Summary
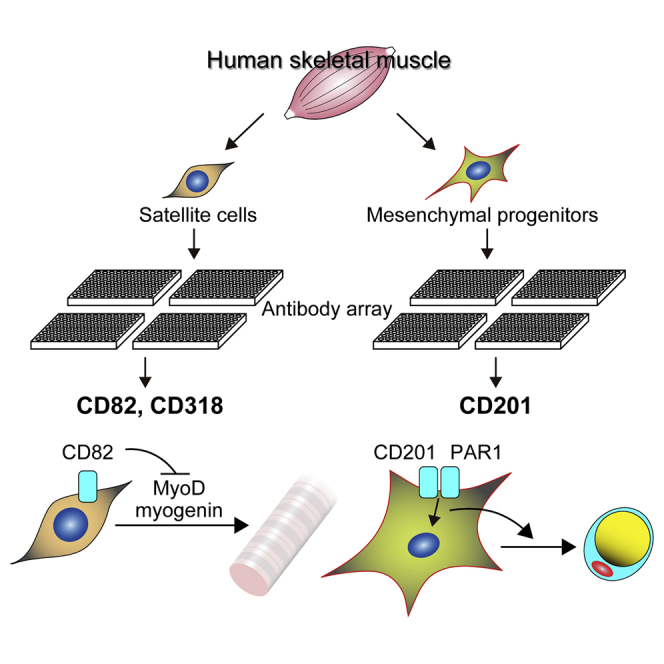
Skeletal muscle contains two distinct stem/progenitor populations. One is the satellite cell, which acts as a muscle stem cell, and the other is the mesenchymal progenitor, which contributes to muscle pathogeneses such as fat infiltration and fibrosis. Detailed and accurate characterization of these progenitors in humans remains elusive. Here, we performed comprehensive cell-surface protein profiling of the two progenitor populations residing in human skeletal muscle and identified three previously unrecognized markers: CD82 and CD318 for satellite cells and CD201 for mesenchymal progenitors. These markers distinguish myogenic and mesenchymal progenitors, and enable efficient isolation of the two types of progenitors. Functional study revealed that CD82 ensures expansion and preservation of myogenic progenitors by suppressing excessive differentiation, and CD201 signaling favors adipogenesis of mesenchymal progenitors. Thus, cell-surface proteins identified here are not only useful markers but also functionally important molecules, and provide valuable insight into human muscle biology and diseases.
Graphical Abstract
Highlights
-
•
CD82 and CD318 are expressed on human muscle satellite cells
-
•
CD201 is expressed on interstitial mesenchymal progenitors in human muscle
-
•
CD82 suppresses premature differentiation of satellite cells
-
•
CD201 signaling favors adipogenesis of mesenchymal progenitors
In this article, Uezumi and colleagues identify previously unrecognized markers of human skeletal muscle-derived progenitors: CD82 and CD318 for satellite cells and CD201 for mesenchymal progenitors. They demonstrate that these markers are not only useful for cell identification and isolation but also functionally important in the regulation of myogenesis and adipogenesis.
Introduction
Skeletal muscle is an organ responsible for movement or physical activity, and therefore is vital for healthy life. Skeletal muscle is mainly composed of multinucleated cylindrical myofibers. Myofibers are terminally differentiated cells, and the cell cycle of their nuclei is irreversibly arrested. However, skeletal muscle regenerates well if myofibers are damaged and undergo necrosis. Skeletal muscle regeneration is attributable to the function of satellite cells that reside between the basal lamina and plasma membrane of myofibers. Satellite cells are normally quiescent, but rapidly become activated after muscle damage and proliferate extensively to produce myoblasts. Myoblasts then differentiate and fuse with each other or damaged myofibers to regenerate muscle. Some myoblasts remain undifferentiated and return to the quiescent state to maintain the satellite cell pool. Thus, satellite cells play a central role in muscle regeneration by acting as muscle stem cells (Bischof, 2004).
Skeletal muscle is also a site where pathological development of ectopic tissues occurs. Adipose tissue, fibrous connective tissue, or even bone can be ectopically formed within muscle not only in muscular disorders but also in other pathological conditions (Uezumi et al., 2014b). Because myofibers are terminally differentiated cells, they cannot be the source of these ectopic tissues. Hence, how these ectopic tissues emerge from skeletal muscle was a long-standing mystery. The identification of mesenchymal progenitors solved this mystery. We and others have identified mesenchymal progenitors distinct from satellite cells in mouse skeletal muscle and have shown that these mesenchymal progenitors contribute to ectopic adipose tissue (Joe et al., 2010, Uezumi et al., 2010), fibrous connective tissue (Uezumi et al., 2011), and heterotopic ossification (Wosczyna et al., 2012). Therefore, satellite cells and mesenchymal progenitors are indispensable cell types for studying skeletal muscle regeneration and pathogenesis, respectively.
Given that satellite cells and mesenchymal progenitors are strongly associated with muscle regeneration and pathogenesis, identifying, distinguishing, and isolating these two progenitor populations in human skeletal muscle are of considerable clinical significance. Compared with mouse, studies dealing with progenitor cells of human skeletal muscle are limited. In human satellite cells, only Pax7, M-cadherin, integrin α7, and CD56 have been considered to be specific markers (Boldrin et al., 2010, Castiglioni et al., 2014). Although Pax7 is a reliable marker for satellite cells in both mouse and human tissues (Boldrin and Morgan, 2012), this marker is not suitable for cell isolation because of its nuclear localization. M-cadherin has been reported to successfully identify human satellite cells (Boldrin and Morgan, 2012, Reimann et al., 2004, Sajko et al., 2004). We also identified satellite cells on human muscle sections using M-cadherin antibody (Uezumi et al., 2014a), but this antibody cannot be used for isolation of human myogenic cells. CD56 is the only marker that enables isolation of human satellite or myogenic cells, as distinguished from mesenchymal progenitors with adipogenic potential, known so far (Agley et al., 2013, Castiglioni et al., 2014, Uezumi et al., 2014a). Several markers have been reported to identify mesenchymal progenitors in human skeletal muscle. CD15 (Lecourt et al., 2010, Pisani et al., 2010a) and CD34 (Pisani et al., 2010b, Vauchez et al., 2009) were used to isolate cells with adipogenic potential, but adipogenic cells were also found in CD15− or CD34− populations of human muscle-derived cells (Agley et al., 2013, Castiglioni et al., 2014). A recent study reported the isolation of a mesenchymal stem cell-like population from human muscle-derived cells as CD73+CD105+CD90− cells (Downey et al., 2015). However, this study did not investigate myogenic cells; thus, whether these markers can isolate mesenchymal cells separately from myogenic satellite cells remains unclear. We know only platelet-derived growth factor receptor α (PDGFRα) as a marker that has been successfully used to isolate mesenchymal progenitors as being distinct from myogenic satellite cells from human skeletal muscle (Arrighi et al., 2015, Uezumi et al., 2014a).
To gain further insight into progenitor cells derived from human skeletal muscle, we performed comprehensive cell-surface protein profiling of two progenitor populations, CD56+ myogenic progenitors and PDGFRα+ mesenchymal progenitors. This comprehensive analysis identified previously unrecognized markers: CD82 and CD318 for satellite cells and CD201 for mesenchymal progenitors. Immunofluorescent staining revealed that CD82 and CD318 are expressed on sublaminar satellite cells, and CD201 is expressed on interstitial mesenchymal progenitors in vivo. Isolated CD82+ or CD318+ cells showed high myogenic potential, while adipogenic potential was enriched exclusively in the CD201+ population. Finally, CD82 knockdown resulted in premature differentiation at the expense of expansion and self-renewal of myogenic progenitors, and stimulation of CD201 signaling facilitated adipogenic differentiation of mesenchymal progenitors. Therefore, cell-surface proteins identified in this study are not only useful markers for cell identification and isolation but also functionally important molecules that regulate myogenesis and adipogenesis of human satellite cells and mesenchymal progenitors, respectively.
Results
Cell-Surface Protein Profiling of Human Skeletal Muscle-Derived Progenitors by Antibody Screening
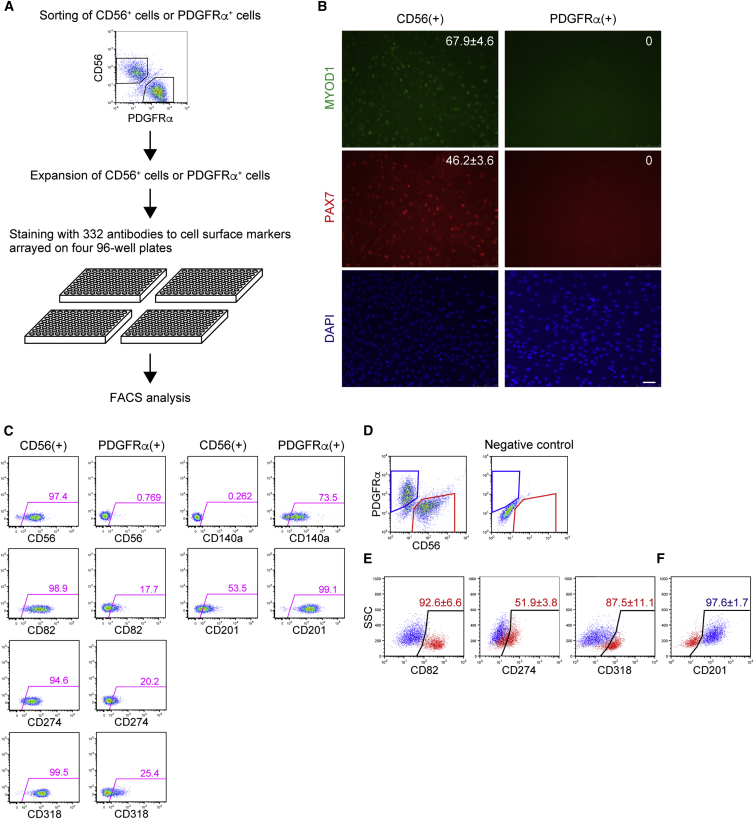
We previously reported the isolation of myogenic and mesenchymal progenitors from human skeletal muscle as CD56+ and PDGFRα+ cells, respectively, and showed that physiological oxygen concentration has significant impact on growth of cells derived from human muscle (Uezumi et al., 2014a). We further optimized culture conditions for efficient expansion of isolated cells and found that collagen I coating and basic fibroblast growth factor (bFGF) promote proliferation of human muscle-derived progenitors (Figure S1). This condition allowed these progenitors to expand in vitro to 1 × 108 cells, which in turn enabled us to perform cell-surface protein profiling by antibody array. This analysis is based on the staining of cell-surface proteins with 332 antibodies and subsequent fluorescence-activated cell sorting (FACS) analysis (Figure 1A). We confirmed that expanded CD56+ cells maintained the myogenic progenitor state, as revealed by PAX7 and myogenic differentiation 1 (MYOD1) positivity, and their surface phenotype as CD56+PDGFRα− (Figures 1B and 1C). On the other hand, PDGFRα+ cells maintained their non-myogenic nature and surface phenotype as CD56−PDGFRα+ cells (Figures 1B and 1C). The entire dataset of CD56+ cells and PDGFRα+ cells is shown in Figures S2 and S3, respectively. We first looked at previously reported markers that were used to isolate myogenic or mesenchymal progenitors from human muscle. Charville et al. (2015) recently reported the isolation of human satellite cells using epidermal growth factor receptor (EGFR) as a marker, but we found that EGFR is expressed in both cultured myogenic and mesenchymal progenitors (Figures S2 and S3). Immunofluorescent staining of human muscle revealed that EGFR is indeed expressed by satellite cells but is also expressed by many interstitial cells (Figure S4A). Furthermore, interstitial PDGFRα+ mesenchymal progenitors do express EGFR (Figure S4B), indicating that this marker cannot distinguish satellite cells from mesenchymal progenitors. CD15 (Lecourt et al., 2010, Pisani et al., 2010a), CD34 (Pisani et al., 2010b, Vauchez et al., 2009), CD73 (Downey et al., 2015), CD90 (Downey et al., 2015), and CD105 (Downey et al., 2015) were used to isolate mesenchymal progenitors, but all of these markers were found to be distributed equally on both cultured myogenic and mesenchymal progenitors (Figures S2 and S3).
Figure 1.
Cell-Surface Protein Profiling of Human Skeletal Muscle-Derived Progenitors by Antibody Screening
(A) Scheme of antibody screening.
(B) Expanded CD56+ cells and PDGFRα+ cells were stained with antibodies against MYOD1 and PAX7. The percentages of positive cells are shown in the panels as means ± SD, n = 5 randomly selected fields. Scale bar, 50 μm.
(C) Distinctive markers identified by antibody screening.
(D) Primary human skeletal muscle-derived cells were stained with antibodies against CD56, PDGFRα, and a newly identified marker. CD56+ (red) and PDGFRα+ (blue) gates were set by analyzing negative control samples stained with an isotype control antibody or secondary reagent only.
(E) Expressions of indicated markers on CD56+ cells were analyzed. The CD56+ population is shown in red. Positive gates were set by using PDGFRα+ cells as the negative population (blue). The percentages of positive cells in the CD56+ population are shown in the panels as means ± SD, n = 13 for CD82, n = 4 for CD271, and n = 15 for CD318.
(F) Expression of CD201 on PDGFRα+ cells was analyzed. The PDGFRα+ population is shown in blue. A positive gate was set by using CD56+ cells as the negative population (red). The percentage of positive cells in the PDGFRα+ population is shown in the panel as means ± SD, n = 6.
See also Figures S1–S4.
We then searched for markers that are expressed differentially between two types of progenitors, and found that CD82, CD274, and CD318 are selectively expressed on cultured myogenic progenitors while CD201 is preferentially expressed on cultured mesenchymal progenitors (Figure 1C). Next, we confirmed results of antibody screening by analyzing primary cells derived from human skeletal muscle. CD82 and CD318 clearly distinguished CD56+ myogenic progenitors from PDGFRα+ mesenchymal progenitors, and the opposite could be achieved by using CD201 (Figures 1D and 1E). Because expression of CD274 was too weak to discriminate the two progenitors, we omitted this marker in the subsequent experiments (Figures 1D and 1E).
Expression of CD82, CD318, and CD201 in Human Skeletal Muscle Tissue
In vivo expression of identified markers was examined by using human muscle sections. Membrane expression of CD82 was clearly observed on PAX7+ or M-cadherin+ satellite cells in normal human muscle (Figures 2A and 2B). CD82 expression was found in almost all satellite cells (97.2% ± 0.6%: mean ± SD, n = 3 different healthy subjects, total number of satellite cells examined = 205). CD82 was predominantly expressed by satellite cells in muscle tissue, although we observed a small number of interstitial cells expressing CD82 (data not shown). We next examined Duchenne muscular dystrophy (DMD) muscle sections to investigate the expression of CD82 on activated satellite cells in vivo. CD82 signals were well overlapped on M-cadherin+ activated satellite cells and also on M-cadherin+ centrally nucleated nascent myofibers in DMD muscles (Figures 2C and 2D). These results indicate that CD82 is expressed on human satellite cells and its expression lasts until the early differentiation phase, but disappears as myofibers mature. A similar expression pattern was observed when human muscle sections were stained with antibody against CD318 (Figures S4C and S4E), although the percentage of positive satellite cells was relatively low (74.6% ± 5.6%; mean ± SD, n = 3 different healthy subjects, total number of satellite cells examined = 253), and cytoplasmic expression was also pronounced. In contrast to CD82 and CD318, CD201 antibody clearly recognized interstitial cells as distinguished from sublaminar PAX7+ satellite cells (Figure 2E). CD201+ cells could be seen with high frequency around blood vessels (Figure 2E). When DMD muscle sections were stained with anti-CD201 antibody, a considerable accumulation of CD201+ cells was conspicuous in the areas of fibrosis where aberrant accumulation of collagen was observed (Figure 2F).
Figure 2.
Expression of Discovered Markers in Adult Human Skeletal Muscle and DMD Muscle
(A and B) Adult human muscle sections were stained with antibodies against CD82, PAX7, and laminin (A) or CD82, M-cadherin, and laminin (B). Arrowheads indicate satellite cells located beneath the basement membrane.
(C and D) DMD muscle sections were subjected to immunofluorescent staining for CD82 and M-cadherin, and subsequently to H&E staining. Arrows indicate centrally nucleated M-cadherin+ myofibers.
(E) Adult human muscle sections were stained with antibodies against CD201, PAX7, and laminin. Arrows indicate CD201+ cells located in interstitial spaces, and arrowheads indicate satellite cell located beneath the basement membrane.
(F) DMD muscle sections were stained with antibodies against CD201 and collagen I.
Scale bars represent 10 μm (A–D) and 20 μm (E and F). See also Figure S4.
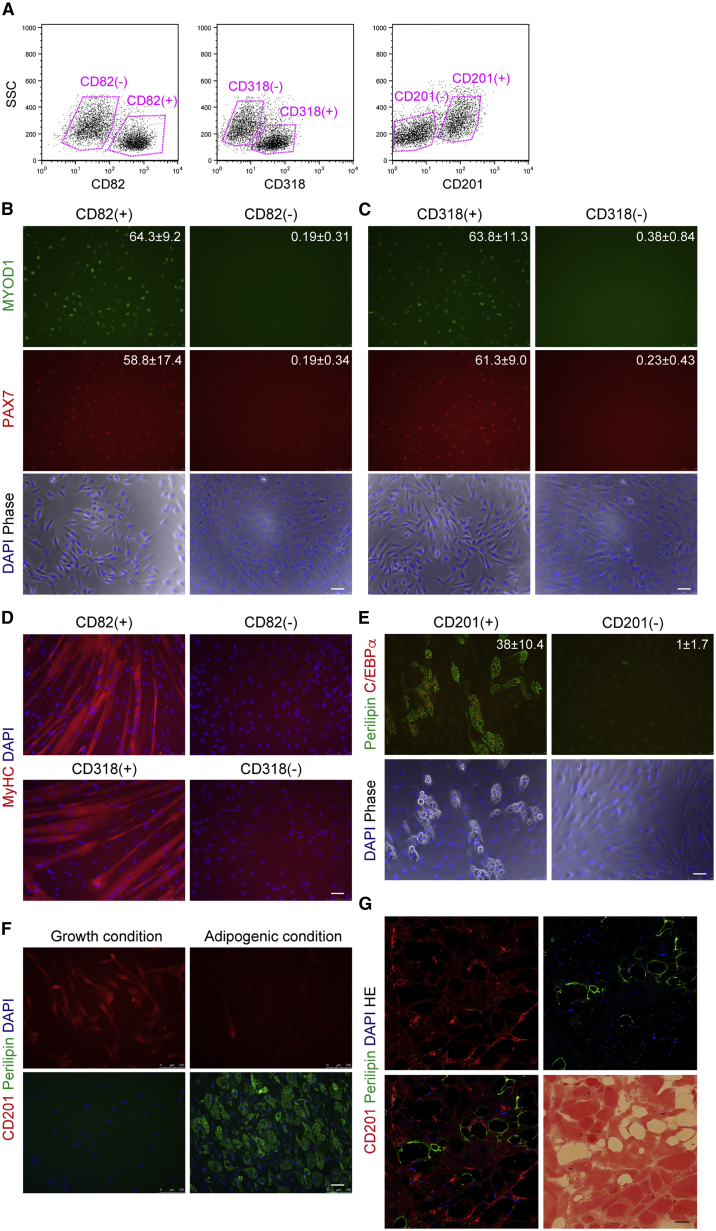
Isolation of Human Myogenic and Mesenchymal Progenitors Using Discovered Markers
To determine the efficacy of identified markers for progenitor cell isolation, we stained human muscle-derived cells with antibodies against CD82, CD318, or CD201. Staining with these markers resulted in clear separation of positive and negative populations (Figure 3A). If cell sorting was performed on the basis of CD82 or CD318 expression, PAX7+MYOD1+ myogenic progenitors were found only in CD82+ or CD318+ fractions (Figures 3B and 3C). When they were induced to differentiate into myotubes, many myosin heavy chain (MyHC)+ myotubes developed only from CD82+ or CD318+ cells (Figure 3D). We next examined adipogenic differentiation after cell sorting based on CD201 expression. We detected adipogenic activity exclusively in CD201+ cells (Figure 3E). Isolated CD201+ cells were uniformly positive for CD201 in the growth condition, but in the adipogenic condition, expression of CD201 disappeared from differentiated adipocytes and was maintained in only a few undifferentiated cells (Figure 3F). Likewise, ectopic adipocytes developed within skeletal muscle were also negative for CD201 (Figure 3G), suggesting a link between expression of CD201 and the undifferentiated state. These results indicate that markers identified by cell-surface protein profiling are useful for efficient isolation of progenitor cells from human muscle. We also examined the expression of identified markers in commercially available human skeletal muscle myoblast (HSMM) cultures. CD56+ cells were strongly positive for CD82, but expression of CD318 was reduced in HSMM cultures (Figure S5A). When cell sorting was done on the basis of CD56 and CD82 expression, myogenic cells were exclusively found in the CD56+CD82+ fraction (Figures S5B–S5D). Surprisingly, many PDGFRα+ non-myogenic cells were present in HSMM cultures and were positive for CD201 (Figure S5E). Adipogenic potential was detected only in this PDGFRα+CD201+ fraction (Figure S5F). Thus, markers described here are also useful in the purification of progenitor cells from commercially available cultures. These results prompted us to use discovered makers for purifying myogenic progenitors from human induced pluripotent stem cells (iPSCs). Human iPSCs (clone 253G4) (Nakagawa et al., 2008) were induced to differentiate into myogenic cells as previously described (Figure S6A) (Hosoyama et al., 2014), and the induced cells were examined for expression of CD82 and CD318. Although CD318 was not detected, a CD82+ fraction was readily identified in iPSC-derived cells (Figure S6B) and CD82+ cells showed considerably high expression of myogenic progenitor-related genes compared with other fractions (Figure S6C), indicating that myogenic progenitors are exclusively enriched in the CD82+ fraction of human iPSC-derived cells. Similar results were obtained when another iPSC line (clone 201B7) (Takahashi et al., 2007) was used (Figure S6D). To further explore the usefulness of CD82 as a myogenic marker, we divided iPSCs (clone 454E2) (Okita et al., 2011) induced to a myogenic lineage into four fractions based on the expression of CD82 and CD56 (Figure S6E). After cell sorting, we found that myotube forming activity was highly enriched in CD82+CD56+ cells (Figure S6F). Thus, CD82 is a useful myogenic marker that is applicable to the isolation of myogenic progenitors not only from human skeletal muscle but also from human iPSCs.
Figure 3.
Efficient Isolation of Myogenic Progenitors by CD82 or CD318 and Mesenchymal Progenitors by CD201
(A) Primary human skeletal muscle-derived cells were stained with antibodies against indicated markers and divided into positive and negative fractions. Sorting gates are shown in the panels.
(B and C) The indicated cell populations were cultured in the growth condition for 3 days and then stained with antibodies against MYOD1 and PAX7. The percentages of positive cells are shown in the panels as means ± SD, n = 15 fields from three independent preparations.
(D) The indicated cell populations were cultured in the myogenic differentiation condition for 5 days and then stained with antibody against myosin heavy chain (MyHC).
(E) The indicated cell populations were cultured in the growth condition for 3 days, then cells were subjected to the adipogenic condition. Cells were stained with antibodies against perilipin and C/EBPα. Numbers of adipocytes per field are shown in the panels as means ± SD, n = 15 fields from three independent preparations.
(F) CD201+ cells grown in growth condition or subjected to the adipogenic condition were stained with antibodies against CD201 and perilipin.
(G) Human muscle samples with ectopic adipocytes were subjected to immunofluorescent staining for CD201 and perilipin, and subsequently to H&E staining.
Scale bars, 50 μm. See also Figures S5 and S6.
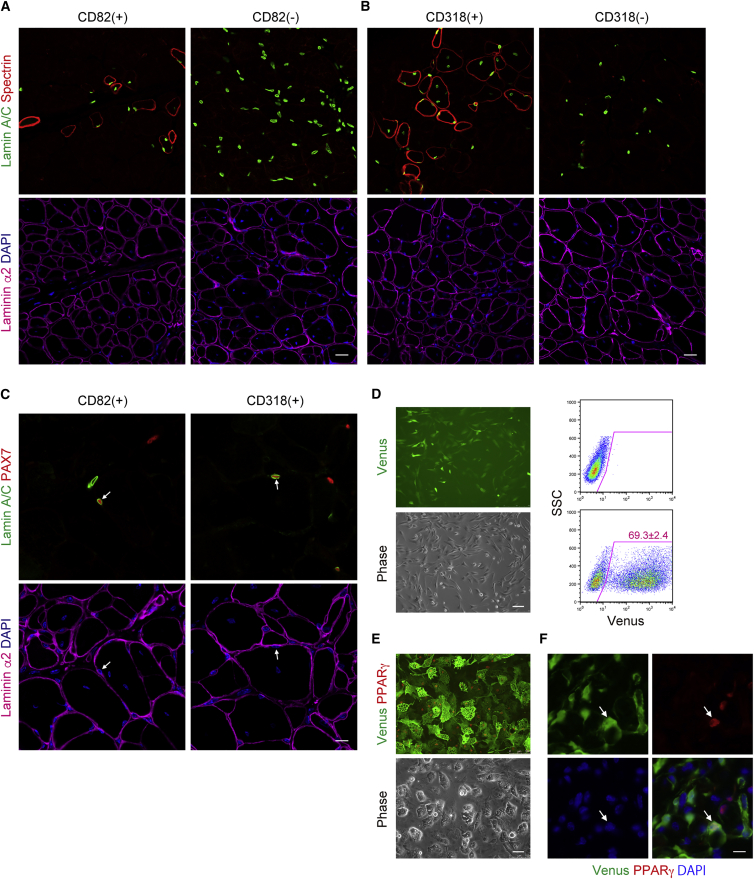
In Vivo Myogenic Potential of CD82+ Cells or CD318+ Cells, and Fibrogenic/Adipogenic Potential of CD201+ Cells
The in vivo myogenic potential of CD82+ or CD318+ cells isolated from human muscle was evaluated by transplantation into injured muscles of immunodeficient mice. Fifteen days after transplantation, many human spectrin+ myofibers that contain human lamin+ nuclei were observed in the muscles that had received CD82+ or CD318+ cells (Figures 4A and 4B; Table 1). In addition to differentiated myofibers, occasional human Lamin+ and PAX7+ sublaminar satellite cells could be detected in CD82+ or CD318+ cell-transplanted muscles (Figure 4C and Table 1), indicating that transplanted CD82+ or CD318+ cells possess self-renewal potential. Such in vivo myogenic activity was detected in all CD82+ or CD318+ populations prepared from four independent samples, but rarely seen in CD82− or CD318− cells (Table 1). To evaluate in vivo differentiation potential of CD201+ cells, we permanently labeled CD201+ cells with the fluorescent protein Venus by using lentiviral vector. We confirmed that CD201+ cells were efficiently transduced with Venus (Figure 4D), and Venus labeling did not affect adipogenic differentiation (Figure 4E). After sorting of Venus-labeled CD201+ cells, the cells were transplanted into glycerol-injured muscles of immunodeficient mice. Two weeks after transplantation, many Venus+ cells were detected in fatty/fibrous degenerated areas and some of them expressed peroxisome proliferator-activated receptor γ (PPARγ) (Figure 4F), suggesting the functional importance of these cells in muscle pathogenesis.
Figure 4.
In Vivo Myogenic Potential of CD82+ Cells or CD318+ Cells, and Fibrogenic/Adipogenic Potential of CD201+ Cells
(A–C) The indicated cell populations were transplanted into CTX-injured muscles of immunodeficient mice. Transplanted muscle sections were subjected to immunofluorescent staining for human lamin A/C, human spectrin, and laminin α2 (A, B), or human lamin A/C, PAX7, and laminin α2 (C). Arrows indicate lamin A/C+PAX7+ human satellite cells located beneath the basement membrane.
(D) CD201+ cells transduced with Venus were analyzed by fluorescent microscope and FACS.
(E) Venus-labeled CD201+ cells were stained with antibodies against GFP and PPARγ.
(F) Venus-labeled CD201+ cells were transplanted into glycerol-injured muscles of immunodeficient mice. Transplanted muscle sections were stained with antibodies against GFP and PPARγ. Arrow indicates Venus+ adipocyte expressing PPARγ.
Scale bars represent 20 μm (A and B), 10 μm (C and F), 100 μm (D), and 50 μm (E).
Table 1.
Summary of Transplantation Experiment
| Patient | Cell Type | Number of Transplanted Cells | Number of Lamin A/C+ Cells/Section | Number of Spectrin+ Myofibers/Section | Number of Lamin A/C+PAX7+ Cells/Section |
|---|---|---|---|---|---|
| Hu36 | CD82+ | 8 × 104 | 45 | 23 | 0 |
| CD82+ | 8 × 104 | 20 | 10 | 3 | |
| CD82+ | 8 × 104 | 35 | 20 | 3 | |
| CD82− | 5 × 104 | 90 | 2 | 0 | |
| CD318+ | 7 × 104 | 125 | 70 | 9 | |
| CD318+ | 7 × 104 | 50 | 28 | 7 | |
| CD318+ | 7 × 104 | 61 | 25 | 3 | |
| CD318− | 5 × 104 | 66 | 0 | 0 | |
| Hu37 | CD82+ | 6 × 104 | 70 | 16 | 0 |
| CD82+ | 6 × 104 | 57 | 13 | 1 | |
| CD82− | 6 × 104 | 352 | 0 | 0 | |
| CD82− | 6 × 104 | 109 | 0 | 0 | |
| CD82− | 6 × 104 | 220 | 0 | 0 | |
| Hu38 | CD82+ | 1 × 105 | 12 | 7 | 1 |
| CD82+ | 1 × 105 | 12 | 3 | 0 | |
| CD82− | 1 × 105 | 26 | 0 | 0 | |
| CD82− | 1 × 105 | 15 | 0 | 0 | |
| Hu39 | CD318+ | 1 × 105 | 55 | 14 | 2 |
| CD318+ | 1 × 105 | 35 | 15 | 1 | |
| CD318− | 1 × 105 | 179 | 1 | 0 | |
| CD318− | 1 × 105 | 19 | 0 | 0 |
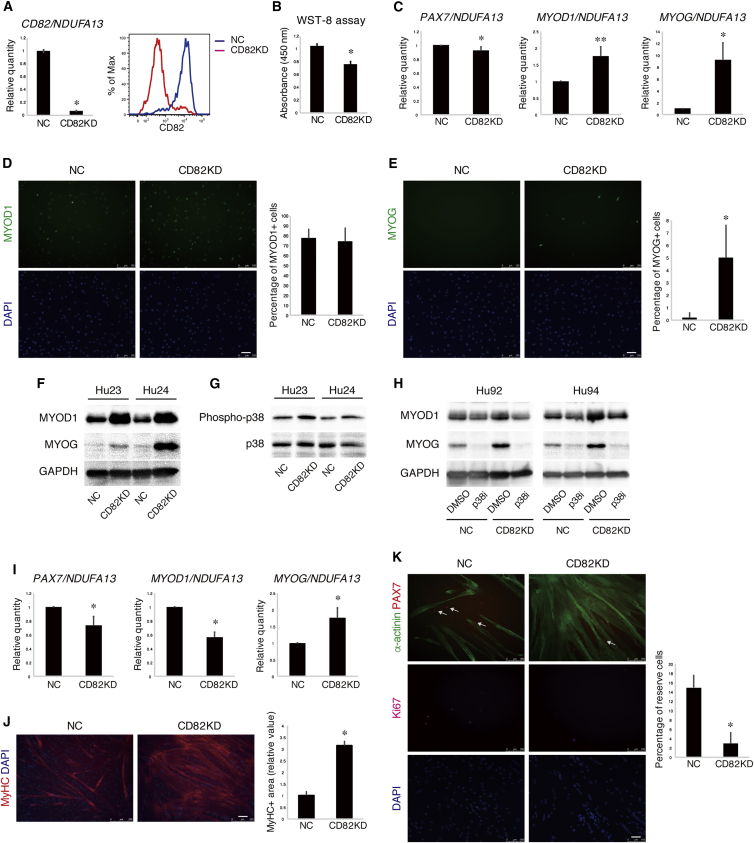
Function of CD82 and CD201 in Human Myogenic and Mesenchymal Progenitors
We explored roles of discovered markers in the regulation of progenitor function. Because CD82 is consistently expressed myogenic cells from human muscle, commercially available cells, and iPSCs, we knocked down CD82 in CD56+CD82+ myogenic cells purified from human muscle by small interfering RNA (siRNA). Almost complete knockdown of CD82 was confirmed at both mRNA and protein levels (Figure 5A). Intriguingly, knocked-down CD82 cells showed reduced proliferation and increased MYOD1 and MYOG transcript level even under growth condition (Figures 5B and 5C). Although the percentage of MYOD1+ cells remained unchanged, the percentage of myogenin (MYOG)+ cells and the total amount of MYOD1 and MYOG protein were dramatically increased in knocked-down CD82 cells (Figures 5D–5F), indicating that CD82 knockdown leads to premature differentiation. Because p38 signaling is well known to be involved in myogenic differentiation, we examined this signaling pathway. Phosphorylation of p38 was increased upon CD82 knockdown (Figure 5G), and the excessive upregulation of MYOD1 and MYOG induced by CD82 knockdown was suppressed by p38 inhibitor (Figure 5H), suggesting that CD82 inhibits premature differentiation at least in part by attenuating the p38 signaling pathway. When induced to differentiate into myotubes, knocked-down CD82 cells showed a significant decrease in the levels of PAX7 and MYOD1 transcript but maintained increased MYOG expression (Figure 5I). Knocked-down CD82 cells exhibited extravagant differentiation (Figure 5J) and reduced the number of reserve cells compared with control cells (Figure 5K). These results suggest that CD82 is required for achieving an appropriate balance between differentiation and self-renewal of human myogenic progenitors.
Figure 5.
Knockdown of CD82 Leads to Premature Differentiation in Myogenic Progenitors at the Expense of Expansion and Reserve Cell Generation
(A) CD56+CD82+ cells were purified and cultured. Cells were transfected with negative control siRNA (NC) or CD82 siRNA (CD82KD). The indicated cells were cultured in the growth condition for 2 days. The expression of CD82 mRNA was quantified by qRT-PCR. Values are represented as the ratio to control cells and shown as means ± SD of three independent preparations. ∗p < 0.01 (left). The expression of CD82 protein was measured by FACS (right).
(B) Cell proliferation was measured by using WST-8 reagent and shown as means ± SD of three independent preparations. ∗p < 0.01.
(C) The expressions of myogenic genes were quantified by qRT-PCR. Values are represented as the ratio to control cells and shown as means ± SD of four independent preparations. ∗p < 0.01, ∗∗p < 0.05.
(D and E) Cells were stained with antibodies against MYOD1 or MYOG. The percentages of positive cells are shown as means ± SD, n = 20 fields from four independent preparations. ∗p < 0.01
(F) Western blot analysis of MYOD1, MYOG, and GAPDH. Results from two independent preparations are shown.
(G) Phosphorylation of p38 was assessed by immunoblotting. Results from two independent preparations are shown.
(H) Control or knocked-down CD82 cells were treated with SB203580 (p38i) or DMSO. The expression of MYOD1, MYOG, and GAPDH was analyzed by immunoblotting. Results from two independent preparations are shown.
(I) After 2 days of culture in growth condition, cells were induced to differentiate into myotubes. The expressions of myogenic genes were quantified by qRT-PCR. Values are represented as the ratio to control cells and are shown as means ± SD of four independent preparations. ∗p < 0.01.
(J) The indicated cells were stained with antibody against MyHC and the MyHC+ area was quantified. Values are represented as the ratio to control cells and are shown as means ± SD of three independent preparations. ∗p < 0.01.
(K) The indicated cells were stained with antibody against sarcomeric α-actinin, PAX7, and Ki67. Arrows indicate PAX7+Ki67− undifferentiated reserve cells. The percentages of reserve cells are shown as means ± SD, n = 9 fields from three independent preparations. ∗p < 0.01.
Scale bars represent 50 μm (D, E, K) and 100 μm (J).
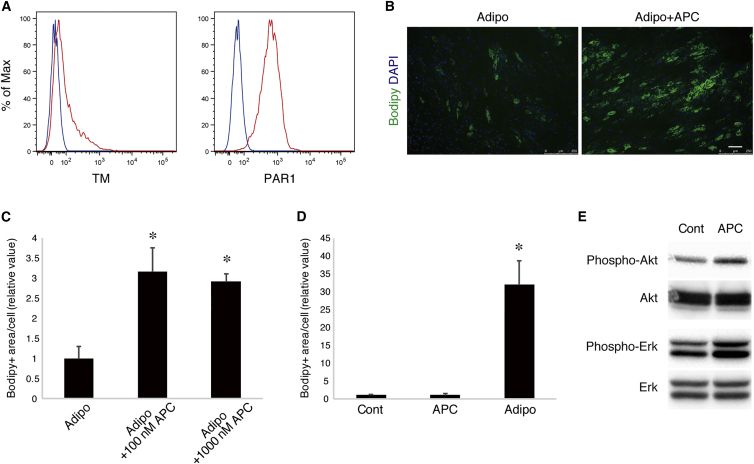
CD201 acts as a receptor for protein C (PC) and activated protein C (APC). CD201 forms a complex with PC and thrombomodulin (TM) to exert its major function, anticoagulation, but also initiates diverse intracellular signaling in the presence of co-receptor protease-activated receptor 1 (PAR1) upon APC binding (Mohan Rao et al., 2014). When analyzed by FACS, PAR1 was found to be expressed strongly on the surface of human mesenchymal progenitors, although only faint expression of TM was detected (Figure 6A), which suggests that mesenchymal progenitors can elicit signals through CD201-PAR1 receptors upon APC binding. To gain insight into the role of CD201 signaling in mesenchymal progenitors, we added APC to the medium during adipogenic differentiation. Although the total number of cells remained unchanged (data not shown), adipocyte formation was significantly enhanced in the presence of APC (Figures 6B and 6C). However, mesenchymal progenitors showed no adipogenesis when treated with the medium containing APC but not adipogenic-inducing reagents (Figure 6D), suggesting that CD201 signaling per se cannot initiate adipogenesis but reinforces the adipogenic program evoked by other inductive cues. Akt and Erk are important signaling pathways that enhance central the adipogenic program governed by PPARγ (Peng et al., 2003, Prusty et al., 2002). When treated with APC, both Akt and Erk pathways were activated in mesenchymal progenitors (Figure 6E). Our data suggest that CD201 signaling favors adipogenesis through activating Akt and Erk pathways in mesenchymal progenitors.
Figure 6.
CD201 Signaling Facilitates Adipogenesis of Mesenchymal Progenitors
(A) The cell-surface expression of TM and PAR1 was analyzed by FACS. Isotype control is shown in blue and antibody-stained sample in red.
(B) Mesenchymal progenitors were induced to differentiate into adipocytes with (Adipo + APC) or without (Adipo) APC. Lipid droplets were stained with Bodipy.
(C) Adipocyte differentiation was quantified by measuring Bodipy+ area per cell. Values are represented as the ratio to cells not treated with APC and shown as means ± SD of three independent preparations. ∗p < 0.01.
(D) Mesenchymal progenitors were cultured in 10% FBS/DMEM (Cont), 10% FBS/DMEM supplemented with APC (APC), or adipogenic differentiation condition (Adipo). Adipocyte differentiation was quantified, and values are represented as the ratio to control cells and shown as means ± SD of three independent preparations. ∗p < 0.01.
(E) Mesenchymal progenitors were serum starved overnight and then stimulated with APC for 30 min. Phosphorylation of Akt and Erk were assessed by immunoblotting.
Discussion
In this study, we comprehensively analyzed the cell-surface phenotype of two types of progenitor cells residing in human skeletal muscle. As a consequence, we identified three previously unknown cell-surface markers: CD82 and CD318 for myogenic progenitors and CD201 for mesenchymal progenitors. In human muscle tissue, sublaminar satellite cells express both CD82 and CD318, and interstitial mesenchymal progenitors express CD201. Intriguingly, these markers can distinguish between myogenic and mesenchymal progenitors, and are applicable to efficient isolation of the two types of progenitor cells. Considering the limited information about progenitor cells residing in human skeletal muscle, our comprehensive analysis provides profound insight into their characteristics by defining the cell-surface phenotype of myogenic progenitors as CD56+CD82+CD318+PDGFRα−CD201− and that of mesenchymal progenitors as CD56−CD82−CD318−PDGFRα+CD201+.
CD82, also known as KAI1, is a member of the tetraspanin family. Although tetraspanins are involved in a wide variety of biological processes such as cell adhesion, migration, proliferation, and signal transduction (Liu and Zhang, 2006), CD82 is probably best known as a metastasis suppressor (Dong et al., 1995). Expression of CD82 was reported in many non-muscle tissues (Custer et al., 2006, Huang et al., 1997, Nagira et al., 1994), and low-level expression of CD82 was also detected in skeletal muscle (Custer et al., 2006, Nagira et al., 1994). However, the detailed expression pattern of CD82 in skeletal muscle has not been examined. In this study, we demonstrated that CD82 is expressed on satellite cells in normal human skeletal muscle and on activated satellite cells or small regenerating myofibers in DMD muscle, but is absent in mature myofibers. Expression of CD82 seems to be specific to immature myogenic cells in human skeletal muscle because only a few CD82+ cells could be seen in the interstitial spaces. Specific expression of CD82 in myogenic cells is further supported by a microarray study of mouse satellite cells (Fukada et al., 2007) that showed high-level expression of CD82 in satellite cells compared with non-myogenic cells (Table S1). Such specificity provides significant value as a marker, because other markers used for the isolation of human satellite cells such as CD29 and EGFR (Charville et al., 2015, Xu et al., 2015) are also expressed in non-myogenic cells. Knockdown experiments revealed that CD82 suppresses premature differentiation by inhibiting MYOD1 and MYOG expression in human myogenic progenitors. We further showed that knocked-down CD82 cells exhibited excessive differentiation at the expense of generation of reserve cells, which represent an in vitro model of self-renewal. Although CD82 has received scant attention in stem cell biology, recent study demonstrated an important role for this cell-surface molecule in maintaining hematopoietic stem cell (HSC) quiescence (Hur et al., 2016), which is suggestive of a general role for CD82 in stem cell maintenance. Our data suggest that CD82 exerts its effects on myogenic progenitors through attenuating p38 signaling, although we cannot exclude other mechanisms that mediate CD82 functions. Precise mechanisms whereby CD82 suppresses MYOD1 and MYOG, and thereby ensures expansion and self-renewal of human myogenic progenitors, are of great interest.
CD318, also known as CUB domain-containing protein 1, acts as a substrate for Src family kinases and is associated with the metastatic potential of cancer cells (Wortmann et al., 2009). In addition to cancer cells, CD318 has been reported to be present in several stem/progenitor populations such as hematopoietic stem/progenitor cells, mesenchymal stem cells, and neural progenitor cells (Buhring et al., 2004, Conze et al., 2003, Takeda et al., 2010). We reported here that CD318 is also expressed on human myogenic progenitors with an expression pattern similar to that of CD82. However, expression of CD318 was diminished in commercially available myoblasts and undetectable in iPSC-derived cells. Although the exact reason for the downregulation of CD318 is currently unknown, the oxygen concentration used in those cultures differed from that in our culture condition. We used a hypoxic condition for cell culture, but commercially available myoblasts or iPSCs were cultured in a normoxic condition. Because expression of CD318 is induced by hypoxia (Cao et al., 2015, Emerling et al., 2013, Razorenova et al., 2011), a different oxygen concentration might lead to a difference in CD318 expression.
CD201, also known as endothelial protein C receptor, is a type 1 transmembrane glycoprotein expressed in the endothelium of large blood vessels and also in many other cell types (Mohan Rao et al., 2014). Its well-known function is anticoagulation. Our analysis revealed that CD201 is specifically expressed in mesenchymal progenitors but not in myogenic satellite cells within human skeletal muscle. While previously reported mesenchymal markers such as CD15 (Lecourt et al., 2010, Pisani et al., 2010a), CD34 (Pisani et al., 2010b, Vauchez et al., 2009), CD73 (Downey et al., 2015), CD90 (Downey et al., 2015), and CD105 (Downey et al., 2015) were distributed equally on both cultured myogenic and mesenchymal progenitors, our comprehensive analysis identifies one of the most powerful markers, which is equivalent to PDGFRα and can distinguish mesenchymal progenitors from myogenic cells. We also found that human mesenchymal progenitors highly express PAR1, a co-receptor of CD201. CD201 is selectively expressed in HSCs (Balazs et al., 2006), and CD201-PAR1 signaling has been recently shown to regulate the retention and recruitment of HSCs within the bone marrow microenvironment (Gur-Cohen et al., 2015). We showed in this study that stimulation of CD201-PAR1 signaling in mesenchymal progenitors leads to enhanced adipogenesis by activating the Akt and Erk pathway. Thus, our study further unveils an additional function of CD201, which is traditionally recognized as a coagulation-related factor, as a regulator of stem/progenitor cell function.
In conclusion, we identified previously unrecognized markers of two types of progenitor cells residing in human skeletal muscle by comprehensive cell-surface protein profiling. These markers are quite unique in being able to distinguish between myogenic and mesenchymal progenitors, and in regulating myogenesis and adipogenesis. Our study provides meaningful information not only for the basic science of human skeletal muscle but also for practical research, including regenerative medicine for muscle diseases.
Experimental Procedures
Human Muscle Samples
Experiments using human samples were approved by the Ethical Review Board for Clinical Studies at Fujita Health University. Non-dystrophic muscle samples were obtained from gluteus medius muscles of subjects undergoing total hip arthroplasty. DMD muscle samples were obtained from muscle (rectus femoris or biceps brachii) biopsies performed for diagnostic purposes. A human myoblast culture was purchased from Lonza.
Dissociation of Cells from Muscle Samples
Muscles were transferred to PBS and digested with 0.2% type II collagenase (Worthington). Muscle slurries were filtered through a cell strainer (BD Biosciences). Cells were resuspended in growth medium (GM) consisting of DMEM supplemented with 20% fetal bovine serum (FBS), 1% penicillin-streptomycin, and 2.5 ng/mL bFGF (Katayama Chemical), seeded onto a collagen I-coated dish (Iwaki), and maintained at 37°C in 5% CO2 and 3% O2.
FACS
Cells were trypsinized and resuspended in washing buffer consisting of PBS with 2.5% FBS, and stained with primary antibodies. Cells were then stained with secondary reagents. Primary antibodies and secondary reagents used are described in Supplemental Experimental Procedures. Stained cells were analyzed by FACSVerse, FACSVantage SE (BD Biosciences), or MoFlo Astrios (Beckman Coulter). Cell sorting was performed on a FACSVantage SE or MoFlo Astrios.
Antibody Screening
After cell sorting, CD56+ cells or PDGFRα+ cells were cultured in GM on a collagen I-coated dish at 37°C in 5% CO2 and 3% O2, and expanded to 1 × 108 cells. Antibody screening was carried out using a LEGENDScreen human cell screening kit (BioLegend).
Cell Culture
Sorted cells were cultured on Matrigel-coated (BD Biosciences) plates in GM at 37°C in 5% CO2 and 3% O2. Ten thousand cells were added per well. Myogenic and adipogenic differentiation were carried out as described in Supplemental Experimental Procedures. CD82 knockdown was performed by transfecting CD82 siRNA (Ambion). Cell proliferation was measured using Cell Counting Kit-8 (Dojindo). SB203580 (Wako), a p38 inhibitor, was used at 10 μM. APC (Haematologic Technologies) was used at 100 or 1,000 nM.
iPSCs and Induction of Myogenic Progenitors
Human iPSCs were provided by the Center for iPS Cell Research and Application. Myogenic cells were induced from iPSCs as previously described (Hosoyama et al., 2014). Induced cells were trypsinized and resuspended in washing buffer, then subjected to FACS sorting.
Generation of Lentiviral Vector and In Vitro Transduction
Plasmids required for generation of lentiviral vector were obtained from RIKEN BRC. The lentiviral vector was generated as described by Ikemoto et al. (2007). Sorted CD201+ cells were infected with 200 MOI of viral vector.
Transplantation Experiment
All procedures using experimental animals were approved by the Institutional Animal Care and Use Committee at Fujita Health University. Immunodeficient NOD/scid or NSG mice were used. One day prior to transplantation, tibialis anterior (TA) muscles were injured with cardiotoxin (CTX) or glycerol as described by Uezumi et al. (2010). Cells in 25 μL of PBS were transplanted into injured TA muscles. Fifteen days after transplantation, TA muscles were sampled and subjected to histological analysis.
Histochemistry, Cytochemistry, and Microscopy
For CD82 and CD318 immunostaining, sections were treated with Antigen Retrieval Reagent-Universal solution (R&D Systems) at 95°C for 5 min. Specimens were blocked with protein-block serum-free reagent (Dako) for 15 min, and incubated with primary antibodies at 4°C overnight, followed by secondary staining. Antibodies used are described in Supplemental Experimental Procedures. Specimens were counterstained with DAPI (Invitrogen) and mounted with SlowFade Gold anti-fade reagent (Invitrogen). Stained samples were photographed using a fluorescence microscope BX51 (Olympus), an inverted fluorescence microscope BZ-9000 (Keyence), or an inverted fluorescence microscope DMI4000B (Leica). Confocal images of muscle sections were taken using the confocal laser scanning microscope system LSM700 (Carl Zeiss).
Immunoblotting
Cells were lysed in lysis buffer containing protease inhibitor cocktail (Roche). For the detection of phosphorylated proteins, phosphatase inhibitor cocktail (Roche) was added. Ten micrograms of protein was separated by SDS-PAGE and transferred onto polyvinylidene difluoride membranes. The membranes were probed with primary antibodies described in Supplemental Experimental Procedures. After incubation with horseradish peroxidase-conjugated secondary antibodies and chemiluminescence reactions, images of the developed immunoblots were captured using a Light-Capture imaging system (ATTO).
Quantitative Analyses of Cultured Cell
Three to five randomly selected fields per well were photographed. Images were collected and pooled from two or three independent experiments. The purity of myogenic progenitors was determined by dividing the number of myogenic marker+ cells by the number of DAPI+ nuclei. To assess the efficiency of adipogenic differentiation, we quantified the number of adipocytes per field or Bodipy-stained area. MyHC+ area per well was quantified to assess the efficiency of myogenic differentiation.
RNA Extraction and RT-PCR
Total RNA was extracted using an RNeasy Micro Kit (Qiagen), and equal amounts of RNA were reverse transcribed into cDNA using a QuantiTect Reverse Transcription Kit (Qiagen). Real-time qPCR was performed on a Thermal Cycler Dice Real Time System (Takara) or LightCycler 480 System (Roche). Specific primer sequences used in this study are described in Supplemental Experimental Procedures.
Statistics
The significance of differences among experimental groups was assessed by Student's t test or one-way ANOVA followed by Tukey's post hoc test.
Author Contributions
A.U. conceived the study. A.U. was responsible for designing and performing experiments, analyzing data, interpreting results, and writing the manuscript. M.N. helped with immunoblotting experiments. M.I.-U. and T.K. helped with immunofluorescent experiments. N.Y. helped with FACS experiments. M.M., A.Y., and H.Y. helped with experiments related to human tissues. S.M., A.N., Y.M.S., S.T., and S.F. performed experiments and analyzed data related to iPSCs. I.N. provided DMD samples. A.U. and K.T. coordinated the project.
Acknowledgments
We thank K. Ono for proofreading the paper. This work was supported by AMED Research Center Network for Realization of Regenerative Medicine, AMED Health and Labor Sciences Research Grants for Comprehensive Research on Persons with Disabilities, Japan Foundation for Aging and Health, JSPS KAKENHI Grant Number 15K12675, Astellas Pharma, and the 24th General Assembly of the Japanese Association of Medical Sciences.
Published: August 9, 2016
Footnotes
Supplemental Information includes Supplemental Experimental Procedures, six figures, and one table and can be found with this article online at http://dx.doi.org/10.1016/j.stemcr.2016.07.004.
Supplemental Information
References
- Agley C.C., Rowlerson A.M., Velloso C.P., Lazarus N.R., Harridge S.D. Human skeletal muscle fibroblasts, but not myogenic cells, readily undergo adipogenic differentiation. J. Cell Sci. 2013;126:5610–5625. doi: 10.1242/jcs.132563. [DOI] [PubMed] [Google Scholar]
- Arrighi N., Moratal C., Clement N., Giorgetti-Peraldi S., Peraldi P., Loubat A., Kurzenne J.Y., Dani C., Chopard A., Dechesne C.A. Characterization of adipocytes derived from fibro/adipogenic progenitors resident in human skeletal muscle. Cell Death Dis. 2015;6:e1733. doi: 10.1038/cddis.2015.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balazs A.B., Fabian A.J., Esmon C.T., Mulligan R.C. Endothelial protein C receptor (CD201) explicitly identifies hematopoietic stem cells in murine bone marrow. Blood. 2006;107:2317–2321. doi: 10.1182/blood-2005-06-2249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bischof R. Satellite and stem cells in muscle regeneration. In: Engel A.G., Franzini-Armstrong C., editors. Myology. McGraw-Hill; New York: 2004. pp. 66–86. [Google Scholar]
- Boldrin L., Morgan J.E. Human satellite cells: identification on human muscle fibres. PLoS Curr. 2012;3:RRN1294. doi: 10.1371/currents.RRN1294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boldrin L., Muntoni F., Morgan J.E. Are human and mouse satellite cells really the same? J. Histochem. Cytochem. 2010;58:941–955. doi: 10.1369/jhc.2010.956201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buhring H.J., Kuci S., Conze T., Rathke G., Bartolovic K., Grunebach F., Scherl-Mostageer M., Brummendorf T.H., Schweifer N., Lammers R. CDCP1 identifies a broad spectrum of normal and malignant stem/progenitor cell subsets of hematopoietic and nonhematopoietic origin. Stem Cells. 2004;22:334–343. doi: 10.1634/stemcells.22-3-334. [DOI] [PubMed] [Google Scholar]
- Cao M., Gao J., Zhou H., Huang J., You A., Guo Z., Fang F., Zhang W., Song T., Zhang T. HIF-2alpha regulates CDCP1 to promote PKCdelta-mediated migration in hepatocellular carcinoma. Tumour Biol. 2015;37:1651–1662. doi: 10.1007/s13277-015-3527-7. [DOI] [PubMed] [Google Scholar]
- Castiglioni A., Hettmer S., Lynes M.D., Rao T.N., Tchessalova D., Sinha I., Lee B.T., Tseng Y.H., Wagers A.J. Isolation of progenitors that exhibit myogenic/osteogenic bipotency in vitro by fluorescence-activated cell sorting from human fetal muscle. Stem Cell Rep. 2014;2:92–106. doi: 10.1016/j.stemcr.2013.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charville G.W., Cheung T.H., Yoo B., Santos P.J., Lee G.K., Shrager J.B., Rando T.A. Ex vivo expansion and in vivo self-renewal of human muscle stem cells. Stem Cell Rep. 2015;5:621–632. doi: 10.1016/j.stemcr.2015.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conze T., Lammers R., Kuci S., Scherl-Mostageer M., Schweifer N., Kanz L., Buhring H.J. CDCP1 is a novel marker for hematopoietic stem cells. Ann. N. Y Acad. Sci. 2003;996:222–226. doi: 10.1111/j.1749-6632.2003.tb03249.x. [DOI] [PubMed] [Google Scholar]
- Custer M.C., Risinger J.I., Hoover S., Simpson R.M., Patterson T., Barrett J.C. Characterization of an antibody that can detect the Kai1/CD82 murine metastasis suppressor. Prostate. 2006;66:567–577. doi: 10.1002/pros.20386. [DOI] [PubMed] [Google Scholar]
- Dong J.T., Lamb P.W., Rinker-Schaeffer C.W., Vukanovic J., Ichikawa T., Isaacs J.T., Barrett J.C. KAI1, a metastasis suppressor gene for prostate cancer on human chromosome 11p11.2. Science. 1995;268:884–886. doi: 10.1126/science.7754374. [DOI] [PubMed] [Google Scholar]
- Downey J., Lauzier D., Kloen P., Klarskov K., Richter M., Hamdy R., Faucheux N., Scime A., Balg F., Grenier G. Prospective heterotopic ossification progenitors in adult human skeletal muscle. Bone. 2015;71:164–170. doi: 10.1016/j.bone.2014.10.020. [DOI] [PubMed] [Google Scholar]
- Emerling B.M., Benes C.H., Poulogiannis G., Bell E.L., Courtney K., Liu H., Choo-Wing R., Bellinger G., Tsukazawa K.S., Brown V. Identification of CDCP1 as a hypoxia-inducible factor 2alpha (HIF-2alpha) target gene that is associated with survival in clear cell renal cell carcinoma patients. Proc. Natl. Acad. Sci. USA. 2013;110:3483–3488. doi: 10.1073/pnas.1222435110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukada S., Uezumi A., Ikemoto M., Masuda S., Segawa M., Tanimura N., Yamamoto H., Miyagoe-Suzuki Y., Takeda S. Molecular signature of quiescent satellite cells in adult skeletal muscle. Stem Cells. 2007;25:2448–2459. doi: 10.1634/stemcells.2007-0019. [DOI] [PubMed] [Google Scholar]
- Gur-Cohen S., Itkin T., Chakrabarty S., Graf C., Kollet O., Ludin A., Golan K., Kalinkovich A., Ledergor G., Wong E. PAR1 signaling regulates the retention and recruitment of EPCR-expressing bone marrow hematopoietic stem cells. Nat. Med. 2015;21:1307–1317. doi: 10.1038/nm.3960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosoyama T., McGivern J.V., Van Dyke J.M., Ebert A.D., Suzuki M. Derivation of myogenic progenitors directly from human pluripotent stem cells using a sphere-based culture. Stem Cells Transl. Med. 2014;3:564–574. doi: 10.5966/sctm.2013-0143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang C., Taki T., Adachi M., Yagita M., Sawada S., Takabayashi A., Inufusa H., Yoshie O., Miyake M. MRP-1/CD9 and KAI1/CD82 expression in normal and various cancer tissues. Int. J. Oncol. 1997;11:1045–1051. doi: 10.3892/ijo.11.5.1045. [DOI] [PubMed] [Google Scholar]
- Hur J., Choi J.I., Lee H., Nham P., Kim T.W., Chae C.W., Yun J.Y., Kang J.A., Kang J., Lee S.E. CD82/KAI1 maintains the dormancy of long-term hematopoietic stem cells through interaction with DARC-expressing macrophages. Cell Stem Cell. 2016;18:508–521. doi: 10.1016/j.stem.2016.01.013. [DOI] [PubMed] [Google Scholar]
- Ikemoto M., Fukada S., Uezumi A., Masuda S., Miyoshi H., Yamamoto H., Wada M.R., Masubuchi N., Miyagoe-Suzuki Y., Takeda S. Autologous transplantation of SM/C-2.6(+) satellite cells transduced with micro-dystrophin CS1 cDNA by lentiviral vector into mdx mice. Mol. Ther. 2007;15:2178–2185. doi: 10.1038/sj.mt.6300295. [DOI] [PubMed] [Google Scholar]
- Joe A.W., Yi L., Natarajan A., Le Grand F., So L., Wang J., Rudnicki M.A., Rossi F.M. Muscle injury activates resident fibro/adipogenic progenitors that facilitate myogenesis. Nat. Cell Biol. 2010;12:153–163. doi: 10.1038/ncb2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lecourt S., Marolleau J.P., Fromigue O., Vauchez K., Andriamanalijaona R., Ternaux B., Lacassagne M.N., Robert I., Boumediene K., Chereau F. Characterization of distinct mesenchymal-like cell populations from human skeletal muscle in situ and in vitro. Exp. Cell Res. 2010;316:2513–2526. doi: 10.1016/j.yexcr.2010.04.020. [DOI] [PubMed] [Google Scholar]
- Liu W.M., Zhang X.A. KAI1/CD82, a tumor metastasis suppressor. Cancer Lett. 2006;240:183–194. doi: 10.1016/j.canlet.2005.08.018. [DOI] [PubMed] [Google Scholar]
- Mohan Rao L.V., Esmon C.T., Pendurthi U.R. Endothelial cell protein C receptor: a multiliganded and multifunctional receptor. Blood. 2014;124:1553–1562. doi: 10.1182/blood-2014-05-578328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagira M., Imai T., Ishikawa I., Uwabe K.I., Yoshie O. Mouse homologue of C33 antigen (CD82), a member of the transmembrane 4 superfamily: complementary DNA, genomic structure, and expression. Cell Immunol. 1994;157:144–157. doi: 10.1006/cimm.1994.1212. [DOI] [PubMed] [Google Scholar]
- Nakagawa M., Koyanagi M., Tanabe K., Takahashi K., Ichisaka T., Aoi T., Okita K., Mochiduki Y., Takizawa N., Yamanaka S. Generation of induced pluripotent stem cells without Myc from mouse and human fibroblasts. Nat. Biotechnol. 2008;26:101–106. doi: 10.1038/nbt1374. [DOI] [PubMed] [Google Scholar]
- Okita K., Matsumura Y., Sato Y., Okada A., Morizane A., Okamoto S., Hong H., Nakagawa M., Tanabe K., Tezuka K. A more efficient method to generate integration-free human iPS cells. Nat. Methods. 2011;8:409–412. doi: 10.1038/nmeth.1591. [DOI] [PubMed] [Google Scholar]
- Peng X.D., Xu P.Z., Chen M.L., Hahn-Windgassen A., Skeen J., Jacobs J., Sundararajan D., Chen W.S., Crawford S.E., Coleman K.G. Dwarfism, impaired skin development, skeletal muscle atrophy, delayed bone development, and impeded adipogenesis in mice lacking Akt1 and Akt2. Genes Dev. 2003;17:1352–1365. doi: 10.1101/gad.1089403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pisani D.F., Clement N., Loubat A., Plaisant M., Sacconi S., Kurzenne J.Y., Desnuelle C., Dani C., Dechesne C.A. Hierarchization of myogenic and adipogenic progenitors within human skeletal muscle. Stem Cells. 2010;28:2182–2194. doi: 10.1002/stem.537. [DOI] [PubMed] [Google Scholar]
- Pisani D.F., Dechesne C.A., Sacconi S., Delplace S., Belmonte N., Cochet O., Clement N., Wdziekonski B., Villageois A.P., Butori C. Isolation of a highly myogenic CD34-negative subset of human skeletal muscle cells free of adipogenic potential. Stem Cells. 2010;28:753–764. doi: 10.1002/stem.317. [DOI] [PubMed] [Google Scholar]
- Prusty D., Park B.H., Davis K.E., Farmer S.R. Activation of MEK/ERK signaling promotes adipogenesis by enhancing peroxisome proliferator-activated receptor gamma (PPARgamma ) and C/EBPalpha gene expression during the differentiation of 3T3-L1 preadipocytes. J. Biol. Chem. 2002;277:46226–46232. doi: 10.1074/jbc.M207776200. [DOI] [PubMed] [Google Scholar]
- Razorenova O.V., Finger E.C., Colavitti R., Chernikova S.B., Boiko A.D., Chan C.K., Krieg A., Bedogni B., LaGory E., Weissman I.L. VHL loss in renal cell carcinoma leads to up-regulation of CUB domain-containing protein 1 to stimulate PKCdelta-driven migration. Proc. Natl. Acad. Sci. USA. 2011;108:1931–1936. doi: 10.1073/pnas.1011777108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reimann J., Brimah K., Schroder R., Wernig A., Beauchamp J.R., Partridge T.A. Pax7 distribution in human skeletal muscle biopsies and myogenic tissue cultures. Cell Tissue Res. 2004;315:233–242. doi: 10.1007/s00441-003-0833-y. [DOI] [PubMed] [Google Scholar]
- Sajko S., Kubinova L., Cvetko E., Kreft M., Wernig A., Erzen I. Frequency of M-cadherin-stained satellite cells declines in human muscles during aging. J. Histochem. Cytochem. 2004;52:179–185. doi: 10.1177/002215540405200205. [DOI] [PubMed] [Google Scholar]
- Takahashi K., Tanabe K., Ohnuki M., Narita M., Ichisaka T., Tomoda K., Yamanaka S. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131:861–872. doi: 10.1016/j.cell.2007.11.019. [DOI] [PubMed] [Google Scholar]
- Takeda H., Fujimori Y., Kai S., Ogawa H., Nakano T. CD318/CUB-domain-containing protein 1 expression on cord blood hematopoietic progenitors. Exp. Ther. Med. 2010;1:497–501. doi: 10.3892/etm_00000078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uezumi A., Fukada S., Yamamoto N., Takeda S., Tsuchida K. Mesenchymal progenitors distinct from satellite cells contribute to ectopic fat cell formation in skeletal muscle. Nat. Cell Biol. 2010;12:143–152. doi: 10.1038/ncb2014. [DOI] [PubMed] [Google Scholar]
- Uezumi A., Ito T., Morikawa D., Shimizu N., Yoneda T., Segawa M., Yamaguchi M., Ogawa R., Matev M.M., Miyagoe-Suzuki Y. Fibrosis and adipogenesis originate from a common mesenchymal progenitor in skeletal muscle. J. Cell Sci. 2011;124:3654–3664. doi: 10.1242/jcs.086629. [DOI] [PubMed] [Google Scholar]
- Uezumi A., Fukada S., Yamamoto N., Ikemoto-Uezumi M., Nakatani M., Morita M., Yamaguchi A., Yamada H., Nishino I., Hamada Y. Identification and characterization of PDGFRalpha+ mesenchymal progenitors in human skeletal muscle. Cell Death Dis. 2014;5:e1186. doi: 10.1038/cddis.2014.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uezumi A., Ikemoto-Uezumi M., Tsuchida K. Roles of nonmyogenic mesenchymal progenitors in pathogenesis and regeneration of skeletal muscle. Front Physiol. 2014;5:68. doi: 10.3389/fphys.2014.00068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vauchez K., Marolleau J.P., Schmid M., Khattar P., Chapel A., Catelain C., Lecourt S., Larghero J., Fiszman M., Vilquin J.T. Aldehyde dehydrogenase activity identifies a population of human skeletal muscle cells with high myogenic capacities. Mol. Ther. 2009;17:1948–1958. doi: 10.1038/mt.2009.204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wortmann A., He Y., Deryugina E.I., Quigley J.P., Hooper J.D. The cell surface glycoprotein CDCP1 in cancer—insights, opportunities, and challenges. IUBMB Life. 2009;61:723–730. doi: 10.1002/iub.198. [DOI] [PubMed] [Google Scholar]
- Wosczyna M.N., Biswas A.A., Cogswell C.A., Goldhamer D.J. Multipotent progenitors resident in the skeletal muscle interstitium exhibit robust BMP-dependent osteogenic activity and mediate heterotopic ossification. J. Bone Miner Res. 2012;27:1004–1017. doi: 10.1002/jbmr.1562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu X., Wilschut K.J., Kouklis G., Tian H., Hesse R., Garland C., Sbitany H., Hansen S., Seth R., Knott P.D. Human satellite cell transplantation and regeneration from diverse skeletal muscles. Stem Cell Rep. 2015;5:419–434. doi: 10.1016/j.stemcr.2015.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.