Abstract
Previous studies have indicated that nonhuman animals might have a capacity for episodic-like recall reflected in memory for “what” events that happened “where” and “when”. These studies did not identify the brain structures that are critical to this capacity. Here we trained rats to remember single training episodes, each composed of a series of odors presented in different places on an open field. Additional assessments examined the individual contributions of odor and spatial cues to judgments about the order of events. The results indicated that normal rats used a combination of spatial (“where”) and olfactory (“what”) cues to distinguish “when” events occurred. Rats with lesions of the hippocampus failed in using combinations of spatial and olfactory cues, even as evidence from probe tests and initial sampling behavior indicated spared capacities for perception of spatial and odor cues, as well as some form of memory for those individual cues. These findings indicate that rats integrate “what,” “where,” and “when” information in memory for single experiences, and that the hippocampus is critical to this capacity.
Tulving (1972) characterized episodic memory as the capacity to recollect where and when past events occurred. Tulving and colleagues have since refined their definition of episodic memory, emphasizing qualities of the subjective consciousness and a sense of time in retrieving past experiences (Tulving and Markowitsch 1998; Tulving 2002). Whether nonhuman animals are able to consciously recollect past experiences and whether they have a sense of time are subjects of ongoing debate (Roberts 2002; Clayton et al. 2003a; Suddendorf and Busby 2003a,b). Although there is no way to directly assess the subjective attributes of episodic memory in animals, Clayton and Dickinson (1998) suggested that the capacity to remember where and when specific events occurred constitutes evidence of “episodic-like” memory which can be tested in animals. A series of experiments on food-caching in scrub jays showed that jays could indeed remember the integrated “what,” “where,” and “when” information from specific past events (Clayton and Dickinson 1998; Clayton et al. 2001). The definition of episodic-like memory has been refined, now also emphasizing the capacity for flexible expression of memory (Clayton et al. 2003b). Those authors argue that the food-caching behavior in scrub jays meets their criteria for episodic-like memory (Clayton et al. 2003b). However, these studies did not identify the brain structures that are critical for this capacity.
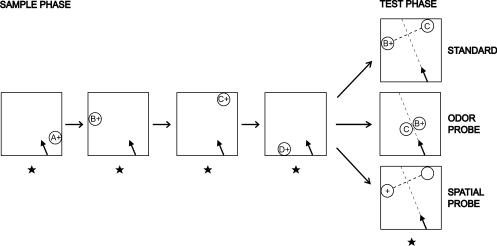
Previous studies with rodents suggest that the hippocampus is involved in memory for the “what” and “when” information in remembering a sequence of odors (Fortin et al. 2002; Kesner et al. 2002), for the “where” and “when” information in remembering a sequence of rewarded places (Kesner and Novak 1982; Chiba et al. 1994), and for the “what” and “where” information in remembering a flavor found in a particular place (Day et al. 2003). In the present study, we developed a novel memory task to assess memory for events from single episodes involving a combination of odors (“what”) presented in unique places (“where”) in a specific order (“when”; Fig. 1). The results indicate that rats can remember the order of events in unique experiences and that they depend on a combination of odor and place information to make accurate judgments about when these events occurred. Additionally, memory expression is flexible in that animals reveal their memory about specific items through a variety of comparison tests that differ in format from the initial learning experience. Furthermore, the hippocampus plays a critical role in this form of “what-where-when” memory for single experiences.
Figure 1.
An example (B vs. C) trial for a standard test and odor and spatial probes. In the sample phase of every trial, the rat is presented with four odors in series (A+ → B+ → C+ → D+), each at a different location on a platform. In the following test phase, odors B and C are presented in their sample locations in the standard choice test, or next to each other in the odor probe, or two nonodorous stimuli are presented in the sample locations of B and C in the spatial probe. “+,” reinforced stimulus; arrow on the platform, position of the rat at the starting point (arrowhead corresponds to the rat's head); star symbol, the experimenter's fixed position throughout testing.
RESULTS
Can Rats Remember the Order of a Sequence of Odors Presented in Different Places?
Fourteen rats were trained on the “what-where-when” task (standard choice tests), using 24 spices as odors and 24 positions at the periphery of a platform as places (Fig. 1). On each trial, the rat sequentially sampled a unique series of four rewarded odors, each presented in a different place on the periphery (A through D). Subsequently, memory was tested in a single choice between an arbitrarily selected pair of the sample odors in their original locations (B vs. D, A vs. C, etc.). The rat was rewarded for the selection of the stimulus that occurred earlier in the sequence.
In five stages of training, rats were initially tested with subsets of the stimulus pairings, and then gradually shifted to a random mixture of all possible choice pairings. In the initial stage, rats reached a criterion of 80% correct over two consecutive sessions on individual types of choice tests in 33.4 ± 2.1 (mean ± SE) trials (A vs. D), 15.0 ± 1.4 trials (B vs. D), and 18.0 ± 2.1 trials (C vs. D). Next, rats reached at least 75% correct over two sessions on mixed presentations of A versus C, A versus D, B versus D, and C versus D tests in 29.6 ± 2.7 trials. In subsequent training on all types of choice tests, rats performed at 71.7% correct (performance vs. chance: t(13) = 10.804, P < 0.0001). Then, in additional training on a mixture of A versus B, B versus C, and C versus D tests, rats performed at 68.8% correct (performance vs. chance: t(13) = 8.232, P < 0.0001). In the final stage of training on all types of choice tests, rats performed at 67.9%, 61.9%, 75%, 76.2%, 75%, and 72.6% correct for six consecutive sessions.
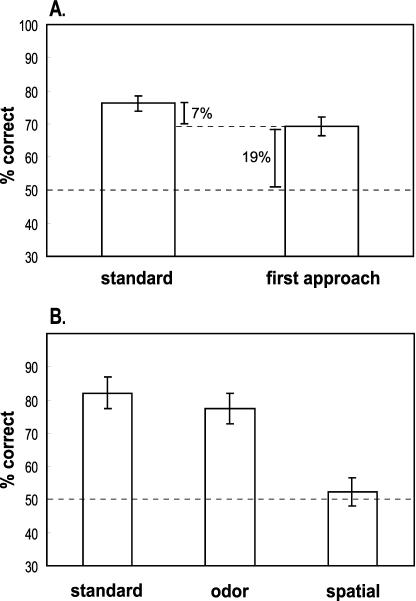
In the last four of these sessions and in a subsequent testing session that accompanied the probe tests, rats performed well above chance on all types of standard choice tests, indicating that normal rats could remember the order of unique sequences of odors and places (t(13) = 11.725, P < 0.0001; Fig. 2A).
Figure 2.
Performance (mean ± SE) of normal rats (n=14). (A) Comparison of performance versus percentage of correct first approaches on standard choice tests. (B) Performance on probe tests and accompanying standard choice tests. Dashed line: chance performance (50%).
We additionally examined performance on choice tests that differed in the separation or lag between initially presented stimuli. Performance was above chance at all lags (all P-values ≤ 0.0001; Table 1), and performance on Lag 2 tests (A vs. D) was superior to that on Lag 0 (A vs. B, B vs. C, C vs. D) and Lag 1 (A vs. C, B vs. D) tests (paired t-tests: t(13) = 5.491, P = 0.0001 and t(13) = 4.387, P = 0.0007, respectively). Performance on Lag 0 and Lag 1 tests did not differ significantly (t(13) = 0.118, P = 0.9079). These findings are consistent with those of previous studies showing that rats and humans have greater difficulty judging the sequential ordering of temporally adjacent items than temporally distant items of studied sequences (Chiba et al. 1994; Madsen and Kesner 1995; Fortin et al. 2002; Kesner et al. 2002).
Table 1.
Performance (% Correct ± SE) on the Standard Task at Different Lags
| Presurgery
|
Postsurgery
|
|||
|---|---|---|---|---|
| Shams | H group | Shams | H group | |
| Lag 0 | 71.4 ± 5.2 | 74.3 ± 4.7 | 73.8 ± 4.2 | 45.2 ± 4.6 |
| Lag 1 | 75.7 ± 7.2 | 68.6 ± 4.0 | 70.2 ± 5.7 | 56.0 ± 5.4 |
| Lag 2 | 94.3 ± 5.7 | 94.3 ± 5.7 | 78.6 ± 7.0 | 61.9 ± 6.0 |
Distinguishing the Use of Spatial and Olfactory Cues in the Standard Choice Tests
In the “what-where-when” task described above, odor and place cues were intentionally confounded, such that accurate judgments about the order of items could be based on only the place cues, the odor cues, or a combination of place and odor cues. We examined which cues were employed by rats, using the following two strategies.
1. First Approach Compared to Final Choice
One possibility is that rats initially use the spatial cues that can easily be seen from anywhere on the maze to approach the earlier visited place. Then they might or might not confirm the initially spatially guided choice by smelling the odor at that location. A comparison of performance on the initial visit and on the final response choice would provide measures of the extent to which overall performance depended on place and odor cues. To the extent that rats use only the spatial cues, one would expect all first visits lead to the same final choice. Alternatively, to the extent that rats use only odor cues, one would expect random selections on the first visit and considerably better performance on the final choice. If, however, rats use a combination of the spatial and odor cues, one would expect better than chance performance on the first choice and a significant improvement above that on the final response.
Odor Detection Tests
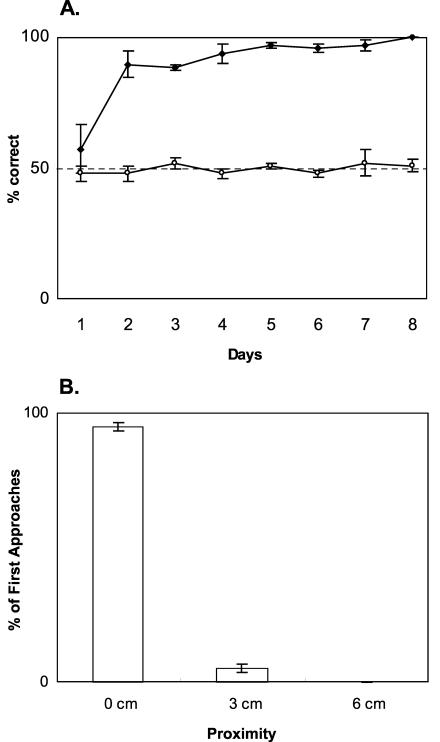
The preceding rationale depends crucially on the assumption that rats use exclusively spatial cues on the initial visit and do not detect the odor of a stimulus before arriving at a stimulus cup. To test this assumption, we performed a separate control experiment that determined the distance from which rats could detect the odor stimuli. A different group of rats was trained to discriminate a scented stimulus from an unscented stimulus, with the two stimuli placed at separate random locations on the periphery of the platform just as in the standard choice tests. Rats learned to choose the scented stimulus within the first day of training (Fig. 3A). If rats could detect the odors at a substantial distance across the platform, then they should be able to directly approach the scented cup first on every trial. However, the incidence of initial visits to the scented cup was not different than chance on any of the test days. Repeated-measures ANOVA indicated a significant interaction between choice performance and initial visit across days (F(7,42) = 6.795, P < 0.0001; Fig. 3A). On Day 1, rats approached both test locations equally often (initial visits vs. chance: t(3) = -0.775, P = 0.495), and the accuracy of choice responses was also not different than chance (t(3) = 0.778, P = 0.4934). From Day 2 through Day 8, however, rats chose accurately everyday (performance vs. chance: all t-values ≥ 7.982, all P-values ≤ 0.0041). In contrast, the incidence of correct initial visits was at chance each day (performance vs. chance: all t-values ≤ 1.732, all P-values ≥ 0.1817), indicating that rats could not detect the odors during the initial approach. Furthermore, rats investigated the first visited stimulus at or beyond a 0-cm-line on 95% of all 192 trials and, on the remaining 5% of the trials, the visit was within 3 cm of the cup (Fig. 3B). Investigation at a distance greater than 3 cm was not observed on any trial. These findings strongly indicate that rats cannot detect the stimulus odors until they approach the cups within 3 cm. Therefore, in standard choice tests, first approach within 3 cm was used as a measure of initial guidance by spatial cues alone.
Figure 3.
Detection of odors on the platform by normal rats (n=4). (A) First approach (○) and choice (♦) responses (mean ± SE). Dashed line: chance. (B) Proximity to cups in the first approach (mean ± SE). 0 cm, rat's nose is over the cup; 3 cm, rat's nose is within 3 cm; 6 cm, rat's nose is within 6 cm.
First Approach in the “What-Where-When” Task
The findings from the control experiment validated our assumption that rats use exclusively spatial cues to guide the initial visit. Therefore, we compared the contribution of place and odor cues by examining the performance of rats on the initial visits and final choices in the “what-where-when” task. Rats first approached the correct choice at well above chance level (t(13) = 7.056, P < 0.0001; Fig. 2A), indicating that they could use their memory for places where the cups were presented to guide the initial visit. However, performance on choice behavior was significantly greater than on initial visits (paired t-test: t(13) = 5.025, P = 0.0002; Fig. 2A), indicating that rats additionally used the scents of the stimuli to make their final choice response.
2. Spatial and Odor Probe Tests
Another way to examine the use of place and odor cues is to eliminate the information from one source of cues and then determine the extent to which rats can use the remaining source of information to make the response choice. Therefore, in an additional series of probe tests, we presented sequences of odor-place cues as in the standard version task, but then on some test trials we provided only the appropriate place cues (spatial probe tests) or only the appropriate odor cues (odor probe tests). Based on the findings from the initial approach analysis, we reasoned that rats would rely on the odor cues obtained following an initial spatially guided approach, and would therefore perform poorly when the confirming odor cues were absent. To the extent that rats could use odor cues alone when spatial cues were absent, one would expect accurate performance on the odor probe tests.
Spatial Probe Tests
We assessed rats' ability to perceive and use spatial cues by examining choice behavior on spatial probe trials when the scents were absent at the test phase (Fig. 1). Standard choice tests, where both the odor and spatial cues were present, were intermixed with the probe trials for comparison of performance. Rats continued to perform significantly above chance on choice tests (t(13) = 6.734, P < 0.0001; Fig. 2B), whereas they chose correctly no more often than predicted by chance on spatial probe tests (t(13) = 0.563, P = 0.5828; Fig. 2B).
Odor Probe Tests
We assessed the ability to perceive and use the odors by examining choice behavior on odor probe trials when the initial spatial cues were absent at the test phase (Fig. 1). Rats performed well above chance on odor probe tests (t(13) = 6.097, P < 0.0001; Fig. 2B), and this performance did not differ significantly from that on standard choice tests (paired t-test: t(13) = -1.075, P = 0.3019; Fig. 2B).
The combination of initial approach and probe test data indicates that rats use the full combination of “what,” “where,” and “when” information in memory for the order of odor-place events. In the standard trials, normal rats initially used exclusively spatial cues to approach the correct place on 69% of the trials, then selected the correct choice on 76% of the trials (Fig. 2A). Thus, it appears that place information alone contributed to an increment of 19% correct responses above the level expected by chance (50%). Olfactory information was used to confirm or disconfirm the initial approach and consequently increased the final choice an additional 7%. Whereas the incremental advantage from the odor cue appears to be relatively small compared to the initial contribution of spatial cues, confirmation by the odor was critical. This conclusion is supported by the observation that removal of the odor cues in the spatial probes severely disrupted performance in normal rats, and indeed rats could use the odor cues alone when the relevant spatial cues were absent. Our interpretation of these findings is that normal rats expected to confirm the initial spatial choice with the earlier sampled odor. When that odor was not found at either place on the spatial probes, rats could not make the critical confirmation. These considerations support the conclusion that rats normally use the combination of “where” and “what” information to make the critical “when” judgment on standard trials, and therefore demonstrate the capacity for integrating these elements of memory for single experiences.
Is the Hippocampus Essential for “What-Where-When” Memory?
Following the testing described above, the same subjects were divided into two equal and matched groups, one group that received bilateral hippocampal lesions (H group) and a sham operated control group. Performance prior to surgery was well matched between the groups on standard choice tests prior to probe testing (unpaired t-test: t(12) = 0.206, P = 0.8406), standard choice tests at each lag (Lag 0: t(12) = -0.408, P = 0.6901, Lag 1: t(12) = 0.866, P = 0.4035, Lag 2: t(12) = 0), first approach (t(12) = -0.595, P = 0.5632), standard choice tests accompanying the probes (t(12) = 0.24, P = 0.8142), spatial probes (t(12) = 1.139, P = 0.277), and odor probes (t(12) = -0.783, P = 0.4487).
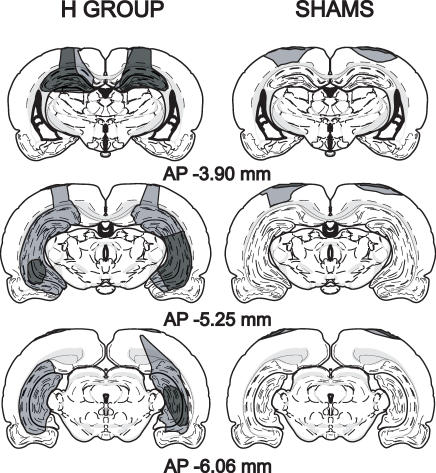
The volume of tissue damage in animals with H lesions was measured at three AP levels: -3.90 mm, -5.25 mm, and -6.06 mm (see examples in Fig. 4). Total volume of the bilateral lesions of the hippocampus proper and dentate gyrus was 35.9%-93.2% (mean ± SE: 71.2% ± 7.8%). The ventral subiculum was partially damaged in all subjects (mean volume damaged ± SE: 26.2% ± 9.9%, range: 3.0%-66.7%). This damage was mostly present at AP -6.06 mm. Minimal damage in the dorsal subiculum was observed in only one subject. Damage to the parahippocampal cortical areas was not observed. In most subjects, unilateral partial damage to the lateral posterior thalamic nucleus and dorsal lateral geniculate nucleus was observed at AP -3.90 mm. In one subject, there was a partial unilateral damage to the dorsal lateral geniculate nucleus and the optic tract at AP -5.25 mm and medial geniculate nucleus (ventral and medial) at AP -6.06 mm. In another subject, the lateral terminal nucleus of accessory optic tract and cerebral peduncle were unilaterally and partially damaged at AP -5.25 mm. Some damage to the cortex overlying the hippocampus was observed in all H subjects as well as sham-operated subjects.
Figure 4.
Reconstructions of the smallest and largest brain lesions for sham-control and H groups. Lesions were reconstructed on coronal sections adapted from Swanson (1992) at AP -3.90 mm, -5.25 mm, and -6.06 mm. The largest lesions are indicated with light gray, smallest lesions with dark gray.
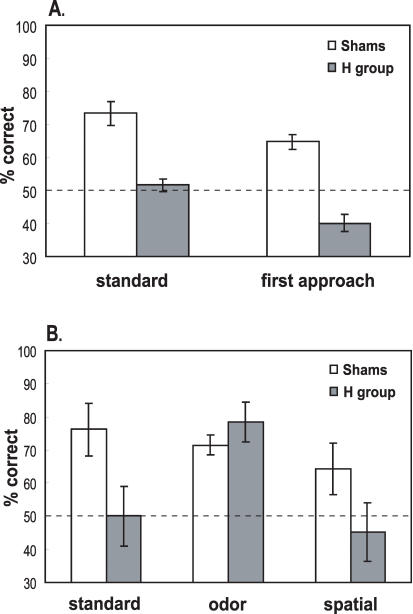
Following surgery, sham-operated control subjects continued to perform well above chance on standard choice tests (t(6) = 6.443, P = 0.0007; Fig. 5A). In contrast, H rats did not (t(6) = 0.787, P = 0.4613; Fig. 5A). The deficit following hippocampal damage was confirmed by a repeated-measures ANOVA indicating a significant group difference in performance between pre- and postoperative testing (group vs. phase interaction: F(1,12) = 8.475, P = 0.0131). Additional repeated-measures ANOVA on postoperative performance did not reveal a significant group difference across lags, but showed a significant group effect (F(1,12) = 24.789, P = 0.0003; Table 1). The loss of a lag effect in postsurgical testing was associated with decreased performance of both groups at Lag 2, and may reflect the effect of increased interference with extended testing. Further post hoc analyses investigated the main effects of group at different lags. In these analyses, a pooled error term was determined depending on the variability of groups across all lags. Because there were three lags to compare, results were considered at α′ = α/3 = 0.017 (Kirk 1982; Girden 1992). These tests indicated that performance between groups differed significantly after surgery at α′ only at Lag 0 (FLag 0 (1,35) = 13.25; P < 0.01). Groups did not differ significantly at Lag 1 (FLag 1 (1,35) = 3.31; P > 0.05) or Lag 2 (FLag 2 (1,35) = 4.51; P < 0.05) at α′. Additionally, separate t-tests showed that the performance of H rats did not differ from chance at any lag (Lag 0: t(6) = -1.034, P = 0.3409, Lag 1: t(6) = 1.109, P = 0.31, Lag 2: t(6) = 1.987, P = 0.0941). In contrast, sham control subjects continued to perform well above chance at all lags (Lag 0: t(6) = 5.739, P = 0.0012, Lag 1: t(6) = 3.545, P = 0.0121, Lag 2: t(6) = 4.076, P = 0.0065), and performance did not differ significantly among lags (paired t-tests: all P-values ≥ 0.343). The mixture of these findings suggests that rats with hippocampal damage were impaired on standard choice tests across all lags, with a stronger impairment at Lag 0 and a milder impairment at Lag 1 and 2.
Figure 5.
Postsurgery performance (mean ± SE) of sham-control and H groups. (A) Comparison of performance vs. percentage of correct first approaches on standard choice tests. (B) Performance on probe tests and accompanying standard choice tests. Dashed line: chance.
How Rats With Hippocampal Damage Use the Place and Odor Cues
The above described findings clearly show that rats with hippocampal damage fail on the “what-where-when” task. It is possible that they fail because they cannot perceive or remember the place cues, or the odors cues, or both. To explore whether and how rats with hippocampal damage use the available cues, we explored the initial approach strategies on the “what-where-when” tests after surgery and then re-examined performance on the spatial and odor probe tests.
First Approach on “What-Where-When” Tests
Whereas sham control subjects performed well on the initial approach during the standard choice tests, performance of the H group was abnormal (repeated-measures ANOVA: F(1,12) = 11.072, P = 0.006; Fig. 5A). Sham controls initially approached the correct stimulus well over chance level (t(6) = 6.542, P = 0.0006). In contrast, and surprisingly, H rats approached the correct choice less often than expected by chance (t(6) = -3.769, P = 0.0093). This observation indicates some form of intact spatial perception and memory in H rats. However, contrary to the strategy of normal rats and to the reinforcement contingency of the standard choice test, H rats were inclined to visit the more recently presented and rewarded place rather than the earlier visited locus.
Spatial and Odor Probe Tests
Sham control subjects also performed well above chance on the standard choice tests that accompanied the spatial and odor probes (t(6) = 3.268, P = 0.0171), whereas H rats did not (t(6) = 0; Fig. 5B). Performance of sham controls was superior to that of H rats (unpaired t-test: t(12) = 2.185, P = 0.0494). Neither group performed better than expected by chance on the spatial probe tests (sham group: t(6) = 1.866, P = 0.1112; H group: t(6) = -0.548, P = 0.6037; Fig. 5B), and performance of the two groups did not differ significantly on these probes (unpaired t-test: t(12) = 1.644, P = 0.126). Performance of both groups was greater than chance on odor probes (sham group: t(6) = 6.975, P = 0.0004; H group: t(6) = 4.768, P = 0.0031; Fig. 5B), and the groups did not differ significantly in performance on these probes (unpaired t-test: t(12) = -1.06, P = 0.3098).
The findings from the initial approach and probe tests indicate that different kinds of memory processing are employed by normal rats and rats with hippocampal damage. As observed prior to surgery, normal rats used the spatial cues available at the outset of the test, then confirmed or disconfirmed their memory of the odor at that place to guide the final choice. Such an interpretation is consistent with a recent observation that rats can conversely use the flavor of a food to remember the location where it was found (Day et al. 2003). Without hippocampal function, other strategies led to misuse of the spatial memory, leading to the initial below-chance approach to the correct place. In rats with hippocampal damage, a “habit” representation mediated by the neostriatum may have guided repetition of the most recently reinforced approach response (Packard et al. 1989). Alternatively, an “emotional” memory mediated by the amygdala may have favored attraction to the most recently rewarded locus (McDonald and White 1993; Eichenbaum and Cohen 2001).
Rats with hippocampal damage succeeded on the odor probes, indicating that they could perceive the odor cues and use them to solve the odor probe tests. Yet they could not use the same available odor cues to solve the standard choice tests. These findings strongly suggest that rats with hippocampal damage use different strategies on these two types of tests. In the odor probes, presentation of the odor cues in close proximity with only a brief memory delay following the initial sampling may have encouraged the comparison of the relative strengths of the memory traces of the two odors. Thus, rats may have simply selected the less familiar of two recently experienced scents, a kind of memory processing that can be accomplished without critical hippocampal function (Dudchenko et al. 2000). On the standard trials and on the spatial probes, presentation of the odors in widely separated places may have made the comparison of relative memory strengths difficult and precluded successful use of this strategy. The findings on the initial approach and probe tests indicate that rats with hippocampal damage could perceive and remember the spatial and odor cues in some way, but could not appropriately apply these cues to solve the “what-where-when” problem.
DISCUSSION
The present findings indicate that normal rats can integrate “what,” “where,” and “when” information to remember the order of a sequence of events, and that the hippocampus is critical to this kind of memory. Our analyses indicate that normal rats used a combination of odor and place cues to guide judgments about temporal order. The probe tests altered how the problem was solved. At the same time, these tests and the findings on initial approaches provide clues about what cues are remembered, how they are normally used, and how they are used or misused following hippocampal damage. In the following discussion, we review other studies that have examined the role of the hippocampus in memory for “what,” “where,” and “when” information presented in single events, and we consider the significance of the present findings for animal models of episodic-like memory.
The Hippocampus and Memory for “What,” “Where,” and “When”
Previous studies have demonstrated a critical role for medial temporal lobe structures in memory for “what” object was presented in a single event. Most prominent of these are studies on delayed matching or nonmatching to sample for once-presented objects (Gaffan 1974; Mishkin and Delacour 1975; for review, see Murray 1996; Eichenbaum et al. 2000; Mumby 2001). Damage to the entire medial temporal lobe region, or to the parahippocampal cortical areas surrounding the hippocampus, produces a severe delay-dependent impairment in memory for objects (Suzuki et al. 1993; Mumby and Pinel 1994; Zola-Morgan et al. 1994) and odors (Otto and Eichenbaum 1992). However, selective damage to or disconnection of the hippocampus results in modest or no deficit on delayed performance on objects (Mumby et al. 1992; Murray and Mishkin 1998; Zola et al. 2000; Clark et al. 2001) and odors (Otto and Eichenbaum 1992), even when the memory load is very high (objects: Murray and Mishkin 1998; odors: Dudchenko et al. 2000), suggesting that parahippocampal cortical areas can support the capacity to remember “what” without critical hippocampal involvement. On the other hand, different results have emerged from studies on another form of recognition for novel objects where monkeys or rats are simply exposed to a novel stimulus and then, following a delay, are tested for time spent investigating that stimulus versus a novel stimulus. In this test, selective damage to the hippocampus produces a severe delay-dependent impairment in both species (Zola et al. 2000; Clark et al. 2001). The mixture of findings in these studies has led to the suggestion that multiple mechanisms can support memory for the prior occurrence of stimuli (Eichenbaum et al. 1994; Brown and Aggleton 2001; Yonelinas et al. 2002). According to this view, the hippocampus supports the capacity for memory of the episode on which the object was experienced, whereas the parahippocampal region supports responses based on stimulus familiarity alone.
Previous studies have also examined the role of the hippocampus in memory for single events based on combinations of “what-where,” “what-when,” and “where-when” information. Blockade of NMDA or AMPA receptors in the hippocampus prevents acquisition of unique flavor-place paired associates, and AMPA receptor blockade also impairs the recall of these “what-where” associations (Day et al. 2003). Also, following fornix transection, monkeys are impaired in rapid acquisition of scene-specific memory for objects (Gaffan 1994). However, monkeys with hippocampal lesions are not impaired in one-trial memory for object-place associations (Malkova and Mishkin 2003), suggesting that different strategies may be used in memory for “what-where” events.
Other studies have indicated that the hippocampus is critical in memory for “where” a recent event occurred. Rats with selective hippocampal damage are severely impaired in finding a location in the Morris water maze following a single exposure to a new locus of escape in a familiar environment (Steele and Morris 1999). In addition, the hippocampus plays a critical role in performance on the radial maze test in which rats must remember which of eight or more locations was visited once in the current testing session (Olton et al. 1979). Also, rats with hippocampal lesions are impaired in remembering the order of once-presented odor sequences (Fortin et al. 2002; Kesner et al. 2002), disambiguation of overlapping odor sequences (Agster et al. 2002), and the order of once-presented place sequences (Kesner and Novak 1982; Chiba et al. 1994). These studies suggest that the hippocampus may be critical in memory for “when” particular “what” or “where” experiences occurred. However, in the present study, rats with hippocampal damage failed on one test of memory for when odors occurred (in the standard choice tests) but succeeded on another test (the odor probe tests), supporting the view that multiple mechanisms may underlie memory for “what” and “when” combinations, just as appears to be the case for “what” and “where” combinations.
Episodic-Like Memory in Rats
What are the implications of the present findings for animal models of episodic memory? Clayton and Dickinson's description of “episodic-like” memory emphasizes the capacity to remember the integrated what-where-when representations from unique past experiences and the flexible use of newly acquired information (Clayton et al. 2003b). Scrub jays displayed this capacity in their food caching behavior (Clayton and Dickinson 1998; Clayton et al. 2003b). In these studies, jays could use the amount of time passed since caching two foods to discriminate which to choose, and they could use the relative time of caching to distinguish between them in a later test (similar to the current protocol). Clayton and colleagues (2003b) expressed concern about whether those animals might have used a sense of time passed (see also Roberts 2002) or relative familiarity of the items, rather than explicit memory for “when” events occurred, to solve these problems. The present findings suggest that under some conditions, such as when two items are closely juxtaposed, spatial cues are eliminated, and little time has passed, even rats with hippocampal damage can use relative recency or differences in familiarity to distinguish the order of two items. However, when spatial cues are provided in addition and more time has passed, those animals could not distinguish the order of the same kinds of items, suggesting that a different strategy is required and used successfully only by normal animals. Fortin et al. (2002) showed that normal rats could remember the order of a series of odors presented without concomitant spatial cues. In that study, both normal rats and rats with hippocampal damage could use familiarity with initially presented items to subsequently recognize individual cues, but only normal rats could judge the order of those items after a substantial delay. These findings suggest that familiarity and relative recency cannot support memory for a sequence of odors. In the present study, where only a very brief delay was interposed between sample presentation and testing, animals with hippocampal damage could judge the order of odors closely juxtaposed in the odor probe tests. We suggest that relative recency may have been sufficient to support this judgment, but memory for the order of events was required when the odors were separated and combined with spatial cues.
The present findings indicate that, like jays, normal rats use a combination of “where” and “what” cues to remember “when” items were presented in a single experience. When probed with special tests, rats with hippocampal lesions demonstrated perception and some form of memory for the odor and place information in isolation. They could use “what” information to make odor judgments, albeit only in a test that facilitates immediate comparisons between odors without interfering spatial cues. They could also use “where” information, albeit inappropriately to approach the most recently rewarded place. The combination of these observations supports the notion that the hippocampus is critical to the integration of “what-where-when” information, and reveals alternative memory strategies that control behavior in the absence of hippocampal processing.
The present findings also indicate that normal rats are capable of flexible expression of the acquired information. On each trial of the what-where-when task, rats acquired the information in a single trial by successively sampling each of four stimuli in a particular order. Then normal rats could flexibly express the acquired sequential information to solve a variety of tests that assessed memory by a choice judgment between any two of the items. Combining these characteristics of memory performance in normal animals, rats show robust memory for a combination of “what-where-when” information and can express these memories flexibly in a variety of tests that are distinct in format from repetition of the learning event. These characteristics satisfy the behavioral criteria for episodic-like memory in animals as defined by Clayton and colleagues (2003b).
The finding that rats can remember the order of events and places is consistent with current characterizations of human episodic memory as the capacity to “replay” memories as a sequence of events and where they occurred in a previous experience (Tulving 2002). The present findings do not inform us about whether rats have a consequent subjective experience of “mental time travel,” characteristic of episodic memory in humans. However, the present observations do suggest that rats have the rudimental capacity to remember the flow of events in single experiences, and that the hippocampus plays a critical role in this form of memory representation.
MATERIALS AND METHODS
Subjects
The odor detection study employed four 2-mo-old male Long-Evans rats weighing 200-250 g prior to training. The subjects for the other studies were 15 male Long-Evans rats of the same age. During training and testing, rats were food-deprived to 80%-90% free-feeding weight and had ad libitum access to water. They were kept on a 12h:12h illumination cycle and tested at the light phase. One rat ceased performing the task after the surgery and was excluded.
Odors
The scents were spices mixed with unscented playground sand up to a final weight of 100 g. Twenty-four different commercially available odorous spices were used: cumin (0.5 g), nutmeg (1 g), orange (1 g), thyme (1 g), lemon (0.7 g), cinnamon (0.3 g), paprika (1 g), anise (1 g), oregano (1 g), garlic (0.75 g), parsley (1 g), white pepper (1 g), marjoram (1 g), ginger (0.5 g), fennel (1 g), garden mint (0.7g), coffee (1 g), dill (1 g), allspice (1 g), sage (1 g), rosemary (1 g), cloves (0.3 g), celery (1 g), cocoa (1 g). Each stimulus was presented in a clear Nalgene cup (7 cm-diameter, 6.3 cm-height) with Velcro strips attached at the bottom. Three duplicate sets of these odors were used.
Locations
Rats were trained and tested on a 0.91-meter-square Plexiglas platform with 2.54-cm-tall borders at the periphery. Twenty-four 3-cm square Velcro strips were attached along the perimeter of the platform to affix the cups and constituted the 24 locations used in the task. There were six strips of Velcro on each side at equal center spacing (7.62 cm), leaving a 15.24-cm Velcro-free space on both ends of each side of the platform.
The platform was located in a dimly illuminated behavioral testing room. One wall of the room contained a rectangular white poster, another wall contained shelving. The third wall had a door and the other wall was blank. The experimenter stood along the wall with the door, and her position was the same throughout training and testing.
Shaping
Initially the rats were handled for 5 min a day for 5 d. Then, they were shaped to dig in cups filled with unscented sand (100 g) to recover buried half Froot Loop rewards (Kellogg's). On day 1, rats were introduced to the reward. Multiple rewards were dropped in the rat's home cage. On day 2, one cup with multiple visible and buried rewards was placed at one end of the cage. The rat was allowed to recover the rewards for 1 h. On day 3, the rat was presented with one cup containing multiple buried rewards for 1 h. On day 4, a cup with a buried reward was placed in the cage until the animal retrieved the reward. This step was repeated three times. On day 5, the rat was placed on the platform and allowed to investigate for 10 min. Multiple rewards were dropped on the platform. On day 6, a cup with a buried reward was placed along a side of the platform, the rat was allowed to recover the reward, and then was returned to its home cage. This procedure was repeated four times with the baited cup presented on different sides of the platform. On day 7, the protocol of day 6 was repeated. Then two cups, only one baited, were presented simultaneously on two different sides. The rat was left on the platform until it retrieved the reward.
The Odor Detection Task
A preliminary experiment was performed to determine the minimum distance away from an odor cup at which rats can detect odors. To facilitate measurement of distances, thin lines were drawn on the platform at 0 cm, 3 cm, and 6 cm from the edge of where the cups would be positioned.
After the rat was shaped as described above, it was presented with two cups placed at different locations on each odor detection trial. One cup contained one of the 24 odors used in the standard task and was rewarded. The other cup contained unscented sand and did not contain a reward. At the beginning of each trial, the rat was placed at the starting point as described below; then it was allowed to choose between the two test stimuli and retrieve the reward. The experimenter noted the cup first approached such that the rat's nose was within 6 cm of the edge of a cup, also noting the minimum distance from the cup during the approach, and scored the first approach as within 6 cm, 3 cm, or 0 cm. In addition, the experimenter scored the choice response as the first cup in which the rat dug. Once the rat ate the reward, it was returned in the home cage. During this time, the cups were removed from the platform and two other cups were placed at different locations for the following trial.
Each rat was tested on 24 trials a day. The stimuli and their locations, and the determination of the starting point, were identical to those employed on the standard trials as described below. The rewarded cup was presented on the right- and left-hand side of the starting point equally often within a day. Testing of each unique odor/place configuration from 28 standard sessions was completed in the first 7 d. Rats were tested for 8 d, such that they received approximately the same number of trials given prior to surgery in the experiment described below.
To confirm that the rat did not smell the reward itself, occasionally no reward was placed in the scented cup until after the rat started digging in. Performance on these trials did not differ from that on other trials.
The What-Where-When Task
Each trial was composed of a sample phase followed by a test phase (see Fig. 1). At the outset of the sample presentation, a randomly selected rewarded odor (A+) was placed in a randomly selected location along one wall of the platform. Then the rat was placed at the starting point on the platform (see below). The rat was allowed to walk to the cup, sample the odor, and dig in the sand to retrieve a buried 1/2 Froot Loop reward. After consumption of the reward, the rat was returned to its home cage for ∼7 sec. During this time, the first odor was removed from the platform, and a second randomly chosen rewarded odor (B+) was placed in a different randomly selected location on a different side of the platform. The rat was then re-placed at the starting point and allowed to sample the second stimulus. After the rat ate the reward, it was again returned to its cage for ∼7 sec. This cycle was repeated with four different rewarded odors placed at four different locations on different sides of the platform (A+ → B+ → C+ → D+). After a final 7-sec delay, two of the sampled odors were randomly chosen (e.g., B and C) and placed in the locations where they had initially been presented, and a reward was buried only in the cup that contained the earlier presented stimulus (B+ vs. C). In the standard choice test, the rat was placed at the starting point and allowed to choose between the two stimuli. Two different scores were observed and noted by the experimenter. The initial visit was scored as the cup initially approached such that the rat's nose was within 3 cm of the edge of a cup. The choice response was scored as the first cup in which the rat began to dig, indicated by touching the sand with its paw.
The Starting Point
The rat began each run towards a cup from the same location on all five runs (four during the sample phase and once during the test) on each trial (see Fig. 1). The starting point was determined as a locus on the periphery at the end of an imaginary line perpendicular to another imaginary line drawn between the two choice test locations. The starting point was always equally distant from both test locations, and was selected to be on the right- and left-hand side of the correct choice equally often. The rat was placed at the starting point facing the midpoint of the line between the cups.
Each session was composed of six trials, presented at a rate of 2-3 trials a day, with an ∼1 h interval between trials within a day. Each of the 24 odors and 24 locations was used only once per session and was assigned pseudorandomly to a position in the sequence (A-D). Each odor and location was associated with a reward in the choice test once over four sequential sessions. Each unique sample sequence was repeated every 28 sessions.
Odor and Spatial Probe Tests
The presentation of sample odors in all probe tests was identical to that for standard trials (see Fig. 1).
Spatial Probes
Cups filled with unscented sand were presented at two of the sampled locations at the test phase. The location presented earlier in the sample series was rewarded. The rewarded location was on the right- and left-hand side of the starting point in pseudorandom order within a session.
Odor Probes
Two of the sampled odors were presented in adjacent locations in the middle of the platform on a 20-cm square Plexiglas plate at the test phase. The odor that was presented earlier in the sample series was rewarded. The rewarded cup was presented on the right- and left-hand side of the starting point in pseudorandom order in a session.
Training and Testing
The sample presentation of each trial always consisted of a unique sequence of four odors (A-D) and the six possible types of test pairings (A vs. D, B vs. D, C vs. D, A vs. C, B vs. C, and A vs. B) were phased in over five stages. Stage 1: The test was always composed of A versus D, and training continued until the rat performed at >80% correct for two consecutive sessions. This protocol was repeated with B versus D and C versus D tests. Stage 2: A versus C tests were used in pseudorandom order among A versus D, B versus D, and C versus D pairs. Each session involved four A versus C tests and two of the other three types of tests. The rat was trained until it performed at >75% correct for two consecutive sessions. Stage 3: Trials with all six types of tests were presented in pseudorandom order for four sessions. Stage 4: Tests with A versus B, B versus C, and C versus D pairs were presented in pseudorandom order for 4-5 sessions to improve performance. Stage 5: Trials with all types of tests were presented in pseudorandom order for six sessions. The data from the last four of these sessions plus the six standard tests that accompanied the odor and place probes (as explained below) were combined to determine the level of performance on standard tests prior to surgery.
Subsequent to training on standard trials, the individual contributions of odor and space in guiding performance were examined in a probe test series. Each session involved two of the standard choice tests, two odor probe tests, and two spatial probe tests, presented in pseudorandom order. All six types of pairings were presented for each type of probe and the standard choice tests over three sessions.
Two weeks following recovery from the surgery, rats were food-deprived and re-shaped as required. Then they were trained with the standard choice tests for six sessions. During postsurgical testing, the observer was blind to the group assignment of the rat. Mean performance of groups was analyzed for each session. Performance of both control rats and rats with hippocampal damage was at chance for the first session and therefore not considered for analysis. The data for the other five sessions and the six standard tests that later accompanied the probes constituted the data for standard task performance after surgery. Subsequently, the contributions of odor and space were examined in a probe test series. To confirm that animals did not smell the reward itself, occasionally no reward was placed in the correct cup until after the rat started digging. Performance on these catch trials did not differ from that on standard choice tests.
Hippocampal Lesions
After presurgery testing, rats were separated into two groups each with seven rats matched for presurgery performance in the standard tests. Each rat was anesthetized with halothane (2%) and nitrous oxide/oxygen (7:3) throughout the surgery. After the rat's head was shaved, it was placed in a stereotaxic instrument (Kopf). A 37°C heating pad was placed under the body to maintain the body temperature. Atropine sulfate (0.081 mg) was intraperitoneally injected to prevent respiratory complications. The skin covering the skull was incised along the midline, and then the skull was exposed and leveled. A section of skull overlying the hippocampus was removed bilaterally. In the surgery of the hippocampal lesion (H) group, a 100-μm nichrome-wire electrode (0.7-mm uninsulated tip) was successively lowered at 12 different locations per hemisphere, and radiofrequency lesions were made within the dentate gyrus and the Ammon's horn. Prior to each lesion, the electrode was allowed to settle for 30 sec, then 8-11 mA radiofrequency current (Radionics RFG-4A) was applied to each location for 1 min. The following coordinates and current levels were used on each side: anteroposterior (AP) -2.2 (from bregma), mediolateral (ML) ±1.0, dorsoventral (DV) -3.5 (from dura measured at AP -4.8, ML ±4.1 ipsilaterally) (8 mA)/ AP -3.2, ML ±1.4, DV -3.3 (8 mA)/ AP -3.2, ML ±3.0, DV -3.3 (8 mA)/ AP -4.0, ML ±2.5, DV -3.3 (8 mA)/ AP -4.0, ML ±3.7, DV -3.3 (8 mA)/ AP -4.8, ML ±4.9, (DV -6.6 (9 mA)/ -5.4 (8 mA))/ AP -4.8, ML ±4.3, (DV -6.9 (9 mA)/ -3.5 (9 mA))/ AP -5.4, ML ±4.2, DV -3.6 (9 mA)/ AP -5.4, ML ±5.0, (DV -6.6 (9 mA)/ -5.5 (11 mA)). Subsequently, the electrode was raised from each spot after a waiting period of 1 min. In the surgery of sham-operated controls, the electrode was lowered to DV -1.6 mm for all nine AP and ML coordinates in each hemisphere, which was above the hippocampal structure. The electrode was left in each location for 2.5 min without passing any radiofrequency current. Then, the wound was sutured and covered with topical antiseptic (Betadine solution, Purdue Frederick) followed by a topical antibiotic (Panolog Cream). The animals then received a subcutaneous injection of 5 mL of saline/dextrose solution to prevent dehydration. Animals were given acetaminophen (suspension liquid, CVS) mixed with drinking water for 5 d to reduce the postsurgery pain and had ad libitum access to food and water for 2 wks after surgery. Rats also received antibiotic (40 mg/kg per day; Cephalexin for oral suspension, Ranbaxy Pharmaceuticals) absorbed in food for 10 d to prevent postsurgery complications.
Histology
After postsurgery testing, animals were injected with an overdose of sodium pentobarbital and perfused with saline, followed by 10% formalin solution. Brains were removed and saturated with 20% sucrose solution overnight. Fifty-μm coronal sections were taken with a microtome. The sections were stained with cresyl violet.
Statistical Analysis
The data were analyzed with statistical software (StatView, SAS Institute). Comparisons of group performance were initially performed using a repeated-measures ANOVA. Additional comparisons were done as follows: Paired t-tests were used to compare group performance at different phases of training. Group performance versus chance level was shown with one-sample t-tests (hypothesized mean: 50). Performance between groups for particular tests and probes was compared with unpaired t-tests.
Lesions of the H and subiculum were determined at AP -3.90 mm, -5.25 mm, and -6.06 mm and reconstructed with the software Canvas 7.0 (Deneba Systems) for each animal. The extent of damage measured as the numerical value of the lesioned area at each of the three AP coordinates was compared with the numerical value of the area of the intact counterpart. The estimated total lesion size was calculated as the sum of the bilateral damage at the three AP coordinates divided by the sum for the bilateral intact structure × 100%.
Acknowledgments
We thank Yadin Dudai for feedback, Norbert J. Fortin for software assistance, and Sule Tinaz for assistance in animal testing. This work was supported by NIH grant MH52090.
The publication costs of this article were defrayed in part by payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 USC section 1734 solely to indicate this fact.
Article published online ahead of print. Article and publication date are at http://www.learnmem.org/cgi/doi/10.1101/lm.73304.
References
- Agster, K.L., Fortin, N.J., and Eichenbaum, H. 2002. The hippocampus and disambiguation of overlapping sequences. J. Neurosci. 22: 5760-5768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown, M.W. and Aggleton, J.P. 2001. Recognition memory: What are the roles of the perirhinal cortex and hippocampus? Nat. Rev. Neurosci. 2: 51-61. [DOI] [PubMed] [Google Scholar]
- Chiba, A.A., Kesner, R.P., and Reynolds, A.M. 1994. Memory for spatial location as a function of temporal lag in rats: Role of hippocampus and medial prefrontal cortex. Behav. Neural Biol. 61: 123-131. [DOI] [PubMed] [Google Scholar]
- Clark, R.E., Zola, S.M., and Squire, L.R. 2001. Impaired recognition memory in rats after damage to the hippocampus. J. Neurosci. 20: 8853-8860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clayton, N.S. and Dickinson, A. 1998. Episodic memory during cache recovery by scrub jays. Nature 395: 272-274. [DOI] [PubMed] [Google Scholar]
- Clayton, N.S., Griffiths, D.P., Emery, N.J., and Dickinson, A. 2001. Elements of episodic-like memory in animals. Philos. Trans. R Soc. Lond. B Biol. Sci. 356: 1483-1491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clayton, N.S., Bussey, T.J., Emery, N.J., and Dickinson, A. 2003a. Prometheus to Proust: The case for behavioural criteria for “mental time travel”. Trends Cog. Sci. 7: 436-437. [DOI] [PubMed] [Google Scholar]
- Clayton, N.S., Bussey, T.J., and Dickinson, A. 2003b. Can animals recall the past and plan for the future? Nat. Rev. Neurosci. 4: 685-691. [DOI] [PubMed] [Google Scholar]
- Day, M., Langston, R., and Morris, R.G.M. 2003. Glutamate-receptor-mediated encoding and retrieval of paired-associate learning. Nature 424: 205-209. [DOI] [PubMed] [Google Scholar]
- Dudchenko, P.A., Wood, E.R., and Eichenbaum, H. 2000. Neurotoxic hippocampal lesions have no effect on odor span and little effect on odor recognition memory, but produce significant impairments on spatial span, recognition and alternation. J. Neurosci. 20: 2964-2977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eichenbaum, H. and Cohen, N.J. 2001. In From conditioning to conscious recollection. Oxford University Press, Oxford.
- Eichenbaum, H., Otto, T., and Cohen, N.J. 1994. Two functional components of the hippocampal memory system. Behav. Brain Sci. 17: 449-518. [Google Scholar]
- Eichenbaum, H., Alvarez, P., and Ramus, S. 2000. Animal models of amnesia. In Handbook of neuropsychology, 2nd ed. (ed. L. Cermak), Vol. 4., pp. 1-24. Elsevier Sciences, New York. [Google Scholar]
- Fortin, N.J., Agster, K.L., and Eichenbaum, H.B. 2002. Critical role of the hippocampus in memory for sequences of events. Nat. Neurosci. 5: 458-462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaffan, D. 1974. Recognition impaired and association intact in the memory of monkeys after transection of the fornix. J. Comp. Physiol. Psychol. 86: 1100-1109. [DOI] [PubMed] [Google Scholar]
- ____. 1994. Scene-specific memory for objects: A model of episodic memory impairment in monkeys with fornix transection. J. Cog. Neurosci. 6: 305-320. [DOI] [PubMed] [Google Scholar]
- Girden, E.R. 1992. ANOVA: Repeated measures. In Sage University paper series on quantitative applications in the social sciences, 07-084. Sage, Newbury Park, CA.
- Kesner, R.P. and Novak, J.M. 1982. Serial position curve in rats: Role of the dorsal hippocampus. Science 218: 173-175. [DOI] [PubMed] [Google Scholar]
- Kesner, R.P., Gilbert, P.E., and Barua, L.A. 2002. The role of the hippocampus in memory for the temporal order of a sequence of odors. Behav. Neurosci. 116: 286-290. [DOI] [PubMed] [Google Scholar]
- Kirk, R.E. 1982. Multiple comparison tests. In Experimental design: Procedures for the behavioral sciences, 2nd ed., pp. 90-133. Brooks/Cole, Belmont, CA.
- Madsen, J. and Kesner, R.P. 1995. The temporal-distance effect in subjects with dementia of the Alzheimer type. Alzheimer Dis. Assoc. Disord. 9: 94-100. [DOI] [PubMed] [Google Scholar]
- Malkova, L. and Mishkin, M. 2003. One-trial memory for object-place associations after separate lesions of hippocampus and posterior parahippocampal region in the monkey. J. Neurosci. 23: 1956-1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald, R.J. and White, N.M. 1993. A triple dissociation of memory systems: Hippocampus, amygdala and dorsal striatum. Behav. Neurosci. 107: 3-22. [DOI] [PubMed] [Google Scholar]
- Mishkin, M. and Delacour, J. 1975. An analysis of short-term visual memory in the monkey. J. Exp. Psychol. Anim. Behav. Proces. 1: 326-334. [DOI] [PubMed] [Google Scholar]
- Mumby, D.G. 2001. Perspectives on object-recognition memory following hippocampal damage: Lessons from studies in rats. Behav. Brain Res. 127: 159-181. [DOI] [PubMed] [Google Scholar]
- Mumby, D.G. and Pinel, J.P.J. 1994. Rhinal cortex lesions and object recognition in rats. Behav. Neurosci. 108: 1-8. [DOI] [PubMed] [Google Scholar]
- Mumby, D.G., Wood, E.R., and Pinel, J.P. 1992. Object recognition memory is only mildly impaired in rats with lesions of the hippocampus and amygdala. Psychobiol. 20: 18-27. [Google Scholar]
- Murray, E.A. 1996. What have ablation studies told us about the neural substrates of stimulus memory? Semin. Neurosci. 8: 13-22. [Google Scholar]
- Murray, E.A. and Mishkin, M. 1998. Object recognition and location memory in monkeys with excitotoxic lesions of the amygdala and hippocampus. J. Neurosci. 18: 6568-6582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olton, D.S., Becker, J.T., and Handelmann, G.E. 1979. Hippocampus, space and memory. Brain Behav. Sci. 2: 313-365. [Google Scholar]
- Otto, T. and Eichenbaum, H. 1992. Complementary roles of the orbital prefrontal cortex and the perirhinal-entorhinal cortices in an odor-guided delayed-nonmatching-to-sample task. Behav. Neurosci. 106: 762-775. [DOI] [PubMed] [Google Scholar]
- Packard, M.G., Hirsh, R., and White, N.M. 1989. Differential effects of fornix and caudate nucleus lesions on two radial maze tasks: Evidence for multiple memory systems. J. Neurosci. 9: 1465-1472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts, W.A. 2002. Are animals stuck in time? Psychol. Bull. 128: 473-489. [DOI] [PubMed] [Google Scholar]
- Steele, R.J. and Morris, R.G.M. 1999. Delay-dependent impairment of a matching-to-place task with chronic intrahippocampal infusion of the NMDA-antagonist D-AP5. Hippocampus 9: 118-136. [DOI] [PubMed] [Google Scholar]
- Suddendorf, T. and Busby, J. 2003a. Mental time travel in animals? Trends Cog. Sci. 7: 391-396. [DOI] [PubMed] [Google Scholar]
- Suddendorf, T. and Busby, J. 2003b. Like it or not? The mental time travel debate: Reply to Clayton et al. Trends Cog. Sci. 7: 437-438. [DOI] [PubMed] [Google Scholar]
- Suzuki, W.A., Zola-Morgan, S., Squire, L.R., and Amaral, D.G. 1993. Lesions of the perirhinal and parahippocampal cortices in the monkey produce long lasting memory impairment in the visual and tactile modalities. J. Neurosci. 13: 2430-2451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson, L.W. 1992. Brain maps: Structure of the rat brain. Elsevier, Amsterdam.
- Tulving, E. 1972. Episodic and semantic memory. In Organization of memory (eds. E. Tulving and W. Donaldson), pp. 381-403. Academic Press, New York.
- ____. 2002. Episodic memory: From mind to brain. Annu. Rev. Psychol. 53: 1-25. [DOI] [PubMed] [Google Scholar]
- Tulving, E. and Markowitsch, H.J. 1998. Episodic and declarative memory: Role of the hippocampus. Hippocampus 8: 198-204. [DOI] [PubMed] [Google Scholar]
- Yonelinas, A.P., Kroll, N.E., Quamme, J.R., Lazzara, M.M., Sauve, M.J., Widaman, K.F., and Knight, R.T. 2002. Effects of extensive temporal lobe damage or mild hypoxia on recollection and familiarity. Nat. Neurosci. 5: 1236-1241. [DOI] [PubMed] [Google Scholar]
- Zola, S.M., Squire, L.R., Teng, E., Stefanacci, L., Buffalo, E.A., and Clark, R.E. 2000. Impaired recognition memory in monkeys after damage limited to the hippocampal region. J. Neurosci. 20: 451-463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zola-Morgan, S., Squire, L.R., and Ramus, S.J. 1994. Severity of memory impairment in monkeys as a function of locus and extent of damage within the medial temporal lobe memory system. Hippocampus 4: 483-495. [DOI] [PubMed] [Google Scholar]