is translated as a 96-amino-acid prohormone, which is processed by intracellular proteases to form the secreted decapeptide. The GnRH decapeptide was clearly detectable in the culture medium of the FGF8-treated cells, but not of the control cells from d25 onward, with the peak concentrations measured on d27 (Figures 3A and S3A). High-magnification images of GnRH immunopositive cells showed a punctate staining pattern in the majority of the cells, indicating vesicular packaging of GnRH decapeptide (Figures 3B and S3B). Activated caspase-3 (Porter and Janicke, 1999) was not present in the GnRH-positive cells (Figure 3C), suggesting that the release of GnRH was not due to cell death. FGF8-treated cells readily responded to KCl-induced depolarization by 1.97-fold higher GnRH decapeptide release (95% confidence interval, 1.62–2.31) when compared with vehicle-treated cells.
Figure 3.
GnRH Decapeptide Secretion in Culture Medium of FGF8-Treated Cells
In the final differentiation step, secretion of GnRH decapeptide was detected in the culture medium of FGF8-treated cells, but not of the control cells.
(A) Four independent experiments using HEL11.4-derived cells (left) and three independent experiments from H9-derived cells (right) are shown. The insets in the control graphs show the same control values with smaller y-axis scale. See also Figure S3A.
(B) At high magnification the GnRH staining appeared in granular spots in both HEL11.4- and H9-derived cells, indicating vesicular packaging of the decapeptide. ROI, region of interest. Scale bars, 50 μm. See also Figure S3B.
(C) GnRH and an apoptosis marker, cleaved caspase-3, were not co-expressed, suggesting that GnRH-release was not due to apoptosis. HEL11.4-derived GnRH-expressing cells display normal nuclear morphology. Arrows indicate cleaved caspase-3 staining and nuclear morphology of apoptotic cell. Scale bars, 20 μm.
Migration of GnRH-Expressing Cells
GABAA receptor stimulation has been shown to decelerate the migration of embryonic mouse GnRH neurons (Casoni et al., 2012). Therefore, we investigated the migratory properties of our FGF8-treated cells with a gap-closure assay, whereby d25 cells were allowed to migrate to an empty area in the middle of the culture wells in the presence or absence of GABAA receptor agonist muscimol (0.1 mM). After 50 hr, significantly fewer cells had migrated to the empty area in muscimol-treated wells than in control wells (paired ratio t test, n = 4; p < 0.05).
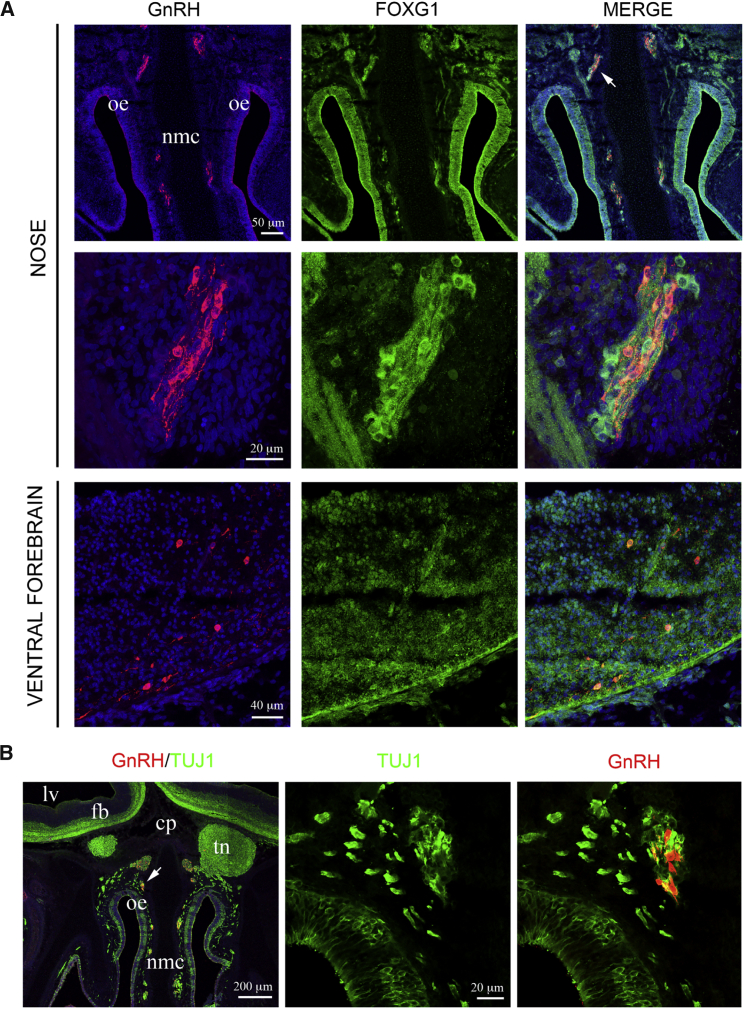
Detection of GnRH-Expressing Neurons in FOXG1-Positive Frontonasal Tissues
As some GnRH cells were positive for FOXG1 in vitro, we investigated whether GnRH neurons express FOXG1 also in vivo. Immunohistochemistry on an 8-gestational-week human fetus showed migrating GnRH neurons that appeared in typical cell clusters, called the migratory mass (Miller et al., 2010, Tarozzo et al., 1995, Wray et al., 1994). The cell clusters contained GnRH neurons, other neurons, and glial cells that delaminated from the olfactory epithelium and migrated toward the forebrain (Figure 4A). Indeed, FOXG1 was expressed in the GnRH neurons, as well as in other cells of the migratory mass and the olfactory epithelium (Figure 4A). Many GnRH-positive cells appeared also to stain with TUJ1 (Figure 4B).
Figure 4.
GnRH, FOXG1, and TUJ1 in an 8-Gestational-Week Human Fetus
(A) Immunohistochemistry of coronal sections in a human fetus. GnRH was expressed in the frontonasal area between the olfactory epithelia (oe) and the nasal medial cartilage (nmc), in migrating cells also known as the migratory mass. These cells were FOXG1-positive, including GnRH-expressing cells. The arrow in the top panel indicates the area magnified in the middle panel. GnRH-FOXG1 double-positive cells were also detected in the ventral forebrain.
(B) TUJ1 was expressed in the migratory mass cells, including GnRH-expressing cells. Arrow indicates the area magnified on the right. cp, cribriform plate; fb, forebrain; lv, lateral ventricle; nmc, nasal medial cartilage; oe, olfactory epithelia; tn, terminal nerve.
Discussion
According to current understanding, GnRH neurons are first formed during early embryogenesis in the olfactory placode area, from where they migrate to the hypothalamus via the vomeronasal/terminal nerve fibers (Wray, 2010). Once at their final destination, they mature and become an integral part of the hypothalamic-pituitary-gonadal axis. While the OP have been generally accepted as a source of GnRH neurons, the exact origin of GnRH neuron progenitors has remained controversial. Our results show that GnRH-secreting cells can be efficiently differentiated from NPCs that express high levels of anterior neural progenitor markers. The induction of neural commitment in hPSCs by using dual SMAD inhibition leads by default to anterior neural cell fates (Chambers et al., 2009, Kirkeby et al., 2012, Pankratz et al., 2007). Indeed, our dual SMAD inhibition-treated NPCs showed high expression of FOXG1 and EMX2. Expressed in the frontonasal area, where GnRH neurons first emerge, FOXG1, DLX2, and DLX5 have been suggested to be involved in GnRH neuron development (Duggan et al., 2008, Givens et al., 2005, Iyer et al., 2010). Indeed, all of these markers were upregulated along our differentiation protocol. Placodal markers SIX1 and EYA1 (Schlosser, 2014) were, however, not upregulated at any time during this protocol.
Our results show that anterior NPCs can give rise to GnRH-expressing cells when cultured with FGF8, which has been shown to promote progenitor cell survival in the anterior neural structures (Forni et al., 2013, Kawauchi et al., 2005). In mouse anterior neural ridge, FGF8 has been shown to positively regulate Foxg1 expression (Shimamura and Rubenstein, 1997), and mouse embryos that carry hypomorphic and conditional Fgf8 mutations display reduced telencephalons, which is suggested to result from a decreased Foxg1 expression (Storm et al., 2006). In our model, FOXG1 expression persisted following FGF8 treatment, and importantly, FOXG1 was also expressed in migrating GnRH neurons of a human fetus.
The final step in our protocol comprised Notch inhibition, which induces neuronal maturation from progenitor cells (Borghese et al., 2010, Imayoshi et al., 2010, Noisa et al., 2014, Yoon and Gaiano, 2005). As expected, the blocking of Notch in our FGF8-treated cells after d20 decelerated cell proliferation, increased the expression of neuron-specific markers TUJ1 and MAP2, and induced GNRH1 expression, with up to 15% of the cells being immunopositive for GnRH. This efficiency is high, given that humans only have few thousand GnRH neurons. Two recent publications have reported hPSC differentiation to hypothalamic-like neuroendocrine cells, such as POMC-, αMSH-, and AGRP-secreting neurons (Merkle et al., 2015, Wang et al., 2015). In both approaches, dual SMAD inhibition in combination with SHH activation led to ventral forebrain progenitor stage, as suggested by marker expression patterns including high NKX2.1 and low FOXG1 expression. It is noteworthy that these two protocols did not report an induction of GNRH1 expression, which highlights the known differences in the embryonic origin of these cells. In contrast, our protocol produced GnRH-expressing cells with long cellular processes consistent with previously described morphology of GnRH neurons (Herde et al., 2013). The GABAA receptor agonist muscimol suppressed the migration of GnRH-expressing cells, in agreement with the studies performed in embryonic mouse GnRH neurons (Casoni et al., 2012). In the majority of GnRH-expressing cells the GnRH staining appeared in punctate structures, indicating GnRH prepropeptide processing to mature decapeptide and packaging into secretory vesicles. The cultures indeed robustly secreted GnRH into culture medium. Future studies are required to further characterize the neuroendocrine properties of these GnRH-expressing cells. Our protocol was reproducible in hiPSCs, which implicates that it can be employed to model diseases that affect GnRH neuron specification, such as hypogonadotropic hypogonadism due to congenital GnRH deficiency.
Experimental Procedures
See also Supplemental Experimental Procedures.
Human Pluripotent Stem Cells
hESC line H9 ((Thomson et al., 1998); Wicell) and two hiPSC lines HEL11.4 (Mikkola et al., 2013) and HEL24.3 (Trokovic et al., 2015) were used in this study. hPSCs were maintained on Geltrex-coated plates with StemPro or Essential 8 medium.
GnRH Neuron Differentiation
N2B27 medium was used throughout the differentiation protocol. For d0–d10, medium was supplemented with dual SMAD inhibitors, 2 μM dorsomorphin, and 10 μM SB431542 (Sigma). At day 11, dual SMAD inhibition was withdrawn and 100 ng/ml FGF8 (Peprotech) was added. From d21 onward, medium was supplemented with both FGF8 and 20 μM Notch inhibitor DAPT (Sigma). Control cells did not receive FGF8 supplementation.
Analysis of GnRH Expression and Secretion
RNA extraction and real-time qPCR were performed using standard protocols. The expression levels of target genes were normalized to cyclophilin G, and compared with undifferentiated hPSCs. For immunocytochemistry, cells were fixed by 4% paraformaldehyde and immunostainings were performed using standard protocols. FOXG1 and GnRH immunopositive cells were quantified by manually counting the cells in images taken with a 40× objective. Secreted GnRH was quantified by a competitive fluorescent enzyme immunoassay.
Human Samples
A human fetus (8 gestational weeks) was obtained from a voluntarily terminated pregnancy with the parent's written informed consent. Permission to utilize human fetal tissues was obtained from the French agency for biomedical research (Agene de la Biomédecine, Saint-Denis la Plaine, France, protocol no. PFS16-002). Tissues were made available in accordance with French bylaws. Tissues were fixed with 4% paraformaldehyde and immunostaining was performed on 20-μm cryosections. Immunostaining was performed according to standard procedures (INSERM).
Statistical Analyses
When used, “n” always stands for number of independent experiments. Statistical comparisons were performed by using paired ratio t test in Prism 5.0 (GraphPad). p < 0.05 was accepted to indicate statistical significance.
Acknowledgments
We thank the Biomedicum Stem Cell Center and the Biomedicum Imaging Unit for advice and assistance. We thank Professor Erik Hrabovszky for the generous gift of GnRH antibody, Professor Timo Otonkoski for valuable comments, Mr. Samuel Malone for the human fetus collection and processing of the samples for immunohistochemical procedures, and M.A. Annika Tarkkanen for linguistic guidance. This work was supported by the Academy of Finland, Foundation for Pediatric Research, Sigrid Juselius Foundation, Novo Nordisk Foundation, Emil Aaltonen Foundation, University of Helsinki, Helsinki University Central Hospital, and Agence Nationale de la Recherche, ANR, France (ANR-14-CE12-0015-01 RoSes and GnRH).
Published: July 14, 2016
Footnotes
Supplemental Information includes Supplemental Experimental Procedures, three figures, and two tables and can be found with this article online at http://dx.doi.org/10.1016/j.stemcr.2016.06.007.
Supplemental Information
References
- Borghese L., Dolezalova D., Opitz T., Haupt S., Leinhaas A., Steinfarz B., Koch P., Edenhofer F., Hampl A., Brustle O. Inhibition of notch signaling in human embryonic stem cell-derived neural stem cells delays G1/S phase transition and accelerates neuronal differentiation in vitro and in vivo. Stem Cells. 2010;28:955–964. doi: 10.1002/stem.408. [DOI] [PubMed] [Google Scholar]
- Casoni F., Hutchins B.I., Donohue D., Fornaro M., Condie B.G., Wray S. SDF and GABA interact to regulate axophilic migration of GnRH neurons. J. Cell Sci. 2012;125:5015–5025. doi: 10.1242/jcs.101675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chambers S.M., Fasano C.A., Papapetrou E.P., Tomishima M., Sadelain M., Studer L. Highly efficient neural conversion of human ES and iPS cells by dual inhibition of SMAD signaling. Nat. Biotechnol. 2009;27:275–280. doi: 10.1038/nbt.1529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung W.C., Tsai P.S. Role of fibroblast growth factor signaling in gonadotropin-releasing hormone neuronal system development. Front. Horm. Res. 2010;39:37–50. doi: 10.1159/000312692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis R.P., Casini S., van den Berg C.W., Hoekstra M., Remme C.A., Dambrot C., Salvatori D., Oostwaard D.W., Wilde A.A., Bezzina C.R. Cardiomyocytes derived from pluripotent stem cells recapitulate electrophysiological characteristics of an overlap syndrome of cardiac sodium channel disease. Circulation. 2012;125:3079–3091. doi: 10.1161/CIRCULATIONAHA.111.066092. [DOI] [PubMed] [Google Scholar]
- Dode C., Levilliers J., Dupont J.M., De Paepe A., Le Du N., Soussi-Yanicostas N., Coimbra R.S., Delmaghani S., Compain-Nouaille S., Baverel F. Loss-of-function mutations in FGFR1 cause autosomal dominant Kallmann syndrome. Nat. Genet. 2003;33:463–465. doi: 10.1038/ng1122. [DOI] [PubMed] [Google Scholar]
- Duggan C.D., DeMaria S., Baudhuin A., Stafford D., Ngai J. Foxg1 is required for development of the vertebrate olfactory system. J. Neurosci. 2008;28:5229–5239. doi: 10.1523/JNEUROSCI.1134-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falardeau J., Chung W.C., Beenken A., Raivio T., Plummer L., Sidis Y., Jacobson-Dickman E.E., Eliseenkova A.V., Ma J., Dwyer A. Decreased FGF8 signaling causes deficiency of gonadotropin-releasing hormone in humans and mice. J. Clin. Invest. 2008;118:2822–2831. doi: 10.1172/JCI34538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forni P.E., Taylor-Burds C., Melvin V.S., Williams T., Wray S. Neural crest and ectodermal cells intermix in the nasal placode to give rise to GnRH-1 neurons, sensory neurons, and olfactory ensheathing cells. J. Neurosci. 2011;31:6915–6927. doi: 10.1523/JNEUROSCI.6087-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forni P.E., Bharti K., Flannery E.M., Shimogori T., Wray S. The indirect role of fibroblast growth factor-8 in defining neurogenic niches of the olfactory/GnRH systems. J. Neurosci. 2013;33:19620–19634. doi: 10.1523/JNEUROSCI.3238-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Givens M.L., Rave-Harel N., Goonewardena V.D., Kurotani R., Berdy S.E., Swan C.H., Rubenstein J.L., Robert B., Mellon P.L. Developmental regulation of gonadotropin-releasing hormone gene expression by the MSX and DLX homeodomain protein families. J. Biol. Chem. 2005;280:19156–19165. doi: 10.1074/jbc.M502004200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hay D.C., Zhao D., Fletcher J., Hewitt Z.A., McLean D., Urruticoechea-Uriguen A., Black J.R., Elcombe C., Ross J.A., Wolf R., Cui W. Efficient differentiation of hepatocytes from human embryonic stem cells exhibiting markers recapitulating liver development in vivo. Stem Cells. 2008;26:894–902. doi: 10.1634/stemcells.2007-0718. [DOI] [PubMed] [Google Scholar]
- Herde M.K., Iremonger K.J., Constantin S., Herbison A.E. GnRH neurons elaborate a long-range projection with shared axonal and dendritic functions. J. Neurosci. 2013;33:12689–12697. doi: 10.1523/JNEUROSCI.0579-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikeda K., Ookawara S., Sato S., Ando Z., Kageyama R., Kawakami K. Six1 is essential for early neurogenesis in the development of olfactory epithelium. Dev. Biol. 2007;311:53–68. doi: 10.1016/j.ydbio.2007.08.020. [DOI] [PubMed] [Google Scholar]
- Imayoshi I., Sakamoto M., Yamaguchi M., Mori K., Kageyama R. Essential roles of Notch signaling in maintenance of neural stem cells in developing and adult brains. J. Neurosci. 2010;30:3489–3498. doi: 10.1523/JNEUROSCI.4987-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iyer A.K., Miller N.L., Yip K., Tran B.H., Mellon P.L. Enhancers of GnRH transcription embedded in an upstream gene use homeodomain proteins to specify hypothalamic expression. Mol. Endocrinol. 2010;24:1949–1964. doi: 10.1210/me.2010-0156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawauchi S., Shou J., Santos R., Hebert J.M., McConnell S.K., Mason I., Calof A.L. FGF8 expression defines a morphogenetic center required for olfactory neurogenesis and nasal cavity development in the mouse. Development. 2005;132:5211–5223. doi: 10.1242/dev.02143. [DOI] [PubMed] [Google Scholar]
- Kim K.H., Patel L., Tobet S.A., King J.C., Rubin B.S., Stopa E.G. Gonadotropin-releasing hormone immunoreactivity in the adult and fetal human olfactory system. Brain Res. 1999;826:220–229. doi: 10.1016/s0006-8993(99)01271-8. [DOI] [PubMed] [Google Scholar]
- Kirkeby A., Grealish S., Wolf D.A., Nelander J., Wood J., Lundblad M., Lindvall O., Parmar M. Generation of regionally specified neural progenitors and functional neurons from human embryonic stem cells under defined conditions. Cell Rep. 2012;1:703–714. doi: 10.1016/j.celrep.2012.04.009. [DOI] [PubMed] [Google Scholar]
- Markakis E.A., Palmer T.D., Randolph-Moore L., Rakic P., Gage F.H. Novel neuronal phenotypes from neural progenitor cells. J. Neurosci. 2004;24:2886–2897. doi: 10.1523/JNEUROSCI.4161-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maroof A.M., Keros S., Tyson J.A., Ying S.W., Ganat Y.M., Merkle F.T., Liu B., Goulburn A., Stanley E.G., Elefanty A.G. Directed differentiation and functional maturation of cortical interneurons from human embryonic stem cells. Cell Stem Cell. 2013;12:559–572. doi: 10.1016/j.stem.2013.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merkle F.T., Maroof A., Wataya T., Sasai Y., Studer L., Eggan K., Schier A.F. Generation of neuropeptidergic hypothalamic neurons from human pluripotent stem cells. Development. 2015;142:633–643. doi: 10.1242/dev.117978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merlo G.R., Mantero S., Zaghetto A.A., Peretto P., Paina S., Gozzo M. The role of Dlx homeogenes in early development of the olfactory pathway. J. Mol. Histol. 2007;38:612–623. doi: 10.1007/s10735-007-9154-x. [DOI] [PubMed] [Google Scholar]
- Mikkola M., Toivonen S., Tamminen K., Alfthan K., Tuuri T., Satomaa T., Natunen J., Saarinen J., Tiittanen M., Lampinen M. Lectin from Erythrina cristagalli supports undifferentiated growth and differentiation of human pluripotent stem cells. Stem Cells Dev. 2013;22:707–716. doi: 10.1089/scd.2012.0365. [DOI] [PubMed] [Google Scholar]
- Miller A.M., Treloar H.B., Greer C.A. Composition of the migratory mass during development of the olfactory nerve. J. Comp. Neurol. 2010;518:4825–4841. doi: 10.1002/cne.22497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noisa P., Lund C., Kanduri K., Lund R., Lahdesmaki H., Lahesmaa R., Lundin K., Chokechuwattanalert H., Otonkoski T., Tuuri T., Raivio T. Notch signaling regulates the differentiation of neural crest from human pluripotent stem cells. J. Cell Sci. 2014;127:2083–2094. doi: 10.1242/jcs.145755. [DOI] [PubMed] [Google Scholar]
- Pankratz M.T., Li X.J., Lavaute T.M., Lyons E.A., Chen X., Zhang S.C. Directed neural differentiation of human embryonic stem cells via an obligated primitive anterior stage. Stem Cells. 2007;25:1511–1520. doi: 10.1634/stemcells.2006-0707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porter A.G., Janicke R.U. Emerging roles of caspase-3 in apoptosis. Cell Death Differ. 1999;6:99–104. doi: 10.1038/sj.cdd.4400476. [DOI] [PubMed] [Google Scholar]
- Rawson N.E., Lischka F.W., Yee K.K., Peters A.Z., Tucker E.S., Meechan D.W., Zirlinger M., Maynard T.M., Burd G.B., Dulac C. Specific mesenchymal/epithelial induction of olfactory receptor, vomeronasal, and gonadotropin-releasing hormone (GnRH) neurons. Dev. Dyn. 2010;239:1723–1738. doi: 10.1002/dvdy.22315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabado V., Barraud P., Baker C.V.H., Streit A. Specification of GnRH-1 neurons by antagonistic FGF and retinoic acid signaling. Dev. Biol. 2012;362:254–262. doi: 10.1016/j.ydbio.2011.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salvi R., Arsenijevic Y., Giacomini M., Rey J.P., Voirol M.J., Gaillard R.C., Risold P.Y., Pralong F. The fetal hypothalamus has the potential to generate cells with a gonadotropin releasing hormone (GnRH) phenotype. PLoS One. 2009;4:e4392. doi: 10.1371/journal.pone.0004392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlosser G. Early embryonic specification of vertebrate cranial placodes. Wiley Interdiscip. Rev. Dev. Biol. 2014;3:349–363. doi: 10.1002/wdev.142. [DOI] [PubMed] [Google Scholar]
- Schlosser G., Awtry T., Brugmann S.A., Jensen E.D., Neilson K., Ruan G., Stammler A., Voelker D., Yan B., Zhang C. Eya1 and Six1 promote neurogenesis in the cranial placodes in a SoxB1-dependent fashion. Dev. Biol. 2008;320:199–214. doi: 10.1016/j.ydbio.2008.05.523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwanzel-Fukuda M., Pfaff D.W. Origin of luteinizing hormone-releasing hormone neurons. Nature. 1989;338:161–164. doi: 10.1038/338161a0. [DOI] [PubMed] [Google Scholar]
- Shimamura K., Rubenstein J.L. Inductive interactions direct early regionalization of the mouse forebrain. Development. 1997;124:2709–2718. doi: 10.1242/dev.124.14.2709. [DOI] [PubMed] [Google Scholar]
- Simeone A., Gulisano M., Acampora D., Stornaiuolo A., Rambaldi M., Boncinelli E. Two vertebrate homeobox genes related to the Drosophila empty spiracles gene are expressed in the embryonic cerebral cortex. EMBO J. 1992;11:2541–2550. doi: 10.1002/j.1460-2075.1992.tb05319.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simeone A., Acampora D., Pannese M., D'Esposito M., Stornaiuolo A., Gulisano M., Mallamaci A., Kastury K., Druck T., Huebner K. Cloning and characterization of two members of the vertebrate Dlx gene family. Proc. Natl. Acad. Sci. USA. 1994;91:2250–2254. doi: 10.1073/pnas.91.6.2250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storm E.E., Garel S., Borello U., Hebert J.M., Martinez S., McConnell S.K., Martin G.R., Rubenstein J.L. Dose-dependent functions of FGF8 in regulating telencephalic patterning centers. Development. 2006;133:1831–1844. doi: 10.1242/dev.02324. [DOI] [PubMed] [Google Scholar]
- Tarozzo G., Peretto P., Fasolo A. Cell migration from the olfactory placode and the ontogeny of the neuroendocrine compartments. Zoolog. Sci. 1995;12:367–383. doi: 10.2108/zsj.12.367. [DOI] [PubMed] [Google Scholar]
- Thomson J.A., Itskovitz-Eldor J., Shapiro S.S., Waknitz M.A., Swiergiel J.J., Marshall V.S., Jones J.M. Embryonic stem cell lines derived from human blastocysts. Science. 1998;282:1145–1147. doi: 10.1126/science.282.5391.1145. [DOI] [PubMed] [Google Scholar]
- Trokovic R., Weltner J., Otonkoski T. Generation of iPSC line HEL24.3 from human neonatal foreskin fibroblasts. Stem Cell Res. 2015;15:266–268. doi: 10.1016/j.scr.2015.05.012. [DOI] [PubMed] [Google Scholar]
- Wang L., Meece K., Williams D.J., Lo K.A., Zimmer M., Heinrich G., Martin Carli J., Leduc C.A., Sun L., Zeltser L.M. Differentiation of hypothalamic-like neurons from human pluripotent stem cells. J. Clin. Invest. 2015;125:796–808. doi: 10.1172/JCI79220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitlock K.E., Wolf C.D., Boyce M.L. Gonadotropin-releasing hormone (GnRH) cells arise from cranial neural crest and adenohypophyseal regions of the neural plate in the zebrafish, Danio rerio. Dev. Biol. 2003;257:140–152. doi: 10.1016/s0012-1606(03)00039-3. [DOI] [PubMed] [Google Scholar]
- Wray S. From nose to brain: development of gonadotrophin-releasing hormone-1 neurones. J. Neuroendocrinol. 2010;22:743–753. doi: 10.1111/j.1365-2826.2010.02034.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wray S., Grant P., Gainer H. Evidence that cells expressing luteinizing hormone-releasing hormone mRNA in the mouse are derived from progenitor cells in the olfactory placode. Proc. Natl. Acad. Sci. USA. 1989;86:8132–8136. doi: 10.1073/pnas.86.20.8132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wray S., Nieburgs A., Elkabes S. Spatiotemporal cell expression of luteinizing hormone-releasing hormone in the prenatal mouse: evidence for an embryonic origin in the olfactory placode. Brain Res. Dev. Brain Res. 1989;46:309–318. doi: 10.1016/0165-3806(89)90295-2. [DOI] [PubMed] [Google Scholar]
- Wray S., Key S., Qualls R., Fueshko S.M. A subset of peripherin positive olfactory axons delineates the luteinizing hormone releasing hormone neuronal migratory pathway in developing mouse. Dev. Biol. 1994;166:349–354. doi: 10.1006/dbio.1994.1320. [DOI] [PubMed] [Google Scholar]
- Yoon K., Gaiano N. Notch signaling in the mammalian central nervous system: insights from mouse mutants. Nat. Neurosci. 2005;8:709–715. doi: 10.1038/nn1475. [DOI] [PubMed] [Google Scholar]
- Zhang X., Huang C.T., Chen J., Pankratz M.T., Xi J., Li J., Yang Y., Lavaute T.M., Li X.J., Ayala M. Pax6 is a human neuroectoderm cell fate determinant. Cell Stem Cell. 2010;7:90–100. doi: 10.1016/j.stem.2010.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.