Abstract
Blood vessels form a highly branched, interconnected and largely stereotyped network of tubes that sustains every organ and tissue in vertebrates. How vessels come to take on their particular architecture, or how they are ‘patterned’, and in turn, how they influence surrounding tissues are fundamental questions of organogenesis. Decades of work have begun to elucidate how endothelial progenitors arise and home to precise locations within tissues, integrating attractive and repulsive cues to build vessels where they are needed. Conversely, more recent findings have revealed an exciting facet of blood vessel interaction with tissues, where vascular cells provide signals to developing organs and progenitors therein. Here, we discuss the exchange of reciprocal signals between endothelial cells (ECs) and neighboring tissues during embryogenesis, with a special focus on the developing pancreas. Understanding the mechanisms driving both sides of these interactions will be crucial to the development of therapies, from improving organ regeneration to efficient production of cell based therapies. Specifically, elucidating the interface of the vasculature with pancreatic lineages, including endocrine cells, will instruct approaches such as generation of replacement beta cells for Type I diabetes.
Keywords: Blood vessel, endothelial cell, organogenesis, patterning, guidance cues, VEGF, pancreas, beta cells, angiocrine
INTRODUCTION
In recent decades, there has been a conceptual shift regarding the relationship between blood vessels and surrounding tissues, both during development and tissue homeostasis. Vessels have been understood to dynamically exchange reciprocal signals with the tissues they perfuse. On the one hand, they receive paracrine cues from tissues that shape the vascular architecture; on the other hand, they provide signals to the surrounding tissue that support growth or homeostasis. The former process is termed vascular patterning and describes how combined signals from the microenvironment guide endothelial progenitors to assemble into vessels at stereotyped locations. In the latter process, blood vessel-derived signals impact different tissues in different ways, including recently identified factors that regulate progenitor cell renewal and differentiation. Mounting evidence posits that these endothelial cell (EC) signals influence adult neurogenesis, osteogenesis, hematopoiesis and further, organogenesis during embryonic development. Therefore, endothelial-tissue crosstalk is an important conversation whereby tissues coordinately grow along with their vasculature (Figure 1).
Figure 1. EC-Tissue crosstalk.
ECs of a vessel dynamically communicate with surrounding tissues. Tissues provide positive and negative patterning cues, such as VEGF or Semaphorins (respectively), which influence EC migration and thereby shape the vasculature. ECs, in turn, provide signals to tissues regulating their growth and homeostasis, which remain largely unknown.
In this review, we discuss both sets of signals: those to and those from blood vessels. We examine how these signals sculpt vessels over the course of embryonic development and into adulthood, as well as how local progenitor niches are sustained by endothelial signals. First, we cover basics of vascular development, and describe formation of the first blood vessels in the embryo. Next, we examine how stereotypy in vascular development is achieved, and bring a set of vascular patterning cues into focus. We also briefly describe vascularization of tissues and organs during embryogenesis, with a special focus on the pancreas. Then, we review how ECs regulate organogenesis or progenitor cell behavior, and consider the candidate EC signals. We further discuss the development of morphological and molecular heterogeneity within the vascular system and its implications for local signaling during organogenesis. Finally, we cover the importance of ECs in regenerative therapies and discuss what we can learn from developmental studies within this context.
DEVELOPING VASCULATURE: PLEXUS TO HIERARCHICAL TREE
Development of the vascular system is initiated early during embryogenesis to enable nutrition and waste removal. This function is crucial for tissues as they grow, when simple diffusion becomes no longer sufficient. Without proper vascular development, murine embryonic development is arrested at embryonic day (E) 10 (E10)1. Therefore, establishment of the vascular system is a key event during embryonic development, where vessels form and adapt to meet local needs of tissues.
Vasculogenesis: From angioblasts to endothelial cells
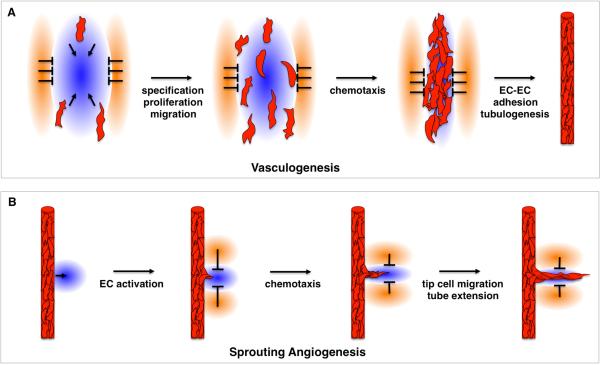
Blood vessels form via a step-wise series of events. Initial embryonic vessels form via vasculogenesis, whereby a simple network connecting the extraembryonic yolk sac and embryonic vessels arises. During this process, angioblasts emerge de novo from the mesoderm, initially as scattered, individual cells. These endothelial progenitors are migratory, amoeboid-like and express the Vascular Endothelial Growth Factor (VEGF) Receptor VEGFR2 (or FLK1), in addition to TIE2 and SCL/TAL1 2. Angioblasts encounter, recognize and adhere to each other, forming lumen-less linear aggregates, called cords. Cords presage functional vessels. As the heart begins to beat, cords almost simultaneously begin to undergo morphogenesis and open central lumens, starting around the heart region. They then progressively open lumens, which come to connect, allowing circulation of blood. Notably, the opening of lumens is not dependent on cardiac function. Once recruited to the vessel wall, angioblasts differentiate into bona fide ECs1.
Vascular patterning: Corralling angioblasts into cords
Formation of vessels at the right times and places, or proper patterning, depends on directed migration and assembly of angioblasts into cords. We have come to understand that this is a tightly regulated process. Early angioblasts are highly motile and respond to cues that dictate their migration. Many of these cues are positive, or attractive, including widely expressed secreted factors, such as VEGF-A and FGF-2 (also called angiogenic factors). Interestingly, other factors do the opposite and repulse angioblasts, directing them away from zones where they are expressed. Together, positive and negative cues corral angioblasts, promoting their aggregation and assembly into vessels at highly reproducible locations3, 4. Investigating where these cues are active, as well as what their cellular effects are on EC precursors, will help us understand how blood vessels form where they do.
Plexus elaboration: Sprouting and remodeling angiogenesis
While the initial system of vessels is established via vasculogenesis, extension of this plexus happens via angiogenesis. This process is the development of new vessels from existing ones. In sprouting angiogenesis, nascent sprouts initiate and branch from an existing vessel into avascular regions. Interestingly, leading ‘tip’ cells of a sprout (cells at the sprout tip that display filopodia and migratory behavior) respond to guidance cues in a manner similar to migrating angioblasts. New vessels can also arise via splitting of a large vessel called intussusceptive angiogenesis, or by coalescence of many smaller vessels into a larger one. In addition, vascular beds can change conformation, with vessels increasing or decreasing in diameter. Vessels can even regress by movement of ECs and disappear altogether. Collectively, these later processes that alter the existing pattern of vessels are called angiogenic remodeling1.
Remodeling angiogenesis generally occurs when the vasculature is still malleable, and is under hemodynamic pressure due to increasing blood flow. As circulation initiates, ECs of a vessel experience shear and laminar stress along their luminal surfaces5. These physical forces have been shown to remodel vessels in many different contexts, including in the developing yolk sac, heart and aortic arches1. In vitro studies point to mechano-transduction pathways as the underlying mechanism, which affect EC migration, proliferation and apoptosis6-8. In particular, hemodynamic forces have been shown to alter the expression of several genes, including those controlling arteriovenous fate, such as Notch19. Through the combined processes of vasculogenesis, lumenogenesis, sprouting and remodeling angiogenesis, the embryonic vasculature is sculpted and adapted into a functional system across the early embryo.
Vessel maturation: Mural cell recruitment
As blood vessels become established, their growth slows, and they mature into largely quiescent structures. A key step in this maturation is the recruitment of supporting mural cells (MCs), such as smooth muscle cells (SMCs) or pericytes. ECs recruit MC precursors primarily through secretion of PDGFB (Platelet-Derived Growth Factor B), which binds to its receptors, PDGFRα and PDGFRβ, on MCs. The resulting cascade of events leads to MC differentiation as well as deposition of extracellular matrix (ECM) by MCs, and thus to structural stabilization of the vessel. Concomitantly, direct EC-MC contact and the produced ECM at their interface, together promote vessel lumen maintenance. Altogether, these generate multiple cellular and matrix layers around the endothelium, stabilizing and strengthening blood vessels. Importantly, the type of recruited MCs and the signaling pathways governing EC-MC communication differ strikingly across vessel types1. This variety of cellular and extracellular components directly contributes to the heterogeneity observed across ECs during vascular development.
VASCULAR PATTERNING: POSITIVE AND NEGATIVE CUES
Blood vessels build an extensive network throughout developing tissues that is remarkably similar between individuals of the same species. How and why this occurs are central questions in vascular biology. Studies in recent decades have shown that blood vessel formation and final architecture are not haphazard. Indeed, vessels develop at genetically hard-wired locations, achieved through guidance cues. These cues are secreted by the microenvironment and impact EC migration, both during de novo vasculogenesis and sprouting angiogenesis3, 4. Integration of attractive and repulsive signaling guides each migrating angioblast and emerging sprout (Figure 2). The resulting patterns are further refined, as vessels experience blood flow. Together, these systems ensure vessel network conformations adapted to carry out optimal circulatory function. Described below are selected chemo-attractive and -repulsive factors, as well as their individual influences on vascular patterning.
Figure 2. Basic principles of cue integration during vessel formation and patterning.
(A) In de novo vasculogenesis, angioblasts migrate and proliferate in a directed manner following their specification. They migrate towards sources of attractive cues (blue) and away from those of negative cues (orange). As a result, they encounter each other, coalesce and assemble into cords at genetically determined locations. They then undergo tubulogenesis to form a functional vessel. (B) Sprouting angiogenesis is similarly governed by attractive and repulsive cues, which direct migration of the activated ECs at the tip of the emerging sprout. This way, a new vessel arises from an existing one at specific genetically determined locations.
Attractive factors
FGF2
One of the first angiogenic signals identified was a Fibroblast Growth Factor, or FGF. FGF family members are expressed throughout embryonic tissues in distinct patterns. While these members drive a variety of cellular events in different organs, the family has been linked to enhancement of endothelial fate as well as migration. FGF2 is expressed ubiquitously in the embryo, and was found to have marked effects on ECs. Addition of FGF2 to a growth factor-rich medium increased de novo angioblast emergence from both cultured quail blastodiscs and differentiating mouse embryonic stem cells (ESCs)10, 11. These findings suggested that it functions during angioblast specification. However, it was also shown to act as a chemotactic cue. When a pellet of cells expressing FGF2 was implanted into the avascular mouse cornea, ingrowth of vessels and angiogenic sprouting were stimulated12. Thus, FGF2 can act as a potent attractant for ECs during vascular patterning in vivo.
VEGF-A
Vascular Endothelial Growth Factor A (VEGF-A) has been known to have profound effects on blood vessel biology and has therefore attracted a dizzying amount of attention, with over 50 thousand publications (http://www.ncbi.nlm.nih.gov/pubmed/). VEGF-A is produced in 4 different isoforms with differing binding characteristics to VEGFR2, a receptor tyrosine kinase largely specific to ECs. Beyond regulating EC specification, proliferation, differentiation, survival and migration, as well as vascular permeability, VEGF-A is also a well-known angiogenic factor. In many instances, localized VEGF-A expression within a tissue will direct vessel growth in response to hypoxia13-16. Its ectopic expression leads to aberrant vascular patterning and hyper-vascularization17, 18. Conversely, Vegf-a null embryos display defects in EC differentiation, vessel tubulogenesis, sprouting angiogenesis and vascular patterning. The severe vascular phenotype following loss of a single Vegf-a allele underlines the importance of its tight regulation during vascular development19, 20. These studies highlight the central role of VEGF-A in vascular patterning.
Bmps
The Bone Morphogenetic Protein (BMP) family of ligands is known to influence the development of multiple organs, including blood vessels. Interestingly, opposing vascular roles have been ascribed to different members of this family. BMP2/4 were identified as attractive factors for vessel patterning in chick embryos21. This study showed that Bmp antagonists, CHORDIN and NOGGIN, are secreted from the notochord during dorsal aorta formation, initially inhibiting angioblast migration to the midline. Bmp inhibition is thus necessary to establish an avascular zone that keeps the aortic primordia as separate tubes during early embryogenesis. Later, as expression of the antagonists is extinguished, the parallel aortae fuse into a single tube that carries blood from the heart to peripheral tissues. By contrast, BMP9/10 have been shown to promote vascular quiescence via ALK1 receptors22, 23. Recent studies showed that while Bmp9 null mice exhibit no vascular defects, injection of BMP9/10 blocking antibodies impaired retinal angiogenic sprouting and remodeling. These findings imply functional redundancy and a positive role for these members during vessel growth24.
CXCL12
CXCL12-CXCR4 is another signaling axis with a significant impact on endothelial patterning. Ablation of either Cxcl12, or its receptor Cxcr4, in mouse results in aberrant alignment of vessels, as well as in arterial differentiation defects25. Signaling is likely critical in endothelial ‘tip’ cells (cells that lead growing vascular sprouts), as CXCR4 is highly enriched in a position to transmit cues to growing vessels26. Interestingly, this signaling has proved important in organ vascularization. Vascular patterning is abnormal in the absence of either factor in the intestine: Veins are present, but arteries absent, along with a failure of EC filopodial formation27. Similarly, CXCL12-expressing podocytes and/or stromal cells direct patterning of ECs through CXCR4 and the more recently identified receptor CXCR7 for vascularization of the mammalian kidney28. Ablation of the ligand or either receptor caused disorganization of the renal vasculature and ballooning of the glomerular tufts29. Signaling through these molecules also direct vascular patterning in the skin25.
Repulsive factors
Bmp antagonists
The notochord has been established as a source of negative cues critical for formation of nearby vessels. Specifically, the notochord has been shown to restrain and thereby pattern the first vertebrate embryonic vessels, the paired dorsal aortae. As explained above, the Bmp antagonists, NOGGIN and CHORDIN, are among the identified notochord-provided repulsive cues21. This principle was demonstrated to be conserved in mouse, where the notochord-less Foxh1 and Foxa2 mutants show dorsal aortae patterning defects similar to those seen in chick embryos. Namely, angioblasts inappropriately appear within normally avascular regions and aortic tubulogenesis fails. The murine notochord similarly expresses repulsive cues, including Bmp antagonists30.
Semaphorins and the PLEXIND1 receptor
Semaphorins have also been shown to act as repulsive cues for ECs. These findings follow a long series of studies showing their roles in neuronal axon growth cone repulsion via collapse of the cytoskeleton31, 32. Interestingly, mouse aorta formation proved particularly sensitive to the absence of SEMA3E. Sema3e null embryos form a disorganized plexus rather than two smooth parallel tubes, despite the presence of notochord-provided repulsive cues discussed above30. SEMA3E was shown to act via PLEXIND1, as PlexinD1 nulls exhibit identical defects33. Other Semaphorins appear to carry out similar functions during vascular patterning. SEMA3A acts as a chemo-repellant for ECs in the developing kidney glomerulus and SEMA3D is similarly repulsive to pulmonary venous ECs 34, 35.
Slits and the ROBO4 receptor
Like the previous families known to inhibit both neuronal and endothelial cell migration, the Slit-Roundabout (ROBO) signaling has also been implicated in vascular patterning36. Out of the 4 Robo receptors identified, ROBO4 is expressed specifically on ECs and ROBO1 has been found in cultured ECs37. Gene ablation studies suggest that ROBO4 promotes vascular stability by counteracting VEGF signaling and angiogenesis 38. In line with this, morpholino disruption of ROBO4 in zebrafish leads to defects in intersomitic vessel sprouting39. Generally, the Slit ligands have been shown to bind and activate Robos, although it is unclear if this is the case for ROBO4. Binding assays have yielded mixed results and additional studies will be required to clarify the molecular pathway40. Slit3 null mice, however, do show reduced vascular branching, suggesting involvement of this family member41.
Netrins and the UNC5B receptor
Netrins represent one more family of repulsive neuronal/endothelial guidance cues 4. UNC5B is a vascular-specific receptor known to bind Netrins, expressed in arteries as well as angiogenic sprouts42, 43. Unc5b null mice display ectopic sprouting and increased vascular density, demonstrating the role of this receptor in vascular patterning42. Similar to the cytoskeletal role of Semaphorin-Plexin signaling, activation of UNC5B causes collapse of EC tip cell lamellipodia, thereby negatively regulating vascular branching44. However, which Netrin acts on this receptor remains unknown. Netrin-1 null mice do not phenocopy mice lacking Unc5b, suggesting either no involvement of this ligand or possible functional redundancy45. Therefore, while UNC5B is understood to mediate EC repulsion, ablation of other Netrins is needed to identify the mechanism of its action.
While we have described here a limited number of cues responsible for patterning the vasculature, it is highly likely that many remain unidentified. Understanding embryonic and organ vascular patterning will require elucidation of the full range of molecules that affect angioblast and tip cell migration during vessel growth.
VASCULARIZATION OF EMBRYONIC ORGANS
Coordinated development
As organs grow in size, so does their vasculature. Generally, blood vessels emerge within the mesoderm, assemble and expand coordinately with organs during organogenesis. In the case of many endodermally derived tree-like organs, such as the lung, pancreas, salivary gland and thyroid, a vascular plexus becomes integrated and remodeled as the organ epithelium branches. Often, ramifying organs will develop co-aligned arterial and venous networks, which follow nearly every branch, large and small. While this co-development remains poorly understood, it is believed that embryonic organs acquire their blood vessels via a combination of peripheral vasculogenesis and angiogenesis. Local vasculogenic ECs differentiate from newly expanding mesoderm, while hypoxic regions within tissues recruit outgrowth of angiogenic ECs from existing vessels46. In either case, new vessels often form juxtaposed to expanding organs47. The question is: How does this occur and how functionally relevant is it?
Patterning cues in organs
Our understanding of how blood vessels and nascent organs grow coordinately is remarkably limited. While the gross anatomy of the developing as well as final vasculature in most organs is generally well-described, we lag in elucidating the intricate patterns of signals that direct stereotyped vessel formation. Many studies have only just begun to identify deviations from normal vessel development in organs, such as lung and kidney, in select mutant mice48-52.
An emerging general theme is that positive patterning cues expressed within tissues help attract and guide ECs to assemble into vessels throughout forming organs. Indeed, many studies have shown that epithelium- or mesenchyme-derived guidance cues, like VEGF-A (Figure 3), are key signals in laying down stereotyped vasculature47. Conversely, EC-derived growth factors, such as Hepatocyte Growth Factor (HGF), are thought to communicate back to cells within growing organs53. These reciprocal interactions, combined with locally expressed common factors that regulate both epithelial and vascular branching, hold the key to coordinated growth. Common factors include the EC guidance signals, Robo/Slits, Netrins, Semaphorins, and Ephrins, as well as growth factor families like Wnts, Bmps and Hedgehogs54,55. For instance, SEMA3A has been shown to inhibit both epithelial and endothelial growth in the kidney56. Altogether, local expression of these factors sets the stage for establishment of organ-specific vascular beds. However, it remains a challenge to map the full landscape of where exactly each cue is expressed, and how overlapping expression fields are regulated, to ensure spatiotemporal precision of coordinated organ and vascular development.
Figure 3. Vascularization of organs.
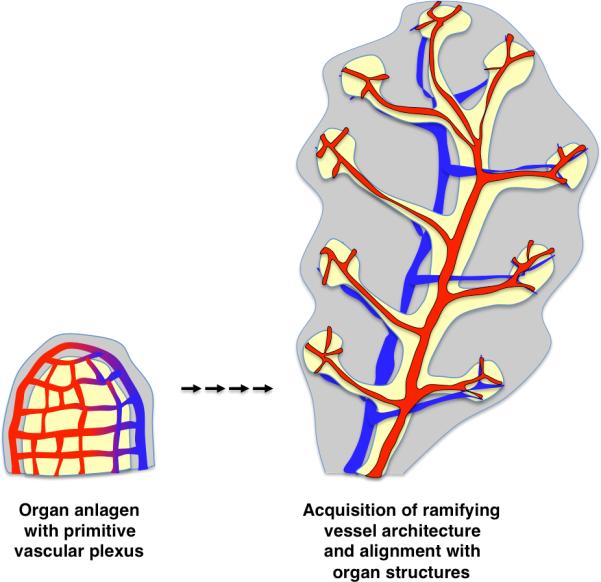
Blood vessels emerging in the mesoderm form an initial net-like plexus around the organ primordium. As development proceeds and organs grow in size, the vasculature is remodeled coordinately. Branching organs of endodermal origin, like pancreas and lung, co-develop epithelial branches with arteriovenous networks. In these organs, arteries and veins align with nearly every branch, both large and small.
Patterning of the vasculature in pancreas
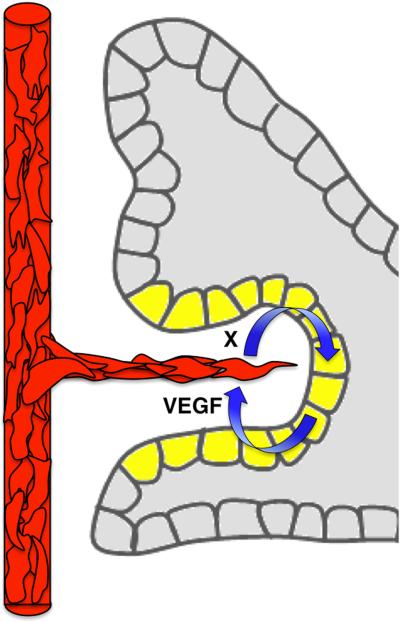
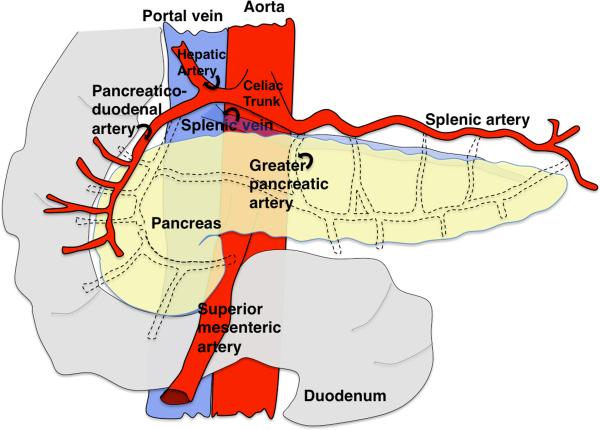
The pancreatic vasculature supplies nutrition and gas exchange to the tree-like gland. In the adult human pancreas, the final architecture of the vasculature has been well characterized (Figure 4). Blood enters the pancreas via two sources of splanchnic arteries, superior mesenteric arteries at the head of the organ and splenic arteries feeding the rest, and then drains into the portal vein via the splenic vein. Within individual branches of the pancreatic gland, arteries and veins run in parallel and in close proximity to major ducts, creating co-aligned tubes. The largest vessels are within central regions of the gland, while the progressively finer microvasculature envelops the terminal acini. Additionally, the endocrine islets, islets of Langerhans, display densely packed capillaries amongst the hormone secreting endocrine cells.
Figure 4. Stereotyped architecture of adult human pancreatic vasculature.
Simplified schematic of major blood vessels in the mature pancreas. Two sources of splanchnic arteries supply blood to the pancreas. The head of the pancreas, derived from the dorsal bud, receives blood from the superior mesenteric arteries. The rest of the pancreas, namely the neck, body and tail, are supplied by the splenic arteries. Blood is then drained via the splenic vein into the portal vein. Blue, veins; Red, arteries.
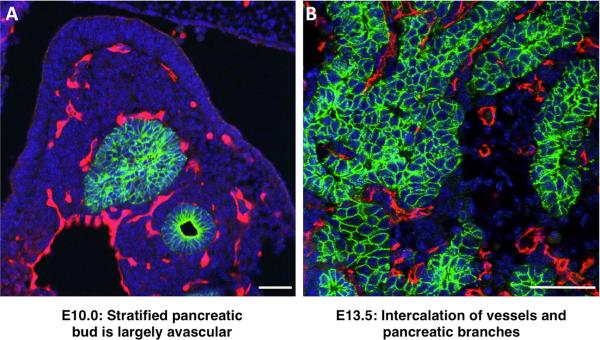
How does this complex architecture of pancreatic vasculature form? We know that vascular progenitors emerge within the pancreatic mesenchyme that surrounds and sustains the branching epithelium. We also know that as epithelial branches grow, they extend between blood vessels (Figure 5). Subsequently, vessels become larger and denser around the central, endocrine-producing areas, but are more sparse around the distal exocrine acini57. Finally, we know that misregulation of vascular development in the pancreas leads to aberrant organ development (discussed in further detail below). Strikingly, however, beyond these basic facts, the signals that drive blood vessels to populate different areas remain completely unknown.
Figure 5. Vascularization of the pancreas during development.
Sections through embryonic mouse pancreas at stages indicated. Blue, DAPI; Green, pancreatic epithelium stained for E-cadherin; Red, blood vessels stained for PECAM. (A) Angioblasts and early vessels arise in the mesoderm which surrounds the pancreatic epithelium. The stratified pancreatic epithelium is initially avascular (E10.0). (B) As the pancreas branches, the mesodermal mesenchyme containing blood vessels associates with the epithelium. ECs become densely packed around the trunk, while tips are relatively devoid of vasculature. Scale bars, 50μm.
To date, no reports have yet examined possible contribution of the endothelial cues and receptors outlined above in the developing pancreas. Only a handful of studies report pancreas-specific manipulation of angiogenic factors, specifically VEGF-A, which leads to effects on acinar and endocrine differentiation, as well as impaired islet vascularization and endothelial fenestration57-59. The field clearly lacks a careful description of how blood vessels emerge and become patterned in the normal pancreas, where major vessels are located, and how they remodel during development. Mesenchymal- or epithelial-specific ablation of known vascular cues, such as the ones listed above, will be crucial in elucidation of the molecular underpinnings.
PARACRINE SIGNALS FROM BLOOD VESSELS
General
Blood vessels grow coordinately with developing tissues, as discussed above. During this coordinated growth, ECs are in a temporally evolving relationship with resident cells, including tissue progenitor cells (PCs). In fact, reciprocal signaling between ECs and organ-specific cell types has been demonstrated in many tissues15, 57, 60-70. However, the underlying molecular mechanisms remain largely unknown.
Identifying paracrine roles for ECs has been challenging due to our inability to uncouple circulation from bona fide endothelial-derived signals. Nonetheless, combinations of ex vivo and in vivo studies have vastly increased our understanding of EC-resident tissue interactions. These studies largely made use of VEGF-A, with gain- or loss-of-function approaches, to manipulate blood vessel growth, in order to assess how organ development is affected by a perturbed endothelium. Generally speaking, ECs appear to influence organogenesis or local progenitor expansion and/or differentiation in a number of systems tested.
Nervous system
EC-tissue interactions have been relatively well-characterized in the nervous system. In many instances, neural structures develop in striking alignment with blood vessels. It has long been noted that ECs are, in fact, very close to the germinal zones of the central nervous system (CNS) where cerebellar cells are produced71, 72. Further investigations demonstrated that this close anatomical arrangement is indeed functionally relevant.
Many studies tested the functional relationship between endothelial and neuronal tissues using loss-of-function approaches. Lowered VEGF-A activity in neuronal precursors in mice or angiogenesis inhibition in rats both cause abnormal cortical brain development 73, 74. One potential explanation was presumed to be an effect on progenitor cells, as ECs have been shown to promote self-renewal of embryonic cortical progenitors. When neuronal progenitors (NPs) from early embryonic mouse cerebral cortex were plated in the presence of ECs, stem cell state was efficiently maintained and subsequent neurogenesis was improved via a Notch-dependent mechanism 65. Interestingly, in contrast to cortical progenitors, neural crest cells instead require ECs for differentiation into autonomic neurons 75. Additionally, ECs have also been shown to drive astrocyte differentiation through Leukemia Inhibitory Factor 76. Thus, ECs have been identified as potent regulators of embryonic NP maintenance and fate determination.
A regulatory role of ECs in neurogenesis is also observed in the adult (Figure 6A). First, in the murine hippocampus, ECs were found to divide coordinately with NPs, a process mediated by VEGF-A77-79. This coordination suggested an EC-NP crosstalk during hippocampal neurogenesis. Second, in the sub-ventricular zone (SVZ), ECs were demonstrated to induce self-renewal of NPs65. Third, in the songbird brain, the neural stem cell pool was found to be maintained via vascular-secreted Brain-Derived Neurotrophic Factor (BDNF)80. In vitro studies have shown that ECs elicit NP proliferation through Placental Growth Factor 2 and Pigment Epithelium-Derived Factor81, 82. Strikingly, ECs isolated from different regions of the brain exhibited different effects on neurogenesis. These studies suggest a role for EC-derived factors in promoting self-renewal of NPs. By contrast, the EC-derived secreted BDNF paralog Neurotrophin-3 (NT-3), the EC surface proteins EPHRINB2 and JAGGED1 were all recently shown to maintain quiescence of NPs in the SVZ83, 84. In addition to self-renewal or quiescence, ECs can promote cell cycle exit of transit amplifying NPs via Bmps85.
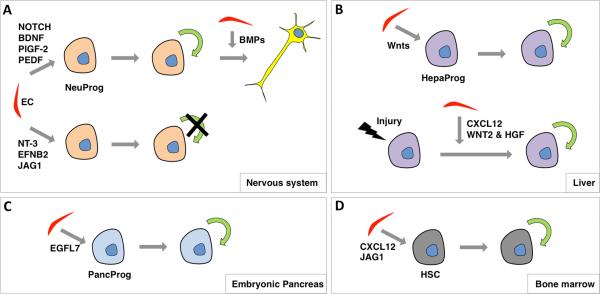
Figure 6. Endothelial niche for stem cell self renewal versus differentiation.
(A) Neuronal progenitor cells receive diverse signals from niche cells, including ECs, which regulate their fate. EC-derived Notch, BDNF, PIGF-2 and PEDF have all been demonstrated to induce self-renewal of neuronal progenitors, while NT-3, EPHRIN-B2 and JAGGED1 promote quiescence. ECs can also prompt cell cycle exit of neuronal progenitors through Bmps. (B) In the liver, Wnt ligands secreted by ECs induce self-renewal of hepatic progenitors during homeostasis. Also under injury, ECs promote hepatic progenitor renewal via WNT2, HGF and CXCL12. (C) During pancreas development, EGFL7 supplied by ECs maintains pancreatic progenitors by promoting their renewal. (D) Hematopoietic stem cells in the bone marrow rely on EC-derived signals, such as CXCL12 and JAGGED1, for renewal and maintenance. BDNF, Brain-Derived Neurotrophic Factor; EFNB2, EPHRIN-B2; HepaProg, hepatic progenitor; HGF, Hepatocyte Growth Factor; HSC, hematopoietic stem cell; JAG1, JAGGED1; NeuProg, neuronal progenitor; NT-3, Neurotrophin-3; PancProg, pancreatic progenitor; PEDF, Pigment Epithelium-Derived Factor; PlGF-2, Placental Growth Factor 2.
Overall, ECs appear to provide different signaling factors to neurogenic niches, at different times or places, and thereby govern NP fate decisions. The nature and interpretations of the signal will be distinct depending on: 1. the type or location of ECs and 2. the targeted NP population. Besides NP fate, vessels can also influence neuronal growth, both in the adult and the embryo86-88. Hence, ECs are proving to be key cellular players that act in a paracrine manner to regulate neurogenesis.
Liver
EC crosstalk has also been observed in the developing liver. Hepatic endoderm is surrounded by ECs following specification of liver progenitor cells, at the very onset of bud formation. As endodermal cells invade the surrounding mesenchyme, they become intermixed with ECs, suggesting a possible functional role for ECs during hepatic development. This was demonstrated using Vegfr2 null mice, which fail to form a proper liver bud, although hepatic specification still occurs63, 64. Anti-angiogenic treatment of embryonic liver explants also impairs hepatic differentiation and albumin expression63, 64. These findings indicate a paracrine role for ECs in hepatic outgrowth and differentiation.
To date, EC-derived molecules governing hepatic development remain largely unknown, although a few candidate molecules have been put forth. Genetic ablation of Bmp4 in mice, or FGF8 inhibition in cultures of murine endoderm, lead to similar liver phenotypes as that observed following Vegfr2 ablation89, 90. In addition, endothelial WNTs and HGF have been proposed to relay EC signals during hepatocyte homeostasis and regeneration (Figure 6B). HGF was found to be secreted from sinusoidal ECs and to promote hepatocyte proliferation in the adult liver91, 92. HGF is also required for proper liver regeneration following partial hepatectomy 93. Conditional ablation of these factors in the hepatic vasculature will be needed to demonstrate a role for ECs in providing these signals.
Lung
ECs in the developing lung also play important roles in formation of this organ. During pulmonary development, ECs increasingly associate with peripheral tubules94. Interestingly, differentiation of lung tubules into saccules coincides with a time point when ECs come into direct contact with the respiratory epithelium. This observation led to further investigations of a possible functional relationship between ECs and the lung epithelium.
A number of studies established that proper vasculature is required for lung development (Figure 7). Inhibition of VEGF through a soluble VEGFR chimeric protein altered alveolar patterning in mice95. Likewise, treatment of lungs with the anti-angiogenic protein Endothelial Monocyte Activating Polypeptide II (EMAPII) resulted in lack of alveolar epithelial type II cells and a decrease in Surfactant Protein C expression 96. Furthermore, lung-specific Vegf-a null mice fail to form primary septae, which normally divide terminal tubules into saccules53. This fits well the previously reported simultaneous occurrence of sacculation with increased vascularization during normal development94. Conversely, lung epithelial-specific Vegf-a transgenic mice exhibit abnormal branching, dilated tubules, reduced saccules and absence of alveolar epithelial type I cells97. A more recent, detailed study by Lazarus et al. demonstrated abnormal and ectopic branching following vascular ablation, as well69. These findings were validated by ex vivo experiments, in which lungs are explanted and grown at the air-fluid interface69, 98. Importantly, branching was abnormal in cultures even when the VEGF inhibitor was removed from the media immediately following vascular ablation. This implies that ECs, but not VEGF itself, are required for normal airway branching. Taken together, these findings suggest that proper airway branching and alveolarization are highly reliant on proper vascularization. However, as in most other systems, the molecular nature of EC-provided cues remains to be determined.
Figure 7. Vascular impact on organogenesis.
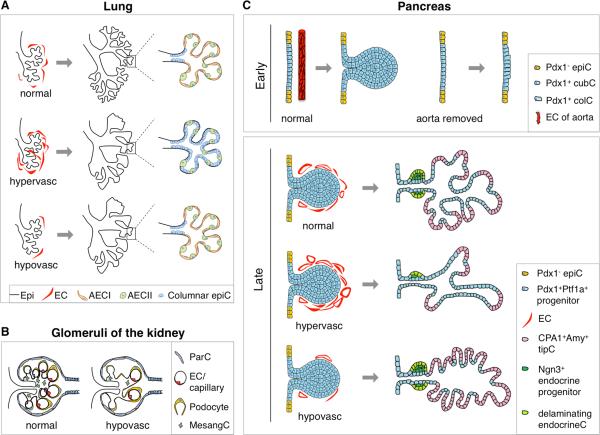
(A) Perturbations in vasculature disrupt lung development. Branching stereotypy is altered, tubules are dilated and sacculation is incomplete in both hyper-vascularized and hypo-vascularized lungs. Hyper-vascularized lungs also fail to differentiate alveolar epithelial type I cells and have immature columnar epithelial cells in their alveoli instead. (B) Hypo-vascularization leads to a failure in glomerular development. Mesangial cells are depleted and the mesangial matrix is altered upon reduction of glomerular capillaries. (C) Early removal of aortic ECs prior to pancreatic specification (E8.25) results in a failure of normal pancreatic bud formation, although the PDX1 domain is specified. Later manipulations of the pancreatic vasculature during epithelial branching also have dramatic effects on pancreas development. Hyper-vascularization results in abnormal branching and reduction of tips. In these pancreata, the undifferentiated trunk epithelium is expanded, while exocrine differentiation as well as endocrine commitment and differentiation are compromised. By contrast, hypo-vascularization during epithelial branching causes ablation of the trunk and expansion of tips. Schematic representation of early pancreatic buds (E10.0, left) and phenotypes at later stages (i.e. E13.5, right). Markers used here to distinguish pancreatic cell lineages: PDX1, PTF1A, CPA1 and NGN3, as described in text. Epi, epithelium; AECI, alveolar epithelial cell type I; AECII, alveolar epithelial cell type II; ColC, columnar cell; CubC, cuboidal cell; EndocrineC, endocrine cell; EpiC, epithelial cell; MesangC, mesangial cell; ParC, parietal cell; TipC, tip cell.
Kidney
Epithelial kidney tubules coordinately develop with peritubular capillaries. ECs initially invade ‘S-shaped bodies’, epithelial tubules formed by progenitor cells following mesenchymal-to-epithelial transition (MET) and aggregation into renal vesicles. The S shaped bodies develop into the filtration unit, called the glomerulus, by further ingrowth of capillaries at the site of podocyte differentiation. Both in vivo and ex vivo work suggests that this organization is critical to normal glomerular development (Figure 7). The zebrafish mutant cloche, which lacks any ECs, fails to form normal glomeruli, although podocyte differentiation does occur99. Likewise, VEGF inhibition in mouse neonates causes abnormal glomerular vascularization and failure of glomerular development, including altered mesangial matrix and degeneration of mesangial cells95. Very similar phenotypes were reported in mice genetically ablated for two out of the three Vegf-a isoforms100. On the other hand, podocyte-specific Vegf-a null mice exhibit deficient mesangial maturation, while podocyte-specific Vegf-a over-expression during development results in a swollen endothelium, abnormally large glomeruli and podocyte depletion 101-103. These studies indicate that endothelial abnormalities lead to failed glomerular morphogenesis, and that VEGF-A expression in podocytes is important for mesangial development. In explant cultures, vascularization induces glomerulogenesis, as well as tubulogenesis, corroborating the circulation-independent role of ECs in kidney development 14, 104. Thus, normal nephron development relies on proper vascularization.
ECs may have additional roles in kidney development prior to glomerulogenesis. In a model proposed by Gao et al., ECs seem to be at the heart of ureteric branching morphogenesis, which initiates nephron progenitor production105. This study demonstrated that VEGFR2 signaling is required to maintain mesenchymal PAX2, which in turn prompts expression of GDNF (Glial cell line-Derived Neurotrophic Factor) and thereby ureteric bud branching. Indeed, mice lacking two different Vegf-a isoforms exhibit ureteric branching defects100. Considering that VEGFR2 expression is largely EC-restricted, these findings indicate a role for ECs in ureteric branching morphogenesis106. Once again, however, molecules and/or pathways that relay the signal from ECs to the mesenchyme, remain unknown. Therefore, early vascular-specific genetic ablation studies will be crucial to determine the role of EC-derived factors in renal development, especially at the stages prior to glomerulus formation that remain rather unexplored.
Pancreas
Given the importance of elucidating pancreatic lineage differentiation for therapeutic applications, the role of ECs during pancreas development has been rigorously examined. This close examination has proved that epithelial-to-endothelial crosstalk is tightly regulated, both spatially and temporally, to ensure proper morphogenesis and differentiation.
From the beginning of organogenesis, the pancreatic primordium is associated with ECs. The pancreas-specific transcription factor PDX1 (Pancreatic and Duodenal Homeobox 1) becomes restricted to the site where vitelline veins and the dorsal aorta physically contact the endoderm62. Following specification, PDX1 expressing cells take a columnar shape, and invade the adjacent mesenchyme, where they form a stratified epithelial bud107-109. This bud represents the pool of progenitors that gives rise to the pancreatic organ and most of its endodermally-derived lineages110. The subsequent early phase of pancreatic development (E9.5-E12.0) has been termed the ‘primary transition’, during which little endocrine differentiation occurs, and that which occurs is towards alpha (glucagon-expressing) cells111, 112. At these stages, capillary ECs surround the outer layer epithelium, while inner layers remain avascular57.
The secondary transition begins around E12.0, when the epithelium starts branching and proliferating rapidly, and the interface with ECs begins to change dramatically. Epithelial protrusions or ‘tips’ of nascent branches arise from a more central trunk epithelium. These structures are characterized by expression of Carboxypeptidase A1 (CPA1). These tips have been suggested to harbor cells with multipotency, or multipotent progenitor cells (MPCs), able to give rise to endocrine, exocrine and ductal lineages113. Tip and trunk epithelial structures display different vascularization patterns and ultimately give rise to different lineages. The bipotential trunk, where endocrine and ductal cells derive, is associated with a greater density of vessels, both large and small. By contrast, the tip cells are relatively devoid of vasculature, and remain so as they give rise to exocrine cells57.
In line with their close anatomical arrangement, perturbation of vasculature affects pancreas development drastically (Figure 7). In Xenopus, pancreatic differentiation fails when dorsal aortic precursors are removed62. Similarly, Vegfr2 null mouse embryos fail to expand the dorsal pancreatic progenitors, even though the PDX1 domain is initiated normally 64. Both the endocrine hormones and expression of Pancreatic Transcription Factor 1A (PTF1A), a pancreatic progenitor marker, are lost in the pancreas of these embryos. Validating a direct, non-secondary effect of ECs, lack of PTF1A expression is reversed when pancreata are cultured in the presence of aortic ECs64. More recently, PDX1 and insulin expression were shown to depend on angioblasts in chick embryos, even before vessel formation114. In this model, recruitment of angioblasts to the endoderm requires expression of the CXCL12 receptor, CXCR4, in angioblasts. PDX1 and insulin are reduced when CXCR4 is inhibited in the embryo114. These findings indicate a requirement for ECs in early pancreas development. Furthermore, multiple studies suggest sufficiency of ECs in pancreatic induction. Aortic ECs are able to elicit insulin expression in isolated mouse endoderm ex vivo62. PDX1-driven VEGF-A over-expression also induces insulin in ectopically hyper-vascularized posterior stomach. Similar to VEGF-A, ectopic CXCL12 expression is sufficient to attract angioblasts and promote PDX1 in the pre-intestinal endoderm, when induced prior to intestinal specification in the chick embryo114. Thus, close proximity of ECs to the endoderm is essential for pancreas development prior to the onset of branching and differentiation, and may be sufficient for pancreatic induction.
Strikingly, concomitant with dynamic vascular reorganization, the role of blood vessel endothelium changes during pancreas development. Pancreas-specific over-expression of VEGF-A, which causes hyper-vascularization, results in suppression of pancreatic branching and a decrease in tip cells. In addition, excess vessels inhibit the expression of the endocrine progenitor marker Neurogenin-3, or NGN3. As a result, hyper-vascularized pancreata have significantly reduced endocrine and exocrine differentiation. Interestingly, these changes are accompanied by persistent expression of undifferentiated trunk markers115. Conversely, VEGFR2 inhibition in E12.5 explants leads to an increased number of tip cells and reduced trunk marker expression. Supporting an inhibitory role for ECs on acinar differentiation, pancreas-specific Vegf-a ablation leads to increased acinar cells in hypo-vascularized regions57. Furthermore, acinar cells over-expressing VEGF-A exhibit reduced amylase expression. Taken together, these studies suggest a negative role for ECs during pancreatic branching and exocrine differentiation at mid-gestation.
How ECs regulate endocrine differentiation is clearly complex. The early requirement of ECs for proper endocrine fate (outlined above) can be explained by an essential role in maintenance of the early pancreatic epithelium. In the later pancreatic bud, ECs appear to support the undifferentiated trunk epithelium, as evidenced by a failure to differentiate toward any lineage upon hyper-vascularization. In support of this idea, a recent study by Kao et al. demonstrated that EC-provided Epidermal Growth Factor Like 7 (EGFL7) maintains pancreatic progenitor renewal116 (Figure 6C). Thus, reduced endocrine differentiation upon hyper-vascularization at these stages may be a result of persistence of an undifferentiated state. Along those lines, ectopic VEGF-A expression in insulin-expressing beta cells during development leads to impaired endocrine differentiation, reduced beta cell mass and perturbed islet morphology117. However, VEGFR2 inhibition leads to a decrease in endocrine progenitors, as well, suggesting that ECs are required for endocrine differentiation57. Overall, endocrine fate may depend on a balanced EC niche, which is permissive to differentiation from the trunk, at the same time repressing acinar differentiation.
After birth, maturation of beta cells is tightly coupled to islet vascularization. Immature beta cells recruit ECs to the islets through VEGF. ECs, in turn, secrete potent factors for beta cell proliferation, maturation or even rejuvenation, via factors such as HGF118, 119. When mature, adult islets can tolerate reduced vascularization and function normally. Ablation of vasculature by VEGF inactivation or soluble VEGFR over-expression in islets only leads to a slight defect in glucose clearance 58, 120, 121. Beta cell regeneration after injury was also found to be normal in hypo-vascularized islets121. On the other hand, hyper-vascularization was shown to reduce beta cell mass, with islets rapidly losing their intact architecture upon increased VEGF expression16. These findings demonstrate the robustness of islet function under vascular perturbations, such as injury, and at the same time suggest EC-derived inhibitory signals acting on beta cell proliferation.
Perspectives on the study of paracrine signals from blood vessels
Seemingly contradictory findings as to how ECs regulate organ development, specifically endocrine differentiation in the pancreas, likely point to the dynamic nature of this regulation. Some discrepancies may result from use of different genetic models, inhibitors, in vivo versus ex vivo systems or different timing in vascular perturbation. On the other hand, the main approach, perturbing VEGF signaling, is an indirect way of manipulating vasculature. VEGF signaling does not only regulate vascularization of tissues, but also acts as a key player in the response of tissues to hypoxia. Similarly, VEGF regulates vascular permeability and thereby access of tissues to factors within the plasma. Thus, it is challenging to interpret the results when this signaling is disturbed and determine to what extent other VEGF-regulated processes are involved in the reported phenotypes. Furthermore, given the heterogeneity of ECs, the extent of perturbation and/or the affected subsets of ECs constitute additional parameters mostly disregarded. Hence, there is an urgent need for clarifying EC-derived signals and studying them individually, as opposed to dependence on pan-endothelial perturbations.
ENDOTHELIAL HETEROGENEITY
Arteriovenous fate: example of early distinctive ECs
One key characteristic of the vasculature that cannot be dissociated from its paracrine interactions with surrounding tissues is its heterogeneity. The blood vascular system is a highly hierarchical tree of large and small vessels. Arteries, and arterioles branching from them, form a closed circulation with venules and veins via capillaries, the finest vessels. Each segment of the vasculature has a different morphology that enables it to serve its specific and local functions122.
Organ-specific structural differences in ECs
Further evidence of vascular heterogeneity can be found in the morphological differences between capillary beds of different organs. Simply put, blood vessels in different tissues must accomplish different functions, and therefore ECs within these tissues take on very different ultrastructural appearances. For instance, organs involved in filtration, such as pancreas and kidney, harbor vessels with pores (fenestrations). Discontinuous capillaries develop much larger pores, or sinusoids, like those in the liver. Furthermore, ECs differ at fundamental levels in their content of a number of subcellular components, such as caveolae, organization of tight junctions, and composition of endocytic machinery122. These organ-specific EC characteristics are essential to enable vessels to serve organ-specific functions.
Transcriptional heterogeneity in ECs
Given the functional and morphological heterogeneity within the vascular system, it is not surprising to find differences at the molecular level across blood vessels. Expression profiling, proteomic approaches and analysis of vascular-specific or -restricted markers demonstrate distinct molecular compositions across vascular beds, arteries, and even cells within an artery123-127 (see reviews128, 129). How and when in the lifespan of an organism do ECs, derived from the same progenitor, give rise to such heterogeneous populations? Do they emerge de novo as distinct cells from different regions of the mesoderm? Do angioblasts give rise to daughter cells with different fates? Studies that compare different vascular beds across different developmental stages will be crucial to answer these questions. Importantly, tissue specificity of EC paracrine signaling is underscored by the inherent heterogeneity of capillary beds in different organs. The challenge ahead is recognizing those differences, understanding how they are molecularly governed and how this could help direct the development of therapeutic applications.
ENDOTHELIAL CELLS IN REGENERATIVE THERAPIES
Regenerative medicine is defined as “the process of replacing, engineering or regenerating human cells, tissues or organs to restore or establish normal function”130. Fundamentally, to create new organs, it will be essential to learn how to build and integrate a functional vasculature with bioengineered tissues, to ensure proper nutrition and gas exchange, but also to provide any signals required by cells within the newly generated tissues. Recently, there has been a tremendous effort to identify these EC-derived signals, referred to as “angiocrine factors” in adult systems124, 131.
ECs in organ regeneration
Perhaps not surprisingly, given their roles in organ development, ECs have proved indispensable for regeneration of tissues in various systems92, 132. Neural regeneration and angiogenesis occur coordinately upon CNS injury, such as stroke or brain trauma133. Moreover, newly generated neurons have been shown to follow paths of the perivascular space to migrate to the site of injury, and also rely on EC-provided angiocrine factors for survival134. Mounting evidence points to a similar role for ECs in the bone marrow (HSCs, Figure 6D) and in the liver. Wang et al. hasrecently demonstrated that central vein ECs supply Wnt signals to the self-renewing hepatocyte population in the uninjured state135. These homeostatic mechanisms are similarly employed during liver regeneration, which has been found to rely on sinusoidal angiocrine factors, WNT2 and HGF, upon hepatectomy, and CXCL12 under acute or chronic injury 92, 136 (Figure 6B). Regeneration of lung upon injury also relies on paracrine signals provided by ECs131, 137. These studies highlight the essential role of ECs in tissue regeneration.
Generating mature beta cells requires ECs
Generating beta cells in vitro is a potential therapy for type I diabetes patients, and thus is an urgent need that regenerative science has been trying to address. Although beta cells can be successfully differentiated using human induced pluripotent stem cells (iPSCs), the resulting cells are far from being mature and fully functional. Incorporating ECs in these step-wise differentiation protocols, at defined stages, has led to a boost in efficiency of functional beta cell production 116. Considering that differentiation and maturation of beta cells depend on ECs, we predict that incorporation of ECs or EC-provided signals will be needed to make fully mature beta cells in vitro. This necessitates a better understanding of how and which EC-signals regulate beta cell differentiation.
EC considerations for organoids and organs-in-a-dish
A major laboratory advance that found its roots in developmental biology has been the establishment of organoid cultures, especially those from human iPSCs. Recapitulating many aspects of organ development in vitro, organoids hold great promise for serving as a tissue source in medical therapies, as well as for providing novel platforms for drug development138. However, non-progenitor cells, including stroma, neurons and ECs, have mostly been ignored in these systems to date. To mimic in vivo processes, incorporating ECs into organoid cultures will be essential. This requires urgent investigation of patterning and heterogeneity of organ-specific ECs. Along those lines, a recent study from Takebe et al. integrated ECs in human iPSC-derived liver buds, which formed a functional vasculature in transplanted animals139. Another study by Takasato et al. recently reported coordinated generation and patterning of renal ducts, tubules and glomerular structures, alongside lumen-containing vasculature, in kidney organoids from iPSCs140. However, whether or not these in vitro generated blood vessels are properly patterned and interfaced in a functionally relevant manner remains to be established.
Overall, these studies suggest importance of coordinated development of tissues and their associated vasculature. Recent work has also demonstrated that prevascularized tissues perfuse more rapidly following transplantation, leading to improved tissue survival and functional outcomes141. Together, these findings draw attention to the contribution of ECs to development within organoid cultures. Understanding how ECs govern organ development will be central to achieving replacement and regenerative therapies.
CONCLUSION
Endothelial cells (ECs) have long been considered passive building blocks of the blood circulatory plumbing. In contrast to long held common belief, studies in the last two decades have revealed functional communication of ECs with surrounding tissues. From these studies, we have learned that reciprocal interactions are essential for the proper patterning of blood vessels throughout the embryo, as well as for organogenesis. While initial events that shape the first vessels in the embryo have begun to be understood, stereotypical organ and tissue vascularization remains unclear. One emerging general theme is that tissues provide an integrated network of local attractive and repulsive cues that dictate patterning of vessels. However, we lack an overall picture of how and where these cues integrate in each organ, as well as what molecules constitute the complete set of cues. Reciprocally, ECs communicate back to resident tissues and regulate their growth during development. This phenomenon has been investigated in a handful of organs, demonstrating crucial paracrine roles for ECs in organogenesis. However, manipulation of vasculature has been mostly indirect, via gain or loss of function of angiogenic molecules such as VEGF, and very few molecular mechanisms have been identified. Strikingly, ECs have organ-specific, and -within an organ- cell type-specific, paracrine functions during organogenesis. Whether these different functions rely on common or distinct cues remains as an outstanding question. Given the final anatomical and molecular heterogeneity across vascular beds, it is probable that organ-specific EC factors are involved. However, this is yet to be demonstrated. Finally, ECs are emerging as key regulators of organ regeneration, yet almost nothing is known about how they communicate with the resident cells and impact their fate. Thus, advancement of current regenerative therapies, as well as making organs-in-a-dish in the near future, will require a deeper, mechanistic understanding of vascular patterning as well as the central instructive role of ECs during organogenesis.
ACKNOWLEDGEMENTS
We are grateful to the Cleaver lab for useful discussions and to Arnaldo Carreira for critical reading of the manuscript. We are grateful to Dr. Alethia Villasenor for images provided (Figure 5). This work is funded by NIH Institutional National Research Service Award (T32) 2T32GM008203-26A1 to DBA, and CPRIT RP110405, R01DK079862, R24DK106743 and R01HL113498 to OC
Footnotes
Related articles
Carolien Wansleeben, Christina E. Barkauskas, Jason R. Rock, Brigid L. M. Hogan (2012) Stem cells of the adult lung: their development and role in homeostasis, regeneration, and disease. WIREs Developmental Biology 2, issue 1 DOI: 10.1002/wdev.58
Ryan S. Udan, James C. Culver, Mary E. Dickinson (2012) Understanding vascular development. WIREs Developmental Biology 2, issue 3 DOI: 10.1002/wdev.91
Teresa L. Mastracci, Lori Sussel (2012) The endocrine pancreas: insights into development, differentiation, and diabetes. WIREs Developmental Biology 1, issue 5 DOI: 10.1002/wdev.44
REFERENCES
- 1.Cleaver O, Krieg PA. Vascular Development. In: Harvey RPaR N, editor. Heart Development and Regeneration. 1 ed. Vol. 1. Academic Press; Amsterdam: 2010. pp. 487–528. [Google Scholar]
- 2.Drake CJ, Fleming PA. Vasculogenesis in the day 6.5 to 9.5 mouse embryo. Blood. 2000;95:1671–1679. [PubMed] [Google Scholar]
- 3.Meadows SM, Cleaver O. Vascular patterning: coordinated signals keep blood vessels on track. Curr Opin Genet Dev. 2015;32:86–91. doi: 10.1016/j.gde.2015.02.002. [DOI] [PubMed] [Google Scholar]
- 4.Adams RH, Eichmann A. Axon guidance molecules in vascular patterning. Cold Spring Harb Perspect Biol. 2010;2:a001875. doi: 10.1101/cshperspect.a001875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ghaffari S, Leask RL, Jones EA. Simultaneous imaging of blood flow dynamics and vascular remodelling during development. Development. 2015 doi: 10.1242/dev.127019. [DOI] [PubMed] [Google Scholar]
- 6.Ziegler T, Nerem RM. Effect of flow on the process of endothelial cell division. Arterioscler Thromb. 1994;14:636–643. doi: 10.1161/01.atv.14.4.636. [DOI] [PubMed] [Google Scholar]
- 7.Galbraith CG, Skalak R, Chien S. Shear stress induces spatial reorganization of the endothelial cell cytoskeleton. Cell Motil Cytoskeleton. 1998;40:317–330. doi: 10.1002/(SICI)1097-0169(1998)40:4<317::AID-CM1>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- 8.Simmers MB, Pryor AW, Blackman BR. Arterial shear stress regulates endothelial cell-directed migration, polarity, and morphology in confluent monolayers. Am J Physiol Heart Circ Physiol. 2007;293:H1937–1946. doi: 10.1152/ajpheart.00534.2007. [DOI] [PubMed] [Google Scholar]
- 9.Jahnsen ED, Trindade A, Zaun HC, Lehoux S, Duarte A, Jones EA. Notch1 is pan-endothelial at the onset of flow and regulated by flow. PLoS One. 2015;10:e0122622. doi: 10.1371/journal.pone.0122622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vittet D, Prandini MH, Berthier R, Schweitzer A, Martin-Sisteron H, Uzan G, Dejana E. Embryonic stem cells differentiate in vitro to endothelial cells through successive maturation steps. Blood. 1996;88:3424–3431. [PubMed] [Google Scholar]
- 11.Risau W, Sariola H, Zerwes HG, Sasse J, Ekblom P, Kemler R, Doetschman T. Vasculogenesis and angiogenesis in embryonic-stem-cell-derived embryoid bodies. Development. 1988;102:471–478. doi: 10.1242/dev.102.3.471. [DOI] [PubMed] [Google Scholar]
- 12.Ausprunk DH, Falterman K, Folkman J. The sequence of events in the regression of corneal capillaries. Lab Invest. 1978;38:284–294. [PubMed] [Google Scholar]
- 13.deMello DE, Sawyer D, Galvin N, Reid LM. Early fetal development of lung vasculature. Am J Respir Cell Mol Biol. 1997;16:568–581. doi: 10.1165/ajrcmb.16.5.9160839. [DOI] [PubMed] [Google Scholar]
- 14.Tufro A, Norwood VF, Carey RM, Gomez RA. Vascular endothelial growth factor induces nephrogenesis and vasculogenesis. J Am Soc Nephrol. 1999;10:2125–2134. doi: 10.1681/ASN.V10102125. [DOI] [PubMed] [Google Scholar]
- 15.Mukouyama YS, Shin D, Britsch S, Taniguchi M, Anderson DJ. Sensory nerves determine the pattern of arterial differentiation and blood vessel branching in the skin. Cell. 2002;109:693–705. doi: 10.1016/s0092-8674(02)00757-2. [DOI] [PubMed] [Google Scholar]
- 16.Brissova M, Shostak A, Shiota M, Wiebe PO, Poffenberger G, Kantz J, Chen Z, Carr C, Jerome WG, Chen J, et al. Pancreatic islet production of vascular endothelial growth factor--a is essential for islet vascularization, revascularization, and function. Diabetes. 2006;55:2974–2985. doi: 10.2337/db06-0690. [DOI] [PubMed] [Google Scholar]
- 17.Cleaver O, Tonissen KF, Saha MS, Krieg PA. Neovascularization of the Xenopus embryo. Dev Dyn. 1997;210:66–77. doi: 10.1002/(SICI)1097-0177(199709)210:1<66::AID-AJA7>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- 18.Flamme I, von Reutern M, Drexler HC, Syed-Ali S, Risau W. Overexpression of vascular endothelial growth factor in the avian embryo induces hypervascularization and increased vascular permeability without alterations of embryonic pattern formation. Dev Biol. 1995;171:399–414. doi: 10.1006/dbio.1995.1291. [DOI] [PubMed] [Google Scholar]
- 19.Carmeliet P, Ferreira V, Breier G, Pollefeyt S, Kieckens L, Gertsenstein M, Fahrig M, Vandenhoeck A, Harpal K, Eberhardt C, et al. Abnormal blood vessel development and lethality in embryos lacking a single VEGF allele. Nature. 1996;380:435–439. doi: 10.1038/380435a0. [DOI] [PubMed] [Google Scholar]
- 20.Ferrara N, Carver-Moore K, Chen H, Dowd M, Lu L, O'Shea KS, Powell-Braxton L, Hillan KJ, Moore MW. Heterozygous embryonic lethality induced by targeted inactivation of the VEGF gene. Nature. 1996;380:439–442. doi: 10.1038/380439a0. [DOI] [PubMed] [Google Scholar]
- 21.Reese DE, Hall CE, Mikawa T. Negative regulation of midline vascular development by the notochord. Dev Cell. 2004;6:699–708. doi: 10.1016/s1534-5807(04)00127-3. [DOI] [PubMed] [Google Scholar]
- 22.David L, Mallet C, Keramidas M, Lamande N, Gasc JM, Dupuis-Girod S, Plauchu H, Feige JJ, Bailly S. Bone morphogenetic protein-9 is a circulating vascular quiescence factor. Circ Res. 2008;102:914–922. doi: 10.1161/CIRCRESAHA.107.165530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.David L, Mallet C, Mazerbourg S, Feige JJ, Bailly S. Identification of BMP9 and BMP10 as functional activators of the orphan activin receptor-like kinase 1 (ALK1) in endothelial cells. Blood. 2007;109:1953–1961. doi: 10.1182/blood-2006-07-034124. [DOI] [PubMed] [Google Scholar]
- 24.Ricard N, Ciais D, Levet S, Subileau M, Mallet C, Zimmers TA, Lee SJ, Bidart M, Feige JJ, Bailly S. BMP9 and BMP10 are critical for postnatal retinal vascular remodeling. Blood. 2012;119:6162–6171. doi: 10.1182/blood-2012-01-407593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li W, Kohara H, Uchida Y, James JM, Soneji K, Cronshaw DG, Zou YR, Nagasawa T, Mukouyama YS. Peripheral nerve-derived CXCL12 and VEGF-A regulate the patterning of arterial vessel branching in developing limb skin. Dev Cell. 2013;24:359–371. doi: 10.1016/j.devcel.2013.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Strasser GA, Kaminker JS, Tessier-Lavigne M. Microarray analysis of retinal endothelial tip cells identifies CXCR4 as a mediator of tip cell morphology and branching. Blood. 2010;115:5102–5110. doi: 10.1182/blood-2009-07-230284. [DOI] [PubMed] [Google Scholar]
- 27.Ara T, Tokoyoda K, Okamoto R, Koni PA, Nagasawa T. The role of CXCL12 in the organ-specific process of artery formation. Blood. 2005;105:3155–3161. doi: 10.1182/blood-2004-07-2563. [DOI] [PubMed] [Google Scholar]
- 28.Haege S, Einer C, Thiele S, Mueller W, Nietzsche S, Lupp A, Mackay F, Schulz S, Stumm R. CXC chemokine receptor 7 (CXCR7) regulates CXCR4 protein expression and capillary tuft development in mouse kidney. PLoS One. 2012;7:e42814. doi: 10.1371/journal.pone.0042814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Takabatake Y, Sugiyama T, Kohara H, Matsusaka T, Kurihara H, Koni PA, Nagasawa Y, Hamano T, Matsui I, Kawada N, et al. The CXCL12 (SDF-1)/CXCR4 axis is essential for the development of renal vasculature. J Am Soc Nephrol. 2009;20:1714–1723. doi: 10.1681/ASN.2008060640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Meadows SM, Fletcher PJ, Moran C, Xu K, Neufeld G, Chauvet S, Mann F, Krieg PA, Cleaver O. Integration of repulsive guidance cues generates avascular zones that shape mammalian blood vessels. Circ Res. 2012;110:34–46. doi: 10.1161/CIRCRESAHA.111.249847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bielenberg DR, Shimizu A, Klagsbrun M. Semaphorin-induced cytoskeletal collapse and repulsion of endothelial cells. Methods Enzymol. 2008;443:299–314. doi: 10.1016/S0076-6879(08)02015-6. [DOI] [PubMed] [Google Scholar]
- 32.Goshima Y, Ito T, Sasaki Y, Nakamura F. Semaphorins as signals for cell repulsion and invasion. J Clin Invest. 2002;109:993–998. doi: 10.1172/JCI15467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Meadows SM, Ratliff LA, Singh MK, Epstein JA, Cleaver O. Resolution of defective dorsal aortae patterning in Sema3E-deficient mice occurs via angiogenic remodeling. Dev Dyn. 2013;242:580–590. doi: 10.1002/dvdy.23949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Aghajanian H, Choi C, Ho VC, Gupta M, Singh MK, Epstein JA. Semaphorin 3d and semaphorin 3e direct endothelial motility through distinct molecular signaling pathways. J Biol Chem. 2014;289:17971–17979. doi: 10.1074/jbc.M113.544833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Degenhardt K, Singh MK, Aghajanian H, Massera D, Wang Q, Li J, Li L, Choi C, Yzaguirre AD, Francey LJ, et al. Semaphorin 3d signaling defects are associated with anomalous pulmonary venous connections. Nat Med. 2013;19:760–765. doi: 10.1038/nm.3185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Stein E, Tessier-Lavigne M. Hierarchical organization of guidance receptors: silencing of netrin attraction by slit through a Robo/DCC receptor complex. Science. 2001;291:1928–1938. doi: 10.1126/science.1058445. [DOI] [PubMed] [Google Scholar]
- 37.Kaur S, Castellone MD, Bedell VM, Konar M, Gutkind JS, Ramchandran R. Robo4 signaling in endothelial cells implies attraction guidance mechanisms. J Biol Chem. 2006;281:11347–11356. doi: 10.1074/jbc.M508853200. [DOI] [PubMed] [Google Scholar]
- 38.Jones EA, Yuan L, Breant C, Watts RJ, Eichmann A. Separating genetic and hemodynamic defects in neuropilin 1 knockout embryos. Development. 2008;135:2479–2488. doi: 10.1242/dev.014902. [DOI] [PubMed] [Google Scholar]
- 39.Bedell VM, Yeo SY, Park KW, Chung J, Seth P, Shivalingappa V, Zhao J, Obara T, Sukhatme VP, Drummond IA, et al. roundabout4 is essential for angiogenesis in vivo. Proc Natl Acad Sci U S A. 2005;102:6373–6378. doi: 10.1073/pnas.0408318102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Suchting S, Heal P, Tahtis K, Stewart LM, Bicknell R. Soluble Robo4 receptor inhibits in vivo angiogenesis and endothelial cell migration. Faseb J. 2005;19:121–123. doi: 10.1096/fj.04-1991fje. [DOI] [PubMed] [Google Scholar]
- 41.Zhang B, Dietrich UM, Geng JG, Bicknell R, Esko JD, Wang L. Repulsive axon guidance molecule Slit3 is a novel angiogenic factor. Blood. 2009;114:4300–4309. doi: 10.1182/blood-2008-12-193326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lu X, Le Noble F, Yuan L, Jiang Q, De Lafarge B, Sugiyama D, Breant C, Claes F, De Smet F, Thomas JL, et al. The netrin receptor UNC5B mediates guidance events controlling morphogenesis of the vascular system. Nature. 2004;432:179–186. doi: 10.1038/nature03080. [DOI] [PubMed] [Google Scholar]
- 43.Bouvree K, Larrivee B, Lv X, Yuan L, DeLafarge B, Freitas C, Mathivet T, Breant C, Tessier-Lavigne M, Bikfalvi A, et al. Netrin-1 inhibits sprouting angiogenesis in developing avian embryos. Dev Biol. 2008;318:172–183. doi: 10.1016/j.ydbio.2008.03.023. [DOI] [PubMed] [Google Scholar]
- 44.Larrivee B, Freitas C, Trombe M, Lv X, Delafarge B, Yuan L, Bouvree K, Breant C, Del Toro R, Brechot N, et al. Activation of the UNC5B receptor by Netrin-1 inhibits sprouting angiogenesis. Genes Dev. 2007;21:2433–2447. doi: 10.1101/gad.437807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Serafini T, Colamarino SA, Leonardo ED, Wang H, Beddington R, Skarnes WC, Tessier-Lavigne M. Netrin-1 is required for commissural axon guidance in the developing vertebrate nervous system. Cell. 1996;87:1001–1014. doi: 10.1016/s0092-8674(00)81795-x. [DOI] [PubMed] [Google Scholar]
- 46.Drake CJ. Embryonic and adult vasculogenesis. Birth Defects Res C Embryo Today. 2003;69:73–82. doi: 10.1002/bdrc.10003. [DOI] [PubMed] [Google Scholar]
- 47.Haigh JJ. Role of VEGF in organogenesis. Organogenesis. 2008;4:247–256. doi: 10.4161/org.4.4.7415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Loscertales M, Mikels AJ, Hu JK, Donahoe PK, Roberts DJ. Chick pulmonary Wnt5a directs airway and vascular tubulogenesis. Development. 2008;135:1365–1376. doi: 10.1242/dev.010504. [DOI] [PubMed] [Google Scholar]
- 49.Okazaki T, Ni A, Baluk P, Ayeni OA, Kearley J, Coyle AJ, Humbles A, McDonald DM. Capillary defects and exaggerated inflammatory response in the airways of EphA2-deficient mice. Am J Pathol. 2009;174:2388–2399. doi: 10.2353/ajpath.2009.080949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mujahid S, Nielsen HC, Volpe MV. MiR-221 and miR-130a regulate lung airway and vascular development. PLoS One. 2013;8:e55911. doi: 10.1371/journal.pone.0055911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Nakagawa N, Xin C, Roach AM, Naiman N, Shankland SJ, Ligresti G, Ren S, Szak S, Gomez IG, Duffield JS. Dicer1 activity in the stromal compartment regulates nephron differentiation and vascular patterning during mammalian kidney organogenesis. Kidney Int. 2015;87:1125–1140. doi: 10.1038/ki.2014.406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hurtado R, Zewdu R, Mtui J, Liang C, Aho R, Kurylo C, Selleri L, Herzlinger D. Pbx1-dependent control of VMC differentiation kinetics underlies gross renal vascular patterning. Development. 2015;142:2653–2664. doi: 10.1242/dev.124776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yamamoto H, Yun EJ, Gerber HP, Ferrara N, Whitsett JA, Vu TH. Epithelial-vascular cross talk mediated by VEGF-A and HGF signaling directs primary septae formation during distal lung morphogenesis. Dev Biol. 2007;308:44–53. doi: 10.1016/j.ydbio.2007.04.042. [DOI] [PubMed] [Google Scholar]
- 54.Hara Y, Nomura T, Yoshizaki K, Frisen J, Osumi N. Impaired hippocampal neurogenesis and vascular formation in ephrin-A5-deficient mice. Stem Cells. 2010;28:974–983. doi: 10.1002/stem.427. [DOI] [PubMed] [Google Scholar]
- 55.Lu P, Werb Z. Patterning mechanisms of branched organs. Science. 2008;322:1506–1509. doi: 10.1126/science.1162783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Tufro A, Teichman J, Woda C, Villegas G. Semaphorin3a inhibits ureteric bud branching morphogenesis. Mech Dev. 2008;125:558–568. doi: 10.1016/j.mod.2007.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Pierreux CE, Cordi S, Hick AC, Achouri Y, Ruiz de Almodovar C, Prevot PP, Courtoy PJ, Carmeliet P, Lemaigre FP. Epithelial: Endothelial cross-talk regulates exocrine differentiation in developing pancreas. Dev Biol. 2010;347:216–227. doi: 10.1016/j.ydbio.2010.08.024. [DOI] [PubMed] [Google Scholar]
- 58.Lammert E, Gu G, McLaughlin M, Brown D, Brekken R, Murtaugh LC, Gerber HP, Ferrara N, Melton DA. Role of VEGF-A in vascularization of pancreatic islets. Curr Biol. 2003;13:1070–1074. doi: 10.1016/s0960-9822(03)00378-6. [DOI] [PubMed] [Google Scholar]
- 59.Magenheim J, Ilovich O, Lazarus A, Klochendler A, Ziv O, Werman R, Hija A, Cleaver O, Mishani E, Keshet E, et al. Blood vessels restrain pancreas branching, differentiation and growth. Development. 2011;138:4743–4752. doi: 10.1242/dev.066548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Tang W, Zeve D, Suh JM, Bosnakovski D, Kyba M, Hammer RE, Tallquist MD, Graff JM. White fat progenitor cells reside in the adipose vasculature. Science. 2008;322:583–586. doi: 10.1126/science.1156232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Jacquemin P, Yoshitomi H, Kashima Y, Rousseau GG, Lemaigre FP, Zaret KS. An endothelialmesenchymal relay pathway regulates early phases of pancreas development. Dev Biol. 2006;290:189–199. doi: 10.1016/j.ydbio.2005.11.023. [DOI] [PubMed] [Google Scholar]
- 62.Lammert E, Cleaver O, Melton D. Induction of pancreatic differentiation by signals from blood vessels. Science. 2001;294:564–567. doi: 10.1126/science.1064344. [DOI] [PubMed] [Google Scholar]
- 63.Matsumoto K, Yoshitomi H, Rossant J, Zaret KS. Liver organogenesis promoted by endothelial cells prior to vascular function. Science. 2001;294:559–563. doi: 10.1126/science.1063889. [DOI] [PubMed] [Google Scholar]
- 64.Yoshitomi H, Zaret KS. Endothelial cell interactions initiate dorsal pancreas development by selectively inducing the transcription factor Ptf1a. Development. 2004;131:807–817. doi: 10.1242/dev.00960. [DOI] [PubMed] [Google Scholar]
- 65.Shen Q, Goderie SK, Jin L, Karanth N, Sun Y, Abramova N, Vincent P, Pumiglia K, Temple S. Endothelial cells stimulate self-renewal and expand neurogenesis of neural stem cells. Science. 2004;304:1338–1340. doi: 10.1126/science.1095505. [DOI] [PubMed] [Google Scholar]
- 66.Shen Q, Wang Y, Kokovay E, Lin G, Chuang SM, Goderie SK, Roysam B, Temple S. Adult SVZ stem cells lie in a vascular niche: a quantitative analysis of niche cell-cell interactions. Cell Stem Cell. 2008;3:289–300. doi: 10.1016/j.stem.2008.07.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Tavazoie M, Van der Veken L, Silva-Vargas V, Louissaint M, Colonna L, Zaidi B, Garcia-Verdugo JM, Doetsch F. A specialized vascular niche for adult neural stem cells. Cell Stem Cell. 2008;3:279–288. doi: 10.1016/j.stem.2008.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Yoshida S, Sukeno M, Nabeshima Y. A vasculature-associated niche for undifferentiated spermatogonia in the mouse testis. Science. 2007;317:1722–1726. doi: 10.1126/science.1144885. [DOI] [PubMed] [Google Scholar]
- 69.Lazarus A, Del-Moral PM, Ilovich O, Mishani E, Warburton D, Keshet E. A perfusion-independent role of blood vessels in determining branching stereotypy of lung airways. Development. 2011;138:2359–2368. doi: 10.1242/dev.060723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Rupnick MA, Panigrahy D, Zhang CY, Dallabrida SM, Lowell BB, Langer R, Folkman MJ. Adipose tissue mass can be regulated through the vasculature. Proceedings of the National Academy of Sciences of the United States of America. 2002;99:10730–10735. doi: 10.1073/pnas.162349799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Leventhal C, Rafii S, Rafii D, Shahar A, Goldman SA. Endothelial trophic support of neuronal production and recruitment from the adult mammalian subependyma. Mol Cell Neurosci. 1999;13:450–464. doi: 10.1006/mcne.1999.0762. [DOI] [PubMed] [Google Scholar]
- 72.Palmer TD, Willhoite AR, Gage FH. Vascular niche for adult hippocampal neurogenesis. J Comp Neurol. 2000;425:479–494. doi: 10.1002/1096-9861(20001002)425:4<479::aid-cne2>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 73.Haigh JJ, Morelli PI, Gerhardt H, Haigh K, Tsien J, Damert A, Miquerol L, Muhlner U, Klein R, Ferrara N, et al. Cortical and retinal defects caused by dosage-dependent reductions in VEGFA paracrine signaling. Dev Biol. 2003;262:225–241. doi: 10.1016/s0012-1606(03)00356-7. [DOI] [PubMed] [Google Scholar]
- 74.Hallene KL, Oby E, Lee BJ, Santaguida S, Bassanini S, Cipolla M, Marchi N, Hossain M, Battaglia G, Janigro D. Prenatal exposure to thalidomide, altered vasculogenesis, and CNS malformations. Neuroscience. 2006;142:267–283. doi: 10.1016/j.neuroscience.2006.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Acevedo LM, Lindquist JN, Walsh BM, Sia P, Cimadamore F, Chen C, Denzel M, Pernia CD, Ranscht B, Terskikh A, et al. hESC Differentiation toward an Autonomic Neuronal Cell Fate Depends on Distinct Cues from the Co-Patterning Vasculature. Stem Cell Reports. 2015;4:1075–1088. doi: 10.1016/j.stemcr.2015.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Mi H, Haeberle H, Barres BA. Induction of astrocyte differentiation by endothelial cells. J Neurosci. 2001;21:1538–1547. doi: 10.1523/JNEUROSCI.21-05-01538.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Cao L, Jiao XY, Zuzga DS, Liu YH, Fong DM, Young D, During MJ. VEGF links hippocampal activity with neurogenesis, learning and memory. Nature Genetics. 2004;36:827–835. doi: 10.1038/ng1395. [DOI] [PubMed] [Google Scholar]
- 78.Schanzer A, Wachs FP, Wilhelm D, Acker T, Cooper-Kuhn C, Beck H, Winkler J, Aigner L, Plate KH, Kuhn HG. Direct stimulation of adult neural stem cells in vitro and neurogenesis in vivo by vascular endothelial growth factor. Brain Pathology. 2004;14:237–248. doi: 10.1111/j.1750-3639.2004.tb00060.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Licht T, Goshen I, Avital A, Kreisel T, Zubedat S, Eavri R, Segal M, Yirmiya R, Keshet E. Reversible modulations of neuronal plasticity by VEGF. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:5081–5086. doi: 10.1073/pnas.1007640108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Louissaint A, Jr., Rao S, Leventhal C, Goldman SA. Coordinated interaction of neurogenesis and angiogenesis in the adult songbird brain. Neuron. 2002;34:945–960. doi: 10.1016/s0896-6273(02)00722-5. [DOI] [PubMed] [Google Scholar]
- 81.Crouch EE, Liu C, Silva-Vargas V, Doetsch F. Regional and Stage-Specific Effects of Prospectively Purified Vascular Cells on the Adult V-SVZ Neural Stem Cell Lineage. Journal of Neuroscience. 2015;35:4528–4539. doi: 10.1523/JNEUROSCI.1188-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Ramirez-Castillejo C, Sanchez-Sanchez F, Andreu-Agullo C, Ferron SR, Aroca-Aguilar JD, Sanchez P, Mira H, Escribano J, Farinas I. Pigment epithelium-derived factor is a niche signal for neural stem cell renewal. Nature Neuroscience. 2006;9:331–339. doi: 10.1038/nn1657. [DOI] [PubMed] [Google Scholar]
- 83.Delgado AC, Ferron SR, Vicente D, Porlan E, Perez-Villalba A, Trujillo CM, D'Ocon P, Farinas I. Endothelial NT-3 Delivered by Vasculature and CSF Promotes Quiescence of Subependymal Neural Stem Cells through Nitric Oxide Induction. Neuron. 2014;83:572–585. doi: 10.1016/j.neuron.2014.06.015. [DOI] [PubMed] [Google Scholar]
- 84.Ottone C, Krusche B, Whitby A, Clements M, Quadrato G, Pitulescu ME, Adams RH, Parrinello S. Direct cell-cell contact with the vascular niche maintains quiescent neural stem cells. Nat Cell Biol. 2014;16:1045–1056. doi: 10.1038/ncb3045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Mathieu C, Sii-Felice K, Fouchet P, Etienne O, Haton C, Mabondzo A, Boussin FD, Mouthon MA. Endothelial cell-derived bone morphogenetic proteins control proliferation of neural stem/progenitor cells. Molecular and Cellular Neuroscience. 2008;38:569–577. doi: 10.1016/j.mcn.2008.05.005. [DOI] [PubMed] [Google Scholar]
- 86.Kokovay E, Goderie S, Wang Y, Lotz S, Lin G, Sun Y, Roysam B, Shen Q, Temple S. Adult SVZ Lineage Cells Home to and Leave the Vascular Niche via Differential Responses to SDF1/CXCR4 Signaling. Cell Stem Cell. 2010;7:163–173. doi: 10.1016/j.stem.2010.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Saito D, Takase Y, Murai H, Takahashi Y. The Dorsal Aorta Initiates a Molecular Cascade That Instructs Sympatho-Adrenal Specification. Science. 2012;336:1578–1581. doi: 10.1126/science.1222369. [DOI] [PubMed] [Google Scholar]
- 88.Won C, Lin ZC, Kumar TP, Li SY, Ding L, Elkhal A, Szabo G, Vasudevan A. Autonomous vascular networks synchronize GABA neuron migration in the embryonic forebrain. Nature Communications. 2013;4 doi: 10.1038/ncomms3149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Jung J, Zheng M, Goldfarb M, Zaret KS. Initiation of mammalian liver development from endoderm by fibroblast growth factors. Science. 1999;284:1998–2003. doi: 10.1126/science.284.5422.1998. [DOI] [PubMed] [Google Scholar]
- 90.Rossi JM, Dunn NR, Hogan BL, Zaret KS. Distinct mesodermal signals, including BMPs from the septum transversum mesenchyme, are required in combination for hepatogenesis from the endoderm. Genes Dev. 2001;15:1998–2009. doi: 10.1101/gad.904601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.LeCouter J, Moritz DR, Li B, Phillips GL, Liang XH, Gerber HP, Hillan KJ, Ferrara N. Angiogenesis-independent endothelial protection of liver: role of VEGFR-1. Science. 2003;299:890–893. doi: 10.1126/science.1079562. [DOI] [PubMed] [Google Scholar]
- 92.Ding BS, Nolan DJ, Butler JM, James D, Babazadeh AO, Rosenwaks Z, Mittal V, Kobayashi H, Shido K, Lyden D, et al. Inductive angiocrine signals from sinusoidal endothelium are required for liver regeneration. Nature. 2010;468:310–315. doi: 10.1038/nature09493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Nejak-Bowen K, Orr A, Bowen WC, Jr., Michalopoulos GK. Conditional genetic elimination of hepatocyte growth factor in mice compromises liver regeneration after partial hepatectomy. PLoS One. 2013;8:e59836. doi: 10.1371/journal.pone.0059836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Burri PH. Fetal and postnatal development of the lung. Annu Rev Physiol. 1984;46:617–628. doi: 10.1146/annurev.ph.46.030184.003153. [DOI] [PubMed] [Google Scholar]
- 95.Gerber HP, Hillan KJ, Ryan AM, Kowalski J, Keller GA, Rangell L, Wright BD, Radtke F, Aguet M, Ferrara N. VEGF is required for growth and survival in neonatal mice. Development. 1999;126:1149–1159. doi: 10.1242/dev.126.6.1149. [DOI] [PubMed] [Google Scholar]
- 96.Schwarz MA, Zhang F, Gebb S, Starnes V, Warburton D. Endothelial monocyte activating polypeptide II inhibits lung neovascularization and airway epithelial morphogenesis. Mech Dev. 2000;95:123–132. doi: 10.1016/s0925-4773(00)00361-0. [DOI] [PubMed] [Google Scholar]
- 97.Zeng X, Wert SE, Federici R, Peters KG, Whitsett JA. VEGF enhances pulmonary vasculogenesis and disrupts lung morphogenesis in vivo. Dev Dyn. 1998;211:215–227. doi: 10.1002/(SICI)1097-0177(199803)211:3<215::AID-AJA3>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 98.Del Moral PM, Sala FG, Tefft D, Shi W, Keshet E, Bellusci S, Warburton D. VEGF-A signaling through Flk-1 is a critical facilitator of early embryonic lung epithelial to endothelial crosstalk and branching morphogenesis. Dev Biol. 2006;290:177–188. doi: 10.1016/j.ydbio.2005.11.022. [DOI] [PubMed] [Google Scholar]
- 99.Majumdar A, Drummond IA. Podocyte differentiation in the absence of endothelial cells as revealed in the zebrafish avascular mutant, cloche. Dev Genet. 1999;24:220–229. doi: 10.1002/(SICI)1520-6408(1999)24:3/4<220::AID-DVG5>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 100.Mattot V, Moons L, Lupu F, Chernavvsky D, Gomez RA, Collen D, Carmeliet P. Loss of the VEGF(164) and VEGF(188) isoforms impairs postnatal glomerular angiogenesis and renal arteriogenesis in mice. J Am Soc Nephrol. 2002;13:1548–1560. doi: 10.1097/01.asn.0000013925.19218.7b. [DOI] [PubMed] [Google Scholar]
- 101.Eremina V, Sood M, Haigh J, Nagy A, Lajoie G, Ferrara N, Gerber HP, Kikkawa Y, Miner JH, Quaggin SE. Glomerular-specific alterations of VEGF-A expression lead to distinct congenital and acquired renal diseases. J Clin Invest. 2003;111:707–716. doi: 10.1172/JCI17423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Eremina V, Cui S, Gerber H, Ferrara N, Haigh J, Nagy A, Ema M, Rossant J, Jothy S, Miner JH, et al. Vascular endothelial growth factor a signaling in the podocyte-endothelial compartment is required for mesangial cell migration and survival. J Am Soc Nephrol. 2006;17:724–735. doi: 10.1681/ASN.2005080810. [DOI] [PubMed] [Google Scholar]
- 103.Veron D, Reidy K, Marlier A, Bertuccio C, Villegas G, Jimenez J, Kashgarian M, Tufro A. Induction of podocyte VEGF164 overexpression at different stages of development causes congenital nephrosis or steroid-resistant nephrotic syndrome. Am J Pathol. 2010;177:2225–2233. doi: 10.2353/ajpath.2010.091146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Robert B, St John PL, Abrahamson DR. Direct visualization of renal vascular morphogenesis in Flk1 heterozygous mutant mice. Am J Physiol. 1998;275:F164–172. doi: 10.1152/ajprenal.1998.275.1.F164. [DOI] [PubMed] [Google Scholar]
- 105.Gao X, Chen X, Taglienti M, Rumballe B, Little MH, Kreidberg JA. Angioblast-mesenchyme induction of early kidney development is mediated by Wt1 and Vegfa. Development. 2005;132:5437–5449. doi: 10.1242/dev.02095. [DOI] [PubMed] [Google Scholar]
- 106.Schrijvers BF, Flyvbjerg A, De Vriese AS. The role of vascular endothelial growth factor (VEGF) in renal pathophysiology. Kidney Int. 2004;65:2003–2017. doi: 10.1111/j.1523-1755.2004.00621.x. [DOI] [PubMed] [Google Scholar]
- 107.Villasenor A, Chong DC, Henkemeyer M, Cleaver O. Epithelial dynamics of pancreatic branching morphogenesis. Development. 2010;137:4295–4305. doi: 10.1242/dev.052993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Hick AC, van Eyll JM, Cordi S, Forez C, Passante L, Kohara H, Nagasawa T, Vanderhaeghen P, Courtoy PJ, Rousseau GG, et al. Mechanism of primitive duct formation in the pancreas and submandibular glands: a role for SDF-1. BMC Dev Biol. 2009;9:66. doi: 10.1186/1471-213X-9-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Kesavan G, Sand FW, Greiner TU, Johansson JK, Kobberup S, Wu X, Brakebusch C, Semb H. Cdc42-mediated tubulogenesis controls cell specification. Cell. 2009;139:791–801. doi: 10.1016/j.cell.2009.08.049. [DOI] [PubMed] [Google Scholar]
- 110.Stanger BZ, Tanaka AJ, Melton DA. Organ size is limited by the number of embryonic progenitor cells in the pancreas but not the liver. Nature. 2007;445:886–891. doi: 10.1038/nature05537. [DOI] [PubMed] [Google Scholar]
- 111.Pictet RL, Rutter WJ. Development of the embryonic pancreas. In: Steiner DF, Frienkel N, editors. Endocrine Pancreas. Handbook of Physiology. Vol. 1. Williams & Wilkins; Baltimore: 1972. pp. 25–66. [Google Scholar]
- 112.Pan FC, Wright C. Pancreas organogenesis: from bud to plexus to gland. Dev Dyn. 2011;240:530–565. doi: 10.1002/dvdy.22584. [DOI] [PubMed] [Google Scholar]
- 113.Zhou Q, Law AC, Rajagopal J, Anderson WJ, Gray PA, Melton DA. A multipotent progenitor domain guides pancreatic organogenesis. Dev Cell. 2007;13:103–114. doi: 10.1016/j.devcel.2007.06.001. [DOI] [PubMed] [Google Scholar]
- 114.Katsumoto K, Kume S. Endoderm and mesoderm reciprocal signaling mediated by CXCL12 and CXCR4 regulates the migration of angioblasts and establishes the pancreatic fate. Development. 2011;138:1947–1955. doi: 10.1242/dev.058719. [DOI] [PubMed] [Google Scholar]
- 115.Magenheim J, Ilovich O, Lazarus A, Klochendler A, Ziv O, Werman R, Hija A, Cleaver O, Mishani E, Keshet E, et al. Blood vessels restrain pancreas branching, differentiation and growth. Development. 2011 doi: 10.1242/dev.066548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Kao DI, Lacko LA, Ding BS, Huang C, Phung K, Gu G, Rafii S, Stuhlmann H, Chen S. Endothelial cells control pancreatic cell fate at defined stages through EGFL7 signaling. Stem Cell Reports. 2015;4:181–189. doi: 10.1016/j.stemcr.2014.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Cai Q, Brissova M, Reinert RB, Pan FC, Brahmachary P, Jeansson M, Shostak A, Radhika A, Poffenberger G, Quaggin SE, et al. Enhanced expression of VEGF-A in beta cells increases endothelial cell number but impairs islet morphogenesis and beta cell proliferation. Dev Biol. 2012;367:40–54. doi: 10.1016/j.ydbio.2012.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Johansson M, Mattsson G, Andersson A, Jansson L, Carlsson PO. Islet endothelial cells and pancreatic beta-cell proliferation: studies in vitro and during pregnancy in adult rats. Endocrinology. 2006;147:2315–2324. doi: 10.1210/en.2005-0997. [DOI] [PubMed] [Google Scholar]
- 119.Almaca J, Molina J, Arrojo EDR, Abdulreda MH, Jeon WB, Berggren PO, Caicedo A, Nam HG. Young capillary vessels rejuvenate aged pancreatic islets. Proc Natl Acad Sci U S A. 2014;111:17612–17617. doi: 10.1073/pnas.1414053111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Reinert RB, Brissova M, Shostak A, Pan FC, Poffenberger G, Cai Q, Hundemer GL, Kantz J, Thompson CS, Dai C, et al. Vascular endothelial growth factor-a and islet vascularization are necessary in developing, but not adult, pancreatic islets. Diabetes. 2013;62:4154–4164. doi: 10.2337/db13-0071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.D'Hoker J, De Leu N, Heremans Y, Baeyens L, Minami K, Ying C, Lavens A, Chintinne M, Stange G, Magenheim J, et al. Conditional hypovascularization and hypoxia in islets do not overtly influence adult beta-cell mass or function. Diabetes. 2013;62:4165–4173. doi: 10.2337/db12-1827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Aird WC. Endothelial cell heterogeneity. Crit Care Med. 2003;31:S221–230. doi: 10.1097/01.CCM.0000057847.32590.C1. [DOI] [PubMed] [Google Scholar]
- 123.Chi JT, Chang HY, Haraldsen G, Jahnsen FL, Troyanskaya OG, Chang DS, Wang Z, Rockson SG, van de Rijn M, Botstein D, et al. Endothelial cell diversity revealed by global expression profiling. Proc Natl Acad Sci U S A. 2003;100:10623–10628. doi: 10.1073/pnas.1434429100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Nolan DJ, Ginsberg M, Israely E, Palikuqi B, Poulos MG, James D, Ding BS, Schachterle W, Liu Y, Rosenwaks Z, et al. Molecular signatures of tissue-specific microvascular endothelial cell heterogeneity in organ maintenance and regeneration. Dev Cell. 2013;26:204–219. doi: 10.1016/j.devcel.2013.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Passerini AG, Polacek DC, Shi C, Francesco NM, Manduchi E, Grant GR, Pritchard WF, Powell S, Chang GY, Stoeckert CJ, Jr., et al. Coexisting proinflammatory and antioxidative endothelial transcription profiles in a disturbed flow region of the adult porcine aorta. Proc Natl Acad Sci U S A. 2004;101:2482–2487. doi: 10.1073/pnas.0305938101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Pusztaszeri MP, Seelentag W, Bosman FT. Immunohistochemical expression of endothelial markers CD31, CD34, von Willebrand factor, and Fli-1 in normal human tissues. J Histochem Cytochem. 2006;54:385–395. doi: 10.1369/jhc.4A6514.2005. [DOI] [PubMed] [Google Scholar]
- 127.Zhang J, Burridge KA, Friedman MH. In vivo differences between endothelial transcriptional profiles of coronary and iliac arteries revealed by microarray analysis. Am J Physiol Heart Circ Physiol. 2008;295:H1556–1561. doi: 10.1152/ajpheart.00540.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Ruoslahti E, Rajotte D. An address system in the vasculature of normal tissues and tumors. Annu Rev Immunol. 2000;18:813–827. doi: 10.1146/annurev.immunol.18.1.813. [DOI] [PubMed] [Google Scholar]
- 129.Simonson AB, Schnitzer JE. Vascular proteomic mapping in vivo. J Thromb Haemost. 2007;5(Suppl 1):183–187. doi: 10.1111/j.1538-7836.2007.02551.x. [DOI] [PubMed] [Google Scholar]
- 130.Mason C, Dunnill P. A brief definition of regenerative medicine. Regen Med. 2008;3:1–5. doi: 10.2217/17460751.3.1.1. [DOI] [PubMed] [Google Scholar]
- 131.Ding BS, Nolan DJ, Guo P, Babazadeh AO, Cao Z, Rosenwaks Z, Crystal RG, Simons M, Sato TN, Worgall S, et al. Endothelial-derived angiocrine signals induce and sustain regenerative lung alveolarization. Cell. 2011;147:539–553. doi: 10.1016/j.cell.2011.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Butler JM, Kobayashi H, Rafii S. Instructive role of the vascular niche in promoting tumour growth and tissue repair by angiocrine factors. Nat Rev Cancer. 2010;10:138–146. doi: 10.1038/nrc2791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Greenberg DA, Jin K. From angiogenesis to neuropathology. Nature. 2005;438:954–959. doi: 10.1038/nature04481. [DOI] [PubMed] [Google Scholar]
- 134.Ohab JJ, Fleming S, Blesch A, Carmichael ST. A neurovascular niche for neurogenesis after stroke. J Neurosci. 2006;26:13007–13016. doi: 10.1523/JNEUROSCI.4323-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Wang B, Zhao L, Fish M, Logan CY, Nusse R. Self-renewing diploid Axin2(+) cells fuel homeostatic renewal of the liver. Nature. 2015;524:180–185. doi: 10.1038/nature14863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Ding BS, Cao Z, Lis R, Nolan DJ, Guo P, Simons M, Penfold ME, Shido K, Rabbany SY, Rafii S. Divergent angiocrine signals from vascular niche balance liver regeneration and fibrosis. Nature. 2014;505:97–102. doi: 10.1038/nature12681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Lee JH, Bhang DH, Beede A, Huang TL, Stripp BR, Bloch KD, Wagers AJ, Tseng YH, Ryeom S, Kim CF. Lung stem cell differentiation in mice directed by endothelial cells via a BMP4-NFATc1-thrombospondin-1 axis. Cell. 2014;156:440–455. doi: 10.1016/j.cell.2013.12.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Rookmaaker MB, Schutgens F, Verhaar MC, Clevers H. Development and application of human adult stem or progenitor cell organoids. Nat Rev Nephrol. 2015;11:546–554. doi: 10.1038/nrneph.2015.118. [DOI] [PubMed] [Google Scholar]
- 139.Takebe T, Zhang RR, Koike H, Kimura M, Yoshizawa E, Enomura M, Koike N, Sekine K, Taniguchi H. Generation of a vascularized and functional human liver from an iPSC-derived organ bud transplant. Nat Protoc. 2014;9:396–409. doi: 10.1038/nprot.2014.020. [DOI] [PubMed] [Google Scholar]
- 140.Takasato M, Er PX, Chiu HS, Maier B, Baillie GJ, Ferguson C, Parton RG, Wolvetang EJ, Roost MS, Chuva de Sousa Lopes SM, et al. Kidney organoids from human iPS cells contain multiple lineages and model human nephrogenesis. Nature. 2015;526:564–568. doi: 10.1038/nature15695. [DOI] [PubMed] [Google Scholar]
- 141.Takebe T, Enomura M, Yoshizawa E, Kimura M, Koike H, Ueno Y, Matsuzaki T, Yamazaki T, Toyohara T, Osafune K, et al. Vascularized and Complex Organ Buds from Diverse Tissues via Mesenchymal Cell-Driven Condensation. Cell Stem Cell. 2015;16:556–565. doi: 10.1016/j.stem.2015.03.004. [DOI] [PubMed] [Google Scholar]
- 142.Ding L, Morrison SJ. Haematopoietic stem cells and early lymphoid progenitors occupy distinct bone marrow niches. Nature. 2013;495:231–235. doi: 10.1038/nature11885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Poulos MG, Guo P, Kofler NM, Pinho S, Gutkin MC, Tikhonova A, Aifantis I, Frenette PS, Kitajewski J, Rafii S, et al. Endothelial Jagged-1 is necessary for homeostatic and regenerative hematopoiesis. Cell Rep. 2013;4:1022–1034. doi: 10.1016/j.celrep.2013.07.048. [DOI] [PMC free article] [PubMed] [Google Scholar]