Abstract
Objectives
This study describes educational placement of school-aged children after spontaneous intracerebral hemorrhage (ICH) and examines whether educational placement is associated with severity of neurological deficits.
Methods
Children with spontaneous ICH presenting from 2007 to 2013 were prospectively enrolled at 3 tertiary children’s hospitals. The pediatric stroke outcome measure (PSOM) and a parental questionnaire gathered information about neurological outcome, school attendance, and educational placement.
Results
The cohort of 92 enrolled children included 42 school-aged children (6–17 years) with ICH. Four died; 1 was excluded due to preexisting cognitive deficits. Thirty-seven completed 3-month follow-up, and 30 completed 12-month follow-up. At 12 months, 14 (46.7%) received regular age-appropriate programming, 12 (40%) attended school with in-class services, 3 (10%) were in special education programs, and one child (3.3%) received home-based services due to ICH-related deficits. Of 30 children with 3-month and 12-month follow-up, 14 children (46.7%) improved their education status, 13 (43.3%) remained at the same education level, and 3 (10%) began to receive in-class services. Increasing PSOM score predicted the need for educational modifications at 3 months (OR 3.3, 95% CI 1.4–7.9, p=0.007) and at 12 months (OR 2.1, 95% CI 1.1–3.9, p=0.025).
Conclusions
Most children returned to school within a year after ICH and many had a reduction in the intensity of educational support. However, a great need for educational services persisted at 12 months after ICH with fewer than half enrolled in regular age-appropriate classes. Worse deficits on the PSOM were associated with remedial educational placement.
Keywords: pediatric ICH, education, PSOM
Introduction
Stroke occurs in 2–13/100,000 children per year in developed countries and is as common as brain tumors in childhood.1,2 Approximately half of strokes in children are hemorrhagic, most of which are spontaneous intracerebral hemorrhage (ICH).1 However, there are few studies on pediatric ICH. More than 50% of children with ICH have neurological disabilities, and up to 50% have cognitive dysfunction.3,4 One report from the United Kingdom of 49 children with arterial ischemic stroke found that at a mean of 6 months after stroke about 20% of children had state funded individual support in mainstream classes and that over half were on a state funded special education register.5 In this study, patient, parent, and teacher-reported measures of health-related quality of life in the school domain were significantly lower than comparative norms. While one study about health status after childhood stroke included children with ICH6, virtually no information exists regarding the educational needs and placement of children who have had ICH.
Schooling is a critical part of any child’s life, both educationally and socially. Therefore understanding the educational needs of children who have suffered ICH may help parents and schools in planning for a child’s effective assimilation into the school environment. Also, improving reintegration strategies and school support could lead to improved school-related quality of life in some children. This report aimed to describe the educational placement of school-aged children with ICH from a prospective cohort of children with spontaneous ICH. We also sought to determine whether degree of neurological dysfunction at 3 months and 12 months was associated with category of school placement.
Methods
Subjects
Children with spontaneous (non-traumatic) intracerebral hemorrhage who presented from 2007 to 2013 were prospectively enrolled at 3 tertiary children’s hospitals (Monroe Carell Jr. Hospital at Vanderbilt, Children’s Hospital of Philadelphia, Johns Hopkins Children’s Center). Only school-aged children (ages 6–17 years) without preexisting cognitive deficits were included in this sub-study. At 3 months and 12 months post-ICH, neurological deficits were assessed with the Pediatric Stroke Outcome Measure (PSOM).7 Hemorrhage pattern was described as isolated intraparenchymal (IPH), isolated intraventricular (IVH), or intraparenchymal with intraventricular extension (IPH+IVH). Intraparenchymal hemorrhage volume was measured using the ABC/XYZ method which estimates hemorrhage volume as a percent of total brain volume.8,9 The study was approved by each site’s institutional review board, and informed consent and child/adolescent assent, when applicable, were obtained for all subjects.
Educational Placement and Neurological Outcome
For each subject, parents were asked whether the child had an individualized education plan (IEP) or 504 plan in place. An IEP is a written document that is developed for a student in public school who requires special education. An IEP is typically prepared by a team that includes teachers, therapists, and the child’s parents. A 504 plan typically is reserved for children who require educational accommodations but who do not require special education (e.g., extra time to walk between classes due to motor impairment). Both IEPs and 504 plans are typically reviewed annually. At the 3 month and 12 month visits, parents were asked whether or not their child was attending school. If the child was attending school, the parent was asked whether the child was attending school in regular age-appropriate grade level programming, attending school at grade level with in-class services, attending school below grade level in a self-contained special education program, or receiving home-based academic services. If the child was not attending school, the parent was asked whether the child was not attending school due to ICH-related deficits or due to medical illness. For the purpose of examining the possible association between neurological outcome and educational placement, educational placement was dichotomized as “normal” defined as attending school in regular age-appropriate grade level programming and “educational modifications” defined as all other categories.
The PSOM is based on the neurological examination and includes 5 subscale scores: sensorimotor left, sensorimotor right, expressive language, receptive language, and cognition/behavior. The subscales are scored as 0 for no deficit, 0.5 for a mild deficit that does not interfere with function, 1 for a moderate deficit that interferes with function, and 2 for a severe deficit. Thus the total PSOM score range is 0 to 10. A “poor neurological outcome” was defined as a total PSOM of ≥1 which indicates moderate deficits or worse.10 This definition has previously been used in the pediatric stroke literature.4,11
Statistical Analysis
STATA 12.0 (StataCorp, College Station, TX) was used for all statistical analyses. Descriptive statistics including counts and percentages for categorical variables and medians with interquartile ranges (IQR) for non-normally distributed continuous variables were used. The Wilcoxon rank-sum test was used to determine if PSOMs at 3 months were different between those with and without 12 month follow-up. The Fisher’s exact test was used to determine whether an association was present between poor neurological outcome and educational modifications. Additionally, logistic regression was used to determine whether the need for educational modifications was associated with total PSOM score when PSOM was treated as a continuous measure or with hemorrhage volumes as a percent of total brain volume. In addition, exploratory analyses were performed to evaluate any association between the need for educational modifications and total scores on the three major categories of the PSOM [sensorimotor (left sensorimotor and right sensorimotor collapsed into a single category), language (expressive and receptive collapsed into a single category), and cognitive/behavior (a single category)]. A probability value of ≤0.05 was considered statistically significant.
Results
Of 92 subjects enrolled, 50 were excluded for this study because they were not school-aged (<6 years). Of 42 school-aged subjects, four died, and 1 with preexisting cognitive impairment and special education needs due to Trisomy 21 was also excluded. The remaining 37 were included. All 37 completed 3-month follow-up, and 30 (81.1%) had 12-month follow-up. Table 1 provides subjects’ demographics and hemorrhage characteristics.
Table 1.
Subject Demographics and Hemorrhage Characteristics (N=37).
| N (%) | |
|---|---|
| Age in years, median (IQR, range) | 13.0 (9.9–14.9, 6.4–17.7) |
| Male | 23 (62.2%) |
| Race and ethnicity | |
| White | 22 (59.5%) |
| African American | 15 (40.5%) |
| Hispanic | 0 (0%) |
| ICH pattern | |
| IPH | 19 (51.4%) |
| IVH | 2 (5.4%) |
| IPH+IVH | 16 (43.2%) |
| ICH etiology | |
| Arteriovenous malformation | 19 (51.4%) |
| Cavernous angioma | 4 (10.8%) |
| Aneurysm | 3 (8.1%) |
| Arteriovenous fistula | 1 (2.7%) |
| Developmental venous anomaly | 1 (2.7%) |
| Coagulopathy | 1 (2.7%) |
| Unknown | 8 (21.6%) |
| ABC//XYZ, median (IQR, range) | 1.2% (0.4–2.7%, 0.006–6.5%) |
N=number, IQR=interquartile range, ICH=intracerebral hemorrhage, IPH=intraparenchymal hemorrhage, IVH=intraventricular hemorrhage.
At 3-month follow-up, median total PSOM score was 2 (IQR 0.5–3, range 0–6), and 26 children (70.3%) had a total PSOM ≥1 (poor neurological outcome). At 12-month follow-up, median total PSOM score was 0.75 (IQR 0.5–3, range 0–5), and 15 children (50%) had a total PSOM ≥1. There was no significant difference in the 3-month total PSOM scores of the children who did and did not have 12-month follow-up (p=0.48, Wilcoxon-rank sum).
At 3 months, 12 (32.4%) had an IEP. At 12 months, 17 (56.7%) had an IEP and 1 had a 504 plan. Twenty-five children (67.6%) had educational modifications at 3 months, and 16 (53.3%) had educational modifications at 12 months. Table 2 describes the subjects’ educational placement. Of 30 children with both 3-month and 12-month follow-up, 14 children (46.7%) improved their education status, 13 children (43.3%) remained at the same education level, and 3 children (10%) began to receive in-class services.
Table 2.
Educational Placement at 3 Months (N=37) and 12 Months (N=30) after ICH.
| Educational Placement | 3 Months | 12 Months |
|---|---|---|
| N (%) | N (%) | |
| Regular classes | 12 (32.4%) | 14 (46.7%) |
| In-class services | 9 (24.3%) | 12 (40%) |
| Below grade level | 1 (2.7%) | 3 (10%) |
| Home based | 8 (21.6%) | 1 (3.3%) |
| Not attending | 7 (19%) | 0 (0%) |
| Accommodation plan | ||
| IEP | 12 (32.4%) | 17 (56.7%) |
| 504 plan | 0 (0%) | 1 (3.3%) |
| None | 23 (62.2%) | 12 (40%) |
| Unknown | 2 (5.4%) | 0 (0%) |
N=Number, IEP=Individualized Education Plan.
Greater deficits on the PSOM correlated with the need for educational modifications at 3 months and 12 months post-ICH. At 3 months, 4 of 26 (15.4%) with a total PSOM ≥1 and 8 of 11 (72.7%) with total PSOM <1 were enrolled in an age-appropriate grade level school program (p=0.001, Fisher’s exact). At 3 months, for every additional 1 point on the total PSOM score, the odds of requiring educational modifications increased 3.3-fold [95% confidence interval (CI) 1.4–7.9, p=0.007]. Intraparenchymal hemorrhage volume as a percent of total brain volume was not associated with the need for educational modifications at 3 months.
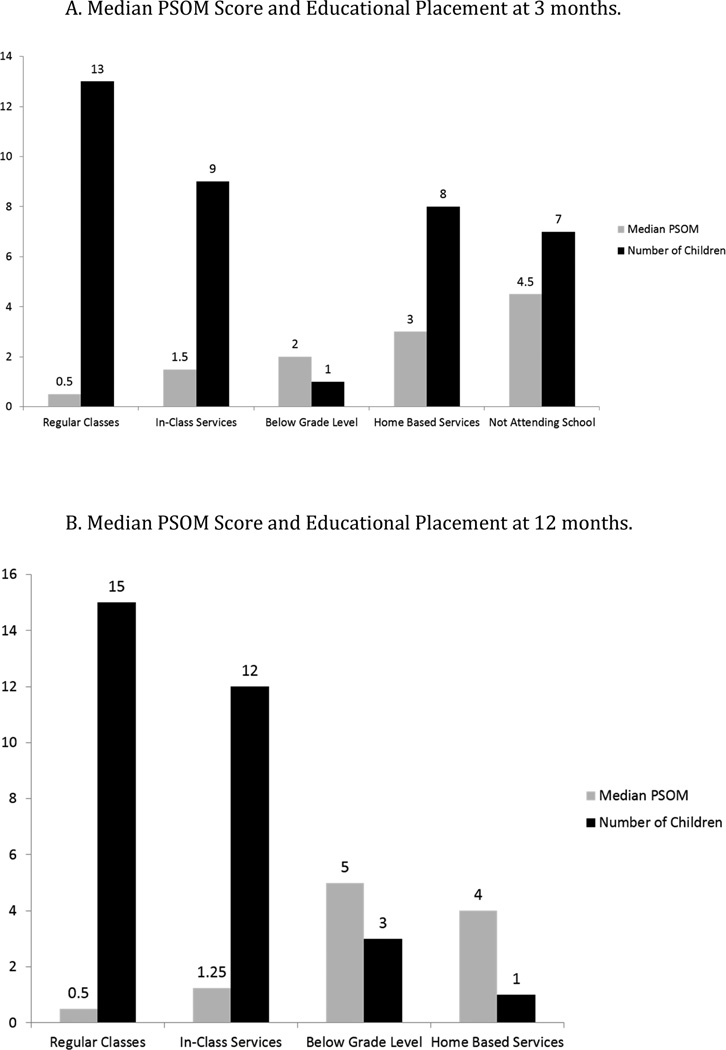
At 12 months, 4 of 15 (26.7%) with total PSOM ≥1 and 10 of 15 (66.7%) with total PSOM <1 were enrolled in an age-appropriate grade level school program (p=0.066, Fisher’s exact). At 12 months, for every additional 1 point on the total PSOM score, the odds of requiring educational modifications increased 2.1-fold [95% CI 1.1–3.9, p=0.025]. Figure 1 shows the median PSOM score for each educational placement level at 3 months (A) and at 12 months (B). Tables 3 and 4 show the exploratory analyses that examine educational placement by PSOM category at 3 months and 12 months after ICH, respectively. Intraparenchymal hemorrhage volume as a percent of total brain volume was not associated with the need for educational modifications at 12 months.
Figure 1.
A. Median PSOM Score and Educational Placement at 3 months.
B. Median PSOM Score and Educational Placement at 12 months.
Table 3.
Educational Modifications by PSOM Subscale Category 3 Months after ICH.
| PSOM Subscale | Neurologic Function Poor* |
Neurologic Function Good |
P-value± | ||
|---|---|---|---|---|---|
| Educational Modifications | |||||
| Present | Absent | Present | Absent | ||
| Cognitive/behavioral | 11/13 | 2/13 | 14/24 | 10/24 | 0.15 |
| Language | 12/14 | 2/14 | 13/23 | 10/23 | 0.08 |
| Sensorimotor | 18/19 | 1/19 | 7/18 | 11/18 | <0.001 |
PSOM=Pediatric Stroke Outcome Measure.
Poor neurological function defined at PSOM subscale score ≥1 for specific function (moderate or severe disability). 8/14 with poor language function had scores >0 on the total of the expressive language and the receptive language subscales. 3/19 with poor sensorimotor function had scores >0 on sensorimotor right and sensorimotor left.
Fisher’s exact test.
Table 4.
Educational Modifications by PSOM Subscale Category 12 Months after ICH.
| PSOM Subscale | Neurologic Function Poor* |
Neurologic Function Good |
P-value± | ||
|---|---|---|---|---|---|
| Educational Modifications | |||||
| Present | Absent | Present | Absent | ||
| Cognitive/behavioral | 8/12 | 4/12 | 8/18 | 10/18 | 0.28 |
| Language | 8/9 | 1/9 | 8/21 | 13/21 | 0.017 |
| Sensorimotor | 7/8 | 1/8 | 9/22 | 13/22 | 0.039 |
PSOM=Pediatric Stroke Outcome Measure.
Poor neurological function defined at PSOM subscale score ≥1 for specific function (moderate or severe disability). 5/9 with poor language function had scores >0 on the total of the expressive language and the receptive language subscales. 1/8 with poor sensorimotor function had scores >0 on sensorimotor right and sensorimotor left.
Fisher’s exact test.
Discussion
In this three-center prospective cohort of children with ICH, while almost all children were able to attend school after ICH, we found that at 3 months, nearly 70% of children had educational modifications. Many children’s educational needs decreased between 3 and 12 months after ICH, but at 12 months after ICH, over half still required modifications to their school programs. These results are similar to a study of children from the United Kingdom with arterial ischemic stroke in which almost 60% were on a state special needs register at 6 months after the stroke.5 To our knowledge, there are no previous descriptions of school placement after pediatric ICH; however, most pediatric quality of life and health status measures include domains that relate to school because school is an integral part of most children’s lives. While some groups have found that school-related quality of life is not diminished after childhood stroke12, other groups have reported decreased school-related quality of life.5,11,13,14 O’Keefe et al found that school-related quality of life was the lowest rated domain by childhood arterial ischemic stroke survivors, their parents, and their teachers.5 Similarly, Friefeld et al found that among 100 children with ischemic stroke, both parents and children rated the school function domain the poorest.13 The strongest predictor of the social domain and school function domains of the quality of life measure was the cognitive/behavioral subscale of the PSOM. In a separate study of over 100 children with ischemic stroke, nearly one third had poor quality life in the “becoming” domain, which includes productive capabilities that are required for school performance. The lowest score on the quality of life measure was that for school and play.11 Comparisons of these quality of life studies5,11,13,14 with the current study are limited because the current study did not directly measure qualify of life. However, the finding that so many children require educational modifications, which is in consistent with studies in pediatric ischemic stroke5, may be related to poor school-related quality of life found in several pediatric stroke studies5,11,13,14.
In the present study, increasing scores on the PSOM were associated with the need for educational modifications. In exploratory analyses, poor outcome (PSOM ≥1) on the sensorimotor subscale of the PSOM was associated with educational modifications at both 3 months and 12 months. Smith et al reported that among 59 children with unilateral stroke of whom 12 had ICH, children with hemiparesis had worse parent-reported and child-reported health status on the school scale of the PedsQL Cerebral Palsy Module compared with children who did not have hemiparesis.6 While the current study cannot be directly compared to that of Smith et al because health status was not measured, it is possible that difficulties with motor functions that affect writing or typing contribute to both worse school-related quality of life and the need for educational modifications, at least in some children. Poor outcome on the combined expressive and receptive language subscale was associated with educational modifications at 12 months. Surprisingly, poor outcome on the cognitive/behavioral subscale of the PSOM was not associated with the need for educational modifications at 3 or 12 months. While it is possible that the power to find an association was different for the cognitive/behavioral subscale than for the language or sensorimotor subscale in which two categories were collapsed, the majority of the children with poor sensorimotor outcome had only unilateral deficits so collapsing the right and left motor scores should not have increased the PSOM subscale for most children with poor outcome on this measure.
There are several limitations to this study. Systematic, formal neuropsychological or cognitive testing was not available in this cohort, so it was not possible to describe the cognitive domains affected by ICH or to explore which types of deficits might most closely relate to school modifications. Additionally, no standardized quality of life assessments were performed. Therefore, we were unable to assess whether the needs for educational modifications or the degree of educational modifications needed were correlated with school-related quality of life measures. Furthermore, educational placement was based on parental report and was in most cases not independently confirmed by the study neurologists. Children were enrolled in many school districts in 3 states, so there was likely variable implementation of educational modifications based on resources available in various geographic regions, differences in school staff expertise in recognizing cognitive deficits, and uncertainties about the persistence of deficits. Thus the association of the PSOM and school placement may be influenced by non-medical factors that were not assessed in this study. While ICH volume was not associated with educational modifications in this sample, the ability to examine this factor is limited by sample size and by the potential variability in which educational services are implemented. Despite its limitations, we believe that this work is helpful because it reflects the educational needs of children with ICH in clinical practice. Additional work is needed to understand the long-term learning difficulties that children with ICH face and to quantify these children’s cognitive and neuropsychological profiles. Future studies should attempt to gather information on neurological function (e.g., PSOM scores), educational placement and modifications, and health status and quality of life measure in order to characterize outcomes of children with spontaneous ICH fully. Also, future studies with a larger sample should examine whether the discharge PSOM or 3-month PSOM is associated with educational modifications at 12 months.
This study highlights the need for educational services for children with intracerebral hemorrhage. While rehabilitation programs often center on motor and language function, attention must also be placed on careful assessments of educational needs and reintegration into the school environment. A partnership among medical professionals, parents, and educators is likely required.
Acknowledgments
This work was funded by NINDS K23-NS062110 (LCJ). DJL is supported by grants from the NIH (1R01NS072338, RO1NS060653, UO1HD087180) and by the Steve and June Wolfson Family Foundation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Fullerton HJ, Wu YW, Zhao S, Johnston SC. Risk of stroke in children: ethnic and gender disparities. Neurology. 2003;61(2):189–194. doi: 10.1212/01.wnl.0000078894.79866.95. [DOI] [PubMed] [Google Scholar]
- 2.Giroud M, Lemesle M, Gouyon JB, Nivelon JL, Milan C, Dumas R. Cerebrovascular disease in children under 16 years of age in the city of Dijon, France: a study of incidence and clinical features from 1985 to 1993. Journal of clinical epidemiology. 1995;48(11):1343–1348. doi: 10.1016/0895-4356(95)00039-9. [DOI] [PubMed] [Google Scholar]
- 3.Blom I, De Schryver EL, Kappelle LJ, Rinkel GJ, Jennekens-Schinkel A, Peters AC. Prognosis of haemorrhagic stroke in childhood: a long-term follow-up study. Developmental medicine and child neurology. 2003;45(4):233–239. doi: 10.1017/s001216220300046x. [DOI] [PubMed] [Google Scholar]
- 4.Beslow LA, Licht DJ, Smith SE, et al. Predictors of outcome in childhood intracerebral hemorrhage: a prospective consecutive cohort study. Stroke. 2010;41(2):313–318. doi: 10.1161/STROKEAHA.109.568071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.O'Keeffe F, Ganesan V, King J, Murphy T. Quality-of-life and psychosocial outcome following childhood arterial ischaemic stroke. Brain injury. 2012;26(9):1072–1083. doi: 10.3109/02699052.2012.661117. [DOI] [PubMed] [Google Scholar]
- 6.Smith SE, Vargas G, Cucchiara AJ, Zelonis SJ, Beslow LA. Hemiparesis and epilepsy are associated with worse reported health status following unilateral stroke in children. Pediatric neurology. 2015;52(4):428–434. doi: 10.1016/j.pediatrneurol.2014.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kitchen L, Westmacott R, Friefeld S, et al. The pediatric stroke outcome measure: a validation and reliability study. Stroke. 2012;43(6):1602–1608. doi: 10.1161/STROKEAHA.111.639583. [DOI] [PubMed] [Google Scholar]
- 8.Beslow LA, Ichord RN, Kasner SE, et al. ABC/XYZ estimates intracerebral hemorrhage volume as a percent of total brain volume in children. Stroke. 2010;41(4):691–694. doi: 10.1161/STROKEAHA.109.566430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kleinman JT, Hillis AE, Jordan LC. ABC/2: estimating intracerebral haemorrhage volume and total brain volume, and predicting outcome in children. Dev Med Child Neurol. 2011;53(3):281–284. doi: 10.1111/j.1469-8749.2010.03798.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.deVeber GA, MacGregor D, Curtis R, Mayank S. Neurologic outcome in survivors of childhood arterial ischemic stroke and sinovenous thrombosis. J Child Neurol. 2000;15(5):316–324. doi: 10.1177/088307380001500508. [DOI] [PubMed] [Google Scholar]
- 11.Friefeld SJ, Westmacott R, Macgregor D, Deveber GA. Predictors of quality of life in pediatric survivors of arterial ischemic stroke and cerebral sinovenous thrombosis. J Child Neurol. 2011;26(9):1186–1192. doi: 10.1177/0883073811408609. [DOI] [PubMed] [Google Scholar]
- 12.Neuner B, von Mackensen S, Krumpel A, et al. Health-related quality of life in children and adolescents with stroke, self-reports, and parent/proxies reports: cross-sectional investigation. Annals of neurology. 2011;70(1):70–78. doi: 10.1002/ana.22381. [DOI] [PubMed] [Google Scholar]
- 13.Friefeld S, Yeboah O, Jones JE, deVeber G. Health-related quality of life and its relationship to neurological outcome in child survivors of stroke. CNS spectrums. 2004;9(6):465–475. doi: 10.1017/s1092852900009500. [DOI] [PubMed] [Google Scholar]
- 14.Gordon AL, Ganesan V, Towell A, Kirkham FJ. Functional outcome following stroke in children. J Child Neurol. 2002;17(6):429–434. doi: 10.1177/088307380201700606. [DOI] [PubMed] [Google Scholar]