Abstract
Background
Multi-drug resistant tuberculosis (MDR-TB) is a major public health problem especially in developing countries. World Health Organization (WHO) recommends use of Xpert MTB/RIF assay to simultaneously detecting Mycobacterium tuberculosis (MTB) and rifampicin (RIF) resistance. The primary objective of this study was to determine the frequency of MDR-TB in patients suspected to have drug resistance in Khyber Pakhtunkhwa. The frequency of probes for various rpoB gene mutations using Xpert MTB/RIF assay within 81 bp RRDR (Rifampicin Resistance Determining Region) was the secondary objective.
Methods
A total of 2391 specimens, received at Programmatic Management of Drug Resistant TB (PMDT) Unit, Lady Reading Hospital (LRH) Peshawar, Pakistan, between October 2011 and December 2014, were analyzed by Xpert MTB/RIF test. MTB positive with rifampicin resistance were further analyzed to first line anti-mycobacterial drug susceptibility testing (DST) using middle brook 7H10 medium. The data was analyzed using statistical software; SPSS version 18.
Results
Out of 2391 specimens, 1408 (59 %) were found positive for MTB and among them, 408 (29 %) showed rifampicin-resistance with four different rpoB gene mutations within 81 bp RRDR. The frequency of various probes among RIF-resistant isolates was observed as: probe E, in 314 out of 408 isolates; B, 44 out of 408; A, 5 out of 408; D, 34 out of 408; and probe C was observed among 6 out of 408 RIF-resistant isolates. The probe A&B and E&D mutation combination was found in only 1 isolate in each case, while B&D mutation combination was detected among 3 out of 408 RIF-resistant isolates.
Conclusions
Hence, it is concluded from our study on a selected population, 29 % of patients had MDR-TB. Probe E related mutations (also known as codon 531and 533) were the most common rpoB genetic mutation [314 (77 %)], acknowledged by Xpert MTB/RIF assay. Least mutation was detected within the sequence 511 (1.2 %).
Keywords: RRDR, RNA polymerase B gene, MDR-TB, Xpert MTB/RIF
Background
Multi-drug resistant tuberculosis (MDR-TB), is defined as a form of TB infection caused by Mycobaterium tuberculosis that is resistant to treatment with at least two of the most powerful first-line anti-TB drugs, isoniazid and rifampicin [1]. Rifampicin (RIF) resistance is of specific epidemiological importance and a valuable surrogate marker for MDR-TB strains as more than 90 % RIF resistant strains are also resistant to isoniazid [2, 3]. Introduction of Xpert MTB/RIF assay (Cepheid, USA) has revolutionized the diagnosis of TB by simultaneously detecting MTB and rifampicin resistance [4, 5]. Rifampicin is one of the key first-line anti-tuberculosis drug, which inhibits DNA-directed ribonucleic acid synthesis of MTB proteins by binding to the β-subunit of bacterial DNA dependent RNA polymerase enzyme (rpoB) protein. In general, rpoB mutations is found in 95–97 % of RIF-resistant MTB strains worldwide and these mutations are typically located in a region at the 507–533 amino acid residuals (81 bp) within the rpoB genetic factor, that is usually referred to as Rifampicin Resistant Determinant Region (RRDR) [6].
Pakistan ranks 5th among 22 high TB burden countries and 4th among MDR-TB countries. WHO report showed that the prevalence of MDR-TB was 4 % and 35 % among all new and previously treated TB cases, respectively [1]. The biological features of bacteria as well as drug resistance typically vary in different geographical areas [7–9]. Data on prevalence of rpoB gene mutations in Pakistan is limited, so this study is aimed to provide reference line data on these mutations using Xpert MTB/RIF assay. The detection of rpoB gene mutations is most important for accurate diagnosis of RIF resistance in MTB strains [10]. The current study was aimed to investigate the frequency of RIF-resistant MTB strains in specimens collected from TB presumptive patients in Khyber Pakhtoonkhwa, Pakistan and also mutations at RRDR within the rpoB gene of RIF-resistant MTB using Xpert MTB/RIF assay.
Methods
We studied 2391 patients in this study. Each patient was required to provide one sputum specimen.
Clinical specimens
Total numbers of 2391 specimens from any drug resistant TB presumptive patients were received at PMDT, LRH between October 2011 and December 2014. All these specimens were investigated for detection of MTB as well as resistance to the first-line anti-TB drug, Rifampicin using Xpert MTB/RIF. The referrals of these specimens were according to the guidelines of NTP for Xpert MTB/RIF.
Criteria for testing on Xpert MTB/RIF
The following criteria was considered for testing specimens on Xpert MTB/RIF: 1. All smear positive new cases that remain positive by the end of 2nd month of treatment; 2. Failure of Cat-I and Cat-II; 3. Re-treated pulmonary cases both smear positive and smear negative;4. Contact with MDR-TB patients; and 5. TB/HIV co-infection cases were considered for testing through Xpert MTB/RIF.
Important details and specimen characteristics (volume and consistence) were recorded in laboratory data assortment forms and later on transferred to access restricted computer-based database. These specimens included sputa, cerebral spinal fluid (CSF), pleural fluid, pus, ascetic fluid, pericardial fluid and bronchial wash (Table 1). TB diagnostics requests ranged from microscopy, culture and Xpert test. In addition to routine analysis, we also performed DST on all MTB positive and rifampicin resistant detected (RRD) cases that were identified by Xpert MTB/RIF test as shown in Fig 1.
Table 1.
Breakdown of samples in extra pulmonary Tuberculosis
| Sample name | Frequency (%) |
|---|---|
| Sputum | 2272 (95) |
| Bronchial Wash | 54 (2.2) |
| Ascetic fluid | 23 (1) |
| CSF | 8 (0.3) |
| PUS | 23 (1) |
| Pericardial fluid | 11 (0.5) |
| Total | 2391 |
Fig. 1.
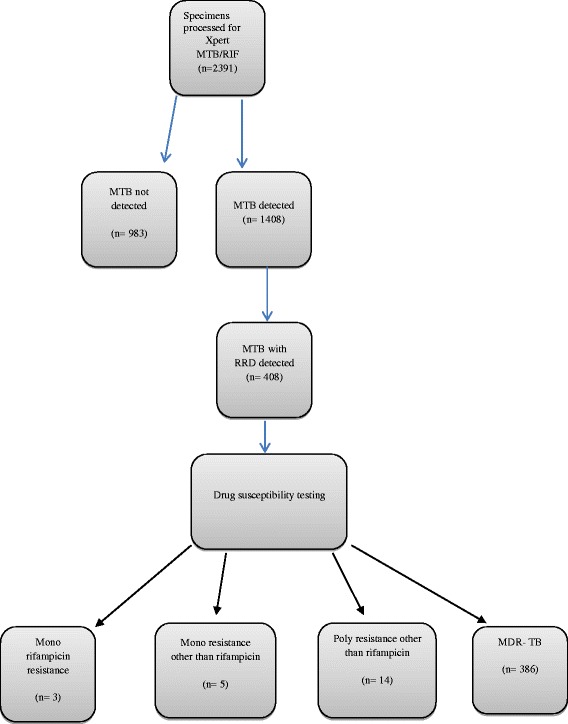
Flow chart of specimens’ analysis (submitted separately via online)
Drug susceptibility testing
Drug susceptibility testing was performed using the standard agar proportion method on enriched Middle brook 7H10 medium (BBL, Bechton Dickinson and Company, New Jersey) at the following drug concentration: Isoniazid 0.2 and 1 μg/ml, rifampicin 1 and 5 μg/ml, ethambutol 5 and 10 μg/ml and streptomycin 2 and 10 μg/ml [11, 12]. Mycobacterium tuberculosis H37Rv (sensitive to all anti-tuberculosis drugs) was used as reference control strain. The drugs were used at critical concentrations.
MTB/RIF assay
Non-sterile clinical specimens were processed by the standard N-acetyl-L-cysteine–NaOH technique. [13] Smears were prepared by the auramine-rhodamine acid-fast staining technique. The MTB/RIF assay was done as described previously [4, 14]. N-acetyl-L-cysteine–NaOH was added to the clinical specimens at a 3:1 ratio, for decontamination in a closed specimen. The container was manually agitated 15-min period at room temperature. 2 ml of inactivated specimen was transferred to the Xpert test cartridge and tested according to manufacturer's guide [4, 14].
Statistical analysis
The data was analyzed by using Statistical Package for Social Sciences (SPSS 18).
Results
Xpert® MTB/RIF assay
Out of RRD cases, 42 (3 %) were new and 366 (26 %) previously treated cases. Females were 243 (59.6 %) and males 165 (40.4 %). Maximum number of RRD patients was found in the age group 15–24 years (38.5 %) as shown in Table 2.
Table 2.
Detection of mutation in rpoB gene of rifampicin in rifampicin resistance detected (RRD) cases with different demographic representation
| Demography | Probe types | Total (%) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| A | B | C | D | E | A&B | B&D | E&D | ||
| Gender | |||||||||
| Female | 3 | 25 | 4 | 22 | 187 | 1 | 0 | 1 | 243 (59.6) |
| Male | 2 | 19 | 2 | 12 | 127 | 0 | 3 | 0 | 165 (40.4) |
| History | |||||||||
| New patients | 1 | 1 | 1 | 4 | 35 | 0 | 0 | 0 | 42 (10.3) |
| Previously treated patients | 4 | 43 | 5 | 30 | 279 | 1 | 3 | 1 | 366 (89.7) |
| Age categories (years) | |||||||||
| <14 | 0 | 2 | 0 | 3 | 24 | 0 | 0 | 0 | 29 (7.1) |
| 15- 24 | 0 | 14 | 3 | 12 | 125 | 1 | 2 | 0 | 157 (38.5) |
| 25- 34 | 3 | 11 | 1 | 7 | 82 | 0 | 0 | 0 | 104 (25.5) |
| 35- 44 | 2 | 9 | 0 | 2 | 36 | 0 | 0 | 0 | 49 (12) |
| 45- 54 | 0 | 3 | 1 | 1 | 25 | 0 | 0 | 1 | 31 (7.6) |
| 55- 64 | 0 | 3 | 1 | 7 | 19 | 0 | 0 | 0 | 30 (7.3) |
| 64< | 0 | 2 | 0 | 2 | 3 | 0 | 1 | 0 | 8 (2) |
Among 2391 specimens tested by Xpert MTB/RIF, MTB was detected in 1408 (58.9 %) specimens. Out of total MTB cases, 408 (29 %) were detected to have RIF-resistance. The resistance was given by four totally different rpoB gene mutations within the 81 bp-RRDR of MTB. These were detected by A, B, C D, E, A&B, B&D and E&D probes. The probe frequency that reflects RIF-resistance was observed as follows: probe A frequency was 1.2 %; B, 10.8 %; C, 1.5 %; D, 8.3 %; and probe E frequency was highest (77 %) among all. Mutation combination of probe A&B and E&D was 0.25 %, while a B&D mutation combination was 0.7 % (Table 2).
408 RRD cases, checked by Xpert MTB/RIF were further subjected to DST. Out of 408 cases, 19 (4.6 %) were failed to show RIF-resistance, 5 (1.2 %) mono resistant and 14 (3.4 %) showed poly resistance to first-line anti-TB drugs other than rifampicin, while 386 (94.6 %) cases were found to be MDR on DST (Table 3). 252 (61.6 %) cases were resistant to all first-line anti-TB drugs while 386 (94.6 %) cases of rifampicin resistant were also found resistant to isoniazid.
Table 3.
Pattern of anti-TB drugs susceptibility testing against RRD cases (n = 408) by Xpert MTB/RIF
| Anti-TB Drugs | Resistance (n) | Resistance (%) |
|---|---|---|
| H | 3 | (0.73) |
| R | 3 | (0.73) |
| S | 2 | (0.5) |
| ES | 2 | (0.5) |
| HE | 3 | (0.73) |
| HS | 2 | (0.5) |
| HZ | 2 | (0.5) |
| HR | 3 | (0.73) |
| HEZ | 2 | (0.5) |
| HRE | 2 | (0.5) |
| HRZ | 34 | (8.3) |
| HEZS | 3 | (0.73) |
| HREZ | 72 | (17.6) |
| HRZS | 23 | (5.6) |
| HREZS | 252 | (61.6) |
H isoniazid, R rifampicin, S streptomycin, E ethambutol, Z pyrazinamide
Discussion
The genetic basis of anti-tuberculosis drug resistant MTB isolates has been widely studied worldwide and commonly believed to be caused by point mutations in some important genes like rpoB, katG, rpsL, embB etc. [6]. Meanwhile globally MDR-TB is big threat to human especially in developing countries, therefore in the present study we use Xpert MTB/RIF to check drug resistant isolates of MTB for mutation in the rpoB gene of rifampicin as more than 90 % strains are also resistant to isoniazid [2, 3]. Khyber Pakhtoonkhwa is one of the four provinces of Pakistan, that share a common border with Afghanistan, and over 30 Million population. To the best of our knowledge, this is the first report ever in this region where Xpert MTB/RIF method has been used for studying over all prevalence of MTB. This study is of great significance because it would provide baseline data in case if the scope is extended to other provinces in Pakistan.
Xpert MTB/RIF test is increasingly used in developing high burden countries to diagnose drug resistant MTB, while conventional test such as culture based DST is considered to be a “gold standard” for MDR-TB. In the present study, 389 (95.4 %) MTB isolates, found RIF resistant by Xpert MTB/RIF test, were confirmed to be resistant to RIF by DST. However the remaining 19 (4.6 %) isolates, initially diagnosed as resistant to RIF by Xpert MTB/RIF, were tested susceptible to RIF by DST. The previous reports indicates that lack of concordance in the results are not due to performance of molecular method [5, 15–18], but this discrepancy may be due to well-known fact that not every genotypic modification of rpoB gene affects phenotypic resistance to RIF correspondingly. It has been observed in the previous studies that the value of the RIF MIC strongly correlates with the mutation in rpoB RRDR. [19, 20] Feuerriegel et al. stated that in Sierra Leone, the isolates from re-treatment cases were re-tested by DNA sequencing, 5 out of 21 (24 %) cases were found RIF susceptible by DST. [21] In our view, the conventional test i.e. DST, fails to detect mutation in 19 (4.6 %) isolates. Present data indicates that these dissimilarities in mutation may be due to poor clinical scenario that is utmost common and undisputed, while few reports had previously suggested that such isolates have clinical relevance [22, 23].
The most common RRDR rpoB gene mutations within the 81 bp were detected in codons 531 (77 %), 513 (10.8 %), 526 (8.3 %), 511 (1.2 %), and 522 (1.5 %) sequences as selected by probes E, B, D, A, and C, respectively, using Xpert MTB/ RIF assay. A study by Yue et al. found these frequencies 531 (41 %), 526 (40 %), and 513 (4 %) in China [24]. Mboowa et al. also reported that 58 % mutations were in probe E in Kampala, Uganda [25]. Khan et al. also described mutations in codons 531 (52 %), 516 (15 %) and 526 (7.0 %) in Punjab, Pakistan [26]. These studies conclude that sequence 531 is the most prevalent codon related to RIF-resistance. According to the previous studies, the sensitivity and specificity of the MTB/RIF for detection of rifampicin resistance was 94.4-100 % and 98.3-100 % [5, 27].
In current study, we have reported 29 % MDR-TB in presumptive drug resistant TB patients in Khyber Pakhtoonkhwa, which is similar to Liu et al. finding as 30.4 % isolates were resistant to at least one first-line anti-TB drug [28]. Luiz et al. reported 44.3 % isolates resistant to at least one first-line anti-TB drug in Brazil which is more than our findings [29]. Ullah et al. [30] and Javaid et al. [11] reported 11.5 and 11.3 % resistance to at least one anti-TB drug in different parts of Punjab, Pakistan. In order to fight the risk MDR TB, a recent Drug Resistant Survey, Study was conducted in Pakistan showed that estimated percentage of MDR TB in new notified TB cases is 4.3 % and in Re-treatment cases is 19.4 % [1]. This difference may be due to geographical location, sample size, and methodology for selection of presumptive patients for MDR-TB.
WHO expanded its MDR-TB detection program in 2012 within the high TB burden countries and use of Xpert MTB/RIF Assay was approved for efficient detection of resistance against anti-TB drugs [1]. Thus the assay could be a useful tool in World’s fight against TB/MDR-TB especially in high TB burden countries such as Pakistan.
Conclusions
In this report, the Xpert MTB/RIF assay detected the RIF-resistance associated mutations in RRDR 81 bp region. Hence, it is concluded from our study on a selected population, 29 % of patients had MDR-TB. Probe E related mutations (also known as codon 531and 533) were the most common rpoB genetic mutation [314 (77 %)], acknowledged by Xpert MTB/RIF assay. Least mutation was detected within the sequence 511 (1.2 %). Further studies ought to be done involving MDR-TB strains isolated from Pakistani population in order and data concerning these mutations would be helpful in development of novel therapies against TB disease.
Abbreviation
CSF, cerebral spinal fluid; DST, drug sensitivity test; LRH, lady reading hospital; MDR-TB, multi drug resistant tuberculosis; PGMI, post graduate medical institute; PMDT, programmatic management of drug resistant tuberculosis; RIF; rpoB gene, RNA Polymerase B gene; RRDR, rifampicin resistant determinant region
Acknowledgment
All the PMDT staff of LRH Peshawar, National TB Control Program of Pakistan, Dr Najma Ayub and Dr Fariha Hasan for their kind support of the conducting this study.
Funding
Funding was not obtained for this study.
Availability of data and materials
All the data supporting our findings is contained within the manuscript.
Authors’ contribution
All authors have read and approve of the final version of the manuscript. Conceived and designed the experiments: IU, AJ. Performed the experiments: IU. Analyzed the data: IU, AK, MA, AB, UU, MI, SM, AM. Wrote the paper: IU, AJ, AAS.
Competing interest
The authors declared that they have no competing interests.
Consent for publish
Our manuscript does not contain any individual persons’ data.
Ethical and Consent to Participate
The present research work was approved by the ethical committee of Post Graduate Medical Institute (PGMI), Lady Reading Hospital (LRH), Peshawar, KPK, Pakistan, in accordance with the ethical standards of the responsible committee on human experimentation and with the latest (2008) version of Helsinki Declaration of 1975. The purpose of study was explained and a written informed consent was obtained from all patients or from next of their kin, caretakers, or guardians/parents on behalf of all child participants.
Contributor Information
Irfan Ullah, Phone: +92 333 8 333 122, Email: irfan_btn@hotmail.com.
Aamer Ali Shah, Email: aamerali.shah@gmail.com.
Anila Basit, Email: zarfishantahir@yahoo.com.
Mazhar Ali, Email: mali_smile2005@yahoo.com.
Afsar khan, Email: afsarafridik@yahoo.com.
Ubaid Ullah, Email: dr_ubaid03@hotmail.com.
Muhammad Ihtesham, Email: ullahobaid445@yahoo.com.
Sumaira Mehreen, Email: sumairapsy@yahoo.com.
Anita Mughal, Email: ahmarahmad46@yahoo.com.
Arshad Javaid, Email: arshadjavaid1@hotmail.com.
References
- 1.Organization WH. World Health Organization Global Tuberculosis Report 2013. Geneva, Switzerland: World Health Organization, WHO Press; 2013. [Google Scholar]
- 2.Drobniewski F, Wilson S. The rapid diagnosis of isoniazid and rifampicin resistance in Mycobacterium tuberculosis—a molecular story. J Med Microbiol. 1998;47(3):189–196. doi: 10.1099/00222615-47-3-189. [DOI] [PubMed] [Google Scholar]
- 3.Mani C, Selvakumar N, Narayanan S, Narayanan P. Mutations in the rpoB gene of multidrug-resistant Mycobacterium tuberculosis clinical isolates from India. J Clin Microbiol. 2001;39(8):2987–2990. doi: 10.1128/JCM.39.8.2987-2990.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Helb D, Jones M, Story E, Boehme C, Wallace E, Ho K, Kop J, Owens MR, Rodgers R, Banada P. Rapid detection of Mycobacterium tuberculosis and rifampin resistance by use of on-demand, near-patient technology. J Clin Microbiol. 2010;48(1):229–237. doi: 10.1128/JCM.01463-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Boehme CC, Nicol MP, Nabeta P, Michael JS, Gotuzzo E, Tahirli R, Gler MT, Blakemore R, Worodria W, Gray C. Feasibility, diagnostic accuracy, and effectiveness of decentralised use of the Xpert MTB/RIF test for diagnosis of tuberculosis and multidrug resistance: a multicentre implementation study. The lancet. 2011;377(9776):1495–1505. doi: 10.1016/S0140-6736(11)60438-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ramaswamy S, Musser JM. Molecular genetic basis of antimicrobial agent resistance inMycobacterium tuberculosis: 1998 update. Tuber Lung Dis. 1998;79(1):3–29. doi: 10.1054/tuld.1998.0002. [DOI] [PubMed] [Google Scholar]
- 7.Li W, Wang S, Li C, Liu Y, Shen G, Zhang X, Niu T, Gao Q, Van Soolingen D, Kremer K. Molecular epidemiology of Mycobacterium tuberculosis in China: a nationwide random survey in 2000. Int J Tuberc Lung Dis. 2005;9(12):1314–1319. [PubMed] [Google Scholar]
- 8.Mokrousov I, Bhanu NV, Suffys PN, Kadival GV, Yap S-F, Cho S-N, Jordaan AM, Narvskaya O, Singh UB, Gomes HM. Multicenter evaluation of reverse line blot assay for detection of drug resistance in Mycobacterium tuberculosis clinical isolates. J Microbiol Methods. 2004;57(3):323–335. doi: 10.1016/j.mimet.2004.02.006. [DOI] [PubMed] [Google Scholar]
- 9.Brossier F, Veziris N, Truffot-Pernot C, Jarlier V, Sougakoff W. Performance of the genotype MTBDR line probe assay for detection of resistance to rifampin and isoniazid in strains of Mycobacterium tuberculosis with low-and high-level resistance. J Clin Microbiol. 2006;44(10):3659–3664. doi: 10.1128/JCM.01054-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ocheretina O, Escuyer VE, Mabou M-M, Royal-Mardi G, Collins S, Vilbrun SC, Pape JW, Fitzgerald DW. Correlation between genotypic and phenotypic testing for resistance to rifampin in Mycobacterium tuberculosis clinical isolates in Haiti: investigation of cases with discrepant susceptibility results. PLoS One. 2014;9(3):e90569. doi: 10.1371/journal.pone.0090569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Javaid A, Hasan R, Zafar A, Ghafoor A, Pathan A, Rab A, Sadiq A, Akram C, Burki I, Shah K. Prevalence of primary multidrug resistance to anti-tuberculosis drugs in Pakistan. Int J Tuberc Lung Dis. 2008;12(3):326–331. [PubMed] [Google Scholar]
- 12.Wayne LG, Krasnow I. Preparation of tuberculosis susceptibility testing mediums by means of impregnated disks. Am J Clin Pathol. 1966;45(6):769. doi: 10.1093/ajcp/45.6_ts.769. [DOI] [PubMed] [Google Scholar]
- 13.Kent PT, Kubica GP, Control CfD. Public health mycobacteriology: a guide for the level III laboratory: US Department of Health and Human Services, Public Health Service, Centers for Disease Control; 1985
- 14.Blakemore R, Story E, Helb D, Kop J, Banada P, Owens MR, Chakravorty S, Jones M, Alland D. Evaluation of the analytical performance of the Xpert MTB/RIF assay. J Clin Microbiol. 2010;48(7):2495–2501. doi: 10.1128/JCM.00128-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Huang W-L, Chen H-Y, Kuo Y-M, Jou R. Performance assessment of the GenoType MTBDRplus test and DNA sequencing in detection of multidrug-resistant Mycobacterium tuberculosis. J Clin Microbiol. 2009;47(8):2520–2524. doi: 10.1128/JCM.02499-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Theron G, Peter J, van Zyl-Smit R, Mishra H, Streicher E, Murray S, Dawson R, Whitelaw A, Hoelscher M, Sharma S. Evaluation of the Xpert MTB/RIF assay for the diagnosis of pulmonary tuberculosis in a high HIV prevalence setting. Am J Respir Crit Care Med. 2011;184(1):132–140. doi: 10.1164/rccm.201101-0056OC. [DOI] [PubMed] [Google Scholar]
- 17.Marlowe EM, Novak-Weekley SM, Cumpio J, Sharp SE, Momeny MA, Babst A, Carlson JS, Kawamura M, Pandori M. Evaluation of the Cepheid Xpert MTB/RIF assay for direct detection of Mycobacterium tuberculosis complex in respiratory specimens. J Clin Microbiol. 2011;49(4):1621–1623. doi: 10.1128/JCM.02214-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Van Rie A, Mellet K, John M, Scott L, Page-Shipp L, Dansey H, Victor T, Warren R. False-positive rifampicin resistance on Xpert® MTB/RIF: case report and clinical implications. Int J Tuberc Lung Dis. 2012;16(2):206. doi: 10.5588/ijtld.11.0395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yang B, Koga H, Ohno H, Ogawa K, Fukuda M, Hirakata Y, Maesaki S, Tomono K, Tashiro T, Kohno S. Relationship between antimycobacterial activities of rifampicin, rifabutin and KRM-1648 and rpoB mutations of Mycobacterium tuberculosis. J Antimicrob Chemother. 1998;42(5):621–628. doi: 10.1093/jac/42.5.621. [DOI] [PubMed] [Google Scholar]
- 20.Campbell EA, Pavlova O, Zenkin N, Leon F, Irschik H, Jansen R, Severinov K, Darst SA. Structural, functional, and genetic analysis of sorangicin inhibition of bacterial RNA polymerase. EMBO J. 2005;24(4):674–682. doi: 10.1038/sj.emboj.7600499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Feuerriegel S, Oberhauser B, George AG, Dafae F, Richter E, Rüsch-Gerdes S, Niemann S. Sequence analysis for detection of first-line drug resistance in Mycobacterium tuberculosis strains from a high-incidence setting. BMC Microbiol. 2012;12(1):90. doi: 10.1186/1471-2180-12-90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Williamson D, Roberts S, Bower J, Vaughan R, Newton S, Lowe O, Lewis C, Freeman J. Clinical failures associated with rpoB mutations in phenotypically occult multidrug-resistant Mycobacterium tuberculosis. Int J Tuberc Lung Dis. 2012;16(2):216–220. doi: 10.5588/ijtld.11.0178. [DOI] [PubMed] [Google Scholar]
- 23.Ioerger TR, Koo S, No E-G, Chen X, Larsen MH, Jacobs WR, Jr, Pillay M, Sturm AW, Sacchettini JC. Genome analysis of multi-and extensively-drug-resistant tuberculosis from KwaZulu-Natal, South Africa. PloS One. 2009;4(11):e7778. doi: 10.1371/journal.pone.0007778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yue J, Shi W, Xie J, Li Y, Zeng E, Wang H. Mutations in the rpoB gene of multidrug-resistant Mycobacterium tuberculosis isolates from China. J Clin Microbiol. 2003;41(5):2209–2212. doi: 10.1128/JCM.41.5.2209-2212.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mboowa G, Namaganda C, Ssengooba W. Rifampicin resistance mutations in the 81 bp RRDR of rpoB gene in Mycobacterium tuberculosis clinical isolates using Xpert MTB/RIF in Kampala, Uganda: a retrospective study. BMC Infect Dis. 2014;14(1):481. doi: 10.1186/1471-2334-14-481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Khan SN, Niemann S, Gulfraz M, Qayyum M, Siddiqi S, Mirza ZS, Tahsin S, Ebrahimi-Rad M, Khanum A. Molecular characterization of multidrug-resistant isolates of Mycobacterium tuberculosis from patients in Punjab, Pakistan. Pakistan J Zool. 2013;45(1):93–100. [Google Scholar]
- 27.Moure R, Muñoz L, Torres M, Santin M, Martín R, Alcaide F. Rapid detection of Mycobacterium tuberculosis complex and rifampin resistance in smear-negative clinical samples by use of an integrated real-time PCR method. J Clin Microbiol. 2011;49(3):1137–1139. doi: 10.1128/JCM.01831-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liu Q, Zhu L, Shao Y, Song H, Li G, Zhou Y, Shi J, Zhong C, Chen C, Lu W. Rates and risk factors for drug resistance tuberculosis in Northeastern China. BMC Public Health. 2013;13(1):1171. doi: 10.1186/1471-2458-13-1171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Luiz RSS, Suffys P, Barroso EC, Kerr LRFS, Duarte CR, Freitas MVC, Mota RMS, Frota CC. Genotyping and drug resistance patterns of Mycobacterium tuberculosis strains observed in a tuberculosis high-burden municipality in Northeast, Brazil. Braz J Infect Dis. 2013;17(3):338–345. doi: 10.1016/j.bjid.2012.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ullah I, Javaid A, Tahir Z, Ullah O, Shah AA, Hasan F, Ayub N. Pattern of Drug Resistance and Risk Factors Associated with Development of Drug Resistant Mycobacterium tuberculosis in Pakistan. PLoS One. 2016;11(1):e0147529. doi: 10.1371/journal.pone.0147529. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All the data supporting our findings is contained within the manuscript.


