Abstract
Proton pump inhibitors (PPIs) are widely used drugs that may increase the cardiovascular risk by mechanisms not entirely known. While PPIs increase asymmetric dimethylarginine (ADMA) levels and inhibit nitric oxide production, it is unknown whether impaired vascular redox biology resulting of increased xanthine oxidoreductase (XOR) activity mediates PPIs-induced endothelial dysfunction (ED). We examined whether increased XOR activity impairs vascular redox biology and causes ED in rats treated with omeprazole. We also examined whether omeprazole aggravates the ED found in hypertension. Treatment with omeprazole reduced endothelium-dependent aortic responses to acetylcholine without causing hypertension. However, omeprazole did not aggravate two-kidney, one-clip (2K1C) hypertension, nor hypertension-induced ED. Omeprazole and 2K1C increased vascular oxidative stress as assessed with dihydroethidium (DHE), which reacts with superoxide, and by the lucigenin chemiluminescence assay. The selective XOR inhibitor febuxostat blunted both effects induced by omeprazole. Treatment with omeprazole increased plasma ADMA concentrations, XOR activity and systemic markers of oxidative stress. Incubation of aortic rings with ADMA increased XOR activity, DHE fluorescence and lucigenin chemiluminescence signals, and febuxostat blunted these effects. Providing functional evidence that omeprazole causes ED by XOR-mediated mechanisms, we found that febuxostat blunted the ED caused by omeprazole treatment. This study shows that treatment with omeprazole impairs the vascular redox biology by XOR-mediated mechanisms leading to ED. While omeprazole did not further impair hypertension-induced ED, further studies in less severe animal models are warranted. Our findings may have major relevance, particularly to patients with cardiovascular diseases taking PPIs.
Keywords: Endothelial dysfunction, Omeprazole, Oxidative stress, Proton pump inhibitors
Graphical abstract
Highlights
-
•
Proton pump inhibitors are widely used and increase the cardiovascular risk by unknown mechanisms.
-
•
Omeprazole increased vascular oxidative stress and caused endothelial dysfunction (ED).
-
•
Those effects were dependent on the prooxidant enzyme xanthine oxidoreductase (XOR).
-
•
XOR inhibition by the selective XOR inhibitor febuxostat blunted both effects induced by omeprazole.
-
•
Omeprazole impairs the vascular redox biology by XOR-mediated mechanisms leading to ED.
1. Introduction
Proton pump inhibitors (PPIs) are over-the-counter drugs widely used in gastroenterology because they inhibit gastric acid secretion. While PPIs have usually been considered relatively safe drugs, recent studies suggest that the use of omeprazole increases the risk of acute myocardial infarction [1], [2], [3]. At least two mechanisms involving impaired nitric oxide (NO) activity have been implicated in the increased cardiovascular risk associated with the use of PPIs. First, these drugs were shown to elevate the concentrations of a NO synthase (NOS) inhibitor, asymmetric dimethylarginine (ADMA) [4], which inhibits NOS. Second, interference with gastric formation of protective NO-related species from dietary nitrite and nitrate may also impair non-enzymatic formation of NO and normal cardiovascular function [5], [6], [7], [8].
Although these mechanisms disturbing NO biology may impair vascular redox biology and cause endothelial dysfunction [9], no previous study has examined the possibility that omeprazole causes endothelial dysfunction by activating other critical mechanisms leading to endothelial dysfunction. Indeed, while omeprazole and other PPIs increase ADMA concentrations [4], no previous study has examined the possibility that PPIs may impair vascular redox biology by mechanisms increasing the formation of reactive oxygen species (ROS), thus promoting endothelial dysfunction. Examining this possibility is important because PPIs used to be accepted as a safe therapy without major adverse effects on the cardiovascular system. However, mounting clinical evidence now challenges this view [1], [2], [3]. Astonishingly, some studies suggest that omeprazole exerts antioxidant effects [10] and may induce vascular relaxation that is partially NO-mediated [11], [12], and therefore no deleterious effects would be expected on the cardiovascular system. However, these previous studies have not assessed in vivo effects of omeprazole on the vascular function, and it is possible that increased ADMA concentrations after treatment with omeprazole [4] decrease NO activity and promote prooxidant mechanisms and vascular dysfunction.
Because NO down-regulates xanthine oxidoreductase (XOR) activity [13], a major contributor to oxidative stress in many cardiovascular diseases [13], [14], we hypothesized that omeprazole increases ADMA concentrations, which impair NO formation and cause endothelial dysfunction by increasing XOR activity and impairing vascular redox biology. While previous studies showed that ADMA promotes tissue oxidative stress [15], a direct relationship between PPIs-induced increases in ADMA concentrations and vascular oxidative stress has not been shown. This mechanism possibly activated by PPIs may be critically involved in the vascular dysfunction and enhanced cardiovascular risk of patients taking PPIs. In addition, given that omeprazole is widely prescribed to hypertensive subjects, we examined whether treatment with omeprazole further impairs hypertension-induced vascular dysfunction.
2. Materials and methods
2.1. Animals, treatment with omeprazole and hypertension model
This study followed the guidelines of the Ribeirao Preto Medical School, University of Sao Paulo, and the animals were handled according to the guiding principles published in the National Institutes of Health Guide for the Care and Use of Laboratory Animals. Male Wistar rats (180–200 g) from the colony at University of São Paulo were maintained at room temperature (22–25 °C) on light/dark cycle (12 h) and had free access to standard rat chow and water.
To assess the cardiovascular effects of omeprazole, the rats were treated with omeprazole 10 mg/kg i.p. (or vehicle) daily [5], [6] for four weeks. This dose significantly impaired antihypertensive effects associated with increased nitric oxide formation [5], [6], [7] . Moreover, to examine the possibility that treatment with omeprazole further impairs the cardiovascular alterations of hypertension, the same treatment with omeprazole (or vehicle) was administered to two kidney, one clip (2K1C)-hypertensive rats. Treatment with omeprazole started after two weeks of hypertension, when the animals were randomly allocated to treatment with omeprazole or control group.
2K1C hypertension was induced as previously described [16], [17]. Systolic blood pressure (SBP) was assessed weekly by tail-cuff plethysmography [18]. By the end of the sixth week of study, the rats were anesthetized with tribromoethanol (250 mg/kg), and arterial blood samples were collected into tubes containing heparin for further biochemical determinations. The thoracic aorta was carefully excised, cleaned of adherent connective tissues and fat, and cut into 4 mm rings for biochemical determinations and vascular reactivity assessment. One ring was embedded in tissue-tek® and used later to prepare cryosections.
2.2. Assessment of changes in vascular reactivity associated with omeprazole treatment and/or hypertension
To assess the effects of omeprazole treatment and/or hypertension on vascular function, the thoracic aorta was carefully excised as described above, and cut into 4 mm rings. The rings were studied as previously detailed [19]. Endothelial integrity was examined by assessing the relaxation in response to acetylcholine (10−6 mol/L) under contractile tone induced by phenylephrine (10−7 mol/L). Thereafter, the aortic rings with intact, functional endothelium were precontracted with phenylephrine (10−7 mol/L) and the relaxing responses to cumulative concentrations (from 10−10 to 10−5 mol/L) of acetylcholine were measured to construct concentration-response curves. These experiments were carried out using aortas from normotensive (or 2K1C hypertensive) rats treated with omeprazole (or vehicle).
2.3. Assessment of gastric washing pH
The effects of omeprazole on gastric pH were assessed by measuring gastric washing pH as previously detailed [6].
2.4. Assessment of vascular reactive oxygen species production
To assess vascular oxidative stress, two independent biochemical assays were used to assess reactive oxygen species (ROS) production. First, superoxide production by the aortas was measured by dihydroethidium (DHE), as previously described [20]. Aortic cryosections (5 µm thick) were incubated with DHE (10 µmol/l) for 30 min and examined by fluorescence microscopy (Leica Imaging Systems Ltd., Cambridge, England) at ×400 using λ=525 nm excitation and λ=605 nm emission, which is not specific to detect only superoxide [21].
In some experiments, the aortas were pretreated for 1 h with Tiron (1 mmol/L, a superoxide scavenger) [22], diphenyl iodonium (DPI 10 µmol/L, a flavoprotein inhibitor) [22], tempol (100 µmol/L, a superoxide dismutase analogue) [23], oxypurinol (300 µmol/L, a XOR inhibitor) [24], febuxostat (50 nmol/L, a selective XOR inhibitor) [25] or asymmetric dimethylarginine (ADMA, 1 µmol/L).
Second, to further explore the effects of omeprazole and/or hypertension on vascular ROS production, NADPH-dependent superoxide production by aortic tissue was measured as previously described [26]. Some experiments were performed in the absence or presence of febuxostat (50 nmol/L) or DPI (10 µmol/L).
2.5. Assessment of vascular and plasma xanthine oxidase activity
Aortic and plasma xanthine oxidoreductase activity were measured as previously described [27], with a commercial fluorometric assay kit (Cayman Chemical Co., Ann Arbor, MI, USA, item number 10010895), following the manufacturer’s instructions. Some experiments were performed in presence of febuxostat (50 nmol/L), a selective inhibitor or xanthine oxide reductase [25].
2.6. Assessment of circulating markers of oxidative stress (plasma lipid peroxide and 8-isoprostanes)
To examine whether treatment with omeprazole affects the circulating levels of markers of oxidative stress, plasma lipid peroxide (determined by measuring thiobarbituric acid reactive substances; TBARS) and 8-isoprostanes concentrations were determined. TBARS were measured by a fluorometric method, as previously detailed [28].
2.7. Assessment of plasma asymmetric dimethylarginine (ADMA) concentrations
Because treatment with omeprazole or other PPIs increases the formation of ADMA, the plasma ADMA concentrations were measured both in normotensive and hypertensive rats treated with omeprazole (or vehicle) with a commercially available enzyme-linked immunosorbent assay kit (MyBioSource, San Diego, CA, USA, item number: MBS703256).
2.8. Assessment of the effects of ADMA on the vascular production of ROS and the possible role of XOR in ADMA-induced oxidative stress
Given the fact that treatment with omeprazole increased circulating ADMA concentrations in association with increased vascular production of ROS, experiments were carried out with aortic rings incubated for three hours with ADMA (1 µM) or vehicle. This concentration is similar to the plasma concentration found after treatment with omeprazole and to those previously used [29]. The incubation conditions were the same as those used in aortic ring reactivity experiments described above. Therefore, to assess the possible role of XOR and other flavoproteins in increasing vascular ROS production by ADMA, incubations with ADMA were carried out in the presence of febuxostat (50 nmol/L) [25] or DPI (10 µmol/L) [22]. After incubation, reactive oxygen species (ROS) production in the aortas were measured by dihydroethidium (DHE) as described above. To further confirm the results obtained with DHE, NADPH-dependent superoxide production by aortic tissue was measured as described above.
To further confirm that XOR activity increases after incubation with ADMA, XOR activity was assessed in aortic rings incubated with ADMA (1 µM) or vehicle, and the effects of febuxostat (50 nmol/L) [25] were also examined.
2.9. Assessment of the role of XOR in the changes in vascular reactivity associated with omeprazole treatment
Given that treatment with omeprazole significantly impaired the endothelial function and increased vascular XOR activity and oxidative stress, experiments designed to assess the role of XOR in the changes in vascular reactivity were carried out using the same methodology as described above. Endothelium-dependent responses were studied in aortic rings from animals treated with omeprazole (or vehicle) in the presence or absence of the XOR inhibitor febuxostat (10−7 mol/L) or vehicle (dimethyl sulfoxide; DMSO 0.0004%).
2.10. Drugs and solutions
All drugs and reagents were purchased from Sigma Chemical Co. (St Louis, MO, USA) and all solutions were prepared immediately before use.
2.11. Statistical analysis
The results are expressed as means±S.E.M. The comparisons between groups were assessed by one-way or two-way analysis of variance followed by the Tukey test. A probability value P<0.05 was considered significant.
3. Results
3.1. Treatment with omeprazole causes endothelial dysfunction without significantly increasing arterial blood pressure
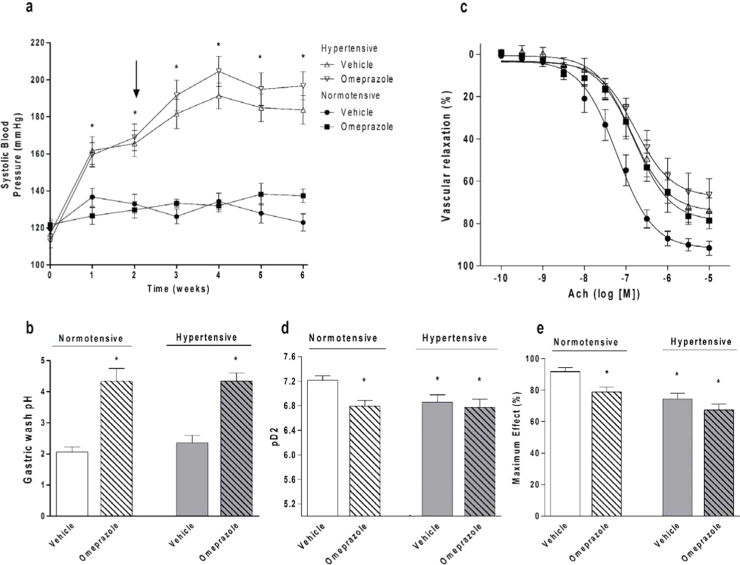
We found that chronic treatment with omeprazole neither increased SBP nor significantly aggravated 2K1C hypertension (Fig. 1a), even though SBP tended to show slightly higher values in 2K1C hypertensive rats treated with omeprazole as compared with hypertensive rats treated with vehicle (196±7 versus 183±7 mmHg, respectively; not significant).
Fig. 1.
Chronic treatment with the proton pump inhibitor omeprazole causes endothelial dysfunction without significantly increasing arterial blood pressure. (a) Systolic blood pressure in normotensive and in 2K1C hypertensive rats treated with omeprazole (or vehicle). The arrow indicates the start of omeprazole or vehicle treatment. (b) Gastric wash pH measured at the end of the study period. (c) Endothelial cell-dependent vasorelaxation induced by acetylcholine (Ach) in aortic rings from normotensive and from 2K1C hypertensive rats treated with omeprazole (or vehicle). (d) pD2 values (−log of the molar concentration of Ach that produces 50% of the maximal effect). (e) Maximal responses to Ach. Data are shown as mean±S.E.M. (n=8–10/group in Fig. 1a and b; n=5/group in Fig. 1c, d, and e). * P<0.05 versus normotensive animals treated with vehicle.
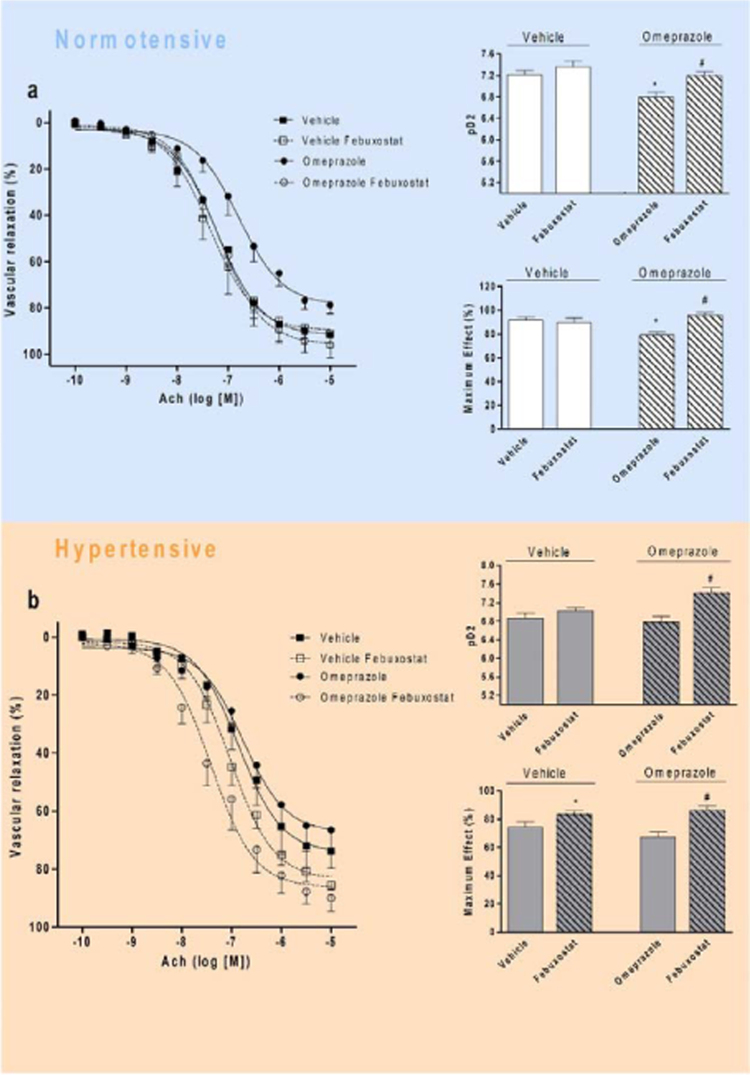
While no major effects of omeprazole on blood pressure were found, chronic treatment with this PPI shifted the concentration-effect curve in response to acetylcholine to the right (pD2=7.2±0.1 and pD2=6.7±0.1, respectively, in controls and in omeprazole-treated animals; P<0.05; Fig. 1c and d). Moreover, treatment with omeprazole decreased the maximum responses to acetylcholine (Emax=92±2% and Emax=79±3%, respectively, in controls and in omeprazole-treated animals; P<0.05; Fig. 1c and e). However, whereas chronic treatment with omeprazole impaired the endothelial-dependent responses to acetylcholine to the same degree as 2K1C hypertension, treatment of 2K1C hypertensive rats with the same dose of omeprazole did not further impair the responses to acetylcholine (P>0.05; Fig. 1c, d, and e). Therefore, omeprazole caused endothelial dysfunction similar to that found in 2K1C hypertension, without aggravating it.
Fig. 1b shows that treatment with omeprazole increased gastric washing pH, both in normotensive and in 2K1C hypertensive rats (both P<0.05; Fig. 1b).
3.2. Treatment with omeprazole increases vascular oxidative stress, which is mostly attributable to increased xanthine oxidase (XOR) activity
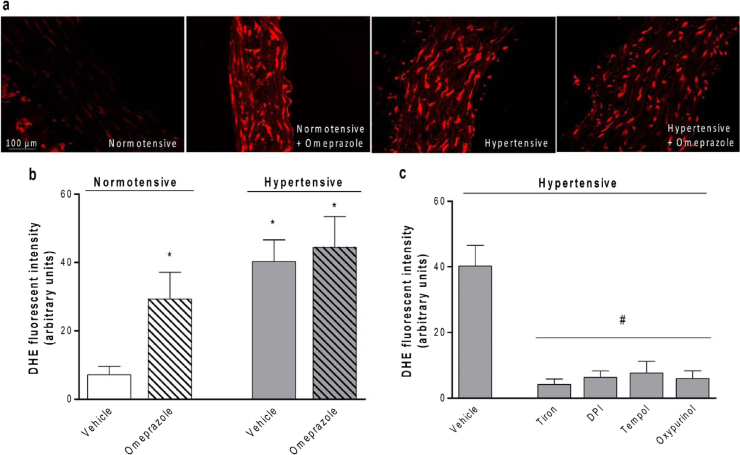
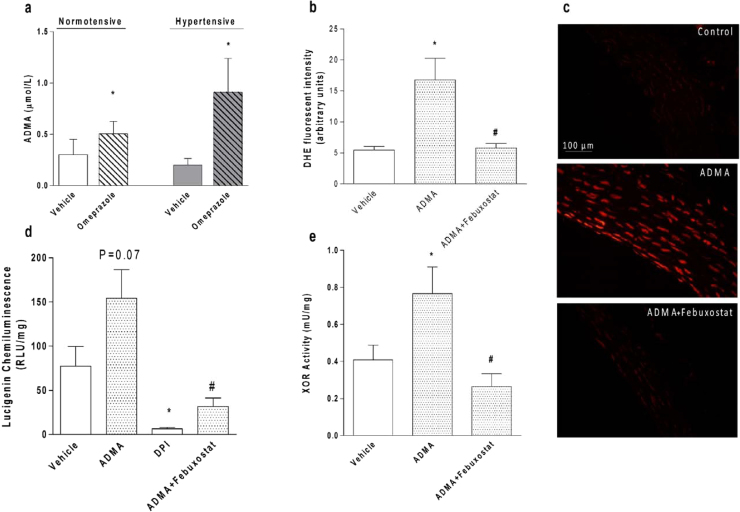
Increased oxidative stress is an important mechanism promoting endothelial dysfunction, and therefore we assessed vascular oxidative stress using two approaches. First, the vascular production of ROS was examined in aortas from rats treated with omeprazole by using the fluorescent superoxide indicator DHE (Fig. 2a). Interestingly, we found a >4-fold increase in vascular ROS levels in normotensive rats treated with omeprazole as compared with vehicle (P<0.05; Fig. 2b). While 2K1C hypertension increased vascular ROS levels by >5-fold (P<0.05; Fig. 2b), treatment with omeprazole did not further increase this parameter. Quantification of DHE fluorescence in control experiments using aortas from hypertensive rats showed that the superoxide scavenger tiron, or the flavoprotein inhibitor DPI, or the superoxide dismutase analogue tempol, or the XOR inhibitor oxypurinol blunted DHE signal by >80% (Fig. 2c), thus suggesting that this signal is attributable to the activity of flavoproteins including NADPH oxidase and XOR.
Fig. 2.
Chronic treatment with the proton pump inhibitor omeprazole increases the vascular formation of reactive oxygen species (ROS). (a) Representative photomicrographs (×400) with red fluorescence of dihydroethidium (DHE)-aortic cryosections. (b) Quantification of aortic DHE fluorescence in each experimental group. (c) Quantification of aortic DHE fluorescence in positive control experiments using aortas from hypertensive rats. Tiron (1 mmol/L, a superoxide scavenger), DPI (10 µmol/L, a flavoprotein inhibitor), tempol (100 µmol/L, a superoxide dismutase analogue), and oxypurinol (300 µmol/L, a XOR inhibitor) blunted DHE signal by >80%. Data are shown as mean±S.E.M. (n=4–8/group). *P<0.05 versus normotensive animals treated with vehicle. #P<0.05 versus Vehicle.
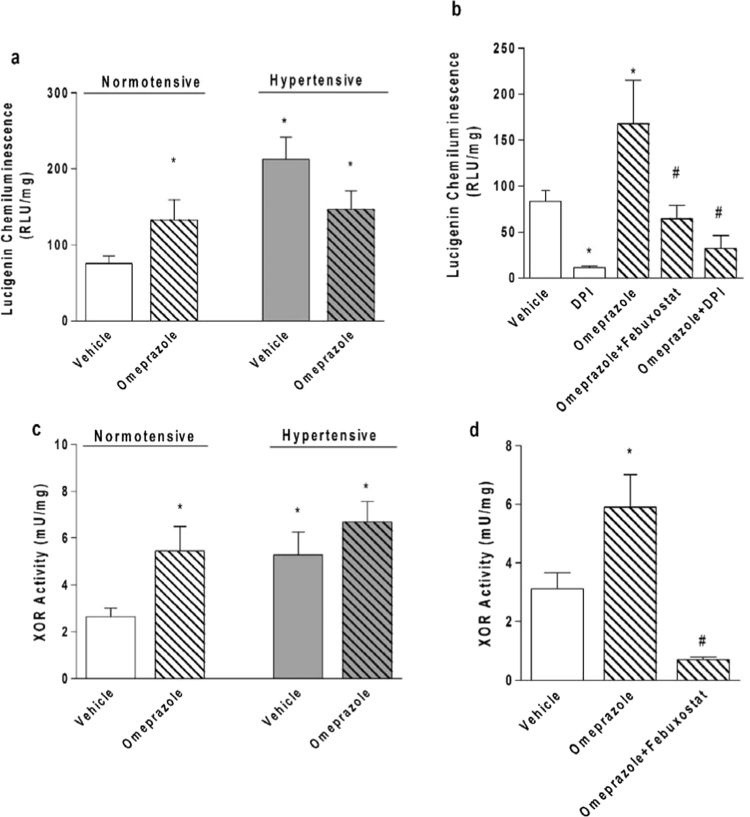
Second, to confirm that omeprazole increases vascular oxidative stress, we measured NADPH-dependent superoxide production by aortas from rats using the lucigenin chemiluminescence assay. In agreement with the DHE results, we found that treatment of normotensive rats with omeprazole increased vascular NADPH-dependent superoxide production by approximately 100%, whereas hypertension increased this parameter by approximately 200% (both P<0.05; Fig. 3a). Treatment of hypertensive rats with omeprazole was associated with lucigenin chemiluminescence similar to that found after treatment of normotensive rats with omeprazole (Fig. 3a). Control experiments with inhibitors of enzymes involved in superoxide production showed that the flavoprotein inhibitor DPI inhibited 70–80% of lucigenin chemiluminescence signal in aortic rings from normotensive rats treated with vehicle or with omeprazole (both P<0.05; Fig. 3b). These results suggest that 70–80% of NADPH-dependent superoxide production by aortas is due to the activity of flavoproteins. Importantly, >55% of the lucigenin chemiluminescence signal was blunted when aortic rings from normotensive rats treated with omeprazole were incubated with the selective XOR inhibitor febuxostat (P<0.05; Fig. 3b), thus suggesting that XOR activity is the major player in this signal.
Fig. 3.
Chronic treatment with the proton pump inhibitor omeprazole increases NADPH-dependent superoxide production by vascular tissue, and this effect involves increased vascular XOR activity. (a) NADPH-dependent superoxide production measured as lucigenin chemiluminescence in the aortic rings from normotensive and in 2K1C hypertensive rats treated with omeprazole (or vehicle) (n=7/group). (b) NADPH-dependent superoxide production by aortic rings from normotensive rats chronically treated with vehicle (open bars) or omeprazole (hatched bars) and incubated with vehicle or DPI (10 µmol/L, a flavoprotein inhibitor), or with febuxostat (50 nmol/L, a selective XOR inhibitor)(n=3–7/group). (c) Xanthine oxidoreductase (XOR) activity measured by a fluorometric assay in the aortic rings from normotensive and in 2K1C hypertensive rats treated with omeprazole (or vehicle)(n=5–7/group). (d) XOR activity measured by a fluorometric assay in the aortic rings from normotensive rats chronically treated with vehicle (open bar) or omeprazole (hatched bars) and incubated (or not) with the selective XOR inhibitor febuxostat (50 nmol/L)(n=5/group). Data are shown as mean±S.E.M. *P<0.05 versus normotensive animals treated with vehicle. #P<0.05 versus normotensive animals treated with Omeprazole.
To confirm that treatment with omeprazole increases vascular XOR activity, we used a fluorometric assay to measure XOR activity in aortic rings from normotensive and in 2K1C hypertensive rats treated with omeprazole (or vehicle). Treatment of normotensive rats with omeprazole increased vascular XOR activity to the same degree as 2K1C hypertension (by approximately>120%; Fig. 3c; both P<0.05). Slightly higher vascular XOR activity was found in hypertensive rats treated with omeprazole (Fig. 3c; not significant). Control experiments using the selective XOR inhibitor febuxostat showed that this inhibitor blunted >85% of vascular XOR activity measured in aortic rings from rats treated with omeprazole (P<0.05; Fig. 3d).
3.3. Treatment with omeprazole increases the plasma XOR activity and the concentrations of systemic markers of oxidative stress
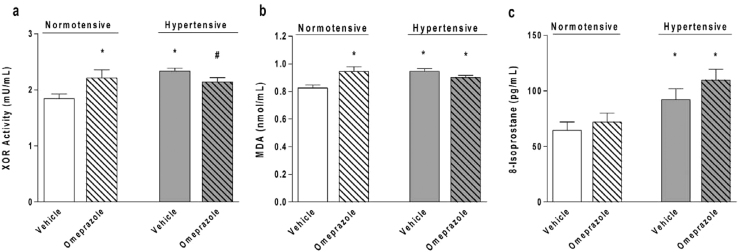
To further validate our results showing increased vascular oxidative stress and XOR activity after treatment with omeprazole, relevant systemic markers oxidative stress were assessed in plasma samples, including plasma XOR activity. As expected, hypertension increased plasma XOR activity and lipid peroxide concentrations measured as MDA concentrations by approximately 15%, and treatment of normotensive rats with omeprazole increased both parameters to the same degree as hypertension (all P<0.05; Fig. 4a and b). However, treatment of hypertensive rats with omeprazole did not further increase plasma XOR activity and MDA concentrations (both P>0.05; Fig. 4a and b). While treatment with omeprazole did not increase plasma 8-isoprostanes concentrations in normotensive rats, higher concentrations of this marker were found in hypertensive rats treated with vehicle or with omeprazole, as compared with their normotensive controls (both P<0.05; Fig. 4c). Together, these results suggest that treatment with omeprazole apparently increases systemic oxidative stress.
Fig. 4.
Treatment with omeprazole increases the circulating concentrations of markers of oxidative stress. (a) Xanthine oxidoreductase (XOR) activity measured by a fluorometric assay (n=8–10/group). (b) Plasma lipid peroxide concentrations measured with a fluorometric assay and expressed as malondialdehyde (MDA) concentrations (n=7/group). (c) Plasma 8-isoprostanes (8-isoPGF2α) concentrations measured with a enzyme-linked immunosorbent assay (n=7/group). Data are shown as mean±S.E.M. *P<0.05 versus normotensive animals treated with vehicle.
3.4. Treatment with omeprazole increases the circulating concentrations of asymmetric dimethylarginine (ADMA), and ADMA increases vascular oxidative stress via XOR activation
Previous studies with PPIs showed that they increase the concentrations of ADMA [4], and therefore plasma ADMA concentrations were measured in the present study. As shown in Fig. 5a, treatment with omeprazole increased ADMA concentrations by approximately 90% and 330% in normotensive and in hypertensive rats, respectively (both P<0.05; Fig. 5a).
Fig. 5.
Treatment with omeprazole increases the circulating concentrations of asymmetric dimethylarginine (ADMA), which increases vascular oxidative stress by mechanisms involving xanthine oxidoreductase (XOR) activation. (a) ADMA concentrations measured with enzyme-linked immunosorbent assay in plasma samples from normotensive and 2K1C hypertensive rats treated with omeprazole (or vehicle) (n=4–7/group). (b) Quantification of dihydroethidium (DHE) fluorescence in aortic rings from normotensive rats, incubated for three hours with ADMA (1 µM) or vehicle, and with ADMA (1 µM) in the presence of the selective XOR inhibitor febuxostat (50 nmol/L)(n=6–8/group). (c) Representative photomicrographs (×400) with red fluorescence of DHE-aortic cryosections after incubation of aortic rings with ADMA (1 µM) or vehicle (Control), and ADMA (1 µM) in the presence of febuxostat. (d) NADPH-dependent superoxide production measured as lucigenin chemiluminescence in the aortic rings from normotensive rats, incubated for three hours with ADMA (1 µM), or vehicle, or with the flavoprotein inhibitor DPI (10 µmol/L), or with ADMA (1 µM) in the presence of febuxostat (50 nmol/L)(n=3–10/group). (e) XOR activity measured by a fluorometric assay in the aortic rings from normotensive rats, incubated for three hours with ADMA (1 µM) or vehicle, and with ADMA (1 µM) in the presence of febuxostat (50 nmol/L)(n=3–8/group). Data are shown as mean±S.E.M. *P<0.05 versus normotensive animals treated with vehicle (panel a). *P<0.05 versus Vehicle (panels b, d, e). #P<0.05 versus ADMA (panels b, d, e).
To examine a possible link between the increases in circulating ADMA concentrations and enhanced vascular XOR activity and oxidative stress associated with omeprazole treatment, aortic rings from normotensive rats were incubated ADMA and vascular ROS levels were determined as described above (DHE and lucigenin assays). Interestingly, the incubation with ADMA (1 µM) increased vascular ROS concentrations by >200%, and this effect was completely blunted by the co-incubation with the selective XOR inhibitor febuxostat (50 nmol/L) (both P<0.05; Fig. 5b; representative DHE-aortic cryosections in Fig. 5c). In agreement with DHE results, NADPH-dependent superoxide production by aortic tissue increased by >100% after incubation with ADMA and this effect was totally inhibited by co-incubation with febuxostat or with DPI, which is a non selective inhibitor of flavoproteins (all P<0.05; Fig. 5d).
To further confirm the involvement of increased XOR activity after incubation with ADMA, XOR activity was assessed in aortic rings incubated with ADMA. In agreement with the other results, ADMA (1 µM) increased aortic XOR activity by approximately 100%, and febuxostat (50 nmol/L) completely blunted this effect (both P<0.05; Fig. 5e).
3.5. The selective XOR inhibitor febuxostat blunts the endothelial dysfunction caused by chronic treatment with omeprazole, both in normotensive and in hypertensive rats
The initial experiments showed that treatment with omeprazole impaired the endothelial function in association with increased vascular XOR activity and oxidative stress. Therefore, the effects of selective XOR inhibition on vascular function were examined. Incubation with febuxostat improved endothelium-dependent responses of aortic rings from animals treated with omeprazole, but not in control rats treated with vehicle, as revealed by the increases in both pD2 values and in the maximal responses to Ach (both P<0.05; Fig. 6a). Very similar effects were found in hypertensive rats treated with omeprazole (or vehicle; Fig. 6b). These results indicate that XOR inhibition with febuxostat blunts the impairment in endothelial-dependent vascular responses caused by treatment with omeprazole.
Fig. 6.
Inhibition of xanthine oxidoreductase (XOR) with febuxostat blunts the endothelial dysfunction caused by chronic treatment with omeprazole, both in normotensive and in hypertensive rats. (a) Endothelial cell-dependent vasorelaxation induced by acetylcholine (Ach) in aortic rings from normotensive rats chronically treated with omeprazole (or vehicle) in the presence of the selective XOR inhibitor febuxostat (10−7 mol/L). The bars show the pD2 values (−log of the molar concentration of Ach that produces 50% of the maximal effect) and the maximal responses to Ach. (b) Endothelial cell-dependent vasorelaxation induced by acetylcholine (Ach) in aortic rings from 2k1C hypertensive rats chronically treated with omeprazole (or vehicle) in the presence of the selective XOR inhibitor febuxostat (10−7 mol/L). The bars show the pD2 values (log of the molar concentration of Ach that produces 50% of the maximal effect) and the maximal responses to Ach. Data are shown as mean±S.E.M. (n=4–5/group). *P<0.05 versus respective Vehicle group. #P<0.05 for Omeprazole+Febuxostat group versus Omeprazole+Vehicle group.
4. Discussion
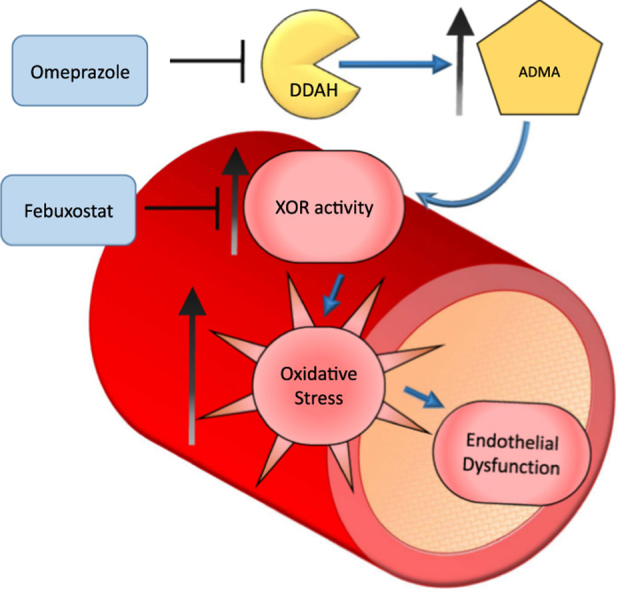
This study showed that treatment with omeprazole causes endothelial dysfunction by mechanisms that involve increased ADMA levels [4] and impaired NO activity. Our results show for the first time that omeprazole impairs vascular redox biology as a result of increased formation of ROS, and this effect is associated with enhanced vascular XOR activity, probably caused by increased ADMA levels. While hypertension does not aggravate the effects of omeprazole, a selective XOR inhibitor prevents the biochemical and functional alterations associated with this PPI.
The wide use of PPIs is now of great concern because growing evidence suggests that they may significantly increase the cardiovascular risk [1], [2], [3], [30] by mechanisms that are independent of their pharmacokinetic interactions with clopidogrel, which could impair the bioactivation of this antiplatelet agent and increase the risk of clinically relevant events [31]. However, there is little information regarding the mechanisms that may explain how PPIs have deleterious effects on the cardiovascular system [30]. As recently shown, PPIs may inhibit the activity of the enzyme that metabolizes ADMA, and therefore enhance ADMA concentrations reducing NOS activity and endogenous NO production [4], [30]. Our results showing increased ADMA levels after treatment with omeprazole support this mechanism. However, omeprazole may also prevent the gastric formation of protective NO-related species from dietary nitrite and nitrate [5], [6], [7], [8] and further deteriorate NO biology. Interestingly, while the effects of omeprazole on NO biology are probably not so severe that hypertension ensues, this PPI clearly induces endothelial dysfunction, as revealed by impaired responses to acetylcholine reported here and in previous studies [4].
Our results strongly suggest that omeprazole may cause endothelial dysfunction by impairing vascular redox biology. To provide evidence that omeprazole induces vascular oxidative stress, we used at least three methods to assess vascular and systemic redox state. We found that treatment with omeprazole caused major increases in systemic and vascular ROS levels, probably as a result of increased vascular XOR activity because treatment with the selective XOR inhibitor completely blunted the vascular increases in ROS and the endothelial dysfunction associated with omeprazole. Because treatment with omeprazole increases ADMA concentrations [4], we hypothesized that ADMA would be critically involved in the activation of vascular XOR, thus leading to increased ROS formation. Our results showing that ADMA increases vascular XOR activity and ROS formation are consistent with this mechanism and agree with previous studies showing that ADMA induces oxidative stress [15], [32].
To support the idea that XOR plays a critical role in ADMA-induced oxidative stress, we used the selective XOR inhibitor febuxostat, which completely blunted the increases in both DHE signal and the lucigenin chemiluminescence induced by ADMA in vascular tissue. Even though a prooxidant mechanism involving ADMA-induced NOS inhibition has been previously suggested [15], febuxostat is not known to affect NOS activity, and therefore its inhibitory effects on both vascular oxidative stress and on endothelial dysfunction are not attributable to interference with NOS activity. Conversely, febuxostat, a XOR-specific inhibitor [25], blunted omeprazole-induced oxidative stress in vivo, thus critically implicating XOR in the vascular oxidative stress associated with omeprazole. To further confirm this new mechanism linking increased ADMA concentrations and enhanced vascular XOR activity/oxidative stress, we incubated aortic rings with ADMA. Interestingly, we found that ADMA increased vascular ROS levels and superoxide production by aortic tissue, and this effect was again completely blunted by febuxostat, therefore critically implicating XOR.
Given that increased vascular oxidative stress is a major factor leading to endothelial dysfunction [33], and that XOR activation may promote both alterations [34], it is reasonable to accept that omeprazole increased ADMA levels which, in turn, promoted XOR-mediated vascular redox imbalance and impaired endothelial-dependent responses to acetylcholine. Although molecular mechanisms have not been investigated in the present study, it is possible that NOS inhibition by ADMA may have increased XOR activity and therefore explain our biochemical and functional findings. This suggestion is supported by previous studies showing that NOS deficiency stimulates XOR activity and depresses myocardial function, an effect that is reversible by XOR inhibition [35], exactly as we have shown in the present study using the selective XOR inhibitor febuxostat. In addition to this mechanism, superoxide produced by increased XOR activity may react with endogenous NO producing peroxynitrite [13], [36], and thus impair NO activity and cause endothelial dysfunction, as we have reported here with omeprazole. This XOR-dependent mechanism agrees with a previous study showing that increased vascular XOR activity could play a critical role in endothelial dysfunction, which is at least partially prevented by XOR inhibition [37].
Hypertensive patients may require the use of PPIs in a number of clinical conditions. Because patients with hypertension or other cardiovascular diseases may be more sensitive to the deleterious effects of PPIs-induced increases in ADMA levels [38], we studied the effects of omeprazole in 2K1C hypertensive rats. While this PPI significantly impaired vascular redox biology and function, it did not aggravate hypertension-induced endothelial dysfunction. It is possible that this hypertension model is so severe that near maximum vascular damage is produced. It should be noted that a pilot study showed that lansoprazole treatment did not affect endothelial function in healthy subjects and in patients [38]. However, this was a short-term study (four weeks of lansoprazole) and the technique used to assess vascular function may offer significant limitations [30]. Further studies are required to confirm that omeprazole does not further deteriorate the vascular function in a less severe hypertension model compared to ours, as well as in patients.
Our results indicate that omeprazole increases vascular XOR activity. Interestingly, our findings may explain the development of acute gout in two patients, as previously reported [39]. The authors suggested a causal relation between the use of omerprazole and acute gout in two patients, without offering a possible mechanism [39]. One of the patients developed a second episode of gout after rechallenge with omeprazole [39]. Our results showing increased XOR activity with omeprazole is consistent with increased conversion of hypoxanthine to xanthine and xanthine to uric acid in patient taking omeprazole, thus increasing the risk of acute gout. Maybe uric acid assessment should be recommended to patients taking omeprazole.
In summary, our results demonstrate that treatment with omeprazole impairs the vascular function to the same extent as renovascular hypertension. This deleterious effect of omeprazole is probably mediated by increased ADMA concentrations leading to enhanced vascular XOR activity and oxidative stress. Our findings show an important mechanism involving impaired redox vascular biology associated with omeprazole treatment, which may be clinically relevant in patients taking other PPIs, particularly those exposed to other factors increasing the cardiovascular risk.
Competing interests
None.
Acknowledgments
This work was supported by Fundação de Amparo a Pesquisa do Estado de São Paulo (FAPESP, Grant number 2014-23946-0), Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), and CAPES (Coordenadoria de Aperfeiçoamento de Pessoal de Nivel Superior).
References
- 1.Shah N.H., LePendu P., Bauer-Mehren A., Ghebremariam Y.T., Iyer S.V., Marcus J., Nead K.T., Cooke J.P., Leeper N.J. Proton pump inhibitor usage and the risk of myocardial infarction in the general population. PLoS One. 2015;10:e0124653. doi: 10.1371/journal.pone.0124653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bateman D.N., Colin-Jones D., Hartz S., Langman M., Logan R.F., Mant J., Murphy M., Paterson K.R., Rowsell R., Thomas S., Vessey M. Mortality study of 18000 patients treated with omeprazole. Gut. 2003;52:942–946. doi: 10.1136/gut.52.7.942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shih C.J., Chen Y.T., Ou S.M., Li S.Y., Chen T.J., Wang S.J. Proton pump inhibitor use represents an independent risk factor for myocardial infarction. Int. J. Cardiol. 2014;177:292–297. doi: 10.1016/j.ijcard.2014.09.036. [DOI] [PubMed] [Google Scholar]
- 4.Ghebremariam Y.T., LePendu P., Lee J.C., Erlanson D.A., Slaviero A., Shah N.H., Leiper J., Cooke J.P. Unexpected effect of proton pump inhibitors: elevation of the cardiovascular risk factor asymmetric dimethylarginine. Circulation. 2013;128:845–853. doi: 10.1161/CIRCULATIONAHA.113.003602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pinheiro L.C., Amaral J.H., Ferreira G.C., Portella R.L., Ceron C.S., Montenegro M.F., Toledo J.C., Jr., Tanus-Santos J.E. Gastric S-nitrosothiol formation drives the antihypertensive effects of oral sodium nitrite and nitrate in a rat model of renovascular hypertension. Free Radic. Biol. Med. 2015;87:252–262. doi: 10.1016/j.freeradbiomed.2015.06.038. [DOI] [PubMed] [Google Scholar]
- 6.Pinheiro L.C., Montenegro M.F., Amaral J.H., Ferreira G.C., Oliveira A.M., Tanus-Santos J.E. Increase in gastric pH reduces hypotensive effect of oral sodium nitrite in rats. Free Radic. Biol. Med. 2012;53:701–709. doi: 10.1016/j.freeradbiomed.2012.06.001. [DOI] [PubMed] [Google Scholar]
- 7.Amaral J.H., Montenegro M.F., Pinheiro L.C., Ferreira G.C., Barroso R.P., Costa-Filho A.J., Tanus-Santos J.E. TEMPOL enhances the antihypertensive effects of sodium nitrite by mechanisms facilitating nitrite-derived gastric nitric oxide formation. Free Radic. Biol. Med. 2013;65C:446–455. doi: 10.1016/j.freeradbiomed.2013.07.032. [DOI] [PubMed] [Google Scholar]
- 8.Pinheiro L.C., Amaral J.H., Tanus-Santos J.E. Letter by Pinheiro et al. regarding article, “unexpected effect of proton pump inhibitors: elevation of the cardiovascular risk factor asymmetric dimethylarginine”. Circulation. 2013;129:e427. doi: 10.1161/CIRCULATIONAHA.113.005307. [DOI] [PubMed] [Google Scholar]
- 9.Hare J.M. Nitroso-redox balance in the cardiovascular system. N. Engl. J. Med. 2004;351:2112–2114. doi: 10.1056/NEJMe048269. [DOI] [PubMed] [Google Scholar]
- 10.Lapenna D., de Gioia S., Ciofani G., Festi D., Cuccurullo F. Antioxidant properties of omeprazole. FEBS Lett. 1996;382:189–192. doi: 10.1016/0014-5793(96)00155-x. [DOI] [PubMed] [Google Scholar]
- 11.Kelicen P., Pekiner C., Sarioglu Y., Uma S. Omeprazole-induced relaxation in rat aorta is partly dependent on endothelium. Pharmacol. Res. 2002;46:321–323. doi: 10.1016/s104366180200124x. [DOI] [PubMed] [Google Scholar]
- 12.Naseri E., Yenisehirli A. Proton pump inhibitors omeprazole and lansoprazole induce relaxation of isolated human arteries. Eur. J. Pharmacol. 2006;531:226–231. doi: 10.1016/j.ejphar.2005.12.025. [DOI] [PubMed] [Google Scholar]
- 13.Berry C.E., Hare J.M. Xanthine oxidoreductase and cardiovascular disease: molecular mechanisms and pathophysiological implications. J. Physiol. 2004;555:589–606. doi: 10.1113/jphysiol.2003.055913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cantu-Medellin N., Kelley E.E. Xanthine oxidoreductase-catalyzed reactive species generation: a process in critical need of reevaluation. Redox Biol. 2013;1:353–358. doi: 10.1016/j.redox.2013.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wells S.M., Holian A. Asymmetric dimethylarginine induces oxidative and nitrosative stress in murine lung epithelial cells. Am. J. Respir. Cell Mol. Biol. 2007;36:520–528. doi: 10.1165/rcmb.2006-0302SM. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ceron C.S., Rizzi E., Guimaraes D.A., Martins-Oliveira A., Gerlach R.F., Tanus-Santos J.E. Nebivolol attenuates prooxidant and profibrotic mechanisms involving TGF-beta and MMPs, and decreases vascular remodeling in renovascular hypertension. Free Radic. Biol. Med. 2013;65C:47–56. doi: 10.1016/j.freeradbiomed.2013.06.033. [DOI] [PubMed] [Google Scholar]
- 17.Martins-Oliveira A., Castro M.M., Oliveira D.M., Rizzi E., Ceron C.S., Guimaraes D., Reis R.I., Costa-Neto C.M., Casarini D.E., Ribeiro A.A., Gerlach R.F., Tanus-Santos J.E. Contrasting effects of aliskiren versus losartan on hypertensive vascular remodeling. Int. J. Cardiol. 2013;167:1199–1205. doi: 10.1016/j.ijcard.2012.03.137. [DOI] [PubMed] [Google Scholar]
- 18.Pinheiro L.C., Amaral J.H., Ferreira G.C., Montenegro M.F., Oliveira-Paula G.H., Tanus-Santos J.E. The antihypertensive effects of sodium nitrite are not associated with circulating angiotensin converting enzyme inhibition. Nitric Oxide. 2014;40C:52–59. doi: 10.1016/j.niox.2014.05.009. [DOI] [PubMed] [Google Scholar]
- 19.Guimaraes D.A., Rizzi E., Ceron C.S., Pinheiro L.C., Gerlach R.F., Tanus-Santos J.E. Atorvastatin and sildenafil lower blood pressure and improve endothelial dysfunction, but only atorvastatin increases vascular stores of nitric oxide in hypertension. Redox Biol. 2013;1:578–585. doi: 10.1016/j.redox.2013.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Montenegro M.F., Neto-Neves E.M., Dias-Junior C.A., Ceron C.S., Castro M.M., Gomes V.A., Kanashiro A., Tanus-Santos J.E. Quercetin restores plasma nitrite and nitroso species levels in renovascular hypertension. Naunyn Schmiedebergs Arch. Pharmacol. 2010;382:293–301. doi: 10.1007/s00210-010-0546-1. [DOI] [PubMed] [Google Scholar]
- 21.Oliveira-Paula G.H., Pinheiro L.C., Guimaraes D.A., Tella S.O., Blanco A.L., Angelis C.D., Schechter A.N., Tanus-Santos J.E. Tempol improves xanthine oxidoreductase-mediated vascular responses to nitrite in experimental renovascular hypertension. Redox Biol. 2016;8:398–406. doi: 10.1016/j.redox.2016.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Montenegro M.F., Amaral J.H., Pinheiro L.C., Sakamoto E.K., Ferreira G.C., Reis R.I., Marçal D.M.O., Pereira R.P., Tanus-Santos J.E. Sodium nitrite downregulates vascular NADPH oxidase and exerts antihypertensive effects in hypertension. Free Radic. Biol. Med. 2011;51:144–152. doi: 10.1016/j.freeradbiomed.2011.04.005. [DOI] [PubMed] [Google Scholar]
- 23.Turoni C.J., Marañón R.O., Proto V., Herrera R., de Bruno M.P. Nitric oxide modulates reactivity to angiotensin II in internal mammary arterial grafts in hypertensive patients without associated risk factors. Clin. Exp. Hypertens. 2011;33:27–33. doi: 10.3109/10641963.2010.503297. [DOI] [PubMed] [Google Scholar]
- 24.Wind S., Beuerlein K., Armitage M.E., Taye A., Kumar A.H.S., Janowitz D., Neff C., Shah A.M., Wingler K., Schmidt H.H.H.W. Oxidative stress and endothelial dysfunction in aortas of aged spontaneously hypertensive rats by NOX1/2 is reversed by NADPH oxidase inhibition. Hypertension. 2010;56:490–497. doi: 10.1161/HYPERTENSIONAHA.109.149187. [DOI] [PubMed] [Google Scholar]
- 25.Cantu-Medellin N., Kelley E.E. Xanthine oxidoreductase-catalyzed reduction of nitrite to nitric oxide: insights regarding where, when and how. Nitric Oxide. 2013;34:19–26. doi: 10.1016/j.niox.2013.02.081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Amaral J.H., Ferreira G.C., Pinheiro L.C., Montenegro M.F., Tanus-Santos J.E. Consistent antioxidant and antihypertensive effects of oral sodium nitrite in DOCA-salt hypertension. Redox Biol. 2015;5:340–346. doi: 10.1016/j.redox.2015.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Montenegro M.F., Pinheiro L.C., Amaral J.H., Ferreira G.C., Portella R.L., Tanus-Santos J.E. Vascular xanthine oxidoreductase contributes to the antihypertensive effects of sodium nitrite in l-NAME hypertension. Naunyn Schmiedebergs Arch. Pharmacol. 2014;387:591–598. doi: 10.1007/s00210-014-0970-8. [DOI] [PubMed] [Google Scholar]
- 28.Montenegro M.F., Pessa L.R., Gomes V.A., Desta Z., Flockhart D.A., Tanus-Santos J.E. Assessment of vascular effects of tamoxifen and its metabolites on the rat perfused hindquarters vascular bed. Basic Clin. Pharmacol. Toxicol. 2009;104:400–407. doi: 10.1111/j.1742-7843.2009.00377.x. [DOI] [PubMed] [Google Scholar]
- 29.Korandji C., Zeller M., Guilland J.C., Collin B., Lauzier B., Sicard P., Duvillard L., Goirand F., Moreau D., Cottin Y., Rochette L., Vergely C. Time course of asymmetric dimethylarginine (ADMA) and oxidative stress in fructose-hypertensive rats: A model related to metabolic syndrome. Atherosclerosis. 2011;214:310–315. doi: 10.1016/j.atherosclerosis.2010.11.014. [DOI] [PubMed] [Google Scholar]
- 30.Sukhovershin R.A., Cooke J.P. How may proton pump inhibitors impair cardiovascular health? Am. J. Cardiovasc. Drugs. 2016;16:153–161. doi: 10.1007/s40256-016-0160-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Charlot M., Ahlehoff O., Norgaard M.L., Jorgensen C.H., Sorensen R., Abildstrom S.Z., Hansen P.R., Madsen J.K., Kober L., Torp-Pedersen C., Gislason G. Proton-pump inhibitors are associated with increased cardiovascular risk independent of clopidogrel use: a nationwide cohort study. Ann. Intern. Med. 2010;153:378–386. doi: 10.7326/0003-4819-153-6-201009210-00005. [DOI] [PubMed] [Google Scholar]
- 32.Sydow K., Munzel T. ADMA and oxidative stress. Atheroscler. Suppl. 2003;4:41–51. doi: 10.1016/s1567-5688(03)00033-3. [DOI] [PubMed] [Google Scholar]
- 33.Li H., Horke S., Forstermann U. Vascular oxidative stress, nitric oxide and atherosclerosis. Atherosclerosis. 2014;237:208–219. doi: 10.1016/j.atherosclerosis.2014.09.001. [DOI] [PubMed] [Google Scholar]
- 34.Landmesser U., Spiekermann S., Dikalov S., Tatge H., Wilke R., Kohler C., Harrison D.G., Hornig B., Drexler H. Vascular oxidative stress and endothelial dysfunction in patients with chronic heart failure: role of xanthine-oxidase and extracellular superoxide dismutase. Circulation. 2002;106:3073–3078. doi: 10.1161/01.cir.0000041431.57222.af. [DOI] [PubMed] [Google Scholar]
- 35.Khan S.A., Lee K., Minhas K.M., Gonzalez D.R., Raju S.V., Tejani A.D., Li D., Berkowitz D.E., Hare J.M. Neuronal nitric oxide synthase negatively regulates xanthine oxidoreductase inhibition of cardiac excitation-contraction coupling. Proc. Natl. Acad. Sci. USA. 2004;101:15944–15948. doi: 10.1073/pnas.0404136101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Houston M., Estevez A., Chumley P., Aslan M., Marklund S., Parks D.A., Freeman B.A. Binding of xanthine oxidase to vascular endothelium. Kinetic characterization and oxidative impairment of nitric oxide-dependent signaling. J. Biol. Chem. 1999;274:4985–4994. doi: 10.1074/jbc.274.8.4985. [DOI] [PubMed] [Google Scholar]
- 37.Mervaala E.M., Cheng Z.J., Tikkanen I., Lapatto R., Nurminen K., Vapaatalo H., Muller D.N., Fiebeler A., Ganten U., Ganten D., Luft F.C. Endothelial dysfunction and xanthine oxidoreductase activity in rats with human renin and angiotensinogen genes. Hypertension. 2001;37:414–418. doi: 10.1161/01.hyp.37.2.414. [DOI] [PubMed] [Google Scholar]
- 38.Ghebremariam Y.T., Cooke J.P., Khan F., Thakker R.N., Chang P., Shah N.H., Nead K.T., Leeper N.J. Proton pump inhibitors and vascular function: a prospective cross-over pilot study. Vasc. Med. 2015;20:309–316. doi: 10.1177/1358863X14568444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kraus A., Flores-Suarez L.F. Acute gout associated with omeprazole. Lancet. 1995;345:461–462. doi: 10.1016/s0140-6736(95)90449-2. [DOI] [PubMed] [Google Scholar]