Abstract
Chronic diarrhea poses a significant threat to the health of NHP research colonies, and its primary etiology remains unclear. In macaques, the clinical presentation of intractable diarrhea and weight loss that are accompanied by inflammatory infiltrates within the gastrointestinal tract closely resembles inflammatory bowel disease of humans, dogs, and cats, in which low serum and tissue cobalamin (vitamin B12) levels are due to intestinal malabsorption. We therefore hypothesized that macaques with chronic idiopathic diarrhea (CID) have lower serum cobalamin concentrations than do healthy macaques. Here we measured serum cobalamin concentrations in both rhesus and pigtailed macaques with CID and compared them with those of healthy controls. Serum cobalamin levels were 2.5-fold lower in pigtailed macaques with CID than control animals but did not differ between rhesus macaques with CID and their controls. This finding supports the use of serum cobalamin concentration as an adjunct diagnostic tool in pigtailed macaques that present with clinical symptoms of chronic gastrointestinal disease. This use of serum vitamin B12 levels has implications for the future use of parenteral cobalamin supplementation to improve clinical outcomes in this species.
Abbreviations: CID, chronic idiopathic diarrhea
Chronic idiopathic diarrhea (CID) is one of the most frequent clinical problems encountered in NHP and is responsible for high levels of morbidity and mortality within captive macaque colonies. Incidence rates as high as 15% have been reported at institutions housing breeding colonies of macaques.4 At our facility, the estimated incidence rate based on clinical records for CID in the last year was 8% in rhesus macaques and 11% in pigtailed macaques. Clinical management of CID in macaques typically includes antiinflammatory or immunosuppressive therapies, and in many cases combinations of these drugs are used. Despite aggressive drug therapy and provision of supportive care, treatment of CID in macaques is largely unrewarding. Animals are often either nonresponsive or become refractory to treatment, and some eventually require euthanasia due to a progressive decline in condition. Chronic diarrhea has been reported to account for 44% of macaque deaths at one institution and is the primary reason for euthanasia of macaques at our facility.25 Review of our institutional pathology database revealed that 38% of all adult macaques submitted for necropsy between August 2014 and August 2015 were diagnosed with CID.
Clinicians who work with NHP have extrapolated from both human and companion animal literature to find alternative treatments for chronic diarrhea that have the potential to improve clinical outcomes. Previous reports of nontraditional treatments include the use of the macrolide antibiotic tylosin, oral fecal bacteriotherapy, therapeutic whipworm infection, and even the addition of dietary coconut.7,8,14,54 The rationale for attempting these treatments may be based on anecdotal reports or proven success in other species. One common practice in the management of human and companion animal patients with chronic gastrointestinal disease is supplementation with parenteral cobalamin (vitamin B12). Hypocobalaminemia (low serum cobalamin levels) and cobalamin deficiency (low tissue levels) are widely reported in cats, dogs, and humans with chronic gastrointestinal disease of various etiologies. Common causes in cats and dogs include inflammatory bowel disease and intestinal lymphoma.3,42 Human inflammatory bowel disease, specifically Crohn disease, is associated with cobalamin deficiency.5 Though the reported prevalence varies according to study, species, and primary disease, there is increasing evidence that a deficiency in cobalamin is a risk factor for negative outcomes.3,28,37 In addition, early identification and treatment of such a deficiency plays a key role in successful management of chronic gastrointestinal disease in these species. Animals and humans that are not concurrently treated with cobalamin are often found to be less responsive to therapies directed at their primary underlying disease.3,42,43
Cobalamin is a water-soluble vitamin that is not synthesized by mammals and therefore must be obtained through the diet. Standard commercial diets fed to captive macaques are supplemented with cobalamin, whereas macaques in the wild may obtain the vitamin through behaviors such as coprophagy, eating insects, and preening.26 The mechanism of dietary cobalamin absorption relies on a series of proteins and enzymes that are produced by the stomach, pancreas, liver, and small intestine. Certain diseases affecting the function of these organs can disrupt this absorptive pathway and lead to a deficiency in cobalamin. When other underlying causes of cobalamin deficiency have been ruled out, serum cobalamin concentration is used as a marker of small intestinal dysfunction, most notably of the ileum.42,53 Ileal enterocytes contain specific receptors responsible for absorption of cobalamin into circulation. Thus, regardless of underlying etiology, ileal mucosal disease resulting in reduced absorptive ability can lead to cobalamin deficiency.
Noninfectious, chronic diarrhea in macaques is traditionally referred to as chronic colitis or chronic idiopathic colitis; however, necropsy findings in macaques with chronic diarrhea at Johns Hopkins University commonly include multifocal to diffuse inflammatory infiltrates consisting predominantly of lymphocytes and plasma cells throughout both the small and large intestine.21,41,45 Given this information, we postulated that macaques with CID, similar to other species with gastrointestinal disease, are at risk for developing a cobalamin deficiency due to malabsorption within the ileum. The presence of such a deficiency has potentially significant clinical implications and would support future investigation into the use of cobalamin as a supplemental treatment for chronic diarrhea in macaques.
We hypothesized that rhesus and pigtailed macaques with CID have lower serum cobalamin concentrations than do control animals. To evaluate this hypothesis, we measured serum cobalamin concentration in both rhesus and pigtailed macaques with CID and compared them with those of healthy conspecifics. In addition, we measured serum cobalamin concentrations in subpopulations of healthy rhesus and pigtailed macaques for the purpose of establishing normal ranges for our breeding colony. Lastly, for animals where histopathologic data was available, we examined the relationship between serum cobalamin concentration and the presence of ileal mucosal disease.
Materials and Methods
Animals.
All macaques used in this study were housed in indoor–outdoor enclosures in harem breeding groups at the Johns Hopkins University Research Farm. Enclosures were either large runs with concrete flooring or corncrib cages that were raised off the ground. Groups varied in composition in regard to age, sex, and total number of animals. Rhesus (Macaca mulatta) and pigtailed macaques (Macaca nemestrina) were housed separately. All macaques were fed a standard commercial diet (rhesus macaques: 2050 Teklad Global 20% Protein Primate Diet, Harlan Laboratories, Madison, WI; pigtailed macaques: 5038 Monkey Diet, LabDiet, Brentwood, MO). The diet was supplemented on a rotating schedule with a variety of food enrichment items, including fresh fruits, vegetables, and forage. Animals were provided water without restriction. Macaques were screened annually and were consistently serologically negative for Macacine herpesvirus 1 (B virus), SIV, simian T-cell leukemia virus, and simian retrovirus. Intradermal tuberculin skin tests were performed semiannually, and all animals in the colony were consistently negative for tuberculosis. All animal work was approved by the Johns Hopkins University IACUC and determined to be in accordance with the guidelines outlined in the Animal Welfare Act, federal regulations, and the Guide for the Care and Use of Laboratory Animals.23
Study groups.
Rhesus and pigtailed macaques were allocated into 2 study groups per species: normal animals (healthy controls) and those with CID. Inclusion criteria for the normal, healthy control groups were adults 3 y and older with no history of diarrhea since birth and no current clinical conditions or ongoing health problems. For the CID groups, macaques were adults 3 y and older and were included when they met at least 1 of the following criteria: (1) diarrhea that recurred and persisted intermittently for no shorter than 6 mo duration after antibacterial or antiprotozoal treatment despite clearance of a previously identified pathogen; (2) intermittent diarrhea of 3 mo or greater in duration for which an infectious cause was not determined; and (3) confirmed CID. The diagnosis of confirmed idiopathic diarrhea was made after exclusion of infectious causes and on the basis of lymphoplasmacytic inflammation in intestinal biopsies taken at endoscopy or laparotomy. Animals were excluded from both the normal and CID groups when they were currently on medication, in menses, pregnant, or lactating or when they had any major wounds, infections, arthritis, or other chronic diseases. Study group demographics are presented in Tables 1 and 2.
Table 1.
Demographics of control macaques
| Age (y) | Sex | Results of fecal culture | Serum cobalamin (ng/L) | |
| Pigtail 1 | 6 | F | Campylobacter spp. | >2000 |
| Pigtail 2 | 7 | F | negative | >2000 |
| Pigtail 3 | 8 | F | negative | 1551 |
| Pigtail 4 | 8 | M | Campylobacter spp. | >2000 |
| Pigtail 5 | 10 | F | negative | >2000 |
| Pigtail 6 | 16 | F | negative | 1699 |
| Rhesus 1 | 6 | F | Campylobacter spp. | 799 |
| Rhesus 2 | 7 | F | negative | >2000 |
| Rhesus 3 | 9 | F | Shigella | 1683 |
| Rhesus 4 | 10 | F | Shigella | >2000 |
| Rhesus 5 | 10 | M | Campylobacter spp. | >2000 |
| Rhesus 6 | 20 | M | negative | 727 |
Table 2.
Demographics of macaques with chronic idiopathic diarrhea
| Age (y) | Sex | Disease duration (mo) | Serum cobalamin (ng/L) | |
| Pigtail 7 | 7 | F | 32 | 921 |
| Pigtail 8 | 8 | F | 8 | 1,070 |
| Pigtail 9 | 8 | F | 1 | 741 |
| Pigtail 10 | 8 | M | 3 | 856 |
| Pigtail 11 | 11 | F | 68 | 403 |
| Pigtail 12 | 16 | F | 44 | 658 |
| Rhesus 7 | 4 | F | 4 | >2,000 |
| Rhesus 8 | 8 | F | 76 | 762 |
| Rhesus 9 | 10 | F | 3 | 1,095 |
| Rhesus 10 | 12 | F | 64 | 786 |
| Rhesus 11 | 16 | M | 43 | >2,000 |
| Rhesus 12 | 17 | M | 80 | 1,802 |
Additional subpopulations of healthy adult rhesus (n = 19; age, 3 to 22 y; 16 female, 3 male) and pigtailed macaques (n = 19; age, 3 to 18 y; 13 female; 6 male) with no history of diarrhea since birth were included in the study for the purpose of establishing normal ranges for serum cobalamin concentration. To establish normal ranges, these subpopulations from our breeding colony were combined with the normal study group animals for a total of 25 rhesus and 25 pigtailed macaques.
Sample collection.
All animals were anesthetized with ketamine HCl (10 to 15 mg/kg IM; Zetamine, VetOne, Boise, ID) for sample collection. Blood was collected and submitted to a commercial laboratory (Idexx Laboratories, Glen Burnie, MD) for CBC (XT-2000iV Automated Hematology Analyzer, Sysmex America, Lincolnshire, IL) and serum chemistry (Beckman Coulter AU5812, Diamond Diagnostics, Holliston, MA) analyses and measurement of serum cobalamin concentration. Each macaque received a physical examination during which weight and body condition were recorded. Feces were collected directly from the rectum of each animal, placed immediately into transport medium (CultureSwab Cary-Blair Agar, Becton Dickinson, Sparks, MD), and cultured for the presence of enteric pathogens (Salmonella, Shigella, Campylobacter spp., Yersinia, Aeromonas, Plesiomonas, E. coli O157, and Vibrio). Fecal samples for culture were submitted to the Johns Hopkins University Clinical Microbiology Lab (Baltimore, MD). Samples were incubated on MacConkey agar and xylose–lysine–desoxycholate agar in a 5% CO2 incubator at 35 °C and read at 24 h. Samples also were plated on Campy CVA agar, incubated at 42 °C and 10% CO2 under microaerophilic conditions in a candle jar, and read at 48 h.
For animals in the CID groups, an additional fecal sample was taken for flotation and direct examination for the presence of protozoan parasites (Giardia lamblia, Balantidium coli, and Entamoeba histolytica), performed inhouse. Fecal samples were placed in a conical tube and mixed with a zinc sulfate solution (400 g/L; Fisher Scientific, Pittsburgh, PA). A coverslip was placed over the samples and allowed to sit for 15 min before examination under a light microscope at 4× and 10×. In addition, fecal samples were smeared onto a slide and mixed with 0.9% saline for direct examination under a light microscope at 10× and 20×. Furthermore, the colony was screened for the presence of Giardia via ELISA (Idexx Laboratories) and found to be negative. Macaques were excluded from the CID study groups when they tested positive for any of the described fecal pathogens by any of the indicated methods. Animals in the control groups that were culture-positive for fecal pathogens but had no history of clinical disease were considered to be chronic carriers of these organisms and were included in the study.
Cobalamin assay.
Blood was collected from fasted animals into serum separator tubes (Covidien, Minneapolis, MN). Serum samples were kept at 2 to 8 °C prior to shipment to the laboratory (Idexx Laboratories), and all samples were submitted on the same day as collection. Serum cobalamin levels were measured by using a solid-phase, competitive chemiluminescent enzyme immunoassay involving an automated alkaline denaturation procedure (IMMULITE 2000 XPi Immunoassay System, Siemens, Tarrytown, NY). According to the manufacturer, this assay has been validated in humans but is used by many laboratories to evaluate samples from a variety of species.19,47 The upper limit of detection for the serum cobalamin assay was 2000 ng/L. For animals with a reported serum cobalamin level greater than 2000 ng/L, values were set at 2000 ng/L for all statistical comparisons.
Tissue biopsy.
After CID and normal study groups were established and study samples obtained, 5 animals (2 rhesus macaques and 3 pigtailed macaques) underwent either endoscopy, laparotomy, or necropsy at different time points. In addition, 1 rhesus macaque underwent endoscopy prior to inclusion in this study. The decision to perform each of these procedures was made by the clinician managing the individual case and as determined necessary at that time for either diagnostic or humane purposes. Biopsies were obtained during endoscopy from 1 pigtailed macaque and 1 rhesus macaque. Animals that underwent endoscopy were anesthetized with ketamine HCl (10 to 15 mg/kg IM; Zetamine), intubated, and placed under general anesthesia with isoflurane gas (Forane, Baxter Healthcare, Deerfield, IL). Upper gastrointestinal endoscopy was performed by using a flexible fiber gastroscope (diameter, 7.9 mm; channel, 2.0 mm; field of view, 100°; GIF XP20, Olympus, Center Valley, PA). Pinch biopsies of the gastric and duodenal mucosa were obtained from each animal (3 from each site) by passing biopsy forceps (Radial Jaw 4, Boston Scientific, Marlborough, MA) through the gastroscope. Pinch biopsies of the distal colonic mucosa (3 per animal) were obtained blindly by inserting biopsy forceps into the colon in a retrograde manner.43 Animals that underwent endoscopy received famotidine (1 mg/kg SC; West-Ward Pharmaceuticals, Eatontown, NJ) on the day of the procedure.
For 1 pigtailed macaque, biopsies were obtained during laparotomy. This animal was placed under general anesthesia as described previously, and laparotomy was performed in a dedicated operating room by using aseptic technique. Single, full-thickness biopsies (approximate diameter, 5 mm) of the jejunum, ileum, and colon were obtained. Postoperatively, this animal received buprenorphine (0.03 mg/kg IM twice daily for 3 d; Reckitt Benckiser Pharmaceuticals, Richmond, VA). In addition, intestinal samples were obtained at necropsy for 1 pigtailed macaque and 2 rhesus macaques that were euthanized due to poor prognosis. To keep interpretation of intestinal biopsies consistent, all samples were resubmitted to a single board-certified veterinary pathologist that had not seen the samples previously. The pathologist was informed of the species and clinical history of each animal but had no knowledge of serum cobalamin concentration or other data obtained from animals in this study. Histologic diagnosis was graded as either mild, moderate, or severe. We used the data obtained from these 6 macaques to examine the relationship between disease location and serum cobalamin level. Amyloidosis was not detected in any of the macaques for which biopsy samples were available.
Statistical analysis.
All results were evaluated by using a commercial statistical analysis software package (Prism version 5.00 for Windows, GraphPad Software, San Diego, CA). Spearman correlation was used to evaluate the relationship between age and serum cobalamin concentration, whereas a 2-tailed, Mann–Whitney test was used to evaluate for sex-associated differences in serum cobalamin levels. To compare serum cobalamin concentrations between control and CID study groups, a 2-tailed, Mann–Whitney test was performed. Spearman correlation was used to evaluate the relationship between disease duration and serum cobalamin concentration. A P value less than 0.05 was considered significant for all comparisons. To compare hematologic parameters between control and CID study groups, a 2-tailed, Mann–Whitney test was performed for each parameter (9 parameters total). Posthoc Bonferroni correction was performed to account for multiple comparisons, and a P value less than 0.0056 was considered significant for all hematologic data analyzed.
Results
Normal ranges for serum cobalamin concentration.
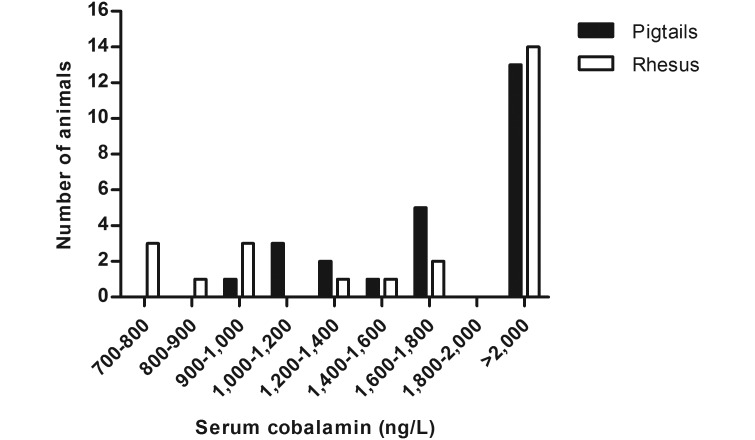
Serum cobalamin concentration was measured in healthy adult pigtailed (n = 25; age, 3 to 18 y; 18 female, 7 male) and rhesus (n = 25; age, 3 to 22 y; 20 female; 5 male) macaques for the purpose of establishing normal ranges in our colony for this study. The range for serum cobalamin concentration in pigtailed macaques was 961 to 2000ng/L, whereas that for rhesus macaques was 727 to 2000 ng/L (Figure 1). Upper values of these ranges were limited due to the number of normal animals whose serum cobalamin concentrations were beyond the upper limit of the detection assay.
Figure 1.
Distribution of serum cobalamin levels in healthy populations of pigtailed and rhesus macaques (n = 25 each group).
Using the same groups of healthy animals, we also evaluated the relationship between age and serum cobalamin concentration. There was no correlation between age and serum cobalamin concentration in pigtailed (Spearman correlation, P = 0.4962, r = 0.1427) or rhesus macaques (Spearman correlation, P = 0.8322, r = 0.0446). The median age of the pigtailed macaques was 7 y, whereas that of rhesus macaques was 6 y. Lastly, sex-associated differences in serum cobalamin concentration were examined. There was no sex-associated difference in serum cobalamin concentration in pigtailed (Mann–Whitney test, P = 0.9220, 2-tailed) or rhesus macaques (Mann–Whitney test, P = 0.8811, 2-tailed).
Serum cobalamin concentrations in control compared with CID macaques.
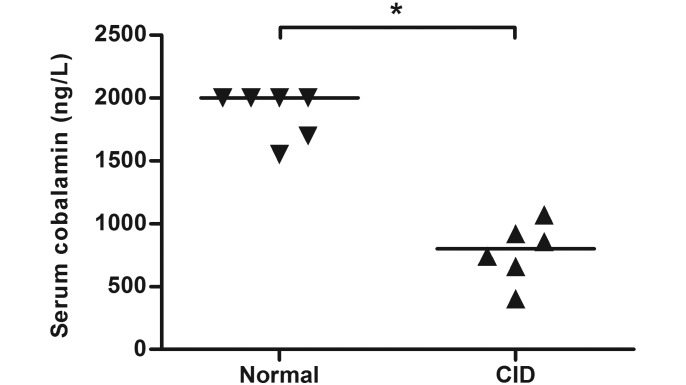
The median serum cobalamin concentration was 799 ng/L in pigtailed macaques with CID and 2000 ng/L in healthy controls. Whereas pigtailed macaques with CID had lower (P = 0.0043) serum cobalamin concentrations than did healthy controls (Figure 2), serum cobalamin concentration did not differ between rhesus macaques with CID and healthy controls (Mann–Whitney test, P = 0.8033, 2-tailed). The median serum cobalamin concentration was 1449 ng/L in rhesus macaques with CID and 1842 ng/L in healthy controls.
Figure 2.
Serum cobalamin concentrations (bar, median) in pigtailed macaques with chronic idiopathic diarrhea (CID) compared with healthy controls. Serum cobalamin levels were significantly (Mann–Whitney test, P = 0.0043, 2-tailed) lower in pigtailed macaques with CID than in control animals.
According to the normal ranges we established for healthy macaques in our colony, 5 of 6 (83%) of pigtailed macaques in the CID study group had serum cobalamin levels below the lowest value observed in our subpopulation of healthy pigtailed macaques. In contrast, none of the 6 rhesus macaques in the CID study group had below-normal serum cobalamin levels. Furthermore, for the current small sample size, disease duration and serum cobalamin level were not significantly correlated in either pigtailed (Spearman correlation, n = 6, P = 0.3556, r = –0.4857) or rhesus macaques (Spearman correlation, n = 6, P = 0.4972, r = –0.3479).
Hematologic parameters in control compared with CID macaques.
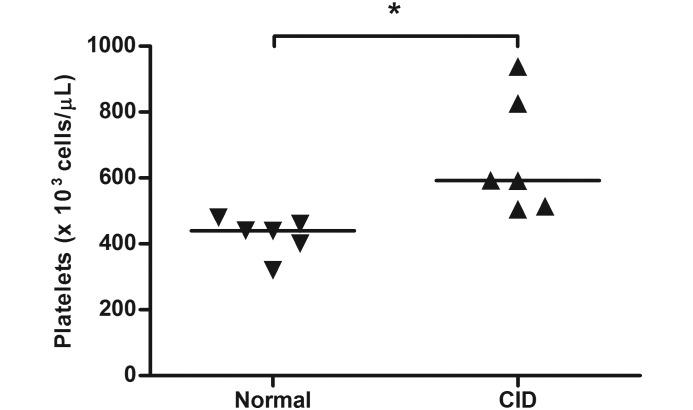
Changes in CBC and serum chemistry parameters occur in other species with chronic gastrointestinal disease.3,11,13,40,51,52 To determine whether there were any comparable changes in macaques with CID, 9 of these hematologic parameters were compared with those of healthy controls. CBC parameters evaluated were WBC count, Hct, MCV, and platelet count. Serum chemistry parameters evaluated were albumin, ALP, phosphorus, potassium, and total calcium. Compared with those of healthy controls, platelet counts were significantly higher in pigtailed macaques with CID (P = 0.0022, Figure 3) but not in rhesus macaques with CID (Mann–Whitney test, P = 0.6991, 2-tailed, data not shown). There were no other significant differences in hematologic parameters between control animals and those with CID for either pigtailed or rhesus macaques.
Figure 3.
Platelet counts (bar, median) in pigtailed macaques with chronic idiopathic diarrhea (CID) compared with healthy controls. Platelet counts were significantly (Mann–Whitney test, P = 0.0022, 2-tailed) higher in pigtailed macaques with CID compared with control animals.
Fecal examination.
All animals in the CID study groups were negative by culture and direct examination for the presence of gastrointestinal pathogens. All macaques in the control groups were negative on direct examination for the presence of gastrointestinal pathogens. In contrast, 2 of the 6 pigtailed macaques in the control group were culture-positive for Campylobacter spp., 2 of the 6 rhesus macaques in the healthy control group were culture-positive for Campylobacter spp., and another 2 of the 6 rhesus macaque controls were culture-positive for Shigella. Given the asymptomatic status of these animals and lack of history of diarrhea, all of the control macaques that were culture-positive were considered to be chronic carriers of those organisms.
Tissue biopsy.
Histopathologic data from 3 rhesus and 3 pigtailed macaques in the CID study groups were examined (Table 3). Biopsies obtained during endoscopy were limited to the mucosal layer; those obtained during laparotomy or at necropsy were full-thickness biopsies. These data indicated that 2 of the 3 pigtailed macaques had confirmed ileal disease; although these animals also had the lowest serum cobalamin levels of the CID group, a conclusive relationship between the presence of ileal disease and serum cobalamin concentration in pigtailed macaques could not be determined due to the small sample size available. In addition, these same animals had inflammatory disease that affected multiple segments of the gastrointestinal tract. Ileal tissue was not available from the third pigtailed macaque. In contrast, examination of the ileal tissue from 2 of the 3 rhesus macaques did not reveal any disease; ileal tissue from the third rhesus macaque was unavailable. Thus, in this small cohort, there is no evidence for a relationship between ileal disease and serum cobalamin concentration in rhesus macaques.
Table 3.
Location of disease and histopathologic diagnoses in macaques with chronic idiopathic diarrhea
| Diagnostic method | Stomach | Duodenum | Jejunum | Ileum | Colon | |
| Pigtail 9 | Endoscopy | mild; LP | moderate; LP | not examined | not examined | moderate; LP |
| Pigtail 11 | Laparotomy | not examined | not examined | mild; LP | mild; LP, N | moderate; LP, H, N |
| Pigtail 12 | Necropsy | moderate; LP | mild; LP, H | mild; LP, H | severe; N, LP, H | mild; LP, H |
| Rhesus 8 | Necropsy | mild; LP | no disease | no disease | no disease | moderate; LP, N |
| Rhesus 9 | Necropsy | no disease | no disease | mild; L | no disease | mild; LP |
| Rhesus 11 | Endoscopy | mild; LP | mild; LP | not examined | not examined | moderate; LP |
H, histiocytic; LP, lymphoplasmacytic; L, lymphocytic; N, neutrophilic
Discussion
One noteworthy finding in the current study was the identification of the lower serum cobalamin levels in pigtailed macaques with CID compared with healthy conspecifics. This result is consistent with that reported in cats, dogs, and humans with chronic gastrointestinal disease.5,6,51 Numerous studies have examined changes in serum cobalamin levels in both humans and companion animals, although the prevalence of cobalamin deficiency varies depending on the underlying etiology and population studied. In cats and dogs with inflammatory bowel disease or intestinal lymphoma, reported prevalence ranges are 5% to 78% and 6% to 36%, respectively.3,6,22,28,31,40 In human patients, Crohn disease is most commonly associated with an increased risk for cobalamin deficiency, and prevalence ranges from 0% to 60% have been reported.5,20 Interestingly, 83% of pigtailed macaques with CID had serum cobalamin levels that were below the lowest level in our normal subpopulation of pigtailed macaques.
Pigtailed macaques with CID may be more susceptible to cobalamin malabsorption than are other species. However, given the wide prevalence ranges reported in other species studied, we cannot make a definitive conclusion based on the single population used in the current study. In addition, false-positive and false-negative rates for measuring serum cobalamin may be as high as 50%.5 This situation might be one reason why reported prevalence differs from study to study. In addition, subject inclusion criteria can influence results. For instance, the presence of concurrent diseases that can lead to cobalamin malabsorption, such as pancreatic and liver disease, may result in reporting a falsely high prevalence of cobalamin deficiency when those diseases go undiagnosed. In contrast, the inclusion of patients that are currently on medication or that were not fasted prior to sample collection may result in reporting a falsely low prevalence of cobalamin deficiency. Therefore, the extent of diagnostic workup performed prior to enrolling patients in a study may have an effect on reported prevalence of cobalamin deficiency in a study population. Future studies in additional larger cohorts of pigtailed macaques with CID likely will provide valuable information on the prevalence of low serum cobalamin levels in this species.
In contrast to pigtailed macaques, rhesus macaques in the CID group did not exhibit decreased serum cobalamin levels. Perhaps rhesus macaques, unlike pigtailed macaques, are less susceptible to developing low serum cobalamin levels. Differences in susceptibility to cobalamin deficiency have been noted in other species. For instance, cats with chronic gastrointestinal disease are thought to be more susceptible to cobalamin deficiency than are dogs, and both cats and dogs are thought to be more susceptible to cobalamin deficiency than are humans.6,30,40,44 Compared with rhesus macaques, pigtailed macaques have an increased susceptibility to diarrhea and other gastrointestinal disease due to a higher degree of inherent dysfunction and damage to the gastrointestinal tract and increased mucosal immune activation.29 Therefore, a physiologic difference in susceptibility to cobalamin deficiency between these 2 species is plausible. Furthermore, the presence of intestinal amyloidosis, a cause of malabsorptive disease in macaques, might also contribute to decreased serum cobalamin levels. The prevalence of amyloid in rhesus and pigtailed macaques in our colony has previously been reported to be 15% and 25%, respectively.41 Although amyloidosis was not detected in any of the biopsied animals in this study, we cannot rule out the presence of amyloid in macaques for which biopsy samples were not available. Therefore, amyloidosis may be a contributing factor to the differential findings between rhesus and pigtailed macaques in this study. In addition, other genetic factors might influence susceptibility to cobalamin deficiency. For example, various dog breeds have genetic defects that result in the inability of receptors in the ileum to efficiently uptake cobalamin.15,33 In addition, several genetic defects that affect the function of proteins involved in cobalamin absorption have been identified in humans.32
Diet is unlikely to account for the difference between study groups detected in pigtailed macaques or the lack of difference detected between rhesus macaque study groups. These 2 species receive distinct diets at our institution within their breeding colonies, and each macaque included in the study had been maintained on its respective diet for its entire life. Although dietary content might directly affect serum cobalamin levels, pigtailed macaques in the CID study group ate the same diet as did pigtailed macaques in the normal study group. Both diets exceeded the minimum recommended amounts of vitamin B12 for NHP, which the National Research Council estimates at 11 μg/kg dietary dry matter and other sources estimate at 30 μg/kg.36,39 Interestingly, given that the vitamin B12 content of our rhesus diet is 36 μg/kg of dry matter—almost half the concentration of our pigtail diet (65 μg/kg of dry matter), we would expect to find lower levels in our rhesus groups if dietary consumption was a factor. In addition, low serum cobalamin levels due to dietary deficiency have been achieved only under extreme conditions. In 2 separate studies, rhesus macaques fed a diet containing 0.0025 μg/kg vitamin B12—well below the recommended amount—exhibited steady decreases in serum cobalamin levels but did not show evidence of true biochemical deficiency until 6 and 16 mo later.1,26 Given this information, even bouts of anorexia from a B12-sufficient diet likely would not result in lower serum cobalamin levels.
Differences in intestinal microflora between our pigtailed and rhesus macaques may offer an alternate explanation for their differing serum cobalamin concentrations. Well documented in both ruminant species as well as humans,2,27,34 vitamin B12 can be synthesized by various microorganisms in the gastrointestinal tract. In addition, baboons fed a vitamin-B12–deficient diet that contained ampicillin exhibited a more severe cobalamin deficiency than did those fed the deficient diet only, suggesting that intestinal flora may influence cobalamin levels.49 Future studies to compare the gut microbiomes of rhesus and pigtailed macaques and their possible influence on vitamin B12 production are warranted.
Macaques were not excluded from our normal study groups if they were culture-positive for either Campylobacter spp. or Shigella as long as they had no history of diarrhea since birth. Animals that are asymptomatic carriers of these organisms, with no evidence of malabsorptive disease, would not be expected to have reduced ability to absorb cobalamin through the gastrointestinal tract. Therefore, the chronic carrier state is unlikely to generate decreased serum cobalamin levels in our normal study group animals. Furthermore, the 2 pigtailed macaques in the normal group that were culture-positive had serum cobalamin concentrations at the upper limit of the assay, and the 4 rhesus macaques in the normal group that were culture-positive had levels distributed throughout the data set (Table 1). Therefore the culture-positive macaques had no evidence of decreased serum cobalamin levels secondary to their status as carriers.
Other variables, such as the specific assay used, may also play a role in differences in vitamin B12 levels between groups.48 Different manufacturers’ assays produce results that differ in reliability; and the chemiluminescent assay used to measure serum cobalamin levels in the current study has been associated with high error rates, including returning false normal values.10,24 Although this assay is used routinely in humans, dogs, and cats and even though we strictly adhered to the sample collection and storage methods indicated by the manufacturer, the assay itself has not been validated specifically for use in macaques. Indeed, the values we obtained in the current study are somewhat higher than those reported for other species.3,31 In addition, although the distribution of serum cobalamin levels in our larger cohorts of normal rhesus and pigtailed macaques suggest that this elevation may in fact be real, it might instead reflect some inaccuracy in the test when it is applied to NHP. Furthermore, fixing values that surpassed the upper limit of the assay to 2000 ng/L may have affected our ability to detect a difference between the rhesus study groups, given that the vitamin B12 levels of some macaques in both the normal and CID groups exceeded the upper limit of the assay. However, many of the normal pigtailed macaques but none of the animals in the CID study group had cobalamin values that exceeded the upper limit. This result emphasizes the importance of the lower serum cobalamin levels in our pigtailed macaque CID study group as compared with the normal study groups, given that a significant difference remains even though reported values for many of the normal pigtails were lower than the actual values. Future studies evaluating test accuracy and comparison with other methods are warranted.
Alternatively, perhaps rhesus macaques with CID are in fact susceptible to low serum cobalamin levels even though this pattern was not exhibited by our study group. Due to various factors that were discussed previously, low serum cobalamin levels may not always be identified readily. A larger sample size might improve the likelihood of seeing a difference; our study was limited by the number of animals from our institution's population that fit the inclusion criteria for the CID group. Additional studies in other populations of rhesus macaques are needed before a definitive conclusion can be made.
In this study, we did not detect an effect of age on serum cobalamin levels in either rhesus or pigtailed macaques. This finding is consistent with reports in cats and dogs.6,44 In contrast, in humans, an age-related decrease in serum cobalamin concentration is reported to affect 10% to 15% of the elderly population.55 This decrease is secondary to reduced production of gastric acid and enzymes responsible for breakdown of cobalamin–protein complexes in the stomach, leading to decreased absorption. In our study, the oldest animals sampled were 18 y (pigtailed) and 22 y (rhesus). A similar change in gastric mucosa with age has not been reported to occur in macaques; however, targeted sampling of geriatric populations of macaques may reveal an effect not seen in this study. Monitoring of vitamin B12 levels in geriatric macaques has been recommended as a precautionary measure.39 In addition, we did not detect an effect of sex on serum cobalamin levels in either rhesus or pigtailed macaques in this study. Additional studies using larger sample sizes may reveal currently unknown effects of age and sex on serum cobalamin level.
In this study, we used histopathologic data available from 3 rhesus and 3 pigtailed macaques in the CID study groups to begin to examine the relationship between disease location and serum cobalamin concentration. We found ileal disease in the 2 pigtailed macaques but not in the 2 rhesus macaques from which ileal tissue was available. Because of the small sample size, we were unable to establish an association between low serum cobalamin levels and location of disease in either rhesus or pigtailed macaques. However, our findings support a connection between ileal disease and lower serum cobalamin levels in pigtailed macaques, similar to that established in other species, which warrants future investigation.
It is interesting to note that all pigtails with lower serum cobalamin levels had gastrointestinal pathology beyond the ileum. The mechanism of cobalamin absorption is complex and relies on normal function of multiple organ systems. Cobalamin ingested in the diet is released from dietary protein by the actions of pepsin and hydrochloric acid in the stomach. Cobalamin then binds to intrinsic factor, produced by the stomach and pancreas, and is transported to the ileum where these complexes are bound by specific receptors in the mucosa and absorbed into circulation.32,53 When certain gastric, pancreatic, and liver diseases have been ruled out, ileal dysfunction is considered the cause of cobalamin deficiency. Still, despite the well-defined role of the ileum for cobalamin absorption in mammals, the presence of ileal disease does not always directly correlate with serum cobalamin level. In one study that examined disease location in humans with Crohn disease, only 42% of patients with confirmed ileal disease showed a deficiency in cobalamin, compared with 21% with disease confined to the colon.37 In addition to the ileum, other portions of the small intestine may contribute to cobalamin absorption. One study suggested that the jejunum may also play a role in vitamin B12 absorption in humans, cats, dogs, and rhesus macaques.16 The jejunum has also been implicated as an important site of cobalamin absorption in the baboon, albeit to a lesser degree than the ileum.50 Thus, it is possible that involvement of other segments of the gastrointestinal tract could also play a role in cobalamin absorption in the macaque. In our study, the jejunum was affected in both pigtailed macaques with confirmed ileal involvement.
Gastric disease that results in a decrease in intrinsic factor production can also lead to cobalamin deficiency. In humans, this is most commonly associated with a disease called pernicious anemia, where the body makes autoantibodies directed against gastric parietal cells, the main site of intrinsic factor production in this species.53 Cobalamin deficiency has also been demonstrated in humans with Helicobacter spp.-associated gastritis and in elderly patients with atrophic gastritis.9,55 In baboons, cobalamin absorption was limited but not completely eliminated after total gastrectomy.18 In our study, 2 pigtailed macaques had confirmed lymphoplasmacytic gastritis. Much remains unknown regarding the contribution of different segments of the gastrointestinal tract to cobalamin absorption in both rhesus and pigtailed macaques. Further examination of this relationship would require biopsy samples from all intestinal segments in each study animal in conjunction with serum cobalamin measurement in a larger cohort.
It is important to note 3 limitations of these data. The 3 pigtailed macaques with biopsy data also had the lowest serum cobalamin concentrations of the CID study group. Biopsy procedures are typically elected for workup of more severe clinical cases at our institution. Thus, the possibility of sampling bias in our histopathology results should be considered. Sampling method is another limitation. With endoscopy, biopsies are small and remain in close proximity to one another within the intestinal segment. During laparotomy, a single biopsy is taken from subjectively abnormal portions of each intestinal segment. At necropsy, again only portions of each intestinal segment are examined histologically. Therefore, any of these biopsy methods could easily result in missing segmental disease in any part of the gastrointestinal tract. A final limitation is the fact that serum samples for cobalamin measurement were not always taken in conjunction with biopsy samples. Cobalamin measurements were taken between 0 and 7 m prior to biopsy samples, and in one animal, cobalamin was measured 15 m after biopsies were taken. Given that cobalamin deficiency is typically reflective of more chronic disease in other species, it is unlikely that this had a significant effect on the results of this study.
There was no significant correlation between disease duration and serum cobalamin concentration in either rhesus or pigtailed macaques, although pigtailed macaques with the longest disease duration also had the lowest serum cobalamin levels. Specifically, a marked decrease is seen in pigtailed macaques with clinical disease for 3.5 y or more. This association is most consistent with reports in humans, in which the liver can store a 3- to 5-y supply of vitamin B12 and thus cobalamin deficiency may take several years to develop.46 Although the extent to which the macaque liver stores and recirculates cobalamin has not been documented, the contribution of these mechanisms to cobalamin recirculation in baboons is similar to that in humans.17 Further study is needed to determine the time required for cobalamin deficiency to develop in macaques.
Consistent with hematologic characteristics of clinical inflammatory bowel disease in humans, pigtailed macaques with CID had higher platelet counts than did healthy controls. In humans, a platelet count that exceeds 450,000 cells/μL is defined as a ‘reactive thrombocytosis’ and is a marker of the active phase of inflammatory bowel disease.11 In fact, the use of platelet count has been proposed to easily distinguish inflammatory bowel disease from infectious diarrhea.11 Although a direct comparison cannot be made, it is interesting to note that all pigtailed macaques in our CID study group had platelet counts that exceeded 450,000 cells/μL (range, 505,000 to 938,000 cells/μL), whereas only 2 of the 6 healthy pigtailed macaques had platelet counts in excess of 450,000 cells/μL (range, 320,000 to 480,000 cells/μL). Platelet count therefore may be a useful parameter to consider during the diagnostic workup of pigtailed macaques with chronic diarrhea.
Macrocytic anemia secondary to cobalamin deficiency is widely reported in humans. Cobalamin plays a critical role in DNA synthesis, and a deficiency can lead to disruptions in cell maturation and ineffective erythropoiesis.52 Even so, it is unsurprising that cobalamin-deficient pigtailed macaques in our study did not develop macrocytic anemia. In previous studies, rhesus macaques with experimentally induced severe cobalamin deficiency did not display the hematologic changes seen in humans, suggesting that the presence of macrocytic anemia may be a major difference in manifestation of cobalamin deficiency between humans and rhesus macaques.1,38 Other hematologic parameters that differ between healthy subjects and those with chronic gastrointestinal disease in other species but not macaques with or without CID include hypoalbuminemia, hypocalcemia, leukocytosis, leukopenia, hypophosphatemia, hypokalemia, and elevated ALP.3,13,40,51,52
In conclusion, this study is the first to examine serum cobalamin concentration in macaques that suffer from CID. The identification of decreased serum cobalamin levels in pigtailed macaques with CID compared with healthy controls has important implications regarding the use of serum cobalamin concentration as a diagnostic measure in this species. Cobalamin deficiency is a serious problem in human and companion animals with chronic gastrointestinal disease. The presence of such a deficiency is an increased risk factor for negative outcomes, is associated with exacerbation of clinical signs including diarrhea and weight loss, and can result in further systemic complications, including neurologic dysfunction and cardiovascular disease.3,28,35,37,52 For these reasons, assessment of serum cobalamin status is a standard component to the diagnostic workup of human and companion animals that present with clinical symptoms of chronic gastrointestinal disease. Furthermore, in addition to therapies targeted toward their primary disease, parenteral cobalamin supplementation is recommended in these species when low serum cobalamin levels are present.12,42,43
It is important to note, however, that measurement of serum cobalamin is a first-line test, and that serum cobalamin levels alone have poor sensitivity and specificity for identification of a biochemical deficiency.5 In humans, it is recommended to perform follow-up tests, including measurement of plasma methylmalonic acid, to help identify a biochemical deficiency. In addition, due to the possibility of assay error, serum cobalamin levels should be considered in context with the presence or absence of clinical signs of deficiency and local reference ranges should be established. Concurrent measurement of serum folate levels is recommended to rule out folate deficiency as a cause of cobalamin deficiency.12 A limitation of our current study is that we did not measure folate levels in our macaques.
The data obtained in our current study provide the first supportive evidence for instituting the same diagnostic measures in pigtailed macaques with CID as those used for humans and companion animals with chronic gastrointestinal disease. The presence of comparable changes in serum cobalamin levels in rhesus macaques with CID as in affected pigtailed macaques cannot be ruled out definitively at this time, and the same diagnostic and treatment recommendations likely should be applied to rhesus macaques until more evidence is gathered. The information available regarding cobalamin deficiency in macaques lags far behind that for other species. Further research into the prevalence of cobalamin deficiency, the precise mechanism of cobalamin absorption, the association with ileal disease, and specific therapeutic protocols is needed.
Acknowledgments
We thank our fellow laboratory animal medicine trainees, especially Cassandra Moats, Anna Goodroe, Peter Otovic, Theresa Meade, and Meghan Vermillion, and the veterinary technical staff at the Johns Hopkins University Research Farm for their aid in sample collection throughout the study. This study was funded in part by NIH ORIP grants R25 OD10913, P40 OD013117, and K01 OD018244.
References
- 1.Agamanolis DP, Chester EM, Victor M, Kark JA, Hines JD, Harris JW. 1976. Neuropathology of experimental vitamin B12 deficiency in monkeys. Neurology 26:905–914. [DOI] [PubMed] [Google Scholar]
- 2.Albert MJ, Mathan VI, Baker SJ. 1980. Vitamin B12 synthesis by human small-intestinal bacteria. Nature 283:781–782. [DOI] [PubMed] [Google Scholar]
- 3.Allenspach K, Wieland B, Grone A, Gaschen F. 2007. Chronic enteropathies in dogs: evaluation of risk factors for negative outcome. J Vet Intern Med 21:700–708. [DOI] [PubMed] [Google Scholar]
- 4.Ardeshir A, Oslund KL, Ventimiglia F, Yee J, Lerche NW, Hyde DM. 2013. Idiopathic microscopic colitis of rhesus macaques: quantitative assessment of colonic mucosa. Anat Rec (Hoboken) 296:1169–1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Battat R, Kopylov U, Szilagyi A, Saxena A, Rosenblatt DS, Warner M, Bessissow T, Seidman E, Bitton A. 2014. Vitamin B12 deficiency in inflammatory bowel disease: prevalence, risk factors, evaluation, and management. Inflamm Bowel Dis 20:1120–1128. [DOI] [PubMed] [Google Scholar]
- 6.Berghoff N, Parnell NK, Hill SL, Suchodolski JS, Steiner JM. 2013. Serum cobalamin and methylmalonic acid concentrations in dogs with chronic gastrointestinal disease. Am J Vet Res 74:84–89. [DOI] [PubMed] [Google Scholar]
- 7.Blackwood RS, Tarara RP, Christe KL, Spinner A, Lerche NW. 2008. Effects of the macrolide drug tylosin on chronic diarrhea in rhesus macaques (Macaca mulatta). Comp Med 58:81–87. [PMC free article] [PubMed] [Google Scholar]
- 8.Broadhurst MJ, Ardeshir A, Kanwar B, Mirpuri J, Gundra UM, Leung JM, Wiens KE, Vujkovic-Cvijin I, Kim CC, Yarovinsky F, Lerche NW, McCune JM, Loke P. 2012. Therapeutic helminth infection of macaques with idiopathic chronic diarrhea alters the inflammatory signature and mucosal microbiota of the colon. PLoS Pathog 8:e1003000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Campuzano-Maya G. 2014. Hematologic manifestations of Helicobacter pylori infection. World J Gastroenterol 20:12818–12838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Carmel R, Agrawal YP. 2012. Failures of cobalamin assays in pernicious anemia. N Engl J Med 367:385–386. [DOI] [PubMed] [Google Scholar]
- 11.Danese S, Motte Cd Cde L, Fiocchi C. 2004. Platelets in inflammatory bowel disease: clinical, pathogenic, and therapeutic implications. Am J Gastroenterol 99:938–945. [DOI] [PubMed] [Google Scholar]
- 12.Devalia V, Hamilton MS, Molloy AM, British Committee for Standards in Haematology. 2014. Guidelines for the diagnosis and treatment of cobalamin and folate disorders. Br J Haematol 166:496–513. [DOI] [PubMed] [Google Scholar]
- 13.Equilino M, Theodoloz V, Gorgas D, Doherr MG, Heilmann RM, Suchodolski JS, Steiner JM, Burgener Dvm IA. 2015. Evaluation of serum biochemical marker concentrations and survival time in dogs with protein-losing enteropathy. J Am Vet Med Assoc 246:91–99. [DOI] [PubMed] [Google Scholar]
- 14.Ferrecchia CE, Hobbs TR. 2013. Efficacy of oral fecal bacteriotherapy in rhesus macaques (Macaca mulatta) with chronic diarrhea. Comp Med 63:71–75. [PMC free article] [PubMed] [Google Scholar]
- 15.Fyfe JC, Ramanujam KS, Ramaswamy K, Patterson DF, Seetharam B. 1991. Defective brush-border expression of intrinsic factor–cobalamin receptor in canine inherited intestinal cobalamin malabsorption. J Biol Chem 266:4489–4494. [PubMed] [Google Scholar]
- 16.Gazet JC, McColl I. 1967. Absorption of vitamin B12 from the small intestine. Study in man, monkey, cat, and dog. Br J Surg 54:128–131. [DOI] [PubMed] [Google Scholar]
- 17.Green R, Jacobsen DW, van Tonder SV, Kew MC, Metz J. 1981. Enterohepatic circulation of cobalamin in the nonhuman primate. Gastroenterology 81:773–776. [PubMed] [Google Scholar]
- 18.Green R, Jacobsen DW, Van Tonder SV, Kew MC, Metz J. 1982. Absorption of biliary cobalamin in baboons following total gastrectomy. J Lab Clin Med 100:771–777. [PubMed] [Google Scholar]
- 19.Grutzner N, Cranford SM, Norby B, Suchodolski JS, Steiner JM. 2012. Evaluation of serum cobalamin concentrations in dogs of 164 dog breeds (2006 to 2010). J Vet Diagn Invest 24:1105–1114. [DOI] [PubMed] [Google Scholar]
- 20.Headstrom PD, Rulyak SJ, Lee SD. 2008. Prevalence of and risk factors for vitamin B12 deficiency in patients with Crohn's disease. Inflamm Bowel Dis 14:217–223. [DOI] [PubMed] [Google Scholar]
- 21.Howell S, White D, Ingram S, Jackson R, Larin J, Morales P, Garcia A, Hicks C, Hopper K, Wagner J. 2012. A biobehavioral study of chronic idiopathic colitis in the rhesus macaque (Macaca mulatta). Appl Anim Behav Sci 137:208–220. [Google Scholar]
- 22.Ibarrola P, Blackwood L, Graham PA, Evans H, German AJ. 2005. Hypocobalaminaemia is uncommon in cats in the United Kingdom. J Feline Med Surg 7:341–348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Institute for Laboratory Animal Research. 2011. Guide for the care and use of laboratory animals, 8th ed Washington (DC): National Academies Press. [Google Scholar]
- 24.Ispir E, Serdar MA, Ozgurtas T, Gulbahar O, Akin KO, Yesildal F, Kurt I. 2015. Comparison of 4 automated serum vitamin B12 assays. Clin Chem Lab Med 53:1205–1213. [DOI] [PubMed] [Google Scholar]
- 25.Kanthaswamy S, Elfenbein HA, Ardeshir A, Ng J, Hyde D, Smith DG, Lerche N. 2013. Familial aggregation of chronic diarrhea disease (CDD) in rhesus macaques (Macaca mulatta). Am J Primatol 76:262–270. [DOI] [PubMed] [Google Scholar]
- 26.Kark JA, Victor M, Hines JD, Harris JW. 1974. Nutritional vitamin B12 deficiency in rhesus monkeys. Am J Clin Nutr 27:470–478. [DOI] [PubMed] [Google Scholar]
- 27.Kau AL, Ahern PP, Griffin NW, Goodman AL, Gordon JI. 2011. Human nutrition, the gut microbiome, and the immune system. Nature 474:327–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kiselow MA, Rassnick KM, McDonough SP, Goldstein RE, Simpson KW, Weinkle TK, Erb HN. 2008. Outcome of cats with low-grade lymphocytic lymphoma: 41 cases (1995 to 2005). J Am Vet Med Assoc 232:405–410. [DOI] [PubMed] [Google Scholar]
- 29.Klatt NR, Harris LD, Vinton CL, Sung H, Briant JA, Tabb B, Morcock D, McGinty JW, Lifson JD, Lafont BA, Martin MA, Levine AD, Estes JD, Brenchley JM. 2010. Compromised gastrointestinal integrity in pigtail macaques is associated with increased microbial translocation, immune activation, and IL17 production in the absence of SIV infection. Mucosal Immunol 3:387–398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kook PH. 2013. Cobalamin deficiency states: a fine example of the 1-medicine concept. Vet J 196:137–138. [DOI] [PubMed] [Google Scholar]
- 31.Kook PH, Lutz S, Sewell AC, Bigler B, Reusch CE. 2013. [[Evaluation of serum cobalamin concentration in cats with clinical signs of gastrointestinal disease]] Schweiz Arch Tierheilkd 154:479–486.[Article in German]. [DOI] [PubMed] [Google Scholar]
- 32.Kozyraki R, Cases O. 2013. Vitamin B12 absorption: mammalian physiology and acquired and inherited disorders. Biochimie 95:1002–1007. [DOI] [PubMed] [Google Scholar]
- 33.Lutz S, Sewell AC, Bigler B, Riond B, Reusch CE, Kook PH. 2012. Serum cobalamin, urine methylmalonic acid, and plasma total homocysteine concentrations in border collies and dogs of other breeds. Am J Vet Res 73:1194–1199. [DOI] [PubMed] [Google Scholar]
- 34.Martens JH, Barg H, Warren MJ, Jahn D. 2002. Microbial production of vitamin B12. Appl Microbiol Biotechnol 58:275–285. [DOI] [PubMed] [Google Scholar]
- 35.McMichael MA, Freeman LM, Selhub J, Rozanski EA, Brown DJ, Nadeau MR, Rush JE. 2000. Plasma homocysteine, B vitamins, and amino acid concentrations in cats with cardiomyopathy and arterial thromboembolism. J Vet Intern Med 14:507–512. [DOI] [PubMed] [Google Scholar]
- 36.National Research Council. 2003. Nutrient requirements of nonhuman primates: second revised ed Washington (DC): National Academies Press. [Google Scholar]
- 37.Oostenbrug LE, van Dullemen HM, te Meerman GJ, Jansen PL, Kleibeuker JH. 2006. Clinical outcome of Crohn's disease according to the Vienna classification: disease location is a useful predictor of disease course. Eur J Gastroenterol Hepatol 18:255–261. [DOI] [PubMed] [Google Scholar]
- 38.Oxnard CE, Smith WT, Torres I. 1970. Vitamin B12 deficiency in captive monkeys and its effect on the nervous system and the blood. Lab Anim 4:1–12. [DOI] [PubMed] [Google Scholar]
- 39.Power M, Toddes B, Koutsos L. 2012. Nutrient requirements and dietary husbandry principles for captive nonhuman primates, p 273–277. In: Abee C, Mansfield K, Tardif S, Morris T. Nonhuman primates in biomedical research: biology and management. New York (NY): Elsevier. [Google Scholar]
- 40.Reed N, Gunn-Moore D, Simpson K. 2007. Cobalamin, folate, and inorganic phosphate abnormalities in ill cats. J Feline Med Surg 9:278–288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rice KA, Chen ES, Metcalf Pate KA, Hutchinson EK, Adams RJ. 2013. Diagnosis of amyloidosis and differentiation from chronic, idiopathic enterocolitis in rhesus (Macaca mulatta) and pigtailed (Macaca nemestrina) macaques. Comp Med 63:262–271. [PMC free article] [PubMed] [Google Scholar]
- 42.Ruaux CG. 2013. Cobalamin in companion animals: diagnostic marker, deficiency states, and therapeutic implications. Vet J 196:145–152. [DOI] [PubMed] [Google Scholar]
- 43.Ruaux CG, Steiner JM, Williams DA. 2005. Early biochemical and clinical responses to cobalamin supplementation in cats with signs of gastrointestinal disease and severe hypocobalaminemia. J Vet Intern Med 19:155–160. [DOI] [PubMed] [Google Scholar]
- 44.Ruaux CG, Steiner JM, Williams DA. 2009. Relationships between low serum cobalamin concentrations and methlymalonic acidemia in cats. J Vet Intern Med 23:472–475. [DOI] [PubMed] [Google Scholar]
- 45.Rubio CA, Hubbard GB. 2002. Chronic colitis in Macaca fascicularis: similarities with chronic colitis in humans. In Vivo 16:191–195. [PubMed] [Google Scholar]
- 46.Rush EC, Katre P, Yajnik CS. 2013. Vitamin B12: 1-carbon metabolism, fetal growth, and programming for chronic disease. Eur J Clin Nutr 68:2–7. [DOI] [PubMed] [Google Scholar]
- 47.Salas A, Manuelian CL, Gargante M, Sanchez N, Fernandez S, Compagnucci M, Ceron JJ, Jeusette I, Vilaseca L, Torre C. 2014. Fat digestibility is reduced in old cats with subnormal cobalamin concentrations. J Nutr Sci 3:e62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Scarpa E, Candiotto L, Sartori R, Radossi P, Maschio N, Tagariello G. 2013. Undetected vitamin B12 deficiency due to false-normal assay results. Blood Transfus 11:627–629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Siddons RC. 1974. The experimental production of vitamin B12 deficiency in the baboon (Papio cynocephalus). A 2-year study. Br J Nutr 32:219–228. [DOI] [PubMed] [Google Scholar]
- 50.Siddons RC, Jacob F. 1975. Vitamin B12 nutrition and metabolism in the baboon (Papio cynocephalus). Br J Nutr 33:415–424. [DOI] [PubMed] [Google Scholar]
- 51.Simpson KW, Fyfe J, Cornetta A, Sachs A, Strauss-Ayali D, Lamb SV, Reimers TJ. 2001. Subnormal concentrations of serum cobalamin (vitamin B12) in cats with gastrointestinal disease. J Vet Intern Med 15:26–32. [DOI] [PubMed] [Google Scholar]
- 52.Stabler SP. 2013. Clinical practice. Vitamin B12 deficiency. N Engl J Med 368:149–160. [DOI] [PubMed] [Google Scholar]
- 53.Suchodolski JS, Steiner JM. 2003. Laboratory assessment of gastrointestinal function. Clin Tech Small Anim Pract 18:203–210. [DOI] [PubMed] [Google Scholar]
- 54.Wilk JL, Maginnis GM, Coleman K, Lewis A, Ogden B. 2008. Evaluation of the use of coconut to treat chronic diarrhea in rhesus macaques (Macaca mulatta). J Med Primatol 37:271–276. [DOI] [PubMed] [Google Scholar]
- 55.Wolters M, Strohle A, Hahn A. 2004. Cobalamin: a critical vitamin in the elderly. Prev Med 39:1256–1266. [DOI] [PubMed] [Google Scholar]