Abstract
Background
After years of periconceptional folic acid supplementation, the prevalence of neural tube defects (NTDs) remains stable following the remarkable reduction observed immediately after the fortification practice. There is accumulating evidence that folate receptor (FR) autoimmunity may play a role in the etiology of folate-sensitive NTDs.
Methods
From 2011 to 2013, 118 NTD cases and 242 healthy controls were recruited from a population-based birth defects surveillance system in Northern China. Enzyme-linked immunosorbent assay was used to measure FR autoantibodies in maternal and cord blood. Logistic regression models were used to estimate the odds ratios (OR) and 95% confidence intervals (95% CI).
Results
Plasma FR autoantibodies levels were significantly elevated in mothers of infants with NTDs compared with mothers of healthy controls. Using the lowest tertile as the referent group, 2.20-fold (95% CI, 0.71–6.80) and 5.53-fold increased odds (95% CI, 1.90–16.08) of NTDs were observed for the second and third tertile of immunoglobulin G (IgG), respectively, and the odds of NTDs for each successive tertile of IgM was 0.98 (95% CI, 0.35–2.75) and 3.49 (95% CI, 1.45–8.39), respectively. A dose–response relationship was found between FR autoantibodies levels and risk of NTDs (P < 0.001 for IgG, P = 0.002 for IgM). The same pattern was observed in both subtypes of spina bifida and anencephaly. No significant difference in levels of cord blood FR autoantibodies was observed.
Conclusion
Higher levels of FR autoimmunity in maternal plasma are associated with elevated risk of NTDs in a dose–response manner.
Keywords: folate receptor, autoantibody, neural tube defects, IgG, IgM
Introduction
Neural tube defects(NTDs), which include spina bifida, anencephaly, craniorachischisis, and encephalocele, are among the most common human birth defects, with a worldwide prevalence of approximately 1 per 1000 births (Frey and Hauser, 2003). The prevalence of NTDs varies considerably by geographic areas, with prevalence reported as 0.2 to 14 per 1000 births (Busby et al., 2005; Li et al., 2006; Allagh et al., 2015; Kishimba et al., 2015). The etiology of NTDs is far from fully understood. All the known risk factors account for less than 50% of NTD cases (Agopian et al., 2013). Both randomized trials and observational studies have shown the effectiveness of periconceptional folate supplementation in the prevention of NTDs (MRC, 1991; Czeizel and Dudas, 1992; Berry et al., 1999). However, a recent report from the U.S. Centers for Disease Control and Prevention shows that since the initial reduction observed immediately after the implementation of the mandatory folic acid fortification policy, the prevalence of NTDs during the postfortification period has remained relatively stable (Williams et al., 2015). In fact, a high proportion of pregnant women carrying a NTD-affected fetus do not show serum folate deficiencies (Yates et al., 1987; Kirke et al., 1993).
The cellular uptake of folic acid is primarily mediated by folate receptor alpha (FRα) (Antony, 1992). This protein is highly expressed in the placenta and plays a vital role in maternal-to-fetal folate transport (Page et al., 1993; Yasuda et al., 2008). Recently, evidence has emerged that immunogenic agents such as nucleoprotein and thyroglobulin can trigger auto-immunity, and maternal immunological responses might have a substantial impact on embryonic development (Coulam, 2000; Arnold et al., 2001; Clark et al., 2001). Animal experiments have found that antibodies to FR administered to pregnant rats caused embryonic damage, and the distribution of antibody to FR on the embryonic and extraembryonic tissues was similar to that of the FR, suggesting that FR antibody can specifically bind to the FR (Da et al., 2003).
Rothenberg and colleagues first reported that serum from women who previously had a pregnancy complicated by NTDs (n = 12) contained autoantibodies that bound to the FR and blocked the cellular uptake of folate in vitro (Rothenberg et al., 2004). This finding was substantiated by findings supporting a 1.4- to 3.2-fold elevated odds of NTDs in women with high titers of FR autoantibodies (Cabrera et al., 2008; Boyles et al., 2011). Although these two studies included only 11 and 29 NTD cases, respectively, they were based on mid-gestational serum samples from women who were currently pregnant with a NTD complicated pregnancy. Molloy et al., used non mid-gestational serum samples and found that maternal FR autoantibodies were not associated with an NTD-affected pregnancy (Molloy et al., 2009). These four studies provided new insight into the etiology of NTDs. However, bovine folate binding protein (FBP) was used in some of these studies to detect the human FR autoantibodies, which may result in biased estimates (Rothenberg et al., 2004; Molloy et al., 2009). The objective of this study was to evaluate the association between maternal and cord blood FR autoantibodies and the risk of NTDs in a large population of Chinese women in the post-folic acid fortification period.
Materials and Methods
STUDY POPULATION
The subjects were recruited from a population-based birth defects surveillance system in five rural counties of Shanxi Province (Taigu, Pingding, Xiyang, Shouyang, and Zezhou) in northern China between 2011 and 2013 (Li et al., 2007; Wang et al., 2014). The surveillance system monitors major external structural birth defects through active case ascertainment. Cases were mothers with NTD-affected pregnancies and their matched fetuses or newborns, while controls were mothers and their term healthy newborns or terminated fetuses without congenital malformations. For each case, a healthy control in the same hospital was selected, matched to the case by place of residence (residing in the same county) and date of last menstrual period (±2weeks). Although the study was designed as a matched case–control study, some blood samples were not available because consent could not be obtained from some women. Therefore, the matched case–control pairs were broken in the present study. The present study included 118 cases and 242 controls. We obtained maternal blood from 109 cases (NTD complicated pregnancy) and 218 controls (unaffected pregnancy). A total of 24 cases and 48 controls were randomly selected and provided cord blood samples. As a pilot study, we measured cord blood FR autoantibodies in these 72 subjects.
In-person interviews were performed by trained local health workers at hospitals within the first week of delivery or pregnancy termination. Information on maternal sociodemographic characteristics, lifestyle, reproductive history, periconceptional folic acid supplementation, drug use, and exposure to domestic fuel use for cooking and heating during pregnancy were collected. Maternal venous blood and cord blood were collected at delivery or termination of the pregnancy. Blood was separated immediately. Aliquoted plasma was frozen at −20°C, transferred on dry ice, and stored at −80°C until it was used for these analyses.
ASSAY FOR FOLATE RECEPTOR AUTOANTIBODIES
Folate receptor autoantibodies in the plasma were measured using an indirect enzyme-linked immunosorbent assay (ELISA) (Cabrera et al., 2008; Bille et al., 2010; Sequeira et al., 2013), with slight modification from the previously published protocols. Briefly, human folate receptors were extracted and purified from placentas of healthy donors as previously described (Antony et al., 1981). Folate receptor protein was diluted with phosphate-buffered saline (pH 7.2) to produce a solution of 5 ng/μl and coated on 96-well plates (#655097, Greiner Bio-one, Monroe, NC) with 4 μl/well. FR has high affinity for both folate acid (Antony, 1992) and FR antibody (Rothenberg et al., 2004).
The nonbound protein was removed by washing the plates with a Tris buffered saline containing Tween-20 solution (1×TNT, PH 7.6). Plasma sample was 1:40 diluted using TNT buffer and applied to the plates with a volume of 50 μl per well. The plates and plasma solutions were sealed with plastic film and incubated overnight at ambient temperature and washed with TNT three times. Goat polyclonal antibody labeled with horseradish peroxidase was used as a secondary conjugate specific for detecting human immunoglobulin G (IgG) or immunoglobulin M (IgM).
After incubation for 1 hr at room temperature, wells were washed with TNT. SuperSignal ELISA Femto Substrate (#37074, Thermo Scientific, Waltham, MA), an ultrasensitive enhanced chemiluminescent substrate for low-femtogram-level detection, was used as the substrate, and the intensity of the luminescence was detected by an automatic microplate reader (Biotek Synergy2, Winooski, VT). Commercially available pooled IgG (#I8640, Sigma Aldrich, St. Louis, MO) and IgM (#I8260, Sigma Aldrich, St. Louis, MO) from human serum were serially diluted to construct the standard curve. The pooled IgG and IgM concentrations in the pooled serum were 5.8 mg/ml and 0.91 mg/ml, respectively.
The standard curve was constructed by initially defining the amount of FR autoantibodies in the pooled IgG and IgM as 1. The curve was used to interpolate the levels of FR autoantibodies present in case and control samples. TNT was used as the negative control. When calculating the levels of FR autoantibodies in samples, the intensity of the luminescence of the negative control was substracted from that of the samples. The lowest FR autoantibody level that can be detected by the assay was 3.12 × 10−4. Thus, any positive value more than 3.12 × 10−4 can indicate the presence of autoantibodies. The measuring range of the standard curve was from 6.25 × 10−4 to 8 × 10−2 (Yang et al., 2014). The intra- and inter-assay coefficients of variation for FR autoantibody IgG assay were 2.74% to 8.07% and 4.16% to 8.23%, respectively. For IgM, the values were 4.54% to 5.49% and 3.50% to 6.61%, respectively.
STATISTICAL ANALYSIS
Distribution of characteristics in cases and controls were compared using a chi-square (χ2) test. Because levels of FR autoantibodies were not normally distributed, geometric mean and median with interquartile range (IQR) was used to describe the distribution of FR autoantibodies in maternal and cord blood. Wilcoxon signed-rank test was used to assess the difference of autoantibodies levels between cases and controls. Tertiles determined by the distribution of autoantibodies in controls were used to categorize subjects. A similar procedure for determining exposure categories for continuous variables has been described by Hsien et al. (1991). Confounders were defined as those factors that were associated with both the risk of NTDs and the levels of autoantibodies. Using the lowest tertile as the nonexposed level, odds ratios (ORs) and 95% confidence intervals (95% CIs) for each higher tertile were estimated from logistic regression procedures after adjusting for confounders. Linear trend between odds ratios for NTD risk was determined by treating the three tertiles as a continuous variable.
Additionally, because there was a difference in gestational age between case and control fetuses, a stratified analysis by gestational age was performed. The control group consists of mothers and their term healthy newborns or terminated fetuses without congenital malformations. All the terminated fetuses in the control group were terminated before 20 weeks. Thus, the stratified analysis was performed based on the cutoff value of 20 weeks to distinguish the terminated fetuses and their mothers from healthy newborns and their mothers in the control group. All the statistical analyses were performed using Stata software version 12.0 (StataCorp LP, College Station, TX). A two-tailed P-value of < 0.05 was considered to be statistically significant.
ETHICAL APPROVAL
The study protocol was approved by the institutional review board of Peking University, and written consent from the mothers was obtained.
Results
The characteristics of the study population are summarized in Table 1. Briefly, case mothers were more likely to be older, less educated, and multiparous compared with control mothers. A higher proportion of case mothers were farmers, and tended to report a history of birth defects, suffering from flu and passive smoking during pregnancy. In addition, a lower proportion of case mothers reported periconceptional folate supplementation compared with control mothers. There were more female fetuses in cases compared with controls. Most of the case fetuses were terminated once identified, therefore, resulting in the reduced gestational age (27.2 ± 7.6 weeks) compared with the control fetuses, most of whom were term births (38.7 ± 4.9 weeks).
TABLE 1.
Characteristics of Women Who Had Pregnancies Affected by Neural Tube Defects (NTDs) and Women Who Gave Birth to Healthy Infants (Controls) in Shanxi Province, China, 2011 to 2013 (N = 360)
| Characteristics | Controls (N = 242) | NTDs (N = 118) | P-Valuea | ||
|---|---|---|---|---|---|
| n | % | N | % | ||
| Maternal age (years)b | 25.7 ± 4.5 | 27.3 ± 5.5 | 0.028 | ||
| Maternal age (years) | |||||
| <25 | 110 | 47.0 | 44 | 38.6 | 0.021 |
| 25–30 | 73 | 31.2 | 31 | 27.2 | |
| 30–35 | 39 | 16.7 | 23 | 20.2 | |
| ≥35 | 12 | 5.1 | 16 | 14.0 | |
| Education | |||||
| Primary or lower | 10 | 4.1 | 11 | 9.5 | <0.001 |
| Junior high | 156 | 64.7 | 90 | 77.6 | |
| High school or above | 75 | 31.1 | 15 | 12.9 | |
| Occupation | |||||
| Farmer | 179 | 74.3 | 101 | 85.6 | 0.015 |
| Non-farmer | 62 | 25.7 | 17 | 14.4 | |
| Prepregnancy body mass index (kg/m2) | |||||
| <18.5 | 64 | 26.7 | 24 | 9.9 | 0.068 |
| 18.5–24.9 | 135 | 56.2 | 56 | 56.4 | |
| ≥25 | 41 | 17.1 | 30 | 33.7 | |
| Previous birth defects history | |||||
| No | 233 | 96.3 | 106 | 89.8 | 0.043 |
| Yes | 4 | 1.7 | 4 | 3.4 | |
| Parity | |||||
| 1 | 127 | 53.4 | 45 | 38.1 | 0.007 |
| ≥2 | 111 | 46.6 | 73 | 61.9 | |
| Gestational age at delivery or termination (weeks)a | 38.7 ± 4.9 | 27.2 ± 7.6 | <0.001 | ||
| Fetal sex | |||||
| Male | 119 | 49.4 | 42 | 35.6 | 0.010 |
| female | 110 | 45.6 | 62 | 52.5 | |
| Passive smoking | |||||
| <1/week | 176 | 75.5 | 34 | 31.2 | <0.001 |
| ≥1/week | 57 | 24.5 | 75 | 68.8 | |
| Alcohol drinking | |||||
| No | 221 | 91.7 | 111 | 95.7 | 0.167 |
| Yes | 20 | 8.3 | 5 | 4.3 | |
| Periconceptional folate supplementation | |||||
| No | 92 | 38.7 | 70 | 59.3 | 0.001 |
| Yes | 143 | 60.1 | 48 | 40.7 | |
| Flu during early pregnancy | |||||
| No | 200 | 82.6 | 75 | 63.6 | <0.001 |
| Yes | 36 | 14.9 | 42 | 35.6 | |
Due to missing data, percent may not add up to 100%.
For continuous variable, P-value was calculated using Wilcoxon rank-sum test. For categorical variable, P-value was calculated using Chi-square test.
Mean ± SD (standard deviation).
The distribution of characteristics of the study population according to tertiles of maternal FR autoantibody IgG levels is summarized in Supplemental Table S1, which is available online. Maternal age, parity, periconceptional folate supplementation, and gestational age at delivery or termination of pregnancies were found to be associated with maternal FR autoantibody IgG levels. A similar distribution pattern of these characteristics according to tertiles of IgM was found. These covariates were defined as confounding factors and included in the final logistic regression model.
As shown in Table 2, significantly higher median concentrations of FR autoantibody IgG (median [IQR]: 1.73 [0.81–4.05] vs. 0.71 [0.34–1.58], P < 0.001) and IgM (median [IQR]: 3.53 [1.65–8.23] vs. 1.43 [0.68–3.21], P < 0.001) were observed in maternal plasma of cases compared with controls. Differences in IgG and IgM concentrations were significant for all three NTDs subtypes. Stratified analysis by gestational age (Table 3) revealed significantly elevated FR autoantibody IgG and IgM levels in case mothers as compared to controls in the subgroup with gestational age >20 weeks. In the subgroup with gestational age ≤20 weeks, only FR autoantibody IgM was significantly higher in case mothers compared with controls. No evidence of significant difference in cord blood FR autoantibodies was observed. We did not perform stratified analysis to cord blood autoantibodies because of the small sample size. Nine cases and 30 controls provided both maternal blood and cord blood samples. There was no evidence of a significant correlation between FR autoantibodies in maternal and cord blood (data not shown).
TABLE 2.
Folate Receptor (FR) Autoantibodies Immunoglobulin G (IgG) and Immunoglobulin M (IgM) Levels in Maternal Plasma and Cord Blood of Neural Tube Defects (NTDs) and Controls, Shanxi Province, China 2011 to 2013 (N = 360)
| Autoantibody | nb | Geometric mean ± SD | Median (IQR) | P-Valuec |
|---|---|---|---|---|
| Plasma IgGa | ||||
| Total NTDs | 109 | 1.65 ± 0.18 | 1.73 (0.81–4.05) | <0.001 |
| Spina bifida | 54 | 1.77 ± 0.31 | 1.54 (0.81–4.24) | <0.001 |
| Anencephaly | 43 | 1.46 ± 0.29 | 1.73 (0.61–3.85) | 0.001 |
| Encephalocele | 12 | 1.92 ± 0.57 | 2.05 (0.97–4.02) | 0.001 |
| Controls | 218 | 0.71 ± 0.09 | 0.71 (0.34–1.58) | |
| Plasma IgMa | ||||
| Total NTDs | 109 | 3.39 ± 0.27 | 3.53 (1.65–8.23) | <0.001 |
| Spina bifida | 54 | 3.08 ± 0.40 | 3.12 (1.65–8.61) | 0.001 |
| Anencephaly | 43 | 3.66 ± 0.47 | 3.53 (1.24–7.81) | <0.001 |
| Encephalocele | 12 | 3.98 ± 0.70 | 4.18 (1.97–8.18) | 0.001 |
| Controls | 218 | 1.99 ± 0.11 | 1.43 (0.68–3.21) | |
| Cord blood IgGa | ||||
| Total NTDs | 24 | 0.65 ± 0.25 | 0.75 (0.32–1.03) | 0.78 |
| Controls | 48 | 0.71 ± 0.09 | 0.77 (0.57–1.06) | |
| Cord blood IgMa | ||||
| Total NTDs | 24 | 0.36 ± 0.09 | 0.28 (0.16–0.62) | 0.65 |
| Controls | 48 | 0.29 ± 0.04 | 0.25 (0.20–0.44) |
Commercialized pooled IgG and IgM from human serum were used to plot the standard curve for detecting FR autoantibody IgG and IgM, respectively; the FR autoantibody IgG and IgM levels in the pooled IgG and IgM were defined as 1.
Maternal blood samples were collected from 327 participants, cord blood samples were collected from 72 participants.
P value was calculated using Wilcoxon sum-rank Test.
IQR, interquartile range; SD, standard deviation.
TABLE 3.
Folate Receptor (FR) Autoantibodies Immunoglobulin G (IgG) and Immunoglobulin M (IgM) Levels in Maternal Plasma of Neural Tube Defects (NTDs) and Controls, Stratified by Gestational Age, Shanxi Province, China 2011–2013 (N = 317a)
| Autoantibody | Gestational age (weeks) | |||||||
|---|---|---|---|---|---|---|---|---|
| ≤20 | >20 | |||||||
| n | Geometric mean ± SD | Median (IQR) | P-Valueb | n | Geometric mean ± SD | Median (IQR) | P-Valueb | |
| Plasma IgGc | ||||||||
| Total NTDs | 17 | 1.73 ± 0.49 | 1.89 (0.9–2.63) | 0.83 | 86 | 1.70 ± 0.23 | 1.75 (0.76–4.16) | <0.001 |
| Controls | 6 | 1.35 ± 2.13 | 2.80 (0.58–3.12) | 208 | 0.70 ± 0.05 | 0.71 (0.34–1.52) | ||
| Plasma IgMc | ||||||||
| Total NTDs | 17 | 4.18 ± 0.71 | 4.11 (2.48–6.17) | 0.008 | 86 | 3.27 ± 0.32 | 4.11 (2.06–6.17) | <0.001 |
| Controls | 6 | 1.81 ± 0.99 | 1.99 (1.29–3.48) | 208 | 2.01 ± 0.11 | 1.93 (1.24–3.36) | ||
Maternal blood samples were collected from 327 participants; 10 participants were excluded due to missing information on gestational age.
P-value was calculated using Wilcoxon sum-rank test.
Commercialized pooled IgG and IgM from human serum were used to plot the standard curve for detecting FR autoantibody IgG and IgM, respectively. The FR autoantibody IgG and IgM levels in the pooled IgG and IgM were defined as 1.
SD, standard deviation; IQR, interquartile range.
Multivariable adjusted ORs for NTDs in relation to maternal plasma FR autoantibody IgG and IgM are shown in Table 4. With the lowest tertile as the referent group, the adjusted OR (95%CI) of total NTDs for each successive tertile was 2.20 (0.71–6.80) and 5.53 (1.90–16.08) for IgG, and 0.98 (0.35–2.75) and 3.49 (1.45–8.39) for IgM. A significant linear trend was observed in OR of total NTDs across increasing tertile of IgG (P < 0.001) and IgM (P = 0.002) (Figs. (1 and 2)). The adjusted OR of spina bifida for the third tertile was 8.70 (2.16–35.01) for IgG, and 2.96 (1.11–7.86) for IgM, and the adjusted OR of anencephaly for the third tertile was 2.28 (0.59–8.79) for IgG, and 3.22 (0.95–10.98) for IgM.
TABLE 4.
Odds Ratio (OR) and 95% Confidence Interval (CI) for Maternal and Cord Blood Folate Receptor (FR) Autoantibodies Immunoglobulin G (IgG) and Immunoglobulin M (IgM) Levels in Relation to Neural Tube Defects (NTDs), Shanxi Province, China, 2011 to 2013 (N = 360a)
| Autoantibody | Control N = 218 n |
Total NTDs N = 109 |
Spina bifida N = 54 |
Anencephaly N = 43 |
||||||
|---|---|---|---|---|---|---|---|---|---|---|
| n | OR (95% CI) |
aORb (95% CI) |
n | OR (95% CI) |
aORb (95% CI) |
n | OR (95% CI) |
aORb (95% CI) |
||
| Maternal IgG | ||||||||||
| Tertile 1 (<0.39) | 73 | 9 | Reference | Reference | 3 | Reference | Reference | 6 | Reference | Reference |
| Tertile 2 (0.39–1.07) | 73 | 28 | 3.11 (1.37–7.05) | 2.20 (0.71–6.80) | 14 | 4.67 (1.29–16.92) | 3.37 (0.78–14.63) | 10 | 1.67 (0.58–4.82) | 0.89 (0.20–3.97) |
| Tertile 3 (>1.07) | 72 | 72 | 8.11 (3.77–17.44) | 5.53 (1.90–16.08) | 37 | 12.50 (3.69–42.39) | 8.70 (2.16–35.01) | 27 | 4.56 (1.78–11.71) | 2.28 (0.59–8.79) |
| P for trend | <0.001 | <0.001 | <0.001 | 0.001 | <0.001 | 0.030 | ||||
| Maternal IgM | ||||||||||
| Tertile 1 (<1.47) | 73 | 15 | Reference | Reference | 9 | Reference | Reference | 6 | Reference | Reference |
| Tertile 2 (1.47–2.79) | 73 | 23 | 1.53 (0.74–3.17) | 0.98 (0.35–2.75) | 14 | 1.56 (0.63–3.82) | 1.09 (0.35–3.35) | 6 | 1.00 (0.31–3.24) | 0.44 (0.09–2.10) |
| Tertile 3 (>2.79) | 72 | 71 | 4.80 (2.52–9.15) | 3.49 (1.45–8.39) | 31 | 3.49 (1.55–7.85) | 2.96 (1.11–7.86) | 31 | 5.24 (2.06–13.31) | 3.22 (0.95–10.98) |
| P for trend | <0.001 | 0.002 | <0.001 | 0.019 | <0.001 | 0.024 | ||||
| Cord blood IgG | 48 | 24 | 1.11 (0.89–1.38) | 1.17 (0.90–1.50) | – | – | – | – | ||
| Cord blood IgM | 48 | 24 | 1.32 (0.68–2.55) | 1.53 (0.77–3.03) | – | – | – | – | ||
Maternal blood samples were collected from 327 participants; cord blood samples were collected from 72 participants.
Adjusted for age, parity, periconceptual folate supplementation, and gestational age.
aOR, adjusted odds ratio.
FIGURE 1.
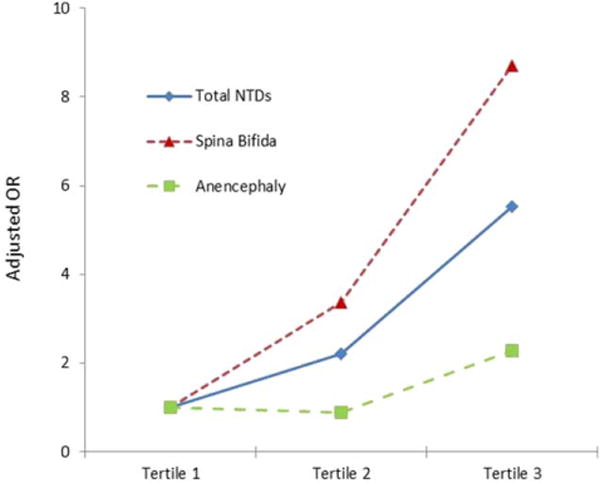
The triangles/squares/diamonds on the lines represent the point estimate of the adjusted odds ratios from the logistic model. A significant linear trend was observed in odds ratio of total NTDs across increasing tertile of IgG (P < 0.001), the same pattern was observed for both subtypes of spinal bifida and anencephaly. NTDs, neural tube defects; OR, odds ratio.
FIGURE 2.
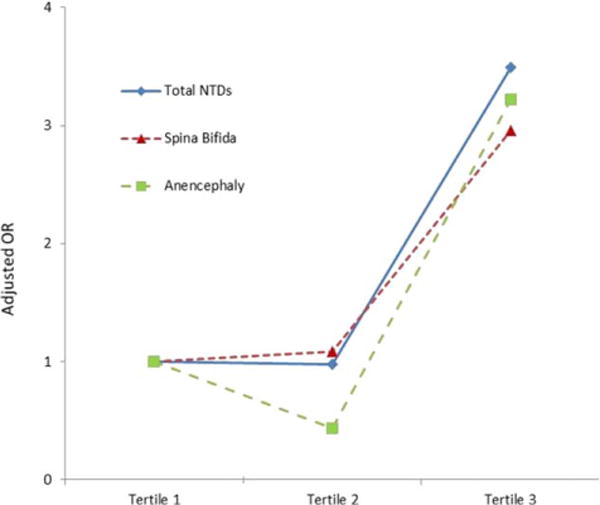
The triangles/squares/diamonds on the lines represent the point estimate of the adjusted odds ratios from the logistic model. A significant linear trend was observed in odds ratio of total NTDs across increasing tertile of IgM (P = 0.002), the same pattern was observed for both subtypes of spinal bifida and anencephaly. NTDs, neural tube defects; OR, odds ratio.
Given the small sample size, the association between FR autoantibodies and anencephaly risk was not statistically significant. However, the same linear pattern was observed in both subtypes of spina bifida (Ptrend = 0.001 for IgG, Ptrend < 0.001 for IgM) and anencephaly (Ptrend = 0.030 for IgG, Ptrend = 0.024 for IgM). Cord blood FR autoantibodies were treated as continuous variables, and no significant association was observed between cord blood FR autoantibodies and the risk of NTDs.
Table 5 shows the results of stratified analysis by gestational age. In the subgroup with gestational age ≤20 weeks, no significant association was found between maternal FR autoantibody IgG levels and risk of NTDs, which might be related to a small sample size. However, an increasing trend in the odds ratios of NTDs did exist with increasing FR autoantibody IgM levels. In subgroup with gestational age > 20 weeks, a gradient increased risk of NTDs was observed with increasing autoantibody levels.
TABLE 5.
Odds Ratio (OR) and 95% Confidence Interval (CI) for Maternal Plasma Folate Receptor (FR) Autoantibodies Immunoglobulin G (IgG) and IgM Levels in Relation to Neural Tube Defects (NTDs), Stratified by Gestational Age, China, 2011–2013 (N = 327a)
| Autoantibody | Gestational age ≤20 weeks | Gestational age >20 weeks | ||||||
|---|---|---|---|---|---|---|---|---|
| Controls n |
NTDs | Controls n |
NTDs | |||||
| n | OR (95% CI) | aOR (95% CI)b | n | OR (95% CI) | aOR (95% CI)b | |||
| IgG | ||||||||
| Tertile 1 (<0.39) | 1 | 2 | Reference | Reference | 70 | 7 | Reference | Reference |
| Tertile 2 (0.39–1.07) | 1 | 3 | 1.50 (0.06–40.63) | 2.70 (0.08–95.00) | 71 | 22 | 3.10 (1.24–7.72) | 3.25 (1.21–8.71) |
| Tertile 3 (> 1.07) | 4 | 12 | 1.50 (0.11–21.31) | 2.01 (0.10–39.90) | 67 | 57 | 8.51 (3.62–19.97) | 8.47 (3.36–21.33) |
| P for trend | 0.794 | 0.751 | <0.001 | <0.001 | ||||
| IgM | ||||||||
| Tertile 1 (<1.47) | 2 | 1 | Reference | Reference | 68 | 14 | Reference | Reference |
| Tertile 2 (1.47–2.79) | 2 | 4 | 4.00 (0.21–75.66) | 8.78 (0.16–488.63) | 70 | 16 | 1.11 (0.50–2.45) | 1.09 (0.47–2.53) |
| Tertile 3 (>2.79) | 2 | 12 | 12.00 (0.71–203.13) | 18.98 (0.51–699.56) | 70 | 56 | 3.89 (1.98–7.62) | 4.50 (2.17–9.31) |
| P for trend | 0.078 | 0.109 | <0.001 | <0.001 | ||||
Twenty-three participants were excluded due to failure to obtain the maternal blood sample.
Adjusted for age, parity, periconceptual folate supplementation, and gestational age.
aOR, adjusted odds ratio.
Discussion
In this study, we examined the association of FR autoantibodies in maternal and cord blood, and the risk of NTDs in a Chinese population with high NTD prevalence. Overall, we found that high levels of maternal plasma FR autoantibodies were associated with an elevated risk of NTDs, and the NTD risk increased with increasing levels of FR autoantibodies. The dose–response relationship was present for both subtypes of spinal bifida and anencephaly. No association between cord blood FR autoantibodies and risk of NTDs was found with the small sample size available for investigation.
Four previous epidemiological studies examined the association between FR autoantibodies and risk of NTDs, and reported inconsistent results. The study by Rothenberg et al. (2004), conducted in an American population with 12 NTD cases, reported a higher proportion of positive FR autoantibodies in maternal serum of NTD cases compared with controls. They did not report the gestational age of the subjects at sample collection (Rothenberg et al., 2004). Cabrera et al. reported a two- and three-fold increased odds of NTDs in an American population at two standard deviations above average control antibody concentrations for IgG and IgM, respectively. Unlike the Rothenberg study, the serum samples in this study were collected during the 15th to 18th weeks of pregnancy (Cabrera et al., 2008).
Boyles et al. measured the amount of folic acid blocked from binding to FR by the presence of FR antibodies in the maternal plasma, and found a 1.4-fold increased risk of NTDs with increased binding inhibition. Only 11 Norwegian women were included in the study and provided mid-gestation blood samples (Boyles et al., 2011). Another study with a larger sample size (103 cases vs. 103 controls) was conducted in samples from an Irish population and reported no association between maternal plasma FR autoantibodies and NTD risk. These samples were analyzed a median of 13 years after the birth of the child with an NTD (Molloy et al., 2009), and there is no indication for the time between sample collection and delivery of the child with an NTD. Specifically, the lack of association may simply be a result of a dynamic immune or autoimmune response changing over time.
Besides the differences in sample size, sample collection time, use of serum or plasma, and the population heterogeneity, the discordant results may also be due to the different sources of coating antigen (bovine FBP or human FR) used when determining the levels of antibodies and autoantibodies to FBP or FR, respectively. Although more than 80% homology between bovine FBP and human FR was present, autoantibodies may bind FR and FBP at different epitopes, thus leading to the biased estimate of the association if FBP was used to measure FR autoantibodies (Cabrera et al., 2008).
The present study used FR isolated from human placentas in the assay, providing a more sensitive, more accurate estimate of the levels of FR autoantibodies with a large sample size. Findings from our study indicated a 5.53- and 3.49-fold increased odds of NTDs in the highest tertile of FR autoantibody IgG and IgM compared with the first tertile, respectively. Our observation adds additional evidence that maternal FR autoantibodies are associated with an increased risk of NTDs in the offspring.
As far as we know, this was the first study that explored this association in subtypes of NTDs. Boyles et al., in their study of 11 NTDs cases, included two anencephaly cases and nine spina bifida cases (Boyles et al., 2011). The other three studies did not provide information about the types of their NTD cases. However, differences in metabolic profiles and one-carbon metabolism among subtypes of NTDs have been observed (Chi et al., 2014), which indicates the physiological and etiological process of these subtypes may be different. Our study observed a dose–response relationship between FR autoantibodies and total NTDs as well as subtypes of spina bifida and anencephaly, which indicates FR autoantibody is a risk factor for these two subtypes of NTDs.
To the best of our knowledge, this is also the first study to examine FR autoantibodies in cord blood of NTDs infants. The levels of FR autoantibodies in cord blood were much lower compared with that of maternal blood, which may reflect the maternal–fetal transport of the autoantibody IgG. Studies have found that IgG transport started in early embryos, at 3.5 to 5 weeks, which is during the critical window of neural tube closure (Gurevich et al., 2001). In a normal, uncomplicated pregnancy, IgM cannot pass through the placenta, the FR autoantibody IgM in cord blood may be produced by the immune system of the fetus. The levels of FR autoantibody IgM was 12% higher in cases compared with controls, but the small sample size limited the power to detect a significant difference in the titer of cord blood FR autoantibodies.
Associations of elevated FR autoantibodies and increased odds of NTDs in the offspring are biologically plausible. Folate participates in numerous single-carbon exchange reactions that are essential for genomic integrity and DNA methylation (Friso and Choi, 2002). Insufficient folate supply during the rapid cell division phase postconception has been associated with NTDs (Smithells et al., 1976; Kirke et al., 1993). Maternal-to-fetal folate transport is mediated by the high-affinity membrane-associated placental FR, and circulating 5-methyltetrahydrofolate from maternal blood is captured by FR on the maternally facing chorionic surface, then passively transferred to the fetal circulation (Weitman et al., 1992; Henderson et al., 1995).
However, FR autoantibodies can bind to the FR and block transplacental folate transport to the developing embryo. Furthermore, FR autoantibodies may exert their effect by an antibody-mediated inflammatory response and disrupt folate transport. FR autoantibodies in cord blood may bind to FR in the embryonic tissues and exaggerate folate deficiency in the developing embryos. In animal models, the effect of low-dose FR antibody administrated to rats can be preventable with large doses of folic acid (Rothenberg et al., 2004).
Animal models have demonstrated increased levels of expression of FR in rat placentas during pregnancy to facilitate maternal–fetal folate transport (Yasuda et al., 2008). Conditions associated with an increased expression of FR can trigger FR autoantibodies production in susceptible persons (Bagnoli et al., 2003; Elnakat and Ratnam, 2004; Parker et al., 2005; Knutson et al., 2006). The conditions will be worse if the mothers are folate-deficient, which can result in an overexpression of the FR (Antony, 2007). Another possible mechanism contributing to the production of FR autoantibodies is that FBP contained in cow’s milk can trigger the human immune system (Berrocal-Zaragoza et al., 2009), and a dairy-free diet downregulates FR immunity (Ramaekers et al., 2008). Recently, a case report described a woman with positive FR antibodies and adverse pregnancy outcomes. Longitudinal follow-up produced a decrease in FR antibody titer with a dairy-free diet. Then she was on the diary-free diet and took certain medications including folic acid. Eventually, the antibody titer dropped to an undetectable level and a healthy baby was delivered, suggesting that FR autoimmunity can have adverse effects on the pregnancy outcomes, which can be prevented by systematically lowering the antibody levels (Shapira et al., 2015).
Denny and colleagues (2015) used a folate-deficient diet in pregnant mice to demonstrate that there is a highly significant correlation between serum homocysteine levels and titers of autoantibodies directed against homocysteinylated proteins, in this case, against the FR. They reported elevated rates of malformations, especially NTDs, in the offspring of dams that were deficient in serum folate. When the dams on a low folate diet received supplementation with folic acid during the pregnancy, it completely rescued the embryos from congenital defects. Furthermore, the now phenotypically normal pups had homocysteinylated FR titers that were comparable to the progeny of dams maintained on a folate-replete diet (Denny et al., 2015). The study supports the hypothesis that homocysteinylation results in neo-self antigen formation and auto-immunity under conditions of limited maternal folate intake.
The strengths of our study include a large sample size, use of FR protein isolated from human placentas, use of well-trained interviewers, and the assessment of FR autoantibody titers in subtypes of NTDs. However, some limitations need to be addressed. The samples were collected at delivery of a term birth or termination of NTD-affected pregnancies. The difference of gestational age at sample collection between cases and controls merits consideration. Unlike several previous studies of FR autoantibodies and birth defects, which did not take into account the effect of gestational age (Rothenberg et al., 2004; Bliek et al., 2006; Molloy et al., 2009), we sought to minimize the effect of gestational age at sample collection by adjusting for gestational age in a multivariable logistic regression model and performing a stratified analysis by gestational age. Another limitation is that NTDs developed in early gestation but the samples were collected later in pregnancy. The case–control design does not allow for an assessment of the temporal relationship of FR autoantibodies and occurrence of NTDs. Longitudinal studies with serial measurement of FR autoantibodies at different times are needed to enhance the causality inference.
In summary, our study found that high levels of FR autoantibodies in maternal plasma were associated with increased odds of having an NTD-affected pregnancy in a dose–response manner. This dose–response relationship was present in both NTD subtypes: spina bifida and anencephaly. Further studies are needed to elucidate the mechanism by which FR autoantibodies are produced, determine why certain women produce antibodies, and develop standard methods to measure this marker in a highly sensitive assay that takes into account the epitope of the binding site. Once the impact of these factors are determined, the cutoff value of FR autoantibodies for an increased risk of NTDs is to be determined. The screening for FR autoantibodies and downregulating the levels of FR immunity is expected to be warranted in women seeking to become pregnant to minimize the risk for NTDs.
Supplementary Material
Acknowledgments
The authors thank Dr. Michelle A. Williams and Dr. Bizu Gelaye for their help with analyzing the data and editing the manuscript.
Supported by grants from the National Natural Science Foundation of China (Grant No. 81202215 and 81472987).
Footnotes
The authors have no actual or potential competing financial interests to disclose.
Additional Supporting information may be found in the online version of this article.
References
- Agopian AJ, Tinker SC, Lupo PJ, et al. Proportion of neural tube defects attributable to known risk factors. Birth Defects Res A Clin Mol Teratol. 2013;1:42–46. doi: 10.1002/bdra.23100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allagh KP, Shamanna BR, Murthy GVS, et al. Birth prevalence of neural tube defects and orofacial clefts in India: a systematic review and meta-analysis. PLoS One. 2015;3:e0118961. doi: 10.1371/journal.pone.0118961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antony AC. The biological chemistry of folate receptors. Blood. 1992;11:2807–2820. [PubMed] [Google Scholar]
- Antony AC. In utero physiology: role of folic acid in nutrient delivery and fetal development. Am J Clin Nutr. 2007;2:598S–603S. doi: 10.1093/ajcn/85.2.598S. [DOI] [PubMed] [Google Scholar]
- Antony AC, Utley C, Van Horne KC, Kolhouse JF. Isolation and characterization of a folate receptor from human placenta. J Biol Chem. 1981;18:9684–9692. [PubMed] [Google Scholar]
- Arnold J, Holmes Z, Pickering W, et al. Anti-beta 2 glycoprotein 1 and anti-annexin V antibodies in women with recurrent miscarriage. Br J Haematol. 2001;4:911–914. doi: 10.1046/j.1365-2141.2001.02812.x. [DOI] [PubMed] [Google Scholar]
- Bagnoli M, Canevari S, Figini M, et al. A step further in understanding the biology of the folate receptor in ovarian carcinoma. Gynecol Oncol. 2003;1(Pt 2):S140–S144. doi: 10.1006/gyno.2002.6705. [DOI] [PubMed] [Google Scholar]
- Berrocal-Zaragoza MI, Murphy MM, Ceruelo S, et al. High milk consumers have an increased risk of folate receptor blocking autoantibody production but this does not affect folate status in Spanish men and women. J Nutr. 2009;5:1037–1041. doi: 10.3945/jn.108.102475. [DOI] [PubMed] [Google Scholar]
- Berry RJ, Li Z, Erickson JD, et al. Prevention of neural-tube defects with folic acid in China. N Engl J Med. 1999;20:1485–1490. doi: 10.1056/NEJM199911113412001. [DOI] [PubMed] [Google Scholar]
- Bille C, Pedersen DA, Andersen AM, et al. Autoantibodies to folate receptor alpha during early pregnancy and risk of oral clefts in Denmark. Pediatr Res. 2010;3:274–279. doi: 10.1203/PDR.0b013e3181cbd564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bliek JB, Rothenberg SP, Steegers-Theunissen RP. Maternal folate receptor autoantibodies and cleft lip and/or palate. Int J Gynaecol Obstet. 2006;2:142–143. doi: 10.1016/j.ijgo.2006.01.021. [DOI] [PubMed] [Google Scholar]
- Boyles AL, Ballard JL, Gorman EB, et al. Association between inhibited binding of folic acid to folate receptor alpha in maternal serum and folate-related birth defects in Norway. Hum Reprod. 2011;8:2232–2238. doi: 10.1093/humrep/der144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Busby A, Abramsky L, Dolk H, Armstrong B. Preventing neural tube defects in Europe: population based study. BMJ. 2005;7491:574–575. doi: 10.1136/bmj.330.7491.574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabrera RM, Shaw GM, Ballard JL, et al. Autoantibodies to folate receptor during pregnancy and neural tube defect risk. J Reprod Immunol. 2008;1:85–92. doi: 10.1016/j.jri.2008.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chi Y, Pei L, Chen G, et al. Metabonomic profiling of human placentas reveals different metabolic patterns among subtypes of neural tube defects. J Proteome Res. 2014;2:934–945. doi: 10.1021/pr4009805. [DOI] [PubMed] [Google Scholar]
- Clark DA, Coulam CB, Daya S, Chaouat G. Unexplained sporadic and recurrent miscarrage in the new millennium: a critical analysis of immune mechanisms and treatments. Hum Reprod Update. 2001;5:501–511. doi: 10.1093/humupd/7.5.501. [DOI] [PubMed] [Google Scholar]
- Coulam CB. Understanding the immunobiology of pregnancy and applying it to treatment of recurrent pregnancy loss. Early Pregnancy. 2000;1:19–29. [PubMed] [Google Scholar]
- Czeizel AE, Dudas I. Prevention of the first occurrence of neural-tube defects by periconceptional vitamin supplementation. N Engl J Med. 1992;26:1832–1835. doi: 10.1056/NEJM199212243272602. [DOI] [PubMed] [Google Scholar]
- Da CM, Sequeira JM, Rothenberg SP, Weedon J. Antibodies to folate receptors impair embryogenesis and fetal development in the rat. Birth Defects Res A Clin Mol Teratol. 2003;10:837–847. doi: 10.1002/bdra.10088. [DOI] [PubMed] [Google Scholar]
- Denny KJ, Kelly CF, Kumar V, et al. Autoantibodies against homocysteinylated protein in a mouse model of folate deficiency-induced neural tube defects. Birth Defects Res A Clin Mol Teratol. 2016;106:201–207. doi: 10.1002/bdra.23483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elnakat H, Ratnam M. Distribution, functionality and gene regulation of folate receptor isoforms: implications in targeted therapy. Adv Drug Deliv Rev. 2004;8:1067–1084. doi: 10.1016/j.addr.2004.01.001. [DOI] [PubMed] [Google Scholar]
- Frey L, Hauser WA. Epidemiology of neural tube defects. Epilepsia. 2003;s3:4–13. doi: 10.1046/j.1528-1157.44.s3.2.x. [DOI] [PubMed] [Google Scholar]
- Friso S, Choi S-W. Gene-nutrient interactions and DNA methylation. J Nutr. 2002;8:2382S–2387S. doi: 10.1093/jn/132.8.2382S. [DOI] [PubMed] [Google Scholar]
- Gurevich P, Ben-Hur H, Moldavsky M, et al. An immunohistochemical study of the secretory immune system in human fetal endocrine glands and their precursors. Early Pregnancy. 2001;3:191–200. [PubMed] [Google Scholar]
- Henderson GI, Perez T, Schenker S, et al. Maternal-to-fetal transfer of 5-methyltetrahydrofolate by the perfused human placental cotyledon: evidence for a concentrative role by placental folate receptors in fetal folate delivery. J Lab Clin Med. 1995;2:184–203. [PubMed] [Google Scholar]
- Hsieh CC, Maisonneuve P, Boyle P, et al. Analysis of quantitative data by quantiles in epidemiologic studies: classification according to cases, noncases, or all subjects? Epidemiology. 1991;2:137–140. doi: 10.1097/00001648-199103000-00008. [DOI] [PubMed] [Google Scholar]
- Kirke P, Molloy A, Daly L, et al. Maternal plasma folate and vitamin B12 are independent risk factors for neural tube defects. Q J Med. 1993;11:703–708. [PubMed] [Google Scholar]
- Kishimba RS, Mpembeni R, Mghamba JM, et al. Birth prevalence of selected external structural birth defects at four hospitals in Dar es Salaam, Tanzania, 2011–2012. J Glob Health. 2015;5:020411. doi: 10.7189/jogh.05.020411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knutson KL, Krco CJ, Erskine CL, et al. T-cell immunity to the folate receptor alpha is prevalent in women with breast or ovarian cancer. J Clin Oncol. 2006;26:4254–4261. doi: 10.1200/JCO.2006.05.9311. [DOI] [PubMed] [Google Scholar]
- Li Z, Ren A, Liu J, et al. Maternal flu or fever, medication use, and neural tube defects: a population-based case-control study in Northern China. Birth Defects Res A Clin Mol Teratol. 2007;4:295–300. doi: 10.1002/bdra.20342. [DOI] [PubMed] [Google Scholar]
- Li Z, Ren A, Zhang L, et al. Extremely high prevalence of neural tube defects in a 4-county area in Shanxi Province, China. Birth Defects Res A Clin Mol Teratol. 2006;4:237–240. doi: 10.1002/bdra.20248. [DOI] [PubMed] [Google Scholar]
- Molloy AM, Quadros EV, Sequeira JM, et al. Lack of association between folate-receptor autoantibodies and neural-tube defects. N Engl J Med. 2009;2:152–160. doi: 10.1056/NEJMoa0803783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MRC VSRG. Prevention of neural tube defects: results of the Medical Research Council Vitamin Study. MRC Vitamin Study Research Group. Lancet. 1991;8760:131–137. [PubMed] [Google Scholar]
- Page ST, Owen WC, Price K, Elwood PC. Expression of the human placental folate receptor transcript is regulated in human tissues. Organization and full nucleotide sequence of the gene. J Mol Biol. 1993;4:1175–1183. doi: 10.1006/jmbi.1993.1116. [DOI] [PubMed] [Google Scholar]
- Parker N, Turk MJ, Westrick E, et al. Folate receptor expression in carcinomas and normal tissues determined by a quantitative radioligand binding assay. Anal Biochem. 2005;2:284–293. doi: 10.1016/j.ab.2004.12.026. [DOI] [PubMed] [Google Scholar]
- Ramaekers VT, Sequeira JM, Blau N, Quadros EV. A milk-free diet downregulates folate receptor autoimmunity in cerebral folate deficiency syndrome. Dev Med Child Neurol. 2008;5:346–352. doi: 10.1111/j.1469-8749.2008.02053.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothenberg SP, Da CMP, Sequeira JM, et al. Autoantibodies against folate receptors in women with a pregnancy complicated by a neural-tube defect. N Engl J Med. 2004;2:134–142. doi: 10.1056/NEJMoa031145. [DOI] [PubMed] [Google Scholar]
- Sequeira JM, Ramaekers VT, Quadros EV. The diagnostic utility of folate receptor autoantibodies in blood. Clin Chem Lab Med. 2013;3:545–554. doi: 10.1515/cclm-2012-0577. [DOI] [PubMed] [Google Scholar]
- Shapira I, Sequeira JM, Quadros EV. Folate receptor autoantibodies in pregnancy related complications. Birth Defects Res A Clin Mol Teratol. 2015;103:1028–1030. doi: 10.1002/bdra.23436. [DOI] [PubMed] [Google Scholar]
- Smithells RW, Sheppard S, Schorah CJ. Vitamin dificiencies and neural tube defects. Arch Dis Child. 1976;12:944–950. doi: 10.1136/adc.51.12.944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang B, Yi D, Jin L, et al. Organochlorine pesticide levels in maternal serum and risk of neural tube defects in offspring in Shanxi Province, China: a case-control study. Sci Total Environ. 2014:1037–1043. doi: 10.1016/j.scitotenv.2014.05.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weitman SD, Lark RH, Coney LR, et al. Distribution of the folate receptor GP38 in normal and malignant cell lines and tissues. Cancer Res. 1992;12:3396–3401. [PubMed] [Google Scholar]
- Williams J, Mai CT, Mulinare J, Isenb, et al. Updated estimates of neural tube defects prevented by mandatory folic Acid fortification -United States, 1995–2011. MMWR Morb Mortal Wkly Rep. 2015;1:1–5. [PMC free article] [PubMed] [Google Scholar]
- Yang N, Wang LL, Yuan Y, et al. Establishment and evaluation of enzyme-linked immunosorbent assay for measuring human IgM autoantibody to folate receptor. Zhongguo Yi Xue Ke Xue Yuan Xue Bao. 2014;4:410–414. doi: 10.3881/j.issn.1000-503X.2014.04.011. [DOI] [PubMed] [Google Scholar]
- Yasuda S, Hasui S, Yamamoto C, et al. Placental folate transport during pregnancy. Biosci Biotechnol Biochem. 2008;9:2277–2284. doi: 10.1271/bbb.80112. [DOI] [PubMed] [Google Scholar]
- Yates J, Ferguson-Smith M, Shenkin A, et al. Is disordered folate metabolism the basis for the genetic predisposition to neural tube defects? Clin Genet. 1987;5:279–287. doi: 10.1111/j.1399-0004.1987.tb02809.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


