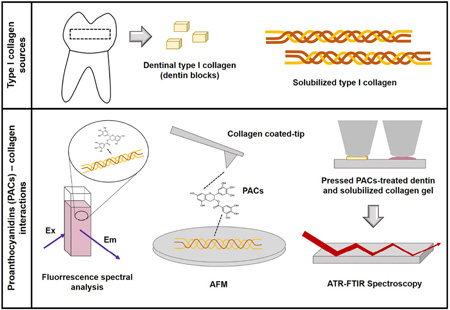
Abstract
Collagen cross-linkings are determinant of biological tissue stability and function. Plant-derived proanthocyanidins (PACs) mimic different hierarchical levels of collagen cross-links by non-enzymatic interactions resulting in the enhancement to the biomechanics and biostability of collagen-rich tissues such as dentin. This study investigated the interaction of PACs from Vitis vinifera grape seed extract with type I collagen in solubilized form and in the demineralized dentin matrix (DDM) by fluorescence spectral analysis; collagen-collagen binding forces in presence of cross-linking solutions by atomic force microscopy (AFM); and spectroscopic analysis of the DDM using attenuated total reflectance Fourier transform-infrared spectroscopy (ATR-FTIR). Glutaraldehyde (GA) and carbodiimide hydrochloride (EDC) with known cross-linking mechanisms were selected for comparative analyses. Changes in fluorescence upon interaction of solubilized type I collagen with PACs, EDC and GA reflected pronounced modifications in collagen conformation. PACs also promoted stronger collagen-collagen fibrils interaction than EDC and GA. A new feature was observed using ATR-FTIR spectroscopic analysis in PACs-treated collagen and DDM. The findings suggest covalent interactions between collagen and PACs. The mechanisms of interaction between PACs-collagen hold attractive and promising tissue-tailored biomedical applications and the binding forces that potentially drive such interaction were characterized.
Keywords: collagen cross-linking, dentin, proanthocyanidins, atomic force microscopy, ATR-FTIR spectroscopy
Graphical Abstract
1. Introduction
Type I collagen is the most abundant structural protein in human body and is the major component of connective tissues such as skin, tendons, bone, and dentin. Collagen molecules are assembled extracellularly to form microfibrils and fibrils. A cascade of reactions initiated by lysyl oxidase on lysine and hydroxylysine aminoacids at the telopeptides regions of the collagen molecules results in the formation of inter-, and intra-molecular and inter-microfibrillar cross-linking [1–4]. These enzymatically mediated cross-links are the tissue foundation for stability, strength and function. The types and amount of cross-links vary among tissues and changes due to physiological and pathological conditions.
Collagen cross-links have inspired novel approaches for tissue repair and regeneration with the ultimate goal to restore function. In dentistry, mimicking cross-linking in mature dentin collagen matrix through non-enzymatic chemical reactions enhances the native tissue’s biomechanics and dramatically reduces enzymatic breakdown [5–8]. Synthetic chemicals such as glutaraldehyde and carbodiimide were explored as non-enzymatic cross-linkers [7,9,10]. Carbodiimide hydrochloride compounds (EDC) are functional coupling reagents that induce cross-linking by activating carboxylic acid groups of glutamic or aspartic acid residues to form stable amide bonds with amines of lysine and hydroxylysine residues in the presence of N-hydroxysuccinimide (NHS) [11]. Glutaraldehyde (GA) effectively cross-links the tissue by forming covalent binding of two amine groups of (hydroxy)-lysine residues with its aldehyde groups, which will further react to form pyridinium compounds [11]. EDC/NHS and GA induce cross-links even in highly cross-linking tissue such as dentin [7]; however clinical usage remains uncertain due to cytotoxic reactions [10].
Plant-derived agents strongly interact with biological tissues and increase their physicochemical properties and stability similar to synthetic agents [10]. In specific, plant-derived proanthocyanidins (PACs) are considered one of the most important classes of secondary metabolites in the plant kingdom, offering a great renewable source of raw material. Naturally occurring chemical compounds such as oligomeric PACs enhance the mechanical properties and decrease degradation of dentin via interaction with type I collagen [6,12]. The specific mechanisms of interaction between PACs-type I collagen from triple helices to fibrils remains largely speculative. The complexity of interactions between PACs and proline rich proteins yielded reports of a variety of molecular bonds, including hydrogen and covalent bonds, hydrophobic interactions and formation of hydrophobic pockets [13–16].
To elucidate the mechanism of interaction of PACs with collagen-rich tissues, we have undertaken spectroscopic and atomic force microscopy analyses in purified type I collagen and correlated them to changes in the spectroscopic analysis of collagen-rich demineralized dentin matrix (DDM) induced by synthetic and naturally occurring chemical cross-linkers. The well-described mechanisms of cross-linking between EDC and GA with collagen-rich tissues were used as a reference to determine the chemical interactions of PACs with dentin collagen. The hypothesis tested was that the interactions of PACs with type I collagen would be of similar chemical functionality as the cross-linking induced by GA and EDC.
2. Material and Methods
2.1. Chemicals and reagents
Cross-linking agents used in this study included: proanthocyanidins [(PACs), Vitis vinifera extract, total proanthocyanidins (PACs) concentration >94%, Lot#31492514-01, MegaNatural Polyphenolics, Madera, CA]; 1-ethyl-3-[3-dimethylaminopropyl] carbodiimide hydrochloride (EDC, Pierce/Thermo Scientific, Rockford, IL), N-hydroxysuccinimide (NHS, Pierce Thermo Fisher Scientific Inc.), and glutaraldehyde (GA, 25% stock solution, Fisher Scientific, Pittsburgh, PA). The Vitis vinifera crude extract contains mainly oligomeric and polymeric molecules, with low content of monomers and other molecules with low degree of polymerization and 80% total polyphenol content [12]. Type I collagen extracted from rat tail tendon and solubilized in acetic acid (BD™ Biosciences, San Jose, CA) was used to evaluate the structural impact of interaction between cross-linking agents and collagen and to determine collagen-collagen interactions.
2.2. Fluorescence spectral analysis
Fluorescence experiments were carried out at room temperature with excitation at 276 nm using a FluoroMax 3 fluorescence spectrophotometer (Horiba Scientific, Edison, New Jersey, NJ) and a 1 cm path length quartz cuvette. Spectra were measured in triplicate by incubating 0.5 g/L of type I collagen with a range of different concentrations of PACS, EDC/NHS and GA: from 0.0065% to 0.065% (w/v) PACS, from 0.0575% to 0.575% (w/v) for EDC with 0.014% to 0.14% (w/v) NHS, and from 0.005% to 0.5% (w/v) GA. Cross-linking solutions were prepared in distilled water (DW) and pH was adjusted to 7.2. Reference spectra of solubilized collagen and of the cross-linking solutions were also recorded.
2.3. Direct measurement of interaction between collagen-collagen fibrils and collagen-collagen molecules
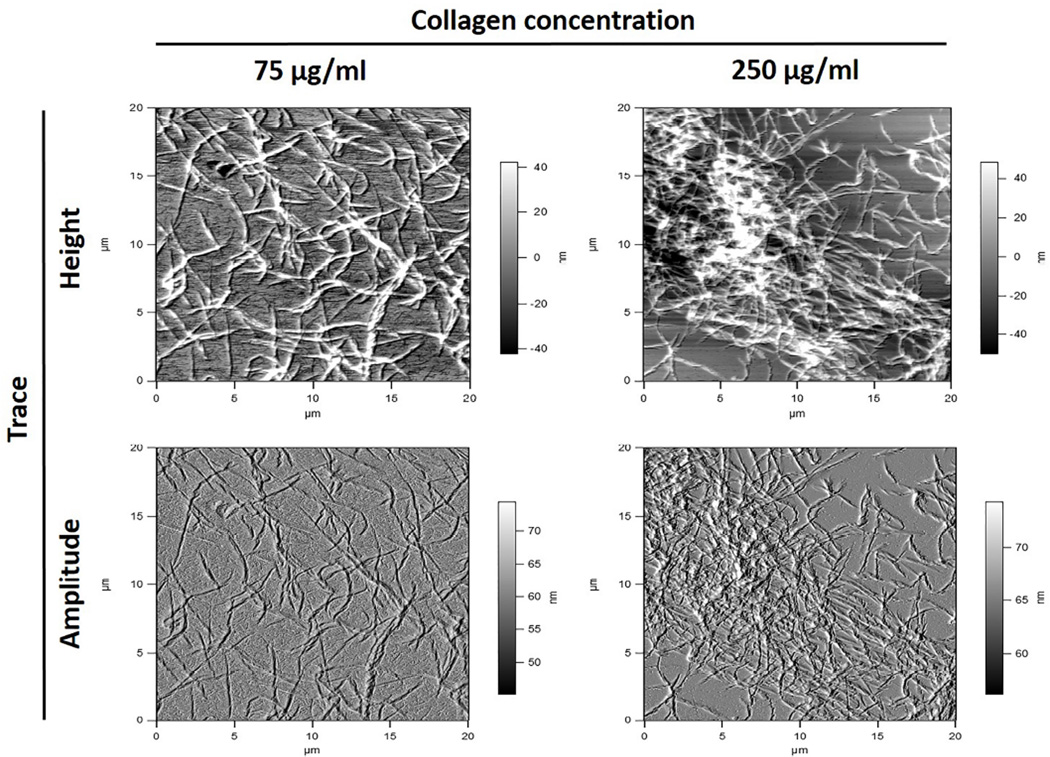
The interaction of collagen-collagen molecules and reconstituted collagen-collagen fibrils and cross-linkers was measured by the pulling forces of a collagen coated tip interaction with a collagen coated substrate while or after exposure to the chemical agents. To determine collagen-collagen molecular interactions, Au-coated AFM probes (Budget Sensors, Inc) were cleaned by exposure to oxygen plasma (PDC-001 plasma cleaner, Harrick Plasma, Ithaca, NY) for 3 minutes on high power and then coated with type I collagen solution diluted to 10 µg/ml using 0.1 M acetic acid. Cleaned tips were placed in collagen solution for 1–2 hours and then thoroughly rinsed in Millipore water followed by drying in nitrogen stream. The coating of Au probes with collagen was confirmed by repeating the coating steps on Au substrates and imaged in an atomic force microscope (Asylum MFP-3D Instrument, Asylum Research, Santa Barbara, CA; Figure 1).
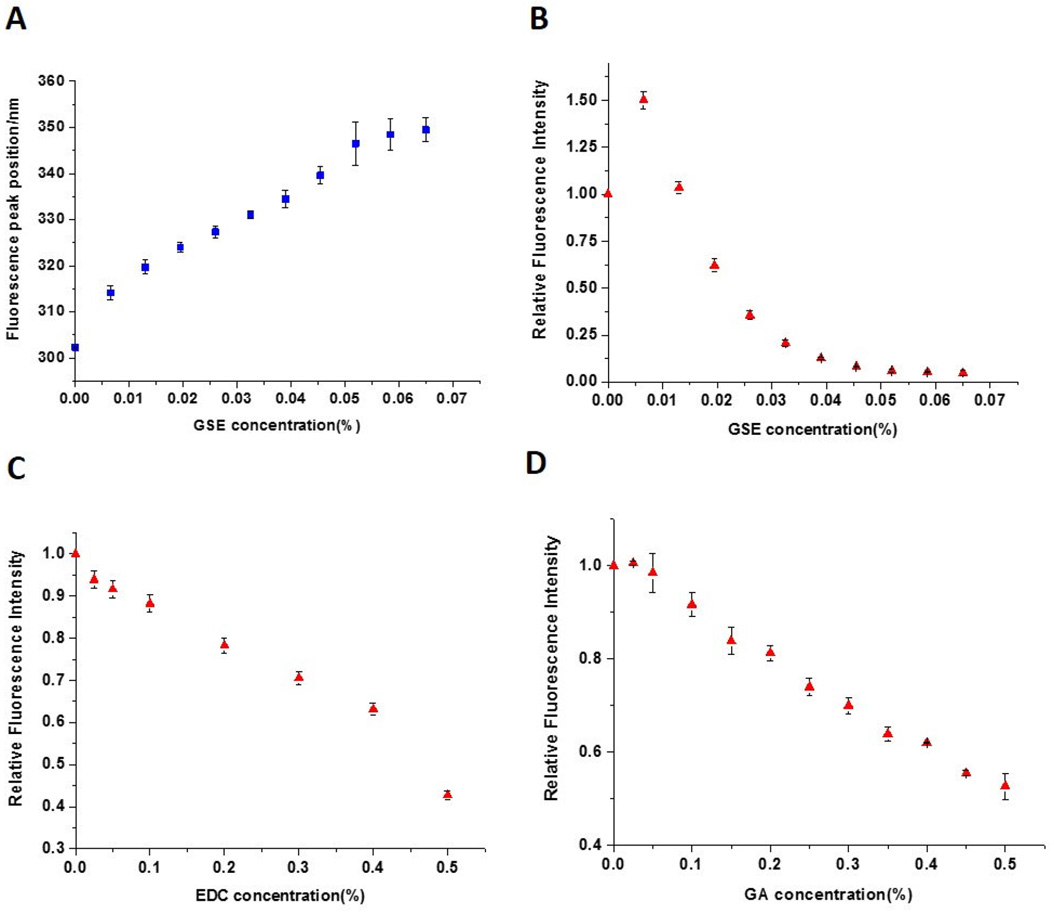
Figure 1.
Collagen fluorescence spectra (nm) and changes in peak fluorescence intensity when treated with different concentrations of PACs, EDC/NHS and GA. (A) Collagen fluorescence peak positions and (B) fluorescence intensities when incubated with 0.0065 – 0.065% PACs. (C) Collagen fluorescence intensities after incubation with 0.0575 – 0.575% EDC containing 0.014 – 0.14% NHS. (D) Fluorescence intensities when collagen was incubated with 0.005 – 0.5% GA.
Force measurements of interaction were performed by placing the collagen-coated AFM tip into contact with the collagen-coated substrate, allowing to dwell (0–3 seconds) and retract away from the surface in presence or absence of cross-linking solutions. All force measurements were performed with Asylum MFP-3D AFM Instrument at room temperature. The substrates were placed in a fluid cell which was then filled with about 2 mL 10 mM PBS (pH 7.2) solution and left to equilibrate the AFM temperature for 10–15 minutes. The spring constant of each AFM tip was calibrated in buffer by the thermal fluctuations method [17]. By measuring the tip deflection and its displacement, force curves (force vs tip separation) were plotted. Experiments were carried out in triplicates. Analysis of force counts was carried out using IGOR Pro software (Wavemetrics, Eugene, OR) and custom written codes in Matlab (Mathworks, Natick, MA).
For collagen-collagen fibrils interactions, gold coated AFM probes were cleaned as described above. Gold substrates (1 × 1 cm2) were cleaned by sonication in isopropyl alcohol for 5–10 min and then plasma cleaned for 3 minutes. Then, substrates were immediately placed in ethanol containing 5 mM octadecanethiol (ODT) for 2 hrs. ODT coated substrates were rinsed in ethanol and water followed by drying in nitrogen stream. Average thickness of ODT layer measured with ellipsometry was 12 Å. Collagen coating of substrates and tip was performed as previously described [18]. Both Au substrates and Au AFM probes were immersed overnight in PBS solution, pH 7.4, containing 75 and 250 µg/ml collagen concentrations respectively. Collagen coated substrates and probes were rinsed several times using distilled water and dried in nitrogen. AFM images were acquired to show coating of Au substrates by collagen fibrils (Figure 2). Then, before interaction with collagen-coated AFM tip, collagen-coated substrate was treated with 0.65% PACs, 0.1% EDC/0.03% NHS or 1% GA solution for one hour, rinsed with PBS buffer and DW, and immediately used in the experiment. Force measurements were performed as described above. Fold increase of high force interactions counts (> 500 pN) were calculated between PBS (control) and cross-linkers and statistically analyzed using paired t-test (p < 0.05)
Figure 2.
Coating of Au substrate with different concentrations of type I collagen characterized by atomic force microscopy and used to determine collagen-collagen molecular interactions.
2.4. ATR-FTIR Spectroscopy
Interactions of type I collagen and dentin collagen (in DDM) with cross-linkers were analyzed using an ATR accessory (single reflection diamond, PIKE MIRacle ATR, Madison, WI) mounted in an FTIR spectrometer (Bruker Vertex 80, Billerica, MA). Solubilized type I collagen (1 mg/ml) was incubated at room temperature for 1 hour with either 0.065% (w/v) PACs, 5.75% (w/v) EDC/1.4% (w/v) NHS or 5% (v/v) GA freshly prepared in DW as described before. Because relatively high concentrations of PACs, EDC or GA were used resulting in strong cross-linking, after mixing the collagen with the cross-linkers the solution became more viscous and eventually a gel phase formed separate from a supernatant liquid phase, especially for the PACs. Since the liquid phase transmission IR spectrum showed mostly peaks from the cross-linkers, ATR-IR spectra were measured for the gel phase to assess changes in the collagen. For that, the gel was pressed onto the ATR plate using a pressure clamp to obtain better contact with the diamond surface. Spectra over the range 4500 cm−1 to 600 cm−1 were collected as an average of 512 scans (10 kHz scan speed with a DTGS detector) and processed with 3-term Blackman-Harris apodization and zero filling of 2. Background transmission spectra collected with same measurement parameters but with no sample on the ATR crystal surface were used to compute absorbance spectra. Repeated spectra were collected until the collagen gels were completely dried and the results were consistent. The ATR absorbance spectra of PACs, EDC/NHS and GA solutions were measured by applying 10 µl of each solution on the crystal surface and drying with a mild flow of nitrogen gas to form a homogenous thin film. Experiments were carried out in triplicate.
The evaluation of collagen cross-linking in the DDM was performed as described for purified type I collagen. Five freshly extracted human molar teeth were collected and stored at −20 °C (IRB, University of Illinois at Chicago, protocol # 2009-0229). Crowns were cut off using a low speed diamond saw (Buehler, Lake Bluff, IL) to obtain dentin specimens with 0.5 mm thickness × 1.5 mm width × 1.5 mm length. Dentin specimens were completely demineralized with 10% phosphoric acid (Ricca Chemical Company, Arlington, TX) for 5 h under agitation [6]. Water-rinsed specimens were pressed onto the diamond crystal surface to better cover the active area and ATR spectra of DDM only were obtained. After that, DDM specimens were re-hydrated and treated with (n = 3): 6.5 % PACs, 5.75% EDC/1.4% NHS or 5% GA, prepared in DW (pH 7.0). Each DDM specimen was treated with 200 µl of fresh cross-linking solution for 1 h at room temperature and washed before re-measurement with ATR-FTIR to obtain a spectrum of cross-linked DDM from the same surface. Reference ATR spectra of the PACs, EDC/NHS, and GA solutions were measured as described above.
Difference spectra were obtained by subtracting the spectra of untreated from treated collagen or DDM with cross-linking agents, using subtraction factors adjusted to minimize the amide I and amide II bands from collagen (in practice the collagen peaks could not be fully eliminated without introducing baseline artifacts into the difference spectra). Spectra from cross-linked collagen or DDM and/or cross-linking solutions were scaled and plotted along with difference spectra for evaluation and comparison. Data were plotted using Origin software (OriginLab, Northampton, MA).
3. Results
The average collagen fluorescence spectra are presented for individual groups in Figure 2. When excited at 276 nm, collagen exhibited emission with a maximum at ~303 nm, characteristic for tyrosine fluorescence [19]. Fluorescence intensity with 276 nm excitation of PACs, EDC and GA solutions alone were much lower than for collagen (not shown), so we could monitor the changes in collagen fluorescence after interaction with such cross-linkers without interference.
When treated with cross-linking agents, collagen fluorescence intensity decreased, with the most prominent reduction observed for addition of PACs (Figure 2B). A large red shift from 303 nm to 352 nm can be seen as PACs concentration increases (Figure 2A). With higher concentrations of PACs, a gel was formed, which impaired the measurements due to higher scatter. Moreover, with addition of low concentrations of PACs, the fluorescence intensity shows an initial increase before falling off. By contrast, collagen fluorescence after cross-linking with EDC and GA showed only very small red shifts, ~1–2 nm (not shown). Somewhat varying from PACs, fluorescence intensity steadily decreased with added EDC and GA, although the latter evidenced a stable intensity for GA additions up to ~0.05 % (Figures 2C and 2D).
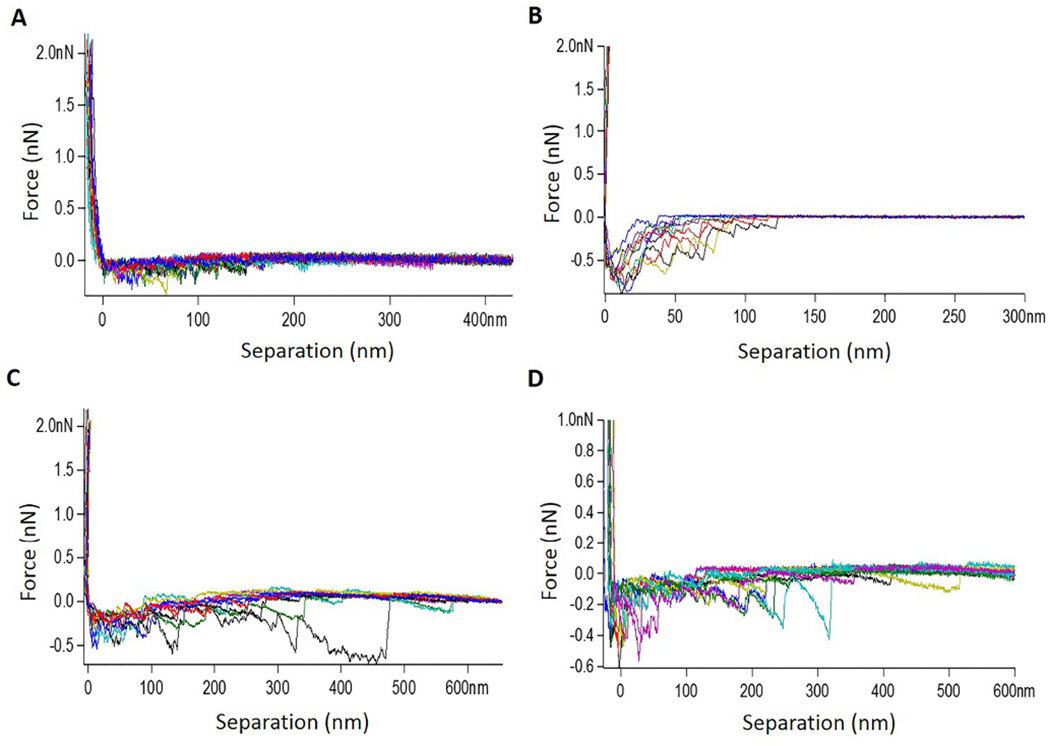
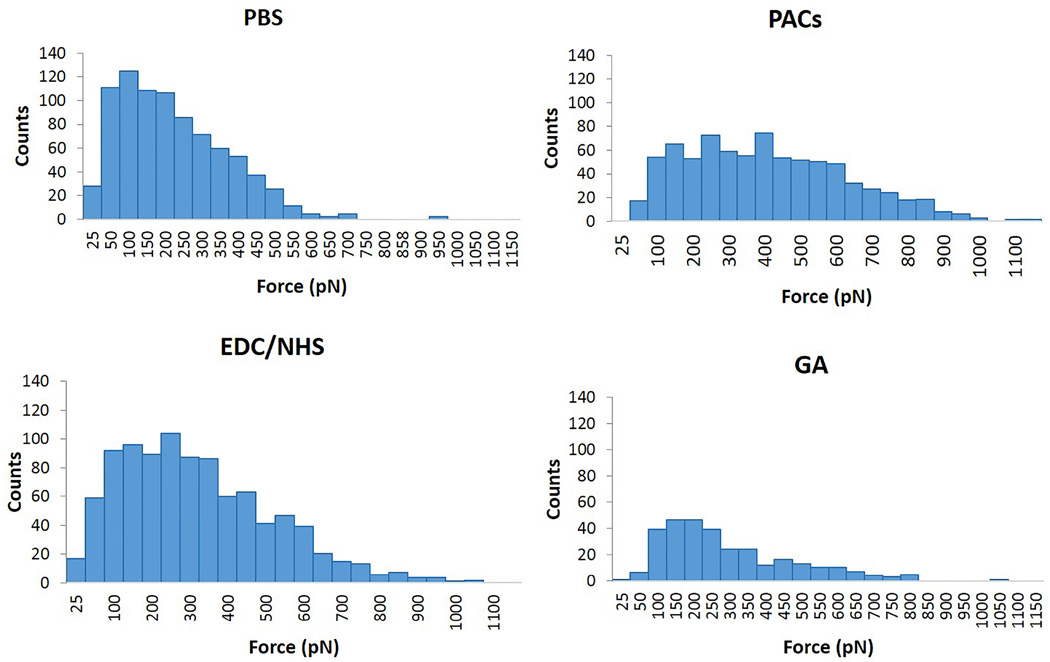
Using AFM, the binding force between collagen-collagen molecules and fibrils in presence or absence of cross-linkers was determined, so the mechanism of interaction could be suggested. AFM images show the substrates coated by collagen fibrils, which are dispersed at the surface. High density of collagen fibrils were found with higher collagen concentration (250 µg/ml) (Figure 1). Collagen-collagen molecular and fibrils interactions were similar. Therefore only reconstituted collagen-collagen fibril interactions in the presence or absence of cross-linkers are described (Figures 3 and 4; note different scales in figures). A control experiment (in the absence of cross-linkers) showed weak collagen-collagen interactions (Figure 3A and 4A, mean force ~ 169 pN). The interaction force experiments were used to plot the force histograms shown in Figure 4. Representative histograms show an increase in the pull-out force intensity and events. High force interaction (> 500 pN) counts were increased for all cross-linkings when compared to PBS. The highest fold increase was observed for PACs (8 ± 7.7) which was statistically higher than EDC/NHS and GA (4.3 ± 3.9 and 1.7 ± 0.8 respectively, p < 0.01); EDC/NHS exhibited significantly higher fold increase than GA (p = 0.003).
Figure 3.
Retract traces between collagen-tip and collagen-substrate treated with (A) PBS, (B) 0.65% PACs in PBS pH 7.4, (C) 0.1%/0.03% EDC/NHS in PBS pH 7.4 and (D) 1% GA in PBS pH 7.4. AFM tip velocity 1000 nm/s. Dwell time 2 s.
Figure 4.
Representative force histograms obtained for PBS (control), PACs, EDC/NHS and GA with dwell 2s. Increased number of interactions in the force range 500–1000 pN indicates the cross-linking between collagen fibrils due to the presence of cross-linker.
Representative ATR-FTIR spectra for cross-linked type I collagen and DDM are presented in Figures 5 and 6. Both DDM and collagen spectra for all specimens showed typical peaks for collagen, such as C=O stretching at ~1632 cm−1 (amide I), out-of-phase combination of N-H bending and C-N stretching at ~1544 cm−1 (amide II), CH2 scissoring at ~1450 cm−1 and some contribution from the mix of Cα-H deformation and N-H bending and C-N stretching between 1350 and 1200 cm−1 (Cα-H and amide III) (Figures 5 and 6).
Figure 5.
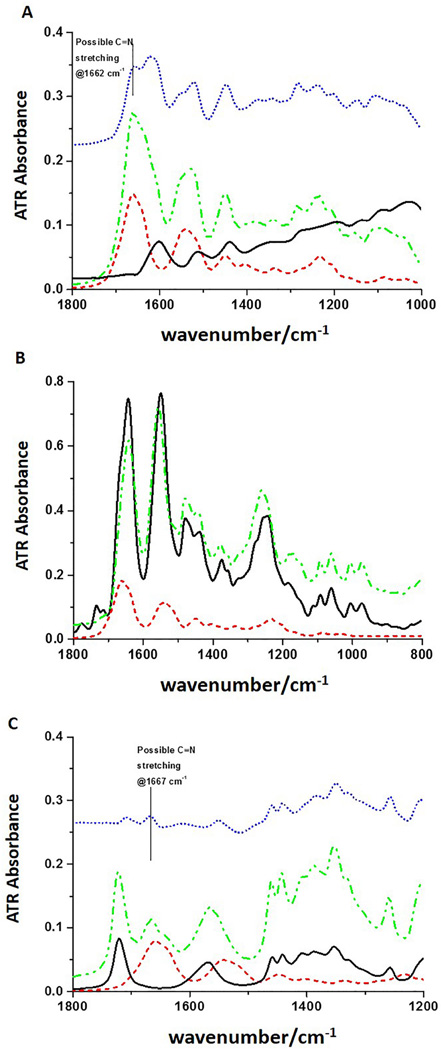
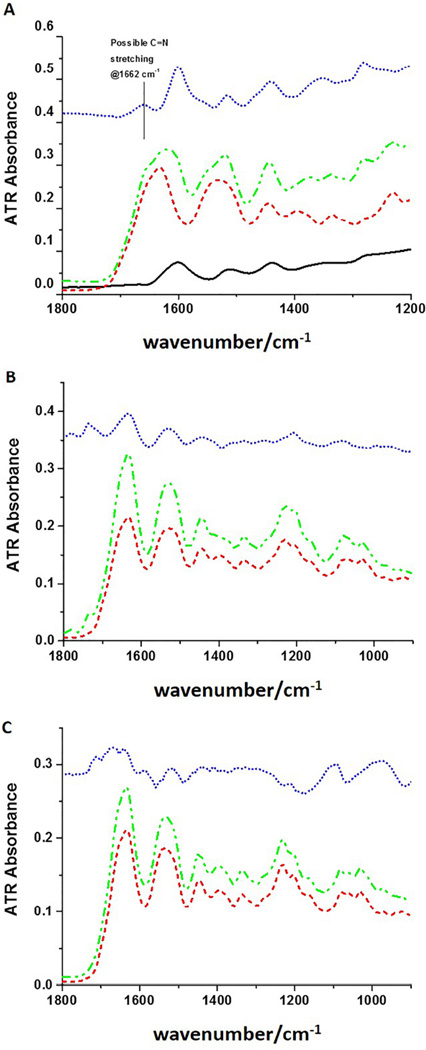
Representative ATR-FTIR spectra of (A) type I collagen treated with 0.065% PACs. Black solid line: PACs; red short dash line: collagen; green dash dot line: collagen + PACs; blue short dot line: difference spectrum; (B) type I collagen treated with 5.75% EDC/1.4% NHS. Black solid line: EDC/NHS; red short dash line: collagen; green dash dot line: collagen + EDC/NHS; and (C) type I collagen treated with 5% GA. Black solid line: 5% GA; red short dash line: collagen; green dash dot line: collagen + GA; blue short dot line: difference spectrum.
Figure 6.
Representative ATR-FTIR spectra of (A) demineralized dentin matrix (DDM) treated with PACs. Black solid line: 6.5% PACs; red short dash line: DDM; green dash dot line: DDM + PACs; blue short dot line: difference spectrum; (B) DDM treated with 5.75% EDC/1.4% NHS. Red short dash line: DDM; green dash dot line: DDM + EDC/NHS; blue short dot line: difference spectrum; and (C) DDM treated with 5% GA. Red short dash line: DDM; green dash dot line: DDM + GA; blue short dot line: difference spectrum (multiplied by three).
In PACs-treated collagen, PACs peaks are aromatic ring C-C stretching at ~1600 cm−1 and ~ 1517 cm−1 and at 1450 cm−1 (Figure 5A, red dash). Difference spectra (blue dots), that subtract collagen spectra from the collagen-PAC complex spectra, bring out the changes in the protein matrix upon addition of the PACs but still evidence considerable collagen features due to inexact match. A possibly new peak at 1662 cm−1 (Figure 5A, blue dots) can be identified in the difference spectrum. For the spectra of EDC-treated collagen (Figure 5B), the gel phase formed after mixing collagen and EDC solution (Figure 5B, green dash-dot) still evidences most of the peaks from EDC/NHS and shows only minor intensity and frequency variations. There are small relative variations in intensities for the mixture as compared to the spectrum of EDC/NHS, however, due to the higher concentrations of EDC/NHS used, our ability to detect its impact on collagen is poor for this ATR-IR test. Similar to EDC-treated collagen, the same sort of difference spectrum for the interaction of GA and collagen is dominated by the peaks from GA, but in this case, by using a double difference method, subtracting both collagen and GA from the spectra of the mixture, a new peak is clearly evident at 1667 cm−1 (Figure 5C, blue dot). In all these cross-linked collagen solution spectra, elimination of the protein spectral bands by simple subtraction should leave behind residual cross-linker plus any new products. Since the added cross-linker is in excess and difficult to separate after reaction, sensitivity to new product in the difference spectra so dominated by cross-linker bands is limited.
In PACs-treated DDM, PACs peaks are similar to the ones described for collagen. Besides the new feature at 1662 cm−1, changes at ~1350 and 1250 cm−1 are observed due to PACs-collagen interaction, which are most evident in the difference spectrum as compared to the spectrum of PACs (Figure 6A). EDC/NHS-treated DDM IR spectrum is almost identical in shape to that of DDM, with the exception of two weak peaks at 1734 cm−1 and 1776 cm−1 (Figure 6B). In addition, there are small absorption increases at 1200 cm−1 and 1079 cm−1 and decrease at 1395 cm−1 when compared to the spectrum of untreated DDM and in the difference spectrum. When DDM was treated with GA, none of the peaks from GA are evident and a very small shoulder at 1658 cm−1 was observed (Figure 6C), which is most obvious in the difference spectrum. The spectra also show a weak feature at ~1715 cm−1, which could be from GA. Other than that, GA peaks are not evident in the difference spectrum and no comparison between difference spectrum and spectrum of GA is made.
4. Discussion
The present study showed marked modifications in collagen fluorescence upon interaction with synthetic and naturally occurring chemical compounds, indicating collagen conformational or morphological changes affecting the Tyr environments promoted by the cross-linkers. Moreover, the mechanism of interaction of agents with the collagen molecule vary as shown by our ATR-FTIR results, although some similarities can be seen for PACs and GA. The exposure of type I collagen and its microfibrils to the cross-linking agents induced strong chemical bonding interactions, with pronounced differences between synthetic or naturally occurring source cross-linkers as shown by AFM studies. The hypothesis that collagen interactions with cross-linking PACs would occur at similar chemical functionalities as induced by synthetic chemical agents was partially rejected.
For the fluorescence spectral analysis, collagen was solubilized at pH 7.2, since at pH higher than 7.4, a precipitate was formed, probably due to fibril or other aggregate formation. After mixing with cross-linking solutions, the pH was dropped to 6.0, so the fluorescence assay could be performed without any distortions from aggregation. With excitation at 276 nm, there was no interference from any of the cross-linking agents. Also, we can clearly observe the tyrosine (Tyr) fluorescence change, since tryptophan is not present in type I collagen [20,21]. The results showed a decrease in collagen fluorescence intensity when any of the cross-linkers were added at relatively high concentrations, with PACs inducing the greatest intensity loss. There were also red shifts of the peak frequencies, but these latter changes were significant only for PACs. Red shifts in protein/Tyr fluorescence generally indicate an increase in solvation or a less hydrophobic environment. The red-shifted tyrosine emission observed for all cross-linkers is not fully understood. One possibility might be that ionization of the phenolic hydroxyl group of Tyr leads to tyrosinate formation and red shift in the emission spectrum, but this seems unlikely at the pH values we used. Another possibility is that the Tyr residue may get trapped in a local conformation and participate in events like excited state proton transfer, leading to a red-shifted fluorescence emission [19]. Since the frequency shift is only significant with PACs, one might assume that the Tyr side chains interact with the aromatic rings of the polyphenolic components of PAC [22], which could result in a red shift, or that the extended linkage exposed the fluorescent Tyr on the telopeptide segments to more solvation.
The fact that the shift is much smaller with EDC/NHS or GA, implies that those cross-linked structures are different, and possibly more tightly packed, at least in the regions of the sequence with a significant Tyr fraction. That would also seem to argue against an aromatic interaction of the PAC with the protein to drive collagen cross-linking with PAC. Some difference in mechanism is indicated here. Loss of intensity is more dependent on quencher availability close by in the sequence. For PAC, there is a gain in intensity at low concentration added, followed by a much larger loss. Charged amino acids such as lysine, aspartic acid, glutamic acid and histidine can quench the fluorescence of tyrosine when they are close. In the sequence of collagen, about 50% of the intensity of the fluorescence is found within the mapped cross-link domain (the cross-linking sites such as lysine) [23]. After addition of low concentrations of cross-linker, many of the lysine residues become cross-linked (thus no longer charged), which could allow the tyrosine fluorescence intensity to increase, as seen with PAC, and possibly, but less so, with GA. This again might argue for a covalent linkage, and not just H-bonding, but could alternatively suggest some aromatic interaction altering quantum yield, although one would expect the PAC to quench Tyr, acting as an acceptor. With higher concentrations of any of the cross-linkers, gel formation causes loss of tyrosine fluorescence intensity due to scatter. The PAC shows a stronger cross-linking effect on the tyrosine fluorescence than GA, as demonstrated from the larger amplitude of tyrosine fluorescence increase at low cross-linker concentrations and decrease at high cross-linker concentrations. On the other hand, the mechanism of cross-linking for EDC is described as “zero-length” since the carbodiimide does not become part of the cross-linked protein [11]. Possibly, the resulting minimal modifications in collagen structure with EDC were reflected in relatively small changes in collagen fluorescence.
AFM measurements can provide clues to the adhesive forces between collagen fibrils without the requirement of further processing steps such as collagen extraction, digestion and fractionation. The detection of a single molecule by AFM and characterization of its specific interaction forces with other molecules is a unique tool to study the dynamics of biological processes [24]. Such technique has been recently used to characterize collagen interactions at the single molecule level in collagen membranes used as scaffolds [25].
When collagen coated AFM tip approaches the collagen coated substrate, the collagen molecules interact through specific bonding reactions. Then the tip is retracted by application of increasing forces until the bond is broken (unbinding force). It is feasible that physical changes to the coated fibrils and collagen mediated by the investigated cross-linkers could affect the tip-substrate interactions resulting in discrete jump-off events. When the collagen substrate is treated with PBS only (control), weak hydrogen-bonding-like interactions are observed (Figure 4, mean force ~ 169 pN). Moreover, it is possible to observe the multi-peak characteristic of the curves (Figure 3A), which are common in multi-domain protein such as collagen and occur due to polypeptide chains stretching and domain unfolding [26]. However, when collagen substrate is treated with the synthetic or naturally occurring chemical cross-linkers, such molecules will mediate the binding between the collagen molecules in the substrate and AFM tip. This is revealed by the increased interaction forces between collagen fibrils after treatment with PAC, EDC and GA (Figure 4). Such an increase is more evident for PAC and is an indication of strong interactions (mean force ~ 700 pN), supporting the ATR-FTIR data for PAC-treated collagen and DDM.
PACs are formed by monomeric building blocks catechins, which are flavan-3-ol units that can be linked by an additional ether bond (C – O) or one or more C – C bonds to form oligomers and polymers. The degree of oligomerization determines PACs interaction with collagen at different hierarchical levels [27]. More specifically, higher oligomeric forms of PACs will mediate both intra-molecular cross-links, that provide biostability to the collagen molecule, and inter-molecular and inter-microfibrillar cross-links, that will enhance mechanical properties [27]. Interestingly, our results showed similar increase in collagen-collagen interaction forces for both molecules (data not shown) and fibrils (Figures 3 and 4), supporting the hypothesis that PAC, which is a mixture of low and high-oligomeric PACs, cross-links collagen at different hierarchical levels.
To better understand the specific mechanisms of interactions between dentin collagen and cross-linking agents, ATR-FTIR analysis was carried out in both type I collagen and DDM. The IR spectra of collagen show its specific feature, the amide bands, which are characteristic of its triple helix conformation. The PAC-treated collagen spectrum shows a combination of IR peaks from the collagen and the PAC spectra, but the difference spectra show evidence for new bands at 1662 cm−1 and possibly 1350 and 1280 cm−1, for example (Figure 5A), which provides evidence of PAC incorporation and interaction with collagen. Similar results were observed for DDM cross-linked with PAC (Figure 6A). A peak at 1450 cm−1 and a loss of intensity at 1400 cm−1 can be noted in the difference spectrum and confirms previous results [28].
Recent studies have suggested that hydrogen bonding is the main mechanism of interaction between hydroxyl groups present in PACs and amino and amide groups of collagen [15,29]. However, hydrophobic interactions were also proposed to explain the binding of polyphenols to proteins, which might occur through the association of their aromatic rings with proline residues [30,31]. In the present study, the comparison of the difference spectrum both for DDM and type I collagen to the PAC ATR spectrum shows the changes after treatment more clearly: exemplified by the new feature at 1662 cm−1 seen in the difference spectrum (top traces, blue dots, Figure 5A and 6A), which may be assigned to a possible C=N stretching. Additionally an enhancement of the CH2 bending is seen at 1442 cm−1, as well as the above noted 1400 cm−1 band, an ester C-O stretching at 1283 cm−1 and a phenol and ether C-C, C-O stretching at 1198 cm−1 and 1038 cm−1. These observations are in agreement with previous studies on the interactions of PACs with collagen and gelatin [16,32]. Based on our data, this new feature can be an indication of covalent or covalent-like bond formation, i.e. Schiff base, between PAC and collagen. The interactions of PACs with both carboxylate groups from glutamic and aspartic acid (ester linkage) and amino groups (Schiff base formation) from lysine are supported in this study by fluorescence spectral analysis as previously discussed.
EDC couples carboxyl groups to primary amines forming amide bonds [33], whose spectral contributions would be obscured by overlap with the collagen amide bands. These crosslinks are called “zero-length” since they directly couple sidechains already in the protein with no added linker and give rise to no added spectral features. The ATR-FTIR data corroborate with such observations. Moreover, both the EDC-treated-collagen and -DDM spectra show peaks at ~1740 and 1780 cm−1, which could correspond to the ester C=O group stretch. Additional ester bonds formed between carboxyl and hydroxyl groups have been previously suggested for EDC-cross-linked collagen [34], but are not evident in the present study. Actually, the asymmetrical and symmetrical stretching frequencies of the C-O ester groups between large molecules are expected in the region of 1185–1277 cm−1 and 1050–1116 cm−1 [35]. While we see intensity changes in this region, overlap with DDM modes makes determination difficult. These ester bonds can be also created with the hydroxyl groups of carbohydrate in glycosaminoglycans [36], which are also present in dentin organic matrix, although in a much smaller amount when compared to collagen. Two weak peaks that differ between EDC/NHS-treated DDM IR spectrum and DDM, are the peaks at 1734 cm−1 and 1776 cm−1. The relative intensities of these features do not correspond to the peak distribution in the EDC/NHS spectrum, so their origin is unclear. They might correspond to impurities, excess of EDC (as seen in EDC/NHS solution Figure 5B, black trace) or ester formation.
The treatment of DDM with GA showed a new peak at 1667 cm−1, which can be seen as a small shoulder in the difference spectrum. The absence of GA peaks in the difference spectrum is probably because GA was completely washed away from the dentin specimen and only the induced changes to DDM could be observed. The mechanism of GA cross-linking has been described in detail and is based on a reaction of its aldehyde groups with free amino groups of lysine or hydroxylysine residues, forming hydrolysable Schiff bases, which are stabilized by further reactions with other GA molecules [37]. While there is evidence for a new band at 1664 cm−1 in the collagen plus GA spectrum, this is less evident in the DDM plus GA spectrum. This is likely due to the presence of endogenous crosslinking and the structural complexity and composition of the dentin matrix limiting extensive incorporation of the GA linkage as compared to the solubilized collagen. A large variety of further reactions can occur forming secondary or tertiary amine groups or pyridinium compounds [11]. Previous spectroscopic changes of GA-treated collagen showed increase of band at 1034 cm−1, suggesting formation of a pyridinium compound, however, same changes were not evident in partially demineralized dentin matrix [38].
5. Conclusion
The findings provide evidence for covalent-like bonds induced by plant PACs in type I collagen as well as complex dental native tissue. A strong collagen-collagen interaction shown by AFM holds key role to the enhanced biostability and biomechanics of PACs-treated demineralized dentin matrices overtime.
Statement of significance.
Connective tissues such as skin bone and dentin are mainly composed of type I collagen, which is cross-linked to promote tissue stability, strength and function. Novel therapies using substances that mimic cross-links have been proposed to promote repair of collagen-based-tissues. In dentistry, naturally occurring proanthocyanidins (PACs) have the potential to enhance dentin mechanical properties and reduce its enzymatic degradation, but their mechanisms of cross-linking are unclear. The present study investigated the specific interactions between PACs-type I collagen in purified and dentin collagen and compared to the well described cross-linking mechanisms promoted by synthetic chemical substances. Our results show that covalent-like bonds are induced by plant PACs in type I collagen as well as in complex dental native tissue, promoting strong collagen-collagen interactions.
Acknowledgments
This study was supported by NIH/NIDCR research grant [DE17740 (ABR), DE021040 (ABR) and R37 DE014193 (PBM)].
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure
The authors confirm that there are no known conflicts of interest associated with this publication and there has been no significant financial support for this work that could have influenced its outcome.
References
- 1.Rivera EM, Yamauchi M. Site comparisons of dentine collagen cross-links from extracted human teeth. Arch. Oral Biol. 1993;38:541–546. doi: 10.1016/0003-9969(93)90118-6. [DOI] [PubMed] [Google Scholar]
- 2.Knott L, Bailey AJ. Collagen cross-links in mineralizing tissues: a review of their chemistry, function, and clinical relevance. Bone. 1998;22:181–187. doi: 10.1016/s8756-3282(97)00279-2. [DOI] [PubMed] [Google Scholar]
- 3.Orgel JPRO, Irving TC, Miller A, Wess TJ. Microfibrillar structure of type I collagen in situ. Proc. Natl. Acad. Sci. U. S. A. 2006;103:9001–9005. doi: 10.1073/pnas.0502718103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bertassoni LE, Orgel JPR, Antipova O, Swain MV. The dentin organic matrix - limitations of restorative dentistry hidden on the nanometer scale. Acta Biomater. 2012;8:2419–2433. doi: 10.1016/j.actbio.2012.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bedran-Russo AKB, Pashley DH, Agee K, Drummond JL, Miescke KJ. Changes in stiffness of demineralized dentin following application of collagen crosslinkers. J. Biomed. Mater. Res. B Appl. Biomater. 2008;86:330–334. doi: 10.1002/jbm.b.31022. [DOI] [PubMed] [Google Scholar]
- 6.Castellan CS, Pereira PN, Grande RHM, Bedran-Russo AK. Mechanical characterization of proanthocyanidin-dentin matrix interaction. Dent. Mater. Off. Publ. Acad. Dent. Mater. 2010;26:968–973. doi: 10.1016/j.dental.2010.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bedran-Russo AKB, Vidal CMP, Dos Santos PH, Castellan CS. Long-term effect of carbodiimide on dentin matrix and resin-dentin bonds. J. Biomed. Mater. Res. B Appl. Biomater. 2010;94:250–255. doi: 10.1002/jbm.b.31649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bedran-Russo AKB, Castellan CS, Shinohara MS, Hassan L, Antunes A. Characterization of biomodified dentin matrices for potential preventive and reparative therapies. Acta Biomater. 2011;7:1735–1741. doi: 10.1016/j.actbio.2010.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sung H-W, Chang W-H, Ma C-Y, Lee M-H. Crosslinking of biological tissues using genipin and/or carbodiimide. J. Biomed. Mater. Res. A. 2003;64:427–438. doi: 10.1002/jbm.a.10346. [DOI] [PubMed] [Google Scholar]
- 10.Han B, Jaurequi J, Tang BW, Nimni ME. Proanthocyanidin: a natural crosslinking reagent for stabilizing collagen matrices. J. Biomed. Mater. Res. A. 2003;65:118–124. doi: 10.1002/jbm.a.10460. [DOI] [PubMed] [Google Scholar]
- 11.Olde Damink LH, Dijkstra PJ, van Luyn MJ, van Wachem PB, Nieuwenhuis P, Feijen J. Cross-linking of dermal sheep collagen using a water-soluble carbodiimide. Biomaterials. 1996;17:765–773. doi: 10.1016/0142-9612(96)81413-x. [DOI] [PubMed] [Google Scholar]
- 12.Aguiar TR, Vidal CMP, Phansalkar RS, Todorova I, Napolitano JG, McAlpine JB, et al. Dentin Biomodification Potential Depends on Polyphenol Source. J. Dent. Res. 2014;93:417–422. doi: 10.1177/0022034514523783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Spencer CM, Cai Y, Martin R, Gaffney SH, Goulding PN, Magnolato D, et al. Polyphenol complexation—some thoughts and observations. Phytochemistry. 1988;27:2397–2409. [Google Scholar]
- 14.Siebert KJ. Effects of protein-polyphenol interactions on beverage haze, stabilization, and analysis. J. Agric. Food Chem. 1999;47:353–362. doi: 10.1021/jf980703o. [DOI] [PubMed] [Google Scholar]
- 15.He L, Mu C, Shi J, Zhang Q, Shi B, Lin W. Modification of collagen with a natural cross-linker, procyanidin. Int. J. Biol. Macromol. 2011;48:354–359. doi: 10.1016/j.ijbiomac.2010.12.012. [DOI] [PubMed] [Google Scholar]
- 16.Liu Y, Chen M, Yao X, Xu C, Zhang Y, Wang Y. Enhancement in dentin collagen’s biological stability after proanthocyanidins treatment in clinically relevant time periods. Dent. Mater. Off. Publ. Acad. Dent. Mater. 2013;29:485–492. doi: 10.1016/j.dental.2013.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hutter JL, Bechhoefer J. Calibration of atomic-force microscope tips. Rev. Sci. Instrum. 1993;64:1868. [Google Scholar]
- 18.Elliott JT, Tona A, Woodward JT, Jones PL, Plant AL. Thin Films of Collagen Affect Smooth Muscle Cell Morphology†. Langmuir. 2003;19:1506–1514. [Google Scholar]
- 19.Alexander Ross JB, Laws WR, Rousslang KW, Wyssbrod HR. Tyrosine Fluorescence and Phosphorescence from Proteins and Polypeptides. In: Lakowicz JR, editor. Top. Fluoresc. Spectrosc. Boston: Kluwer Academic Publishers; 2002. [accessed June 12, 2015]. pp. 1–64. http://link.springer.com/10.1007/0-306-47059-4_1. [Google Scholar]
- 20.Eastoe JE. The amino acid composition of mammalian collagen and gelatin. Biochem. J. 1955;61:589–600. doi: 10.1042/bj0610589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nuytinck L, Tükel T, Kayserili H, Apak MY, De Paepe A. Glycine to tryptophan substitution in type I collagen in a patient with OI type III: a unique collagen mutation. J. Med. Genet. 2000;37:371–375. doi: 10.1136/jmg.37.5.371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Quideau S, Deffieux D, Douat-Casassus C, Pouységu L. Plant polyphenols: chemical properties, biological activities, and synthesis. Angew. Chem. Int. Ed Engl. 2011;50:586–621. doi: 10.1002/anie.201000044. [DOI] [PubMed] [Google Scholar]
- 23.Lutz V, Sattler M, Gallinat S, Wenck H, Poertner R, Fischer F. Impact of collagen crosslinking on the second harmonic generation signal and the fluorescence lifetime of collagen autofluorescence. Skin Res. Technol. Off. J. Int. Soc. Bioeng. Skin ISBS Int. Soc. Digit. Imaging Skin ISDIS Int. Soc. Skin Imaging ISSI. 2012;18:168–179. doi: 10.1111/j.1600-0846.2011.00549.x. [DOI] [PubMed] [Google Scholar]
- 24.Bozec L, Horton M. Topography and mechanical properties of single molecules of type I collagen using atomic force microscopy. Biophys. J. 2005;88:4223–4231. doi: 10.1529/biophysj.104.055228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Huang X, Li X, Wang Q, Dai J, Hou J, Chen L. Single-molecule level binding force between collagen and collagen binding domain-growth factor conjugates. Biomaterials. 2013;34:6139–6146. doi: 10.1016/j.biomaterials.2013.04.057. [DOI] [PubMed] [Google Scholar]
- 26.Zlatanova J, Lindsay SM, Leuba SH. Single molecule force spectroscopy in biology using the atomic force microscope. Prog. Biophys. Mol. Biol. 2000;74:37–61. doi: 10.1016/s0079-6107(00)00014-6. [DOI] [PubMed] [Google Scholar]
- 27.Vidal CMP, Aguiar TR, Phansalkar R, McAlpine JB, Napolitano JG, Chen S-N, et al. Galloyl moieties enhance the dentin biomodification potential of plant-derived catechins. Acta Biomater. 2014;10:3288–3294. doi: 10.1016/j.actbio.2014.03.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liu Y, Wang Y. Proanthocyanidins’ efficacy in stabilizing dentin collagen against enzymatic degradation: MALDI-TOF and FTIR analyses. J. Dent. 2013;41:535–542. doi: 10.1016/j.jdent.2013.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Canon F, Giuliani A, Paté F, Sarni-Manchado P. Ability of a salivary intrinsically unstructured protein to bind different tannin targets revealed by mass spectrometry. Anal. Bioanal. Chem. 2010;398:815–822. doi: 10.1007/s00216-010-3997-9. [DOI] [PubMed] [Google Scholar]
- 30.Baxter NJ, Lilley TH, Haslam E, Williamson MP. Multiple interactions between polyphenols and a salivary proline-rich protein repeat result in complexation and precipitation. Biochemistry (Mosc.) 1997;36:5566–5577. doi: 10.1021/bi9700328. [DOI] [PubMed] [Google Scholar]
- 31.Hagerman AE, Rice ME, Ritchard NT. Mechanisms of Protein Precipitation for Two Tannins, Pentagalloyl Glucose and Epicatechin16 (4→8) Catechin (Procyanidin) J. Agric. Food Chem. 1998;46:2590–2595. [Google Scholar]
- 32.Kim S, Nimni ME, Yang Z, Han B. Chitosan/gelatin-based films crosslinked by proanthocyanidin. J. Biomed. Mater. Res. B Appl. Biomater. 2005;75:442–450. doi: 10.1002/jbm.b.30324. [DOI] [PubMed] [Google Scholar]
- 33.Zeeman R, Dijkstra PJ, van Wachem PB, van Luyn MJ, Hendriks M, Cahalan PT, et al. Successive epoxy and carbodiimide cross-linking of dermal sheep collagen. Biomaterials. 1999;20:921–931. doi: 10.1016/s0142-9612(98)00242-7. [DOI] [PubMed] [Google Scholar]
- 34.Everaerts F, Torrianni M, Hendriks M, Feijen J. Biomechanical properties of carbodiimide crosslinked collagen: influence of the formation of ester crosslinks. J. Biomed. Mater. Res. A. 2008;85:547–555. doi: 10.1002/jbm.a.31524. [DOI] [PubMed] [Google Scholar]
- 35.Socrates G. Infrared and Raman characteristic group frequencies: tables and charts, 3. ed., repr. as paperback. Chichester: Wiley; 2010. [Google Scholar]
- 36.Gratzer PF, Lee JM. Control of pH alters the type of cross-linking produced by 1-ethyl-3-(3-dimethylaminopropyl)-carbodiimide (EDC) treatment of acellular matrix vascular grafts. J. Biomed. Mater. Res. 2001;58:172–179. doi: 10.1002/1097-4636(2001)58:2<172::aid-jbm1004>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 37.Olde Damink LHH, Dijkstra PJ, Van Luyn MJA, Van Wachem PB, Nieuwenhuis P, Feijen J. Glutaraldehyde as a crosslinking agent for collagen-based biomaterials. J. Mater. Sci. Mater. Med. 1995;6:460–472. [Google Scholar]
- 38.Qin C, Xu J, Zhang Y. Spectroscopic investigation of the function of aqueous 2-hydroxyethylmethacrylate/glutaraldehyde solution as a dentin desensitizer. Eur. J. Oral Sci. 2006;114:354–359. doi: 10.1111/j.1600-0722.2006.00382.x. [DOI] [PubMed] [Google Scholar]