Abstract
Demodex musculi, a prostigmatid mite that has been reported infrequently in laboratory mice, has been identified with increasing frequency in contemporary colonies of immunodeficient mice. Here we describe 2 episodes of D. musculi infestation with associated clinical signs in various genetically engineered mouse strains, as well as treatment strategies and an investigation into transmissibility and host susceptibility. The first case involved D. musculi associated with clinical signs and pathologic lesions in BALB/c-Tg(DO11.10)Il13tm mice, which have a defect in type 2 helper T cell (Th2) immunity. Subsequent investigation revealed mite transmission to both parental strains (BALB/c-Tg[DO11.10] and BALB/c-Il13tm), BALB/c-Il13/Il4tm, and wild-type BALB/c. All Tg(DO11.10)Il13tm mice remained infested throughout the investigation, and D. musculi were recovered from all strains when they were cohoused with BALB/c-Tg(DO11.10)Il13tm index mice. However, only Il13tm and Il13/Il4tm mice demonstrated persistent infestation after index mice were removed. Only BALB/c-Tg(DO11.10)Il13tm showed clinical signs, suggesting that the phenotypic dysfunction of Th2 immunity is sufficient for persistent infestation, whereas clinical disease associated with D. musculi appears to be genotype-specific. This pattern was further exemplified in the second case, which involved NOD.Cg-PrkdcscidIl2rgtm1Wjl/SzJ (NSG) and C;129S4 Rag2tm1.1Flv Il2rgtm1.1Flv/J mice with varying degrees of blepharitis, conjunctivitis, and facial pruritis. Topical amitraz decreased mite burden but did not eliminate infestation or markedly ameliorate clinical signs. Furthermore, mite burden began to increase by 1 mo posttreatment, suggesting that topical amitraz is an ineffective treatment for D. musculi. These experiences illustrate the need for vigilance regarding opportunistic and uncommon pathogens in rodent colonies, especially among mice with immunologic deficits.
Abbreviations: Th2, type 2 helper T cell; NSG, NOD SCID Gamma (NODCg-PrkdcscidIl2rgtm1Wjl/SzJ)
Demodex are small prostigmatid mites belonging to the family Demodicidae. Prostigmata is a suborder within the order Acari and comprises a large, diverse group of mites that show great variation in morphology, biology, and habitat selection.3,10 Members of the genus Demodex inhabit the epidermis, hair follicles, sebaceous glands, and excretory portion of specialized glands of several mammalian hosts, including humans,48 NHP,36 dogs, cats, horses, livestock,20 rodents,6,8,41 and numerous other animal species and are readily identified by their small size, cigar-shaped bodies, and short stubby legs.10,22 Demodex typically shows host preference but not necessarily strict host specificity, as demonstrated by the ability of D. canis to infest hamster and canine skin but not murine skin engrafted onto SCID mice.51 In addition, when multiple Demodex species parasitize the same host species, each may inhabit a specific anatomic region of the host. For example, humans typically are inhabited by D. folliculorum and D. brevis, with the former occupying hair follicles and the latter residing in sebaceous glands.1,19
Demodex mites have been reported in several murine rodents, including Mus musculus,6,27 although only rarely in laboratory mice. Reports of infestation in murine rodents other than Mus musculus include descriptions of Demodex species in the oral cavity and esophagus of grasshopper mice (Onychomys leucogaster),47 D. agrarii in sebaceous glands of the ear in striped field mice (Apodemus agrarius),8,29 and D. lacrimalis in the meibomian glands of European wood mice (A. sylvaticus).41 Recently, D. corniculatus was newly described after its identification on yellow-necked mice (A. flavicollis), as was D. auricularis sp. nov., which was noted in the ear canal of A. sylvaticus.30,41 Reports of Demodex infestation in wild Rattus norvegicus include D. ratti from the back, eyelids, and external ear; D. norvegicus from the anal and genital areas; and D. ratticola from the muzzle.9 In addition, D. nanus has been reported in both R. norvegicus and R. rattus.9,14,28 Until recently, the only reports of Demodex species recovered from M. musculus involved D. flagellurus, which was recovered from the preputial and clitoral glands of wild populations of M. musculus,6,7,27 and D. musculi, which primarily has affected laboratory mice.25,39 However, recent work32-34 revealed 3 new species of demodecid mites in M. musculus, as well as a redescription of D. musculi. In particular, D. conicus sp. nov. was recovered from the ear canal, D. marculus sp. nov. was recovered from the skin in the abdomen, back, limbs, and anal area, and D. fusiformis sp. nov. was found in the skin of the abdomen, back, and limbs.32-34
D. musculi affecting M. musculus from laboratory colonies has been reported only 3 times in almost a century, and a single report describes infestation in 2 pet house mice.25,26,39 D. musculi is a small mite that has the cigar-shaped body and short stout legs typical of this genus. Adult male mites measure approximately 130 μm in length, whereas female mites are as long as 150 μm in length. This relatively small size immediately distinguishes D. musculi from the much larger D. flagellurus (length, 476 to 689 μm) and moderately larger D. conicus (199 to 300 μm). Although D. musculi is larger than D. marculus (99 μm) and D. fusiformis (111 μm), these species are close enough in size that additional morphologic features, such as podosomal and opisthosomal size and shape, should be used to make a definitive distinction.4,32,34 Little is known regarding the life cycle of D. musculi, but it is presumed to be similar to other Demodex species, with female mites laying as many as 24 eggs within hair follicles, followed by the hatching of eggs, progression through larval and nymphal stages, and development to the adult stage within 18 to 24 d.4
Here we describe 2 cases of D. musculi infestation in laboratory mice, which were associated with clinical lesions in a subset of the mice, as well as various treatment regimens. In addition, we discuss the transmission of infestation among mice of various immunodeficient genotypes and host immunologic conditions that permit increased mite burden and, in some cases, the manifestation of clinical signs in immunocompromised compared with immunocompetent animals.
Case Reports
All mice described in this report were housed in autoclaved IVC with 1/8-in. corncob bedding (no. 7092, Harlan, South Easton, MA) and nesting material (Nestlets, Ancare, Bellmore, NY). Autoclaved rodent chow (no. 5010, Purina Mills, St Louis, MO, or Teklad no. 2018, Harlan, Indianapolis, IN) and hyperchlorinated water were provided without restriction. Room conditions included temperature of 72 ± 2 °F (22.2 ± 1.1 °C), relative humidity of 50% ± 10%, a 12:12-h photoperiod; and 10 to 15 room air changes hourly. IVC were maintained under positive pressure, and the room air pressure differential relative to the corridor was positive for standard mouse rooms and negative in the quarantine facility. Cages were changed biweekly in a class II biosafety cabinet within the animal rooms. All mice were free of mouse parvovirus, mouse minute virus, mouse hepatitis virus, murine rotavirus, Sendai virus, pneumonia virus of mice, lymphocytic choriomeningitis virus, ectromelia virus, mouse encephalomyelitis virus, Mycoplasma pulmonis, and endo- and ectoparasites (with the exception of D. musculi as described following), according to testing of individual experimental animals or recent sentinel data. Experimental procedures were approved by the Yale University IACUC and were in accordance with all federal policies and guidelines governing the use of vertebrate animals.
Case 1.
Several adult BALB/c-Tg(DO11.10)Il13tm mice of various ages presented with dermatitis, which progressed from mild erythema to severe ulceration. This mouse strain was produced inhouse by crossing BALB/c-Tg(DO11.10) mice, a transgenic line that expresses a T-cell receptor specific for an MHCII-restricted ovalbumin peptide, and BALB/c-Il13tm, a strain with a targeted mutation that results in a deficiency of IL13, a key mediator of the Th2 immune response. The Tg(DO11.10) transgene contains rearranged Tcra and Tcrb genes and is expressed in the majority of T cells; these rearranged transgenes encode a chicken ovalbumin-specific MHC class II (I-Ad)-restricted T-cell receptor.40,50 The initial lesions noted involved the pinnae, but subsequent examinations revealed mice with similar lesions on the face, dorsal cervical region, and intrascapular region (Figure 1 A and B). No other strains were clinically affected, and sentinel mice were free of common murine pathogens, including ectoparasites. Consultation with lab personnel suggested that the clinical signs represented a strain-specific phenotype, and affected mice were treated symptomatically by using topical antibiotic preparations.
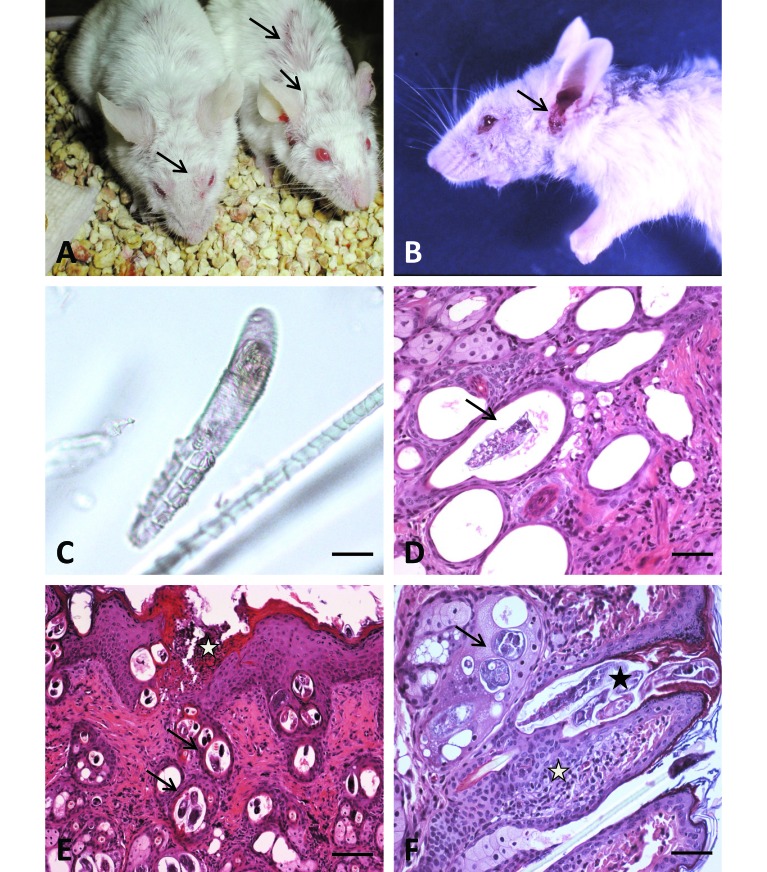
Figure 1.
Gross and histologic findings in BALB/c-Tg(DO11.10)IL13tm mice. (A and B). Gross lesions associated with Demodex musculi infestation in BALB/c-Tg(DO11.10)IL13tm mice. (A) Alopecia and excoriations in the dorsal cervical, intrascapular and periocular regions (arrows). (B) Facial alopecia and severe excoriation of the external ear canals and perioccular regions (arrows). (C) Hair plucks from affected mice. Hair plucks were taken from the head and dorsal cervical region of Tg(DO11.10)IL13tm mice, placed on a slide with mineral oil, and examined by light microscopy. The size and morphology of the mites identified in these samples are consistent with Demodex musculi (arrow). Bar, 20 μm. (D through F). Photomicrographs of skin sections from BALB/c-Tg(DO11.10)IL13tm mice infested with Demodex musculi. Sections of mites were present within dilated hair follicles (arrows, panels D and E; black asterisk, panel F) and sebaceous glands (arrow, panel F). Mite infestation was accompanied by epidermal hyperplasia with superficial erosions and cellular crusts (white asterisk, panel E). Superficial periadnexal inflammation was evident (white asterisk, panel F). Bar: 50 μm (D), 100 μm (E, F)
When treatment failed to produce significant improvement, representative mice were necropsied. Histopathology revealed mites consistent with Demodex in hair follicles of the pinnae, head, neck, and intrascapular region (Figure 1 D through F). Infested skin was accompanied by epidermal hyperplasia with superficial erosions and cellular crusts, and superficial periadnexal inflammation was evident (Figure 1 E and F). Subsequent fur plucks from the face, head, dorsal neck, and intrascapular region of 20 BALB/c-Tg(DO11.10)Il13tm mice revealed mites that were classified further as D. musculi, according to their size and morphology32 (Figure 1 C). Fur plucks were performed by using thumb forceps and gentle manual restraint. Extensive sampling (hair plucks) of additional mice housed in the same room (50 mice representing 17 genotypes on 3 different background strains) failed to reveal infestation. Caesarian rederivation was performed to eradicate D. musculi from the affected strain, but the discontinuation of this mouse strain in the lab shortly thereafter precluded long-term follow-up to determine whether rederivation for this purpose was successful.
Prior to rederivation, several affected mice were moved to our quarantine facility for further investigation of the transmission of D. musculi to mice of various genotypes, including the 2 parental strains used to produce the clinically affected genotype and wild-type BALB/c. These strains were not present in the room at the time of detection and therefore not represented in the sampling described earlier. To determine transmissibility, a single infested BALB/c-Tg(DO11.10)Il13tm mouse was cohoused with 4 presumably mite-free mice (according to the failure to detect mites on repeated fur plucks) of each of the parental strains, DO11.10+/+ and BALB/c-IL13tm; wild-type BALB/c mice; and BALB/c-IL13/IL4tm (IL13–/–/IL4−/−) mice deficient in both IL13 and IL4. The BALB/c-IL13/IL4tm mice were not present in the room when the infestation was detected but were added to the transmission study to further elucidate the role of Th2 immunity in the transmissibility of D. musculi and the associated clinical manifestations. Fur plucks were performed weekly on all of the mice, and the number of positive mice in each group and a subjective assessment of mite burden according to the relative number of mites per low-power (4×) field were recorded. Mice were monitored daily for clinical signs.
One week after introduction of the mite-positive BALB/c-Tg(DO11.10)Il13tm index mice, 25% (1 of 4) DO11.10+/+, 25% (1 of 4) IL13−/−, 50% (2 of 4) of IL13/IL4−/−, and 25% (1 of 4) BALB/c mice were mite-positive on the basis of fur-pluck results. Sampling continued intermittently over 6 mo of contact exposure to index mice, and the number of mite-positive mice in each group ranged from 25% to 75% for DO11.10+/+, 25% to 100% for IL13−/−, 50% to 100% for IL13/IL4−/−, and 25% to 50% for BALB/c, although mite burden was variable. BALB/c-Tg(DO11.10)Il13tm index mice consistently demonstrated the highest level of infestation, with mites detected in 100% of the mice at each time point and numerous mites per low-power field in each case. By comparison, at no time point during exposure to infested index mice were mites detected in more than 2 of 4 BALB/c mice, and in each case, fur plucks yielded very few mites (often fewer than 1 mite per low-power field).
After 6 mo of continuous exposure, the BALB/c-Tg(DO11.10)Il13tm index mice were removed from each cage, and fur plucks and clinical monitoring were continued to determine whether detectable infestation was maintained in the absence of the known source. In other words, we wanted to determine whether the various strains of mice maintained detectable levels of infestation or whether they were repeatedly ‘seeded’ with mites from the index mice during cohousing. At 1 wk after the removal of the index mice, none of test cages showed further evidence of infestation in the DO11.10+/+ or BALB/c mice. All mice of both strains failed to yield mites on fur plucks for the remainder of the study (3 mo after removal of the index mice). In addition, 1 of 4 (25%) IL13−/− and 2 of 4 (50%) IL13/IL4−/− mice had evidence of low-level infestation at 3 mo after the removal of the index mice. All BALB/c-Tg(DO11.10)Il13tm mice remained infested at variable (low, moderate, or high) levels throughout the course of the experiment and were the only strain to consistently manifest clinical signs, which included dermal erythema, pruritus, and excoriations of the pinna and around the head.
Case 2.
Several animals from a colony of genetically engineered mice were reported to have dorsal hair loss and varying degrees of blepharitis, conjunctivitis, and facial pruritis. (Figure 2 A and B). All affected mice had various immunologic alterations backcrossed onto NOD.Cg-PrkdcscidIl2rgtm1Wjl/SzJ (NSG) or C;129S4-Rag2tm1.1Flv Il2rgtm1.1Flv/J genetic backgrounds, both of which express severe generalized immunodeficiency. Affected animals included male and female mice ranging in age from 25 to 29 wk. Primary differentials included ectoparasites and bacterial conjunctivitis and dermatitis. Fur plucks were performed, sampling regions of the face and neck, which revealed presence of large numbers of D. musculi, identified on the basis of size and morphology (Figure 2 C). In addition, keratin follicular plugs, which are characteristic of Demodex infestation, were identified on fur plucks. Histopathology confirmed the presence of intrafollicular mites associated with minimal or no inflammation (Figure 2 D). Mite burden was generally reflective of the severity of clinical signs, with heavier infestation corresponding with more severe clinical signs. Management options discussed with the lab included rederivation to eliminate the mites from the colony and parasiticidal treatment of selected animals to help reduce mite burden and alleviate clinical signs. The lab elected to attempt treatment, and a course of amitraz was administered as previously described.39
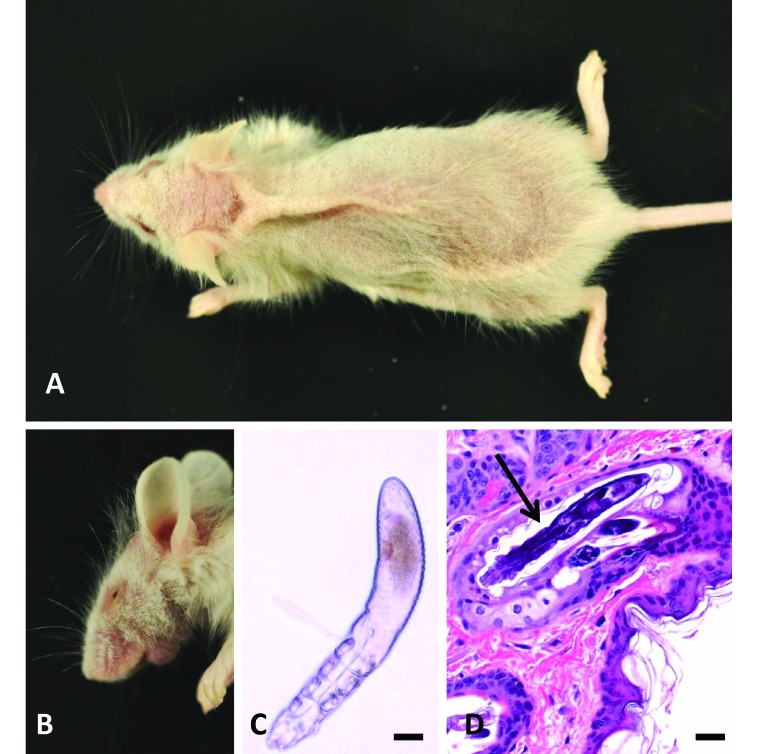
Figure 2.
Gross and histologic findings in NOD.Cg-PrkdcscidIl2rgtm1Wjl/SzJ (NSG) and C;129S4-Rag2tm1.1Flv Il2rgtm1.1Flv/J mice. (A and B) Affected mice had variable degrees of (A) dorsal hypotrichosis, scaling, and erythema and (B) accompanied by blepharitis. (C) Fur plucks revealed an arthropod with the elongated abdominal morphology consistent with Demodex musculi . Mineral oil mount; Bar, 20 μm. (D) Histopathology of the dorsal interscapular region revealed mites within follicles. In many infested follicles, inflammation was minimal to absent. Hematoxylin and eosin stain; bar, 50 μm.
A warm bath of dilute amitraz was prepared by adding 0.6 mL of amitraz liquid concentrate (Mitaban, Pfizer, New York) to 500 mL warmed sterile water in a sterile empty mouse-cage bottom to achieve a 250 ppm concentration. The amitraz bath and recovery cages were kept warm with the use of a heat lamp and microwaveable heating pads. Mice were placed into the bath, and a disposable towel (Wypall, Kimberly-Clark, Irving, TX) was used to saturate the entire coat, after which the mice were removed from the bath, dried with a new disposable towel, and placed into a warmed cage for recovery. Once fully recovered (cessation of intense grooming, coat mostly dry, resumption of normal behaviors), the cage was returned to the rack. Two modifications to the previous procedure39 were incorporated. First, ocular lubricant was not used, because we observed that the mice closed their eyes whenever the wet towel was placed near the face and because our technique of using a moistened towel did not result in splashing, we became less concerned about ocular exposure. In addition, application of the lubricant generally results in inadvertent application of the lubricant to the periocular skin; we were concerned that the extraneous lubricant might prevent amitraz from accessing the underlying skin and associated glands in the periorbital region, one of the preferred locations of Demodex spp.21,41 The second modification was that we did not change the amitraz bath after every mouse. Because all of the treated mice originated from the same colony and were therefore considered to microbiologically equivalent, changing the bath between mice was determined unnecessary. We bathed cagemates together and prepared a fresh bath after every 3 to 5 cages of mice, due to soiling of the water. Treatments were performed on approximately 60 mice every 2 wk for a total of 4 treatments.
Data to determine mite burden before and after treatment were collected from 20 mice. As such, fur plucks were obtained at baseline, 2 wk after the second treatment, and 2 and 4 wk after the last treatment. Fur plucks were performed under gentle manual restraint by using a hemostat to pluck fur from 3 locations on the head: right cheek, left cheek, and dorsal head and neck. As much as possible, a standard amount of fur was plucked from each area. The fur from each mouse was placed in mineral oil on a glass slide and cover-slipped for counting. The total number of mites per slide was then determined by visualization under light microscopy at 40×.
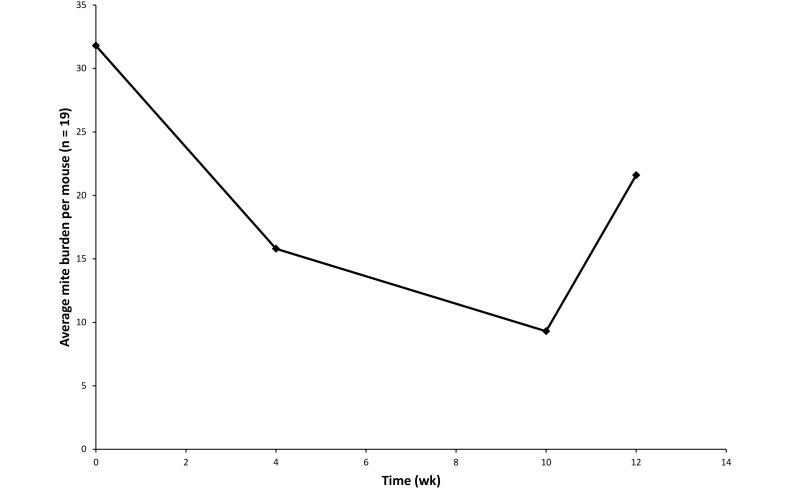
After 2 treatments, the mite burden was reduced by approximately 50%, with a further moderate reduction after the final treatment. However, within a month after treatment was discontinued, mite burden began to increase (Figure 3). Despite the reduction in mite burden, clinical signs in affected mice did not abate markedly. No adverse effects of amitraz were observed, including eye injury due to the lack of ocular lubricant, or sedation, which is a known side effect of this drug, according to the manufacturer's package insert. During the fourth week of treatment (11 d after the second treatment), one male mouse was euthanized due to severe fight wounds, and another male mouse in the same cage was found dead. The cause of death in this second mouse (age, 6 mo) was unknown; however, occasional deaths in adult mice of this colony are not uncommon and are generally due to complications secondary to the severely immunocompromised phenotype.
Figure 3.
Mite burden at baseline, after the second bath, and at 2 and 4 wk after the final (that is, 4th) treatment with amitraz. Mite numbers declined in response to amitraz but started to return to pretreatment levels after discontinuation.
Discussion
The ancient relationship between demodicid mites and their mammalian hosts dates back as many as 200 million years to the appearance of mammals on earth, when haired skin first appeared on the evolutionary scene. This long-standing relationship between Demodex mites and mammals has been proposed as an explanation for the relative tolerance of the immune system to this parasite.16,42 In most mammalian species Demodex mites have historically been considered commensal organisms, although their pathogenicity and appropriate classification as a commensal or pathogen has been a topic of discussion in both the human and veterinary medical literature.2,16,49 Some authors suggest that the mites should be considered commensals, because they inhabit the pilosebaceous unit of mammalian skin and benefit from the human sebum in their sheltered ecological follicular niche.37 However, in humans and other mammals, a close link between Demodex infestation and skin diseases has been well documented, suggesting pathogenic potential. Canine demodicosis is probably the best and most important example of Demodex overgrowth-induced disease in veterinary medicine. In canine demodicosis, cutaneous inflammation is associated with excessive numbers of proliferating mites, including immature forms (eggs, larvae, and nymphs), and the clinical cure is clearly associated with reduction of mite burden after acaricidal treatment; this pattern suggests that Demodex mites are more accurately described as parasites that can act as opportunistic pathogens in certain circumstances, most notably host immunosuppression or immunodeficiency.16 Host immunity is responsible for the control of mite populations, and a normal immune system appears to detect and tolerate the presence of these mites and has an inhibitory effect on mite proliferation, keeping numbers low without inducing an inflammatory response.1,16 As previous authors have noted, the mechanism of the immune response and the control of D. canis populations in healthy dogs merit further investigation.16 The assumption that the immune system plays a key role in the control of D. canis originated from studies on clinical demodicosis and is based on the following: 1) the possibility of inducing demodicosis by suppressing the immune response; 2) the development of demodicosis in strains of immunodeficient mice; and 3) numerous clinical observations of demodicosis in immunosuppressed people and animals.16
D. musculi has been reported infrequently in laboratory mice, with the first report occurring in 1917.26 The second case report was published over 80 y later,25 and only one additional case has been published since.39 The paucity of reports describing D. musculi in laboratory mice, both in the published literature and anecdotally within the laboratory animal community, suggests that this parasite either occurs very rarely in research colonies, or perhaps more likely, infestation or colonization is relatively common but is typically undetected, leading to underreporting of its occurrence in contemporary colonies. In this regard, as with fur mites, relying on bedding transfer sentinels for detection will undoubtedly result in underdiagnosis in the laboratory environment, because like fur mites, these follicle-dwelling mites are not readily spread by indirect means. D. musculi is believed to be transmitted from dam to pups during nursing and primarily by direct contact thereafter, in similar fashion to other Demodex species.3 This is supported by a study that demonstrated transmission of D. flagellurus to mite-free laboratory mice (M. musculus) by breeding them with naturally infested house mice (M. musculus). Consistent with what is known about transmission in other species, mites were transmitted between breeders and from dam to offspring.7
Typically, mite burden is greatly restricted by the immune system of the host, only achieving readily detectable levels or manifesting clinically when host immunity is compromised. Therefore, healthy, immunocompetent laboratory mice presumably harbor Demodex in their hair follicles, although skin scrapings and hair plucks rarely yield mites.22 Overgrowth of D. musculi has been reported in SCID mice, CD3E transgenic mice (a strain lacking mature T lymphocytes and natural killer cells), and Prad1 transgenic mice (a strain overexpressing human cyclin D1 and manifesting severe thymic hyperplasia).12 Not surprisingly, recent reports of D. musculi have described infestation with or without clinical signs in genetically engineered mice with deficiency in one or more aspects of their immune system.27,39 Another report39 describes demodicosis in an immunodeficient mouse strain, with alopecia and severe dermatitis. The affected mice were double-knockout mice lacking CD28 and STAT6, a model developed to study immune response to Nippostrongylus braziliensis.39 Interestingly, in that colony, neither of the single-knockout siblings lacking either CD28 or STAT6, both of which are involved in Th2 signaling, had increased numbers of D. musculi or clinical signs even when they were housed in the same cages, suggesting that control of D. musculi infestation is lost only when the functions of both molecules are inhibited.16
Although multiple immunologic defects may predispose to demodicosis in mice, the role of Th2 immunity in controlling D. musculi populations is further supported by our first case, in which mice deficient in the Th2 cytokines IL4 and IL13 (alone or in combination) as well as IL13-deficient mice transgenic for DO11.10 consistently demonstrated increased mite burden. This association is not surprising, given the Th2 immune response is the stereotypic response to parasitic infections. The unique and critical role of IL13 in T-cell–mediated expulsion of intestinal nematodes has been demonstrated by the inability of mice deficient in IL13 (but not wild-type mice or IL4-deficient mice) to expel Nippostrongylus braziliensis and suggests that the roles of IL13 and IL4 in Th2 immunity are not redundant.43 Furthermore, and perhaps more relevant in the context of this report, recent studies suggest that IL13 plays a major role in the generation of Th2 immune responses in the cutaneous microenvironment.18,24 Therefore, the various Th2 deficiencies of the host strains in our first case report likely provided conditions permissive to proliferation of this parasite and, in some cases, progression to pruritis and clinical disease.
In our first case, the most severely affected mice were deficient in IL13 as well as transgenic for DO11.10 expression. DO11.10 transgenic mice have a defective Th2 response, which may have further contributed to the phenotype observed in the double-mutant mice.38 Interestingly, this mutation alone was insufficient to maintain detectable mite burden in the transmission study described in the current report, although when combined with the IL13−/− mutation, the resulting phenotype was consistently more severe, both in terms of mite burden and clinical manifestation, than that of the other strains included in the study. Our second case involved NOD.Cg-PrkdcscidIl2rgtm1Wjl/SzJ (NSG) and C;129S4-Rag2tm1.1Flv Il2rgtm1.1Flv/J mice, which are characterized by severe generalized immunodeficiency. Not surprisingly, these mice were highly permissible to heavy mite burdens, and some mice developed associated clinical signs.
The fact that all recent reports of D. musculi in laboratory mice involve immunodeficient hosts suggests that, as with other Demodex species, immunocompetent mice can prevent these mites from attaining a population sufficient for detection by conventional means or to cause clinical disease. The manifestation of increased mite burden and, in some cases, dermatitis in various strains of genetically engineered mice lends itself to further investigation into the underlying mechanism of demodicosis in M. musculus and may provide opportunities to develop mouse models of human demodicosis. Although the role of the immune system in control of Demodex spp. appears to be multifaceted, our first case offers further evidence that impaired Th2 immunity alone is sufficient to render a host permissive to increased mite burden and, in some cases, to result in clinical disease. Our second case, involving mice with severe generalized immune deficiency, demonstrated that mite burden and severity of clinical manifestations may correlate with the degree of immune dysfunction.
In veterinary medicine, the treatment of Demodex spp. has been described most commonly in the context of canine generalized demodicosis, although demodicosis is becoming more frequently identified as a clinical problem in cats.52 A variety of treatment regimens have been tried, but the most successful treatment strategies consist of either biweekly amitraz rinses or daily oral administration of macrocyclic lactones (ivermectin, moxidectin, milbemycin oxime, and doramectin). Because secondary skin infections are common, treatment with systemic or topical antimicrobials (or both) is an important aspect of therapy.44,45 In cats, the treatment of choice for D. gatoi is a series of lime sulfur dips, whereas for D. cati, either lime sulfur dips or oral ivermectin is recommended.5,52 Reports in pet rodents and other companion small-mammal species are sparse, and amitraz is the most commonly reported treatment.11,13,23,35,46 The only published treatment regimen for Demodex mites in laboratory rodents is biweekly amitraz baths.39
Because amitraz successfully improved the clinical condition of the laboratory mice affected by D. musculi in a previous report,39 we chose this treatment method over other options we considered (for example, macrocyclic lactone therapy). However, we found a number of disadvantages to the amitraz method. First, precautions must be taken when handling amitraz because it is a health hazard, and exposure can occur through inhalation, ingestion, and absorption through the skin.15 In addition to human risk, there is the potential for the effects of amitraz to interfere with certain types of research studies. For example, amitraz has been shown to alter serum biochemical parameters in mice.17 In addition, the drug may cause transient sedation after treatment, and because amitraz can alter the ability to maintain homeostasis, the product insert contains a warning that animals should not be subjected to stress for at least 24 h after treatment. Logistically, implementation of this method on a large scale would be impractical due to the labor-intensive procedure of individually dipping all affected mice.
The main advantage we found to the amitraz treatment was that the mite burden was reduced to approximately half that of pretreatment burden. However, in these particular mice, clinical improvement was not observed. Taken together, we conclude that the amitraz treatment was neither an effective nor practical means of treating clinical cases of Demodex in this colony of affected mice. Worth noting is that we have subsequently switched to the use of either long-term oral moxidectin treatment provided in gel cups (MediGel Sucralose, Clear H2O, Westbrook, ME) at a concentration of 0.12 mg per cup to achieve a target dose of 0.4 mg/kg, gel cups preformulated with 8 ppm ivermectin (MediGel IVR, Clear H2O), or as medicated diet containing 12 ppm ivermectin (Teklad TD.130755, Harlan) for animals showing clinical signs and preemptively for breeder mice of lines known to be prone to developing clinical signs as they age. Although data regarding mite burden were not collected, clinical observations suggest that both ivermectin and moxidectin are effective in the prevention and amelioration of clinical signs attributed to D. musculi.
Recent reports of D. musculi in laboratory mice prompt the question of whether this mite, as with other Demodex species in their respective hosts, should be considered a commensal organism or an opportunistic pathogen that occasionally causes clinical disease in a small subset of immunocompromised hosts and whether it should be excluded from laboratory colonies. Like other organisms whose pathogenicity is similarly ambiguous, each institution must establish its level of tolerance for this organism. At our institution, we currently consider D. musculi a commensal organism that achieves increased burdens in many immunodeficient mouse strains and that may act as an opportunistic pathogen resulting in clinical disease in a small subset of these strains. We have attributed similar pathologic behavior to commensal bacteria in severely immunocompromised mice. As such, although we have attempted treatment and eradication in select colonies, we do not generally exclude Demodex from our facilities. This position, of course, is subject to change as we learn more about the behavior of this mite and its effects on health and research in laboratory mouse colonies
Acknowledgment
We thank Fu-Chen Yang for microbiologic support, including the morphologic identification of Demodex musculi, and Daniel Martin Escalante and Alison Faruolo for technical support and sample collection.
References
- 1.Akilov OE, Mumcuoglu KY. 2004. Immune response in demodicosis. J Eur Acad Dermatol Venereol 18:440–444. [DOI] [PubMed] [Google Scholar]
- 2.Badescu AC, Iancu LS, Statescu L. 2013. Demodex: commensal or pathogen? Rev Med Chir Soc Med Nat Iasi 117:189–193. [PubMed] [Google Scholar]
- 3.Baker DG.2007. Arthropods, p 568–569. In: Fox J, Barthold SW, Davisson MT, Newcmer CE, Quimby FW, Smith AL. The mouse in biomedical research, 2nd ed: vol. 2 DiseasesNew York (NY):Elsevier. [Google Scholar]
- 4.Baker DG. 2007. Parasites of rats and mice, p356–357 In: Flynn RJ, Baker DG, Flynn RJ. Flynn's parasites of laboratory animals, second ed Ames (IA): Blackwell Publishing. [Google Scholar]
- 5.Beale K. 2012. Feline demodicosis: a consideration in the itchy or overgrooming cat. J Feline Med Surg 14:209–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bukva V. 1985. Demodex flagellurus sp. n. (Acari: Demodicidae) from the preputial and clitoral glands of the house mouse, Mus musculus L. Folia Parasitol (Praha) 32:73–81, passim. [PubMed] [Google Scholar]
- 7.Bukva V. 1990. Transmission of Demodex flagellurus (Acari: Demodicidae) in the house mouse, Mus musculus, under laboratory conditions. Exp Appl Acarol 10:53–60. [DOI] [PubMed] [Google Scholar]
- 8.Bukva V. 1994. Demodex agrarii sp. n. (Acari: Demodecidae) from cerumen and the sebaceous glands in the ears of the striped field mouse, Apodemus agrarius (Rodentia). Folia Parasitol (Praha) 41:305–311. [PubMed] [Google Scholar]
- 9.Bukva V. 1995. Demodex species (Acari:Demodecidae) parasitizing the brown rat, Rattus norvegicus (Rodentia): redescription of Demodex ratti and description of D. norvegicus sp. n. and D. ratticola sp. n. Folia Parasitol (Praha) 42:149–160. [PubMed] [Google Scholar]
- 10.Byford R, Craig ME. 2007. Biology of arthropods, p 60 In: Baker DG. Flynn's parasites of laboratory animals. Ames (IA): Blackwell Publishing. [Google Scholar]
- 11.Capello V. 2002. Pet hamster medicine and surgery part III: infectious, parasitic and metabolic diseases. Exotic DVM 3:27–32. [Google Scholar]
- 12.Caswell JL, Yager JA, Barta JR, Parker W. 1996. Establishment of Demodex canis on canine skin engrafted onto scid-beige mice. J Parasitol 82:911–915. [PubMed] [Google Scholar]
- 13.Clyde VL. 1996. Practical treatment and control of common ectoparasites in exotic pets. Vet Med 91:632–637. [Google Scholar]
- 14.Desch CE., Jr 1987. Redescription of Demodex nanus (Acari: Demodicidae) from Rattus norvegicus and R. rattus (Rodentia). J Med Entomol 24:19–23. [DOI] [PubMed] [Google Scholar]
- 15.Elinav E, Shapira Y, Ofran Y, Hassin T, Ben-Dov IZ. 2005. Near-fatal amitraz intoxication: the overlooked pesticide. Basic Clin Pharmacol Toxicol 97:185–187. [DOI] [PubMed] [Google Scholar]
- 16.Ferrer L, Ravera I, Silbermayr K. 2014. Immunology and pathogenesis of canine demodicosis. Vet Dermatol 25:427–e65. [DOI] [PubMed] [Google Scholar]
- 17.Filazi A, Sireli M, Kalkan F. 2003. The influence of amitraz on biochemical parameters in mice. Hum Exp Toxicol 22:99–101. [DOI] [PubMed] [Google Scholar]
- 18.Finkelman FD, Urban JF., Jr 2001. The other side of the coin: The protective role of the TH2 cytokines. J Allergy Clin Immunol 107:772–780. [DOI] [PubMed] [Google Scholar]
- 19.Forton FMN. 2011. Papulopustular rosacea, skin immunity and Demodex: pityriasis folliculorum as a missing link. J Eur Acad Dermatol Venereol 26:19–28. [DOI] [PubMed] [Google Scholar]
- 20.Fryderyk S, Izdebska JN. 2001. Demodex phylloides (Acari, Demodecidae) as a specific parasite of Sus scrofa (Mammalia, Artiodactyla). Wiad Parazytol 47:797–800. [PubMed] [Google Scholar]
- 21.Bowman DD. 1990. Georgis’ parasitology for veterinarians, 5th ed Philadelphia (PA): WB Saunders. [Google Scholar]
- 22.Harkness JE, Wagner JE. 1989. Specific diseases and conditions: acariasis, p 111–115. The biology and medicine of rabbits and rodents, 3rd ed Philadelphia (PA): Lea and Febiger. [Google Scholar]
- 23.Hasegawa T. 1995. A case report of the management of demodicosis in the golden hamster. J Vet Med Sci 57:337–338. [DOI] [PubMed] [Google Scholar]
- 24.Herrick CA, Xu L, McKenzie ANJ, Tigelaar RE, Bottomly K. 2003. IL-13 is necessary, not simply sufficient, for epicutaneously induced Th2 responses to soluble protein antigen. J Immunol 170:2488–2495. [DOI] [PubMed] [Google Scholar]
- 25.Hill LR, Kille PS, Weiss DA, Craig TM, Coghlan LG. 1999. Demodex musculi in the skin of transgenic mice. Contemp Top Lab Anim Sci 38:13–18. [PubMed] [Google Scholar]
- 26.Hirst S. 1917. Remarks on certain species of the genus Demodex, Owen (the Demodex of man, the horse, dog, rat, and mouse). Annals and Magazine of Natural History: Series 8 20:233–235. [Google Scholar]
- 27.Izdebska JN. 2000. [Demodex ssp. (Acari, Demodecidae) in Mus musculus from Poland.] Wiad Parazytol 46:277–280.[Article in Polish]. [PubMed] [Google Scholar]
- 28.Izdebska JN. 2004. Demodex spp. (Acari: Demodecidae) in brown rat (Rodentia: Muridae) in Poland. Wiad Parazytol 50:333–335. [PubMed] [Google Scholar]
- 29.Izdebska JN, Cydzik K. 2010. Occurrence of demodex spp. (Acari, Demodecidae) in the striped field mouse Apodemus agrarius (Rodentia, Muridae) in Poland. Wiad Parazytol 56:59–61. [PubMed] [Google Scholar]
- 30.Izdebska JN. 2012. A new demodecidae species (Acari) from the yellow-necked mouse Apodemus flavicollis (Rodentia: Muridae)-description with data on parasitism. J Parasitol 98:1101–1104. [DOI] [PubMed] [Google Scholar]
- 31.Izdebska JN, Rolbiecki L. 2013. A new species of Demodex (Acari: Demodecidae) with data on topical specificity and topography of demodectic mites in the striped field mouse Apodemus agrarius (Rodentia: Muridae). J Med Entomol 50:1202–1207. [DOI] [PubMed] [Google Scholar]
- 32.Izdebska JN, Rolbiecki L. 2015. Two new species of Demodex (Acari: Demodecidae) with a redescription of Demodex musculi and data on parasitism in Mus musculus (Rodentia: Muridae).J Med Entomol 52:604–613. [DOI] [PubMed] [Google Scholar]
- 33.Izdebska JN, Rolbiecki L. 2015. New data on occurrence of Demodex flagellurus (Acari, Demodecidae) - rarely recorded parasite from the house mouse Mus musculus (Rodentia, Muridae). Ann Parasitol 61:37–41. [PubMed] [Google Scholar]
- 34.Izdebska JN, Rolbiecki L. 2015. A new species of the genus Demodex Owen, 1843 (Acari: Demodecidae) from the ear canals of the house mouse Mus musculus L. (Rodentia: Muridae). Syst Parasitol 91:167–173. [DOI] [PubMed] [Google Scholar]
- 35.Jekl V, Hauptman K, Jeklova E, Knotek Z. 2006. Demodicosis in 9 prairie dogs (Cynomys ludovicianus). Vet Dermatol 17:280–283. [DOI] [PubMed] [Google Scholar]
- 36.Karjala Z, Desch CE, Starost MF. 2005. First description of a new species of Demodex (Acari: Demodecidae) from rhesus monkey. J Med Entomol 42:948–952. [DOI] [PubMed] [Google Scholar]
- 37.Lacey N, Ní Raghallaigh S, Powell FC. 2011. Demodex mites - commensals, parasites or mutualistic organisms? Dermatology 222:128–130. [DOI] [PubMed] [Google Scholar]
- 38.Lemaire MM, Dumoutier L, Warnier G, Uyttenhove C, Van Snick J, de Heusch M, Stevens M, Renauld JC. 2011. Dual TCR expression biases lung inflammation in DO11.10 transgenic mice and promotes neutrophilia via microbiota-induced Th17 differentiation. J Immunol 187:3530–3537. [DOI] [PubMed] [Google Scholar]
- 39.Liu Q, Arseculeratne C, Liu Z, Whitmire J, Grusby MJ, Finkelman FD, Darling TN, Cheever AW, Swearengen J, Urban JF, Gause WC. 2004. Simultaneous deficiency in CD28 and STAT6 results in chronic ectoparasite-induced inflammatory skin disease. Infect Immun 72:3706–3715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lucas PJ, Negishi I, Nakayama KI, Fields LE, Loh DY. 1995. Naive CD28-deficient T cells can initiate but not sustain an in vitro antigen-specific immune response. J Immunol 154:5757–5768. [PubMed] [Google Scholar]
- 41.Lukoschus FS, Jongman RH. 1974. Demodex lacrimális spec. nov. (Demodicidae: Trombidiformes) from the Meibomian glands of the European wood mouse Apodemus sylváticus. Acarologia 16:274–281. [PubMed] [Google Scholar]
- 42.Maderson PFA. 2003. Mammalian skin evolution: a reevaluation. Exp Dermatol 12:233–236. [DOI] [PubMed] [Google Scholar]
- 43.McKenzie GJ, Bancroft A, Grencis RK, McKenzie ANJ. 1998. A distinct role for interleukin-13 in Th2-cell-mediated immune responses. Curr Biol 8:339–342. [DOI] [PubMed] [Google Scholar]
- 44.Mueller RS. 2012. An update on the therapy of canine demodicosis. Compend Contin Educ Vet 34:E1–E4. [PubMed] [Google Scholar]
- 45.Mueller RS, Bensignor E, Ferrer L, Holm B, Lemarie S, Paradis M, Shipstone MA. 2012. Treatment of demodicosis in dogs: 2011 clinical practice guidelines. Vet Dermatol 23:86–96. [DOI] [PubMed] [Google Scholar]
- 46.Noli C, van der Horst HHA, Willemse T. 1996. Demodicosis in ferrets (Mustela putorius furo). Vet Q 18:28–31. [DOI] [PubMed] [Google Scholar]
- 47.Nutting WB, Satterfield LC, Cosgrove GE. 1973. Demodex sp. infesting tongue, esophagus, and oral cavity of Onychomys leucogaster, the grasshopper mouse. J Parasitol 59:893–896. [PubMed] [Google Scholar]
- 48.Rather PA, Hassan I. 2014. Human demodex mite: the versatile mite of dermatological importance. Indian J Dermatol 59:60–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ravera I, Altet L, Francino O, Sánchez A, Roldán W, Villanueva S, Bardagí M, Ferrer L. 2013. Small demodex populations colonize most parts of the skin of healthy dogs. Vet Dermatol 24:168–172.e37. [DOI] [PubMed] [Google Scholar]
- 50.Szabo SJ, Kim ST, Costa GL, Zhang X, Fathman CG, Glimcher LH. 2000. A novel transcription factor, T-bet, directs Th1 lineage commitment. Cell 100:655–669. [DOI] [PubMed] [Google Scholar]
- 51.Tani K, Une S, Hasegawa A, Adachi M, Kanda N, Watanabe SI, Nakaichi M, Taura Y. 2005. Infestivity of demodex canis to hamster skin engrafted onto scid mice. J Vet Med Sci 67:445–448. [DOI] [PubMed] [Google Scholar]
- 52.Tater KC, Patterson AP. 2008. Canine and feline demodicosis. Vet Med 103:444–461. [Google Scholar]