Abstract
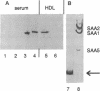
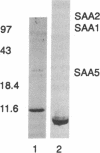
The serum amyloid A (SAA) proteins make up a multigene family of apolipoproteins associated with high density lipoproteins. They are of ancient origin; the finding of a highly homologous protein in mammals and ducks indicates that SAAs have been in existence for at least 300 million years. The interspecies similarity among the SAAs makes the mouse, in which they have been most thoroughly studied, a reasonable model to use for defining the function(s) of this family of proteins in humans. Originally it was observed that the SAA proteins were made in the liver and represented a set of proteins belonging to acute-phase reactants. SAA3 is a unique member of the SAA multigene family in mice in that its mRNA is also expressed in extrahepatic tissues by a variety of cell types, mainly macrophages and adipocytes. To date, nothing has been reported regarding the fate or function of the SAA3 translation product. To identify the SAA3 protein, we developed SAA3-specific antibodies by immunizing rabbits against a portion of SAA3 protein synthesized in a bacterial fusion protein expression system. Electroimmunoblot analysis of serum and lipoprotein fractions of it showed SAA3 to be associated with high density lipoproteins of mice treated with lipopolysaccharide. Furthermore, a continuous mouse macrophage cell line (J-774.1), when exposed to lipopolysaccharide, expressed SAA3 mRNA in a dose-dependent manner and secreted SAA3 protein. The expression and secretion of SAA3 by macrophages stimulated with lipopolysaccharide suggest a role for this SAA in local responses to injury and inflammation.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Benditt E. P., Eriksen N. Amyloid protein SAA is associated with high density lipoprotein from human serum. Proc Natl Acad Sci U S A. 1977 Sep;74(9):4025–4028. doi: 10.1073/pnas.74.9.4025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benditt E. P., Eriksen N., Hanson R. H. Amyloid protein SAA is an apoprotein of mouse plasma high density lipoprotein. Proc Natl Acad Sci U S A. 1979 Aug;76(8):4092–4096. doi: 10.1073/pnas.76.8.4092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benditt E. P., Meek R. L. Expression of the third member of the serum amyloid A gene family in mouse adipocytes. J Exp Med. 1989 May 1;169(5):1841–1846. doi: 10.1084/jem.169.5.1841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Betts J. C., Edbrooke M. R., Thakker R. V., Woo P. The human acute-phase serum amyloid A gene family: structure, evolution and expression in hepatoma cells. Scand J Immunol. 1991 Oct;34(4):471–482. doi: 10.1111/j.1365-3083.1991.tb01570.x. [DOI] [PubMed] [Google Scholar]
- Bond H. M., Morrone G., Venuta S., Howell K. E. Characterization and purification of proteins which bind high-density lipoprotein. A putative cell-surface receptor. Biochem J. 1991 Nov 1;279(Pt 3):633–641. doi: 10.1042/bj2790633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Coetzee G. A., Strachan A. F., van der Westhuyzen D. R., Hoppe H. C., Jeenah M. S., de Beer F. C. Serum amyloid A-containing human high density lipoprotein 3. Density, size, and apolipoprotein composition. J Biol Chem. 1986 Jul 25;261(21):9644–9651. [PubMed] [Google Scholar]
- Ericsson L. H., Eriksen N., Walsh K. A., Benditt E. P. Primary structure of duck amyloid protein A. The form deposited in tissues may be identical to its serum precursor. FEBS Lett. 1987 Jun 22;218(1):11–16. doi: 10.1016/0014-5793(87)81008-6. [DOI] [PubMed] [Google Scholar]
- Eriksen N., Benditt E. P. Trauma, high density lipoproteins, and serum amyloid protein A. Clin Chim Acta. 1984 Jul 16;140(2):139–149. doi: 10.1016/0009-8981(84)90338-3. [DOI] [PubMed] [Google Scholar]
- Eriksen N., Ericsson L. H., Pearsall N., Lagunoff D., Benditt E. P. Mouse amyloid protein AA: Homology with nonimmunoglobulin protein of human and monkey amyloid substance. Proc Natl Acad Sci U S A. 1976 Mar;73(3):964–967. doi: 10.1073/pnas.73.3.964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Fong B. S., Salter A. M., Jimenez J., Angel A. The role of apolipoprotein A-I and apolipoprotein A-II in high-density lipoprotein binding to human adipocyte plasma membranes. Biochim Biophys Acta. 1987 Jul 31;920(2):105–113. doi: 10.1016/0005-2760(87)90249-9. [DOI] [PubMed] [Google Scholar]
- Hoffman J. S., Benditt E. P. Changes in high density lipoprotein content following endotoxin administration in the mouse. Formation of serum amyloid protein-rich subfractions. J Biol Chem. 1982 Sep 10;257(17):10510–10517. [PubMed] [Google Scholar]
- Kushner I. The phenomenon of the acute phase response. Ann N Y Acad Sci. 1982;389:39–48. doi: 10.1111/j.1749-6632.1982.tb22124.x. [DOI] [PubMed] [Google Scholar]
- Leblond L., Marcel Y. L. The amphipathic alpha-helical repeats of apolipoprotein A-I are responsible for binding of high density lipoproteins to HepG2 cells. J Biol Chem. 1991 Apr 5;266(10):6058–6067. [PubMed] [Google Scholar]
- Lowell C. A., Potter D. A., Stearman R. S., Morrow J. F. Structure of the murine serum amyloid A gene family. Gene conversion. J Biol Chem. 1986 Jun 25;261(18):8442–8452. [PubMed] [Google Scholar]
- Lowell C. A., Stearman R. S., Morrow J. F. Transcriptional regulation of serum amyloid A gene expression. J Biol Chem. 1986 Jun 25;261(18):8453–8461. [PubMed] [Google Scholar]
- Meek R. L., Benditt E. P. Amyloid A gene family expression in different mouse tissues. J Exp Med. 1986 Dec 1;164(6):2006–2017. doi: 10.1084/jem.164.6.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meek R. L., Benditt E. P. Rat tissues express serum amyloid A protein-related mRNAs. Proc Natl Acad Sci U S A. 1989 Mar;86(6):1890–1894. doi: 10.1073/pnas.86.6.1890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meek R. L., Eriksen N., Benditt E. P. Serum amyloid A in the mouse. Sites of uptake and mRNA expression. Am J Pathol. 1989 Aug;135(2):411–419. [PMC free article] [PubMed] [Google Scholar]
- Oram J. F., Johnson C. J., Brown T. A. Interaction of high density lipoprotein with its receptor on cultured fibroblasts and macrophages. Evidence for reversible binding at the cell surface without internalization. J Biol Chem. 1987 Feb 15;262(5):2405–2410. [PubMed] [Google Scholar]
- Ordovas J. M., Litwack-Klein L., Wilson P. W., Schaefer M. M., Schaefer E. J. Apolipoprotein E isoform phenotyping methodology and population frequency with identification of apoE1 and apoE5 isoforms. J Lipid Res. 1987 Apr;28(4):371–380. [PubMed] [Google Scholar]
- Ramadori G., Sipe J. D., Colten H. R. Expression and regulation of the murine serum amyloid A (SAA) gene in extrahepatic sites. J Immunol. 1985 Dec;135(6):3645–3647. [PubMed] [Google Scholar]
- Rokita H., Shirahama T., Cohen A. S., Meek R. L., Benditt E. P., Sipe J. D. Differential expression of the amyloid SAA 3 gene in liver and peritoneal macrophages of mice undergoing dissimilar inflammatory episodes. J Immunol. 1987 Dec 1;139(11):3849–3853. [PubMed] [Google Scholar]
- Segrest J. P., De Loof H., Dohlman J. G., Brouillette C. G., Anantharamaiah G. M. Amphipathic helix motif: classes and properties. Proteins. 1990;8(2):103–117. doi: 10.1002/prot.340080202. [DOI] [PubMed] [Google Scholar]
- Stearman R. S., Lowell C. A., Pearson W. R., Morrow J. F. Regulation of synthesis of amyloid A-related protein. Ann N Y Acad Sci. 1982;389:106–115. doi: 10.1111/j.1749-6632.1982.tb22128.x. [DOI] [PubMed] [Google Scholar]
- Werb Z., Chin J. R. Endotoxin suppresses expression of apoprotein E by mouse macrophages in vivo and in culture. A biochemical and genetic study. J Biol Chem. 1983 Sep 10;258(17):10642–10648. [PubMed] [Google Scholar]
- Yamamoto K., Migita S. Complete primary structures of two major murine serum amyloid A proteins deduced from cDNA sequences. Proc Natl Acad Sci U S A. 1985 May;82(9):2915–2919. doi: 10.1073/pnas.82.9.2915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto K., Shiroo M., Migita S. Diverse gene expression for isotypes of murine serum amyloid A protein during acute phase reaction. Science. 1986 Apr 11;232(4747):227–229. doi: 10.1126/science.3456645. [DOI] [PubMed] [Google Scholar]
- de Beer M. C., Beach C. M., Shedlofsky S. I., de Beer F. C. Identification of a novel serum amyloid A protein in BALB/c mice. Biochem J. 1991 Nov 15;280(Pt 1):45–49. doi: 10.1042/bj2800045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Hodenberg E., Heinen S., Howell K. E., Luley C., Kübler W., Bond H. M. Cholesterol efflux from macrophages mediated by high-density lipoprotein subfractions, which differ principally in apolipoprotein A-I and apolipoprotein A-II ratios. Biochim Biophys Acta. 1991 Nov 5;1086(2):173–184. doi: 10.1016/0005-2760(91)90005-3. [DOI] [PubMed] [Google Scholar]