Abstract
Concentric left ventricular hypertrophy (LVH) is a hallmark finding in hypertrophic cardiomyopathy that leads to diastolic dysfunction and variable cardiac consequences as severe as congestive heart failure and sudden cardiac death. LVH was diagnosed postmortem in a large colony of rhesus macaques (Macaca mulatta), but methods to screen and diagnose LVH in living animals are desired. We hypothesized that targeted echocardiography of macaques with a familial association of LVH would yield antemortem LVH diagnoses. We also hypothesized that cardiac biomarker levels would be higher in sudden-death LVH or occult LVH than controls and that cardiac troponin I (cTnI) levels would be higher in macaques housed outdoors than indoors. Sera were assayed for cardiac biomarkers (cTnI, C-reactive protein, creatinine kinase-MB, creatine phosphokinase, and LDH), in conjunction with echocardiography, after diagnosis by postmortem exam or from animals with different levels of exercise due to indoor compared with outdoor housing. None of the investigated biomarkers were associated with LVH. cTnI levels were significantly higher in serum collected from outdoor than indoor macaques. In addition, LVH was diagnosed in 29.4% of subjects with a familial association of LVH. These findings suggest that exercise may increase cTnI levels in rhesus macaques and that targeted echocardiography of rhesus macaques with a familial association of LVH was the most useful variable examined for disease surveillance.
Abbreviations: BNP, brain natriuretic peptide; CK-MB, creatine kinase MB; cTnI, cardiac troponin I; CV, coefficient of variation; hsCRP, high-sensitivity C-reactive protein; IVSd, interventricular septum thickness at end-diastole; LVH, left ventricular hypertrophy; LVPWd, left ventricular posterior wall thickness at end-diastole
Left ventricular hypertrophy (LVH) is a potentially fatal heart condition that was identified in 1.3% of postmortem exams of rhesus macaques from a large colony (Figure 1)33 . Nearly 50% of LVH cases manifested as sudden death, whereas the remainder showed no clinical signs prior to presentation (occult LVH). Many of the sudden deaths were linked to acute precipitating events such as sedation, anesthesia, or trauma that may have exacerbated undetected LVH. It is well established that concentric LVH leads to deficiencies in LV relaxation and filling, resulting in decreased stroke volume and diastolic dysfunction.13,36 Myocardial perfusion occurs during diastole and, in the setting of LVH, the myocardial oxygen demand is increased. This increased oxygen demand in a setting of poor diastolic function establishes an environment that favors myocardial ischemia and arrhythmogenesis, which can result in sudden death.18,24 In addition to rhesus macaques, sudden death with LVH has been documented in owl monkeys (Aotus spp.), captive western lowland gorillas (Gorilla gorilla gorilla), captive chimpanzees (Pan troglodytes), and humans.12,22,37 A neural-based essential hypertension that perhaps is driven by hyperreactive responses of the sympathetic nervous system to environmental events is thought to lead to compensatory concentric LVH in owl monkeys.12,40 Causes of LVH in gorillas and chimpanzees remain undiscovered. Several conditions may result in concentric LVH in humans. In the absence of precipitating cardiovascular abnormalities, LVH is generally considered to be a manifestation of primary hypertrophic cardiomyopathy, a condition that occurs due to mutations typically found in sarcomeric genes.25 In the rhesus macaque colony at our facility, the distribution of LVH cases in 108 probands was previously attributed to a founder effect that supports an inherited cause of LVH.17
Figure 1.
Example images of rhesus macaque hearts at necropsy. Hearts from a (A) 4-mo-old, healthy female and (B) 2-y-old, spontaneous-death male macaque with left ventricular hypertrophy. Hearts were transversely sectioned midway between the heart apex and the base. When the ratio of ventricular diameter to lumen diameter was greater than 3, the animal was diagnosed with left ventricular hypertrophy. The line scale at the lower right represents 1 cm.
Despite detailed pedigree analysis of past LVH occurrences, LVH has remained utterly undetected in living rhesus macaques. Antemortem diagnoses in rhesus macaques are critical to adopt interventions prior to advanced heart disease or death and to maintain the scientific integrity of research studies. Echocardiography is the ‘gold standard’ LVH diagnostic tool for humans, and the first-degree relatives of patients diagnosed with LVH typically undergo echocardiography screening as well.26 Adoption of a similar approach in rhesus macaques is possible, but echocardiography with rhesus macaques is challenging because it requires chemical restraint and often the temporary removal of animals from social groups.
Serum cardiac biomarker testing could provide a safe and efficient method of LVH screening that avoids the technical challenges associated with echocardiography. Serum cardiac biomarkers are naturally occurring proteins that are released into the blood stream after acute or chronic heart insults, and assays for these markers usually require only a blood sample. Our facility maintains a large program of colony-wide blood collection and long-term serum banking, during which a serologic screening test could be adopted for general-population LVH testing without disrupting scientific studies or social groups.
Several biomarkers have shown promising abilities to aid in diagnoses of cardiac disease in both NHP and humans. High-sensitivity C-reactive protein (hsCRP) is an acute inflammatory protein that rises with inflammation or infection and is elevated during LVH in humans.8,31 Creatine kinase MB (CK-MB), CPK, and LDH are released after insult to cardiomyocytes, although they also are released during damage of skeletal muscle cells.42,43 More specific for cardiomyocyte injury, cardiac troponin I (cTnI) levels are prominent and sustained after cardiac injury with necrosis and are less pronounced after reversible cardiac injury.1,14,16,38 In humans, cTnI is elevated with LVH, but mild elevations after exercise can interfere with the discrimination abilities of this biomarker.38,46 The effects of exercise on cardiac biomarkers in rhesus macaques are unknown; in addition, the amount of exercise that rhesus macaques experience varies with different housing types. Exercise is limited by routine husbandry practices for indoor-housed rhesus macaques; however, exercise is a normal activity of rhesus macaques group-housed outdoors in half-acre field pens.
The aim of the current study was to assess the utility of cardiac biomarkers and echocardiography for LVH screening in a large colony of rhesus macaques to diagnose LVH antemortem. First, we performed targeted echocardiographic surveillance and cTnI assays on animals with a familial association of LVH. Then, we assayed banked serum from necropsy-diagnosed sudden-death LVH, occult LVH, and control cases for cTnI and the additional cardiac biomarkers hsCRP, CK-MB, CPK, and LDH. Finally, we collected serum from healthy groups of indoor- and outdoor-housed macaques to assess biomarker levels in subject groups with or without exercise. We postulated that: 1) targeted echocardiographic surveillance with LVH familial association would result in antemortem LVH diagnoses; 2) macaques diagnosed with LVH or equivocal disease by echocardiography would have higher cTnI levels than would healthy animals; 3) cardiac biomarker levels would be higher in banked sera from macaques with sudden-death LVH than in occult LVH cases; 4) cardiac biomarker levels would be higher in banked sera from both sudden-death LVH and occult LVH groups than in control sera; and 5) cTnI levels would be higher in outdoor macaques than indoor macaques, whereas the other biomarkers (hsCRP, CK-MB, CPK, and LDH) would remain comparable between groups.
Materials and Methods
Subjects and housing.
All study procedures and methods were conducted at the California National Primate Research Center and preapproved by the University of California–Davis IACUC. All animals were cared for in accordance with the Animal Welfare Act and Guide for the Care and Use of Laboratory Animals.3,15 The animal care and use program at the University of California–Davis is USDA-registered, maintains a Public Health Services Assurance, and is fully AAALAC-accredited. Indoor animals were housed in stainless steel cages sized in accordance with or in excess of primary cage-space regulations and pair-housed unless medical, behavioral, or scientifically justified reasons precluded pair housing. Routine husbandry parameters included 12:12-h light:dark cycles and controlled temperature, humidity, and ventilation. Outdoor macaques were group-housed in 0.5-acre rectangular enclosures (field pens). All macaques were housed with species-appropriate environmental enrichment, fed commercial primate chow twice daily (LabDiet Monkey Diet 5047, Purina Laboratory, St. Louis, MO), offered water without restriction through automatic watering devices, and supplemented with fruits and vegetables biweekly. All rhesus macaques were sedated (approximately 10 mg/kg ketamine IM) at least annually to undergo complete physical examination, weighing, tuberculosis testing, dental prophylaxis, and occasional serum collection for the institutional frozen serum bank.
Blood samples for serum banking were collected into red-top tubes and stored at –70 °C. Trypanosoma cruzi has been shown to induce cTnI autoantibodies that might interfere with cardiac biomarker assays used in this study.32 However, northern California is not considered an endemic region of this organism, and Trypanosoma cruzi has not been detected in rhesus macaques at our facility. All animals included in these studies were born and raised at the center, thus precluding the necessity of screening for Trypanosoma cruzi.
Targeted echocardiographic surveillance study.
The echocardiography study was performed to diagnose LVH in living macaques and to compare the cardiac biomarkers levels with echocardiography findings. All animals (n = 17) included in this study had a familial association of LVH (16 full siblings and 1 half sibling) and available serum samples in the institutional serum bank. Serum samples were obtained from the serum bank and assayed for cTnI, after which echocardiography was performed under sedation with ketamine (10 mg/kg IM; Ketaject, Phoenix Pharmaceutical, St Joseph, MO) or ketamine (10 mg/kg)–midazolam (0.5 mg/kg IM; APP Pharmaceuticals, Schaumburg, IL) in combination. The addition of midazolam to ketamine sedation extended the sedation time for macaques when prolonged cardiac evaluations were necessary. Midazolam has minimal effects on the cardiovascular system and minimizes the risk of cardiac consequences secondary to high-dose ketamine. A combination of midazolam with ketamine was previously described as an ideal sedation combination for echocardiography of laboratory animals.34 Echocardiography (CX50 Ultrasound System, Philips, Best, Netherlands) using a 4- to 12-mHz sector array transducer was performed by an ACVIM board-certified cardiologist, and data analysis was completed by the same cardiologist using standard off-line analysis software (Syngo Dynamics, Siemens, Erlangen, Germany). A complete 2D, m-mode, color, spectral, and tissue Doppler analysis was performed from the right and left hemithorax in each subject. None of the subjects in this study underwent necropsy.
To be diagnosed with occult LVH by echocardiography, subjects had to meet established criteria of LVH and diastolic dysfunction. Subjects possessing either diastolic dysfunction alone with an age younger than 16 y or LVH alone were considered to be disease equivocal. All macaques that lacked any clinical signs of cardiovascular disease on echocardiography were classified as healthy.
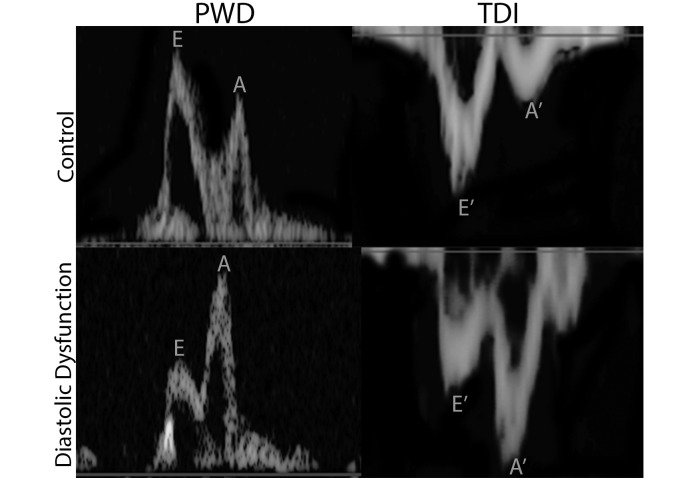
Diastolic dysfunction was evaluated according to the following criteria: spectral Doppler transmitral flow pattern and passive filling velocity (E wave) to atrial contraction velocity (A wave) ratios and spectral tissue Doppler lateral or medial E′ wave to A′ wave ratios. All images were gathered from the left apical 4-chamber view and measured on 3 to 5 consecutive heart cycles in the absence of ectopy. Subjects that were 16 y or older and had diastolic dysfunction were considered normal due to the reported geriatric changes to diastolic function in other species.28 Criteria for diagnosing diastolic dysfunction were either a transmitral E:A ratio less than or equal to 0.9 or a transmitral E:A ratio greater than or equal to 0.9 with a lateral or medial E′:A′ ratio less than or equal to 0.9 (pseudonormalization pattern).
LVH was evaluated according to the diastolic m-mode measurements of the LV posterior wall thickness at diastole (LVPWd) and interventricular septum thickness at diastole (IVSd) taken from the right parasternal short-axis view at the level of the papillary muscles. Measures were obtained during 3 to 5 consecutive heart cycles in the absence of ectopy by using a leading-edge-to-leading-edge technique. Age-defined disease cut-off criteria were derived as described previously.19,41 LVH was defined as LVPWd or IVSd measures that exceeded the following cut-off values: subjects younger than 9 y of age with IVSd greater than or equal to 6.5 mm or LVPWd greater than or equal to 6.5 mm and subjects older than or equal to 9 y of age with IVSd greater than or equal to 8.8 mm or LVPWd greater than or equal to 7.4 mm. The cutoffs presented represent values greater than at least 2 SD from the means for healthy rhesus macaques.19,41
The age cut-offs for diastolic dysfunction evaluations and diastolic m-mode measurements conservatively identify a ‘geriatric time point’ within the framework of the published echocardiography normal values. Due to the absence of published heart measurements from healthy 9- to 16-y-old macaques, subjects in this age group in the current study were diagnosed according to criteria from available published means of 17- to 20-y-old macaques. The geriatric time point criteria of macaques aged 17 to 20 y are conservative for diagnostic criteria of LVH and minimize the risk of false-positive diagnoses.
Necropsy-diagnosis study.
Serum samples of control (n = 10), occult LVH (n = 10), and sudden death LVH (n = 10) cases were obtained from the institutional serum bank. These cases did not overlap those in the targeted echocardiographic surveillance study and were diagnosed previously at necropsy by veterinary pathologists. Control sera were from macaques that lacked any clinical or postmortem signs of cardiovascular disease and were presumed to be healthy. None of the subjects in this study underwent echocardiograms. Age- and freezer-time–matched sera from 5 female and 5 male macaques were used in each group. Serum samples were assayed for cTnI, hsCRP, CK-MB, CPK, and LDH.
Exercise study.
Serum samples were obtained from rhesus macaques housed in an outdoor half-acre field pen (n = 30) and from age- and sex-matched, indoor-housed macaques (n = 30). Subjects were sedated with ketamine (10 mg/kg IM), and 3 mL blood was obtained for serum collection. Animals that shared the same parents as those diagnosed with LVH on necropsy were excluded from either group, as were macaques with any signs of potential cardiovascular disease. None of the subjects in this study group underwent echocardiogram or necropsy.
Laboratory methods.
All serum samples were frozen at −70 °C before analysis and thawed at room temperature (18 to 20 °C) for assays. Samples containing gross hemolysis or lipemia were excluded. Sera were assessed for circulating levels of cardiac biomarkers by using standard assays (cTnI: ADVIA Centaur CP TnI-Ultra, Siemens Healthcare Diagnostics, Tarrytown, NY; hsCRP, CK-MB, CPK, and LDH: model AU480, Beckman Coulter, Brea, CA). The detection limit of cTnI was 0.006 ng/mL and the upper limit was 50 ng/mL. According to the manufacturer, the 10% coefficient of variation (CV) was 0.03 ng/mL, with a 99th percentile of 0.04 ng/mL. CPK was detected by using the rate-reaction method of the International Federation of Clinical Chemistry with a detection limit of 10 to 2000 U/L and precision specification less than 10% CV. CK-MB was measured by the modified rate-reaction method of the International Federation of Clinical Chemistry with a detection limit of 10 to 2000 U/L and precision specification less than 6.5% CV. LDH was measured according to the lactate–pyruvate method of the International Federation of Clinical Chemistry, with a detection limit of 25 to 1200 U/L and precision specification less than 10% CV. hsCRP was measured by using the latex particle immunoturbidimetric method, with a detection range of 0.2 to 160 mg/L and a precision specification less than 10% CV. hsCRP, CPK, and LDH were validated in rhesus macaques and specific reference ranges were compiled based on analysis of a significant number of clinically healthy animals (n ≥ 40; Table 1).
Table 1.
Cardiac biomarker details
| Limit of detection | Validated for rhesus macaques? | |
| Cardiac troponin I | 0.006–50 ng/mL | Yes (reference 4) |
| Creatine kinase-MB | 10–2000 U/L | No |
| High-sensitivity C-reactive protein | 0.2–160 mg/L | Yes (inhouse) |
| Lactate dehydrogenase | 25–1200 U/L | Yes (inhouse) |
| Creatine phosphokinase | 10–2000 U/L | Yes (inhouse) |
All assays were performed on a model AU480 (Beckman Coulter) clinical analyzer except for cardiac troponin I (Advia Centuar TnI-Ultra, Siemens Healthcare Diagnostics). High-sensitivity C-reactive protein, lactate dehydrogenase, and creatine phosphokinase assays were validated in rhesus macaques in the clinical diagnostic laboratory of the California National Primate Research Center, and specific reference ranges were compiled based on results from a significant number of clinically healthy animals (n ≥ 40).
Statistics.
All statistical analyses were conducted by using the R statistical program (R Foundation for Statistical Computing, Vienna, Austria). Sample size calculations for the serum cardiac biomarker tests estimated a minimum of 10 subjects per group to detect biomarker differences with α = 0.05 and β = 0.2. Normality of data was determined by inspection of data presented in histograms. Parametric data analysis of the exercise study started with F-tests to determine significantly different variances between groups, followed by either the Welch t test or Student t test for significance mean differences. One-way ANOVA was used to determine whether significant differences in mean biomarker levels existed between groups in the necropsy-diagnosis study and the targeted echocardiographic surveillance study. When significant differences were found by ANOVA, Tukey posthoc testing was used to discern differences between groups. Nonparametric data of the exercise study were analyzed by using the Mann-Whitney U test, and the Kruskal–Wallis test was used to evaluate significantly different distributions in the necropsy-diagnosis and targeted echocardiographic surveillance studies. A P value of less than 0.05 was considered significant in all tests.
Results
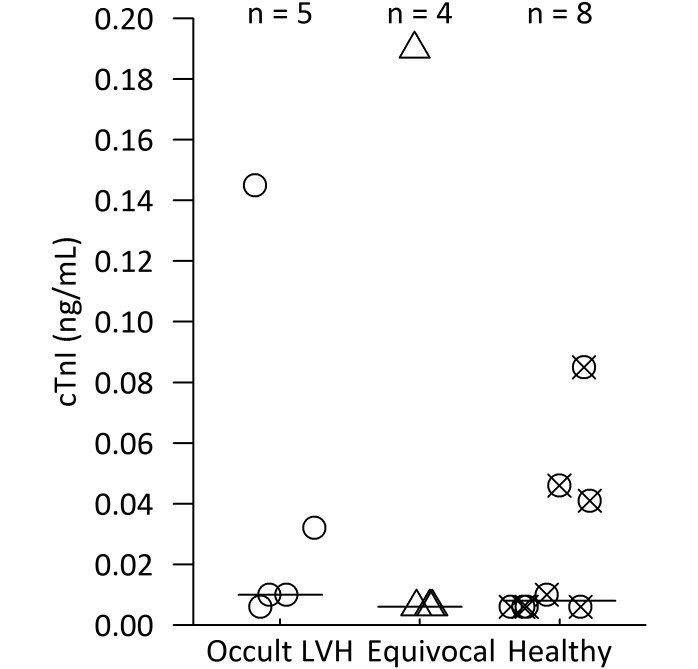
Occult LVH was diagnosed in 5 of the 17 (29.4%) rhesus macaques evaluated, and another 4 (23.5%) macaques were diagnosed with equivocal disease. The remaining 8 subjects had unremarkable echocardiography exams and were deemed healthy. Example images from healthy and LVH-affected subjects in the study are provided in Figure 2. Example pulsed-wave Doppler (PWD) and tissue Doppler (TDI) images from a control animal and a macaque that meets study criteria of diastolic dysfunction comprise Figure 3. Data for hsCRP, CK-MB, CPK, and LDH but not cTnI were normally distributed in histograms.
Figure 2.
Example images from subjects included in the targeted echocardiographic surveillance study. Echocardiography views are provided from a control macaque and one with left ventricular hypertrophy. (A) 2-Dimensional right parasternal 4-chamber long-axis image at end diastole. (B) m-Mode right parasternal short-axis image at the level of the papillary muscles. (C) 2-Dimensional right parasternal short-axis image at the level of the papillary muscles at end diastole. (D) 2-Dimensional right parasternal short-axis image at the level of the papillary muscles at end systole. Note the end-systolic cavity obliteration that occurs with left ventricular hypertrophy.
Figure 3.
Example pulsed-wave Doppler and tissue Doppler images from the targeted echocardiographic surveillance study. Doppler images are provided for a control macaque and one that meets study criteria of diastolic dysfunction. The transmitral flow waves are depicted as early filling (E) and atrial contraction (A). Note the normal pattern (higher E and lower A waves) of the control and the reversed pattern in the rhesus macaque with diastolic dysfunction. The tissue Doppler sampled at the lateral mitral valve annulus is again labeled as the early filling motion (E′) and atrial contraction motion (A′). Note the normal pattern (deep E′ and shallow A′ waves) of the control animal and the reversed pattern in the rhesus macaque with diastolic dysfunction.
The limit of detection for cTnI was 0.006 ng/mL and resulted in a large right-skewed distribution curve. cTnI data were analyzed nonparametrically because transformation by multiple methods failed to yield a normal distribution. cTnI levels did not differ significantly between groups (occult LVH, equivocal disease, and healthy) that underwent targeted echocardiographic surveillance (Figure 4; Kruskal–Wallis test, χ2 = 0.8489, df = 2, P = 0.6541). Nor did cTnI differ significantly between groups (sudden -death LVH, occult LVH, and control) in the necropsy-diagnosis study (Table 2; Kruskal–Wallis test, χ2 = 0.5519, df = 2, P = 0.7588). The additional cardiac biomarkers assessed in the necropsy-diagnosis study (CK-MB, hsCRP, CPK, and LDH) did not differ between groups (one-way ANOVA; hsCRP, P = 0.082; CK-MB, P = 0.780; CPK, P = 0.889; LDH, P = 0.270; Table 2).
Figure 4.
Cardiac troponin I (cTnI) in rhesus macaques with a familial association of left ventricular hypertrophy (LVH). Circles represent cases of occult LVH—subjects had to meet previously defined criteria of LVH and diastolic dysfunction as determined by echocardiography. Triangles represent animals that had either diastolic dysfunction alone with an age less than 16 y or LVH alone and thus were considered disease equivocal. Crosses indicate macaques that met neither of these criteria and were deemed healthy. Both indoor and outdoor animals were included in each group. Group median cTnI levels are represented by black bars. cTnI levels did not differ (Kruskal–Wallis test) between groups.
Table 2.
Cardiac biomarker levels in banked serum from rhesus macaques diagnosed with left ventricular hypertrophy (LVH) at necropsy
| Cardiac troponin I (ng/mL) | High-sensitivity C-reactive protein (mg/mL) | Creatine phosphokinase -MB (U/L) | Creatine phosphokinase (U/L) | Lactate dehydrogenase (U/L) | |
| Sudden-death LVH (n = 10) | 0.008 ± 0.016 | 1.40 ± 1.51 | 192.6 ± 131.2 | 643.5 ± 369.1 | 449.6 ± 228.2 |
| Occult LVH (n = 10) | 0.006 ± 0.041 | 5.50 ± 7.74 | 199.8 ± 98.8 | 747.0 ± 626.3 | 502.1 ± 220.4 |
| Control (n = 10) | 0.007 ± 0.001 | 1.30 ± 0.82 | 167.2 ± 89.9 | 714.7 ± 427.7 | 359.0 ± 116.8 |
Data are shown as mean ± 1 SD for high-sensitivity C-reactive protein, creatine phosphokinase-MB, creatine phosphokinase, and lactate dehydrogenase and as median ± 1 SD for cardiac troponin I. None of the cardiac biomarkers differed significantly between groups according to necropsy diagnosis (1-way ANOVA: hsCRP, CPK, CK-MB, and LDH; Kruskal–Wallis test: cTnI).
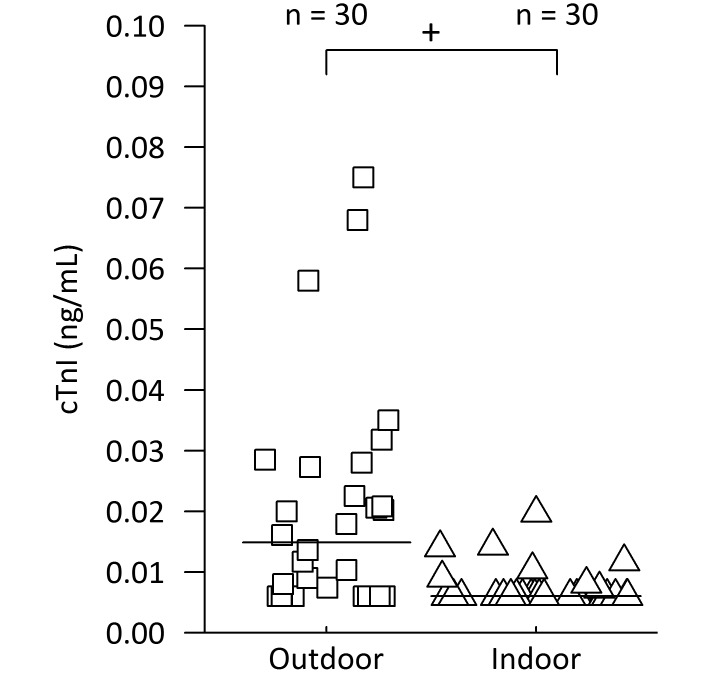
In the exercise study, we ran a Mann–Whitney U test to evaluate differences in cTnI between indoor and outdoor groups and found a significant effect. The cTnI medians of the indoor and outdoor groups were 0.006 ng/mL and 0.015 ng/mL, respectively, and the mean ranks of indoor and outdoor groups were 22.4 and 38.1, respectively (U = 222, Z = –3.35, P = 0.0004, r = 0.4325; Figure 5). In addition, LDH levels differed significantly between outdoor (mean ± 1 SD, 357.9 ± 26.9 U/L) and indoor (264.7 ± 21.1 U/L; Welch t test, P = 0.025; Table 3) macaques, but hsCRP, CPK, and CK-MB concentrations did not differ between groups (Welch t test; hsCRP, P = 0.209; CK-MB, P = 0.144; CPK, P = 0.052; Table 3).
Figure 5.
Cardiac troponin I (cTnI) in healthy rhesus macaques housed either indoors or outdoors. Exercise is limited by routine husbandry practices for indoor rhesus macaques (triangles); however, exercise is a normal activity of rhesus macaques group-housed outdoors (squares) in half-acre field pens. Group median cTnI levels are represented by black bars. The cTnI levels of indoor and outdoor animals were significantly different (+, P = 0.0004; Mann–Whitney U test).
Table 3.
Cardiac biomarker levels in rhesus macaques from different housing types (n= 30 per group)
| Cardiac troponin I (ng/mL) | High-sensitivity C-reactive protein (mg/mL) | Creatine phosphokinase-MB (U/L) | Creatine phosphokinase (U/L) | Lactate dehydrogenase (U/L) | |
| Outdoor | 0.015 ± 0.018a | 3.57 ± 7.89 | 165.6 ± 46.0 | 852.5 ± 722.5 | 357.9 ± 142.3b |
| Indoor | 0.006 ± 0.005 | 1.73 ± 1.41 | 146.4 ± 40.9 | 485.0 ± 409.5 | 264.7 ± 115.6 |
Data are shown as mean ± 1 SD for high-sensitivity C-reactive protein, creatine phosphokinase-MB, creatine phosphokinase, and lactate dehydrogenase and as median ± 1 SD for cardiac troponin I.
P < 0.001 between the distribution of values for indoor and outdoor rhesus macaques (Mann–Whitney U test).
P = 0.025 between values for indoor and outdoor rhesus macaques (Welch t test).
Cumulative posthoc evaluations of cTnI were performed in light of the results of the exercise study. Two groups of results were formed: healthy, indoor macaques from the echocardiography and exercise studies and LVH-equivocal animals from the echocardiography study. A Mann–Whitney U test found a significant effect of cTnI level between the 2 groups (Figure 6; Mann–Whitney U test: U = 97, Z = 1.66, P = 0.0485, r = 0.2447). The median cTnI value was 0.006 ng/mL for healthy, indoor macaques (n = 34) and 0.01 ng/mL for LVH-equivocal animals (n = 9); the means for the healthy indoor and LVH-equivocal groups were 0.008 ng/mL and 0.046 ng/mL, respectively.
Figure 6.
Posthoc assessment of cardiac troponin I (cTnI) as a cardiac biomarker of left ventricular hypertrophy (LVH) in rhesus macaques. LVH-equivocal group (circles) combined both LVH and equivocal animals diagnosed by echocardiography. The healthy-indoor (triangles) group combined healthy animals diagnosed by echocardiography and indoor-housed, healthy animals. Group median cTnI levels are represented by black bars. cTnI levels differed significantly (+, P = 0.0485; Mann–Whitney U test) between groups.
Discussion
This study is the first to provide antemortem assessments of LVH in rhesus macaques. Approximately 29% of rhesus macaques with a familial association of LVH were diagnosed with occult LVH, and this high prevalence in related macaques exceeds the reported inhouse prevalence17 from postmortem exam surveillance. Our findings support a previous hypothesis that the LVH in rhesus macaques at our facility might represent an inherited form of hypertrophic cardiomyopathy.17 Obviously the significance of LVH prevalence in macaques with a familial association depends in part of the frequency of LVH in nonrelated subjects. Unfortunately, definitive data from normal subjects are sparse, but the general frequency of LVH is about 1.3% colony-wide on postmortem exams since 1992.17,33 Using echocardiography to screen the entire general rhesus macaque population was not cost-effective; instead, we used both LVH familial association and cardiac biomarkers to target LVH screening in the current study. In the future, antemortem echocardiographic diagnosis of LVH can be used to support clinical decisions, careful selection of appropriate research subjects, and subsequent in vivo studies of the LVH cohort.
Our study showed that CK-MB, hsCRP, CPK, and LDH were not useful as screening tools for LVH in rhesus macaques. Previous studies have shown that CK-MB, hsCRP, CPK, and LDH levels are elevated during LVH in multiple other species.8,9,27,35,46 Although hsCRP, CPK, and LDH in macaques have been reliably measured by the inhouse clinical laboratory, CK-MB assays have not been validated in rhesus macaques. Therefore, we cannot rule out the possibility that rhesus macaque CK-MB does not crossreact with reagents of CK-MB assays designed for human sera. This possible limitation was suggested by the situation with another biomarker for human LVH. That is, assays designed to detect brain natriuretic peptide (BNP) in human LVH studies also detect BNP in chimpanzee serum,20,46 but BNP's utility in rhesus macaques is limited due to its species-specific sequence and the absence of validated tests in macaques.23,44 At least one study evaluated BNP assays with macaque serum, and the results could not be correlated with severe cardiac disease.44 Perhaps macaque-specific BNP and CK-MB assays are warranted as a future direction for the study of cardiac biomarkers and their relationship to LVH in rhesus macaques.
cTnI may be a useful biomarker to discriminate between healthy macaques and those with LVH, but our exercise study indicated that exercise and, indirectly, housing type may interfere with cTnI interpretation. cTnI is a serum cardiac biomarker that is considered more specific 1and sensitive than other available cardiac biomarkers for the detection of cardiomyocyte injury. Severe and prolonged elevations of cTnI are seen after myocardial infarction, and assays that are designed for human serum detect increases in cTnI in the sera from macaques with experimentally induced myocardial infarctions.4,11 In addition, minor elevations in cTnI are strongly related to poor clinical outcomes.30 In contrast, exercise is a known stimulus of cTnI release that likely reflects reversible myocardial injury and not poor prognosis.38 Exercise-induced cTnI release is due to a physiologic process that does not warrant undue concern.39 There can be detectable, normally distributed, cTnI elevations in healthy subjects and a cTnI rise in all humans during exercise studies.39,45 Whereas myocardial necrosis-induced cTnI release typically lasts longer than 7 d, exercise-induced cTnI release returns to baseline in less than 24 h, thus suggesting an alternative mechanism for cTnI release during exercise in the absence of cardiomyocyte death.39 At this time, the mechanisms for exercise-induced cTnI release are unclear. However, clearly exercise-induced elevations in cTnI can cloud decisions about cardiac disease in algorithms using cTnI assays unless specific reference ranges are developed for exercising persons.39 The same may be true for rhesus macaques.
Exercise may have obscured our ability to detect differences between LVH and healthy animals. Both the echocardiography surveillance study and the necropsy-diagnosis study included both indoor- and outdoor-housed animals with variable amounts of exercise prior to blood collection. Our serum sample sizes were limited by what was available from LVH macaques, which subsequently included animals from all housing types. Future studies might assess the cTnI levels associated with LVH once exercise is eliminated as a confounding variable. Exercise-induced cTnI release might easily mask the cTnI release associated with LVH. Reasonably, we may expect only minor cTnI elevations when LVH in rhesus macaques leads to reversible injury, mirroring exercise-induced injury and subsequent cTnI release, rather than the prolonged and severe cTnI release seen with myocardial infarction and necrosis. In a previous study, histopathology showed little evidence of myocardial necrosis in rhesus macaques with LVH.33
If we consider only indoor-housed and healthy animals as our comparison group for our LVH studies, then we minimize the exercise-associated confounding of cTnI. In fact, posthoc analysis of cTnI levels between healthy, indoor animals and LVH-equivocal animals revealed a significant effect from cTnI that otherwise did not show evidence of a difference (Figure 6). Although not conclusive, this analysis highlights the role exercise plays as a confounding variable of cTnI and the importance of eliminating exercise in subsequent evaluations. In addition, LDH was significantly elevated in outdoor-housed macaques, but the importance of this finding in our studies is limited by the low sensitivity of LDH as a cardiac biomarker.43
Other limitations in our studies should be noted. If the criteria for the diagnosis of LVH by echocardiography or necropsy are inaccurate, misclassification bias could affect our study. The definition of LVH is controversial, and definitive criteria for macaques have not been established. Fortunately, LVH diagnoses made previously by necropsy in our studies were outlined in a systematic, uniform procedure developed by inhouse veterinary pathologists.33 On gross exam, rhesus macaque hearts were transversely sectioned midway between the apex and the base, and when the ratio of ventricular diameter to lumen diameter was greater than 3, the animal was diagnosed with LVH (Figure 1). Macaques that died suddenly without evidence of other life-threatening lesions were identified as cases of sudden-death LVH, whereas other animals in which LVH was an incidental finding were identified as cases of occult LVH for the current study.
Parameters for echocardiographic diagnosis of LVH in rhesus macaques have not been established, but this study is the first to describe—antemortem—enlarged hearts in rhesus macaques. We are at the forefront of diagnosing LVH, and we have developed LVH diagnosis criteria that can be obtained through echocardiography and that were sensitive to the established methods for measuring normal rhesus macaque hearts.19,41 LV measurements exceeding 2 SD above the mean for healthy macaques were not the only criteria considered for LVH diagnosis: both gross enlargement of the left ventricle myocardium and a LV physiologic deficit (diastolic dysfunction) were required for LVH diagnosis by echocardiography. The dysfunction occurs when hypertrophied LV myocardium impairs LV relaxation, consequently reducing early diastolic filling. In healthy individuals, the speed at which blood enters the left ventricle during early diastole usually reflects passive ventricle-filling blood velocity (E wave velocity), and the speed at which blood enters the left ventricle during late diastole usually reflects the pressure induced by atrial contraction (A-wave velocity). In cases of LVH, LV relaxation is impaired, thus dampening passive filling velocities (E wave velocity depression) and necessitates increased force of atrial contraction to complete LV filling (A wave velocity elevation). As a result, in cases of LVH, diastolic dysfunction (decreased E:A ratio) is expected. Tissue Doppler echocardiography can be used to obtain a similar measurement, which is often expressed as the E′:A′ ratio. In our study, diastolic dysfunction was gauged according to both early and late transmitral velocities (E:A) and tissue Doppler (E′:A′). We considered tissue Doppler findings to be more reliable than transmitral flow velocities, because a process known as pseudonormalization can produce normal E:A ratios in the face of abnormal tissue Doppler assessments.
Diastolic dysfunction is a risk factor of detrimental cardiovascular events in humans.21 Astonishingly, diastolic dysfunction is a relatively common finding in geriatric men and women.28 To avoid confounding due to age-related changes in heart structure and function in our current study, age-appropriate indices of LVPWd, IVSd, E:A, and E′:A′ for healthy macaques were developed from published heart measurements obtained by echocardiography of young and geriatric macaques.19,41 However, due to the absence of published heart measurements from healthy 9- to 16-y-old macaques, our subjects in this age group were diagnosed according to criteria from available published means of 17- to 20-y-old macaques. Rather than extrapolating data from younger macaques, we thus developed conservative and reliable LVH diagnostic criteria for echocardiography. These tools would make our confidence in disease positive subjects high and limit the possibility of false positives. Studies that follow animals antemortem and postmortem, involving clinical and pathology teams working collaboratively, could refine LVH criteria in the future.
Banked serum provides a cost-effective option for large-scale screening studies, but the varying effects of storage time, temperature, and number of freeze–thaw cycles can be problematic. The banked sera assayed for the targeted echocardiographic surveillance and necropsy-diagnosis studies had not undergone a freeze-thaw cycle and were stored at –70 °C for different amounts of time. We accounted for variations in storage time by matching controls and test groups with approximately equivalent storage times so that no group was overrepresented by old or new sera, and the possible confounding with biomarker degradation over time would be distributed among groups evenly. Freezer storage time (mean ± 1 SD) did not differ significantly between groups in either the targeted echocardiographic surveillance study (healthy, 1.1 ± 0.7 y; equivocal disease, 1.5 ± 0.9 y; occult LVH = 1.1 ± 0.7 y; ANOVA, P = 0.566) or necropsy-diagnosis study (control, 8.0 ±4.4 y; occult LVH, 7.5 ± 3.5 y; sudden-death LVH, 8.0 ±5.0 y; ANOVA, P = 0.961).
The degradation of biomarkers during storage at –70 °C can vary depending on the analyte. When human sera were stored at –70 °C, the analytes CPK and LDH were stable for 5 y.7 CK-MB, a specific isotype and constituent of CPK, has only been assessed after 1 mo of storage at –20 °C, and there was no effect of storage time.6 CRP levels remained stable for 11 y after collection when samples were stored at –70 °C.29 Assay manufacturers report that cTnI is stable for at least 6 mo when stored at –80 °C.10 Stability testing beyond 6 mo has shown that cardiac troponins undergo only small decreases over time. One study concluded cTn levels dropped approximately 0.007 ng/mL over 2 y at –70 °C,5 and another reported that samples testing below the limit of detection (0.006 ng/mL) in large studies would remain unaffected even after 8 y.2 Therefore, some biomarker degradation might have occurred in our samples before being assayed, but that possibility should not detract from our finding that cTnI may be a valuable screening biomarker when exercise is a controlled variable.
In summary, we developed the first echocardiographic set of antemortem diagnostic criteria for LVH in rhesus macaques. The prevalence of LVH was much higher in macaques with a familial association of the disease than in the general population. This finding supports the previously published conclusion that LVH might represent an inherited cardiomyopathy in rhesus macaques. Using LVH familial association to drive targeted echocardiography proved to be superior when compared with the serum cardiac biomarkers that we assessed. Finally, our exercise study of cardiac biomarkers confirms that exercise and, indirectly, housing type are potential confounding factors for cardiac biomarker assessment in macaques. Ultimately our study confirms the ability of echocardiography to diagnose LVH in living macaques. Future studies of LVH likely will improve the health and wellbeing of affected animals, prevent confounding factors in studies, and enable the development of an NHP model of hypertrophic cardiomyopathy in humans.
Acknowledgments
We acknowledge the expertise of Nancy Gee and the CNPRC Endocrine Core, who performed the cTnI assays in this report. This study was supported by the Office of Research Infrastructure Programs of the NIH through the California National Primate Research Center Award Number P51OD011107.
References
- 1.Adams JE, 3rd, Bodor GS, Dávila-Román VG, Delmez JA, Apple FS, Ladenson JH, Jaffe AS. 1993. Cardiac troponin I. A marker with high specificity for cardiac injury. Circulation 88:101–106. [DOI] [PubMed] [Google Scholar]
- 2.Agarwal SK, Avery CL, Ballantyne CM, Catellier D, Nambi V, Saunders J, Sharrett AR, Coresh J, Heiss G, Hoogeveen RC. 2011. Sources of variability in measurements of cardiac troponin T in a community-based sample: the atherosclerosis risk in communities study. Clin Chem 57:891–897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Animal Welfare Act as Amended. 2013. 7 USC §2131–2159.
- 4.Apple FS, Murakami MM, Ler R, Walker D, York M, HESI Technical Committee of Biomarkers Working Group on Cardiac Troponins 2008. Analytical characteristics of commercial cardiac troponin I and T immunoassays in serum from rats, dogs, and monkeys with induced acute myocardial injury. Clin Chem 54:1982–1989. [DOI] [PubMed] [Google Scholar]
- 5.Basit M, Bakshi N, Hashem M, Allebban Z, Lawson N, Rosman HS, Maciejko JJ. 2007. The effect of freezing and long-term storage on the stability of cardiac troponin T. Am J Clin Pathol 128:164–167. [DOI] [PubMed] [Google Scholar]
- 6.Buttery JE, Stuart S, Pannall PR. 1992. Stability of the CK-MB isoenzyme on routine storage. Clin Biochem 25:11–13. [DOI] [PubMed] [Google Scholar]
- 7.Clark S, Youngman LD, Palmer A, Parish S, Peto R, Collins R. 2003. Stability of plasma analytes after delayed separation of whole blood: implications for epidemiological studies. Int J Epidemiol 32:125–130. [DOI] [PubMed] [Google Scholar]
- 8.Conen D, Zeller A, Pfisterer M, Martina B. 2006. Usefulness of B-type natriuretic peptide and C-reactive protein in predicting the presence or absence of left ventricular hypertrophy in patients with systemic hypertension. Am J Cardiol 97:249–252. [DOI] [PubMed] [Google Scholar]
- 9.Cottone S, Nardi E, Mulè G, Vadalà A, Lorito MC, Riccobene R, Palermo A, Arsena R, Guarneri M, Cerasola G. 2007. Association between biomarkers of inflammation and left ventricular hypertrophy in moderate chronic kidney disease. Clin Nephrol 67:209–216. [DOI] [PubMed] [Google Scholar]
- 10.Dunn ME, Coluccio D, Zabka TS, Gopalakrishnan G, Hirkaler G, Geng W, Nicklaus R, Lipshultz SE, Doessegger L, Saladino BH, Singer T, Mikaelian I. 2012. Myocardial mononuclear cell infiltrates are not associated with increased serum cardiac troponin I in cynomolgus monkeys. Toxicol Pathol 40:647–650. [DOI] [PubMed] [Google Scholar]
- 11.Fredericks S, Merton GK, Lerena MJ, Heining P, Carter ND, Holt DW. 2001. Cardiac troponins and creatine kinase content of striated muscle in common laboratory animals. Clin Chim Acta 304:65–74. [DOI] [PubMed] [Google Scholar]
- 12.Gozalo A, Dagle DE, Montoya E, Weller RE, Málaga CA. 1992. Spontaneous cardiomyopathy and nephropathy in the owl monkey (Aotus sp.) in captivity. J Med Primatol 21:279–284. [PubMed] [Google Scholar]
- 13.Hanrath P, Mathey DG, Siegert R, Bleifeld W. 1980. Left ventricular relaxation and filling pattern in different forms of left ventricular hypertrophy: an echocardiographic study. Am J Cardiol 45:15–23. [DOI] [PubMed] [Google Scholar]
- 14.Hessel MHM, Atsma DE, van der Valk EJM, Bax WH, Schalij MJ, van der Laarse A. 2007. Release of cardiac troponin I from viable cardiomyocytes is mediated by integrin stimulation. Pflugers Arch 455:979–986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Institute for Laboratory Animal Research. 2011. Guide for the care and use of laboratory animals, 8th ed Washington (DC): National Academies Press. [Google Scholar]
- 16.Jaffe AS, Babuin L, Apple FS. 2006. Biomarkers in acute cardiac disease: the present and the future. J Am Coll Cardiol 48:1–11. [DOI] [PubMed] [Google Scholar]
- 17.Kanthaswamy S, Reader R, Tarara R, Oslund K, Allen M, Ng J, Grinberg C, Hyde D, Smith DG, Lerche N. 2014. Large-scale pedigree analysis leads to evidence for founder effects of hypertrophic cardiomyopathy in rhesus macaques (Macaca mulatta). J Med Primatol 43:288–291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Karamitsos TD, Dass S, Suttie J, Sever E, Birks J, Holloway CJ, Robson MD, Jerosch-Herold M, Watkins H, Neubauer S. 2013. Blunted myocardial oxygenation response during vasodilator stress in patients with hypertrophic cardiomyopathy. J Am Coll Cardiol 61:1169–1176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Korcarz CE, Padrid PA, Shroff SG, Weinert L, Lang RM. 2011. Doppler echocardiographic reference values for healthy rhesus monkeys under ketamine hydrochloride sedation. J Med Primatol 26:287–298. [DOI] [PubMed] [Google Scholar]
- 20.Kubo T, Kitaoka H, Okawa M, Yamanaka S, Hirota T, Baba Y, Hayato K, Yamasaki N, Matsumura Y, Yasuda N, Sugiura T, Doi YL. 2011. Combined measurements of cardiac troponin I and brain natriuretic peptide are useful for predicting adverse outcomes in hypertrophic cardiomyopathy. Circ J 75:919–926. [DOI] [PubMed] [Google Scholar]
- 21.Kuznetsova T, Thijs L, Knez J, Herbots L, Zhang Z, Staessen JA. 2014. Prognostic value of left ventricular diastolic dysfunction in a general population. J Am Heart Assoc 3:e000789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lammey ML, Lee DR, Ely JJ, Sleeper MM. 2008. Sudden cardiac death in 13 captive chimpanzees (Pan troglodytes). J Med Primatol 37 Suppl 1:39–43. [DOI] [PubMed] [Google Scholar]
- 23.Liu ZL, Wiedmeyer CE, Sisson DD, Solter PF. 2002. Cloning and characterization of feline brain natriuretic peptide. Gene 292:183–190. [DOI] [PubMed] [Google Scholar]
- 24.Maron BJ, Maron MS. 2013. Hypertrophic cardiomyopathy. Lancet 381:242–255. [DOI] [PubMed] [Google Scholar]
- 25.Maron BJ. 2002. Hypertrophic cardiomyopathy: a systematic review. JAMA 287:1308–1320. [DOI] [PubMed] [Google Scholar]
- 26.Michels M, Hoedemaekers YM, Kofflard MJ, Frohn-Mulder I, Dooijes D, Majoor-Krakauer D, Ten Cate FJ. 2007. Familial screening and genetic counselling in hypertrophic cardiomyopathy: the Rotterdam experience. Neth Heart J 15:184–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Monfared A, Salari A, Kazemnezhad E, Lebadi M, Khosravi M, Mehrjardi NK, Rahimifar S, Amini N. 2013. Association of left ventricular hypertrophy with high-sensitive C-reactive protein in hemodialysis patients. Int Urol Nephrol 45:1679–1686. [DOI] [PubMed] [Google Scholar]
- 28.Nagueh SF, Appleton CP, Gillebert TC, Marino PN, Oh JK, Smiseth OA, Waggoner AD, Flachskampf FA, Pellikka PA, Evangelista A. 2009. Recommendations for the evaluation of left ventricular diastolic function by echocardiography. J Am Soc Echocardiogr 22:107–133. [DOI] [PubMed] [Google Scholar]
- 29.Nilsson TK, Boman K, Jansson JH, Thogersen AM, Berggren M, Broberg A, Granlund A. 2005. Comparison of soluble thrombomodulin, von Willebrand factor, tPA/PAI-1 complex, and high-sensitivity CRP concentrations in serum, EDTA plasma, citrated plasma, and acidified citrated plasma (Stabilyte) stored at -70 degrees C for 8 to11 y. Thromb Res 116:249–254. [DOI] [PubMed] [Google Scholar]
- 30.Olatidoye AG, Wu AHB, Feng YJ, Waters D. 1998. Prognostic role of troponin T compared with troponin I in unstable angina pectoris for cardiac events with meta-analysis comparing published studies. Am J Cardiol 81:1405–1410. [DOI] [PubMed] [Google Scholar]
- 31.Pepys MB, Hirschfield GM. 2003. C-reactive protein: a critical update. J Clin Invest 111:1805–1812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pisharath H, Zao CL, Kreeger J, Portugal S, Kawabe T, Burton T, Tomaeck L, Shoieb A, Campbell BM, Franco J. 2013. Immunopathologic characterization of naturally acquired Trypanosoma cruzi infection and cardiac sequalae in cynomolgus macaques (Macaca fascicularis). J Am Assoc Lab Anim Sci 52:545–552. [PMC free article] [PubMed] [Google Scholar]
- 33.Reader JR, Canfield DR, Lane JF, Kanthaswamy S, Ardeshir A, Allen AM, Tarara RP. 2016. Left ventricular hypertrophy in rhesus macaques (Macaca mulatta) at the California National Primate Research Center 1992 to 2014. Comp Med 66: 162–169. [PMC free article] [PubMed] [Google Scholar]
- 34.Sabatini CF, O'Sullivan ML, Valcour JE, Sears W, Johnson RJ. 2013. Effects of injectable anesthetic combinations on left ventricular function and cardiac morphology in Sprague-Dawley rats. J Am Assoc Lab Anim Sci 52:34–43. [PMC free article] [PubMed] [Google Scholar]
- 35.Salles GF, Fiszman R, Cardoso CRL, Muxfeldt ES. 2007. Relation of left ventricular hypertrophy with systemic inflammation and endothelial damage in resistant hypertension. Hypertension 50:723–728. [DOI] [PubMed] [Google Scholar]
- 36.Sanderson JE, Traill TA, Sutton MG, Brown DJ, Gibson DG, Goodwin JF. 1978. Left ventricular relaxation and filling in hypertrophic cardiomyopathy. An echocardiographic study. Br Heart J 40:596–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schulman FY, Farb A, Virmani R, Montali RJ. 1995. Fibrosing cardiomyopathy in captive Western Lowland Gorillas (Gorilla gorilla gorilla) in the United States: a retrospective study. J Zoo Wildl Med 26:43–51. [Google Scholar]
- 38.Shave R, Baggish A, George K, Wood M, Scharhag J, Whyte G, Gaze D, Thompson PD. 2010. Exercise-induced cardiac troponin elevation: evidence, mechanisms, and implications. J Am Coll Cardiol 56:169–176. [DOI] [PubMed] [Google Scholar]
- 39.Shave R, Oxborough D. 2012. Exercise-induced cardiac injury: evidence from novel imaging techniques and highly sensitive cardiac troponin assays. Prog Cardiovasc Dis 54:407–415. [DOI] [PubMed] [Google Scholar]
- 40.Smith OA, Astley CA. 2006. Naturally occurring hypertension in new world nonhuman primates: potential role of the perifornichypothalamus. Am J Physiol Regul Integr Comp Physiol 292:R937–R945. [DOI] [PubMed] [Google Scholar]
- 41.Tang HL, Wang LL, Cheng G, Wang L, Li S. 2008. Evaluation of the cardiovascular function of older adult rhesus monkeys by ultrasonography. J Med Primatol 37:101–108. [DOI] [PubMed] [Google Scholar]
- 42.Tsung JS, Tsung SS. 1986. Creatine kinase isoenzymes in extracts of various human skeletal muscles. Clin Chem 32:1568–1570. [PubMed] [Google Scholar]
- 43.Wagner GS, Roe CR, Limbird LE, Rosati RA, Wallace AG. 1973. The importance of identification of the myocardial-specific isoenzyme of creatine phosphokinase (MB Form) in the diagnosis of acute myocardial infarction. Circulation 47:263–269. [DOI] [PubMed] [Google Scholar]
- 44.Wescott D, Hennan J, Pavlock D, Dipiero J, Bounous D. 2012. NT pro-BNP method comparison in cynomolgus monkeys with congestive heart failure. Clin Chem 58 Suppl 10: A50 [Google Scholar]
- 45.Wu AHB, Fukushima N, Puskas R, Todd J, Goix P. 2006. Development and preliminary clinical validation of a high sensitivity assay for cardiac troponin using a capillary flow (single molecule) fluorescence detector. Clin Chem 52:2157–2159. [DOI] [PubMed] [Google Scholar]
- 46.Xanthakis V, Larson MG, Wollert KC, Aragam J, Cheng S, Ho J, Coglianese E, Levy D, Colucci WS, Felker GM, Benjamin EJ, Januzzi JL, Wang TJ, Vasan RS. 2013. Association of novel biomarkers of cardiovascular stress with left ventricular hypertrophy and dysfunction: implications for screening. J Am Heart Assoc 2:e000399. [DOI] [PMC free article] [PubMed] [Google Scholar]