Abstract
Cynomolgus macaques (CYNO; Macaca fascicularis) are a well-established NHP model used for studies in immunology. To provide reference values on the baseline cell distributions in the hematopoietic and lymphoid organs (HLO) of these animals, we used flow cytometry to analyze the peripheral blood, bone marrow, mesenteric lymph nodes, spleen, and thymus of a cohort of male, adult, research-naïve, Mauritian CYNO. Our findings demonstrate that several cell distribution patterns differ between CYNO and humans. First, the CD4+:CD8+ T-cell ratio is lower in CYNO compared with humans. Second, the peripheral blood of CYNO contains a population of CD4+CD8+ T cells. Third, the CD31 level was elevated in all organs studied, suggesting that CD31 may not be an accurate marker of recent thymic emigrants within the CD4+ T cells of CYNO. Finally the B-cell population is lower in CYNO compared with humans. In summary, although the majority of immune cell populations are similar between cynomolgus macaques and humans, several important differences should be considered when using CYNO in immunologic studies. Our current findings provide valuable information to not only researchers but also veterinarians working with CYNO at research centers, in zoos, or in the wild.
Abbreviations: BM, bone marrow; CYNO, cynomolgus macaque; HLO, hematopoietic and lymphoid organs; IBM, ilial bone marrow; MLN, mesenteric lymph node; NK, natural killer; PB, peripheral blood; SBM, spinal bone marrow; Treg, regulatory T cell
Animal models play a crucial role in scientific discoveries and in applying those discoveries to humans and animals. In studies of the immune system, various mammalian models have been used as surrogates for humans, ranging from small (mice, 10 to 20 g) to large (for example, pigs and NHP) animals. Mice have the benefit of being relatively inexpensive, and their genomes can be manipulated to develop inbred, knockin, knockout, and transgenic strains. Furthermore, mice have been key in the field of bone marrow transplantation.11 However, discoveries in mouse models do not always successfully translate to use in humans.20 Although NHP are expensive and require specialized veterinary and husbandry care,10,64 as model animals, they have the advantages of being evolutionarily closely related to humans. Importantly many reagents used in humans crossreact with NHP, making them highly valuable for the testing of such reagents to be used clinically.13,32,72
NHP have a complex genome, and inbred strains are currently unavailable. Cynomolgus macaques (Macaca fascicularis; CYNO) have been used extensively in transplant and SIV studies for the past 2 decades.14,30,31 A population of CYNO has been naturally isolated on the small island of Mauritius. This geographic isolation has allowed for natural inbreeding to occur, narrowing their genetic MHC diversity to 6 different haplotypes.50,62 This limited genetic diversity makes the Mauritian CYNO a particularly attractive model for immunologic studies.
To date several studies have evaluated immune cells in CYNO; most of these have focused on T or B cells (or both) in the peripheral blood, with some studies also including the lymph nodes.28,54 To our knowledge, we are the first to characterize multiple immune cell populations (T cells, regulatory T [Treg] cells, B cells, natural killer [NK] cells, monocytes, CD34+ hematopoietic stem cells, and granulocytes) in diverse hematopoietic and lymphoid organs (HLO; peripheral blood [PB], spleen, mesenteric lymph nodes [MLN], thymus, and bone marrow [BM]) of adult, male Mauritian CYNO. Because our lab is using CYNO for BM transplantation, we also examined the CD3+ (that is, T cell) and CD34+ (that is, hematopoietic stem cell) yields from BM harvested from 2 anatomic locations.
Materials and Methods
Animals.
Adult, male, Mauritian-origin CYNO (age, 7 to 10 y; weight, 4 to 8 kg) were obtained from Charles River Laboratories (Wilmington, MA). All animal work was approved by the Columbia University IACUC. All animals were housed at the Institute of Comparative Medicine within the Columbia University College of Physicians and Surgeons at Columbia University Medical Center (New York, NY). This facility holds a current USDA assurance and is an AAALAC-accredited institution. All animals were negative for B virus, simian T-lymphotropic virus, simian retrovirus, SIV, simian varicella virus, and malaria.
Tissue collection and harvesting.
For PB collection, CYNO were sedated with ketamine or ketamine–dexdormitor; PB was drawn from a total of 27 macaques, but because multiple samples were collected from some animals, we obtained a total of 45 samples. No single animal provided more than 4 samples of peripheral blood for these studies. Not every tissue sample was stained with every cell maker; the numbers of samples evaluated are provided in Tables 1 through 5.
Table 1.
T-cell populations (mean ± SEM) in the hematopoietic and lymphoid organs of Mauritian cynomolgus macaques
| CD3+ | CD4+CD8– | CD4–CD8+ | CD4+CD8+ | CD4+CD8+ | CD4-CD8- | |
| Peripheral blood | ||||||
| % | 28.6 ± 2.2 | 39.0 ± 1.7 | 47.9 ± 1.5 | 0.90 ± 0.07 | 5.37 ± 0.67 | 3.36 ± 0.33 |
| No. of samples | 45 | 43 | 43 | 43 | 43 | 43 |
| Spinal bone marrow | ||||||
| % | 6.70 ± 1.72 | 17.3 ± 1.8 | 51.4 ± 4.2 | 0.36 ± 0.04 | 3.59 ± 0.49 | 23.6 ± 5.4 |
| No. of samples | 11 | 11 | 11 | 11 | 11 | 11 |
| Ilial bone marrow | ||||||
| Cell count | 7.83 ± 2.20 | 30.8 ± 4.9 | 44.9 ± 4.7 | 0.68 ± 0.09 | 3.69 ± 1.10 | 17.7 ± 7.6 |
| No. of samples | 6 | 6 | 6 | 6 | 6 | 6 |
| Mesenteric lymph nodes | ||||||
| % | 66.9 ± 3.8 | 62.8 ± 2.5 | 24.3 ± 1.8 | 2.82 ± 0.34 | 2.02 ± 0.32 | 7.32 ± 1.09 |
| No. of samples | 11 | 11 | 11 | 11 | 11 | 11 |
| Spleen | ||||||
| % | 34.8 ± 3.7 | 31.7 ± 2.1 | 50.2 ± 2.9 | 0.65 ± 0.06 | 3.80 ± 0.67 | 9.49 ± 2.3 |
| No. of samples | 11 | 9 | 9 | 9 | 9 | 9 |
| Thymus (all cells) | ||||||
| % | 41.6 ± 4.3 | 7.36 ± 0.80 | 13.6 ± 2.6 | 0.61 ± 0.08 | 48.6 ± 6.0 | 28.4 ± 6.8 |
| No. of samples | 10 | 9 | 9 | 9 | 9 | 9 |
| Thymus (CD3+) | ||||||
| % | not applicable | 17.2 ± 1.4 | 20.1 ± 2.4 | 0.91 ± 0.08 | 53.5 ± 5.0 | 7.20 ± 1.3 |
| No. of samples | not applicable | 9 | 9 | 9 | 9 | 9 |
| Thymus (CD3low/negative) | ||||||
| % | not applicable | 0.73 ± 0.29 | 9.85 ± 3.12 | 0.16 ± 0.07 | 47.9 ± 7.8 | 39.7 ± 8.6 |
| No. of samples | not applicable | 9 | 9 | 9 | 9 | 9 |
Table 5.
Granulocytes, monocytes, B cells, and hematopoietic stem cells in the hematopoietic and lymphoid organs of Mauritian cynomolgus macaques
| Granulocytes | B cells | T:B cell ratio | Monocytes | CD34+ progenitors | |
| Peripheral blood | |||||
| %a | 48.7 ± 3.2 | 3.1 ± 1.0 | 21.3 ± 8.5 | 4.1 ± 0.7 | 0.1 ± 0.1 |
| No. of samples | 45 | 16 | 16 | 17 | 5 |
| Spinal bone marrow | |||||
| %a | 53.0 ± 5.7 | 2.4 ± 0.8 | 8.8 ± 6.0 | 4.8 ± 1.1 | 1.6 ± 0.2 |
| No. of samples | 11 | 10 | 9 | 6 | 11 |
| Ilial bone marrow | |||||
| %a | 70.1 ± 4.5 | 0.8 ± 0.3 | 12.9 ± 3.7 | 7.3 ± 3.0 | 1.1 ± 0.5 |
| No. of samples | 6 | 5 | 5 | 4 | 6 |
| Mesenteric lymph nodes | |||||
| %a | not applicable | 12.7 ± 2.3 | 14.2 ± 6.4 | 0.5 ± 0.3 | not applicable |
| No. of samples | not applicable | 10 | 10 | 10 | not applicable |
| Spleen | |||||
| %a | 19.1 ± 3.3 | 15.5 ± 2.5 | 4.0 ± 1.4 | 2.9 ± 1.1 | not applicable |
| No. of samples | 11 | 11 | 11 | 5 | not applicable |
| Thymus | |||||
| %a | not applicable | 1.4 ± 0.3 | not applicable | 2.81 ± 0.71 | not applicable |
| No. of samples | not applicable | 10 | not applicable | 10 | not applicable |
Except for T:B cell ratio
Table 2.
CD45RA and CD31 expression (mean ± SEM) within T-cell populations in the hematopoietic and lymphoid organs of Mauritian cynomolgus macaques
| CD31+ |
CD45RA+ |
|||||||
| CD4+CD8– | CD4–CD8+ | CD4+CD8+ | CD4–CD8– | CD4+CD8– | CD4–CD8+ | CD4+CD8+ | CD4–CD8– | |
| Peripheral blood | ||||||||
| % | 73.7 ± 4.9 | 47.7 ± 7.5 | 35.6 ± 6.9 | 55.7 ± 8.4 | 61.7 ± 3.8 | 86.5 ± 1.7 | 78.3 ± 3.3 | 66.1 ± 4.2 |
| No. of samples | 11 | 11 | 11 | 11 | 33 | 33 | 33 | 33 |
| Spinal bone marrow | ||||||||
| % | 58.2 ± 5.29 | 44.9 ± 4.3 | 44.4 ± 10.7 | 25.6 ± 6.5 | 46.7 ± 5.1 | 78.2 ± 3.3 | 62.3 ± 6.0 | 48.8 ± 11.3 |
| No. of samples | 5 | 5 | 5 | 5 | 6 | 6 | 6 | 6 |
| Ilial bone marrow | ||||||||
| % | 71.0 ± 6.8 | 37.9 ± 17.6 | 39.5 ± 13.0 | 38.7 ± 16.9 | 54.8 ± 17.5 | 86.3 ± 6.8 | 62.2 ± 14.2 | 62.8 ± 28.5 |
| No. of samples | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 |
| Mesenteric lymph nodes | ||||||||
| % | 74.9 ± 2.5 | 79.8 ± 4.5 | 74.1 ± 4.5 | 50.8 ± 6.7 | 45.6 ± 7.3 | 76.4 ± 6.5 | 65.4 ± 5.5 | 60.6 ± 7.6 |
| No. of samples | 7 | 7 | 7 | 7 | 8 | 8 | 8 | 8 |
| Spleen | ||||||||
| % | 54.1 ± 10.2 | 58.7 ± 5.7 | 43.5 ± 5.2 | 40.8 ± 8.4 | 54.9 ± 11.6 | 77.6 ± 7.6 | 79.5 ± 4.7 | 54.8 ± 10.7 |
| No. of samples | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 |
| Thymus (all cells) | ||||||||
| % | 64.4 ± 6.2 | 64.6 ± 8.4 | 85.6 ± 5.4 | 15.6 ± 8.7 | 28.1 ± 7.8 | 38.5 ± 8.1 | 16.9 ± 4.1 | 20.4 ± 7.7 |
| No. of samples | 6 | 6 | 6 | 6 | 6 | 6 | 6 | 6 |
| Thymus (CD3+) | ||||||||
| % | 71.1 ± 6.1 | 72.1 ± 7.2 | 88.4 ± 5.1 | 28.6 ± 8.5 | 31.0 ± 8.1 | 39.7 ± 10.0 | 16.0 ± 4.8 | 41.6 ± 12.1 |
| No. of samples | 6 | 6 | 6 | 6 | 6 | 6 | 6 | 6 |
| Thymus (CD3low/negative) | ||||||||
| % | 36.2 ± 7.5 | 49.2 ± 10.6 | 84.3 ± 6.2 | 15.4 ± 8.8 | 28.0 ± 6.9 | 36.6 ± 8.3 | 13.7 ± 4.7 | 16.8 ± 6.2 |
| No. of samples | 6 | 6 | 6 | 6 | 6 | 6 | 6 | 6 |
Table 3.
Regulatory T-cell populations in the hematopoietic and lymphoid organs of Mauritian cynomolgus macaques
| CD4+FoxP3+ | CD4+CD25+ | CD25+FoxP3+ | |
| Peripheral blood | |||
| % | 3.91 ± 0.092 | 9.43 ± 2.19 | 2.40 ± 0.40 |
| n | 30 | 42 | 30 |
| Spinal bone marrow | |||
| % | 2.28 ± 0.89 | 8.25 ± 2.75 | NA |
| n | 4 | 4 | NA |
| Ilial bone marrow | |||
| % | 7.40 ± 5.51 | 7.59 ± 3.24 | NA |
| n | 3 | 4 | NA |
| Mesenteric lymph nodes | |||
| % | 10.7 ± 3.9 | 14.4 ± 3.0 | 8.80 ± 5.25 |
| n | 5 | 10 | 5 |
| Spleen | |||
| % | 7.11 ± 2.44 | 8.78 ± 1.78 | 6.35 ± 2.88 |
| n | 6 | 10 | 6 |
| Thymus | |||
| % | 4.33 ± 1.18 | 2.81 ± 0.71 | 1.87 ± 0.72 |
| n | 5 | 10 | 5 |
NA, not applicable
Table 4.
NK cells and NK T cells (mean ± SEM) in the hematopoietic and lymphoid organs of Mauritian cynomolgus macaques
| NK | CD3+CD56+ | CD56+ | CD8+CD56+ | CD8–CD56+ | CD3–CD8+ | CD56+ in CD3–CD8+ | CD56+ in CD3–CD8– | |
| Peripheral blood | ||||||||
| % | 3.28 ± 0.57 | 0.38 ± 0.10 | 0.10 ± 0.03 | 0.21 ± 0.07 | 0.03 ± 0.02 | 4.75 ± 0.49 | 2.77 ± 0.81 | 35.2 ± 6.3 |
| No. of samples | 17 | 17 | 17 | 17 | 17 | 45 | 16 | 16 |
| Spinal bone marrow | ||||||||
| % | 5.85 ± 0.82 | 0.48 ± 0.12 | 5.78 ± 2.00 | 2.38 ± 1.00 | 4.33 ± 1.67 | 0.29 ± 0.09 | 14.20 ± 1.60 | 20.80 ± 6.10 |
| No. of samples | 8 | 8 | 7 | 7 | 7 | 11 | 7 | 7 |
| Ilial bone marrow | ||||||||
| % | 4.94 ± 0.90 | 0.126 ± 0.095 | 5.01 ± 2.69 | 1.18 ± 1.14 | 4.25 ± 4.25 | 0.44 ± 0.27 | 5.47 ± 4.19 | 32.50 ± 6.20 |
| No. of samples | 4 | 4 | 4 | 4 | 3 | 4 | 4 | 4 |
| Mesenteric lymph nodes | ||||||||
| % | 0.65 ± 0.30 | 1.95 ± 1.23 | 2.96 ± 1.66 | 1.55 ± 0.92 | 2.22 ± 1.45 | 1.13 ± 0.95 | 9.82 ± 3.03 | 1.89 ± 0.90 |
| No. of samples | 10 | 10 | 10 | 10 | 10 | 10 | 10 | 10 |
| Spleen | ||||||||
| % | 1.18 ± 0.38 | 0.53 ± 0.19 | 2.72 ± 0.80 | 1.31 ± 0.41 | 0.80 ± 0.44 | 3.70 ± 0.88 | 3.12 ± 0.98 | 3.64 ± 1.45 |
| No. of samples | 6 | 6 | 6 | 6 | 6 | 10 | 6 | 6 |
| Thymus (all cells) | ||||||||
| % | 0.62 ± 0.34 | 0.65 ± 0.30 | 1.84 ± 0.66 | 1.84 ± 0.66 | 0.88 ± 0.38 | 23.0 ± 3.2 | 1.85 ± 0.72 | 3.13 ± 1.02 |
| No. of samples | 10 | 10 | 9 | 9 | 9 | 9 | 9 | 8 |
| Thymus (CD3+) | ||||||||
| % | NA | NA | 2.64 ± 0.96 | 2.98 ± 0.84 | 1.32 ± 0.67 | NA | NA | NA |
| No. of samples | NA | NA | 9 | 9 | 9 | NA | NA | NA |
| Thymus (CD3low/negative) | ||||||||
| % | NA | NA | 1.26 ± 0.80 | 1.38 ± 0.57 | 0.98 ± 0.58 | NA | NA | NA |
| No. of samples | NA | NA | 9 | 9 | 9 | NA | NA | NA |
NA, not applicable
For tissue collection of thymus, lymph nodes, bone marrow, blood and spleen, all animals were sedated with ketamine or ketamine–dexdormitor; they then were anesthetized with isoflurane by mask prior to intubation, or they received a small bolus of propofol (6mg/kg IV to effect). The animals were maintained under deep anesthesia during BM aspiration. Central venous (external or internal jugular) and arterial (carotid) catheters were inserted prior to euthanasia. Macaques were exsanguinated and then given an overdose of KCl, according to the experimental protocol. Once the animal was confirmed to be dead, tissue harvest was initiated. Spinal bone marrow (SBM) was collected from vertebrae. The bone pieces were stored in BM media (95% RPMI [Life Technologies, Grand Island, NY] and 5% DNase (Roche Applied Sciences, Penzberg, Germany). These pieces were rocked for 60 min, after which the media was passed through 70-μm filters. The marrow was pelleted, and the supernatant was collected and centrifuged. The resulting pellets were resuspended in 99.5% HBSS (Life Technologies) and 0.5% human serum albumin (Grifols, Raleigh, NC); and the number of live cells was determined by using trypan blue staining. Ilial BM (IBM) was collected by aspiration of the iliac crest; aspirates were collected in volumes of 2 mL or less. IBM was processed in the same manner as SBM.
All organs and tissues were processed and analyzed within 24 h from the time of procurement. All tissues were fresh at time of analysis.
Organ and tissue processing.
The spleen, thymus, and MLN were diced into small pieces by using a scalpel and then mechanically dissociated. The tissues were washed with PBS and filtered through 70-μm filters, and live cells were counted by using trypan blue staining.
RBC in PB, BM, and spleen were lysed by using a buffer consisting of water with ammonium chloride (8.26 g/L; Sigma Aldrich), potassium bicarbonate (1.0 g/L; Sigma Aldrich), and EDTA (0.037 g/L; Sigma Aldrich). Briefly, the single-cell suspensions of PB and splenocytes were gently rocked in the lysis buffer for 8 min at 7 revolutions per minute and then centrifuged for 10 min at 800 × g; BM was rocked for 4 min before the same centrifugation.
Antibodies and flow cytometry.
Cells were stained in FACS media (95% PBS [Corning, Manassas, VA], 5% fetal bovine Serum [Gemini Bioproducts], and sodium azide [1 g/L; Sigma Aldrich]). Cells were incubated for 1 h with antibodies. Cells were permeabilized by using Fix–Perm Buffer and Permeabilization Buffer (BioLegend, San Diego, CA) according to the manufacturer's directions. A comprehensive list of markers examined in each tissue is listed in Tables 1 through 5; the antibodies used are listed in Table 6. Samples were analyzed on a CANTO II (BD Biosciences, Franklin Lakes, NJ) multicolor flow cytometer. Data was analyzed on FlowJo (version X; FlowJo, Ashland, OR) flow cytometry data analysis software.
Table 6.
Antibodies used during analysis of hematopoietic and lymphoid organs of Mauritian cynomolgus macaques
| Antibody | Fluorochrome | Clone | Manufacturer |
| CD3 | PerCP Cy5.5 | SP34-2 | BD Biosciences |
| CD4 | FITC | L200 | BD Biosciences |
| CD4 | PE | L200 | BD Biosciences |
| CD4 | AmCyan | L200 | BD Biosciences |
| CD8 | APC | BW135/80 | Miltenyi |
| CD11b | VioGreen | M1/70.15.11.5 | Miltenyi |
| CD20 | APC-Cy7 | LT20 | Miltenyi |
| CD25 | Pacific Blue | BC96 | BioLegend |
| CD31 | PE | WM59 | BD Biosciences |
| CD34 | PE | 563 | BD Biosciences |
| CD45RA | Vio770 | T6D11 | Miltenyi |
| CD45RA | APC-H7 | 5H9 | BD Biosciences |
| CD56 | PE | AF12-7HE | Miltenyi |
| FoxP3 | PE | 236A/E7 | eBiosciences |
Gating strategy.
Granulocytes were defined as a distinct population of highly granular cells. T cells were defined as nongranular cells that were CD3+. We noticed that the thymus contained CD3low and CD3neg cells that expressed CD4 and CD8. To ensure that these cells were not lost during analysis, the CD3+ and CD3low/negative populations were each analyzed separately, and the thymus was also analyzed as a whole. Gating on CD4 and CD8 was determined by the isotype control. Within the T-cell subsets, CD45RA and CD31 gating was based on isotype controls. Treg cells were defined in 1 of 3 ways: CD25+ cells within CD4+ T cells; FoxP3+ cells within CD4+ T cells; or CD25+FoxP3+ cells within the CD4+ T-cell population. Natural killer cells were defined as CD3–CD56+ cells. CD56 expression and CD56–CD8 coexpression in T cells was evaluated. A population of CD3–CD8+ cells was observed also. All gates within T-cell subsets were drawn according to isotype controls. B cells were defined as CD3–CD20+ cells, with gates drawn based by using isotype controls. Monocytes were defined as nongranular CD3–CD11b+ cells. CD34+ progenitor cells were defined as nongranular cells expressing CD34.
Results
Granular cells in CYNO HLO.
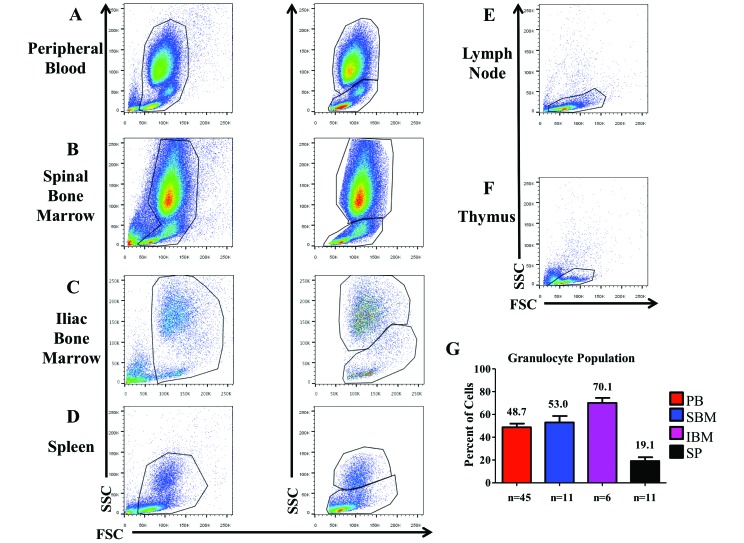
Neutrophils, basophils, and eosinophils comprise the granulocytic compartment, a component of the innate immune system. Large populations of granular cells were present in the PB, SBM, IBM, and spleen (Figure 1 A through D) of CYNO but not the MLN or thymus (Figure 1 E and F). The BM and PM contained more granulocytes than did the spleen, thymus, or MLN (Figure 1 G).
Figure 1.
Size and granularity of cells in each of the HLO. (A) Peripheral blood (PB), (B) spinal bone marrow (SBM), (C) ilial bone marrow (IBM), and (D) spleen all have large populations of granular cells. These populations were not seen in the (E) mesenteric lymph nodes or (F) thymus, which contain only populations with less granularity. (G) We assessed the percentage (mean ± SEM) of high side-scatter (granular) cells in PB, SBM, IBM, and spleen.
Distribution of T cells across HLO.
T cells are the main cell population that participates in the adaptive immune response. These cells have receptor that binds to either MHC-I (in the case of CD8+ T cells) or to MHC-II (CD4+ T cells). T cells are educated in the thymus (i.e. are exposed to self-antigen found in the surface of thymic epithelial cells and dendritic cells), which contains multiple subsets of T cells. Once in the periphery, T cells further differentiate and mature in the circulation. Some of these subpopulations have been immunophenotyped and are discussed later.
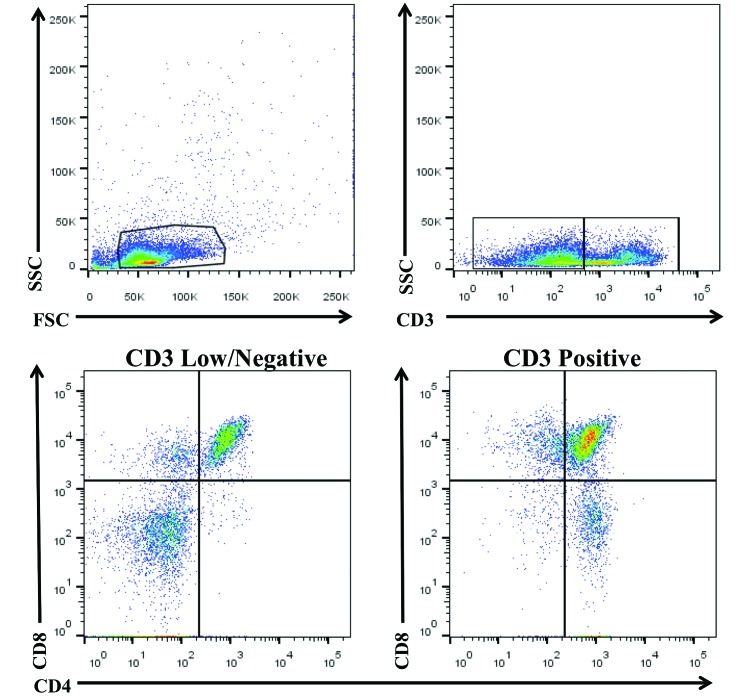
For the T-cell analysis, cells with the pan-T cell marker CD3 were selected and further analyzed for expression of CD4 and CD8. In the thymus of CYNO, CD3low/negative cells expressed both CD4 and CD8. To study the distribution of T cells and of CD4 and CD8 T cells within the thymus, thymocytes were assessed as a whole and then divided into CD3high and CD3low/negative subsets for further analysis. This approach allowed us to examine ‘traditional’ CD3+ T cells as well as the unexpected CD3low/negative cells that coexpressed CD4 and CD8. By analyzing the thymus as a whole, we were able to determine how each subset contributes to the thymic T-cell distribution (Figure 2).
Figure 2.
Analysis of CD3+, CD4+, and CD8+ T cells in thymus. When gating on thymocytes, the CD3 marker emerges as a smear that appears to comprise high and low/negative populations. When further analyzed, the CD3low/negative population is shown to contain CD4+ and CD8+ single- and double-positive cells.
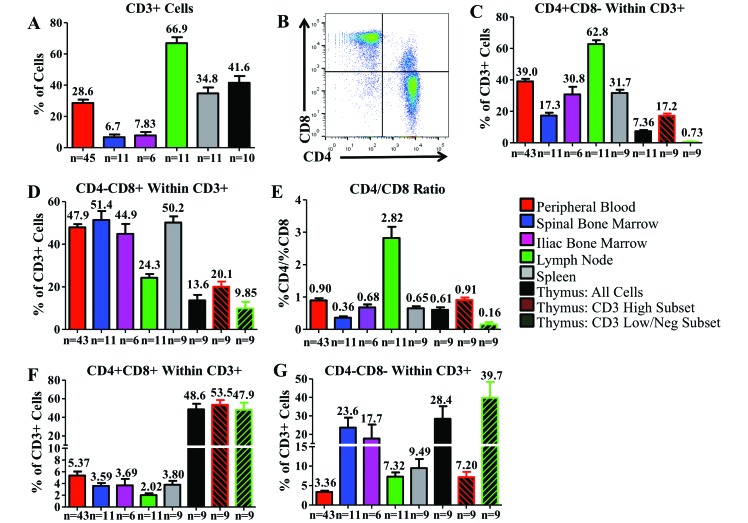
CD3+ cell counts were highest in the MLN of CYNO; high in the thymus, spleen, and PB; and low in the IBM and SBM (Figure 3 A). CD3+ T cells fell into 1 of 4 categories: CD4+ single-positive cells, CD8+ single-positive cells, CD4+CD8+ double-positive cells, and CD4–CD8– double-negative cells (Figure 3 B). CD4+CD8+ cells are consistently present in the peripheral blood of CYNO1 but are rarely seen outside of the thymus in humans, except during infection.18 CD4+ single-positive cell were most numerous in the MLN, PB, and spleen, with lower counts in the IBM, SBM, and thymus. Within the thymus, the CD3high population had a higher percentage of CD4+ cells than did the CD3low/negative population (Figure 3 C). The percentages of CD8+ single-positive cells were comparable among the PB, SBM, IBM, and spleen; fewer cells in the MLN and thymus were CD8+. In the thymic CD3high and CD3low/negative populations, fewer than 20% of cells were CD8+ (Figure 3 D). The CD4+:CD8+ ratio was calculated by dividing the percentage of CD4+ single-positive cells by the percentage of CD8+ single-positive T cells. Only in the MLN was the CD4+:CD8+ ratio greater than 1:1; all other organs of CYNO had at least a slightly higher percentage of CD8+ T cells than CD4+ (Figure 3 E).
Figure 3.
Distributions of T-cell populations within the hematopoietic and lymphoid organs of cynomolgus macaques. (A) Comparison of the percentage of cells in each organ that are CD3+. (B) CD3+ cells from PB are subdivided into 4 populations (from top left, clockwise: CD4–CD8+, CD4+CD8+, CD4+CD8–, and CD4–CD8–). Between the studied organs, we compared the percentages of (C) CD4+CD8– and (D) CD4–CD8+. CD4+ and CD8+ single-positive cells can be directly compared by calculating (E) the CD4+CD8–: CD4–CD8+ T cell ratio. We examined the distribution of (F) CD4+CD8+and (G) CD4–CD8– cells in each organ. Data are given as mean ± SEM.
In CYNO, the CD4+CD8+ population was largest in the thymus, comprising about 50% of total cells, as expected; this pattern remained the case for both the thymic CD3high and CD3low/negative populations. In all other tissues, the CD4+CD8+ population was much lower, comprising fewer than 6% of T cells (Figure 3 F). The CD4–CD8– population was most prevalent in the thymus, particularly among CD3low/negative cells. Double-negative T cells also were common in the IBM and SBM but accounted for less than 10% of T cells in all other organs (Figure 3 G).
CD31 and CD45RA expression in CYNO T cells.
CD31 is a platelet–endothelial cell adhesion molecule (PECAM1), and its expression on CD4+ T cells has been associated with recent thymic emigration.61 CD45RA is a naïve (nonactivated) T-cell marker in humans that is often used in other mammalian species. These 2 populations are important in the understanding of the aging of the T-cell population in PB. These markers can provide information on whether T cells are antigen-experienced, whether the thymus is active and releasing new naïve cells, and how the phenotypes of thymic emigrants change as they migrate to and through the blood and secondary lymphoid organs. As part of our studies, we assessed the expression of CD31 and CD45RA in the PB and HLO of CYNO.
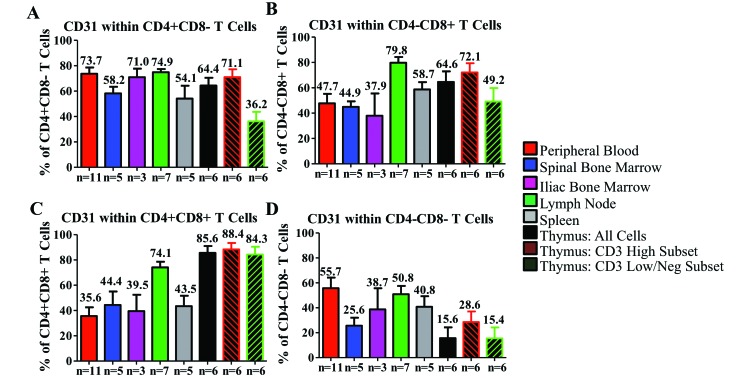
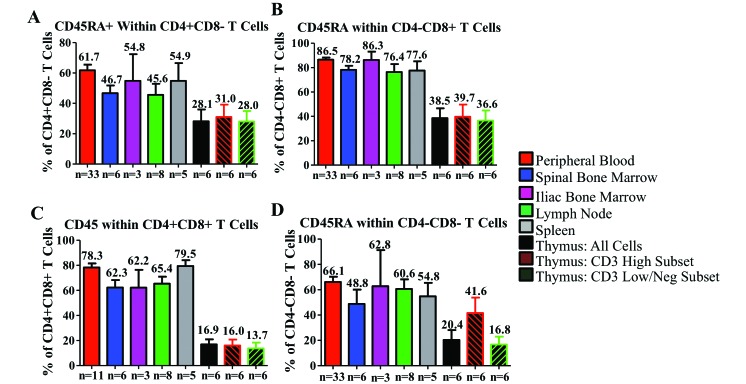
Within the CD4+ single-positive cell population, CD31 is relatively highly expressed (more than 50%) in all tissues of CYNO, with the exception of the thymic CD3low/negative population (Figure 4 A). To our surprise, CD31 expression was not highest in thymus-derived CD4+ T cells. Similar to the CD4+ population, CD8+ T cells highly expressed CD31 in the thymus, MLN, and spleen but markedly less frequently in the PB, IBM, and SBM (Figure 4 B). Among CD4+CD8+ T cells, CD31 was highest in the thymus and MLN and noticeably lower in all other tissues (Figure 4 C). In the double-negative T-cell population, the expression of CD31 in thymus is far lower than in any other T-cell subset, with expression being highest in the PB, MLN and spleen (Figure 4 D). CD45RA expression was relatively consistent among tissues and subsets, with 50% to 65% of CD4+ single-positive T cells expressing CD45RA, 80% to 90% of CD8+, 60% to 80% of CD4+CD8+, and 55% to 65% of CD4–CD8– in all tissues except the thymus. The thymic CD3high and CD3low/negative cells expressed only about half the levels of CD45RA seen in other tissues (Figures 5 A through D).
Figure 4.
Distribution of CD31 in T-cell populations within the hematopoietic and lymphoid organs of cynomolgus macaques. We examined the expression of CD31 in (A) CD4+ single-positive T cells, (B) CD8+ single-positive T cells, (C) CD4+CD8+ T cells, and (D) double-negative T cells. Data are given as mean ± SEM.
Figure 5.
Distribution of CD45RA in T-cell populations within the hematopoietic and lymphoid organs of cynomolgus macaques. Expression of CD45RA was determined among (A) CD4+ single-positive T cells, (B) CD8+ single-positive T cells, (C) CD4+CD8+ T cells, and (D) double-negative T cells. Data are given as mean ± SEM.
Expression of CD25 and Foxp3 in CYNO HLO.
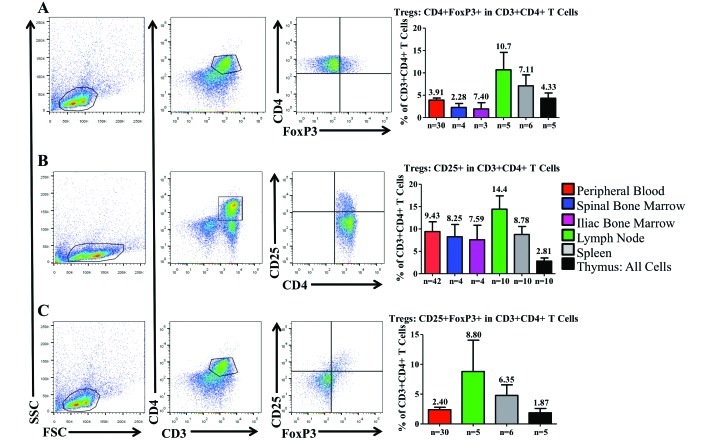
Treg cells can be challenging to identify, because no phenotypic marker is unique to this cellular subset. The combination of CD25 and FoxP3 has become the most reliable set of markers for the identification of Treg cells within CD4+ populations. FoxP3 is constitutively expressed in natural Treg cells, but other populations, such as activated T cells, can also express FoxP3, albeit transiently and at lower levels. For our current purpose, we sought to identify the percentage of Treg cells (that is, CD25highFoxP3highCD4+ T cells) within each HLO of CYNO.
Among CD3+CD4+ cells, we first focused on the expression of FoxP3 alone. FoxP3 was most highly expressed in the MLN and spleen, whereas FoxP3 expression was between 2% to 5% in all other tissues (Figure 6 A). We then assessed the average percentage of CD4+ T cells that expressed CD25, the high-affinity IL2 receptor chain; CD25 is highly expressed on Treg cells48 but is also expressed on activated T cells.2 Within the CD4+ T-cell population, CD25 was most highly expressed in the MLN, SBM, and IBM; CD25 expression was lowest in the thymus (Figure 6 B).
Figure 6.
Distribution of regulatory T cells within the hematopoietic and lymphoid organs of cynomolgus macaques. All flow data are representative plots derived from the mesenteric lymph nodes. Regulatory T-cell populations are enriched in the mesenteric lymph nodes. Regulatory T cells were assessed in multiple ways. (A) Initially the percentage of CD4+ T cells expressing FoxP3 was determined in each organ. (B) When assessing nonpermeabilized CD4+ T cells, the expression of high levels of CD25 can be used as a proxy for FoxP3. (C) Costaining of CD25 and FoxP3 is a more reliable method for the identification of regulatory T cells. Data are given as mean ± SEM.
Finally, we examined CD4+ T cells for coexpression of CD25 and FoxP3, which is the most reliable definition of Treg cells. As expected, we found that coexpression of CD25 and FoxP3 was highest in the MLN and spleen but much lower in PB and thymus; we did not assess BM for CD25 and FoxP3 (Figure 6 C).
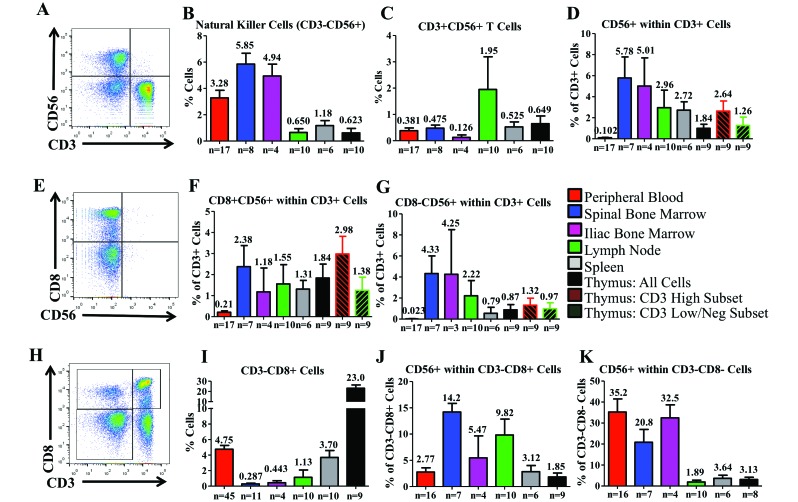
Expression of CD56 within CYNO HLO.
CD56, the neural cell adhesion marker (NCAM), is a marker used to define NK cells in humans.58 NK cells are potentially cytolytic cells and are often highly involved in antiviral immunity; in CYNO, we defined NK cells as CD3–CD56+ cells (Figure 7 A). NK cells were most prevalent in PB and BM. In the MLN and thymus, NK cells made up less than 1% of the total cell population (Figure 7 B). When we examined CD56 expression among CD3+ T cells (the subpopulation known as NK T cells), we found that coexpression of CD3+ and CD56+ was very low: the MLN had the highest levels, at 1.95% of all cells (Figure 7 C). When CD56 expression was assessed within the CD3+ population instead of all cells overall, expression was highest in the BM and lowest in the PB and thymus (Figure 7 D). Because expression of CD8 has been observed on CD56+ human NKT cells, we assessed the coexpression of CD8 and CD56 within the T cells of CYNO (Figure 7 E). Again, expression was high in the SBM but highest in the CD3+ thymic population (Figure 7 F). Furthermore, the CD3high population expressing CD56 but not CD8 (that is, CD3high CD56+CD8–) was largest in the SBM and IBM but was 2% or less in other tissues (Figure 7 G).
Figure 7.
Distribution of NK and CD56 across the hematopoietic and lymphoid organs of cynomolgus macaques. (A) Initially we defined NK cells as CD3–CD56+ cells. In each organ, we determined the presence of (B) NK cells and (C) CD3+CD56+ cells. We also examined (D) CD56 expression within CD3+ T cells. (E) Within T cells, we assessed the relationship between CD8 and CD56 expression. We examined both (F) coexpression of CD8 and CD56 and (G) expression of CD56 in the absence of CD8. (H) A population of CD3–CD8+ cells that has been suggested to have NK-like function in NHP was (I) observed and quantified in each tissue and (J) further examined for CD56 expression. (K) CD56+ expression within the CD3–CD8– population was evaluated as well. Data are given as mean ± SEM.
Another population of interest in CYNO was CD3–CD8+ cells (Figure 7 H), because they have been hypothesized to be another subset of NHP NK cells.26 These cells were quite prevalent in the PB, comprising 4.75% of the total cell population. On average, CD3–CD8+ cells were observed at lower percentages elsewhere, comprising 0.29% of SBM cells, 0.44% of IBM, 1.13% of the MLN, and 3.70% of the spleen. In the thymus, 23.0% of cells were CD3–CD8+ cells, the highest proportion among all tissues (Figure 7 I). CD56 was not expressed on the majority of CD3–CD8+ cells, and the highest incidences were 14.20% in SBM and 9.82% in MLN. In other CYNO tissues, fewer than 5.5% of CD3–CD8+ cells expressed CD56 (Figure 7 J). In comparison, the expression of CD56 among CD3–CD8– cells was high in PB, SBM, and IBM and lower in MLN, spleen, and thymus (Figure 7 K).
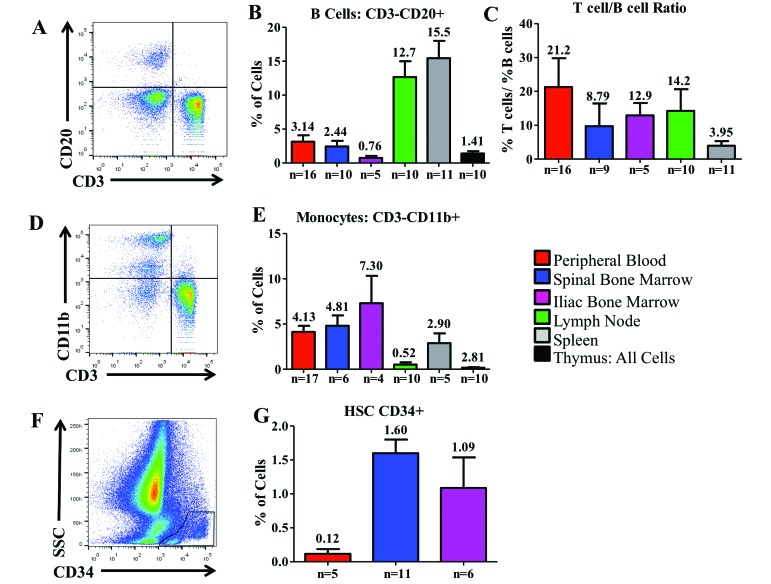
Presence of B cells in HLO.
B cells in CYNO were classified as cells that were CD3–CD20+ (Figure 8 A). As expected, B cells were most prevalent in MLN and spleen, with very few B cells in the thymus and SBM (Figure 8 B). We calculated the T:B cell ratio for each animal and averaged those ratios (Figure 8 C).
Figure 8.
Distribution of nonT-cell lymphocytes across the hematopoietic and lymphoid organs of Mauritian cynomolgus macaques. Cells shown in (E) are from Iliac Bone Marrow. (A) Using peripheral blood, we defined B cells as CD3-CD20+ cells and (B) determined their distribution. (C) The T:B cell ratio was calculated for each tissue. (D) Monocytes were defined as less granular cells that were CD3–CD11b+. (E) Their distribution was determined in each tissue. (F) We identified CD34+ hematopoietic stem cells and determined (G) the mean percentage of CD34+ hematopoietic stem cells acquired by harvesting bone marrow from either the spinal vertebrae or the iliac crest of Mauritian cynomolgus macaques; we compared that value with the proportion of CD34+ cells circulating in the peripheral blood. Gates were drawn on the basis of an isotype control and on cell size. Data are given as mean + SEM.
Distribution of monocytes across HLO.
Monocytes are myeloid cells that are excellent antigen-presenting cells. Nongranular cells (that is, with low side scatter) that were CD3–CD11b+ were classified as monocytes in CYNO (Figure 8 D). None of the tissues had large percentages of monocytes, which were highest in the SBM and IBM and comprised less than 1% of cells in MLN and thymus (Figure 8 E).
Expression of CD34 in PB and BM.
CD34 is considered to be a marker of hematopoietic stem cells in humans and NHP.5 To compare CD34+ populations across different methods of collecting BM, we compared PB CD34+ levels with CD34+ levels in SBM and IBM (Figure 8 F). We found that SBM had a mean of 1.60% CD34+ cells, whereas IBM had a mean of 1.09% CD34+ cells. In PB, CD34+ cells comprised only 0.12% (Figure 8 G).
Discussion
Our study provides the first comprehensive descriptive study of the immunophenotype of CYNO HLO. Our findings support an immunophenotype that is similar between CYNO and humans, with a few noteworthy differences.
Compared with humans, CYNO have more CD3–CD4+ and CD3–CD8+ T cells in thymus. According to murine and human fetal studies,55 thymocytes entering the cortex of the thymus experience various developmental checkpoints, at which a variety of cell surface markers are expressed. A population of CD3low/negativeCD4+ cells emerges in humans when cells enter the preT-cell stage.55 The fact that we note the same phenotype in our model suggests that the thymic function and phenotype of CYNO is comparable to that of humans. Interestingly, a thymic population present in CYNO but not in humans is CD3–CD4–CD8+ cells. CD8 expression only occurs after CD4 expression, and the coexpression of these 2 markers disappears during positive selection, by which point CD3 is fully expressed.33,55 The fact that CYNO have a CD3– population within the thymus that expresses CD8 leads us to hypothesize that this population may potentially be a subset of CYNO NK cells that is resident in the thymus or developing there.
Outside of the CYNO thymus is another population of note, CD4+CD8+ double-positive T cells, which are present throughout the lymphopoietic organs. The traditional T cell development model states that no T cells outside of the thymus should be positive for both of these markers, having been permanently delineated during thymic selection. However, CD4+CD8+ cells are consistently present across several species and do exist in healthy humans, although only as a small fraction of circulating lymphocytes.63 Previous studies of CYNO T cells overlooked this population, analyzing only CD3+CD8+ and CD3+CD4+ T cells. We are the first to characterize CD4+CD8+ T cells cross a wide range of CYNO HLO.28 In humans, increases in the percentage of CD4+CD8+ T cells occur only during autoimmune disease or viral infection, such HIV–AIDS.25,45,69 CYNO are widely used as a model in SIV and HIV studies, and this cell population is present in many patients with HIV. It previously was reported that CD4+CD8+ T cells are not positive for the entire CD8 receptor heterodimer but instead for a homodimer of CD8α.1 Our current findings differ from a previous study in CYNO,1 in which CD4+CD8+ T cells comprised 19.5% of PB cells, 4.5% of MLN cells, and 10.7% of splenic cells. The exact approach used to select these cells in the previous study1 is not described, making comparison with our analysis difficult. However, the fraction of PB T cells that are CD4+CD8+ double-positive in CYNO is known to increase with age.1,47 Whereas the CYNO in the earlier study were 10 to 17 y old, and none of our macaques was older than 12 y, it is possible that the age difference may account for the discrepancy between the 2 data sets.
Functional proliferative assays assessing the alloresponses of CD4+CD8+ T cells in CYNO have not been reported to our knowledge. However, in pigs, another commonly used large animal model, both the CD4 and CD8 coreceptors contribute to the proliferative response.63 Blocking of either coreceptor of the double-positive cells led to a decreased alloresponse, and blocking of CD4 had a greater effect than did that of CD8.63 A similar assessment has not been performed in CYNO, but their activity likely will resemble that of pigs. CD4+CD8+ T cells appear to functionally overlap both CD4+ and CD8+ T cells. In CYNO, CD4+CD8+ T cells have been demonstrated to have cytotoxic activity, contain granzyme, and express a pattern of cytokine production and receptor overlap that is indicative of a closer functional relationship to CD4+ single-positive T cells than to CD8+ cells.46
Some evidence suggests that CD4+CD8+ T cells play a role in the immune response to viruses. The increased presence of CD4+CD8+ T cells in CYNO may be an evolutionary adaptation, given that in their natural environment these animals are exposed to many viruses and depend on T-cell mediated clearance. In conjunction with conventional CD8+ cytotoxic T lymphocytes and NK cells, CD4+CD8+ T cells may enhance the likelihood of survival in the wild. This role is supported by the observation that CD4+CD8+ T cells appear during the onset of AIDS in HIV-positive human patients.25 No experimental studies have been performed to date in which CD4+CD8+ T cells are selectively eliminated from CYNO that subsequently were virally challenged.
Although the CD4:CD8 ratio of CYNO has been studied previously, the studies examined PB only, and not all included the CD3 marker to aid in the selection of T cells.4,7 We assessed the CD4:CD8 ratio in the various HLO of CYNO and compared these data with those in human tissues. A recent study of human tissues found that the CD4:CD8 ratio was approximately 4 in MLN, 2.5 in PB, and 1.7 in spleen.53 In CYNO, the ratio followed the same trend as in humans but to be lower overall, being 2.8 in MLN, 0.90 in PB, and 0.65 in spleen. This difference is noteworthy, because it indicates a markedly higher fraction of CD8+ T cells in each of these CYNO tissues. These ratios were calculated from CD4+ and CD8+ single-positive T cells, excluding the CD4+CD8+ T cells that are prominent in CYNO. Some previous studies of CYNO T cells have failed to account for this double-positive population, potentially skewing any calculated T cell ratios or numbers.54,65 In view of the additional presence of the CD4+CD8+ T cell population, our findings further support the hypothesis that Mauritian CYNO evolved an immune system that carries an increased emphasis on cytotoxic cells capable of responding to viral challenges.
B cells in CYNO play a crucial role in the adaptive immune system, just as in their human counterparts. Although the B cells of the axillary and inguinal lymph nodes of CYNO have been evaluated,28 no study has comprehensively studied the B cells throughout the HLO. In our current study, we observed a key difference in the number of B cells compared with the number of T cells. In humans, the ratio of T:B cells is approximately 4 in PB, 3 in MLN, and 1 in spleen;44 The ratios in CYNO are 21.3, 14.2, and 3.95 respectively. Although the ratios in CYNO do follow the same trend as in humans—with the ratio lowest in spleen, increasing in MLN, and highest in PB—the ratios in CYNO are clearly skewed toward T cells. The lower number of B cells and more prevalent T cells suggests that a Th1/Th17 cellular response may dominate over a humoral Th2 response in CYNO and further support the preference for a cell-mediated (cytotoxic) response. These differences between humans and NHP might be, in part, explained by differences in environment and by the fact that immunizations are used readily in humans. Because NHP receive few vaccines (at most), it is possible that these immunizations may contribute to these differences.
NK cells are a subset of the innate immune system and are derived from the same progenitors as T and B cells. NK cells undergo differentiation and selection in the BM,58 diverging from T and B cells at an early stage. In humans, NK cells have been characterized into multiple subsets, according to their expression of various combinations of markers.67 For the purpose of our current study, we initially assessed CD3– cells that expressed CD56. Our findings in CYNO differed from those in humans, in whom a lower percentage of NK cells was found in BM (0.5%) and MLN (0.2%) and a higher percentage in spleen (15%).70
Currently there is some question regarding whether CD56 is a comprehensive marker of NK cells in NHP as it is in humans. A previous study in rhesus macaques suggested that CD56 is expressed on monocytes but not on NK cells.71 These results are not definitive and have been contradicted in the same model.59 At least 2 NK populations are hypothesized to exist in NHP, one CD56+ and the other CD16+, corresponding with the human CD56bright and CD56dim NK cell populations, respectively.24
Because the majority of the work regarding NK cells was done in rhesus macaques (Macaca mulatta), further characterization of these cells is needed in Mauritian CYNO. The CD56–CD3–CD8+ population of CYNO may parallel the CD56dim NK cell subpopulation in humans.24 This hypothesis is supported by one study in CYNO that demonstrated that CD3–CD8+ cells do have cytotoxic activity associated with NK cells (in response to K562 cells).66 This hypothesis also provides an explanation for the CD3–CD8+ cells in the thymus of CYNO. According to these cited studies,24, ,66 further refinement of the definition of the NK population of Mauritian CYNO may be necessary. More reagents to NK markers are needed, specifically to CD16 and KIR, and, in conjunction with additional functional assays, will provide valuable insight into the nature of the innate immune system of Mauritian CYNO.
Because our laboratory is interested in developing novel BM transplantation approaches across MHC barriers without graft-versus-host disease, we examined different methods with which to harvest bone marrow. An optimal graft has a high percentage of CD34+ cells; successful transplantation of CD34+ cells leads to immune reconstitution of all hematopoietic lineages in humans, mice, and NHP,5,15,37 whereas failure to obtain a sufficient number of CD34+ hematopoietic stem cells may lead to delayed or failed engraftment.49,56 We also examined the T cells in the BM grafts, because CD3+ T cells play a major part in graft-versus-host disease and are involved in facilitating engraftment by ‘making space.’23,40,68 Marrow harvested from the vertebrae of CYNO (that is, SBM) had a higher overall number of nucleated cells, a higher percentage of CD34+ cells, and a smaller CD3+ population than did BM aspirates obtained by iliac puncture (that is, IBM). The procedure of spinal bone marrow harvest requires the euthanasia and exsanguination of the donor and it is less preferred because it is only applicable to cadaveric organ donors. SBM also requires more time and manpower for aseptic collection compared with BM aspiration.
Among human CD4+ T cells, CD31 has been used in conjunction with CD45RA as a marker of recent thymic emigrants.39 Therefore, coexpression of CD31 and CD45RA (a marker of naïve cells) is useful for identifying the level of maturity and relative age of CD4+ T cells.34 However, CD31 has also been found on numerous other cells, including but not limited to NK cells, granulocytes, and monocytes.41 In humans, the expression of CD31 on CD4+ T cells only persists for about 3 wk after the cells leave the thymus.39 In CYNO, CD4+ T cells that highly express CD31 occur throughout the tissues, leading us to question whether this marker in CYNO is as specific for the identification of CD4+ recent thymic emigrants as it is in humans. Perhaps CD31 is not down-regulated on CD4+ T cells after they leave the CYNO thymus.
CD31 is considered a questionable marker of human CD8+ recent thymic emigrants.39,61 Currently human CD8+ recent thymic emigrants are best described as CD8+ T cells that express a naïve phenotype (that is, CD45RO–CD45RA+CD62L+CD27brightCD11a+CD95dim) and which are positive for CD103.42 Interestingly, we saw that expression of CD31 on CD8+ T cells in CYNO may be indicative of recent thymic emigration. In the CYNO thymus, the expression of CD31 on CD8+ T cells was very high, whereas outside the thymus, CD31 expression was high only in MLN, a secondary lymph organ and a target of recent thymic emigrants.39 Perhaps most telling is that when CYNO are used as a BM transplantation model with a nonmyeloablative conditioning regimen, CD31 expression on CD8+ T cells is remarkably high immediately after transplantation in both donor and recipient cells. However, over time, this level drops toward the average.19 These findings suggest that CD31 may indeed have some utility as a marker of CD8+ recent thymic emigrants in CYNO. Further studies are needed to confirm these findings.
In addition to CD4+ and CD8+ single-positive T cells, CYNO have a population of T cells that is double negative, expressing neither of the 2 coreceptors. Although this population is un surprising in the thymus, where T cells develop and undergo multiple double-negative stages,33 CD3+CD4–CD8– cells elsewhere require further examination. There are 3 types of double-negative T cells described in healthy humans: γδ T cells, NK T cells, and the recently described double-negative suppressive T-cell subset. γδ T cells are a population of T cells that express CD3, but instead of the standard αβ T-cell receptor, they express a γδ receptor. These cells play a large role in the initiation of inflammatory responses, secreting IL179,60 and have only a relatively small role in the adaptive immune system.12,16,21 γδ T cells have been documented in many mammals including CYNO.8
NK T cells are a small subset of CD3+ T cells that do not express either CD4 or CD8. NK T cells have an αβ T-cell receptor with limited variability and only oligoclonality. These cells also straddle the border of innate and adaptive immunity and play a role in both.35 Human NK T cells are defined by invariant T-cell receptor chains. Analyzing NK T cells according to the receptor chain expression is problematic, given that this expression differs between individuals and between species. Previous studies have shown that individual NHP do not express these markers, and expression was observed only when larger populations were studied.38 Because of this situation, it may be reasonable to use a combination of markers to define NK T cells instead of just a single marker. We plan to include additional markers such as CD16 and NKG2D, perhaps allowing us to better define NK T cells in Mauritian CYNO.
The final type of double-negative T cells is a recently described population of CD4–CD8– double-negative Treg cells. These cells are distinct from CD4+ Treg cells, they exhibit a FoxP3–CD25lowCTLA4–CD69low phenotype, and, like Treg cells, cannot produce IL2. Currently CD4–CD8– double-negative Treg cells can only be distinguished through exclusion, by absence of markers.17,22 In summary, Mauritian CYNO has a considerable double-negative cell population that requires special attention and more in-depth study. The use of a γδ T-cell receptor marker in conjunction with markers specific for NK T cells would allow us to distinguish among the 3 CD4–CD8– T-cell subsets that have been described. Currently we can show that large populations of CD4–CD8– T cells are present in spleen and BM compared with PB, but we do not know which of these subsets is responsible for the difference.
Regulatory T cells are a subset of CD4+ T cells that play a role in suppressing the immune response and in self-tolerance.51 These cells are well characterized in mice and humans and help to prevent autoimmune disease. Deficiencies or defects in Treg cells have been implicated in various autoimmune diseases, including Type 1 diabetes.36 Therapeutically, Treg cells have been used to promote transplant tolerance under regimens that would normally fail, in both mouse27 and NHP (manuscript in preparation). Definitive extracellular marker that uniquely identify Treg cells are unavailable currently, and multiple markers are needed. Our current definition of Treg cells in Mauritian CYNO includes CD4+ T cells that express high levels of CD25 and are FoxP3 positive. Unfortunately, in both humans and Mauritian CYNO, activated T cells can express CD25 and FoxP3,2 and peripheral T Cells can transform temporarily into induced Treg cells in the presence of TGFβ.6 Other markers, such as CD127, GITR, and CTLA4, are indicative of natural Tregs.52 Although the PB of CYNO has been reported previously to contain Treg cells,29 this current study is the first to examine and report the Treg populations throughout the HLO of CYNO.
In humans, thymic Treg cells have been isolated, characterized phenotypically, and found to have immunosuppressive functions.3 These cells comprise 9% of CD4+CD8– cells in humans,57 which is higher than what we report in CYNO, but the discrepancy may be due to differences in the markers used during analysis. In human PB, 2.9% of CD4+ T cells are classified as activated Treg cells (defined as CD25highFoxP3+ cells).43 This proportion is comparable to our current results in CYNO, again supporting the strength of CYNO as a model system.
Overall, due to differences in gating strategy and tissue processing methods, comparing the distribution of Treg cells between CYNO and humans has been difficult, but we have shown here that Treg cells are present in comparable numbers in the PB of CYNO and humans. We also have demonstrated for the first time the distribution of Treg cells across all CYNO lymphopoietic organs.
One limitation of our study is the narrow age and sex ranges of the CYNOs used. Our animals were purpose-bred with specific MHC genotypes to minimize variability for transplantation studies. In addition, our animals were all adult male macaques younger than 12 y. Future studies should expand the cohort to include female macaques and a wider range of ages. Furthermore, the CYNO studied were all from a Mauritian colony, which may differ in cell distribution from Indian- or Chinese-origin CYNO.
In conclusion, CYNO are a well-established model that is well suited for immunologic and transplant research. A more detailed understanding of how the cell distributions in CYNO resemble or differ from those of humans will help researchers to draw informed and meaningful conclusions from experimental outcomes. Here for the first time we have comprehensively described the immunologic makeup of the HLO of adult male Mauritius CYNO and have identified some differences between CYNO and humans, providing valuable information for research immunologists and veterinarians who use CYNO in research. Further assessment of animals of different ages and sexes and of additional organs, including the gastrointestinal tract, skin, lung, and liver, will be necessary to complete the picture of the CYNO immunophenotype.
Acknowledgments
We acknowledge Drs Sulemon Chaudry and Adam Griesemer for the use of their animals and the staff of the Columbia University Institute of Comparative Medicine for technical assistance during the organ procurements. This study was supported by internal funds (to RDS) of the Columbia Center for Translational Immunology.
References
- 1.Akari H, Terao K, Murayama Y, Nam KH, Yoshikawa Y. 1997. Peripheral blood CD4+CD8+ lymphocytes in cynomolgus monkeys are of resting memory T lineage. Int Immunol 9:591–597. [DOI] [PubMed] [Google Scholar]
- 2.Allan SE, Crome SQ, Crellin NK, Passerini L, Steiner TS, Bacchetta R, Roncarolo MG, Levings MK. 2007. Activation-induced FOXP3 in human T effector cells does not suppress proliferation or cytokine production. Int Immunol 19:345–354. [DOI] [PubMed] [Google Scholar]
- 3.Annunziato F, Cosmi L, Liotta F, Lazzeri E, Manetti R, Vanini V, Romagnani P, Maggi E, Romagnani S. 2002. Phenotype, localization, and mechanism of suppression of CD4+CD25+ human thymocytes. J Exp Med 196:379–387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Baroncelli S, Panzini G, Geraci A, Pardini S, Corrias F, Iale E, Varano F, Turillazzi PG, Titti F, Verani P. 1997. Longitudinal characterization of CD4+CD8+ T-cell subsets and of haematological parameters in healthy newborns of cynomolgus monkeys. Vet Immunol Immunopathol 59:141–150. [DOI] [PubMed] [Google Scholar]
- 5.Berenson RJ, Andrews RG, Bensinger WI, Kalamasz D, Knitter G, Buckner CD, Bernstein ID. 1988. Antigen CD34+ marrow cells engraft lethally irradiated baboons. J Clin Invest 81:951–955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Beres A, Komorowski R, Mihara M, Drobyski WR. 2011. Instability of Foxp3 expression limits the ability of induced regulatory T cells to mitigate graft compared with host disease. Clin Cancer Res 17:3969–3983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bleavins MR, Brott DA, Alvey JD, de la Iglesia FA. 1993. Flow cytometric characterization of lymphocyte subpopulations in the cynomolgus monkey (Macaca fascicularis). Vet Immunol Immunopathol 37:1–13. [DOI] [PubMed] [Google Scholar]
- 8.Cendron D, Ingoure S, Martino A, Casetti R, Horand F, Romagne F, Sicard H, Fournie JJ, Poccia F. 2007. A tuberculosis vaccine based on phosphoantigens and fusion proteins induces distinct g d and αβ T cell responses in primates. Eur J Immunol 37:549–565. [DOI] [PubMed] [Google Scholar]
- 9.Chien YH, Zeng X, Prinz I. 2013. The natural and the inducible: interleukin (IL)-17-producing γδT cells. Trends Immunol 34:151–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Coleman K. 2011. Caring for nonhuman primates in biomedical research facilities: scientific, moral and emotional considerations. Am J Primatol 73:220–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Culver KW, Smith WL, Cowan MJ. 1989. Allogeneic bone marrow transplantation in newborn mice treated pre- and postnatally with antithymus 1.2 monoclonal antibody. Transplant Proc 21:2939. [PubMed] [Google Scholar]
- 12.Dar AA, Patil RS, Chiplunkar SV. 2014. Insights into the relationship between toll-like receptors and γδT cell responses. Front Immunol 5:366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Daubenberger CA, Spirig R, Patarroyo ME, Pluschke G. 2007. Flow cytometric analysis on crossreactivity of human-specific CD monoclonal antibodies with splenocytes of Aotus nancymaae, a nonhuman primate model for biomedical research. Vet Immunol Immunopathol 119:14–20. [DOI] [PubMed] [Google Scholar]
- 14.Diedrich CR, Mattila JT, Klein E, Janssen C, Phuah J, Sturgeon TJ, Montelaro RC, Lin PL, Flynn JL. 2010. Reactivation of latent tuberculosis in cynomolgus macaques infected with SIV is associated with early peripheral t cell depletion and not virus load. PLoS One 5:e9611.(Erratum in PLoS One, 2015, 10: e0124221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Donnelly DS, Zelterman D, Sharkis S, Krause DS. 1999. Functional activity of murine CD34+ and CD34– hematopoietic stem cell populations. Exp Hematol 27:788–796. [DOI] [PubMed] [Google Scholar]
- 16.Ferreira LM. 2013. γδT cells: innately adaptive immune cells? Int Rev Immunol 32:223–248. [DOI] [PubMed] [Google Scholar]
- 17.Fischer K, Voelkl S, Heymann J, Przybylski GK, Mondal K, Laumer M, Kunz-Schughart L, Schmidt CA, Andreesen R, Mackensen A. 2005. Isolation and characterization of human antigen-specific TCRαβ+ CD4–CD8– double-negative regulatory T cells. Blood 105:2828–2835. [DOI] [PubMed] [Google Scholar]
- 18.Frahm MA, Picking RA, Kuruc JD, McGee KS, Gay CL, Eron JJ, Hicks CB, Tomaras GD, Ferrari G. 2012. CD4+CD8+ T cells represent a significant portion of the antiHIV T cell response to acute HIV infection. J Immunol 188:4289–4296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Guallart PA, Zitsman J, Sondermeijer H, Woodland D, Kato Y, Weiner J, Griesemer A, Buhler L, McMurchy A, Levings M, Sykes M, Duran-Struuck R. 2015. Induction of durable mixed hematopoietic chimersism and immune tolerance in monkeys. Biol Blood Marrow Transplant 21 Suppl: S46–S47. [Google Scholar]
- 20.Hammer C. 1998. [[Potential barriers in xenogenic organ transplantation]] Schweiz Med Wochenschr 128:931–934.[Article in German]. [Google Scholar]
- 21.Hara H, Koike N, Long C, Piluek J, Roh DS, SundarRaj N, Funderburgh JL, Mizuguchi Y, Isse K, Phelps CJ, Ball SF, Ayares DL, Cooper DK. 2011. Initial in vitro investigation of the human immune response to corneal cells from genetically engineered pigs. Invest Ophthalmol Vis Sci 52:5278–5286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hillhouse EE, Lesage S. 2013. A comprehensive review of the phenotype and function of antigen-specific immunoregulatory double-negative T cells. J Autoimmun 40:58–65. [DOI] [PubMed] [Google Scholar]
- 23.Ho VT, Soiffer RJ. 2001. The history and future of T-cell depletion as graft compared with host disease prophylaxis for allogeneic hematopoietic stem cell transplantation. Blood 98:3192–3204. [DOI] [PubMed] [Google Scholar]
- 24.Hong HS, Rajakumar PA, Billingsley JM, Reeves RK, Johnson RP. 2013. No monkey business: why studying NK cells in nonhuman primates pays off. Front Immunol 4:32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Howe R, Dillon S, Rogers L, Palmer B, MaWhinney S, Blyveis N, Schlichtemeier R, D'Souza M, Ingoldby L, Harwood JE, Rietmeijer C, Ray G, Connick E, Wilson CC. 2009. Phenotypic and functional characterization of HIV1-specific CD4+CD8+ double-positive T cells in early and chronic HIV1 infection. J Acquir Immune Defic Syndr 50:444–456. [DOI] [PubMed] [Google Scholar]
- 26.Ibegbu C, Brodie-Hill A, Kourtis AP, Carter A, McClure H, Chen ZW, Nahmias AJ. 2001. Use of human CD3 monoclonal antibody for accurate CD4+ and CD8+ lymphocyte determinations in macaques: phenotypic characterization of the CD3–CD8+ cell subset. J Med Primatol 30:291–298. [DOI] [PubMed] [Google Scholar]
- 27.Joffre O, Santolaria T, Calise D, Al Saati T, Hudrisier D, Romagnoli P, van Meerwijk JP. 2007. Prevention of acute and chronic allograft rejection with CD4+CD25+Foxp3+ regulatory T lymphocytes. Nat Med 14:88–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Karlsson I, Malleret B, Brochard P, Delache B, Calvo J, Le Grand R, Vaslin B. 2007. Dynamics of T-cell responses and memory T cells during primary simian immunodeficiency virus infection in cynomolgus macaques. J Virol 81:13456–13468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Karlsson I, Malleret B, Brochard P, Delache B, Calvo J, Le Grand R, Vaslin B. 2007. FoxP3+CD25+ CD8+ T-cell induction during primary simian immunodeficiency virus infection in cynomolgus macaques correlates with low CD4+ T-cell activation and high viral load. J Virol 81:13444–13455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kawai T, Cosimi AB, Colvin RB, Powelson J, Eason J, Kozlowski T, Sykes M, Monroy R, Tanaka M, Sachs DH. 1995. Mixed allogeneic chimerism and renal allograft tolerance in cynomologous monkeys. Transplantation 59:256–262. [PubMed] [Google Scholar]
- 31.Kawai T, Cosimi AB, Wee SL, Houser S, Andrews D, Sogawa H, Phelan J, Boskovic S, Nadazdin O, Abrahamian G, Colvin RB, Sach DH, Madsen JC. 2002. Effect of mixed hematopoietic chimerism on cardiac allograft survival in cynomolgus monkeys. Transplantation 73:1757–1764. [DOI] [PubMed] [Google Scholar]
- 32.Kimikawa M, Sachs DH, Colvin RB, Bartholemew A, Kawai T, Cosimi AB. 1997. Modifications of the conditioning regimen for achieving mixed chimerism and donor-specific tolerance in cynomolgus monkeys. Transplantation 64:709–716. [DOI] [PubMed] [Google Scholar]
- 33.Koch U, Radtke F. 2011. Mechanisms of T cell development and transformation. Annu Rev Cell Dev Biol 27:539–562. [DOI] [PubMed] [Google Scholar]
- 34.Kohler S, Thiel A. 2008. Life after the thymus: CD31+ and CD31– human naïve CD4+ T-cell subsets. Blood 113:769–774. [DOI] [PubMed] [Google Scholar]
- 35.Kronenberg M. 2005. Toward an understanding of NKT cell biology: progress and paradoxes. Annu Rev Immunol 23:877–900. [DOI] [PubMed] [Google Scholar]
- 36.Lindley S, Dayan CM, Bishop A, Roep BO, Peakman M, Tree TI. 2005. Defective suppressor function in CD4+CD25+ T-cells from patients with type 1 diabetes. Diabetes 54:92–99. [DOI] [PubMed] [Google Scholar]
- 37.Link H, Arseniev L, Bahre O, Kadar JG, Diedrich H, Poliwoda H. 1996. Transplantation of allogeneic CD34+ blood cells. Blood 87:4903–4909. [PubMed] [Google Scholar]
- 38.Liu Y, Goff RD, Zhou D, Mattner J, Sullivan BA, Khurana A, Cantu C, 3rd, Ravkov EV, Ibegbu CC, Altman JD, Teyton L, Bendelac A, Savage PB. 2006. A modified α-galactosyl ceramide for staining and stimulating natural killer T cells. J Immunol Methods 312:34–39. [DOI] [PubMed] [Google Scholar]
- 39.Ljungman P, Deliliers GL, Platzbecker U, Matthes-Martin S, Bacigalupo A, Einsele H, Ullmann J, Musso M, Trenschel R, Ribaud P, Bornhauser M, Cesaro S, Crooks B, Dekker A, Gratecos N, Klingebiel T, Tagliaferri E, Ullmann AJ, Wacker P, Cordonnier C. 2001. Cidofovir for cytomegalovirus infection and disease in allogeneic stem cell transplant recipients. The Infectious Diseases Working Party of the European Group for Blood and Marrow Transplantation. Blood 97:388–392. [DOI] [PubMed] [Google Scholar]
- 40.Martin PJ, Hansen JA, Buckner CD, Sanders JE, Deeg HJ, Stewart P, Appelbaum FR, Clift R, Fefer A, Witherspoon RP, Kennedy MS, Sullivan KM, Flournoy N, Storb R, Thomas ED. 1985. Effects of in vitro depletion of T cells in HLA-identical allogeneic marrow grafts. Blood 66:664–672. [PubMed] [Google Scholar]
- 41.Matar AJ, Pathiraja V, Wang Z, Duran-Struuck R, Gusha A, Crepeau R, Tasaki M, Sachs DH, Huang CA. 2012. Effect of preexisting antidiphtheria toxin antibodies on T cell depletion levels following diphtheria toxin-based recombinant antimonkey CD3 immunotoxin treatment. Transpl Immunol 27:52–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.McFarland RD, Douek DC, Koup RA, Picker LJ. 2000. Identification of a human recent thymic emigrant phenotype. Proc Natl Acad Sci USA 97:4215–4220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Miyara M, Yoshioka Y, Kitoh A, Shima T, Wing K, Niwa A, Parizot C, Taflin C, Heike T, Valeyre D, Mathian A, Nakahata T, Yamaguchi T, Nomura T, Ono M, Amoura Z, Gorochov G, Sakaguchi S. 2009. Functional delineation and differentiation dynamics of human CD4+ T cells expressing the FoxP3 transcription factor. Immunity 30:899–911. [DOI] [PubMed] [Google Scholar]
- 44.Monticelli LA, Sonnenberg GF, Abt MC, Alenghat T, Ziegler CG, Doering TA, Angelosanto JM, Laidlaw BJ, Yang CY, Sathaliyawala T, Kubota M, Turner D, Diamond JM, Goldrath AW, Farber DL, Collman RG, Wherry EJ, Artis D. 2011. Innate lymphoid cells promote lung–tissue homeostasis after infection with influenza virus. Nat Immunol.12:1045–1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Munschauer FE, Stewart C, Jacobs L, Kaba S, Ghorishi Z, Greenberg SJ, Cookfair D. 1993. Circulating CD3+CD4+CD8+ T-lymphocytes in multiple sclerosis. J Clin Immunol 13:113–118. [DOI] [PubMed] [Google Scholar]
- 46.Nam K, Akari H, Terao K, Shibata H, Kawamura S, Yoshikawa Y. 2000. Peripheral blood extrathymic CD4+CD8+ T cells with high cytotoxic activity are from the same lineage as CD4+CD8– T cells in cynomolgus monkeys. Int Immunol 12:1095–1103. [DOI] [PubMed] [Google Scholar]
- 47.Nam KH, Akari H, Terao K, Itagaki S, Yoshikawa Y. 1998. Age-related changes in major lymphocyte subsets in cynomolgus monkeys. Exp Anim 47:159–166. [DOI] [PubMed] [Google Scholar]
- 48.Nelson BH. 2004. IL2, regulatory T cells, and tolerance. J Immunol 172:3983–3988. [DOI] [PubMed] [Google Scholar]
- 49.Newman PJ. 1997. The biology of PECAM1. J Clin Invest 100:S25–S29. [PubMed] [Google Scholar]
- 50.O'Connor SL, Blasky AJ, Pendley CJ, Becker EA, Wiseman RW, Karl JA, Hughes AL, O'Connor DH. 2007. Comprehensive characterization of MHC class II haplotypes in Mauritian cynomolgus macaques. Immunogenetics 59:449–462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sakaguchi S, Sakaguchi N, Shimizu J, Yamazaki S, Sakihama T, Itoh M, Kuniyasu Y, Nomura T, Toda M, Takahashi T. 2001. Immunologic tolerance maintained by CD25+ CD4+ regulatory T cells: their common role in controlling autoimmunity, tumor immunity, and transplantation tolerance. Immunol Rev 182:18–32. [DOI] [PubMed] [Google Scholar]
- 52.Sakaguchi S, Vignali DA, Rudensky AY, Niec RE, Waldmann H. 2013. The plasticity and stability of regulatory T cells. Nat Rev Immunol 13:461–467. [DOI] [PubMed] [Google Scholar]
- 53.Sathaliyawala T, Kubota M, Yudanin N, Turner D, Camp P, Thome JJ, Bickham KL, Lerner H, Goldstein M, Sykes M, Kato T, Farber DL. 2013. Distribution and compartmentalization of human circulating and tissue-resident memory T-cell subsets. Immunity 38:187–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sato R, Tomita K, Sano H, Ichihashi H, Yamagata S, Sano A, Yamagata T, Miyara T, Iwanaga T, Muraki M, Tohda Y. 2009. The strategy for predicting future exacerbation of asthma using a combination of the asthma control test and lung function test. J Asthma 46:677–682. [DOI] [PubMed] [Google Scholar]
- 55.Spits H. 2002. Development of αβ T cells in the human thymus. Nat Rev Immunol 2:760–772. [DOI] [PubMed] [Google Scholar]
- 56.Steffens HP, Podlech J, Kurz S, Angele P, Dreis D, Reddehase MJ. 1998. Cytomegalovirus inhibits the engraftment of donor bone marrow cells by downregulation of hemopoietin gene expression in recipient stroma. J Virol 72:5006–5015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Stephens LA, Mottet C, Mason D, Powrie F. 2001. Human CD4+CD25+ thymocytes and peripheral T cells have immune suppressive activity in vitro. Eur J Immunol 31:1247–1254. [DOI] [PubMed] [Google Scholar]
- 58.Sun JC, Lanier LL. 2011. NK cell development, homeostasis and function: parallels with CD8+ T cells. Nat Rev Immunol 11:645–657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sun Y, Asmal M, Lane S, Permar SR, Schmidt SD, Mascola JR, Letvin NL. 2011. Antibody-dependent cell-mediated cytotoxicity in simian immunodeficiency virus-infected rhesus monkeys. J Virol 85:6906–6912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sutton CE, Mielke LA, Mills KH. 2012. IL17-producing γδ T cells and innate lymphoid cells. Eur J Immunol 42:2221–2231. [DOI] [PubMed] [Google Scholar]
- 61.Tanaskovic S, Fernandez S, Price P, Lee S, French MA. 2010. CD31 (PECAM1) is a marker of recent thymic emigrants among CD4+ T cells, but not CD8+ T cells or γδ T cells, in HIV patients responding to ART. Immunol Cell Biol 88:321–327. [DOI] [PubMed] [Google Scholar]
- 62. United States Centers for Disease Control. [Internet]. 2001. Public Health Service Guideline on Infectious Disease Issues in Xenotransplantation. MMWR Morb Mortal Wkly. [Cited 01 June 2015]. Available at: http://www.cdc.gov/mmwr/preview/mmwrhtml/rr5015a1.htm.
- 63.United States Department of Health and Human Services. [Internet]. 2004.Secretary's Advisory Committee on Xenotransplantation (SACX). Report on the state of the science in xenotransplantation, Draft. [Cited 01 June 2015]. Available at: http://www.nelsonerlick.com/PDF/NIH%20Report%20on%20State%20of%20Xenotransplantation%202005.pdf.
- 64.Velaga S, Ukena SN, Dringenberg U, Alter C, Pardo J, Kershaw O, Franzke A. 2015. Granzyme A is required for regulatory T-cell–mediated prevention of gastrointestinal graft-versus-host disease. PLoS One 10:e0124927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Verdier F, Aujoulat M, Condevaux F, Descotes J. 1995. Determination of lymphocyte subsets and cytokine levels in cynomolgus monkeys. Toxicology 105:81–90. [DOI] [PubMed] [Google Scholar]
- 66.Vieillard V, Habib RE, Brochard P, Delache B, Bovendo HF, Calvo J, Morin J, Picq I, Martinon F, Vaslin B, Le Grand R, Debre P. 2008. CCR5 or CXCR4 use influences the relationship between CD4 cell depletion, NKp44L expression, and NK cytotoxicity in SHIV-infected macaques. AIDS 22:185–192. [DOI] [PubMed] [Google Scholar]
- 67.Vivier E, Tomasello E, Baratin M, Walzer T, Ugolini S. 2008. Functions of natural killer cells. Nat Immunol 9:503–510. [DOI] [PubMed] [Google Scholar]
- 68.Wagner JE, Santos GW, Noga SJ, Rowley SD, Davis J, Vogelsang GB, Farmer ER, Zehnbauer BA, Saral R, Donnenberg AD. 1990. Bone marrow graft engineering by counterflow centrifugal elutriation: results of a phase I–II clinical trial. Blood 75:1370–1377. [PubMed] [Google Scholar]
- 69.Weiss R, Huhn D, Mitrou P, Nerl C, Schurmann D, Scheidegger C, Knauf W, Trenn G, Kronawitter U, Van Lunzen J, Arasteh K, Herbst H. 1998. HIV-related nonHodgkin's lymphoma: CHOP induction therapy and interferon α2b–zidovudine maintenance therapy. Leuk Lymphoma 29:103–118. [DOI] [PubMed] [Google Scholar]
- 70.Westermann J, Pabst R. 1992. Distribution of lymphocyte subsets and natural killer cells in the human body. Clin Investig 70:539–544. [DOI] [PubMed] [Google Scholar]
- 71.World Health Organization. [Internet] 2001. Organisation for Economic Co-operation and Development (OECD)/World Health Organization (WHO), Consultation on Xenotransplantation Surveillance: Summary. Geneva, WHO,[Cited 01 June 2015]. Available at: http://whqlibdoc.who.int/hq/2001/WHO_CDS_CSR_EPH_2001.1.pdf.
- 72.Yoshino N, Ami Y, Terao K, Tashiro F, Honda M. 2000. Upgrading of flow cytometric analysis for absolute counts, cytokines, and other antigenic molecules of cynomolgus monkeys (Macaca fascicularis) by using antihuman crossreactive antibodies. Exp Anim 49:97–110. [DOI] [PubMed] [Google Scholar]