Abstract
OBJECTIVE
Sturge-Weber syndrome (SWS) is often accompanied by seizures and neurocognitive deterioration, although previous studies have suggested that early functional brain reorganization may diminish the cognitive sequelae in some children with unilateral SWS. The ‘rules’ governing these plasticity mechanisms are poorly understood. In this study we evaluated longitudinal changes of cognitive functioning (IQ) and assessed the performance of clinical, EEG, and MRI variables for predicting IQ in children with SWS.
METHODS
Thirty-three young children (mean age: 3.3 years at baseline) with unilateral SWS underwent MRI, scalp EEG and neuro-psychology evaluation twice, with a median follow-up of two years. None of the children had epilepsy surgery. Longitudinal IQ changes were calculated. Seizure variables, interictal EEG abnormalities, as well as extent and location of MRI brain involvement were correlated with IQ assessed at follow-up.
RESULTS
Global IQ showed a highly variable course with both increases and decreases over time. Lower IQ at baseline was associated with interval IQ increase. In univariate analyses, lower outcome IQ was associated with baseline EEG abnormalities (p<0.001), young age at seizure onset (p=0.001), high seizure frequency (p=0.02), and early frontal lobe involvement on MRI (p=0.01). In multivariate analysis EEG abnormalities at baseline remained a robust, independent predictor of outcome IQ.
CONCLUSIONS
The early trajectory of cognitive changes in children with unilateral SWS is highly variable; children with improving IQ likely undergo effective unimpeded functional reorganization. Early onset, frequent seizures and interictal epileptiform abnormalities on EEG likely interfere with this process resulting in poor cognitive functions. Future studies assessing interventions should target this high-risk subgroup to optimize cognitive outcome in SWS.
Keywords: Sturge-Weber syndrome, epilepsy, cognitive functions, longitudinal study, EEG, MRI
INTRODUCTION
Sturge-Weber syndrome (SWS) is a congenital neurocutaneous disorder characterized by facial venous capillary malformation (port wine stain), leptomeningeal venous malformation (which is unilateral in most cases) and glaucoma1. SWS brain involvement is typically diagnosed by magnetic resonance imaging (MRI), where post-contrast images show leptomeningeal enhancement, enlarged deep veins and other venous vessel and parenchymal abnormalities2. Most common neurological manifestations of SWS are seizures, stroke-like episodes, hemiparesis and visual field deficit3. In addition, about half of the patients develop cognitive impairment4–6, which is a major factor affecting quality of life in these patients. Although SWS is often a progressive disorder, the natural course of neurocognitive changes and factors governing these changes are poorly understood.
Previous retrospective and cross-sectional studies have identified a number of potential clinical and imaging variables associated with cognitive impairment in SWS. Among these, bilateral brain involvement, early seizure onset, and high seizure frequency were related to poor cognitive functions7,8. In patients with unilateral SWS brain involvement, frontal lobe damage and extent of cortical atrophy on MRI were also implicated in cognitive impairment8,9. In a quantitative MRI study of children with unilateral SWS, decreased white matter volume in the affected hemisphere was associated with impaired cognitive functions10. Cortical and thalamic hypometabolism, measured by 2-[18F]fluoro-2-deoxy-D-glucose positron emission tomography (FDG-PET), was also associated with poor cognitive functions11,12. Our group has reported surprisingly preserved cognitive functions (IQ) in a subset of children with SWS and early, extensive unilateral hemispheric damage (corresponding to severe hypometabolism on PET)12,13; such children likely underwent early, effective functional reorganization in the unaffected hemisphere due to extensive unilateral damage. However, predictors of cognitive outcome in SWS have not been identified in prospective, longitudinal studies.
Electroencephalography (EEG) is a simple, inexpensive, outpatient clinical test to evaluate epileptiform abnormalities and brain dysfunction. In SWS, EEG background activity is often attenuated and/or slowed in the affected hemisphere; interictal epileptiform activity is also not uncommon3,14. A study utilizing quantitative EEG analysis found a positive correlation between the EEG asymmetry and severity of neurological as well as MRI abnormalities15. Another study showed that quantitative EEG correctly identified infants with SWS brain involvement even before the MRI revealed abnormalities16. A recent EEG study in SWS patients found a positive correlation between the severity of EEG abnormalities and age, suggesting a progressive evolution of EEG abnormalities, although there was no correlation with severity of neurological symptoms or seizure frequency scores17. Whether simple EEG variables can contribute to prediction of neurocognitive functions remains to be determined.
The main purpose of the present study was to evaluate longitudinal changes of cognitive functions in children with unilateral SWS. In particular, we assessed if simple, readily obtainable clinical, EEG and/or neuroimaging variables could predict the severity of epilepsy and cognitive outcome at follow-up during the early clinical course of SWS.
SUBJECTS AND METHODS
Data were collected from children with SWS who were enrolled in a prospective, longitudinal clinical and neuroimaging study between July 1, 2003 and December 31, 2015 at the Children's Hospital of Michigan, Wayne State University School of Medicine in Detroit. Inclusion criteria: i. evidence of unilateral SWS brain involvement, such as leptomeningeal venous malformation and/or enlarged deep medullary veins (with or without enlarged choroid plexus) on contrast-enhanced MRI; ii. age between 6 months and 10 years. Exclusion criteria: i. bilateral brain involvement; ii. previous epilepsy surgery. Thirty-three children (mean age: 3.3 years; age range: 0.5–8.2 years at baseline; 21 girls) fulfilled these criteria and were included in the study. Thirty-one of these 33 children had a history of epilepsy, with a mean age of seizure onset at 1.2 years (range: 0–6 years). Five children were not on any antiepileptic drug (AED) at baseline. Four of these five patients were not on AEDs at follow-up either, while the fifth child (#5) was on two AEDs at follow-up (see data in Table 1.). Five patients were on phenobarbital (PHB) therapy at baseline but only one child was still on PHB at follow-up from these five patients, while another child was started on PHB between baseline and follow-up. Eleven of the 33 children received multiple AEDs at baseline and 10 children at follow-up (Table 1.). All patients had MRI and scalp EEG performed at baseline. The median follow-up period was 2 years (range: 1–8 years). Patients underwent formal neurocognitive assessment performed by a licensed neuropsychologist both at baseline and follow-up. The study was approved by the Human Investigation Committee at Wayne State University, and written informed consent of the parent or legal guardian and verbal assent (age 7 years and above) was obtained.
Table 1.
Clinical data of 33 children with unilateral Sturge-Weber syndrome. Patients are listed from the youngest to the oldest.
| Patient no. |
Gender | Age at baseline (year) |
Age at follow-up (year) |
AAO (year) |
Affected Side |
Lobe(s) involved |
Baseline EEG severity score |
Outcome seizure frequency score |
Baseline GIQ |
Outcome GIQ |
GIQ Change |
AEDs at baseline |
AEDs at follow-up |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | F | 0.5 | 3.8 | 0 | L | Fr | 1 | 0 | 92 | 91 | −1 | OXC | OXC |
| 2 | F | 0.6 | 2.6 | 0.1 | R | FrTPO | 3 | 1 | 70 | 72 | 2 | OXC,LEV | OXC,PHB,LAC |
| 3 | F | 0.7 | 8.9 | 0.3 | L | TPO | 1 | 0 | 82 | CBZ,LAM | None | ||
| 4 | M | 0.9 | 2.7 | 0.2 | R | FrTPO | 1 | 2 | 53 | 60 | 7 | PHB | OXC |
| 5 | F | 0.9 | 6.0 | 2.5 | R | FrTPO | 0 | 1 | 111 | None | OXC,GBP | ||
| 6 | F | 1.0 | 3.4 | 0.1 | L | PTO | 1 | 1 | 78 | CBZ | CBZ | ||
| 7 | F | 1.3 | 4.5 | 0.3 | L | FrTPO | 1 | 1 | 55 | PHB | OXC,VPA,TPX | ||
| 8 | F | 1.4 | 3.4 | no seizure | L | PO | 0 | 0 | 75 | 104 | 29 | None | None |
| 9 | F | 1.5 | 2.5 | 0.7 | L | FrTPO | 1 | 1 | 89 | OXC | OXC | ||
| 10 | F | 1.8 | 3.8 | 1.5 | R | FrTPO | 1 | 0 | 76 | 118 | 42 | OXC | LEV |
| 11 | F | 1.8 | 3.0 | 0.4 | R | FrTPO | 0 | 1 | 88 | LEV, OXC | LEV,OXC | ||
| 12 | M | 1.9 | 3.0 | 0.5 | R | PFr | 1 | 0 | 58 | 92 | 34 | CBZ | CBZ |
| 13 | M | 2.3 | 4.3 | 0.4 | L | FrTPO | 2 | 1 | 71 | 78 | 7 | PHB, OXC | OXC |
| 14 | M | 2.3 | 3.7 | 0.6 | L | TPO | 0 | 1 | 70 | 86 | 16 | LEV, ZON | LEV,ZON |
| 15 | F | 2.5 | 4.5 | 2.4 | L | P | 0 | 0 | 85 | 90 | 5 | OXC | None |
| 16 | F | 2.5 | 4.5 | 2.0 | L | P | 0 | 0 | 102 | 96 | −6 | None | LAM |
| 17 | F | 2.6 | 4.1 | 0.5 | L | TPO | 0 | 0 | 102 | 87 | −15 | VPA | VPA |
| 18 | M | 2.8 | 3.8 | 1.6 | R | TPO | 0 | 0 | 92 | LEV | OXC | ||
| 19 | M | 3.3 | 5.4 | 0.9 | R | P | 0 | 1 | 101 | 101 | 0 | LEV, OXC | LEV,OXC,LAM |
| 20 | M | 3.5 | 6.3 | 1.8 | R | FrTPO | 2 | 0 | 55 | CBZ,LAM,CLO | CBZ,LEV | ||
| 21 | F | 3.5 | 5.5 | 3.5 | R | P | 0 | 0 | 100 | 116 | 16 | None | None |
| 22 | F | 3.6 | 6.0 | 0.9 | R | P | 0 | 1 | 128 | 110 | −18 | OXC,LEV | OXC,LEV |
| 23 | F | 3.9 | 6.0 | 0.3 | L | FrTPO | 1 | 0 | 60 | 69 | 9 | OXC | OXC |
| 24 | M | 3.9 | 4.9 | 3.5 | L | TPO | 1 | 3 | 110 | 92 | −18 | OXC | VPA |
| 25 | F | 4.5 | 5.5 | 0.3 | R | FrTPO | 1 | 0 | 94 | 90 | −4 | LEV,OXC | LEV,OXC |
| 26 | F | 5.0 | 8.2 | 4.5 | R | O | 0 | 1 | 98 | 102 | 4 | TPX | LEV |
| 27 | F | 5.5 | 7.5 | 0.5 | L | TPO | 0 | 0 | 117 | 107 | −10 | PHB | PHB |
| 28 | M | 6.0 | 8.7 | 0.5 | L | FrO | 3 | 3 | 45 | 45 | 0 | VPA,LEV | VPA,CLO,ZON |
| 29 | M | 6.2 | 7.1 | 1.5 | L | FrP | 0 | 2 | 81 | 78 | −3 | CBZ | CBZ |
| 30 | F | 7.0 | 9.0 | 6 | L | FrP | 2 | 1 | 90 | 87 | −3 | LEV | OXC |
| 31 | M | 7.4 | 10.3 | no seizure | R | PO | 2 | 0 | 69 | 73 | 4 | None | None |
| 32 | F | 8.1 | 10.7 | 0.2 | L | FrTPO | 2 | 0 | 55 | 52 | −3 | CBZ | LAM |
| 33 | M | 8.2 | 12.3 | 0.6 | L | PO | 0 | 1 | 100 | 93 | −7 | PHB,LEV | OXC |
Abbreviations: F=female, M=male; L=left, R=right; Fr=frontal, T=temporal, P=parietal, O=occipital; AAO=age at epilepsy onset, GIQ=Global IQ (estimated from Index of Mental Development from BSID2 in children <2.5 years of age), AEDs=antiepileptic drugs; OXC=oxcarbazepine, LEV=levetiracetam, PHB=phenobarbital, LAC=lacosamide, CBZ=carbamazepine, VPA=valproic acid, LAM=lamotrigine, GBP=gabapentin, ZON=zonisamide, CLO=clonazepam, TPX= topiramate
Assessment of cognitive functioning
Neurocognitive assessment was performed within 24 hours of the MRI and EEG studies. Children 6–30 months of age completed the Bayley Scales of Infant Development-2nd Edition (BSID2); children 30–87 months of age were administered the Wechsler Pre-primary and Pre-school Scale of Intelligence-III (WPPSI-III), Third Edition, and children older than 87 months, the Wechsler Intelligence Scales for Children-III (WISC-III), Third Edition. The BSID2 provides an overall index of mental development (MDI), which was used for a global IQ measure for the youngest patients. Both of the above Wechsler scales provide indices of global (GIQ), verbal (VIQ), and nonverbal (PIQ) cognitive functioning. Reliable baseline IQ could be obtained from 25 patients and outcome IQ from all patients. In patients with both baseline and outcome IQ, an IQ change was calculated for the GIQ (BSID2 MDI or Wechsler GIQ [Time 2−Time 1]). Each of the above measures has been demonstrated to have good psychometric properties and is widely used in clinical and research populations, and reliability estimates for both Wechsler measures have been shown to be good18. For the WPPSI-III, test-retest estimates for preschool age children (age 2:6 to 3:11 years old) for verbal, performance, and full scale IQ were .90, .84, .92, respectively; estimates are slightly higher for 4–7.3 year old age group19, and for the WISC-III verbal, performance and full scale indices20.
Seizure frequency scores
Clinical seizure frequency was evaluated by parent interviews and medical charts, and a seizure frequency score was assigned to each patient based on clinical seizures occurring during the one year period prior to the imaging study (or since seizure onset, if seizures started less than one year before the study). The scoring system was slightly modified from a previous study on children with SWS13, and the scores were determined as follows: 0: no seizure in the last 1 year; 1: 1–11 seizures per year; 2: 1–4 seizures per month; 3: >4 seizures per month. Similar scores were also determined at follow-up. The two patients with no seizures at all were scored with 0. Since most patients presented with focal motor seizures, seizure type was not separately included. Two patients had a history of infantile spams but presented with partial seizures at the time of the evaluation. One patient (#26 in Table 1) with pure occipital involvement on MRI had simple partial seizures without a clear motor component.
EEG acquisition and assessment
Scalp electrodes were applied according to the International 10/20 system electrode placement, and EEG was recorded for a 30-min period using a Nihon Kohden digital system (Nihon Kohden America Inc, Foothill Ranch, CA, USA). EEG was reviewed and reported by a board certificated pediatric neurophysiologist [E.A.]. EEG abnormalities were then assessed in two ways: i. by an EEG spike frequency score, 0–3 (0: none; 1: rare [<1/min]; 2: occasional [1–10/min]; 3: frequent epileptiform activity [>10/min]); ii. EEG severity score, adopted from a previous study17: scores were 0–3 (where 0: normal EEG; 1: focal voltage asymmetry [with or without slowing] without epileptiform discharges; 2: sporadic, unilateral epileptiform activity [≤10/min]; 3: frequent epileptiform activity [>10/min]). The frequency of epileptiform discharges was based on the area/side showing the highest spike frequency. In case of bilateral epileptiform activity, higher frequency typically occurred on the side of the SWS brain lesion (except two cases), and none of the EEGs showed generalized epileptiform discharges.
MRI acquisition and assessment
During MRI acquisition, children were sedated with pentobarbital (1.5–3 mcg/kg) and midazolam (0.1–0.2 mcg/kg); or by midazolam (0.1 – 0.2 mcg/kg) followed by dexmedetomidine (1–2 mcg/kg), titrated slowly to achieve mild to moderate sedation. In some cases, fentanyl (1 mcg/kg) was used as necessary in conjunction with either pentobarbital or midazolam. All sedated subjects were continuously monitored by a pediatric nurse with special training in the sedation of children for radiological procedures, and physiological parameters (heart rate, pulse oximetry) were monitored during the study. The typical MRI acquisition included an axial T1 3D magnetization-prepared rapid gradient-echo (MPRAGE, with 1mm slice thickness), an axial fluid attenuated inversion recovery (FLAIR), an axial T2-weighted turbo spin-echo acquisition, susceptibility weighted imaging, diffusion tensor imaging (DTI), followed by dynamic contrast enhanced MR perfusion-weighted imaging and a post-gadolinium axial 3D MPRAGE acquisition (using the same imaging parameters as the first MPRAGE acquisition). The total scanning time was kept below 60 minutes. Gadolinium-DTPA (Magnevist, Berlex, USA) was injected in a bolus via a peripheral vein with a dose of 0.1 mmol/kg of body weight. No adverse events associated with sedation were reported.
The hemispheric extent of MRI brain involvement was determined by the baseline MRI and was expressed as the number of affected lobes (ranging 1–4). Lobe(s) showing leptomeningeal enhancement, and/or deep transmedullary veins (with or without enlarged choroid plexus, atrophy and calcification) were considered to be involved.
Statistical Analysis
After performing descriptive statistical analyses, first univariate correlations (using Spearman’s rank correlation) and group comparisons (using Mann-Whitney U test for binary outcome variables such as side and frontal lobe involvement) were performed to assess the predictive value of clinical, EEG and MRI variables on cognitive functions (IQ) and seizure frequency assessed at follow-up. In the univariate correlations, predictors included age (at enrollment), follow-up time, baseline epilepsy variables (age at seizure onset, duration of epilepsy, seizure frequency score), number of AEDs, extent of brain involvement on MRI, and EEG variables (EEG severity score and spike frequency score). Outcome variables included the seizure frequency score and IQ measures obtained at follow-up. We evaluated the role of early frontal lobe damage based on our previous cross-sectional study showing that frontal lobe white matter abnormalities on diffusion MRI were commonly associated with low IQ in SWS children21. In a second step, a binary logistic regression analysis was performed including predictors that showed significant (p<0.05) correlations or group differences in the univariate analyses. For this analysis, the outcome IQ was dichotomized using IQ=85 (i.e., 1 standard deviation below normal mean) as the cutoff threshold. A p value of less than 0.05 was considered to be significant.
RESULTS
The median number of affected lobes on MRI was 3 (range: 0–4), the median seizure frequency score at baseline was 1 (see individual data in Table 1). Mean baseline GIQ was 84 (range: 45–128), and outcome IQ was 86 (range: 45–118). There were no gender differences in baseline or outcome IQs. Baseline EEG was normal in 14 children (42%), showed only background asymmetry (score 1) in 12, and showed occasional or frequent epileptiform activity (scores 2–3) in the remaining 7.
Longitudinal changes in cognitive functions
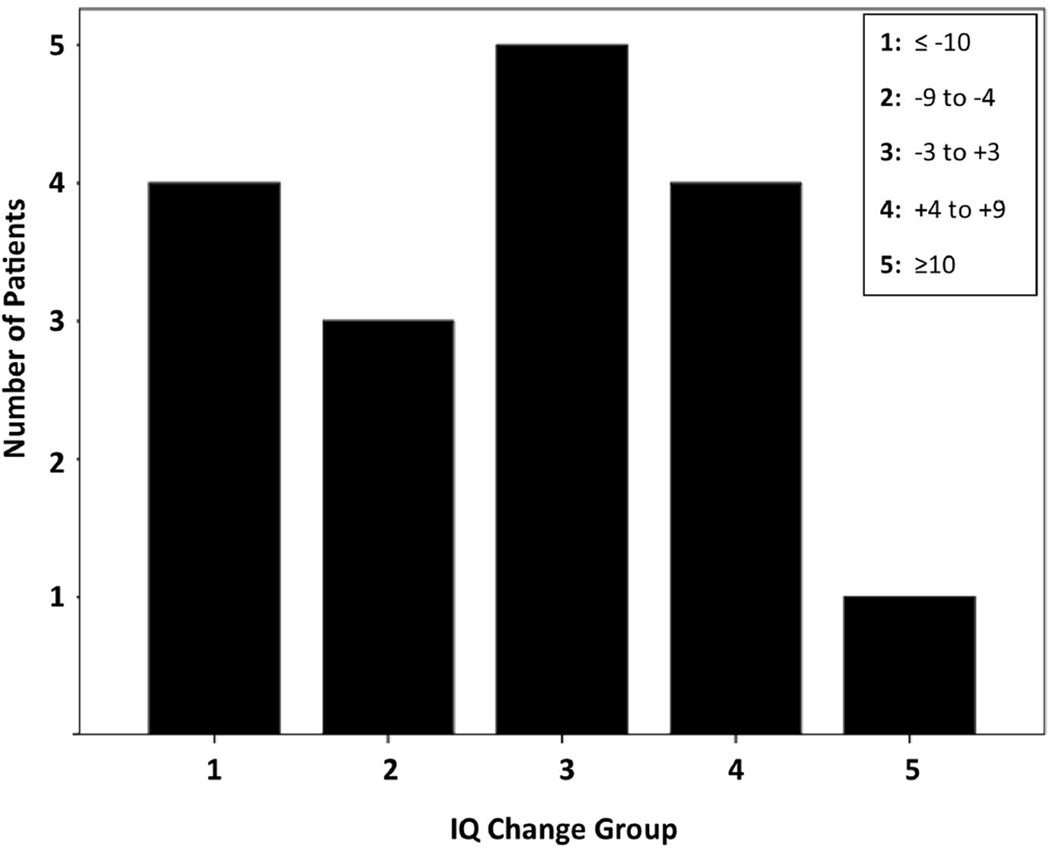
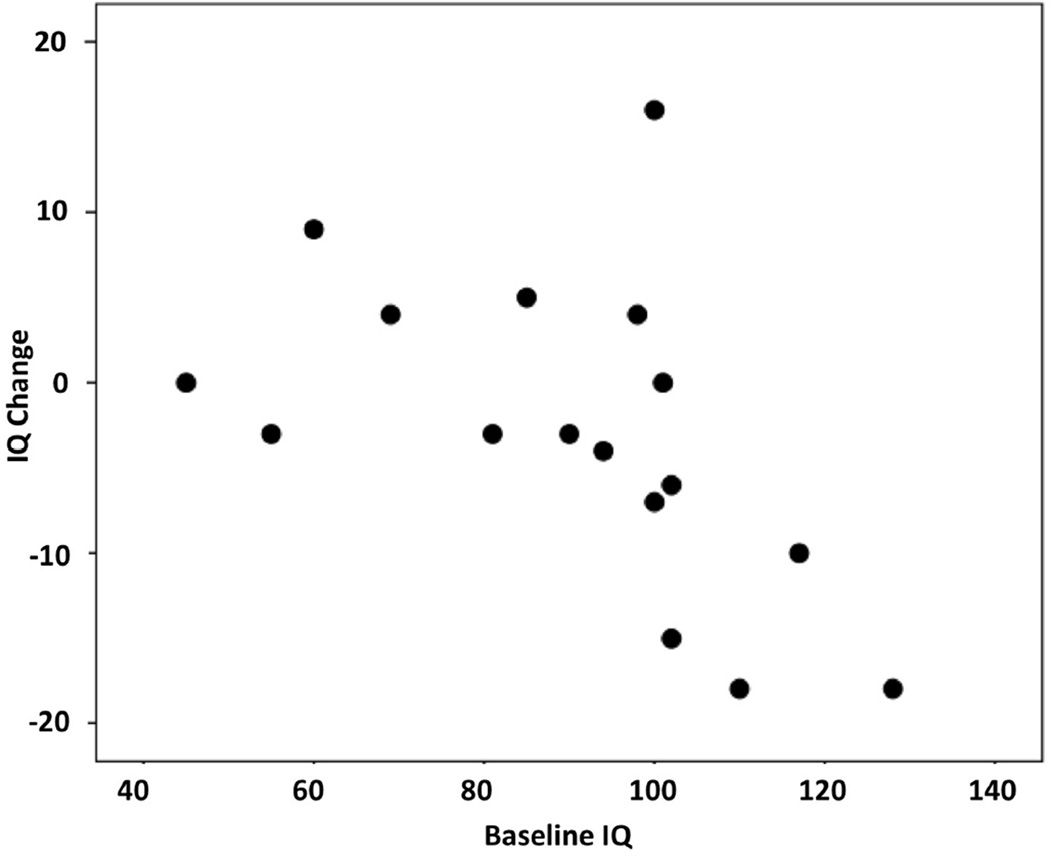
In patients where both baseline and outcome GIQ were available based on WPPSI-III or WISC-III (n=17), GIQ showed both increases and decreases over time; interestingly, only a minority of the patients (n=4) showed a robust (at least 10 points) GIQ decrease during the follow-up period, while several patients showed no/minor changes, and five patients actually showed a GIQ increase (see IQ change distribution on Figure 1). Interval change in GIQ showed a negative correlation with baseline GIQ (Spearman’s rho= −0.66, p=0.004), indicating that lower baseline GIQ was associated with a GIQ increase during the follow-up period (Figure 2). Age, follow-up time, number of AEDs or clinical seizure variables did not correlate with interval IQ change.
Figure 1.
Distribution of global IQ (GIQ) changes [Time 2−Time 1] in 17 patients. GIQ changes are categorized into five groups (shown in the five columns). The first and second columns include patients with declining GIQ, the third with minimal/no changes, and the fourth and fifth columns are patients with improving GIQ during follow-up. Only 4 of the 17 patients showed a robust GIQ decrease (−10 or more), while 5 patients showed a moderate or robust increase.
Figure 2.
Negative correlation between baseline global IQ (GIQ) and interval GIQ change (r=−0.66, p=0.004). Low baseline IQ was associated with increasing outcome IQ.
Predictors of outcome IQ and seizure frequency scores
Univariate analyses
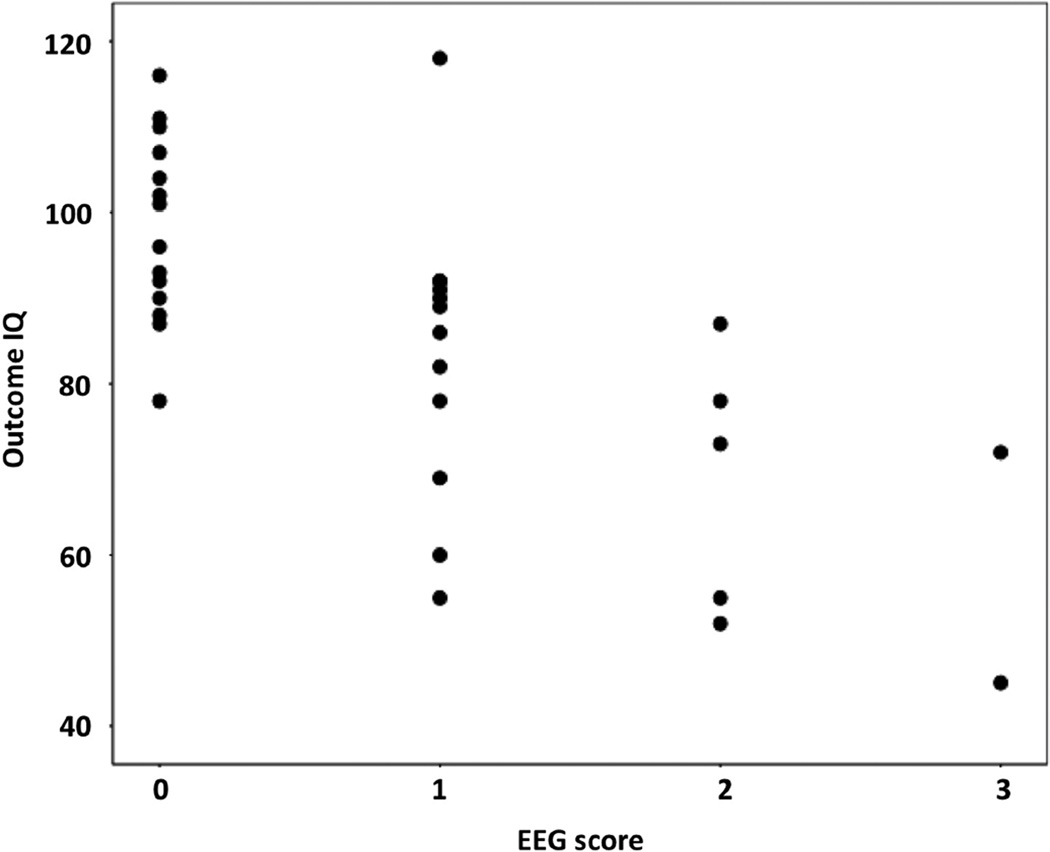
Outcome GIQs were significantly correlated with several baseline predictors, including the EEG severity score (Figure 3), the EEG spike frequency score, the seizure frequency score and age at seizure onset (see details in Table 2). Outcome VIQ and PIQ values showed similar correlations (data not shown). Thirteen of 14 (93%) children with normal baseline EEG (score 0) had outcome GIQ above 80 (mean: 98). In contrast, in patients with an EEG severity score 2–3, only 1 out of 7 (14%) had outcome GIQ above 80 (mean: 66). Although extent of MRI brain involvement showed no relation to cognitive functions, patients with frontal lobe involvement on baseline MRI had lower outcome GIQ and VIQ compared to those with no frontal lobe MRI abnormality (mean GIQ 94 vs. 78, p=0.01; VIQ 101 vs. 84, p=0.009; PIQ 87 vs. 77, p=0.12). Both baseline and outcome GIQs were similar in patients with left and right-sided lesions (mean baseline GIQ 84 and 85; mean outcome GIQ 91 vs. 82, respectively; p>0.1). Mean GIQ change was 0 (±12) in patients with left and +9 (±18) in patients with right-sided lesions, but the difference was also not significant (p=0.35).
Figure 3.
Negative correlation between EEG severity scores at baseline and outcome global IQ (GIQ). Patients with worse baseline EEG scores had lower GIQ at follow up (r=−0.67; p<0.001).
Table 2.
Predictors of outcome GIQ and seizure frequency scores in univariate (Spearman’s) correlations and group comparisons (Mann-Whitney U for binary predictor variables).
| Predictor variable | GIQ | Outcome seizure frequency | ||
|---|---|---|---|---|
| Age at baseline | r = −0.03 | p = 0.86 | r = 0.02 | p = 0.92 |
| Age at seizure onset | r = 0.55 | p = 0.001 | r = 0.05 | p = 0.79 |
| Duration of epilepsy | r = −0.14 | p = 0.46 | r = 0.26 | p = 0.16 |
| Number of antiepileptic drugs | r = −0.31 | p = 0.08 | r = 0.28 | p = 0.12 |
| Extent of MRI brain involvement | r = −0.34 | p = 0.05 | r = 0.05 | p = 0.76 |
| Seizure frequency score | r = −0.4 | p = 0.02 | r = 0.71 | p < 0.001 |
| EEG severity score | r = −0.67 | p < 0.001 | r = 0.08 | p = 0.68 |
| Spike frequency score | r = −0.57 | p < 0.001 | r = 0.11 | p = 0.54 |
| Frontal lobe involvement on MRI (yes vs. no) | p = 0.01 | p = 0.38 | ||
| Affected side (left vs. right) | p = 0.12 | p = 0.47 | ||
Significant correlations and group differences are bolded.
Seizure frequency at follow-up was only predicted by the baseline seizure frequency scores (r=0.71, p<0.001) but no other potential predictors (Table 2).
Binary logistic regression analysis for predictors of IQ
Four predictors were used in the multivariate analysis for IQ: the EEG severity score (which was the EEG variable showing the stronger correlation in the univariate analysis), the seizure frequency score, age at seizure onset and frontal lobe involvement on MRI. Among these, only the EEG severity score predicted the outcome GIQ category (Table 3). Age at seizure onset predicted outcome PIQ, but not GIQ or VIQ (Table 3). The predictive value of the EEG severity score remained significant when age or follow-up time were entered in the regression analysis.
Table 3.
Results of binary logistic regressions to identify predictors of outcome IQs.
| Predictor variable | GIQ | VIQ | PIQ |
|---|---|---|---|
| EEG severity score | p = 0.028 | p = 0.19 | p = 0.11 |
| Age at seizure onset | p = 0.10 | p = 0.17 | p = 0.02 |
| Seizure frequency score | p = 0.10 | p = 0.19 | p = 0.08 |
|
Frontal lobe involvement on MRI |
p = 0.70 | p = 0.99 | p = 0.19 |
P values less than 0.05 (bolded) indicate significant independent predictor(s).
DISCUSSION
In this prospective, longitudinal study to evaluate formal IQ changes and cognitive outcome in a non-surgical group of children with unilateral SWS, the majority of the patients had stable or improved IQ measures during follow-up, and only four patients (among those enrolled at age 2.5 years above) showed a robust IQ decline. Improvement in global intellect was associated with lower baseline IQs. We have identified several predictors of IQ, including EEG and seizure variables in the univariate analyses. In multivariate analyses the EEG severity score remained a strong, independent predictor of global IQ at follow-up. Altogether, these results suggest that interictal EEG abnormalities, along with early onset frequent seizures are important contributors of cognitive functions in children with SWS during the early disease course.
Longitudinal changes of cognitive functions in SWS
Although SWS is often considered to be a progressive disease, most studies performed to date have been retrospective, cross-sectional, and rarely utilized formal cognitive testing. Some previous studies included longitudinal imaging, which demonstrated progressive brain abnormalities. For example, in a study of 20 patients with SWS, progressive hypoperfusion and glucose hypometabolism detected in subsets of patients were associated with neurologic deterioration22. In a subsequent MRI study of nine children with SWS, progressive atrophy was observed in those with early seizure onset23. In a longitudinal PET study, our group reported that progressive glucose metabolic changes were associated with high seizure frequency24; however, some of the metabolic abnormalities were reversible in patients with well-controlled seizures. This finding was consistent with results from a previous longitudinal FDG-PET study of children with non-lesional epilepsy: patients with persistent refractory seizures between scans showed an expanding cortical hypometabolic area, whereas those achieving seizure control showed an improved glucose metabolic pattern on repeated scan25. These studies provided imaging evidence for reversible functional brain abnormalities as a result of effective seizure control. In other, cross-sectional FDG-PET studies of children with unilateral SWS, we reported a subgroup of children where large unilateral hemispheric damage was associated with relatively preserved IQ12,13. These data suggest a possible role of effective brain plasticity in children with early, extensive unilateral damage, where functional reorganization likely shifted to the contralateral hemisphere.
The current study provides evidence that there is a subgroup of SWS patients who show improved cognitive functions over time during the early disease course. Indeed, progressive cognitive deterioration was uncommon in the present cohort. To interpret these findings, one needs to consider that the mean age of our study group was 3.3 years, while the mean age of seizure onset was 1.2 years. Thus, in several cases (15 of the 33 children), baseline testing was performed more than 1 year after initial seizure onset. Therefore, it is likely that patients with lower baseline IQ had already undergone cognitive deterioration before being enrolled in our study. Interestingly, most patients showing a marked IQ decline had a relatively high baseline IQ while their outcome IQs fell in the normal range; while some of these decreases may reflect clinical decline, this finding could be explained, at least partially, by a statistical artifact (i.e., regression toward the mean26); some of the variance in this finding may also reflect some error associated with reduced reliability in assessment of preschool-aged children.
It should be also noted that the present cohort was a biased, non-surgical SWS patient group, since several patients (n=13 in the study period) with severe, medically refractory seizures (that likely affect cognitive functions), who were initially enrolled in our longitudinal study, underwent epilepsy surgery after the baseline studies and were excluded from the present analysis. We also excluded children with bilateral brain involvement, who often have severe epilepsy and cognitive impairment27. Nevertheless, the present findings provide an insight into the natural course of neurocognitive trajectories in SWS children where no surgery is performed. Thus, the results will be useful in future studies evaluating the effects of surgery on cognitive functions.
Predictors of cognitive functions
Several predictors of cognitive outcome identified in this study, such as seizure variables and frontal lobe involvement, are consistent with results from previous cross-sectional studies7,8,28. In addition, a novel finding was that interictal EEG abnormalities are a strong predictor of cognitive functions both in univariate and multivariate analyses, even though the outcome IQ was assessed at least 1 year after the baseline EEG. An even tighter correlation between EEG abnormalities and IQ might be present with shorter follow-up. On the other hand, only baseline seizure frequency predicted seizure frequency at follow-up, while no other predictors were useful in this respect. The latter result is consistent with a recent EEG study of 44 children with SWS, where the EEG score did not correlate with seizure frequency17.
Cognitive functions could also be affected by AED treatment. In our study, the majority of the patients were on a variety of AEDs, but only 10 children (30%) took multiple AEDs at follow-up (and 11 at baseline), and the number of AEDs showed no significant correlation with outcome IQ (Table 2). It should be also noted that 6 patients took phenobarbital (at baseline and/or follow-up), a drug with potentially severe cognitive side effects. Although the low number of this subgroup precluded a meaningful statistical analysis, mean baseline IQ was similar (85 vs. 84, respectively) and outcome IQ was only slightly lower (78 vs. 86, respectively) in the phenobarbital-treated group as compared to the whole group. Altogether these data suggest that AED treatment was unlikely to have a robust effect on the overall results.
Interestingly, frontal lobe abnormality rather than side or hemispheric extent of brain involvement on MRI was associated with IQ in our study. The findings regarding side are not novel, because previous pediatric studies on cognitive functions, particularly language functions, have not supported strong associations between lesion side or location and outcomes29. Also, our previous cross-sectional study in SWS children did not find a robust difference in IQ abnormalities based on lesion side13. The prognostic implication of frontal lobe involvement is also consistent with previous, cross-sectional studies involving advanced MRI and PET techniques. In an MRI study using DTI in children with unilateral SWS, focal decreases in white matter fractional anisotropy in the prefrontal region were associated with lower IQ21. In our previous study of children with bilateral SWS brain involvement, bifrontal hypometabolism on FDG-PET was associated with the most severe developmental impairment27. In the present study, we did not include any advanced imaging variables but assessed the value of simple, clinically practical prognostic variables including imaging without complicated post-processing or quantitative analysis. Thus, the results can be highly relevant in the clinical evaluation of children with SWS, who almost invariable undergo EEG and MRI assessment during the early disease course; thus, the results may be applied also in developing nations.
In summary, despite the relatively wide age range and limited length of follow-up, these results provide an insight into the natural course of cognitive changes in young children with unilateral SWS. While some of our findings may reflect increasing reliability of assessment in older children, such improvements are also likely explained by brain functional reorganization, as hypothesized by previous cross-sectional studies12,13. Early, frequent seizures with interictal EEG abnormalities, particularly epileptiform discharges, likely interfere with such reorganization and are associated with poor cognitive functions at follow-up. Such patients are at high risk for poor cognitive outcome and should be considered for epilepsy surgery early in their disease course. Future studies evaluating effects of early interventions (such as surgery) should target this high-risk patient group to optimize cognitive outcome in SWS.
Acknowledgments
We thank Cathie Germain, MA and Cynthia Burnett, BA, for assisting patient recruitment and scheduling; Jane Cornett, RN and Anne Deboard, RN, for performing sedation; and Xuan Yang, BS, for assisting with MR imaging data acquisition. We are also grateful to the Sturge-Weber Foundation and the families who participated in these studies. This study was partially funded by a grant from the National Institutes of Health (R01 NS041922 to C.J.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Bodensteiner JB, Roach ES. Overview of Sturge-Weber syndrome. In: Bodensteiner JB, Roach ES, editors. Sturge-Weber syndrome. Mt Freedom, NJ: The Sturge-Weber Foundation; 2010. pp. 19–32. [Google Scholar]
- 2.Hu J, Lu Y, Juhász C, Kou Z, Xuan Y, Latif Z, Kudo K, Chugani HT, Haacke EM. MR susceptibility weighted imaging (SWI) complements conventional contrast enhanced T1 weighted MRI in characterizing brain abnormalities of Sturge-Weber syndrome. J Magnetic Res Imaging. 2008;28:300–307. doi: 10.1002/jmri.21435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Comi A, Roach ES, Bodensteiner JB. Neurological manifestations of Sturge-Weber syndrome. In: Bodensteiner JB, Roach ES, editors. Sturge-Weber syndrome. Mt Freedom, NJ: The Sturge-Weber Foundation; 2010. pp. 69–93. [Google Scholar]
- 4.Peterman AF, Hayles AB, Dockerty MB, Love JG. Encephalotrigeminal angiomatosis (Sturge–Weber disease); clinical study of thirty-five cases. JAMA. 1958;30:2169–2176. doi: 10.1001/jama.1958.02990350007002. [DOI] [PubMed] [Google Scholar]
- 5.Aicardi J, Arzimanoglou A. Sturge–Weber syndrome. Int Pediatr. 1991;6:129–134. [Google Scholar]
- 6.Sujansky E, Conradi S. Outcome of Sturge–Weber syndrome in 52 adults. Am J Med Genet. 1995;57:35–45. doi: 10.1002/ajmg.1320570110. [DOI] [PubMed] [Google Scholar]
- 7.Kramer U, Kahana E, Shorer Z, Ben-Zeev B. Outcome of infants with unilateral Sturge-Weber syndrome and early onset seizures. Dev Med Child Neurol. 2000;42:756–759. doi: 10.1017/s0012162200001407. [DOI] [PubMed] [Google Scholar]
- 8.Zabel TA, Reesman J, Wodka EL, Gray R, Suskauer SJ, Turin E, Ferenc LM, Lin DD, Kossoff EH, Comi AM. Neuropsychological features and risk factors in children with Sturge-Weber syndrome: four case reports. Clin Neuropsychol. 2010;24:841–859. doi: 10.1080/13854046.2010.485133. [DOI] [PubMed] [Google Scholar]
- 9.Kelley TM, Hatfield LA, Lin DD, Comi AM. Quantitative analysis of cerebral cortical atrophy and correlation with clinical severity in unilateral Sturge-Weber syndrome. J Child Neurol. 2005;20:867–870. doi: 10.1177/08830738050200110201. [DOI] [PubMed] [Google Scholar]
- 10.Juhasz C, Lai C, Behen ME, Muzik O, Helder EJ, Chugani DC, Chugani HT. White matter volume as a major predictor of cognitive function in Sturge-Weber syndrome. Arch Neurol. 2007;64:1169–1174. doi: 10.1001/archneur.64.8.1169. [DOI] [PubMed] [Google Scholar]
- 11.Alkonyi B, Chugani HT, Behen M, Halverson S, Helder E, Makki MI, Juhász C. The role of the thalamus in neuro-cognitive dysfunction in early unilateral hemispheric injury: a multimodality imaging study of children with Sturge-Weber syndrome. Eur J Paediatr Neurol. 2010;14:425–433. doi: 10.1016/j.ejpn.2010.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lee JS, Asano E, Muzik O, Chugani DC, Juhász C, Pfund Z, Philip S, Behen ME, Chugani HT. Sturge-Weber syndrome: correlation between clinical course and FDG PET findings. Neurology. 2001;57:189–195. doi: 10.1212/wnl.57.2.189. [DOI] [PubMed] [Google Scholar]
- 13.Behen ME, Juhász C, Wolfe-Christensen C, Guy W, Halverson S, Rothermel R, Janisse J, Chugani HT. Brain damage and IQ in unilateral Sturge-Weber syndrome: support for a"fresh start” hypothesis. Epilepsy Behav. 2011;22:352–357. doi: 10.1016/j.yebeh.2011.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brenner RP, Sharbrough FW. Electroencephalographic evaluation in Sturge-Weber syndrome. Neurology. 1976;26:629–632. doi: 10.1212/wnl.26.7.629. [DOI] [PubMed] [Google Scholar]
- 15.Hatfield LA, Crone NE, Kossoff EH, Ewen JB, Pyzik PL, Lin DD, Kelley TM, Comi AM. Quantitative EEG asymmetry correlates with clinical severity in unilateral Sturge-Weber syndrome. Epilepsia. 2007;48:191–195. doi: 10.1111/j.1528-1167.2006.00630.x. [DOI] [PubMed] [Google Scholar]
- 16.Ewen JB, Kossoff EH, Crone NE, Lin DD, Lakshmanan BM, Ferenc LM, Comi AM. Use of quantitative EEG in infants with port-wine birthmark to assess for Sturge-Weber brain involvement. Clin Neurophysiol. 2009;120:1433–1440. doi: 10.1016/j.clinph.2009.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kossoff EH, Bachur CD, Quain AM, Ewen JB, Comi AM. EEG evolution in Sturge-Weber syndrome. Epilepsy Res. 2014;108:816–819. doi: 10.1016/j.eplepsyres.2014.01.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sattler J, Dumont R. In: Assessment of children, WISC-IV and WPPSI-III Supplement. Jerome M, editor. La Mesa, CA: Sattler Publisher; 2004. [Google Scholar]
- 19.Wechsler D. The Wechsler Preschool and Primary Scale of Intelligence, Third Edition (WPPSI-III) San Antonio, TX: The Psychological Corporation; 2002. [Google Scholar]
- 20.Wechsler D. The Wechsler Intelligence Scale for Children—third edition. San Antonio, TX: The Psychological Corporation; 1991. [Google Scholar]
- 21.Alkonyi B, Govindan RM, Chugani HT, Behen ME, Jeong JW, Juhász C. Focal white matter abnormalities related to neurocognitive dysfunction: an objective diffusion tensor imaging study of children with Sturge-Weber syndrome. Pediatr Res. 2011;69:74–79. doi: 10.1203/PDR.0b013e3181fcb285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Maria BL, Neufeld JA, Rosainz LC, Drane WE, Quisling RG, Ben-David K, Hamed LM. Central nervous system structure and function in Sturge-Weber syndrome: evidence of neurologic and radiologic progression. J Child Neurol. 1998;13:606–618. doi: 10.1177/088307389801301204. [DOI] [PubMed] [Google Scholar]
- 23.Udani V, Pujar S, Munot P, Maheshwari S, Mehta N. Natural history and magnetic resonance imaging follow-up in 9 Sturge-Weber syndrome patients and clinical correlation. J Child Neurol. 2007;22:479–483. doi: 10.1177/0883073807300526. [DOI] [PubMed] [Google Scholar]
- 24.Juhász C, Batista EA, Chugani DC, Muzik O, Chugani HT. Evolution of cortical metabolic abnormalities and their clinical correlates in Sturge-Weber syndrome. Eur J Paediatr Neurol. 2007;11:277–84. doi: 10.1016/j.ejpn.2007.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Benedek K, Juhász C, Chugani DC, Muzik O, Chugani HT. Longitudinal changes in cortical glucose hypometabolism in children with intractable epilepsy. J Child Neurol. 2006;21:26–31. doi: 10.1177/08830738060210011101. [DOI] [PubMed] [Google Scholar]
- 26.Bland JM, Altman DG. Regression towards the mean. BMJ. 1994;308:1499. doi: 10.1136/bmj.308.6942.1499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Alkonyi B, Chugani HT, Karia S, Behen ME, Juhász C. Clinical outcomes in bilateral Sturge-Weber syndrome. Pediatr Neurol. 2011;44:443–449. doi: 10.1016/j.pediatrneurol.2011.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sujansky E, Conradi S. Sturge-Weber syndrome: age of onset of seizures and glaucoma and the prognosis for affected children. J Child Neurol. 1995;10:49–58. doi: 10.1177/088307389501000113. [DOI] [PubMed] [Google Scholar]
- 29.Bates E, Roe K. Language development in children with unilateral brain injury. In: Nelson CA, Luciana M, editors. Handbook of developmental cognitive neuroscience. Cambridge, MA: MIT Press; 2001. pp. 281–308. [Google Scholar]