Abstract
Mitral regurgitation (MR) is a common heart-valve lesion after myocardial infarction in humans. Because it is considered a risk factor for accelerated heart failure and death, various surgical approaches and catheter-based devices to correct it are in development. Lack of a reproducible animal model of MR after myocardial infarction and reliable techniques to perform open-heart surgery in these diseased models led to the use of healthy animals to test new devices. Thus, most devices that are deemed safe in healthy animals have shown poor results in human efficacy studies, hampering progress in this area of research. Here we report our experience with a swine model of postinfarction MR, describe techniques to induce regurgitation and perform open-heart surgery in these diseased animals, and discuss our outcomes, complications, and solutions.
Abbreviations: CPB, cardiopulmonary bypass; MI, myocardial Infarction; MR, mitral regurgitation; MV, mitral valve; PMPM, posteromedial papillary muscle
Mitral regurgitation (MR) developing secondary to a myocardial infarction (MI) is associated with accelerated heart failure and death, yet conclusive evidence that its timely repair can benefit patients’ quality of life and cardiac function remains unavailable.17,28 Mortality with uncorrected MR is high, with 35% of patients alive at 5 y with severe MR, 44% alive with moderate MR, and 61% alive without MR;10 yet at 1 y, repair of MR conferred no significant survival benefit.29 Divergent findings in these patients warrant careful mechanistic investigations into cardiac function and remodeling after repair of the mitral valve (MV). Human clinical trials do not provide the vehicle for such mechanistic insights, due to interpatient variability in their history, disease processes, infarct size, frailty, and comorbidities.4,5 Preclinical animal models that mimic human disease play an important role in developing mechanistic insights, guide appropriate selection of patients for clinical trials, and help translate new therapies and devices toward clinical application. The development of new MV surgical and transcatheter approaches urgently requires a reproducible animal model of functional MR that accommodates surgical repair techniques that mimic clinical reality as closely as possible, in light of the covariates induced by cardiac surgery, such as trauma, myocardial stunning, ischemia–reperfusion injury, and perioperative complications of surgery.
Some animal models of postinfarction functional MR have previously been reported; in these models, surgical ligation of the obtuse marginal 2 and obtuse marginal 3 arteries is the most common.18 Left ventricular dilatation and contractile dysfunction occur over an 8-wk period, but reproducibility of MR is poor.18 We recently reported that, in humans, infarction of the posteromedial papillary muscle and the underlying myocardium is essential to perturb the interpapillary muscle dynamics and cause functional MR.14 Targeted infarction of this region in swine caused functional MR.14 Targeted infarction in surgical models is challenging, and imaging-guided interventions that map the perfusion patterns to the MV and left ventricular complex are necessary. Surgical repair of MR, using cardiac arrest and support from cardiopulmonary bypass (CPB), is the procedure of choice in human patients with functional MR. However, CPB is challenging to perform in animal models, given that myocardial protection strategies for the ischemic and failing ventricle and protocols for perioperative surgical critical care have not been well defined. One group circumvented this limitation by using an extracardiac ventriculo–atrial shunt to mimic MR2,23,31 and its repair without the need for open surgery. However, exacerbated volume overload from an open conduit and little relevance to clinical surgery with suspended animation limits the model's capacity for translational research. Another group achieved a different approach by implanting an annuloplasty ring in normal sheep before inducing ischemic cardiomyopathy and subsequently inducing the infarction to mimic ventricular remodeling11—but this technique has little relevance to the clinical setting.
Clinical interest in treating postinfarction MR continues to increase, and an appropriate translational platform to investigate disease mechanisms and new therapies in a randomized fashion (as other fields17) is needed urgently. Here we report our experience with a novel, minimally invasive swine model of postinfarction MR and the techniques to perform surgical mitral repair after the onset of heart failure equivalent to that in human patients. We describe stepwise protocols for anesthesia, cardioplegic arrest of the heart, MV access route for repair, postoperative reanimation of the heart, and postsurgical recovery. Short- and long-term survival, the complications associated with each mortality, and the measures adopted to adequately prepare for these events are reported.
Materials and Methods
Animal selection.
The IACUC-approved procedures were performed at an AAALAC-accredited facility. Female Yorkshire farm swine (weight, 38.0± 5.1 kg; n = 26) were acquired from USDA-approved vendors (Palmetto Research Swine, Reevesville, SC, or Valley Brook Research, Madison, GA) where swine were bred for research purposes in a closed colony. The health status of each pig was described by the vendor to be SPF, that is, pigs were raised free of diseases that interfere with weight gain and production efficiency. On their arrival at our facility, the pigs were pair-housed in quarantine and received vaccinations against Pasturella spp. (atrophic rhinitis), Bordetella spp. (atrophic rhinits), Actinobacillus spp., Haemophilus spp., and Erysiplethrix rhusiopathiae (Parapleuro Shield P+BE; Novartis, Basel, Switzerland); swine influenza H1N1, H1N2, and H2N2 and Mycoplasma hyopneumonieae (FluSure XP and Respisure, Zoetis Animal Health, Florham Park, NJ); porcine circovirus (Ingelvac CircoFLEX, Boehringer Ingleheim Vetmedica, St Joseph, MO); porcine reproductive and respiratory syndrome (Ingelvac PRRS MLV, Boehringer Ingleheim Vetmedica); and Salmonella choleraesuis (Salmo Shield Live, Novartis). Swine were maintained in quarantine for 5 d until stress from transportation was reduced and they were acclimated to their new surroundings and veterinary staff. Personnel with experience in cardiothoracic surgery and animal care performed the procedures. Swine were pair-housed prior to surgery but individually caged after the first procedure. All animals were allowed continuous access to drinking water and were fed daily with standard chow containing a minimum of 14% crude protein, 3% crude fat, 0.6% calcium, 0.65% phosphorus, 0.3% salt, and 166 ppm zinc (catalog no. 61114, 14% Hog Finisher, Tucker Milling, Guntersville, AL).
Myocardial infarction procedure.
Preoperative preparation.
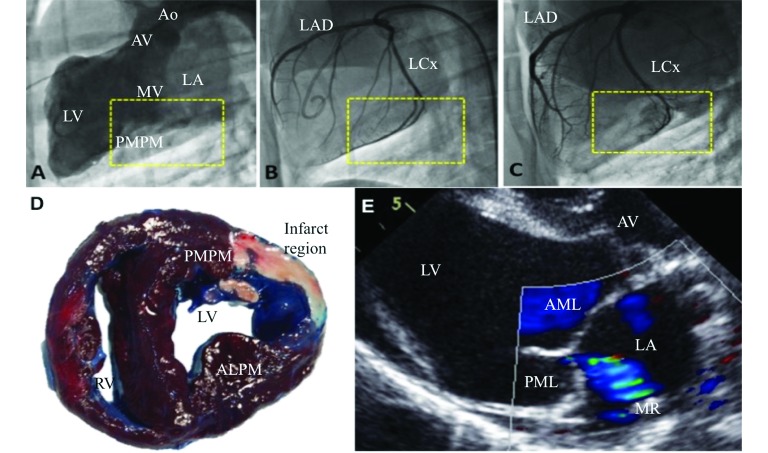
Beginning 3 d prior to the infarction procedure, swine received amiodarone (600 mg PO daily; aspirin (81 mg PO daily), and clopidogrel (75 mg PO daily; Plavix, Bristol–Myers Squibb, New York, NY; Figure 1). Pigs were fasted overnight to reduce the weight of the gastrointestinal tract, prevent reflux, and minimize compression of abdominal organs on the diaphragm. Access to water was unrestricted, although other authors have suggested restriction of water for 8 to 12 h.8 The animals were sedated with ketamine (22 mg/kg IM), acepromazine (1.1 mg/kg IM), and atropine (0.05 mg/kg IM), by kneeling by the animal to reduce stress. Other drug combinations have been used in literature,3,8,12,21,32 but we chose this combination on the basis of our past experience with swine.14 The pig was further anesthetized with isoflurane by mask, transported to the prep area in sternal recumbency, and intubated. A long-blade (at least 20 to 22 cm) laryngoscope was used to visualize the epiglottis, and an appropriately sized endotracheal tube (inner diameter, typically 8 or 9 mm) with an inflatable cuff was inserted into the trachea. An auricular vein was accessed and cannulated with an 18-gauge catheter (catalog number 381706, BD Angiocath Autoguard Shielded IV Catheter, Becton Dickinson, Franklin Lakes, NJ) and wrapped with veterinary tape around the ear, for administration of intravenous drugs. Use of sedatives in swine can cause excessive salivation and laryngeal secretions, which can be countered with glycopyrrolate (0.004 mg/kg IV), an anticholinergic agent; although cardiac arrhythmias, gastric stasis, and ileus can occur with the use of glycopyrrolate, no such side effects occurred in this study. Each pig was positive-pressure–ventilated (8 to 12 breaths per minute; tidal volume, 10 mL/kg; peak inpiratory pressure, 18 to 22 cm H2O, and peak expiratory pressure, 3 to 5 cm H2O) and received 1% to 2% isoflurane in 100% oxygen to ensure a deep plane of anesthesia. When additional anesthetic support was necessary for induction, propofol (2 to 4 mg/kg IV) was administered. The pig was then transported to the cardiac catheterization laboratory and secured in dorsal recumbency.
Figure 1.
Drug regimen for the myocardial infarction procedure in swine.
Cardiac catheterization and MI.
Three-lead dermal EKG electrodes (Kendall Medi-Trace 530 Foam ECG Electrode, Covidien, Dublin, Ireland) were applied on the skin of the right and left front legs and the left rear leg. A single dose of cefazolin (22 to 25 mg/kg IV) and carprofen (3 mg/kg IV) was administered. Carprofen is a noncontrolled NSAID that offers a maximum of 24 h of analgesic effects; other drugs including phenylbutazone, enteric-coated aspirin, ketoprofen, ketorolac, flunixine, and meloxicam are viable alternatives.27,34-36 The groin and neck were shaved, cleaned with ethanol and povidone–iodine, and sterile-draped. By using aseptic techniques, the right femoral and carotid arteries were exposed, and arterial sheaths (8 to 9 French; Super Sheath, Boston Scientific, Natick, MA) were inserted for catheterization according to the Seldinger technique.25 Complete percutaneous access to the femoral vessels is less invasive than surgical exposure, but we exposed the vessels to ensure complete closure and prevent the risk of bleeding. A 6-French pigtail catheter (Site Seer, Medtronic, Minneapolis, MN) was inserted through the right carotid artery and advanced into the left ventricle for a ventriculography, and a 7-French ‘hockey stick’ catheter (Vista Brite Tip, Cordis, Miami Lakes, FL) was inserted into the right femoral artery and positioned at the ostium of the left main coronary artery. A slow drip of amiodarone (8 mg/kg IV) was administered to avoid arrhythmias, after which baseline transthoracic and transesophageal cardiac echocardiograms were obtained.
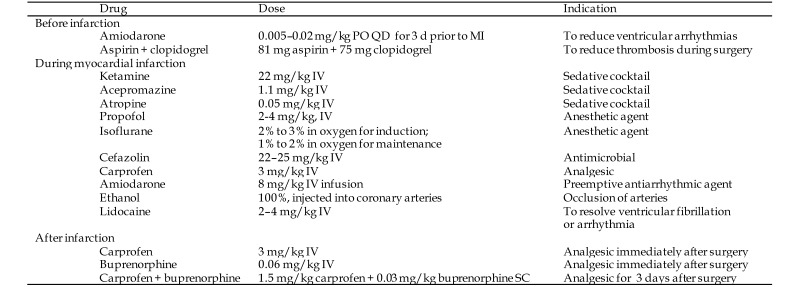
Under fluoroscopic imaging (GE Innova, GE Medical, Fairfield, CT), the location of the posteromedial papillary muscle (PMPM) was identified by injecting contrast simultaneously into the left ventricle and into the coronary arteries (Figure 2). The typical camera angle was 65 to 70° left anterio oblique and 5 to 8o caudally to visualize the PMPM and the lateral myocardial wall. A balloon was inflated in the identified artery to isolate the vascular bed to the PMPM and the underlying myocardium, and 100% ethanol (200 proof; 1 to 2 mL; Sigma, St Louis, MO) was injected, followed by 1 mL saline to flush the catheter into the coronary artery. Elevation of the ST segment was observed in all animals, and the balloon was deflated after 15 min and coronary angiography repeated to verify occlusion. When the occlusion was incomplete, additional 0.5-mL doses of ethanol were injected and angiography repeated as needed. On average, 2 or 3 arterial branches were occluded to create the PMPM infarction and MR (Figure 2).14 Ventricular wall dyskinesia, MR, and ejection fraction were measured from the ventriculogram, and postoperative transthoracic and transesophageal echocardiography confirmed mitral valvar perturbations.
Figure 2.
(A–C) Fluoroscopic images of the heart with the camera in LAO 65-70 degrees and CAU 6-8 degrees. In (A), the yellow inset indicates the location of the posteromedial papillary muscle as visualized on a ventriculogram. In (B), the yellow inset indicates the branches of the left circumflex artery that perfuse the identified papillary muscle. In (C), the inset depicts complete occlusion of the identified coronary branches. (D) A representative myocardial infarct in a swine after explantation of the heart at the end of the study. (E) Representative mitral regurgitation visualized on color Doppler imaging. AML, anterior mitral leaflet; AMPM, anterolateral papillary muscle; Ao, aorta; AV, aortic valve; LA, left atrium; LAD, left anterior descending artery; LCx, left circumflex artery; LV, left ventricle; MR, mitral regurgitation; MV, mitral valve; PML, posterior mitral leaflet; PMPM, posteromedial papillary muscle; RV, right ventricle.
The catheters were removed, the vessels were ligated, and groin and neck arteriotomies were closed with 3-0 poly-glycolic acid suture for the muscle and subcutaneous layers, and 2-0 polydioxanone suture (Ethicon, Somerville, NJ) for the skin. Isoflurane was discontinued, and the pig was ventilated until spontaneous breathing was observed, after which ventilation was halted and the animal transported to the intensive care unit. The pig was placed in sternal recumbency, leaning against the wall of the kennel, because pigs demonstrate better recovery when prone. Tissue oxygenation and heart rate were measured for 1 to 2 h (PalmSAT 2500, Portable Handheld Pulse Oximeter, Nonin, Plymouth, MN), and nasal oxygen was provided when SpO2 was less than 90%. When labored breathing was observed, furosemide (Lasix, Validus Pharmaceuticals, Parsippany, NJ) was given as necessary to clear pulmonary edema. Once ambulatory, the swine was transported to its pen, and a single dose of buprenorphine (0.06 mg/kg IM) was administered. Buprenorphine is an excellent long-term analgesic that does not cause significant respiratory depression after the perioperative period; fentanyl has been reported for analgesia but led to relatively poor outcomes in our laboratory.8 The pig was provided food and water and activity was monitored carefully until 6 to 12 h after surgery. Thereafter, daily observations were performed, with the body weight, activity, feces and urine, dryness of the wound sites, pain and anxiety, rectal temperature, and food intake noted. Chest auscultation and abdominal palpation were performed to assess cardiac and pulmonary function.
CPB procedure.
Preoperative preparation.
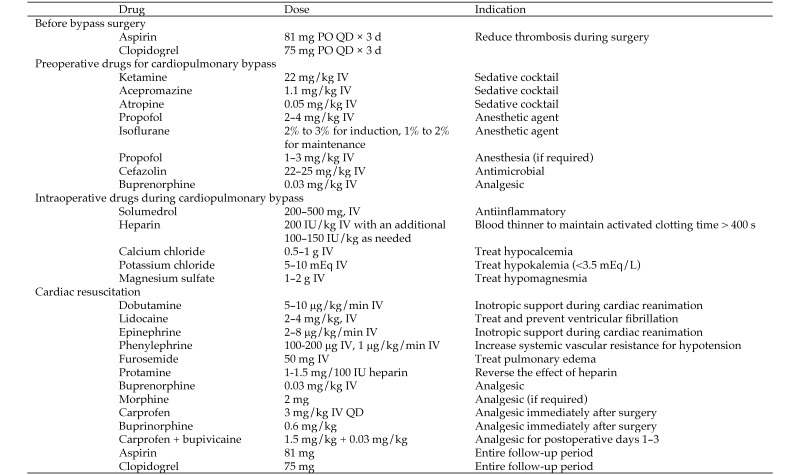
At 7 to 10 wk after MI, mitral valve repair was performed in the surviving swine with CPB support. Animals were pretreated with aspirin (81 mg PO) and clopidogrel (75 mg PO; Plavix, Bristol–Myers Squibb) daily for 3 d prior to the surgery day (Figure 3). Swine were fasted overnight but had access to drinking water. On the day of surgery, swine received methylprednisolone sodium succinate (200 to 500 mg IV, Solumedrol, Pfizer, New York, NY) to reduce systemic inflammatory response and then were sedated according to the same protocol as used for infarction. Once anesthetized, the pig was moved to the operating room, secured in right lateral recumbency, and draped sterilely; a rectal temperature probe was inserted, and the grounding pad for electro cautery was attached to the chest. An auricular venous line (18-gauge, BD Angiocath, Becton Dickinson) was used for fluid administration, and single doses of cefazolin (22 to 25 mg/kg IV) and carprofen (3 mg/kg IV) were administered. The EKG was monitored by using a 3-lead dermal probe.
Figure 3.
Drugs used in swine with mitral regurgitation and heart failure for cardiopulmonary bypass and mitral valve repair.
By using aseptic technique, either the left carotid or femoral artery was exposed, and a high-fidelity blood pressure measurement catheter (Millar Instruments, Houston, TX) was advanced into the aortic arch or descending aorta. Femoral access was deemed a better choice, because carotid access positioned the tip of the transducer at the exit of the arterial cannula, where flow stagnation and high jet velocities are common. The central venous pressure was measured by using a catheter advanced through the external jugular vein or femoral vein. Arterial blood sampling was performed, and acid–base balance, Hct, electrolyte concentrations, Hgb concentration, ionic balance, and activated clotting time were recorded. The CPB circuit length was shortened and primed with 1000 to 1200 mL of Plasma-Lyte (Baxter Healthcare, Deerfield, IL) or Normosol (Hospira, Lake Forest, IL). Systemic heparinization was achieved through intravenous administration of heparin (loading dose, 200 IU/kg; additional doses of 100 to 200 IU/kg as required) to prevent thrombosis and maintain the activated clotting time at greater than 400 s.
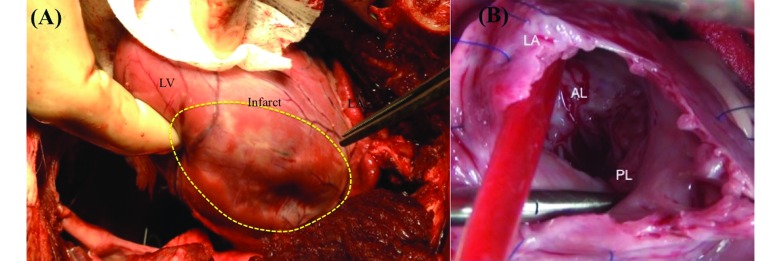
A left thoracotomy through the fourth intercostal space was performed to expose the heart, and a pericardial cradle was made to expose the cardiac structures sufficiently (Figure 4). Although median sternotomy has been recommended by others, we find that it reduces access to the left atrium.15 In addition, being quadrupeds, swine experience severe pain from sternotomy, which hampers their recovery. Baseline transesophageal and direct epicardial echocardiography was performed to confirm MR and measure the left ventricular ejection fraction and volumes. Cannulation was performed sequentially, with aortic cannulation in the midarch by using a 21-French right-angled cannula (EZ Glide, Edwards Lifesciences, Irvine, CA) performed first. The fatty tissue surrounding the aortic arch was carefully dissected, and a pursestring suture (3-0 Ethibond suture with pledgets, Ethicon) was placed for arterial cannulation. The cannula was connected to the CPB circuit and was left clamped. Other cannulation strategies, such as of the descending aorta or carotid artery can be used, but direct aortic arch cannulation similar to that in humans is feasible in swine. For venous cannulation, a purse string suture was placed on the right atrial appendage, and a 29- to 37-French 2-stage venous cannula (Medtronic, Minneapolis, MN) was inserted, connected to the bypass circuit, and clamped. Individual caval cannulation can be performed also but was deemed unnecessary in our study.
Figure 4.
(A) Exposure of the heart by using left thoracotomy and a pericardial cradle, depicting the left ventricular wall with a myocardial infarction (area enclosed by the yellow dotted line). To the right of the dotted area is the left atrium. (B) Exposure of the mitral valve after left atriotomy, exposing the anterior and posterior mitral valve leaflets. AL, anterior mitral leaflet; LA, left atrium; LV, left ventricle; PL, posterior mitral leaflet.
A standard CPB circuit was used with a pediatric oxygenator (catalog no. 050713, Inspire 6 LPM, Sorin, Arvada, CO; catalog no. 1CXFX15RE40, Capiox, Terumo, Tokyo, Japan). A cardioplegia catheter (12-gauge DLP catheter, Medtronic) with a root vent was inserted into the ascending aorta and connected to a specialized cardioplegia delivery system (MPS2 myocardial Protection System, Quest Medical, Allen, TX) that enabled flow- and temperature-controlled delivery of the cardioplegic solution. A hemoconcentrator (Hemocor HPH 400, Minntech Therapeutic Technologies, Minneapolis, MN) was included in the circuit to reduce hemodilution. CPB was initiated gradually, and appropriate right-ventricular emptying was achieved. Positive-pressure ventilation was stopped when full CPB support was achieved and isoflurane was introduced into the oxygenator. The mean arterial pressure decreased acutely after the induction of CPB and was countered by increasing the pump speed and providing a continuous drip of phenylephrine (2 to 3 μg/kg/min) to maintain a mean arterial pressure of 40 to 45 mm Hg. Balancing the pump flow rate along with phenylephrine-induced vasoconstriction increased the mean arterial pressure sufficiently. The activated clotting time and Hct were measured every 30 min during CPB; once the pump parameters were adjusted appropriately, blood was cooled to 34 °C.
At full CPB flow (2 to 3 L/min), the ascending aorta was cross-clamped, and adenocaine cardioplegic solution24 was injected into the aortic root. Dilatation of the aortic root and coronary arteries was monitored to ensure adequate perfusion of cardioplegic solution into the myocardium; when aortic regurgitation occurred, the catheter was repositioned gently. Ice-cold saline slush was applied topically to the heart to further reduce myocardial metabolism. A vent and a suction line were established to drain blood from the surgical field into the venous reservoir and to avoid the need for transfusions. A vacuum line was used when necessary, to increase venous return.
Mitral valvar repair.
The MV was exposed through left atriotomy, on the roof of the left atrium. A vent was placed in the pulmonary vein to create a bloodless field and was shifted into the left ventricle whenever necessary. MV leaflets were exposed and examined (Figure 4), and the site of MR was confirmed by distending the ventricle with saline to identify the site of the regurgitation. The MV was repaired in various ways: annular repair by implanting a ring and fixing it with 2-0 Ethibond (Ethicon) suture (n = 7); papillary muscle approximation by using pledgeted 2-0 Prolene (Ethicon) suture (n = 6); and combination of both techniques in the remaining animals (n = 12; Figure 5). These different repairs were performed to assess whether annular and subannular techniques were feasible and to determine the complexity of the surgery and associated survival. Antegrade cardioplegic solution was redosed every 20 min or when myocardial activity occurred.
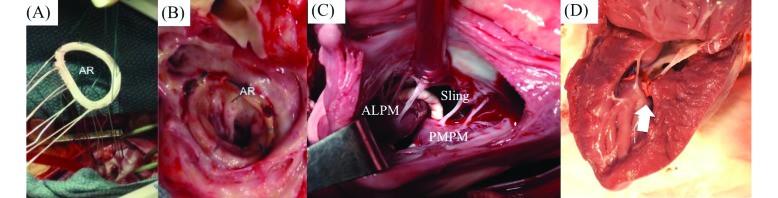
Figure 5.
(A) Implantation of a standard annuloplasty ring onto the mitral annulus by using sutures around the circumference of the mitral annulus. (B) Example of a mitral annuloplasty ring at 3 mo after implantation in the pig. (C) A papillary muscle approximating sling procedure, where the papillary muscles are accessed through the left atrium, and an expanded polytetrafluoroethylene loop is placed around the muscles to draw them together. The photograph was taken from a left atriotomy. (D) A different approach to papillary muscle approximation, where the tips of the muscles were drawn together by using a pledgeted suture. The photograph depicts the approximation of the 2 heads at 3 mo after the procedure was performed. ALPM, anterolateral papillary muscle; AR, annuloplasty ring; PMPM, posteromedial papillary muscle; sling, expanded polytetrafluoroethylene sling.
Once the repair was completed, reduction of MR was confirmed with a saline test, and the left atriotomy was closed using 4-0 Prolene (Ethicon) suture, first in a horizontal mattress pattern and then as a running suture. To avoid embolic stroke, adequate de-airing of the left atrium was achieved before closure, and a warm bolus of cardioplegic solution that was free of K+ was administered to prepare the heart for reanimation, followed by warm blood only. In most pigs, normal sinus rhythm was established without the need for cardioversion, but in the animals that fibrillated during reanimation, cardioversion was performed by using epicardial paddles at 30 J. In our experience, the final bolus of warm cardioplegic solution was essential to provide sufficient circulating lidocaine to reduce ventricular fibrillation during reanimation. The aortic cross-clamp was removed, and the pig was warmed and placed on a slow drip of dobutamine (5 to 10 μg/kg/min) for inotropic support. Maintaining ionic balance is of utmost importance at this stage. Calcium determines myocardial contractility, and hypocalcemia was treated with supplemental calcium chloride (0.5 g) or calcium gluconate injected directly into the bypass circuit.
Once adequate cardiac contractility and output were established, the pig was weaned off CPB by gradually clamping the arterial inflow and venous outflow from the bypass circuits. Ventilation was restarted, and the lungs were mechanically inflated to clear lung atelectasis and improve oxygenation. The cannulae were removed, and sufficient protamine was administered to reverse the heparin dose, and all incisions were examined for hemostasis. Protamine could cause anaphylaxis and acute pulmonary vasoconstriction and hypertension in swine, but we did not encounter these side effects in this study. The remaining blood in the bypass circuit was pumped into a heparinized blood bag for intravenous infusion. This practice helped to maintain an adequate Hct level and to avoid transfusions of donor blood. A chest tube was placed into the thoracic cavity and connected to a chest drainage system (Pleur-evac S-1100-08LF, Teleflex, Morrisville, NC) to enable negative-pressure evacuation of effusions. The thoracotomy was closed with 1-0 polyglycolic acid suture in interrupted pattern of figure-8 sutures, the muscle was closed in layers, and the skin closed with absorbable polydioxanone sutures. During skin closure, an additional dose of both cefazolin (22 to 25 mg/kg IV) and carprofen (3 mg/kg IV) was administered, and a final arterial blood gas measurement was performed. All exposed vessels were closed, and all catheters were removed, except for the auricular venous catheter. The chest tube was removed once the perfusate from the chest was minimal, and the incision was closed.
Postoperative care.
The isoflurane concentration and ventilation rate were reduced gradually; at the onset of spontaneous breathing, mechanical ventilation was ceased, and the pig was moved to the postoperative recovery area. The pig was secured in sternal recumbency, and tissue oxygenation and heart rate were monitored. Rectal temperature was monitored, and the pig was covered in warming blankets when necessary to maintain normal body temperature. In addition, 100% oxygen was provided by facemask when necessary. Maintaining an intravenous line is essential throughout this period, to quantify ionic balance and supplement with ionized elements as necessary. Once the pig was standing and eating, buprenorphine (0.03 mg/kg IM) was given for analgesia. All pigs received buprenorphine and carprofen for 3 to 5 d after surgery, and additional doses were used whenever a pig demonstrated signs of pain.
Cardiac imaging.
Echocardiographic imaging of the MV and left ventricle was performed at baseline, after MI, prior to surgery, after surgery, and at the time of euthanasia. The MR fraction was obtained by measuring the ratio of the regurgitant jet area to the left atrial area. Ventricular function was measured from M-mode echo imaging, and ejection fraction, end-diastolic volume, and end-systolic volume were calculated at each time point.
Statistical analysis.
All statistical analyses were performed by using SPSS 21 software (IBM, Armonk, NY). Data are expressed as mean ± 1 SD, and a P value less than 0.05 was considered to be statistically significant.
Results
MR model outcomes.
The weight (mean ± 1 SD) of the 26 pigs at the time of MI was 38.0 ± 5.1 kg. Acute survival (24 h after the MI) was 100% but decreased to 96% by the time of CPB. One pig died on day 10 after infarction, possibly from a fatal arrhythmia, given that findings from necropsy were unremarkable, but the exact cause of death remains unknown. At the time of infarction, complete occlusion of the targeted vessels was possible in all pigs, with delayed filling of contrast in the occluded vessels in 2 animals. Acute ST segment elevation was evident on EKG after coronary occlusion, and by the time of bypass, T-wave inversion was seen. Representative EKG imaging of a pig before infarction, acutely after infarction, and after several weeks after infarction is shown in Figure 6. The MR index (regurgitant jet area to left atrial area; mean ± 1 SD) at baseline was 13% ± 4%, which increased to 27% ± 3% by the time of CPB and fell to 0% after surgery. The ejection fraction was 55% ± 7% at baseline and 34% ± 4% prior to MV surgery. The end-diastolic volume was 64 ± 13 mL at baseline and increased to 159 ± 39 mL at the time of surgery. The end-systolic volume was was 30 ± 7 mL at baseline and increased to 99 ± 24 mL by the time of surgery. In summary, we successfully established a human-like model of postinfarction MR with poor ejection fraction in swine.
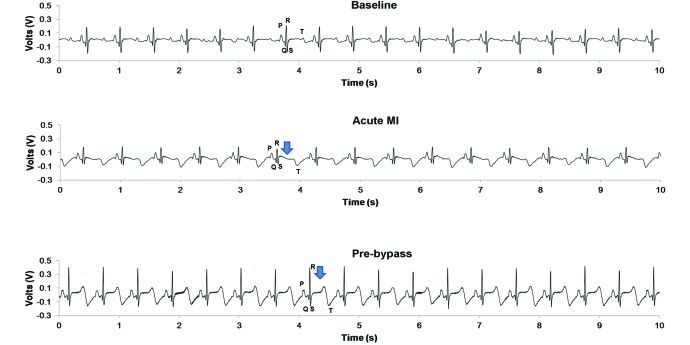
Figure 6.
EKG characteristics before and after various interventions. The top EKG trace is a normal baseline trace in a pig prior to any interventions, where the PQRST waves are vivid. The middle EKG trace corresponds to postmyocardial infarction, where T-wave inversion and ST elevation are visible. The bottom EKG trace was recorded several weeks after infarction, while the pig was anesthetized for mitral repair, and shows persistent T-wave inversion and ST elevation.
CPB outcomes.
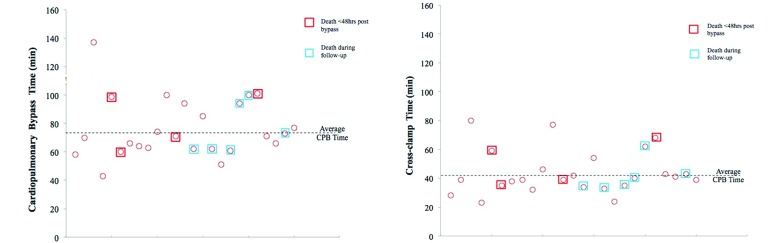
Pigs weighed an average of 60.7 ± 8.6 kg (n = 25) at the time of CPB. The activated clotting time was 133 ± 13 s at baseline and 1242 ± 260 s after heparinization. The highest activated clotting time was achieved during CPB. The mean CPB time was 76 ± 21 min, and the mean cross-clamp time was 44 ± 15 min. An average 1744 ± 911 mL of cardioplegic solution was used, and the total volume of Plasma-lyte or Normosol during bypass was 1350 ± 631 mL. Hemodynamic parameters at baseline, before CPB, and after surgery are reported in Table 1, and the distribution of cardioplegia times and cross-clamp times for all pigs relative to their survival in the study is shown in Figure 7. Heart rate did not differ significantly between the different time points. Systolic, diastolic, and mean blood pressures were comparable between preinfarction, postinfarction, and preCPB time points but were significantly different from those after CPB (P < 0.05). Both systolic and diastolic blood pressures were lower after CPB than before, possibly due to the pain-reducing drugs used in the perioperative period. Of the 25 pigs that underwent surgery, 3 died immediately after surgery (12% perioperative mortality rate), another animal died the first week after surgery (4% acute postoperative mortality), and an additional 6 pigs died between 30 to 90 d after surgery (28% chronic postoperative mortality). Therefore, 15 pigs survived the entire 90-d study period. Of the 3 pigs that died during the perioperative period, necropsy revealed cerebral hemorrhage in 2 pigs, and the third pig could not be revived from CPB. During the first week after surgery, one pig appeared depressed with severe leg pain and was thus immobile; this animal was euthanized according to IACUC guidelines, and its death was recorded as a mortality of the procedure. Of the 4 pigs that died during the first 40-d after CPB, 2 were found dead in their cages (postoperative days 30 and 33); the third animal was euthanized on postoperative day 30 due to poor health, loss of appetite, and weakness; and the remaining pig developed severe pain and high fever (on postoperative day 30) due to ingestion of a foreign object, which caused bowel perforation, and was euthanized. The mean survival time after MV repair was 61.2 ± 7.5 d, with an 84% acute survival rate and 86% survival at 5 wk after CPB.
Table 1.
Invasive hemodynamics (mean ± 1 SD) measured
| Before MI | After MI | Before bypass | After bypass | At termination | |
| Heart rate (bpm) | 86.6 ± 13.9 | 84.0 ± 12.6 | 90.3 ± 14.4 | 102.6 ± 18.2 | 92.7 ± 29.1 |
| Peak systolic blood pressure (mm Hg) | 95.6 ± 14.0 | 90.7 ± 14.3 | 101.8 ± 14.4 | 82.5 ± 12.5 | 91.3 ± 15.6 |
| Peak diastolic blood pressure (mm Hg) | 67.0 ± 12.5 | 61.7 ± 14.5 | 66.8 ± 12.9 | 49.6 ± 8.0 | 63.1 ± 11.2 |
| Mean arterial pressure (mm Hg) | 86.1 ± 13.3 | 81.0 ± 14.2 | 90.1 ± 13.2 | 71.5 ± 9.6 | 81.7 ± 13.7 |
Figure 7.
(Left) Distribution of total cardiopulmonary bypass time. Red indicates animals that died acutely; blue indicates pigs that died during the follow-up period. (Right) Distribution of the aortic crossclamp times.
Discussion
We here report our ability to use a transcatheter approach to induce MI and postinfarction MR, perform open-heart surgical repair of the MV at several weeks after infarction and onset of heart failure, and conduct cardiac echocardiography to assess cardiac function in pigs. The influence of timely repair of MR in halting adverse left ventricular remodeling and the molecular momentum toward congestive heart failure is a topic of significant clinical controversy.1,2,9,19,22,26,29 Although human trials continue to provide some insights into the pathologic processes, differences between patients regarding their clinical history, infarct size, timing of MV repair, and genetic predispositions introduce multiple covariates into the investigations. The need for highly controlled preclinical trials of mitral repair in which these factors can be controlled precisely are necessary. In addition, such a model would provide a reliable platform for testing the efficacy of novel therapies in treating MR and heart failure in this setting and potentially would guide correct selection of patients for a given therapy. The complexity of performing cardiac surgery in animal models of heart failure has caused most research studies to be performed in healthy animals or as acute studies in swine with heart failure swine. To date, only one group, using a sheep model of MI, has reported the ability to perform MV repair with an acute success rate of 54.8% and poor chronic survival, with the major mortality associated with the stress of CPB and postoperative pericardial effusion.20 Here, in our swine model, we report a 96% survival after infarction, an 88% acute survival rate, and an 84% chronic survival rate at 30-d after infarction. The ability to conduct cardiac echocardiography in these sick animals is a step forward in assessing cardiac mechanics, ventricular remodeling, and cardiac function.
Sheep (Ovis aries) have been widely used as the model of choice for CPB procedures, because of their historical use in cardiovascular research and because of their suitability to investigate pathways involved in pediatric heart-valve calcification.8 However, in our experience, the 2 primary drawbacks of sheep are the risk of pulmonary failure in the perioperative period after CPB and the lack of adequate imaging windows for transthoracic imaging. Being ruminants, sheep have large stomachs, which preclude adequate transgastric or transthoracic imaging windows for the heart. In contrast, swine make an excellent model for cardiac preclinical studies due to the similarities between porcine and human cardiac anatomy and the cardiac echocardiographic windows available for imaging because of their smaller stomach. Postinfarction myocardial remodeling has been well studied in swine, and the feasibility of testing therapies to improve cardiac function increases the translational potential of the studies.13 Finally, swine are easier to handle in a research laboratory and do not develop stress from individual housing, compared with sheep, which are herd animals that become stressed when separated from their herdmates.
MR is diagnosed often in patients who have survived acute MI or chronic coronary artery disease. We recently reported that, in humans, the risk of developing MR is increased in patients with targeted infarction of the lateral myocardial wall involving the PMPM.14 A minimally invasive approach to induce MR has several benefits: small incisions reduce the chance of infection, pain, and complications; mapping the coronary architecture and targeted occlusion of the branches to the PMPM can reproducibly produce MR; and ease of performing subsequent surgeries, such as the MV repair we performed in the current study. In this study, we used ethanol to cause acute thrombosis of blood in the coronary arteries, which was easy and highly reproducible. Injecting ethanol not only induced acute coagulation of the blood in the targeted vessel but also caused necrosis of the RBC and platelets in the coagulating blood, which might underlie the minimal ventricular fibrillation that occurred in these pigs. A recent study indicated that the secretome from platelets trapped between the coronary occlusion and the myocardium contribute to ventricular fibrillation,7 and necrosis of the captured cells with ethanol may have reduced this risk. Other materials, such as collagen, high-density bead suspensions, and embolization coils or stents have been used, but with higher incidence of ventricular fibrillation than what occurred here.6,30,33
Our pigs tolerated CPB very well, and data from this study validates their use in future studies. In the current study, we used high potassium induction (15 mEq/L), followed by adenocaine, a normokalemic, nondepolarizing, cardioplegic solution, which our laboratory has used previously in swine. The data from this study do not confirm a definitive benefit of adenocaine over commonly used hyperkalemic cardioplegic solutions, but the theoretical benefits of adenocaine in failing hearts are several. The nondepolarizing arrest induced by adenocaine might reduce Ca2+ accumulation in the myocytes and thus inhibit ischemia–reperfusion injury due to cardiac arrest; normokalemia might minimize coronary artery vasoconstriction and thus improve the supply of the cardioplegic solution to subendocardial regions of the ventricle; and reduced K+ concentrations may decrease coronary endothelial cell damage, lipid uptake, and cholesterol deposition in the subintimal space.
In the perioperative period, the key determinants of recovery in our swine model seem to be body temperature and pain management. Hypothermia often manifests in the perioperative recovery period, despite rapid rewarming of the animal after reanimation of the heart and completion of CPB. This phenomenon, known as ‘after drop,’ is attributed to the redistribution of heat from the warmer inner core to the cooler distal tissues. To combat such hypothermia, we covered our pigs with warm blankets. Pain management and relief is of utmost importance too, with painful recovery often associated with poor outcomes. Pigs demonstrate pain through changes in gait or posture, unwillingness to move, and anorexia. According to our observations, the use of preemptive analgesia was effective in managing postoperative pain in our animals. After recovery, success as long-term survival is largely governed by the progression of heart failure and associated cardiac events. Because of our interest in the natural progression of heart failure after MV repair, we did not treat our pigs with any heart failure drugs, and this decision might have increased mortality and morbidity. In preclinical device trials, the use of heart-failure drugs may further improve the outcomes and survival in this swine model.
In conclusion, we here report a new technique for transcatheter induction of post infarction MR and heart failure. Open-heart surgery for MV repair in swine with advanced heart failure is feasible, with reasonable success regarding chronic survival. This model in swine of human-like cardiothoracic surgery is a first step toward translational research in developing novel surgical and transcatheter therapies.
Acknowledgments
The authors thank Susan Schmarkey, Trisha Bruce, Adam Cook, Milton Wilkes, and Roberto Hernandez-Merlo for their assistance during this study. This work was supported by the American Heart Association Scientist Development Grant (14SDG20380081) and the Carlyle Fraser Heart Center at Emory University. The authors declare no conflicts of interest, financial or otherwise, regarding this work.
References
- 1.Beaudoin J, Levine RA, Guerrero JL, Yosefy C, Sullivan S, Abedat S, Handschumacher MD, Szymanski C, Gilon D, Palmeri NO, Vlahakes GJ, Hajjar RJ, Beeri R. 2013. Late repair of ischemic mitral regurgitation does not prevent left ventricular remodeling: importance of timing for beneficial repair. Circulation 128:S248–S252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Beeri R, Yosefy C, Guerrero JL, Abedat S, Handschumacher MD, Stroud RE, Sullivan S, Chaput M, Gilon D, Vlahakes GJ, Spinale FG, Hajjar RJ, Levine RA. 2007. Early repair of moderate ischemic mitral regurgitation reverses left ventricular remodeling: a functional and molecular study. Circulation 116:I288–I293. [DOI] [PubMed] [Google Scholar]
- 3.Belanger M, Wittnich C, Torrance S, Juhasz S. 2002. Model of normothermic long-term cardiopulmonary bypass in swine weighing more than 80 kilograms. Comp Med 52:117–121. [PubMed] [Google Scholar]
- 4.Byrne JG, Aranki SF, Cohn LH. 2000. Repair compared with replacement of mitral valve for treating severe ischemic mitral regurgitation. Coron Artery Dis 11:31–33. [DOI] [PubMed] [Google Scholar]
- 5.Cohn LH, Kowalker W, Bhatia S, DiSesa VJ, St John-Sutton M, Shemin RJ, Collins JJ. 1995. Comparative morbidity of mitral valve repair compared with replacement for mitral regurgitation with and without coronary artery disease. 1988. Updated in 1995. Ann Thorac Surg 60:1452–1453. [DOI] [PubMed] [Google Scholar]
- 6.Crisostomo V, Maestre J, Maynar M, Sun F, Baez-Diaz C, Uson J, Sanchez-Margallo FM. 2013. Development of a closed chest model of chronic myocardial infarction in swine: magnetic resonance imaging and pathological evaluation. ISRN Cardiol 2013:781762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dhanjal TS, Medina RA, Leem J, Clark JE, Southworth R, Curtis MJ. 2013. Trapped platelets activated in ischemia initiate ventricular fibrillation. Circ Arrhythm Electrophysiol 6:995–1001. [DOI] [PubMed] [Google Scholar]
- 8.DiVincenti L, Jr, Westcott R, Lee C. 2014. Sheep (Ovis aries) as a model for cardiovascular surgery and management before, during, and after cardiopulmonary bypass. J Am Assoc Lab Anim Sci 53:439–448. [PMC free article] [PubMed] [Google Scholar]
- 9.Goland S, Czer LS, Siegel RJ, DeRobertis MA, Mirocha J, Zivari K, Kass RM, Raissi S, Fontana G, Cheng W, Trento A. 2009. Coronary revascularization alone or with mitral valve repair: outcomes in patients with moderate ischemic mitral regurgitation. Tex Heart Inst Jl 36:416–424. [PMC free article] [PubMed] [Google Scholar]
- 10.Grigioni F, Enriquez-Sarano M, Zehr KJ, Bailey KR, Tajik AJ. 2001. Ischemic mitral regurgitation: long-term outcome and prognostic implications with quantitative Doppler assessment. Circulation 103:1759–1764. [DOI] [PubMed] [Google Scholar]
- 11.Guy TS, 4th, Moainie SL, Gorman JH, 3rd, Jackson BM, Plappert T, Enomoto Y, St John-Sutton MG, Edmunds LH, Jr, Gorman RC. 2004. Prevention of ischemic mitral regurgitation does not influence the outcome of remodeling after posterolateral myocardial infarction. J Am Coll Cardiol 43:377–383. [DOI] [PubMed] [Google Scholar]
- 12.Hosking MC, Keaney MA, Lebeau J, Redmond ML. 1995. Cardiopulmonary bypass with circulatory arrest in swine: echocardiographic evaluation of left ventricle function and pulmonary vein flow. Lab Anim Sci 45:427–431. [PubMed] [Google Scholar]
- 13.Ishikawa K, Ladage D, Takewa Y, Yaniz E, Chen J, Tilemann L, Sakata S, Badimon JJ, Hajjar RJ, Kawase Y. 2011. Development of a preclinical model of ischemic cardiomyopathy in swine. Am J Physiol Heart Circ Physiol 301:H530–H537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kalra K, Wang Q, McIver BV, Shi W, Guyton RA, Sun W, Sarin EL, Thourani VH, Padala M. 2014. Temporal changes in interpapillary muscle dynamics as an active indicator of mitral valve and left ventricular interaction in ischemic mitral regurgitation. J Am Coll Cardiol 64:1867–1879. [DOI] [PubMed] [Google Scholar]
- 15.Katz MG, Kendle AP, Fargnoli AS, Mihalko KL, Bridges CR. 2015. Sheep (Ovis aries) as a model for cardiovascular surgery and management before, during, and after cardiopulmonary bypass. J Am Assoc Lab Anim Sci 54:7–8. [PMC free article] [PubMed] [Google Scholar]
- 16.Lefer DJ, Bolli R. 2011. Development of an NIH consortium for preclinicAl AssESsment of CARdioprotective therapies (CAESAR): a paradigm shift in studies of infarct size limitation. J Cardiovasc Pharmacol Ther 16:332–339. [DOI] [PubMed] [Google Scholar]
- 17.Levine RA, Hung J. 2003. Ischemic mitral regurgitation, the dynamic lesion: clues to the cure. J Am Coll Cardiol 42:1929–1932. [DOI] [PubMed] [Google Scholar]
- 18.Llaneras MR, Nance ML, Streicher JT, Lima JA, Savino JS, Bogen DK, Deac RF, Ratcliffe MB, Edmunds LH., Jr 1994. Large animal model of ischemic mitral regurgitation. Ann Thorac Surg 57:432–439. [DOI] [PubMed] [Google Scholar]
- 19.Maltais S, Schaff HV, Daly RC, Suri RM, Dearani JA, Sundt TM, 3rd, Enriquez-Sarano M, Topilsky Y, Park SJ. 2011. Mitral regurgitation surgery in patients with ischemic cardiomyopathy and ischemic mitral regurgitation: factors that influence survival. J Thorac Cardiovasc Surg 142:995–1001. [DOI] [PubMed] [Google Scholar]
- 20.Matsuzaki K, Morita M, Hamamoto H, Noma M, Robb JD, Gillespie MJ, Gorman JH, 3rd, Gorman RC. 2010. Elimination of ischemic mitral regurgitation does not alter long-term left ventricular remodeling in the ovine model. Ann Thorac Surg 90:788–794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mickelson HE, Qayumi AK, Jamieson WR, Smith CL, Gillespie KD, van den Broek JE. 1990. Swine as a model for cardiovascular research: improved cardiopulmonary bypass techniques. J Invest Surg 3:253–260. [DOI] [PubMed] [Google Scholar]
- 22.Mustonen J, Suurmunne H, Kouri J, Pitkanen O, Hakala T. 2011. Impact of coronary artery bypass surgery on ischemic mitral regurgitation. Scand J Surg 100:114 –119. [DOI] [PubMed] [Google Scholar]
- 23.Rankin JS, Nicholas LM, Kouchoukos NT. 1975. Experimental mitral regurgitation: effects on left ventricular function before and after elimination of chronic regurgitation in the dog. J Thorac Cardiovasc Surg 70:478–488. [PubMed] [Google Scholar]
- 24.Rudd DM, Dobson GP. 2009. Toward a new cold and warm nondepolarizing, normokalemic arrest paradigm for orthotopic heart transplantation. J Thorac Cardiovasc Surg 137:198–207. [DOI] [PubMed] [Google Scholar]
- 25.Seldinger SI. 1953. Catheter replacement of the needle in percutaneous arteriography; a new technique. Acta radiol 39:368–376. [DOI] [PubMed] [Google Scholar]
- 26.Silberman S, Eldar O, Oren A, Tauber R, Fink D, Klutstein MW, Bitran D. 2011. Surgery for ischemic mitral regurgitation: should the valve be repaired? J Heart Valve Dis 20:129–135. [PubMed] [Google Scholar]
- 27.Smith AC, Swindle MM. 2006. Preparation of swine for the laboratory. ILAR J 47:358–363. [DOI] [PubMed] [Google Scholar]
- 28.Smith PK, Michler RE, Woo YJ, Alexander JH, Puskas JD, Parides MK, Hahn RT, Williams JB, Dent JM, Ferguson TB, Jr, Moquete E, Rose EA, Page P, Jeffries NO, O'Gara PT, Ascheim DD. 2012. Design, rationale, and initiation of the Surgical Interventions for Moderate Ischemic Mitral Regurgitation Trial: a report from the Cardiothoracic Surgical Trials Network. J Thorac Cardiovasc Surg 143:111–117e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Smith PK, Puskas JD, Ascheim DD, Voisine P, Gelijns AC, Moskowitz AJ, Hung JW, Parides MK, Ailawadi G, Perrault LP, Acker MA, Argenziano M, Thourani V, Gammie JS, Miller MA, Page P, Overbey JR, Bagiella E, Dagenais F, Blackstone EH, Kron IL, Goldstein DJ, Rose EA, Moquete EG, Jeffries N, Gardner TJ, O'Gara PT, Alexander JH, Michler RE, Cardiothoracic Surgical Trials Network Investigators. 2014. Surgical treatment of moderate ischemic mitral regurgitation. N Engl J Med 371:2178–2188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Song W, Lee J, Kim H, Shin J, Oh D, Tio F, Wong SC, Hong MK. 2005. A new percutaneous porcine coronary model of chronic total occlusion. J Invasive Cardiol 17:452–454. [PubMed] [Google Scholar]
- 31.Spratt JA, Olsen CO, Tyson GS, Jr, Glower DD, Jr, Davis JW, Rankin JS. 1983. Experimental mitral regurgitation. Physiological effects of correction on left ventricular dynamics. J Thorac Cardiovasc Surg 86:479–489. [PubMed] [Google Scholar]
- 32.Suematsu Y, Martinez JF, Wolf BK, Marx GR, Stoll JA, DuPont PE, Howe RD, Triedman JK, del Nido PJ. 2005. Three-dimensional echo-guided beating heart surgery without cardiopulmonary bypass: atrial septal defect closure in a swine model. J Thorac Cardiovasc Surg 130:1348–1357. [DOI] [PubMed] [Google Scholar]
- 33.Suzuki K, Saito N, Zhang G, Conditt G, McGregor J, Flynn AM, Leahy D, Glennon P, Leon MB, Hayase M. 2008. Development of a novel calcified total occlusion model in porcine coronary arteries. J Invasive Cardiol 20:296–301. [PubMed] [Google Scholar]
- 34.Swindle MM, Makin A, Herron AJ, Clubb FJ, Jr, Frazier KS. 2011. Swine as models in biomedical research and toxicology testing. Vet Pathol 49:344–356. [DOI] [PubMed] [Google Scholar]
- 35.Swindle MM, Smith AC. 2013. Best practices for performing experimental surgery in swine. J Invest Surg 26:63–71. [DOI] [PubMed] [Google Scholar]
- 36.Swindle MM, Smith AC, Hepburn BJ. 1988. Swine as models in experimental surgery. J Invest Surg 1:65 –79. [DOI] [PubMed] [Google Scholar]