Abstract
Posttraumatic stress disorder (PTSD) has been associated with sleep disturbances including alterations in sleep stages and recently, elevated nocturnal autonomic nervous system (ANS) arousal (i.e., dominance of the sympathetic nervous system over the parasympathetic nervous system). Data suggest that sleep contributes to the regulation of ANS activity. In our previous ambulatory heart rate variability (HRV) monitoring study, strong relationships between sleep and nocturnal ANS activity in resilient participants (i.e., individuals who had never had PTSD despite exposure to high-impact trauma) were not seen with PTSD. In this study, we examined the impact of PTSD vs. resilience on ANS activity as a function of sleep stage and time of sleep. Participants (age 18–35) with current PTSD (n = 38) and resilience (n = 33) completed two overnight polysomnography recordings in a lab setting. The second night electrocardiogram was analyzed for frequency domain HRV parameters and heart rate within rapid-eye-movement (REM) and non-REM (NREM) sleep periods. Results indicated that ANS arousal indexed by HRV was greater during REM compared with NREM sleep and that the REM–NREM difference was greater in the PTSD than in the resilient participants. This effect of PTSD was reduced to non-significance when analyses controlled for REM sleep percentage, which was lower with PTSD. Exploratory analyses revealed that the REM–NREM difference in HRV was correlated with REM sleep percentage in resilient participants, but not with PTSD. In contrast with our data from home settings, the present study did not find increased overall nocturnal ANS arousal with PTSD. Analyses did reveal higher heart rate during initial NREM sleep with more rapid decline over the course of NREM sleep with PTSD compared with resilience. Findings suggest that elevated ANS arousal indexed by heart rate with PTSD is specific to the early part of sleep and possible impairment in regulating ANS activity with PTSD related to REM sleep.
Keywords: Posttraumatic stress disorder, Sleep, Autonomic nervous system, Heart rate variability, Resilience
1. Introduction
Insomnia and recurrent nightmares related to trauma are common symptoms of posttraumatic stress disorder (PTSD) [1,2] and are included in the hyperarousal and re-experience symptom clusters of its diagnostic criteria [3]. Despite the prominence of those symptoms, objective indices of nocturnal hyperarousal have been elusive. Polysomnographic (PSG) studies have not consistently documented impaired sleep initiation and maintenance in PTSD [4]. Studies examining nocturnal arousal in PTSD have utilized indices of autonomic nervous system (ANS) activity including heart rate (HR) and HR variability (HRV). Some, but not all, studies have found evidence for heightened ANS arousal [i.e., dominance of the sympathetic nervous system over the parasympathetic nervous system indexed by increased HR and a decreased high-frequency (HF) component of HRV] during sleep with PTSD [5–8].
Studies have also suggested associations of PTSD with sleep-stage specific alterations, including increased shallow sleep (Stage 1) and reduced slow-wave sleep [4], increased rapid-eye-movement (REM) density, and fragmented patterns of REM sleep [9–11]. Trauma-related nightmares are prominent symptoms of PTSD [12], and dreams are associated with REM sleep [13]. ANS activity changes as a function of sleep stage in normal sleepers [14,15]. We could only identify two studies that examined sleep-stage specific ANS activity in trauma-exposed individuals, one with developing PTSD [16] and the other with established PTSD [7]. The study by Mellman et al. [16] examined nocturnal ANS activity within a month of a traumatic injury using ratios of low frequency to high frequency components (LF/HF) of HRV as an index of sympathetic tone [17]. They found elevated LF/HF during REM sleep in injured patients who subsequently developed PTSD compared with those who did not develop the disorder. Consistent with studies of normal sleepers [14,15], LF/HF and HR were greater during REM sleep than during non-REM (NREM) sleep in this study (LF/HF) and the study by Woodward et al. [7] (HR). The study by Woodward et al. further suggested that PTSD and the percentage of sleep comprised of REM sleep (REM%) moderate this REM–NREM difference in ANS activity since the veterans with PTSD had smaller REM–NREM differences in HR than controls and that REM% was negatively correlated with REM–NREM HR differences in those with PTSD. HRV was not reported in the study. Therefore, no study has reported HRV during sleep as a function of sleep stage in established PTSD.
Sleep appears to have a critical role in regulating ANS activity. Normalized LF (also an index of sympathetic tone) and HR have been found to be reduced during both nighttime and daytime sleep compared with wake [18]. Reductions from sleep onset to the morning in HR and increase in pre-ejection period (consistent with decreased sympathetic tone) have been documented in normal sleepers [19–21], and these effects were also observed after controlling for sleep stage [15]. However, effects of time of sleep on ANS activity indexed primarily by HF measures have not been as consistent with findings of both increased and decreased parasympathetic tone as well as no change across sleep time [14,15,19,22,23]. In the aforementioned study by Mellman et al. of recently injured patients [16], LF/HF was higher during the first than the last REM period in both injured patients who subsequently did and did not develop PTSD. Bertram et al. [24] examined HR change over the course of sleep measured by actigraphy in individual with and without PTSD and found a reduction of HR across sleep time in both groups; however, this study did not control for the effects of sleep stages on HR, nor did it report HRV as a function of time of sleep. Therefore, influences of PTSD on ANS activity during REM and NREM sleep over the course of sleep have not been examined in established PTSD.
Arousal during sleep could also be affected by the sleep environment. Most PSG studies in PTSD have been conducted in laboratory settings, and it has been suggested that these environments contributed to the lack of consistent evidence for impaired sleep initiation and maintenance [25,26]. Researchers have anecdotally reported that participants with PTSD reported having slept better in the lab than their homes with a technician being “on guard” outside their sleeping room [25]. In fact, the two home PSG studies found longer sleep latency, reduced total sleep time and sleep maintenance in individuals with PTSD compared with those without PTSD [26,27]. We recently reported that individuals with PTSD had lower parasympathetic tone indexed by normalized high frequency (nHF) during sleep at home compared with resilient individuals (i.e., individuals who had never had PTSD despite exposure to high-impact trauma) [28].
In summary, PTSD has been associated with elevated ANS arousal during sleep and alterations in sleep stages; however, investigation into ANS activity as a function of sleep stage in PTSD and resilience has been limited despite connections between ANS activity and sleep stages. In addition, ANS arousal decreases over the course of sleep in normal sleepers. The reduction of HR across time asleep was also observed in individuals with PTSD in an actigraphic study; however, effects of sleep stage were not controlled in this study. Further, HRV as a function of time of sleep was not reported in this study despite that there have been discrepancies in findings between studies examining HR and HRV across sleep time in normal sleepers. The purpose of this report is to add the literature on nocturnal ANS activity in PTSD by describing the impact of PTSD vs. resilience on ANS activity as functions of sleep stage and time of sleep using HRV parameters and HR. Due to the suggestion of influences of the sleep environment on nocturnal arousal, we also examined whether the lower parasympathetic tone with PTSD compared with resilience found in our previous ambulatory study manifests in a lab setting.
2. Materials and methods
2.1. Participants and procedure
Participants of the present report were the subsample of physically healthy young adult African Americans (age 18–35 years) who met criteria for current full or subthreshold PTSD or resilience (the criteria for these groups are described below) and completed laboratory PSG as a part of a larger study on PTSD, sleep, neighborhood stress, and nocturnal blood pressure and ANS activity. Participants were recruited from the Washington, DC metropolitan area through flyers and referral from prior participants. During the initial phone or in-person screening, potential participants were excluded if they were found to have a body mass index ≥40, ongoing medical disorder that can affect blood pressure, use of medications that can affect blood pressure or sleep, severe mental disorders (psychotic disorders, bipolar disorder, severe recurrent depression), consuming >5 cups of coffee per day or its equivalent, smoking >20 cigarettes per day, drinking >14 alcoholic drinks/week in men or >7 drinks/week in women, and habitual bedtime and rise time after 2 AM and 10 AM, respectively, or habitual napping >1 h/day. Following the screening, participants were invited to the Howard University Clinical Research Unit where they completed informed consent and self-report surveys that included a demographic questionnaire, a checklist of traumatic experiences, and a measure of PTSD symptom severity (N = 543).
A subset of participants selected from those who filled out the self-report surveys were invited to the laboratory phase and completed a clinical interview, urine toxicology screening, height and weight measurement, and two consecutive-night PSG recordings in the research unit (n = 185). Participants for the laboratory phase were selected based on their answers to the self-report surveys to balance the sample by trauma exposure and PTSD status and gender and to increase representation of community residents as oppose to students living on campus. Additional exclusion criteria evaluated during the laboratory phase were sleep apnea defined as an apnea/hypopnea index of ≥10 on a screening sleep recording, positive urine toxicology for illicit drugs, current alcohol or drug dependence, and current psychiatric disorder other than PTSD, phobic disorder, generalized anxiety disorder, or depression that was secondary to PTSD evaluated through the structured clinical interview. Participants received $25 for completing the self-report survey, another $25 for the clinical interview, and $125 for each PSG recording. The study procedure was approved by the Howard University Institutional Review Board.
For the present analyses, we selected only participants who met the criteria for current PTSD (n = 53) or resilience (n = 43) and completed the second PSG recording. The PTSD participants met the Diagnostic and Statistical Manual of Mental Disorders – Fourth Edition [29] criteria for current PTSD or subthreshold criteria (meeting the criteria for at least two of the three symptom clusters). The resilient participants had never met PTSD criteria despite exposure to a high-impact traumatic event. High-impact trauma was designated for traumatic events that have been associated with high risk for engendering PTSD in prior studies. They included abuse during childhood, sexual assault, physical assault, and trauma associated with injury [30–33]. Of those PSG completers who had never met PTSD criteria (n = 60), 43 (72%) had trauma determined to be “high impact.” This determination preceded analysis of HRV. Participants were excluded for positive toxicology results (3 PTSD, 2 resilient), apnea/hypopnea index of ≥10 (1 PTSD, 3 resilient), abnormal electrocardiogram (ECG) results (1 PTSD, 1 resilient), late discovered exclusion criteria (high body mass index, 1 PTSD), and <4 h of PSG recording which was significantly shorter than their reported habitual time in bed (2 PTSD, 1 resilient). PSG data of 7 PTSD and 3 resilient participants were lost due to technical problem or unable to be scored due to poor signal quality. Therefore, our final sample included 38 PTSD (18 with full PTSD criteria and 20 with subthreshold criteria) and 33 resilient participants. Participant characteristics are presented in Table 1.
Table 1.
Participant characteristics.
| PTSD (n = 38) |
Resilient (n = 33) |
||||
|---|---|---|---|---|---|
| M | SD | M | SD | t | |
| Age | 22.5 | 4.7 | 22.7 | 3.8 | –0.19 |
| Body mass index | 25.7 | 4.9 | 25.8 | 3.8 | –0.11 |
| Current CAPS scores | 41.5 | 17.7 | 8.2 | 7.7 | 10.5*** |
| Duration of full or subthreshold PTSD (months) | 67.0 | 63.8 | - | - | |
| n | n | ||||
| Gender (female) | 25 | 17 | X2 = 1.49 | ||
| Smoker | 7 | 4 | p = 0.527 | ||
| Comorbid psychiatric disorders | |||||
| Anxiety disorder | 7 | 1 | p = 0.060 | ||
| Major depressive disorder | 5 | 1 | p = 0.206 | ||
| Cannabis abuse | 0 | 1 | p = 0.465 | ||
| Types of index trauma | |||||
| Non-sexual violent crime | 6 | 11 | |||
| Childhood physical and/or sexual abuse | 7 | 6 | |||
| Sudden, unexpected death of someone close | 6 | 3 | |||
| Witnessing violent death | 4 | 3 | |||
| Accident | 2 | 3a | |||
| Sexual assault | 5 | 0 | |||
| Intimate partner violence | 3 | 2 | |||
| Otherb | 5 | 5 | |||
Note. CAPS = Clinician Administered PTSD Scale.
Car or pedestrian accidents associated with serious injury or death.
Other categories include family member being injured in violence, witnessing violent acts, and being raised by parents with drug addiction.
p < 0.001.
2.2. Measures
2.2.1. Clinical interviews
The Clinician Administered PTSD Scale [34] is a structured clinical interview designed to assess PTSD diagnostic status and symptom severity. The frequency and intensity of each of DSM-IV PTSD symptoms associated with a participant's index event (i.e., the most distressing traumatic event that met Criterion A) were assessed. Cronbach's α was 0.94 for current and 0.93 for lifetime assessment in this study. Current and lifetime diagnoses of mood disorders, anxiety disorders other than PTSD, substance abuse and dependence, eating disorders, and psychotic disorders were assessed using the Structured Clinical Interview for the DSM-IV [35]. All clinical interviews were conducted by trained staff members (psychology graduate students, medical students, and clinical psychology postdoctoral fellows), and a board certified psychiatrist (TAM) reviewed all cases.
2.2.2. PSG sleep measure
Participants underwent two consecutive-overnight PSG recordings in the Howard University Clinical Research Unit. Recordings were conducted using an Embla (Denver, Colorado, USA) Titanium portable unit. PSG collection included a standard electroencephalogram montage with bilateral frontal, central, and occipital leads, two electrooculograms, and chin electromyograms and limb electromyograms and respiratory monitors on the first night only. Study staff, who were blind to participants’ PTSD diagnostic status, visually scored sleep records on a computer monitor applying the American Academy of Sleep Medicine scoring rules [36]. All scorers had demonstrated >90% concordance for scoring epochs with reference records. Participants were instructed to go to bed and get up near their habitual bedtime and rise time. First-night recordings were used to exclude participants with the apnea and hypopnea index of ≥10. Sleep measures from the second-night recordings were used for these analyses.
2.2.3. ECG monitoring
ECGs were recorded as a part of the PSGs at a 256 Hz sampling rate. ECG data obtained during the second-night PSG recording were imported to LabChart Pro (ADInstruments, Colorado Springs, CO) to compute HR and frequency domain HRV parameters, including low-frequency (LF: 0.04–0.15 Hz) power, HF (0.15–0.4 Hz) power, and nHF [HF / (total power – very low frequency)]* 100 [17]], for each non-overlapping 5-min epoch during the 5-min wake period prior to the first sleep epoch and during consecutive REM and NREM sleep periods, separately, using the Lomb periodogram. No interruptions by other sleep stages were included in each 5-min epoch, and HRV parameters were not computed for REM or NREM periods shorter than 5 min. The Lomb periodogram is a spectral power density estimation method that allows for missing observation points and was shown to produce more accurate HRV parameter estimations than Fast Fourier Transformation with ectopic beat removal and resampling [37]. All heart beats within each epoch were visually inspected on a computer monitor before computing HRV parameters to confirm the correct identification of R-peaks or manually edit if peaks were not correctly identified by the computer program and correct R-peaks were discernible to the researcher. Segments with ectopic beats and artifacts that did not allow for correctly identifying R-peaks were marked as missing before the computation. The average numbers of epochs within NREM and REM sleep included in analyses were not significantly different between the PTSD and resilient groups (NREM sleep: 53.32 epochs for PTSD and 49.58 for resilience, t = 1.70, p = 0.095; REM sleep: 12.21 for PTSD and 13.79 for resilience, t = –1.37, p = 0.176). The LF/HF ratio was interpreted as the index of sympathetic tone, and nHF as the index of parasympathetic tone [17].
2.3. Analyses
An alpha level of 0.05 was used in all analyses. Initially, distributions of data were examined. To normalize the positively skewed distributions, square root transformation was performed for sleep onset latency and wake after sleep onset data. Inverse [(–1 / (x + 1)) + 1] transformation was necessary to normalize LF/HF [38,39]. To examine differences between the PTSD and resilient groups in participant characteristics and sleep parameters, t-tests were performed for continuous variables, and chi-square or Fisher's exact tests were performed for categorical variables.
To examine the impact of PTSD vs. resilience on ANS activity changes as functions of sleep stage and time of sleep, hierarchical linear modeling (HLM) analyses were performed using HLM7 (SSI, Skokie, IL). HLM is suitable for analyzing the hierarchically structured data collected in this study [40] and is also able to handle missing data without excluding subjects [41]. To examine the impact of PTSD vs. resilience on ANS activity changes as a function of sleep stage, we tested a model in which the subject-level equation was ANSij = β0j + β1j(sleep stage) + rij, and the group-level equations were β0j = γ00 + γ01(PTSD) + u0j and β1j = γ10 + γ11(PTSD), where ANS was nHF, LF/HF, or HR, i was subject-level units (i.e., 5-min epochs of ANS, i = 1, ...., nj), j was group-level units (i.e., participants, j = 1, ..., 71), rij and u0j were subject-level and group-level random effects, respectively, representing the error of estimation. Resilient participants were assigned 0, and participants with PTSD were assigned 1. For sleep stage, 0 and 1 were assigned to epochs within NREM and REM sleep periods, respectively. As we found that REM sleep percentage (REM%) was significantly different between the PTSD and resilient groups, we also tested a model in which grand-mean-centered REM% was added to these group-level equations as a predictor. The full maximum likelihood method was used. The subject-level N for these analyses was 4579.
The impact of PTSD vs. resilience on ANS activity as a function of time of sleep was examined by testing longitudinal HLM models separately for REM and NREM sleep periods since ANS activity changes as a function of sleep stage. To prepare for the HLM analyses, epochs were sorted into 30-min bins, separately for REM and NREM periods applying Trinder et al.'s methodology in which epochs during the first 7 h of sleep were sorted into 30-min bins separately for N2, N3, and REM sleep [15]. Given that the mean total sleep time was 6 h 20 min in this study, only epochs within the first 6.5 h from the first sleep epoch were sorted into up to a total of thirteen 30-min bins for each participant. If there were multiple epochs in a bin, epochs were averaged within the bin following Trinder et al.'s methodology. In the models, the subject-level equation was ANSti = π0i + π1i(time) + eti, and the group-level equations were π0i = β00 + β01(PTSD) + r0i and π1i = β10 + β11(PTSD), where time was the 30-min bins numbered 0 through 6.0 (in increments of 0.5) for the analyses of ANS during NREM sleep and numbered –1.0 through 5.0 for the analyses of ANS during REM sleep. This numbering of bins for REM sleep analyses was chosen because the average REM latency was 80.1 min and numbering the third bin (60–90 min after sleep onset) as “0” allows for meaningful interpretation of the subject-level intercept, π0i. The subject-level Ns were 812 for the NREM and 339 for the REM period analyses, respectively.
Exploratory analyses were performed to examine whether PTSD symptom severity moderates ANS activity changes as functions of sleep stage and time of sleep. We modified the aforementioned HLM models by replacing PTSD with grand-mean-centered current CAPS scores, and analyses were conducted with only participants in the PTSD group.
Because results of the models with LF/HF as a subject-level criterion were similar but the inverse of those for nHF, we only presented nHF results.
3. Results
3.1. Participant characteristics
There were no significant differences between PTSD and resilient groups in terms of gender, age, body mass index, comorbidity, and smoking status (Table 1). As expected, current CAPS scores were significantly higher in the PTSD group than the resilient group. Means and standard deviations of PSG sleep parameters are presented in Table 2. The PTSD group had significantly lower REM sleep percent (REM%) than the resilient group (20.8 vs. 23.7%, t(69) = –2.28, p = 0.026).
Table 2.
Polysomnography sleep characteristics.
| PTSD (n = 38) |
Resilient (n = 33) |
||||
|---|---|---|---|---|---|
| M | SD | M | SD | t (69) | |
| Total sleep time (minutes) | 385.9 | 60.2 | 372.4 | 67.9 | 0.89 |
| Sleep onset latency (minutes)a | 33.7 | 44.7 | 39.9 | 59.9 | –0.46 |
| Wake after sleep onset (minutes)a | 31.4 | 28.7 | 25.3 | 31.9 | 1.30 |
| Sleep efficiency (minutes) | 86.4 | 9.3 | 86.2 | 11.7 | 0.07 |
| N1 (%) | 2.2 | 1.1 | 2.2 | 1.6 | –0.17 |
| N2 (%) | 52.0 | 8.5 | 50.7 | 7.8 | 0.65 |
| N3 (%) | 25.1 | 9.1 | 23.4 | 8.9 | 0.82 |
| Rapid-eye-movement sleep (%) | 20.8 | 5.7 | 23.7 | 4.9 | –2.28* |
Note.
Values were square-root transformed before t-tests were performed.
p < 0.05.
3.2. The impact of PTSD status on ANS activity as a function of sleep stage
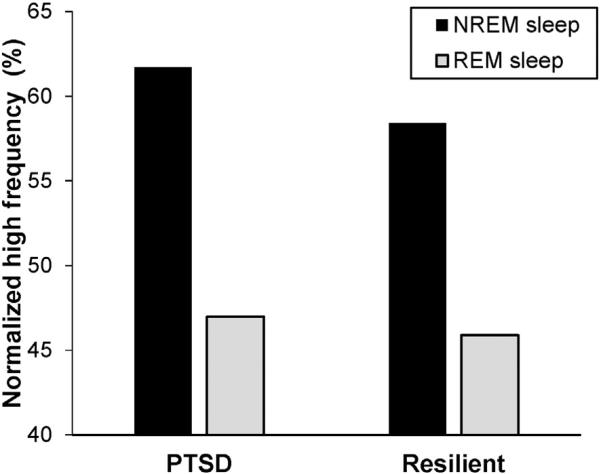
The final estimation of fixed effects of the model with nHF as a subject-level outcome is presented in Table 3. In this model, the group-level coefficient for PTSD, γ01, was not significant, indicating that PTSD was not related to individual's mean nHF. The group-level coefficient, γ10, was significant (γ10 = –12.50, p < 0.001), indicating a significant main effect of sleep stage with lower nHF during REM compared with NREM sleep. The significant coefficient for PTSD, γ11, indicates a cross-level interaction between PTSD and sleep stage. Participants with PTSD had significantly steeper sleep stage slopes, β1j, in the negative direction (–12.50–2.23 = –14.73) (i.e., greater REM–NREM difference) than those who were resilient (–12.50). The graph for this HLM model is presented in Fig. 1. In Table 3, we also presented the results of the model that included REM% as a group-level predictor. The coefficient for REM%, γ12, was significant (γ12 = 0.25, p = 0.023), but the coefficient for PTSD, γ11, was no longer significant. These findings indicate that participants with lower REM% had steeper sleep stage slopes in the negative direction than participants with higher REM% (i.e., the lower REM% was, the greater the REM–NREM differences became).
Table 3.
HLM final estimation of fixed effects.
| Subject-level outcome → |
nHF |
Heart rate |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Initial model |
Model with REM sleep |
Initial model |
Model with REM sleep |
|||||||||
| Fixed effect | Coefficient | SE | p | Coefficient | SE | p | Coefficient | SE | p | Coefficient | SE | p |
| Intercept, β0j | ||||||||||||
| Intercept, γ00 | 58.39 | 2.19 | <0.001 | 58.56 | 2.23 | <0.001 | 63.75 | 1.41 | <0.001 | 64.20 | 1.40 | <0.001 |
| PTSD, γ01 | 3.32 | 2.99 | 0.271 | 2.98 | 3.10 | 0.340 | 3.21 | 1.92 | 0.100 | 2.37 | 1.95 | 0.228 |
| REM Sleep (%), γ02 | –0.12 | 0.28 | 0.677 | –0.29 | 0.18 | 0.113 | ||||||
| Sleep Stage slopes, β1j | ||||||||||||
| Intercept, γ10 | –12.50 | 0.76 | <0.001 | –13.08 | 0.80 | <0.001 | 1.45 | 0.22 | <0.001 | 1.70 | 0.23 | <0.001 |
| PTSD, γ11 | –2.23 | 1.06 | 0.035 | –1.62 | 1.09 | 0.137 | –0.09 | 0.30 | 0.767 | –0.36 | 0.31 | 0.253 |
| REM sleep (%), γ12 | 0.25 | 0.11 | 0.023 | –0.11 | 0.03 | <0.001 | ||||||
Note. The number of subject-level units = 4579; the number of group-level units = 71. nHF = normalized high frequency; PTSD = posttraumatic stress disorder; REM = rapid eye movement.
Fig. 1.
Normalized HF predicted by the HLM model. PTSD = posttraumatic stress disorder, REM = rapid eye movement, NREM = non-rapid eye movement.
For the model with HR as a subject-level outcome, the coefficient for PTSD, γ01, was not significant, indicating that PTSD was not significantly related to individual's mean HR. The group-level coefficient, γ10, was significant (γ10 = 1.45, p < 0.001), indicating that overall HR was higher during REM compared with NREM sleep. The PTSD × Sleep stage cross-level interaction, γ11, was not significant. For the model that included REM% as a group-level predictor, REM% significantly contributed to sleep stage slopes (γ12 = –0.11, p < 0.001), indicating that sleep stage slopes were steeper in the positive direction for participants with lower REM% compared with those with higher REM% (i.e., the lower REM% was, the greater the REM–NREM difference became).
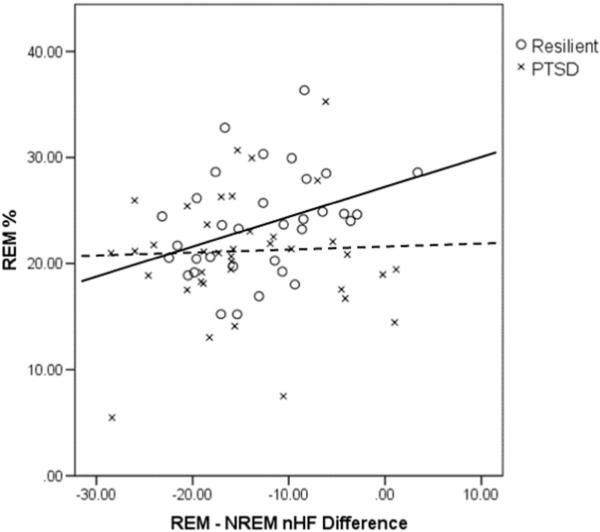
To examine if the relationships between REM% and REM–NREM differences in ANS activity were different between groups, exploratory analyses were performed adding an interaction between PTSD and REM% (PTSD × REM%) to the group-level equations of the model. Results indicated that both coefficients for REM% and PTSD × REM% in the sleep stage slope equation were significant in the models with nHF (γ12 = 0.25, p = 0.021 for REM%; γ13 = –1.28, p = 0.033 for PTSD × REM%) and HR (γ12 = –0.11, p < 0.001; γ13 = 0.50, p = 0.003) as a subject-level outcome, indicating that relationships between REM% and REM–NREM differences in these ANS indices were different between PTSD and resilience. To unpack the significant PTSD × REM% interaction effects on REM–NREM differences in ANS activity, we performed correlations (Pearson's r) between average REM–NREM differences in HRV and HR (i.e., individual average of HRV parameters and HR during NREM sleep subtracted from individual average of HRV parameters and HR during REM sleep) and REM% in the PTSD and resilient groups separately. Results showed that REM–NREM nHF was significantly correlated with REM% in the resilient group (r = 0.345, p = 0.049), but not in the PTSD group (r = 0.063, p = 0.708). Fig. 2 presents a scatter plot showing these associations between REM–NREM nHF and REM% and regression lines for each group. REM–NREM HR was not correlated with REM% in either group (r = –0.248, p = 0.164 for resilience; r = 0.040, p = 0.810 for PTSD).
Fig. 2.
Associations between REM–NREM differences in nHF in PTSD and resilience. The solid regression line applies to resilient participants, the dashed line to PTSD participants. PTSD = posttraumatic stress disorder, REM = rapid eye movement, NREM = non-rapid eye movement, nHF = normalized high frequency.
3.3. The impact of PTSD status on ANS activity as a function of time of sleep
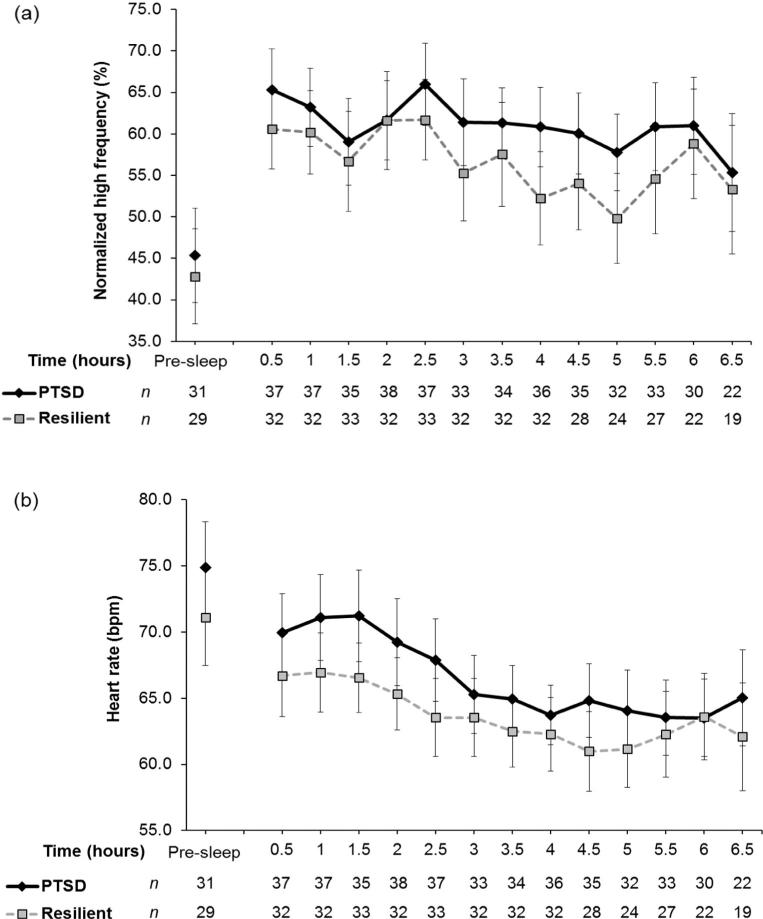
In the longitudinal HLM model with nHF during NREM sleep as an outcome, the intercept of the group-level equation for time was significant (β10 = –1.21, p < 0.001), but neither group-level coefficient for PTSD was significant (β01 = 3.09, p = 0.310; β11 = 0.49, p = 0.241). These results show that nHF during NREM sleep period at the beginning of sleep did not differ between the groups and that nHF declined across the night, but the rates for nHF changes for the two groups were not different. To illustrate the change across sleep time, we plotted group mean nHF during NREM sleep for each bin in Fig. 3a. For nHF during REM sleep, none of the group-level coefficients, except for β00, was significant (β01 = 0.48, p = 0.897; β10 = 0.64, p = 0.197; β11 = 0.28, p = 0.681), indicating that nHF during REM sleep did not linearly change over the course of sleep.
Fig. 3.
(a) Normalized high frequency during non-rapid eye movement (NREM) sleep periods across the night; (b) Heart rate during NREM sleep periods across the night. PTSD = posttraumatic stress disorder. The error bars indicate 95% CIs.
For HR during NREM sleep, all of the group-level coefficients were significant (β00 = 66.51, p < 0.001; β01 = 4.38, p = 0.030; β10 = –1.08, p < 0.001; β11 = –0.37, p = 0.002). These results indicate that the PTSD group had on average 4.38 beats per minute (bpm) higher NREM HR during the first 30 min of sleep (the length of the first bin) than the resilient group, and the PTSD group had a steeper declining slope over the course of sleep (1.45 bpm decrease each half-hour for the PTSD group vs. 1.08 bpm per half-hour decrease for the resilience group). To illustrate this group difference, we plotted group mean HR during NREM sleep for each bin in Fig. 3b. For HR during REM sleep, the intercept of group-level equation for time was significant (β10 = –1.19, p < 0.001), but neither group-level coefficient for PTSD was significant (β01 = 3.54, p = 0.072; β11 = –0.26, p = 0.201). These results indicate that REM HR at the average first REM sleep onset was higher at a trend level (on average 3.54 bpm higher) with PTSD and that HR during REM declined across the night (1.19 bpm/half-hour for resilience; 1.45 bpm/half-hour for PTSD), but the rates of HR change for PTSD and resilience were not significantly different.
3.4. The impact of PTSD severity on ANS activity as functions of sleep stage and time of sleep
The HLM models examining effects of PTSD severity on nHF changes as a function of sleep stage showed that PTSD severity measured by CAPS scores was not associated with either individual's mean nHF (γ01 = –0.10, p = 0.422) or nHF differences between REM and NREM sleep (γ11 = –0.03, p = 0.533). The models examining effects of PTSD severity on HR changes showed that PTSD severity was not associated with individual's mean HR (γ01 = 0.06, p = 0.456), but PTSD severity significantly moderated REM–NREM differences in HR (γ11 = –0.03, p = 0.011), indicating that the more severe PTSD was, the smaller REM–NREM HR difference became. The longitudinal models indicated that PTSD severity did not moderate effect of time of sleep on nHF or HR during NREM or REM sleep (all p > 0.451).
We also examined whether HRV and HR are different between participants who met the full PTSD criteria and those who met only the subthreshold criteria within the PTSD group. Results of HLM models examining nHF changes as a function of sleep stage showed that full PTSD and subthreshold participants were not different in either mean nHF (γ01 = –6.30, p = 0.133) or nHF differences between REM and NREM sleep (γ11 = 0.96, p = 0.514). The HLM models examining HR changes as a function of sleep stage indicated that full PTSD and subthreshold participants were not different in mean HR (γ01 = 2.85, p = 0.305), but the full PTSD participants had significantly smaller REM–NREM HR differences than the subthreshold participants (γ11 = –1.59, p < 0.001). The longitudinal HLM models showed that effects of time of sleep on nHF and HR were not different between full PTSD and subthreshold participants (all p > 0.265).
4. Discussion
We compared people with PTSD vs. resilience with regard to ANS activity indexed by HRV and HR as functions of sleep stage and time of sleep and found that 1) individuals with PTSD had greater REM–NREM differences in ANS activity compared with resilient individuals and that the percentage of REM sleep accounted for this group difference, 2) associations between REM% and REM–NREM differences in ANS activity were significant in resilience, but not in PTSD, 3) HR was elevated with PTSD compared with resilience during NREM and REM sleep in the early part of sleep, and 4) HR during NREM and REM sleep diminished over the course of sleep and the rate of reduction of HR during NREM sleep was faster with PTSD than resilience. We previously reported heightened overall nocturnal ANS arousal indexed by reduced nHF in people with PTSD compared with resilience from ambulatory HRV monitoring [28]. The present study with participants largely overlapping those of the previous study did not find elevated nocturnal ANS arousal, except for higher HR in the early part of sleep. This discrepancy suggests that the environment (home versus lab) has an effect on ANS activity.
Our findings of greater ANS arousal during REM compared with NREM sleep with both HRV and HR are consistent with prior studies of normal sleepers, and extend Woodward et al.s' [7] finding of elevated HR during REM sleep in veterans with PTSD. In addition, the present findings of associations between REM% and REM–NREM differences in ANS activity indexed by HRV only in the resilient group are parallel to our prior findings of a strong inverse relationships between total sleep time and overall ANS arousal indexed by HRV with resilience that was absent with PTSD [28]. It has been suggested that sleep has a role in regulating ANS activity [18]. Both our prior finding and the present finding could reflect a function of sleep in regulating ANS activity that is compromised with PTSD. Woodward et al. [7] also found an association between REM% and REM–NREM differences with HR and speculated possible coupling between HR and mechanisms regulating REM sleep. However, unlike our study, they observed the association only in veterans with PTSD, but not in controls possibly due to a lack of statistical power for the control group. Characteristics of research participants might have influenced the differences in these studies’ findings. Participants in Woodward et al.s’ study were middle-aged inpatient Vietnam War veterans with chronic severe PTSD, whereas participants of this report were young adults with mild to moderate PTSD severity recruited from non-clinical settings.
The resilient group in this study had higher REM% than the PTSD group; however, in our prior report [42], the broadly defined trauma control group (i.e., trauma-exposed individuals without past or current PTSD whose index trauma was not necessarily “high impact”) did not have significantly higher REM% compared to those with current full or subthreshold PTSD. This difference in findings suggests that increased REM sleep might be more pronounced among those who are resilient to high-impact trauma.
This report is the first to our knowledge to examine the influence of PTSD vs. resilience on ANS activity indexed by both HR and HRV during REM and NREM sleep over the course of sleep. The present study revealed that HR during NREM sleep was higher at the beginning of the night with PTSD and declined at a faster rate over the night compared with resilience. These findings show that elevated ANS arousal at the beginning of sleep in people with PTSD, which was likely to be carried over from pre-sleep arousal, diminished over sleep time. We recently reported associations between PTSD severity and pre-sleep cognitions and behaviors related to fear of sleep (e.g., fear of loss of vigilance, fear of re-experiencing trauma) [43]. It is possible that these pre-sleep factors contribute to the elevated ANS arousal at the beginning of sleep in individuals with PTSD.
The HR reduction over the course of sleep in both PTSD and resilient participants is consistent with prior studies of normal sleepers and the study by Bertram et al. [24] of people with and without PTSD. However, unlike the present study, Bertram et al. did not find a faster rate of HR reduction with PTSD, rather consistently higher HR during sleep with PTSD compared with controls. Methodological differences could have contributed to this difference. Bertram et al. did not differentiate REM and NREM sleep periods as they used actigraphy to measure sleep, and their recordings were performed in home settings. Considering the present finding of HR reduction over sleep time in both of the groups and the dissociation between REM% and REM–NREM differences in HRV in the PTSD group, we speculate that PTSD-related impairment in the function of sleep in regulating ANS activity could be related to factors regulating REM sleep rather than the time course of sleep, such as circadian systems.
As noted in the Introduction, prior findings for HRV are not as consistent as findings for HR, which declined over sleep time. Similarly, the present findings include some differences between HR and HRV related findings, mainly those that relate to time of sleep. For example, analyses of HRV did not indicate effects of PTSD either on ANS arousal in the early part of sleep or the rate of ANS changes over the course of sleep. In addition, analyses revealed a pattern of HRV changes indicative of increase in ANS arousal during NREM sleep over the course of sleep (i.e., decrease in NREM nHF and increase in NREM LF/HF). Given that the discrepancies between HR and HRV changes over sleep time were found in both prior studies with normal sleepers and the present study, it is possible that the discrepancies found in this study are not entirely due to effects of PTSD but also influenced by differences in characteristics of these measures. For instance, there are differences in physiological processes that contribute to HR and HRV. HRV can be utilized to separate effects of multiple factors influencing HR as the sympathetic and parasympathetic nervous systems and other factors such as thermoregulatory mechanisms and chemoreceptor activity [44] are indexed by different frequencies, though overlap exists. In addition, the proportion of sleep stages within NREM sleep might have affected the present finding. Trinder et al. [15] found that HF expressed in the proportion of the total sleep time was, on average, higher during N3 than N2, whereas HR was not different between these stages. Therefore, reduction of N3 over the course of sleep time might have influenced nHF and LF/HF, but not HR in the present study.
Interpretations of present findings are limited by the cross-sectional and correlational research design. The study's focus on a physically healthy young adult nonclinical sample and moderate symptom severity of the PTSD group limit its generalizability. Reliance on a single night recording of PSG did not allow for capturing night-to-night variability in sleep parameters, though one night of recording was probably sufficient for HRV parameters given high short-term stability of HRV [45]. Results of HRV analyses should be interpreted with caution, as it has been suggested that the LF component is influenced not only by the sympathetic nervous system, but also the parasympathetic nervous system [46]. We used LF/HF to control for parasympathetic influence on LF.
Despite these limitations, our analyses address some of the methodological limitations of prior studies and provide novel information that furthers our understanding of the nature of nocturnal hyperarousal in PTSD and resilience. Participants of this report included urban-residing minorities with PTSD who had shown elevated nocturnal ANS arousal in their home environments. We observed elevated HR specific to the early part of sleep with PTSD. This difference in findings may indicate influences of sleep environments on ANS activity in individuals with PTSD. Our prior study suggested impairment in the function of sleep in regulating ANS activity with PTSD. The present analysis provides a further support and additional information for this hypothesis. The associations between REM% and REM–NREM differences in ANS activity with resilience and lack thereof with PTSD suggest that PTSD-related impairment in regulating ANS activity may be related to REM sleep. Future studies should examine whether treatment for PTSD and trauma-related sleep disturbances would reduce early evening ANS arousal and align REM sleep and ANS activity while exploring mechanisms underlying the possible impairment in the function of sleep in regulating ANS activity.
HIGHLIGHTS.
Autonomic activity as a function of sleep stage was associated with PTSD status.
Heart rate was elevated in PTSD compared to resilience at the beginning of sleep.
Heart rate declined over time of sleep at a faster rate in PTSD than resilience.
Acknowledgements
This study was supported by NHLBI grant 5R01HL087995 to Dr. Mellman, and NCRR grant 1UL1RR031975, now NCATS grant UL1TR000101 for the Georgetown Howard Universities Center for Clinical and Translational Science. The authors wish to thank Bryonna Wilson, BS, and the nursing staff of the Howard University Clinical Research Unit for their assistance.
References
- 1.Neylan TC, Marmar CR, Metzler TJ, Weiss DS, Zatzick DF, Delucchi KL, et al. Sleep disturbances in the Vietnam generation: findings from a nationally representative sample of male Vietnam veterans. Am. J. Psychiatry. 1998;155(7):929–933. doi: 10.1176/ajp.155.7.929. [DOI] [PubMed] [Google Scholar]
- 2.Ohayon MM, Shapiro CM. Sleep disturbances and psychiatric disorders associated with posttraumatic stress disorder in the general population. Compr. Psychiatry. 2000;41(6):469–478. doi: 10.1053/comp.2000.16568. [DOI] [PubMed] [Google Scholar]
- 3.American Psychiatric Association . Diagnostic and Statistical Manual of Mental Disorders. fifth ed. American Psychiatric Association; Washington, DC: 2013. [Google Scholar]
- 4.Kobayashi I, Boarts JM, Delahanty DL. Polysomnographically measured sleep abnormalities in PTSD: a meta-analytic review. Psychophysiology. 2007;44(4):660–669. doi: 10.1111/j.1469-8986.2007.537.x. [DOI] [PubMed] [Google Scholar]
- 5.Muraoka MY, Carlson JG, Chemtob CM. Twenty-four-hour ambulatory blood pressure and heart rate monitoring in combat-related posttraumatic stress disorder. J. Trauma. Stress. 1998;11(3):473–484. doi: 10.1023/A:1024400628342. [DOI] [PubMed] [Google Scholar]
- 6.van Liempt S, Arends J, Cluitmans PJM, Westenberg HGM, Kahn RS, Vermetten E. Sympathetic activity and hypothalamo–pituitary–adrenal axis activity during sleep in post-traumatic stress disorder: a study assessing polysomnography with simultaneous blood sampling. Psychoneuroendocrinology. 2013;138(1):155–165. doi: 10.1016/j.psyneuen.2012.05.015. [DOI] [PubMed] [Google Scholar]
- 7.Woodward SH, Murburg MM, Bliwise DL. PTSD-related hyperarousal assessed during sleep. Physiol. Behav. 2000;70:197–203. doi: 10.1016/s0031-9384(00)00271-7. [DOI] [PubMed] [Google Scholar]
- 8.Woodward SH, Arsenault NJ, Voelker K, Nguyen T, Lynch J, Skultety K, et al. Autonomic activation during sleep in posttraumatic stress disorder and panic: a mattress actigraphic study. Biol. Psychiatry. 2009;7/166(1):41–46. doi: 10.1016/j.biopsych.2009.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Breslau N, Roth T, Burduvali E, Kapke A, Schults L, Roehrs T. Sleep in lifetime post-traumatic stress disorder a community-based polysomnographic study. Arch. Gen. Psychiatry. 2004;61(5):508–516. doi: 10.1001/archpsyc.61.5.508. [DOI] [PubMed] [Google Scholar]
- 10.Habukawa M, Uchimura N, Maeda M, Kotorii N, Maeda H. Sleep findings in young adult patients with posttraumatic stress disorder. Biol. Psychiatry. 2007;62(10):1179–1182. doi: 10.1016/j.biopsych.2007.01.007. [DOI] [PubMed] [Google Scholar]
- 11.Woodward SH, Arsenault NJ, Michel GE, Santerre CS, Groves WK, Stewart WK. Polysomnographic characteristics of trauma-related nightmares. Sleep. 2000;23(S2):A356–A357. [Google Scholar]
- 12.Ross RJ, Ball WA, Sullivan KA, Caroff SN. Sleep disturbance as the hallmark of posttraumatic stress disorder. Am. J. Psychiatry. 1989;146(6):697–707. doi: 10.1176/ajp.146.6.697. [DOI] [PubMed] [Google Scholar]
- 13.Hobson JA, Pace-Schott EF, Stickgold R. Dreaming and the brain: toward a cognitive neuroscience of conscious states. Behav. Brain Sci. 2000;23(6):793–842. doi: 10.1017/s0140525x00003976. [DOI] [PubMed] [Google Scholar]
- 14.Bonnet MH, Arand DL. Heart rate variability: sleep stage, time of night, and arousal influences. Electroencephalogr. Clin. Neurophysiol. 1997;102:390–396. doi: 10.1016/s0921-884x(96)96070-1. [DOI] [PubMed] [Google Scholar]
- 15.Trinder J, Kleiman J, Carrington M, Smith S, Breen S, Tan N, et al. Autonomic activity during human sleep as a function of time and sleep stage. J. Sleep Res. 2001;10(4):253–264. doi: 10.1046/j.1365-2869.2001.00263.x. [DOI] [PubMed] [Google Scholar]
- 16.Mellman TA, Knorr BR, Pigeon WR, Leiter JC, Akay M. Heart rate variability during sleep and the early development of posttraumatic stress disorder. Biol. Psychiatry. 2004;55(9):953–956. doi: 10.1016/j.biopsych.2003.12.018. [DOI] [PubMed] [Google Scholar]
- 17.Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology Heart rate variability: standards of measurement, physiological interpretation and clinical use. Circulation. 1996;93(5):1043–1065. [PubMed] [Google Scholar]
- 18.Viola AU, Simon C, Ehrhart J, Geny B, Piquard F, Muzet A, et al. Sleep processes exert a predominant influence on the 24-h profile of heart rate variability. J. Biol. Rhythm. 2002;17(6):539–547. doi: 10.1177/0748730402238236. [DOI] [PubMed] [Google Scholar]
- 19.Burgess HJ, Penev PD, Schneider R, Van Cauter E. Estimating cardiac autonomic activity during sleep: impedance cardiography, spectral analysis, and Poincare plots. Clin. Neurophysiol. 2004;115(1):19–28. doi: 10.1016/s1388-2457(03)00312-2. [DOI] [PubMed] [Google Scholar]
- 20.Burgess HJ, Holmes AL, Dawson D. The relationship between slow-wave activity, body temperature, and cardiac activity during nighttime sleep. Sleep. 2001 May 1;24(3):343–349. doi: 10.1093/sleep/24.3.343. [DOI] [PubMed] [Google Scholar]
- 21.Kerkhof GA, Van Dongen HPA, Bobbert AC. Absence of endogenous circadian rhythmicity in blood pressure? 1. Am. J. Hypertens. 1998;311(3):373–377. doi: 10.1016/s0895-7061(97)00461-5. [DOI] [PubMed] [Google Scholar]
- 22.Hall M, Vasko R, Buysse D, Ombao H, Chen Q, Cashmere JD, et al. Acute stress affects heart rate variability during sleep. Psychosom. Med. 2004;66(1):56–62. doi: 10.1097/01.psy.0000106884.58744.09. [DOI] [PubMed] [Google Scholar]
- 23.Burgess HJ, Trinder J, Kim Y, Luke D. Sleep and circadian influences on cardiac autonomic nervous system activity. Am. J. Phys. 1997 Oct;273:H1761–H1768. doi: 10.1152/ajpheart.1997.273.4.H1761. [DOI] [PubMed] [Google Scholar]
- 24.Bertram F, Jamison AL, Slightam C, Kim S, Roth HL, Roth WT. Autonomic arousal during actigraphically estimated waking and sleep in male veterans with PTSD. J. Trauma. Stress. 2014;27(5):610–617. doi: 10.1002/jts.21947. [DOI] [PubMed] [Google Scholar]
- 25.Woodward SH, Bliwise DL, Friedman MJ, Gusman DF. Subjective versus objective sleep in Vietnam combat veterans hospitalized for PTSD. J. Trauma. Stress. 1996;9(1):137–143. doi: 10.1007/BF02116839. [DOI] [PubMed] [Google Scholar]
- 26.Germain A, Hall M, Shear MK, Nofzinger EA, Buysse DJ. Sleep disruption in PTSD: a pilot study with home-based polysomnography. Sleep Biol. Rhythms. 2006;4(3):286–289. [Google Scholar]
- 27.Herbst E, Metzler TJ, Lenoci M, McCaslin SE, Inslicht S, Marmar CR, et al. Adaptation effects to sleep studies in participants with and without chronic posttraumatic stress disorder. Psychophysiology. 2010;1147(6):1127–1133. doi: 10.1111/j.1469-8986.2010.01030.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kobayashi I, Lavela J, Mellman TA. Nocturnal autonomic balance and sleep in PTSD and resilience. J. Trauma. Stress. 2014;27(6):712–716. doi: 10.1002/jts.21973. [DOI] [PubMed] [Google Scholar]
- 29.American Psychiatric Association . Diagnostic and Statistical Manual of Mental Disorders — Forth Edition — Text Revision. American Psychiatric Association; Washington, DC: 2000. [Google Scholar]
- 30.Kessler RC, Sonnega A, Bromet E, Hughes M, Nelson CB. Posttraumatic stress disorder in the national comorbidity survey. Arch. Gen. Psychiatry. 1995;52:1048–1060. doi: 10.1001/archpsyc.1995.03950240066012. [DOI] [PubMed] [Google Scholar]
- 31.Breslau N, Kessler RC, Chilcoat HD, Schultz LR, Davis GC, Andreski P. Trauma and posttraumatic stress disorder in the community: the 1996 Detroit area survey of trauma. Arch. Gen. Psychiatry. 1998 Jul 1;55(7):626–632. doi: 10.1001/archpsyc.55.7.626. [DOI] [PubMed] [Google Scholar]
- 32.Ursano RJ, Fullerton CS, Epstein RS, Crowley B, Kao TC, Vance K, et al. Acute and chronic posttraumatic stress disorder in motor vehicle accident victims. Am. J. Psychiatry. 1999;156(4):589–595. doi: 10.1176/ajp.156.4.589. [DOI] [PubMed] [Google Scholar]
- 33.Koren D, Norman D, Cohen A, Berman J, Klein EM. Increased PTSD risk with combat-related injury: a matched comparison study of injured and uninjured soldiers experiencing the same combat events. Am. J. Psychiatry. 2005;162(2):276–28. doi: 10.1176/appi.ajp.162.2.276. [DOI] [PubMed] [Google Scholar]
- 34.Blake DD, Weathers FW, Nagy LM, Kaloupek DG, Gusman FD, Charney DS. The development of a clinician-administered PTSD scale. J. Trauma. Stress. 1995;8(1):75–90. doi: 10.1007/BF02105408. [DOI] [PubMed] [Google Scholar]
- 35.First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV-TR Axis I Disorders, Research Version. Patient ed. Biometrics Research; New York: 2002. [Google Scholar]
- 36.American Academy of Sleep Medicine . AASM Manual for the Scoring of Sleep and Associated Events. American Academy of Sleep Medicine; 2007. [Google Scholar]
- 37.Clifford GD, Tarassenko L. Quantifying errors in spectral estimates of HRV due to beat replacement and resampling. Biomed. Eng., IEEE Transactions on. 2005;52(4):630–638. doi: 10.1109/TBME.2005.844028. [DOI] [PubMed] [Google Scholar]
- 38.Evans J. Your Psychology Project: The Essential Guide. Sage; Thousand Oaks, CA: 2007. [Google Scholar]
- 39.Tabachnick BG, Fidell LS. Using Multivariate Statistics. 4th Edition ed. Allyn & Bacon; Needham Heights, MA: 2000. [Google Scholar]
- 40.Raudenbush SW, Bryk AS. Hierarchical Linear Models: Applications and Data Analysis Methods. second ed. Sage; Thousand Oaks, CA: 2002. [Google Scholar]
- 41.Kwok O, Underhill AT, Berry JW, Luo W, Elliott TR, Yoon M. Analyzing longitudinal data with multilevel models: an example with individuals living with lower extremity intra-articular fractures. Rehabil Psychol. 2008;53(3):370. doi: 10.1037/a0012765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mellman TA, Kobayashi I, Lavela J, Wilson B, Wilson HBTS. A relationship between REM sleep measures and the duration of posttraumatic stress disorder in a young adult urban minority population. Sleep. 2014;37:1321–1326. doi: 10.5665/sleep.3922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Huntley ED, Hall Brown TS, Kobayashi I, Mellman TA. Validation of the Fear of Sleep Inventory (FOSI) in an urban young adult African American sample. J. Trauma. Stress. 2014;103-7 doi: 10.1002/jts.21882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pumprla J, Howorka K, Groves D, Chester M, Nolan J. Functional assessment of heart rate variability: physiological basis and practical applications. Int. J. Cardiol. 2002;784(1):1–14. doi: 10.1016/s0167-5273(02)00057-8. [DOI] [PubMed] [Google Scholar]
- 45.Israel B, Buysse DJ, Krafty RT, Begley A, Miewald J, Hall M. Short-term stability of sleep and heart rate variability in good sleepers and patients with insomnia: for some measures, one night is enough. Sleep. 2012 Sep 1;35(9):1285–1291. doi: 10.5665/sleep.2088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Reyes del Paso GA, Langewitz W, Mulder LJM, van Roon A, Duschek S. The utility of low frequency heart rate variability as an index of sympathetic cardiac tone: a review with emphasis on a reanalysis of previous studies. Psychophysiology. 2013;50(5):477–487. doi: 10.1111/psyp.12027. [DOI] [PubMed] [Google Scholar]