Abstract
Photodynamic therapy (PDT) offers a new approach to selective tumor eradication. Modifications designed to increase and optimize efficacy continue to emerge. Selective photodamage to malignant cells and their environment can bring about tumor cell destruction, shutdown of the tumor vasculature, stimulation of immunologic anti-tumor effects and potentiation of other therapeutic effects. Current development of combination protocols may provide a better rationale for integration of PDT into clinical practice. An example described here is the ability of a sequential (two-sensitizer) PDT protocol to enhance the efficacy of photokilling. The first step involves low-level lysosomal photodamage that has been shown to promote the apoptotic response to subsequent photodynamic effects directed at mitochondria. In this report, we demonstrate the ability of Photofrin, an FDA-approved photosensitizing agent, to serve as either the first or second element of the sequential protocol.
Keywords: photosensitizer, porphyrin, lysosomes, mitochondria, apoptosis, autophagy
INTRODUCTION
Surgery and ionizing radiation are often used in cancer treatment protocols but have limitations. Surgery can leave malignant cells at the margins [1] while radiation requires careful targeting to avoid unnecessary damage to normal tissues. A more selective approach to cancer control is photodynamic therapy (PDT), a procedure that involves the preferential photosensitization of neoplastic tissues and their vasculature [2, 3]. Irradiation at visible wavelengths of light can bring about a significant degree of tumor eradication. There is also an immunologic element: PDT efficacy is sub-optimal without a functional immune system [4]. It has been shown that killing of tumor cells by PDT can lead to the creation of cancer vaccines [5, 6], suggesting the possibility of effects on tumor at remote sites. If the PDT can also initiate an enhanced immunologic reaction, this could have implications with regard to distant metastasis. A protocol designed to explore this possibility has been reported [7].
Direct photokilling results from the triggering of an apoptotic response [8, 9], circumventing the need for drug activation, availability of indirect pathways to cell death or other processes that can be impaired or absent in some tumor phenotypes. Cells deficient in apoptosis may be spared from photokilling, but clinical experience indicates that this is rare [2, 3]. Apoptosis is a highly-conserved and irreversible pathway to cell death [8]. Depending on the particular sub-cellular organelles where the photosensitizer has localized, apoptosis may be initiated by photodamage to the anti-apoptotic protein Bcl-2 or to the mitochondrial membrane leading to loss of cytochrome c [10–12]. Lysosomal photodamage leads to a more circuitous pathway to apoptosis: release of proteases that cleave the protein Bid, resulting in formation of a pro-apoptotic ‘truncated’ protein termed t-Bid [13].
Lysosomes are also an essential element of the process of autophagy whereby cellular elements are digested and recycled [14]. Autophagy can offer partial protection from PDT, perhaps via recycling of photodamaged mitochondria before apoptosis can occur [15]. Initiation of autophagy by PDT has other implications. Autophagy has been shown to enhance immune recognition, a possible route to an enhanced eradication of remote neoplastic loci after primary photodamage [16, 17]. An examination of the role of autophagy in photokilling has indicated a cytoprotective effect in cell lines capable of carrying out the apoptotic program: see Ref. 18 for a recent review on this topic. Autophagy can, however, represent a “death pathway” when apoptosis is unavailable: perhaps the most unambiguous report on this topic was published by Oleinick’s group [19]. A recent “commentary” on the subject has also appeared [20].
Most chemotherapy involves drug combinations, often with a view toward targeting both rapidly and slowly-dividing malignant cells, promoting responses in tumors that may be unresponsive to single agents and minimizing host toxicity. Combinations that include PDT are currently being explored. A 1996 report showed that PDT sequentially directed against lysosomes and mitochondria could result in enhanced photokilling in a mouse sarcoma model [21]. Another report indicated a similar result when lysosomal and golgi were the PDT targets [22]. A potential mechanism for this result has been suggested: lysosomal photodamage can release calcium stores [23] which, in turn, activate the protease calpain leading to cleavage of ATG5 to a pro-apoptotic fragment [24]. This effect could be reproduced in a cell-culture system using any of three lysosomal-targeting agents [25]. Another report indicated successful promotion of photokilling by this model in a 3D cell-culture model of inflammatory breast cancer [26].
This study reports on the ability of Photofrin to be successfully utilized for either the first or the second photosensitization step in the sequential protocol. This likely derives from the broad spectrum of photodamage produced by this agent upon irradiation in a cellular environment.
EXPERIMENTAL
Cell lines
Growth of murine hepatoma 1c1c7 cells and procedures for clonogenic assays have been described; sub-lines lacking autophagy-associated proteins were prepared as previously described [27].
Photosensitizers
NPe6 was provided by Dr. Kevin M. Smith, Louisiana State University. BPD (benzoporphyrin derivative, Verteporfin) was purchased from VWR (Cat No 1711461), Photofrin was generously provided by Pinnacle Inc. Other reagents were obtained from Sigma-Aldrich and were of the highest available purity. Fluorescent probes were provided by Life Technologies, Inc.
Irradiation procedures
Cell cultures in 35 mm diameter plastic dishes were incubated at 37 °C with 0.5 µM BPD + 40 µM NPe6 for 1 h or with 10 µg/mL Photofrin for 16 h. NPe6 (40 µM) was added during the final hour. The medium was then replaced and the dishes irradiated using a 600-watt quartz-halogen source filtered through 10 cm of water to remove wavelengths of light > 900 nm. The bandwidth was further confined by interference filters (Oriel, Stratford CT). Based on absorbance spectra, wavelengths of 690 nm were used with BPD, 660 nm for NPe6, 630 nm for Photofrin. Action spectra for Photofrin were determined using a series of interference filters of varying optimum transmission properties (±10 nm). For the acquisition of dose-response data, light doses were varied so as to induce 10–90% photokilling.
Microscopy
Fluorescence microscopy was used to monitor effects of photodamage by Photofrin-induced photodamage on the mitochondrial membrane potential (ΔΨm) and the ability of the lysosome to maintain a pH gradient, using techniques described in Ref. 28. To avoid interference from Photofrin fluorescence, Rhodamine 123 was used as a probe for ΔΨm [29] and Lysotracker Green for lysosomal integrity [30].
Absorbance spectra
To approximate the absorbance spectra of Photofrin in cellular environment, 1c1c7 cells were incubated with the photosensitizer for 16 h at 37°. The cells were then collected and dispersed in 10 mM Triton-X100 and the absorbance spectrum determined using a dual-beam Shimadzu BioSpec 1601 instrument.
Clonogenic assays
Viability was determined by colony counting using an Oxford Optronix GelCount device.
An algorithm was used to confine the colony identification to groups of 30 cells or more. Although this device can detect colonies without additional staining, crystal violet was used as a colony marker. All experiments were performed in triplicate.
Statistical analysis
Analysis of data was carried out using the independent groups t-test. We indicate where differences in values were statistically different (p < 0.05) from untreated controls.
RESULTS AND DISCUSSION
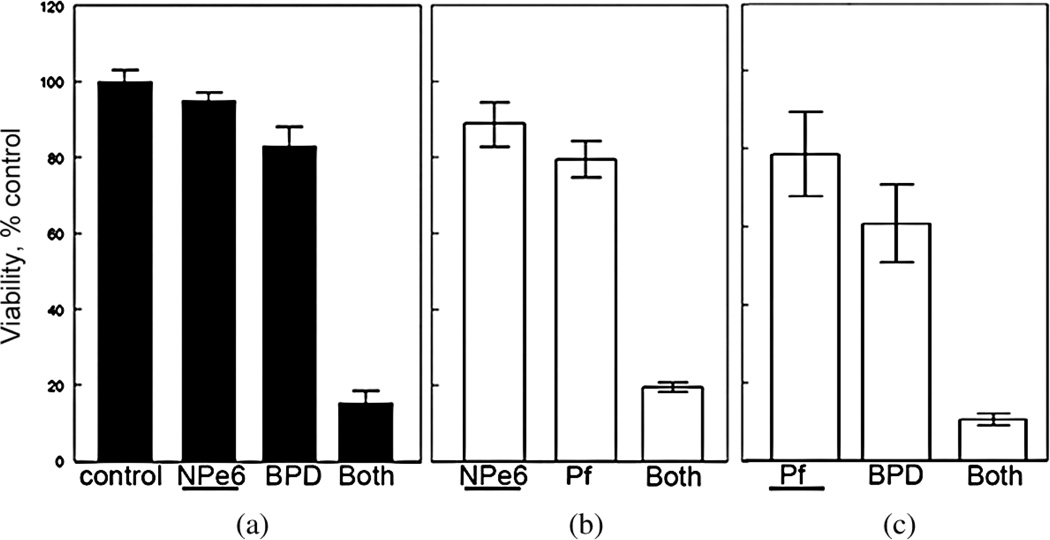
Photofrin is a widely used photosensitizing agent known to target multiple subcellular organelles [28]. Figure 1 shows a comparison of the effects of sequential PDT protocols on photokilling of murine hepatoma 1c1c7 cells. In this figure, the photosensitizer activated first is indicated by underlining in the legend. Panel A summarizes a prior report [31] involving NPe6 and BPD. The data clearly indicate a marked enhancement of photokilling when low-dose lysosomal photodamage (NPe6) is followed by mitochondrial photodamage (BPD). Panel B indicates the results of a similar protocol involving NPe6 and Photofrin. In Panel C, the photosensitizers were Photofrin, followed by BPD. In all three cases, a marked potentiation of photokilling was produced by the sequential protocol. We had previously proposed a mechanism for these results [31] as outlined above. Data shown in panels B and C suggest that Photofrin can substitute for either NPe6 or BPD in the protocol.
Fig. 1.
Promotion of photokilling by a sequential PDT protocol. (a) NPe6 followed by BPD; (b) NPe6 followed by Photofrin; (c) Photofrin followed by BPD. The sensitizer first activated in the combination study is indicated by an underline. Data indicate results of clonogenic assays; average ± SD for three determinations
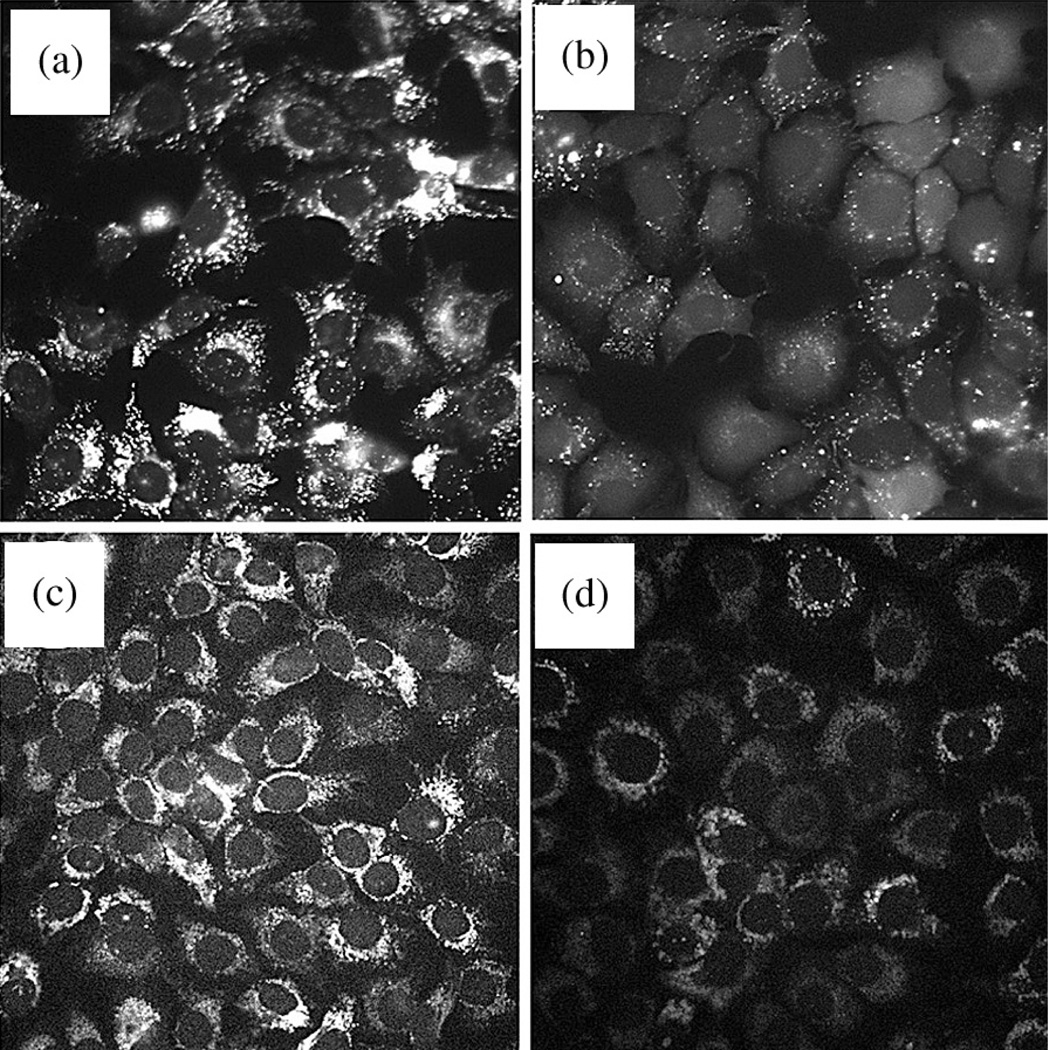
While mitochondrial localization of Photofrin has been described [32], evidence of lysosomal photodamage dates from 1993 [33]. It should be noted that these experiments were carried out with an earlier variant of Photofrin that may not represent the product being produced today. In the present work, the current Photofrin formulation was employed as provided by Pinnacle Biologics Inc. Studies involving fluorescent probes for photodamage revealed that the earliest detectable effect, directly following an L90 PDT dose, was loss of both the lysosomal pH gradient and ΔΨm within 10 min as shown in Fig. 2.
Fig. 2.
Effects of an LD90 PDT dose using Photofrin. Fluorescence microscopy was used to assess photodamage to specific organelles 10 min after irradiation. (a, b) Lysotracker Green (LTG) fluorescence assessing the lysosomal pH gradient. (c, d) Rhodamine 123 (R123) reporting on the mitochondrial membrane potential (ΔΨm). (a, c) controls; (b, d) effects of photodamage
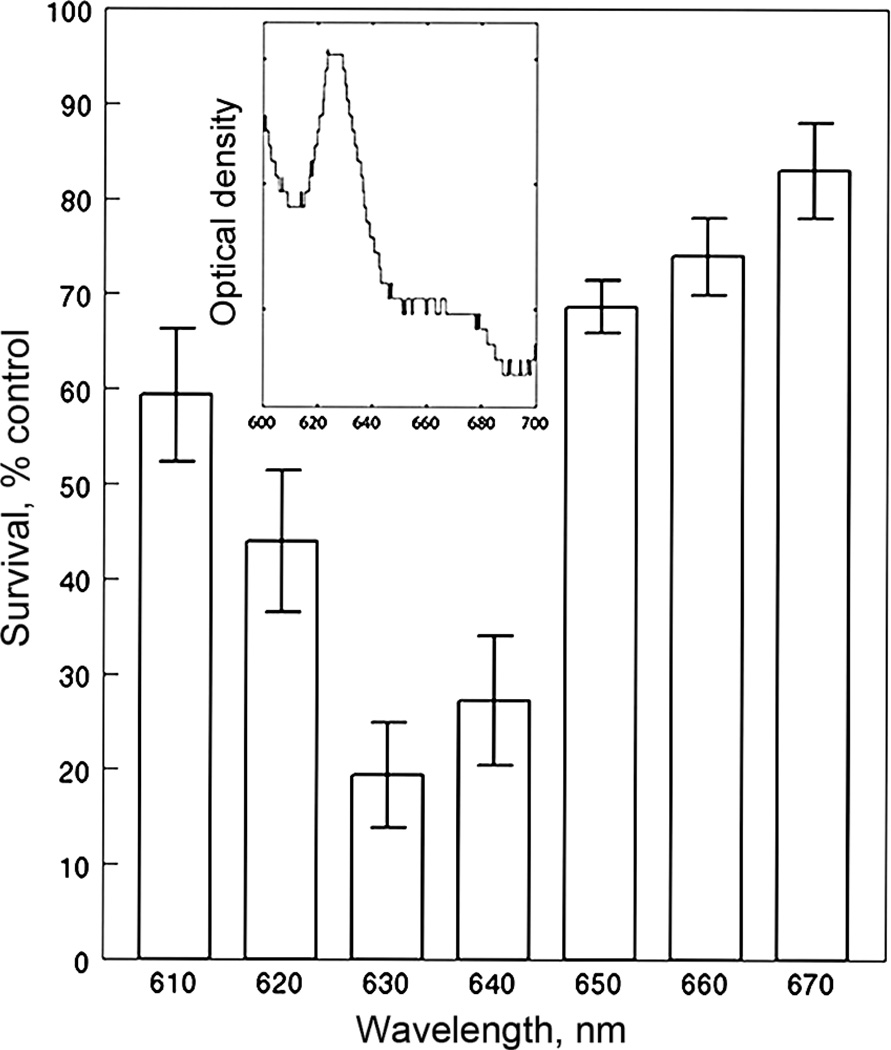
Perhaps because of its complex composition, Photofrin has relatively broad absorbance spectrum, as shown in the inset to Fig. 3. Spectra for NPe6 and BPD are shown in Ref. 28; this indicates minimal overlap. A potential concern with the sequential protocol involving Photofrin is the possibility for eliciting additional (unwanted) photodamage during irradiation at 660 or 690 nm. With NPe6 and BPD, the spectra are sufficiently different so that this is not a problem. Photofrin does appear to be minimally photodynamically active at wavelengths > 650 nm (Fig. 3).
Fig. 3.
Action spectrum of Photofrin. Irradiation wavelengths were selected by interference filters as described in the text. Data represent results of clonogenic assays (average ± SD for three experiments). Inset: absorbance spectrum of Photofrin in 1c1c7 cells after solubilization with 10 mM Triton-X100 as described in the text
CONCLUSION
Most current chemotherapy protocols involve drug combinations with a view toward targeting both rapidly and slowly-dividing malignant cells, promoting responses in tumors that may be unresponsive to single agents while minimizing host toxicity. PDT combinations are now being explored. Hasan’s group has demonstrated successes in combining PDT with conventional chemotherapy in both cell culture and animal studies [34, 35]. The former often involves 3D cultures where tumors are allowed to grow under conditions that more closely recapitulate conditions that occur in vivo. It is also feasible to carry out co-culture studies where tumor cells are grown together with normal cell-types to further increase the mimicking of the tumor microenvironment that exists in cancer. It had been demonstrated in 1996 that a sequential PDT protocol was able to provide a much higher degree of tumor eradication in vivo than could be provided by either agent alone [21]. We propose that the present study, together with two prior reports [25, 31] provides an explanation for this effect. A protocol that sequentially evokes lysosomal, followed by mitochondrial photodamage shows promise for promoting tumor eradication where single agents are less effective.
PDT can be a useful approach to cancer control: minimal toxicity to normal tissues and organs, no cross-resistance with other modalities, no significant adverse reactions, ability to retreat without development of ‘resistance’, feasibility for identification of neoplasia loci via fluorescence of photosensitizing agents, a concomitant shutdown of tumor vasculature adding to the anti-tumor effect and the possibility of enhancing immune responses. Clinical use of PDT is, however, lagging. Adequate delivery of light is a complex prospect with special devices often required for mapping of evenness of illumination [36]. But this is not significantly more complex than, e.g., procedures involved in the use of ionizing radiation. It is proposed that continued investigation into combination protocols involving PDT may promote tumor responses and minimize incomplete tumor control deriving from inadequate light doses and/or, perhaps, hypoxia.
Acknowledgments
Work in my laboratory has been supported since 1980 by a grant from the National Cancer Institute; NIH R-01 CA 23378. Excellent technical assistance was provided by Ann Marie Santiago.
REFERENCES
- 1.Höckel M, Dornhöfer N. Cancer Res. 2005;15:2997–3002. doi: 10.1158/0008-5472.CAN-04-3868. [DOI] [PubMed] [Google Scholar]
- 2.Dougherty TJ, Gomer CJ, Henderson BW, Jori G, Kessel D, Korbelik M, Moan J, Peng Q. J. Natl. Cancer Inst. 1998;90:889–905. doi: 10.1093/jnci/90.12.889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Agostinis P, Berg K, Cengel KA, Foster TH, Girotti AW, Gollnick SO, Hahn SM, Hamblin MR, Juzeniene A, Kessel D, Korbelik M, Moan J, Mroz P, Nowis D, Piette J, Wilson BC, Golab J. CA Cancer J. Clin. 2011;61:250–281. doi: 10.3322/caac.20114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Korbelik M. Lasers Surg. Med. 2006;38:500–508. doi: 10.1002/lsm.20337. [DOI] [PubMed] [Google Scholar]
- 5.Korbelik M. Photochem. Photobiol. Sci. 2011;10:664–669. doi: 10.1039/c0pp00343c. [DOI] [PubMed] [Google Scholar]
- 6.Gollnick SO, Vaughan L, Henderson BW. Cancer Res. 2002;62:1604–1608. [PubMed] [Google Scholar]
- 7.Shams M, Owczarczak B, Manderscheid-Kern P, Bellnier DA, Gollnick SO. Cancer Immunol. Immunother. 2015;64:287–297. doi: 10.1007/s00262-014-1633-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Agarwal ML, Clay ME, Harvey EJ, Evans HH, Antunez AR, Oleinick NL. Cancer Res. 1991;51:5993–5996. [PubMed] [Google Scholar]
- 9.Luo Y, Chang CK, Kessel D. Photochem. Photobiol. 1996;63:528–534. doi: 10.1111/j.1751-1097.1996.tb03079.x. [DOI] [PubMed] [Google Scholar]
- 10.Kim HR, Luo Y, Li G, Kessel D. Cancer Res. 1999;59:3429–3432. [PMC free article] [PubMed] [Google Scholar]
- 11.Xue LY, Chiu SM, Oleinick NL. Oncogene. 2001;20:3420–3427. doi: 10.1038/sj.onc.1204441. [DOI] [PubMed] [Google Scholar]
- 12.Kim J, Rodriguez ME, Oleinick NL, Anderson VE. Free Radic. Biol. Med. 2010;49:718–725. doi: 10.1016/j.freeradbiomed.2010.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Reiners JJ, Jr, Caruso JA, Mathieu P, Chelladurai B, Yin XM, Kessel D. Cell Death Differ. 2001;9:934–944. doi: 10.1038/sj.cdd.4401048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Feng Y, He D, Yao Z, Klionsky DJ. Cell Res. 2014;24:24–41. doi: 10.1038/cr.2013.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kim I, Lemasters JJ. Antioxid. Redox. Signal. 2011;14:1919–1928. doi: 10.1089/ars.2010.3768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Garg AD, Maes H, Romano E, Agostinis P Autophagy. Photochem. Photobiol. Sci. 2015;14:1410–1424. doi: 10.1039/c4pp00466c. [DOI] [PubMed] [Google Scholar]
- 17.Deretic V, Kimura T, Timmins G, Moseley P, Chauhan S, Mandell M. J. Clin. Invest. 2015;125:75–84. doi: 10.1172/JCI73945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Reiners JJ, Jr, Agostinis P, Berg K, Oleinick NL, Kessel D. Autophagy. 2010;6:7–18. doi: 10.4161/auto.6.1.10220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Xue LY, Chiu SM, Oleinick NL. Autophagy. 2010;6:248–255. doi: 10.4161/auto.6.2.11077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kessel D. Autophagy. 2015;11:1941–1943. doi: 10.1080/15548627.2015.1078960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cincotta L, Szeto D, Lampros E, Hasan T, Cincotta AH. Photochem. Photobiol. 1996;63:229–237. doi: 10.1111/j.1751-1097.1996.tb03019.x. [DOI] [PubMed] [Google Scholar]
- 22.Acedo P, Stockert JC, Cañete M, Villanueva A. Cell Death. Dis. 2014;13(5):e1122. doi: 10.1038/cddis.2014.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Medina DL, Di Paola S, Peluso I, Armani A, De Stefani D, Venditti R, Montefusco S, Scotto-Rosato A, Prezioso C, Forrester A, Settembre C, Wang W, Gao Q, Xu H, Sandri M, Rizzuto R, De Matteis MA, Ballabio A. Nat. Cell Biol. 2015;17:288–299. doi: 10.1038/ncb3114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yousefi S, Perozzo R, Schmid I, Ziemiecki A, Schaffner T, Scapozza L, Brunner T, Simon HU. Nat. Cell Biol. 2006;8:1124–1132. doi: 10.1038/ncb1482. [DOI] [PubMed] [Google Scholar]
- 25.Kessel D, Reiners JJ., Jr Photochem. Photobiol. 2015;91:931–936. doi: 10.1111/php.12456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Aggarwal N, Santiago AM, Kessel D, Sloane B. Breast Cancer Res. Treat. 2015 doi: 10.1007/s10549-015-3618-6. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Andrzejak M, Price M, Kessel DH. Autophagy. 2011;7:979–984. doi: 10.4161/auto.7.9.15865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Morgan J, Potter WR, Oseroff AR. Photochem. Photobiol. 2000;71:747–757. doi: 10.1562/0031-8655(2000)071<0747:coptia>2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 29.Baracca A, Sgarbi G, Solaini G, Lenaz G. Biochim. Biophys. Acta. 2003;1606:137–146. doi: 10.1016/s0005-2728(03)00110-5. [DOI] [PubMed] [Google Scholar]
- 30.Mwakwari SC, Wang H, Jensen TJ, Vicente MG, Smith KM. J. Porphyrins Phthalocyanines. 2011;15:918–929. doi: 10.1142/S108842461100380X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kessel D, Reiners JJ., Jr Photochem. Photobiol. 2014;90:889–895. doi: 10.1111/php.12270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yeh SC, Diamond KR, Patterson MS, Nie Z, Hayward JE, Fang Q. Theranostics. 2012;2:817–826. doi: 10.7150/thno.4479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gèze M, Morlière P, Mazière JC, Smith KM, Santus R. J. Photochem. Photobiol. B. 1993;20:23–35. doi: 10.1016/1011-1344(93)80128-v. [DOI] [PubMed] [Google Scholar]
- 34.Spring BQ, Rizvi I, Xu N, Hasan T. Photochem. Photobiol. Sci. 2015;14:1476–1491. doi: 10.1039/c4pp00495g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Celli JP, Solban N, Liang A, Pereira SP, Hasan T. Lasers Surg. Med. 2011;43:565–574. doi: 10.1002/lsm.21093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jarvi MT, Patterson MS, Wilson BC. Biophys. J. 2013;102:661–671. doi: 10.1016/j.bpj.2011.12.043. [DOI] [PMC free article] [PubMed] [Google Scholar]