Abstract
Background
Many methods have been used for preventing and reducing recurrences of bladder cancers. In recent years, some investigators have examined the use of metformin for this purpose. First lines of evidence have shown that metformin inhibits cancer cell growth and prevents cancer occurrence in patients with type 2 diabetes.
Objectives
This study is designed to assess metformin usage in the prevention of bladder cancer recurrence after the trans-urethral resection of a bladder tumor (TUR-T).
Patients and Methods
In the present study, metformin was administered in the treatment of 32 patients with a history of bladder cancer, and their results were compared with those of 33 patients with bladder cancer recurrence (placebo group). Patients in the metformin group received 1000 mg metformin (2 tablets 500 mg) for 1 year. Frequency of tumor recurrence was calculated and compared with the placebo group.
Results
There was no statistical difference between the 2 groups with respect to the recurrence rate (P > 0.05). Although the recurrence interval was longer for the metformin group, this increase was not statistical significant (P > 0.05). Furthermore, tumor recurrence had no correlation with sex or the grade of the tumors.
Conclusions
According to our findings, it seems that metformin has no considerable inhibitory effect on the recurrence rate of bladder cancer, but that it can delay tumor recurrence.
Keywords: Bladder Tumor, Metformin, Tumor Recurrence
1. Background
Bladder cancer is the second most frequent cancer of the genitourinary system, the fourth most common cancer in men, and the seventh most common of all (1). Hematuria is the most prevalent symptom that occurs in 80% - 90% of cases and is usually presented without other urinary symptoms (called painless hematuria) (2). After diagnosis, the disease is treated with surgery, chemotherapy, or radiotherapy based on the patient’s particular condition, but recurrence is very common (3-5). Many drugs have been used in recent decades to lower the recurrence rate, such as bacillus Calmette-Guerin (BCG), mitomycin C, doxorubicin, and thiotepa.
About forty years ago, Heuson indicated that there is a relationship between diabetes mellitus (DM) and cancer (6), and other investigators claimed that disorders of intracellular signaling pathways, insulin resistance, and treatment with anti-diabetic drugs can affect cancer incidence and progression (7-10). One such drug is metformin. Metformin is a semi-synthetic agent that is derived from galegine and is administered to lower blood sugar. Today, this drug is used for the treatment of diabetes mellitus and the prevention of cardio-vascular disease and polycystic ovarian syndrome (PCOS) (11, 12).
Preliminary evidence shows that metformin can lower the incidence rate of some cancers (including those of the breast, colon, liver, lung, prostate, and pancreas) in type 2 diabetic patients (13-15). Metformin activates protein kinases and decreases rapamycin signaling (mTOR) which can then lower blood sugar (16). Based on current evidence, cancer cell growth in bladder cancer requires activation of mTOR (17, 18), so it seems that inhibition of this pathway could be an effective way to treat or prevent bladder cancer.
2. Objectives
This study was designed for the assessment of metformin usage in the prevention of bladder cancer recurrence after the trans-urethral resection of a bladder tumor (TUR-T).
3. Patients and Methods
3.1. Patient Selection and Data Collection
From April 2013 to September 2014, all patients referred to Baqiyatallah hospital with the diagnosis of a bladder tumor who underwent TUR-T were included in this study. There were 65 patients and they were divided into two groups: group 1 included 32 patients who were administered metformin as part of their treatment plan, and group 2 included 33 patients who did not take the drug. Patients with hepatic dysfunction and a history of chemotherapy or radiation were excluded. Data on sex, age, history of smoking, diagnosis of DM, size and number of tumors, histology of tumor cells, and grade and stage of resected tumors were collected before surgery.
3.2. Metformin Administration
Metformin was administered in doses of 1000 mg per day (2 doses 500 mg) for the 32 patients in group 1.
3.3. Follow Up
All patients were followed up with ultrasonography and cystoscopy every 3 months for a 1 year period.
3.4. Statistics
For analyzing the data, SPSS software version 20 was employed. Then, a chi-square test and Spearman’s rank correlation coefficient were used to determine qualitative parameters, and an independent t-test was used for assessing the quantitative parameters. In all tests, a P value of less than 0.05 was considered significant.
4. Results
4.1. Demographic and Past Medical History
Group 1 (metformin) included 32 patients with an age range between 46 and 91 years and a mean age of 63.44 ± 11.71 years. In this group, 11 patients were female (34.4%) and 21 were male (65.6%). Group 2 (control) included 33 patients with an age range between 23 - 83 years and a mean age of 62.06 ± 14.88 years. Of these patients, 8 were female (24.2%) and 25 were male (75.8%).
Other demographic characteristics are shown in Table 1. According to this it it is evident that smoking, DM, previous surgery on the genitourinary tracts, tumor histology, and stage and grade were not statistically different between the 2 groups, but the tumor pedicle type (papillary or sessile) was statistically different (P value < 0.05).
Table 1. Demographic Parametersa.
| Group 1 | Group 2 | P Value | |||
|---|---|---|---|---|---|
| Female | Male | Female | Male | ||
| Smoking | 0.148 | ||||
| + | 0 | 9 (43) | 1 (13) | 14 (56) | |
| - | 11 (100) | 12 (57) | 7 (87) | 11 (44) | |
| DM | 0.423 | ||||
| + | 2 (18) | 3 (14) | 2 (25) | 1 (4) | |
| - | 9 (82) | 14 (86) | 6 (75) | 24 (96) | |
| Previous surgery | 0.857 | ||||
| + | 6 (55) | 4 (19) | 2 (25) | 9 (36) | |
| - | 5 (45) | 17 (81) | 6 (75) | 16 (64) | |
| Number of tumors | 0.138 | ||||
| Single | 9 (82) | 15 (71) | 7 (87) | 12 (48) | |
| Multiple | 2 (18) | 6 (29) | 1 (13) | 13 (52) | |
| Pedicle | 0.016 | ||||
| Papillary | 9 (82) | 9 (43) | 7 (87) | 20 (80) | |
| Sessile | 1 (9) | 9 (43) | 1 (13) | 3 (12) | |
| Both | 1 (9) | 3 (14) | 0 (0) | 2 (8) | |
| Stage | 0.810 | ||||
| T1 | 6 (55) | 9 (43) | 6 (75) | 12 (48) | |
| T2 | 1 (9) | 9 (43%) | 0 (0) | 8 (32) | |
| Ta | 4 (36) | 1 (5%) | 2 (25) | 2 (8) | |
| CIS | 0 | 0 | 0 | 1 (4) | |
| Undefined | 0 | 2 (9) | 0 | 2 (8) | |
| Grade | 0.803 | ||||
| Low | 8 (73) | 9 (43) | 5 (62) | 14 (56) | |
| High | 1 (9) | 9 (43) | 2 (25) | 8 (32) | |
| PUNLMP | 2 (18) | 1 (5) | 1 (13) | 1 (4) | |
| Chronic cystitis | 0 | 0 | 0 | 1 (4) | |
| Undefined | 0 | 2 (9) | 0 | 1 (4) | |
aValues are expressed as No. (%).
None of patients showed any side effects of metformin use, including abdominal or stomach discomfort, cough or hoarseness, decreased appetite, diarrhea, fast or shallow breathing, fever or chills, general feeling of discomfort, lower back or side pain, muscle pain or cramping, painful or difficult urination, or drowsiness.
4.2. Tumor Size
In group 1, the tumor size was between 0.5 - 5 cm with a mean size of 1.90 ± 1.13, and in group 2, it was 0.5 - 11 cm with a mean of 2.14 ± 1.79. There was no statistical differences between the 2 groups (P value = 0.535).
4.3. Tumor Recurrence
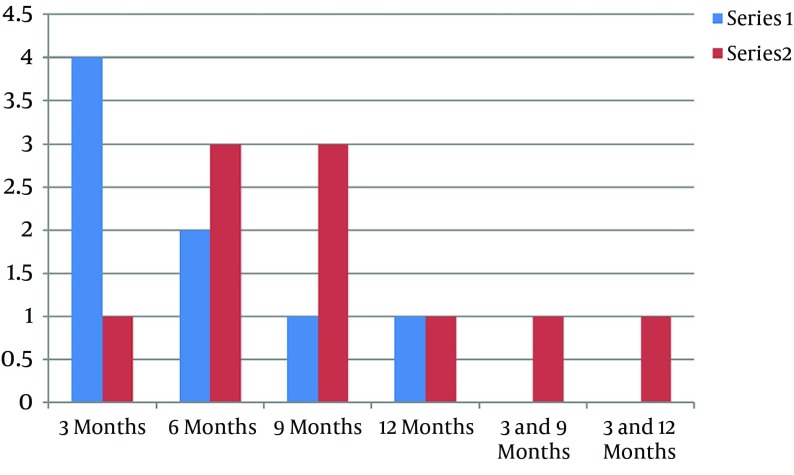
It was revealed that 8 (25%) patients in group 1 and 10 (30.3%) in group 2 had tumor recurrences during the 1 year follow-up period, and this rate is not statistically different (P value = 0.633). The time of recurrence (as shown in Figure 1) is longer in group 1 but the prevalence of the recurrence time is not different (P value = 0.5430).
Figure 1. Time of Recurrence.
The results of a correlation test between recurrence and demographic characteristics are shown in Table 2. There was no correlation between sex, DM, and tumor grade with tumor recurrence, but age and tumor size had a weak positive correlation with recurrence in group 1, and a weak negative correlation with recurrence in group 2. However, neither of these correlations were statistically significant (P value > 0.05).
Table 2. Correlation Test Results for Tumor Recurrence and Demographic Characteristics.
| Sex | Age | Smoking | DM | Tumor Grade | Tumor Stage | Tumor Size | |
|---|---|---|---|---|---|---|---|
| Group 1 | |||||||
| Correlation | 0.190 | 0.231 | -0.040 | -0.050 | -0.121 | -0.017 | 0.275 |
| P Value | 0.298 | 0.204 | 0.827 | 0.287 | 0.510 | 0.927 | 0.127 |
| Group 2 | |||||||
| Correlation | 0.089 | -0.288 | -0.205 | 0.021 | 0.094 | 0.275 | -0.336 |
| P Value | 0.624 | 0.104 | 0.253 | 0.908 | 0.603 | 0.122 | 0.056 |
5. Discussion
To our knowledge, the present study is the first one to evaluate the efficacy of metformin on bladder tumor recurrence after TUR-T. Our results show that metformin cannot reduce bladder tumor recurrence, but it can prolong the time to recurrence, although such intervals are not statistically significant. This result is in contrast to those of many other studies (13, 19-22).
Currie in 2009 claimed that in diabetic patients using insulin and metformin, the incidence of colorectal and pancreatic cancer was lower than the corresponding rates among the normal population (13). In similar studies, the survival rates of diabetic patients who were treated for colorectal or pancreatic cancer were 30% higher than those of diabetic patients who received other anti-diabetic agents (19-21). Furthermore, Wright and colleagues found in their study on Caucasian men that metformin can reduce the risk of prostate cancer by up to 44% (22). This discrepancy between our results and other studies may be due to our small sample size or the relatively short time of metformin administration and follow up (13, 19-22).
In 2010, Patel studied diabetic patients who had undergone radical prostatectomy and claimed that there is no association between metformin usage and the recurrence of prostate cancer; indeed, the recurrence rate was 55% higher in diabetic patients (23). These differences in the results may be due to the various risk factors that can affect incidence, progression, and recurrence of cancers, such as age, sex, obesity, smoking, genetics, and the environment. For example, it has been shown that smoking can increase the risk of developing bladder cancers by 3.89% and 4.65% in men and women, respectively (24), but this correlation was not shown in our study.
The precise effects of metformin on cancer are unclear, but some suggestions include the following: (1) it might stop the mTOR signaling pathway through AMP-activated protein kinase (AMPK) (25-27); and (2) it might decrease the insulin level by way of reducing the insulin-like growth factor-1 (IGF-1) (26, 28). Furthermore, it is clear that metformin can increase poly (ADP-ribose) polymerase (PARP)-dependent cell death and caspase-dependent apoptosis in breast cancer. Metformin has also been shown to decrease the activity and expression of HER2 in cancer cells, which is dose dependent and can be seen in higher administered doses of metformin (29-31). This may be the cause of the ineffectiveness of metformin in our study.
5.1. Conclusions
It seems that metformin can prolong the recurrence interval of bladder cancer, but the recurrence rate itself is not affected. This may be due to the small sample size and the short time of both administration and follow up in our study. Therefore, more studies with a greater sample size and longer administration and follow up times are needed.
Footnotes
Authors’ Contribution:Fatemeh Heidari and Shahin Abbas Zade carried out the operations, Seyed Hassan Mir Hosseini collected the data information, and Alireza Ghadian contributed to the analysis and the writing of the manuscript.
References
- 1.Zaitsu M, Toyokawa S, Tonooka A, Nakamura F, Takeuchi T, Homma Y, et al. Sex differences in bladder cancer pathology and survival: analysis of a population-based cancer registry. Cancer Med. 2015;4(3):363–70. doi: 10.1002/cam4.379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Park JC, Hahn NM. Bladder cancer: a disease ripe for major advances. Clin Adv Hematol Oncol. 2014;12(12):838–45. [PubMed] [Google Scholar]
- 3.Kitamura H, Kakehi Y. Treatment and management of high-grade T1 bladder cancer: what should we do after second TUR? Jpn J Clin Oncol. 2015;45(4):315–22. doi: 10.1093/jjco/hyu219. [DOI] [PubMed] [Google Scholar]
- 4.Nativ O, Witjes JA, Hendricksen K, Cohen M, Kedar D, Sidi A, et al. Combined thermo-chemotherapy for recurrent bladder cancer after bacillus Calmette-Guerin. J Urol. 2009;182(4):1313–7. doi: 10.1016/j.juro.2009.06.017. [DOI] [PubMed] [Google Scholar]
- 5.Seisen T, Granger B, Colin P, Leon P, Utard G, Renard-Penna R, et al. A Systematic Review and Meta-analysis of Clinicopathologic Factors Linked to Intravesical Recurrence After Radical Nephroureterectomy to Treat Upper Tract Urothelial Carcinoma. Eur Urol. 2015;67(6):1122–33. doi: 10.1016/j.eururo.2014.11.035. [DOI] [PubMed] [Google Scholar]
- 6.Heuson JC, Legros N, Heimann R. Influence of insulin administration on growth of the 7,12-dimethylbenz(a)anthracene-induced mammary carcinoma in intact, oophorectomized, and hypophysectomized rats. Cancer Res. 1972;32(2):233–8. [PubMed] [Google Scholar]
- 7.Krone CA, Ely JT. Controlling hyperglycemia as an adjunct to cancer therapy. Integr Cancer Ther. 2005;4(1):25–31. doi: 10.1177/1534735404274167. [DOI] [PubMed] [Google Scholar]
- 8.Dankner R, Chetrit A, Segal P. Glucose tolerance status and 20 year cancer incidence. Isr Med Assoc J. 2007;9(8):592–6. [PubMed] [Google Scholar]
- 9.Kasper JS, Giovannucci E. A meta-analysis of diabetes mellitus and the risk of prostate cancer. Cancer Epidemiol Biomarkers Prev. 2006;15(11):2056–62. doi: 10.1158/1055-9965.EPI-06-0410. [DOI] [PubMed] [Google Scholar]
- 10.Evans JM, Donnelly LA, Emslie-Smith AM, Alessi DR, Morris AD. Metformin and reduced risk of cancer in diabetic patients. BMJ. 2005;330(7503):1304–5. doi: 10.1136/bmj.38415.708634.F7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.El Messaoudi S, Rongen GA, de Boer RA, Riksen NP. The cardioprotective effects of metformin. Curr Opin Lipidol. 2011;22(6):445–53. doi: 10.1097/MOL.0b013e32834ae1a7. [DOI] [PubMed] [Google Scholar]
- 12.Kirpichnikov D, McFarlane SI, Sowers JR. Metformin: an update. Ann Intern Med. 2002;137(1):25–33. doi: 10.7326/0003-4819-137-1-200207020-00009. [DOI] [PubMed] [Google Scholar]
- 13.Currie CJ, Poole CD, Gale EA. The influence of glucose-lowering therapies on cancer risk in type 2 diabetes. Diabetologia. 2009;52(9):1766–77. doi: 10.1007/s00125-009-1440-6. [DOI] [PubMed] [Google Scholar]
- 14.Tan BX, Yao WX, Ge J, Peng XC, Du XB, Zhang R, et al. Prognostic influence of metformin as first-line chemotherapy for advanced nonsmall cell lung cancer in patients with type 2 diabetes. Cancer. 2011;117(22):5103–11. doi: 10.1002/cncr.26151. [DOI] [PubMed] [Google Scholar]
- 15.Zhuang Y, Miskimins WK. Metformin induces both caspase-dependent and poly(ADP-ribose) polymerase-dependent cell death in breast cancer cells. Mol Cancer Res. 2011;9(5):603–15. doi: 10.1158/1541-7786.MCR-10-0343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lage R, Dieguez C, Vidal-Puig A, Lopez M. AMPK: a metabolic gauge regulating whole-body energy homeostasis. Trends Mol Med. 2008;14(12):539–49. doi: 10.1016/j.molmed.2008.09.007. [DOI] [PubMed] [Google Scholar]
- 17.Gibbons JJ, Abraham RT, Yu K. Mammalian target of rapamycin: discovery of rapamycin reveals a signaling pathway important for normal and cancer cell growth. Semin Oncol. 2009;36 Suppl 3:S3–S17. doi: 10.1053/j.seminoncol.2009.10.011. [DOI] [PubMed] [Google Scholar]
- 18.Jung I, Messing E. Molecular mechanisms and pathways in bladder cancer development and progression. Cancer Control. 2000;7(4):325–34. doi: 10.1177/107327480000700401. [DOI] [PubMed] [Google Scholar]
- 19.Garrett CR, Hassabo HM, Bhadkamkar NA, Wen S, Baladandayuthapani V, Kee BK, et al. Survival advantage observed with the use of metformin in patients with type II diabetes and colorectal cancer. Br J Cancer. 2012;106(8):1374–8. doi: 10.1038/bjc.2012.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lee JH, Kim TI, Jeon SM, Hong SP, Cheon JH, Kim WH. The effects of metformin on the survival of colorectal cancer patients with diabetes mellitus. Int J Cancer. 2012;131(3):752–9. doi: 10.1002/ijc.26421. [DOI] [PubMed] [Google Scholar]
- 21.Li D, Yeung SC, Hassan MM, Konopleva M, Abbruzzese JL. Antidiabetic therapies affect risk of pancreatic cancer. Gastroenterology. 2009;137(2):482–8. doi: 10.1053/j.gastro.2009.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wright JL, Stanford JL. Metformin use and prostate cancer in Caucasian men: results from a population-based case-control study. Cancer Causes Control. 2009;20(9):1617–22. doi: 10.1007/s10552-009-9407-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Patel T, Hruby G, Badani K, Abate-Shen C, McKiernan JM. Clinical outcomes after radical prostatectomy in diabetic patients treated with metformin. Urology. 2010;76(5):1240–4. doi: 10.1016/j.urology.2010.03.059. [DOI] [PubMed] [Google Scholar]
- 24.Freedman ND, Silverman DT, Hollenbeck AR, Schatzkin A, Abnet CC. Association between smoking and risk of bladder cancer among men and women. JAMA. 2011;306(7):737–45. doi: 10.1001/jama.2011.1142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dowling RJ, Zakikhani M, Fantus IG, Pollak M, Sonenberg N. Metformin inhibits mammalian target of rapamycin-dependent translation initiation in breast cancer cells. Cancer Res. 2007;67(22):10804–12. doi: 10.1158/0008-5472.CAN-07-2310. [DOI] [PubMed] [Google Scholar]
- 26.Woodard J, Joshi S, Viollet B, Hay N, Platanias LC. AMPK as a therapeutic target in renal cell carcinoma. Cancer Biol Ther. 2010;10(11):1168–77. doi: 10.4161/cbt.10.11.13629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zakikhani M, Dowling R, Fantus IG, Sonenberg N, Pollak M. Metformin is an AMP kinase-dependent growth inhibitor for breast cancer cells. Cancer Res. 2006;66(21):10269–73. doi: 10.1158/0008-5472.CAN-06-1500. [DOI] [PubMed] [Google Scholar]
- 28.Memmott RM, Dennis PA. LKB1 and mammalian target of rapamycin as predictive factors for the anticancer efficacy of metformin. J Clin Oncol. 2009;27(34):e30261. doi: 10.1200/JCO.2009.25.3963. author reply e227. [DOI] [PubMed] [Google Scholar]
- 29.Alimova IN, Liu B, Fan Z, Edgerton SM, Dillon T, Lind SE, et al. Metformin inhibits breast cancer cell growth, colony formation and induces cell cycle arrest in vitro. Cell Cycle. 2009;8(6):909–15. doi: 10.4161/cc.8.6.7933. [DOI] [PubMed] [Google Scholar]
- 30.Gong J, Robbins LA, Lugea A, Waldron RT, Jeon CY, Pandol SJ. Diabetes, pancreatic cancer, and metformin therapy. Front Physiol. 2014;5:426. doi: 10.3389/fphys.2014.00426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vazquez-Martin A, Oliveras-Ferraros C, Menendez JA. The antidiabetic drug metformin suppresses HER2 (erbB-2) oncoprotein overexpression via inhibition of the mTOR effector p70S6K1 in human breast carcinoma cells. Cell Cycle. 2009;8(1):88–96. doi: 10.4161/cc.8.1.7499. [DOI] [PubMed] [Google Scholar]