Abstract
Parkinson’s disease has long been known to involve the loss of dopaminergic neurons in the substantia nigra and the coincidental appearance of Lewy bodies containing oligomerized forms of α-synuclein. The ‘catecholaldehyde hypothesis’ posits a causal link between these two central pathologies mediated by 3,4-dihydroxyphenylacetaldehyde (DOPAL), the most toxic dopamine metabolite. Here we determine the structure of the dominant product in reactions between DOPAL and α-synuclein, a dicatechol pyrrole lysine adduct. This novel modification results from the addition of two DOPAL molecules to the Lys sidechain amine through their aldehyde moieties and the formation of a new carbon-carbon bond between their alkyl chains to generate a pyrrole ring. The product is detectable at low concentrations of DOPAL and its discovery should provide a valuable chemical basis for future studies of DOPAL-induced crosslinking of α-synuclein.
Keywords: Parkinson’s disease, amyloid disease, covalent modification, oxidative stress, Paal-Knorr
Parkinson’s disease (PD) is a neurodegenerative disorder that causes debilitating loss of motor control. The two pathological hallmarks of PD are the death of dopaminergic neurons in the substantia nigra region of the brain and the appearance in those neurons of proteinaceous aggregates called Lewy bodies, the major component of which is α-synuclein, a 140-residue neuronal protein abundant at presynaptic termini. α-Synuclein is intrinsically disordered under physiological conditions, but binds to negatively charged lipid membranes through an amphipathic helical structure formed by its N-terminal domain, comprised of seven imperfect 11-residue repeats.[1] Although there is increasing evidence for roles as a chaperone in synaptic vesicle fusion and as an antioxidant,[2-4] the exact function of α-synuclein and the cause of its aggregation in the disease state are unclear. Its connection to PD, however, has been solidified by the discovery of multiple mutations of its gene that cause early-onset disease.[5-6]
Given the well-established association of dopaminergic nerve loss and Lewy body formation, the potential for a connection between them has inspired considerable research effort and vigorous debate.[7-10] The ‘catecholaldehyde hypothesis’ suggests that an endogenous neurotoxin unique to dopamine-producing cells causes or contributes to their death in PD.[10-12] Following biosynthesis or synaptic transmission and reuptake of dopamine, it is either sequestered into storage vesicles or catabolized to prevent its accumulation in the cytosol. The first and obligate step in dopamine metabolism is conversion of the amine group to an aldehyde, giving 3,4-dihydroxyphenylacetaldehyde (DOPAL), by monoamine oxidase A. The aldehyde is then enzymatically oxidized to a carboxylic acid or reduced to a hydroxyl.[8-9]
Of dopamine’s metabolites, DOPAL is the likeliest source of toxicity. This would be expected on the basis of its chemical structure alone, which like dopamine and its other metabolites contains a catechol group that is prone to auto-oxidize to a reactive quinone, but also features an aldehyde, a functional group that is known to cause cellular damage by reacting with nucleophilic groups in macromolecules to generate covalent adducts that in many cases are considered to be markers of oxidative stress.[13] Extensive experimental evidence for DOPAL’s toxicity and potential involvement in PD has been summarized in recent work and points to its role in covalently crosslinking the highly abundant α-synuclein as a possible etiological culprit.[10, 12, 14]
Despite clear evidence for DOPAL’s neurotoxicity, its reactivity toward proteins has been investigated only recently. Doorn et al. first showed that Lys is the only residue type with significant reactivity towards DOPAL.[15-16] In a reaction with a Lys-containing model peptide, mass spectrometry (MS) identified a product with a mass corresponding to a DOPAL Schiff base adduct, formed by reaction of the aldehyde with a peptidyl amine group.[15] Although this was attributed to Lys, the model peptide also contained an unprotected amine at its N-terminus. New work by Follmer et al. using DOPAL and α-synuclein reported MS/MS data of products formed after a prolonged (24 hour) reaction time, including Lys adducts through both a Schiff base linkage and a Michael addition to the DOPAL catechol group.[17] On the basis of these two late-stage products, the authors proposed a DOPAL-based crosslinking mechanism.
In this study, we sought to characterize the intial adduct formed between DOPAL and α-synuclein and accordingly, we focus on the early period (<5 hours) of the reaction. We confirm Lys residues as the primary site of reactivity, and use NMR and MS to characterize the predominant covalent adduct, revealing a surprising dicatechol pyrrole lysine structure.
To study the reactivity of DOPAL towards α-synuclein, we set up reactions with DOPAL (2 mM) and 1H/15N-enriched wild-type α-synuclein (100 μM), using the N-terminally acetylated form found in mammalian cells (Ac-WT aS).[18-19] Extensive loss of signal intensity in the 1H-15N HSQC spectra is observed for the N-terminal half of the protein that includes 11 Lys residues (Supporting Information (SI) Figure S1). The decrease in signal intensity for the N-terminus is attributable to the loss of native signal for Lys and its neighboring residues due to modification by DOPAL averaged over many different sites, resulting in heterogeneous line broadening (SI Figure S2). This latter effect is caused by modification of any of the Lys residues causing small chemical shift changes throughout the protein, analogous to what is observed upon mutating a residue.[20]
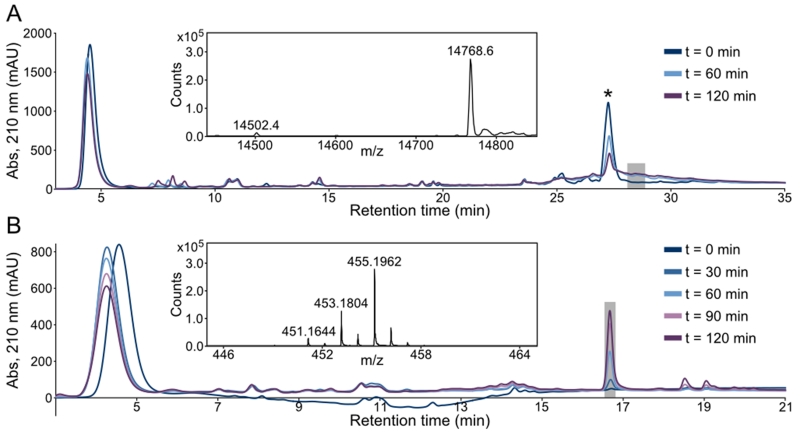
Analysis of DOPAL reaction products with Ac-WT aS by liquid chromatography-mass spectrometry (LC-MS) was carried out using protein at natural isotopic abundance, and aliquots were withdrawn from the reaction at 60 minute intervals and subjected to LC-MS without prior purification. The chromatograms show the progressive loss of native protein over time, and the appearance of a relatively heterogeneous species that elutes at higher acetonitrile concentration (Figure 1A). Based on an extensive literature of reactions between Lys and aldehyde groups, we expected to observe a mass increase of +134 Da for the modified synuclein, corresponding to the formation of a SB between a Lys sidechain amine group and the DOPAL aldehyde.[15] Surprisingly, the modified protein showed a clear and homogeneous difference of +266 Da over the native mass, with no detectable SB adduct. By contrast, the same analysis for α-synuclein lacking N-terminal acetylation shows, in addition to the +266 Da product, a +134 Da modification (SI Figure S3), indicating that significant SB base adduct formation in the early stage of the reaction monitored here occurs only at the N-terminal amine, which is not freely available in the physiological, N-terminally acetylated form of the protein.[18-19]
Figure 1.
LC-MS analysis of DOPAL reactions with (A) Ac-WT aS and (B) Ac-Lys. Samples were taken from reactions at 30 or 60 minute intervals and subjected to reverse-phase chromatography and electrospray ionization mass spectrometry (ESI-MS). (A) DOPAL reacts with Ac-WT aS, leading to the progressive loss of native protein (marked by an asterisk in the chromatogram, m/z = 14502) and appearance of product at later retention time with a heterogeneous elution profile. The inset shows the deconvoluted mass spectrum extracted from the latest reaction time point and the LC region indicated by the shaded square, illustrating the homogeneous mass of the protein adduct, with a difference of +266 Da over native Ac-WT aS. (B) DOPAL reacts with Ac-Lys to give a single major product. The deconvoluted mass spectrum from the latest time point and the LC region denoted by the shaded box is shown as an inset, with the predominant observed mass corresponding to a +266 Da increase over unmodified Ac-Lys.
As synuclein contains 15 lysine residues, detailed analysis of its reaction products with DOPAL is complicated, and we therefore continued our investigations with the simplest possible model system: Nα-acetylated lysine (Ac-Lys). Reactions were carried out at a concentration (1.5 mM) equivalent to the reactions with 100 μM Ac-WT aS (15 Lys per protein) described above and the same DOPAL concentration. As for Ac-WT aS, the reaction with Ac-Lys shows the progressive increase of a product eluting at higher acetonitrile concentration than the reactants, with a mass of 454.1735 Da (corresponding to an m/z ratio of 455.1962 in positive ion mode), or a +266.0574 Da increase over the Ac-Lys mass of 188.1161 Da (Figure 1B).
Purification from larger-scale reactions by reverse-phase chromatography enabled the use of standard small molecule NMR techniques to determine the structure of the reaction product. LC-MS on the purified material showed the expected elution time and +266 Da mass increase over Ac-Lys (SI Figure S4). Purity of the final sample was >90%, as judged by its 1D 1H NMR spectrum and the LC-MS chromatogram.
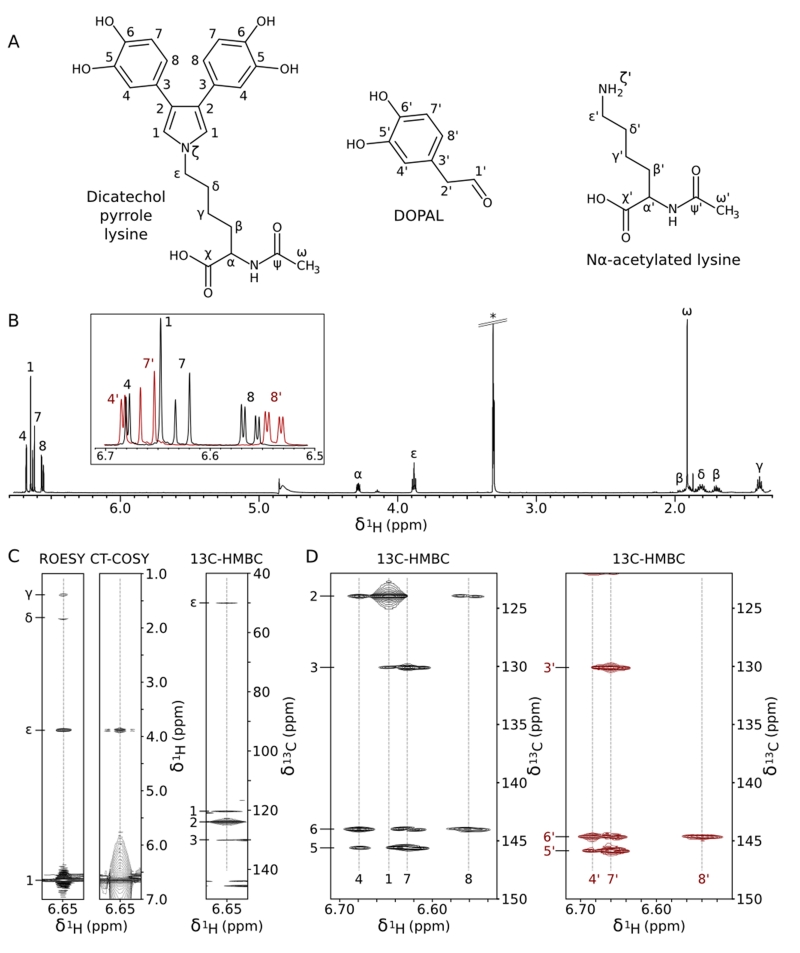
Analysis of the NMR data led to the unambiguous determination of the product’s structure (Figure 2A), which results from the addition of two DOPAL molecules to the Lys sidechain amine and formation of a pyrrole ring containing the Lys Nζ and the carbon atoms of the two DOPAL alkyl groups. Chemical shifts and scalar couplings are listed in Table S1, full NMR spectra are shown in SI Figures S5 and S6, and the key features of the dicatechol pyrrole lysine (DCPL) adduct are illustrated in Figure 2B-D and described below.
Figure 2.
NMR structure determination of purified dicatechol pyrrole lysine. (A) Annotated structures of DCPL and reactants. (B) 1D 1H spectrum of DCPL. An asterisk marks the methanol solvent peak. The inset is an overlay of the aromatic regions of DCPL (black) and DOPAL (red), illustrating the similarities between the catechol signals. (C) Connectivity of the pyrrolic singlet (labeled 1 in B) to the Lys sidechain terminus and the catechol rings. Strips are shown from the indicated spectra. (D) Comparison of the 1H-13C HMBC patterns for the catechol rings of DOPAL (red) and DCPL (black). Correlations to the hydroxyl-bearing carbons (~145 ppm) and the carbon at the base of the ring (~130 ppm) are very similar for the two compounds. The 4 and 8 positions of the DCPL catechol show additional crosspeaks to the pyrrole carbon at 123.9 ppm (labeled 2). Full spectra are shown in SI Figures S5 and S6.
There are 7 aliphatic signals in the DCPL 1D 1H spectrum (Figure 2B) that were readily connected in 1H-1H TOCSY, 1H-1H ROESY, and 1H-13C HMBC spectra, and assigned to the Ac-Lys portion of the molecule. Comparison of data collected for the modified and native Ac-Lys (Table S1) shows that the 1H and 13C chemical shifts are nearly identical for the backbone and the beginning portion of the sidechain, with deviations increasing to a nearly 1 ppm downfield shift for the Hε protons. The aromatic region of the 1D 1H spectrum contains four signals, three of which closely resemble the three catechol signals of unmodified DOPAL in their 1H and 13C chemical shifts, 1H-1H and 1H-13C J-couplings, and 1H-13C HMBC correlations to the hydroxyl-bearing ring carbons and the carbon at the base of the ring (Figure 2B and D). Integration of the signals in the 1D 1H spectrum confirms that there are two of each ring proton for each lysine molecule (SI Table S1).
The pyrrole structure of the DCPL adduct is revealed most clearly by the downfield 1H singlet without an analog in the spectrum of unmodified DOPAL or Ac-Lys (labeled 1 in Figure 2). This pyrrolic singlet shows a strong NOE to Hε of the modified Ac-Lys sidechain and 1H-13C HMBC correlations to the C3 carbon at the base of the catechol ring and Cε of the modified Ac-Lys, marking its role as the connective moiety (Figure 2C). Despite its lack of observable 1H-1H splitting, we were able to measure a connection to the modified Ac-Lys Hε using a 150 ms constant-time (CT) 1H-1H COSY experiment designed to measure long-range scalar coupling transfers (Figure 2C). In the 1H-13C HMBC spectrum (Figure 2C), the predominant feature is a crosspeak to a carbon at 123.9 ppm (labeled 2). This carbon lacks an attached proton, is weakly coupled to the catechol ring protons (Figure 2D), and has no analog in the spectra of the reactants. In addition, the pyrrolic singlet shows a unique auto-correlation in the 1H-13C HMBC spectrum, a crosspeak at the position of a diagonal, representing magnetization transfer from 1H to 13C but without the large splitting from one-bond 1H-13C scalar coupling. This crosspeak arises from the correlation of the signal through multiple bonds to its symmetric partner, and confirms the stoichiometry of two catechol rings to one Ac-Lys deduced from integration of the 1D 1H peaks. Finally, the 184 Hz 1JHC splitting of the pyrrolic signal is consistent with an alkene carbon.
We were able to confirm the DCPL structure by MS/MS spectra collected in both positive and negative ion mode (SI Figure S7). DCPL is formally named 2-acetamido-6-[3,4-bis(3,4-dihydroxyphenyl)pyrrol-1-yl]hexanoic acid and has a formula of C24H26N2O7. Its calculated mass is 454.1740 Da, in excellent agreement with the experimentally observed mass of 454.1735 Da. The UV-Vis spectrum of purified DCPL is shown in SI Figure S8.
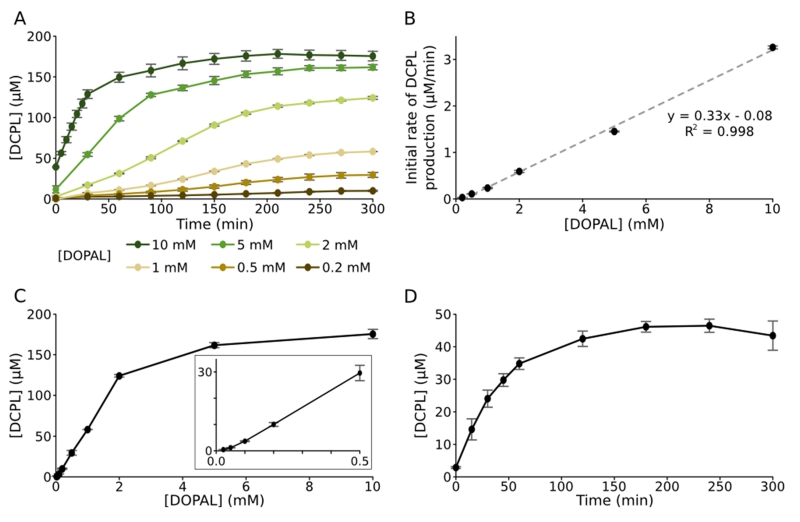
Formation of DCPL in live reactions can be monitored directly from 1D 1H NMR spectra (SI Figure S9). Integration of its NMR signals shows that the concentration of DCPL produced in our standard reaction with 2 mM DOPAL and 1.5 mM Ac-Lys increases roughly linearly over the first 2.5 hours before slowing to reach a maximal value of 124 μM at 5 hours (Figure 3A). The plateau in DCPL formation presumably results from a combination of pathways – oxidative degradation, further reactions with DOPAL, and reactions between DCPL molecules – that compete with its production. While two DOPAL molecules are required in the formation of DCPL, the initial rates are linearly dependent on the DOPAL concentration over the NMR-accessible range (Figure 3B). Maximal amounts of DCPL also increase linearly with DOPAL concentration (Figure 3C), except at the high and very low ends of the observed range where they increase more slowly. At lower concentrations of DOPAL, this nonlinear increase may reflect the buildup of an intermediate species that is more transient when DOPAL is not a limiting reactant. Notably, we are able to observe DCPL formation by NMR in reactions with only 25 μM DOPAL. With LC-MS, DCPL is observable in extracted ion chromatograms for reactions at 10 μM DOPAL (data not shown), near the physiological 2.3 μM concentration for healthy cells. DOPAL levels are much higher in diseased cells, owing to a 70% loss in DOPAL breakdown by aldehyde dehydrogenase and up to a 90% decrease in the vesicular uptake of dopamine.[10]
Figure 3.
Quantification of dicatechol pyrrole lysine. (A) DCPL formation over time was monitored by NMR for reactions with 1.5 mM Ac-Lys and varying concentrations of DOPAL. (B) Initial rates of DCPL formation for the reactions in (A) are plotted against DOPAL concentration and fit to a linear regression. (C) DCPL concentrations after 5 hour incubation of reactions with 1.5 mM Ac-Lys are plotted versus DOPAL concentration. The inset is a magnified view of the lower concentrations. (D) DCPL adduct formation in reactions between 2 mM DOPAL and 100 μM Ac-WT aS was quantified with Ehrlich’s reagent. In (A), (C), and (D), error bars denote standard deviations from three independent reactions and in (B) they denote standard errors for the linear fitting of initial rates.
Formation of DCPL in reactions with Ac-WT aS was confirmed and quantified by Ehrlich’s assay, a simple and specific colorimetric method for measuring pyrrole groups. In reactions with 2 mM DOPAL and 100 μM Ac-WT aS, DCPL production increases nearly linearly at first, followed by a plateau at later time points (Figure 3D), as seen with Ac-Lys. The maximal amount of DCPL observed is 46 μM, or 37% of that observed in reactions with an equivalent concentration of Ac-Lys, which may reflect an intrinsically lower reactivity of the Lys residues of α-synuclein or an increase in the rate of breakdown and/or crosslinking due to the high effective local concentration of Lys and other possible reactive residues. We also analyzed DOPAL-reacted Ac-WT aS by MS/MS of trypsin-digested peptides. With overnight proteolysis of the modified protein, we were able to identify DCPL adducts at two Lys residues, Lys12 and Lys23 (SI Figures S10 and S11). By shortening the trypsin digestion to 1 hour, we were able to confirm these adducts, and identify a third DCPL site at Lys21 (data not shown). The preferential detection of DCPL adducts at N-terminal Lys residues agrees well with the pattern of amide signal loss in SI Figure S1, and suggests that the reactivities of individual Lys residues in α-synuclein may vary, possibly as a result of the local primary sequence or differences in accessibility due to transient conformations. Alternatively, DCPL adduct stability may vary by location, which could bias the results of assays that detect DCPL rather than a loss of native Lys.
Two possible mechanisms that provide chemically reasonable explanations for DCPL pyrrole ring formation are shown in SI Figure S12. Both are driven by auto-oxidation of the DOPAL catechol ring followed by isomerization to a quinone methide and have a similarity to the well-known Paal-Knorr pyrrole synthesis.
The DCPL adduct is highly reactive and a potential scaffold for DOPAL-induced crosslinking. In reactions with DOPAL and Ac-Lys, DCPL reaches a maximum level at 6 hours and then decreases by half over the next 18 hours (SI Figure S13), presumably through oxidative decomposition and the formation of crosslinked species. In the case of a DCPL adduct on synuclein, where the effective concentration of proximate reactive residues in the intrinsically disordered protein is orders of magnitude higher, further reaction of DCPL should be significantly more efficient, and may explain why it has escaped detection in an earlier study by Follmer et al. that used much longer incubations with DOPAL.[17] Under the buffer conditions used by Follmer et al., we still find that DCPL is the only observable initial adduct in reactions with Ac-WT aS (SI Figure S14), Ac-Lys (data not shown), and for an Nα-acetylated Lys-Lys dipeptide (SI Figure S15). DCPL is also highly light sensitive (SI Figure S16), and reactions in our study were performed in the dark to prevent photodegradation. Our ready detection of a SB adduct at synuclein’s unprotected N-terminal amine (SI Figure S3) suggests that this group behaves distinctly from the lysine amine, perhaps owing to their very different pKa’s,[21] and may explain the prior detection of a SB adduct in reactions with a non-acetylated peptide.[15]
The DCPL structure provides a valuable chemical basis for future studies of DOPAL-induced aggregation and crosslinking of α-synuclein. There is increasing evidence that α-synuclein oligomers play a more important role in PD etiology than fibrils, either by proving toxic to cells directly or by providing nucleation points for the formation of smaller and less homogeneous aggregates that are more deleterious.[22] The pyrrole and catechol rings of the DCPL adduct and their potential to polymerize after further oxidation suggest an interesting potential mechanism of α-synuclein oligomer formation in dopaminergic neurons. It also raises the possibility that the widely used clinical monoamine oxidase inhibitors, which can delay the onset of PD, may function by suppressing the production of toxic DOPAL-induced oligomers, in addition to the accepted mechanism of increasing the levels of biogenic amine neurotransmitters.[10]
Supplementary Material
Acknowledgements
We thank Jinfa Ying for technical help, Daniel Mulvihill (University of Kent) for the NatB acetyltransferase construct, the NIDDK Advanced Spectrometry Core for preliminary data collection, and Richard Glass (University of Arizona), S. Bruce King (Wake Forest University), Kenneth Kirk, Vincent Cole, and Rolf Swenson (National Institutes of Health), and Sanford Markey, W. Gary Mallard, and Edward White (National Institutes of Standards and Technology) for their advice. This work was supported by the Intramural Research Programs of the National Institute of Diabetes and Digestive and Kidney Diseases and the National Heart, Lung, National Institutes of Health.
References
- [1].Dikiy I, Eliezer D. Biochim Biophys Acta. 2012;1818:1013–1018. doi: 10.1016/j.bbamem.2011.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Auluck PK, Caraveo G, Lindquist S. Annu Rev Cell Dev Biol. 2010;26:211–233. doi: 10.1146/annurev.cellbio.042308.113313. [DOI] [PubMed] [Google Scholar]
- [3].Burre J, Sharma M, Tsetsenis T, Buchman V, Etherton MR, Sudhof TC. Science. 2010;329:1663–1667. doi: 10.1126/science.1195227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Schildknecht S, Gerding HR, Karreman C, Drescher M, Lashuel HA, Outeiro TF, Di Monte DA, Leist M. J Neurochem. 2013;125:491–511. doi: 10.1111/jnc.12226. [DOI] [PubMed] [Google Scholar]
- [5].Cookson MR. Annu Rev Biochem. 2005;74:29–52. doi: 10.1146/annurev.biochem.74.082803.133400. [DOI] [PubMed] [Google Scholar]
- [6].Trinh J, Farrer M. Nat Rev Neurol. 2013;9:445–454. doi: 10.1038/nrneurol.2013.132. [DOI] [PubMed] [Google Scholar]
- [7].Ahlskog JE. Neurology. 2007;69:1701–1711. doi: 10.1212/01.wnl.0000296942.14309.4a. [DOI] [PubMed] [Google Scholar]
- [8].Burke WJ, Li SW, Chung HD, Ruggiero DA, Kristal BS, Johnson EM, Lampe P, Kumar VB, Franko M, Williams EA, Zahm DS. Neurotoxicology. 2004;25:101–115. doi: 10.1016/S0161-813X(03)00090-1. [DOI] [PubMed] [Google Scholar]
- [9].Burke WJ, Li SW, Williams EA, Nonneman R, Zahm DS. Brain Res. 2003;989:205–213. doi: 10.1016/s0006-8993(03)03354-7. [DOI] [PubMed] [Google Scholar]
- [10].Goldstein DS, Kopin IJ, Sharabi Y. Pharmacol Ther. 2014;144:268–282. doi: 10.1016/j.pharmthera.2014.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Blaschko H. Pharmacol Rev. 1952;4:415–458. [PubMed] [Google Scholar]
- [12].Panneton WM, Kumar VB, Gan Q, Burke WJ, Galvin JE. PLoS One. 2010;5:e15251. doi: 10.1371/journal.pone.0015251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Negre-Salvayre A, Coatrieux C, Ingueneau C, Salvayre R. Br J Pharmacol. 2008;153:6–20. doi: 10.1038/sj.bjp.0707395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Burke WJ, Kumar VB, Pandey N, Panneton WM, Gan Q, Franko MW, O’Dell M, Li SW, Pan Y, Chung HD, Galvin JE. Acta Neuropathol. 2008;115:193–203. doi: 10.1007/s00401-007-0303-9. [DOI] [PubMed] [Google Scholar]
- [15].Rees JN, Florang VR, Anderson DG, Doorn JA. Chem Res Toxicol. 2007;20:1536–1542. doi: 10.1021/tx700248y. [DOI] [PubMed] [Google Scholar]
- [16].Rees JN, Florang VR, Eckert LL, Doorn JA. Chem Res Toxicol. 2009;22:1256–1263. doi: 10.1021/tx9000557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Follmer C, Coelho-Cerqueira E, Yatabe-Franco DY, Araujo GD, Pinheiro AS, Domont GB, Eliezer D. J Biol Chem. 2015 doi: 10.1074/jbc.M115.686584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Anderson JP, Walker DE, Goldstein JM, de Laat R, Banducci K, Caccavello RJ, Barbour R, Huang J, Kling K, Lee M, Diep L, Keim PS, Shen X, Chataway T, Schlossmacher MG, Seubert P, Schenk D, Sinha S, Gai WP, Chilcote TJ. J Biol Chem. 2006;281:29739–29752. doi: 10.1074/jbc.M600933200. [DOI] [PubMed] [Google Scholar]
- [19].Bartels T, Choi JG, Selkoe DJ. Nature. 2011;477:107–110. doi: 10.1038/nature10324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Kang L, Wu KP, Vendruscolo M, Baum J. J Am Chem Soc. 2011;133:13465–13470. doi: 10.1021/ja203979j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Grimsley GR, Scholtz JM, Pace CN. Protein Sci. 2009;18:247–251. doi: 10.1002/pro.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Winner B, Jappelli R, Maji SK, Desplats PA, Boyer L, Aigner S, Hetzer C, Loher T, Vilar M, Campioni S, Tzitzilonis C, Soragni A, Jessberger S, Mira H, Consiglio A, Pham E, Masliah E, Gage FH, Riek R. Proc Natl Acad Sci U S A. 2011;108:4194–4199. doi: 10.1073/pnas.1100976108. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.