Abstract
Nervous system development is a process that integrates cell proliferation, differentiation and programmed cell death (PCD). PCD is an evolutionary conserved mechanism and a fundamental developmental process by which the final cell number in a nervous system is established. In vertebrates and invertebrates, PCD can be determined intrinsically by cell lineage and age, as well as extrinsically by nutritional, metabolic and hormonal states. Drosophila has been an instrumental model for understanding how this mechanism is regulated. We review the role of PCD in Drosophila central nervous system development from neural progenitors to neurons, its molecular mechanism and function, how it is regulated and implemented, and how it ultimately shapes the fly central nervous system from the embryo to the adult. Finally, we discuss ideas that emerge while integrating this information.
Apoptosis shapes Drosophila neural development from the emergence of neuroectoderm in the embryo to the eclosing adult. The earliest indication of programmed cell death (PCD) during neurodevelopment is at embryonic stages 11-12 (Truman, Thorn et al. 1992, Abrams, White et al. 1993, Sprecher, Urbach et al. 2006), when the first neurons and epidermal cells die (Rogulja-Ortmann, Luer et al. 2007, Lin, Zhang et al. 2009). Neuronal apoptosis then increases dramatically peaking at embryonic stages 16-17, when the ventral nerve cord (VNC) condenses (Truman, Thorn et al. 1992) and the embryo produces its first twitching movements (Abrams, White et al. 1993). Although the observed amount of neuronal death differs from embryo to embryo (Rogulja-Ortmann, Luer et al. 2007), symmetry in neuronal PCD is often observed between the right and left sides of a single embryo (Abrams, White et al. 1993), indicating that both deterministic and “random” processes participate in the selection of surviving neurons.
PCD in Drosophila neural development comes in different flavors. It can be triggered by spatial, temporal, nutritional, hormonal, and metabolic signals; it can be regulated by Hox genes, Notch and other signaling pathways; it can be mediated by one or more pro-apoptotic genes; and it can act at the stage of neural precursors, of newly born or of mature neurons/glia. What is clear is that dying is not trivial for a cell. There exist many layers of regulation that ensure both proper timing of death and effective killing without sacrificing specificity for the cells that should (or should not) die. Here we review in detail these different levels of regulation during Drosophila neurodevelopment. After introducing the mechanisms of apoptosis (Section 1) and how the Drosophila central nervous system develops (Section 2), we describe in detail the occurrence and regulation of apoptosis in progenitors (Section 3) and neurons (Section 4) throughout the different stages of Drosophila neurodevelopment. Finally (Section 5), we discuss emerging ideas and perspectives in the field.
1. Mechanisms of apoptosis
The effectors of the apoptotic process are the caspases, which are cysteine proteases that, at the onset of apoptosis, undergo a cascade of catalytic activation reactions. Seven caspases have been identified in Drosophila: the initiator regulatory caspases Dronc, Dredd and Strica, and the effector caspases DrICe, Dcp-1, Decay and Damm (Fraser, McCarthy et al. 1997, Song, McCall et al. 1997, Chen, Rodriguez et al. 1998, Dorstyn, Colussi et al. 1999, Dorstyn, Read et al. 1999, Doumanis, Quinn et al. 2001, Harvey, Daish et al. 2001, Kornbluth and White 2005). Once activated, effector caspases cleave their cellular targets, including structural proteins and enzymes, disrupting DNA replication and cellular metabolism.
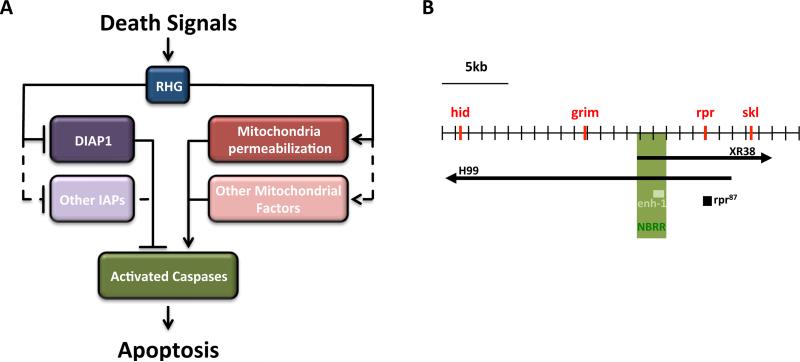
Apoptosis in Drosophila is initiated by the pro-apoptotic genes reaper (rpr), head involution defective (hid), grim and sickle (skl), collectively known as RHG (Cashio, Lee et al. 2005) (Figure 1A). Although the four genes are clustered in a small genomic region on chromosome III (Figure 1B), their protein sequences are unrelated, only sharing weak homology in the first 15 amino acids (Bangs and White 2000, Christich, Kauppila et al. 2002, Wing, Karres et al. 2002). Deletion of reaper, grim and hid (but not sickle) in the def(3)H99 deletion (Figure 1B) blocks developmental apoptosis, while overexpression of each gene is often sufficient to induce apoptosis in a caspase dependent manner in both insects and mammals (Grether, Abrams et al. 1995, Hay, Wassarman et al. 1995, Chen, Nordstrom et al. 1996, Pronk, Ramer et al. 1996, White, Tahaoglu et al. 1996, Evans, Kuwana et al. 1997, Claveria, Albar et al. 1998, McCarthy and Dixit 1998, Haining, Carboy-Newcomb et al. 1999). In the fly, three Inhibitor of Apoptosis Proteins (IAPs) have been identified – DIAP1, DIAP2 and Deterin (Hay, Wassarman et al. 1995, Jones, Jones et al. 2000). IAPs bind to caspases and inhibit their activity (Kaiser, Vucic et al. 1998, Meier, Silke et al. 2000). Part of the RHG proteins proaptotic activity is due to their ability to bind and inactivate IAPs and in this way regulate caspase activity. In addition, Rpr and Grim have been shown to suppress DIAP1 translation (Yoo, Huh et al. 2002, Orme and Meier 2009) (Figure 1A).
Figure 1. Reaper, Hid, Grim and Sickle orchestrate apoptosis in Drosophila.
(A) Reaper, Hid, Grim, and Sickle (RHG) integrate signals from different sources, and trigger apoptosis. RHG act through two independent pathways. They either inhibit DIAP1, and/or other inhibitors of apoptosis, or they act by promoting mitochondria permeabilization. Both pathways ultimate lead to the activation of caspases, cysteine proteases that implement the apoptotic program. Solid arrows represent proven interactions and dashed lines represent unproven interactions.
(B) Schematic representation of the genomic locus of the third chromosome where RHG are located. The neuroblast regulatory region (NBRR) is situated between rpr and grim and is responsible for the integration of developmental signals that control reaper, grim and sickle expression, and, hence, apoptosis. A cis-regulatory element (enh-1) is responsible for the restricted expression of the pro-apoptotic genes in the abdominal neuroblasts. Three main genomic deletions that have been used to study apoptosis in flies. In deficiency Df(3l)H99, rpr, hid, and grim are removed, while in Df(3l)XR38, rpr and skl are not present. Finally, rpr87 only removes rpr. Black arrows represent the deleted regions, and red bars the pro-apoptotic genes. The NBRR is represented in green and, within it, the enh-1 enhancer in light green.
RHG activity is combinatorial and synergistic (Srinivasula, Datta et al. 2002, Wing, Karres et al. 2002, Tan, Yamada-Mabuchi et al. 2011). For instance, the expression of hid or rpr alone in the CNS midline glia is insufficient to induce apoptosis; however, when both genes are expressed, they trigger extensive glial apoptosis. The expression of grim alone is sufficient to induce midline glial apoptosis, but its activity is also synergistic with that of rpr and hid (Bangs, Franc et al. 2000).
While RHG proteins share similar downstream mechanisms, they are not functionally equivalent to each other and they do not share the same activation pathways; hid expression and activity are negatively regulated both transcriptionally and post-translationally by the Ras/MAPK pathway, while the p53/DNA damage pathway and ecdysone receptor mediated signaling directly regulate rpr expression (Brodsky, Nordstrom et al. 2000, Jiang, Lamblin et al. 2000). As a consequence RHG are differentially expressed in dying cells in response to different signals they are competent to receive. In both the embryo and adult CNS, rpr and grim are broadly expressed in dying cells while hid is limited to the dying midline glia and is also expressed in some cells that do not undergo PCD (White, Grether et al. 1994, Grether, Abrams et al. 1995, Robinow, Draizen et al. 1997). sickle was identified as a damage-responsive gene. Like the other RHG proteins, Sickle binds DIAP1 but neither its overexpression induces apoptosis nor its removal prevents it (Christich, Kauppila et al. 2002, Srinivasula, Datta et al. 2002, Wing, Karres et al. 2002, Tan, Yamada-Mabuchi et al. 2011). Instead, sickle activity appears to potentiate the activity of the other RHG genes (Srinivasula, Datta et al. 2002, Wing, Karres et al. 2002). The coordinated expression of RHG genes is thus crucial in regulating PCD, although it is still unclear how distinct combinations of RHG proteins impact IAPs function in the fly. RHG transcription in neuroblasts is in part achieved by a NeuroBlast Regulatory Region (NBRR) that lies between the rpr and grim loci. This genomic region integrates multiple developmental signals to control the spatio-temporal pattern of apoptosis in neuroblasts through the expression of grim, reaper and sickle (Tan, Yamada-Mabuchi et al. 2011) (Figure 1B).
MicroRNAs are also involved in apoptotic death regulation (Jovanovic and Hengartner 2006). Different microRNAs have been shown to act at different levels of the apoptotic cascade. Members of the evolutionarily conserved miR-2 family (miR-2/13, miR-6, miR-11, and miR-308) exert their anti-apoptotic activity by downregulating reaper, hid, and grim (Leaman, Chen et al. 2005). Moreover, bantam, which encodes a microRNA with multiple roles during development, is able to regulate cell number by increasing cell proliferation and decreasing cell death by blocking hid-induced apoptosis during development (Hipfner, Weigmann et al. 2002, Brennecke, Hipfner et al. 2003). The role of microRNAs in regulating cell death during Drosophila neurodevelopment is yet to be shown.
During PCD Rpr, Hid, and Grim localize to mitochondria (Haining, Carboy-Newcomb et al. 1999, Claveria, Caminero et al. 2002, Olson, Holley et al. 2003). Inhibition of Rpr or Hid mitochondrial localization prevents full caspase activation (Olson, Holley et al. 2003, Abdelwahid, Yokokura et al. 2007, Thomenius, Freel et al. 2011) suggesting a role for this organelle in the apoptotic process. In agreement with this, mutants for the gene encoding the dynamin-related protein Drp1, which regulates the rate of mitochondrial fission and fusion (Yaffe 1999, Meeusen and Nunnari 2005, Goyal, Fell et al. 2007), show reduced mitochondrial fragmentation and decreased caspase activation (Goyal, Fell et al. 2007). Mitochondria are permeabilized in response to reaper and hid expression (Abdelwahid, Yokokura et al. 2007). Interestingly, mitochondrial membrane permeabilization is independent of DIAP1 activity (Abdelwahid, Yokokura et al. 2007). Hence, RHG may act in two independent pathways: a) binding DIAP1 and relieving caspase inhibition and b) permeabilizing mitochondria and increasing caspase activation (Figure 1A). Altogether these observations show that that the survival of a cell is regulated by the interplay between RHG pro-apoptotic genes and the mitochondrial fusion machinery. Why mitochondrial localization of RHG pro-apoptotic proteins is significant for caspase activation, and what is the role of mitochondrial fragmentation and permeabilization remains unknown (Clavier, Ruby et al. 2015, Clavier, Rincheval-Arnold et al. 2016).
2. Drosophila neurodevelopment: from the embryo to the adult
The Drosophila central nervous system can be divided into optic lobes (OL), central brain (CB) and ventral nerve cord (VNC). In the embryo, the head neuroectoderm generates the brain structures while the ventral neuroectoderm gives rise to the VNC, composed of segmental units called neuromeres (3 gnathal, 3 thoracic and 7 abdominal) (Skeath and Thor 2003, Egger, Chell et al. 2008, Neriec and Desplan 2016) (Figure 2A). Four additional segments and a non segmented telson constitute the most posterior part of the VNC (‘tail region’) (Jürgens 1987). The optic lobes develop later, during larval stages, from neuroepithelial placodes (Green, Hartenstein et al. 1993, Nassif, Noveen et al. 2003, Neriec and Desplan 2016).
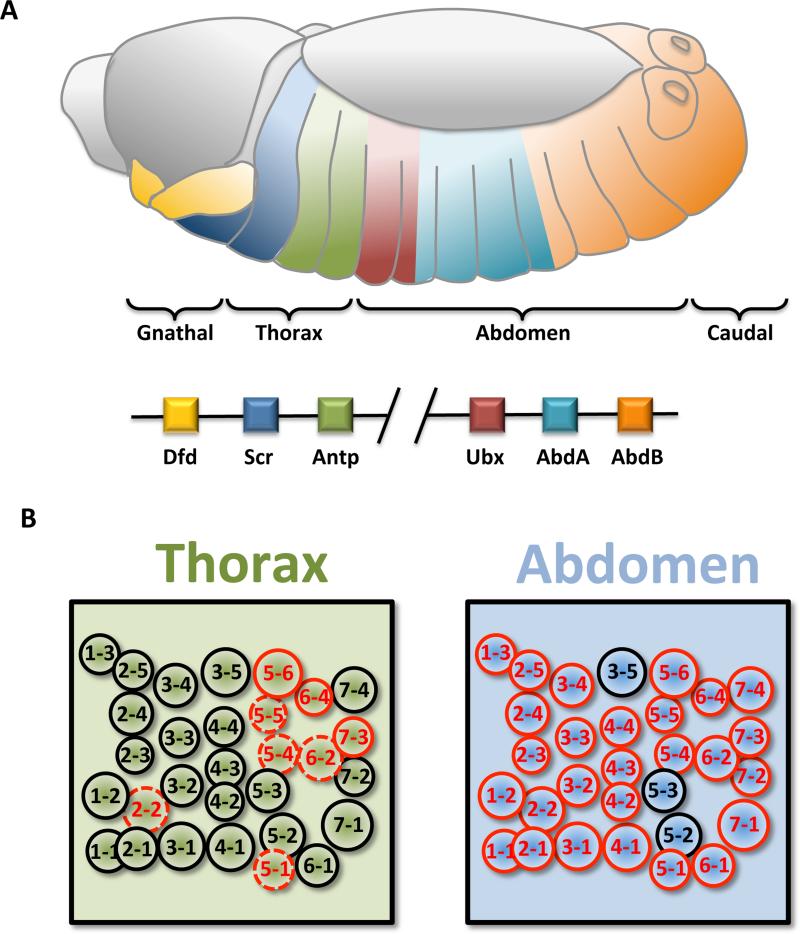
Figure 2. Neuroblast fate is regulated by Hox gene expression and differs between abdominal and thoracic segments.
(A) In the embryo, the identity of each segment depends on Hox gene activity. Sex combs reduced (Scr) and Antennapedia are active in the thoracic segments, while Ubx, Abdominal-A, and Abdominal-B are active in the abdominal and caudal segments, in a colinear manner with respect to their positions in the Hox cluster. The ventral nerve chord (VNC) is similarly subdivided into 17 neuromeres, 3 gnathal, 3 thoracic, 7 abdominal, and 4 caudal.
(B) Upon delamination, 30 neuroblasts are observed in each thoracic and abdominal hemisegment. At the end of embryogenesis some thoracic and most of the abdominal neuroblasts are eliminated by PCD. When the larva hatches, 23 post embryonic neuroblasts per hemisegment are found in the thorax, 12 in A1, 4 in A2, and 3 in each of the A3-A8 abdominal hemisegments. Neuroblasts that are maintained to the larvae are labeled in black, apoptotic neuroblasts are labeled in red, and potential thoracic apoptotic neuroblasts are labeled in dashed red.
2.1 Neuroblast generation and proliferation
Drosophila neurogenesis starts at early stages of embryogenesis, with the regional specification of neurogenic vs. non-neurogenic regions, determined by the early activity of the proneural genes and by Notch signaling (Jimenez and Campos-Ortega 1990, Skeath and Carroll 1992, Campos-Ortega 1994). In each equivalent group of neurogenic cells, one cell acquires a neuronal progenitor fate known as neuroblast and inhibits its neighbors that remain epidermal cells. Neuroblasts delaminate to the interior of the embryo (Wheeler 1891, Poulson 1950). Each neuroblast typically divides asymmetrically to self-renew and to produce a ganglion mother cell (GMC), which divides once, asymmetrically, to produce two post-mitotic neurons or glia (neuroblast type I) (Poulson 1950). During germ band elongation (stages 8-11), five sequential waves of neuroblast delamination in the embryonic VNC produce an invariant pattern of 30 neuroblasts per hemisegment (Hartenstein and Campos-Ortega 1984, Truman and Bate 1988, Doe 1992, Doe and Technau 1993) (Figure 2B). Elegant heterotopic transplantation experiments between thoracic and abdominal sites of early gastrula neuroepithelium have shown that neuroblasts proliferate according to their domain of origin in a cell autonomous manner, demonstrating that segment specific identity is programmed early during development at the level of the neuroepithelium (Udolph, Luer et al. 1995, Prokop, Bray et al. 1998). Such specification is established by the early expression of spatial patterning genes including the segment polarity genes (e.g. runt, wingless (wg), gooseberry (gsb)), which are stereotypically expressed in segmental stripes that subdivide each neuromere along the antero-posterior axis, and columnar patterning genes (e.g. ventral nervous system defective (vnd), intermediate neuroblasts defective (ind), muscle specific homeobox (msh)) that act along the dorso-ventral axis. The superimposition of these expression patterns establishes an almost invariant cartesian grid of positional information (McGinnis and Krumlauf 1992, Dormand and Brand 1998, Bhat 1999, Skeath 1999).
In each hemisegment, homologous neuroblasts under the same positional cues produce similar embryonic lineages (Bossing, Udolph et al. 1996, Schmidt, Rickert et al. 1997, Schmid, Chiba et al. 1999). However, homologous lineages do show some variations, in particular between thoracic and abdominal segments, reflecting the different requirements of each segment (Bossing, Udolph et al. 1996, Schmidt, Rickert et al. 1997). Segmental specificity is defined by the expression of Hox genes that act in the neuroepithelium, neuroblasts, neurons and glia to control cell specification and proliferation/apoptosis in a segment specific manner (McGinnis and Krumlauf 1992, Rogulja-Ortmann and Technau 2008, Tsuji, Hasegawa et al. 2008, Karlsson, Baumgardt et al. 2010, Reichert and Bello 2010) (Figure 2A). For example, Hox genes from the Antennapedia complex (ANT-C) control the differentiation of the head, gnathal and anterior thorax (Kaufman, Lewis et al. 1980) while those from the Bithorax complex (BX-C) define the identity of the posterior thorax, abdomen and caudal segments (Lewis 1978, Birkholz, Vef et al. 2013) (Figure 2A).
As VNC neuroblasts age, they progress through a well-defined ‘series’ of temporal transcription factors (tTFs) that dictate the identity of the neurons/glia produced by the neuroblasts based on their time of birth (Brody and Odenwald 2000, Isshiki, Pearson et al. 2001, Pearson and Doe 2003, Kohwi and Doe 2013, Li, Chen et al. 2013, Allan and Thor 2015). In the VNC, embryonic neuroblasts progress through the tTF series Hunchback (Hb)→ Krüppel (Kr)→ POU domain proteins 1/2 (Pdm) → Castor (Cas) (Isshiki, Pearson et al. 2001, Pearson and Doe 2003, Cleary and Doe 2006, Grosskortenhaus, Robinson et al. 2006, Li, Chen et al. 2013). The temporal series continues during postembryonic VNC development, with postembryonic neuroblasts expressing Castor→Seven-Up (Svp) (Almeida and Bray 2005, Maurange, Cheng et al. 2008, Bayraktar and Doe 2013). Interestingly, distinct sequences of transcription factors are used in different contexts (Bayraktar and Doe 2013, Li, Chen et al. 2013, Li, Erclik et al. 2013, Bertet, Li et al. 2014), likely reflecting a universal strategy in the establishment of neuronal diversity. Thus, spatio-temporal patterns of neuroblast delamination, together with a neuroblast intrinsic tTF series define their unique identities and the lineages they produce (neuronal/glial types and cell number) (Doe 1992, Udolph, Prokop et al. 1993, Bossing, Udolph et al. 1996, Schmidt, Rickert et al. 1997, Berger, Urban et al. 2001). A more derived, yet similar, neuroblast distribution pattern is observed in the 3 gnathal neuromeres that lie anterior to the thorax (28 neuroblasts per labial hemisegment, 26 in maxillary and 22 neuroblasts in mandibular hemisegments) (Urbach, Jussen et al. 2016) and in the most posterior caudal neuromeres (30 in A8, 29 neuroblasts in A9, 11 neuroblasts in A10 and no neuroblasts in A11) (Birkholz, Vef et al. 2013).
The pre-gnathal segments that form the embryonic head give rise to the brain, which is structurally more complex than the VNC. It arises from a bilaterally symmetric procephalic neurogenic region (pNR). While most of the brain structures are formed during larval stages, the adult brain bauplan is laid out early during embryogenesis. About 100 neuroblasts per hemisphere are generated, each with a unique molecular identity (Nassif, Noveen et al. 1998, Kurusu, Nagao et al. 2000, Noveen, Daniel et al. 2000, Urbach, Schnabel et al. 2003, Urbach and Technau 2003, Reichert and Bello 2010). A close inspection of the combination of markers expressed in brain neuroblasts and their relative positions suggests that several of them might be homogolous to VNC neuroblasts (Doe 1992, Urbach and Technau 2003, Urbach, Jussen et al. 2016).
2.2 Ending proliferation
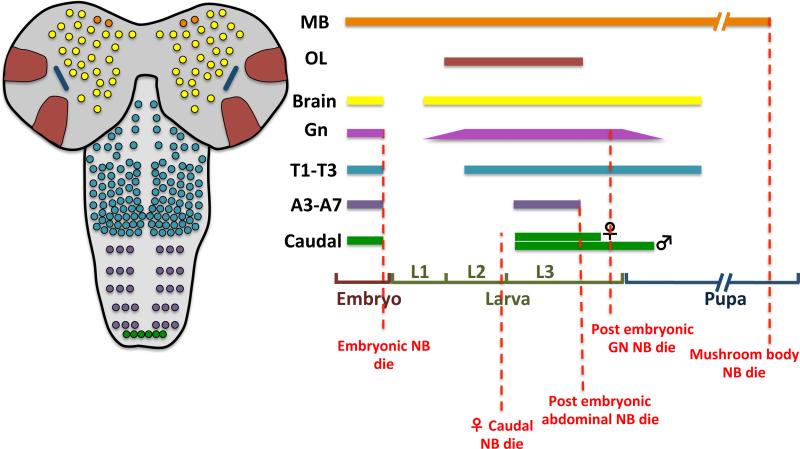
During embryonic neurogenesis, the larval CNS and about 10% of the adult neurons are produced. Before larval hatching, neuroblasts stop proliferating by either entering a quiescent state or by committing PCD. The majority of brain neuroblasts stop dividing by embryonic stage 14, with only one lateral neuroblast and the four mushroom body neuroblasts per hemisphere escaping quiescence and dividing throughout development until late pupae (Ito and Hotta 1992) (Figure 3).
Figure 3. Patterning of neuroblast mitotic activity and apoptosis throughout development.
Patterning of neuroblast activity and apoptosis during embryonic, larval and pupal development. Colored bars represent the mitotic activity of the neuroblasts depicted in the left cartoon (gnathal neuroblasts are not visible). Red dashed lines indicate the timing of neuroblast apoptosis. Note that gnathal neuroblasts division is approximate and deduced based on limited description in the literature (see main text). The timing of the female caudal neuroblast apoptosis and gnathal neuroblasts is approximate (see main text). Neuroblast number is representative and does not correspond to the real number. Abbreviations: MB, Mushroom body; OL, Optic Lobe; Gn, Gnathal; T,Thorax; A, Abdominal.
Neuroblasts in the VNC also stop proliferating at the end of embryogenesis. While most of the thoracic neuroblasts enter quiescence, the majority of gnathal and abdominal neuroblasts are eliminated by PCD (Truman and Bate 1988, Prokop and Technau 1991, Maurange and Gould 2005, Tan, Yamada-Mabuchi et al. 2011, Kuert, Hartenstein et al. 2014) (Figure 3). Thus, when the larva hatches, a total of 19 postembryonic neuroblasts are found in the gnathal segments, 23 postembryonic neuroblasts per hemisegment are found in the thorax, 12 in A1, 4 in A2, and 3 in each of the A3-A7 abdominal hemisegments.
Central brain and VNC neuroblasts resume proliferation during larval stages, triggered by increased levels of circulating amino acids as the 1st instar larva starts feeding (Britton and Edgar 1998, Chell and Brand 2010, Sousa-Nunes, Yee et al. 2011). It is during the larval and pupal periods that 90% of adult neurons are produced (White and Kankel 1978, Truman and Bate 1988, Truman, Taylor et al. 1993). At the same time, the differences between serially homologous lineages become even more pronounced, matching the transition from a crawling larva to an adult fly with much more complex behaviors and different requirements for the different adult segments. Neurogenesis progresses until the pupal stage, when postembryonic neuroblasts stop dividing either by undergoing a Prospero-dependent cell cycle exit (terminally differentiating into a GMC) or by PCD (Maurange and Gould 2005, Maurange, Cheng et al. 2008) (Figure 3).
3. Apoptotic death of neuroblasts
3.1 Embryonic apoptosis
The mechanism by which most abdominal neuroblasts die at the end of embryogenesis was only recently elucidated. A cis-regulatory element (enh-1) was identified within the NBRR that restricts expression of RGH genes to abdominal neuroblasts (Arya, Sarkissian et al. 2015) (Figure 1B). This element is activated by a pulse of Abdominal-A (AbdA) at the end of embryogenesis. AbdA expression is the result of Notch being activated by the Delta ligand, which is expressed by the neuroblast's glial progeny. Therefore, it is the progeny of the neuroblast that induce its apoptosis, assuring its death only after it has produced the proper array of neurons and glia. Thus, Hox gene expression controls neuroblast apoptosis at two levels: early in embryogenesis by specifying the neuroblast's lineage, and at the end of embryogenesis by promoting its apoptosis. Upregulation of AbdA occurs upon ectopic overexpression of the Notch intracellular domain (NotchICD), but only in segments that expressed AbdA early in development. This suggests that early AbdA expression induces chromatin conformational changes that facilitate its later activation by Notch. Broad ectopic expression of AbdA only regulates RHG expression later in embryogenesis, and AbdA is also upregulated upon Notch ectopic expression only in late embryogenesis. This indicates that neuroblast competence to respond to both signals is established later during embryogenesis, preventing precocious neuroblast apoptosis.
Extensive neuroblast apoptosis is also observed at the end of embryogenesis in the gnathal segments (Figure 3). While ~80 embryonic neuroblasts are observed at stage 12, only 24 are observed at stage 16 and ~19 in the 1st instar larva (Kuert, Hartenstein et al. 2014). PCD also occurs at the level of the neuroectoderm. The Hox gene deformed regulates PCD in the neuroectodermal progenitors that constitute the neuroblast 6-4 proneural cluster of the gnathal maxillary and mandibular hemisegments, revealing yet another level where PCD acts to regulate cell number in the developing CNS (Urbach, Jussen et al. 2016).
Most of the head and thoracic neuroblasts exit the cell cycle and stop proliferating in a Prospero-dependent manner in the pupae. Exceptions are observed in the thorax; neuroblasts 5-6, 6-4 and 7-3 die at stage 16 (Baumgardt, Karlsson et al. 2009, Lacin and Truman 2016). Also, the lineages of neuroblasts 2-2, 5-1, 5-4, 5-5 and 6-2 differ between wild type and H99 deficiency clones (Rogulja-Ortmann, Luer et al. 2007, Birkholz, Rickert et al. 2015, Lacin and Truman 2016). Such differences might arise due to neuroblast or neuronal apoptosis.
3.2 Postembryonic apoptosis
The end of neuroblast proliferation in the VNC occurs at different times in different regions, being complete by the end of metamorphosis (Ito and Hotta 1992, Maurange, Cheng et al. 2008). In the central brain and thorax, most postembryonic neuroblasts cease dividing around 20 hours after pupal formation (Truman and Bate 1988). Removing RHG activity does not prevent or delay loss of postembryonic neuroblasts, suggesting that they end proliferation by terminal differentiation (Maurange, Cheng et al. 2008). This is not the case for abdominal postembryonic neuroblasts, which stop proliferation by apoptosis in the late larvae (Prokop, Bray et al. 1998, Allan and Thor 2015) (Figure 3).
Ventral nerve cord
In postembryonic neuroblasts of the ventral nerve cord, tTFs (Hb→ Kr→ Pdm→ Castor→ Svp) not only specify neuronal identity but they also schedule the end of postembryonic divisions via Prospero-dependent cell-cycle exit for thoracic neuroblasts and via apoptosis for abdominal neuroblasts (Maurange, Cheng et al. 2008) (Figure 4A). Castor simultaneously promotes Grainyhead (Grh) expression and the down regulation of Dichaete (D). This bestows neuroblasts with the competence to respond to a burst of AbdA in the late larvae. This intercepts the default terminal differentiation program and promotes apoptosis (Figure 4A). If Grh expression is repressed or D is not silenced, AbdA cannot trigger apoptosis. Removal of Svp or persistent Cas expression prevents abdominal neuroblast apoptosis, despite normal AbdA expression (Maurange, Cheng et al. 2008). This suggests that postembryonic neuroblasts have to transit through the temporal series to establish the competence to die when they assume the D− Grh+ Cas− AbdA+ code. Therefore, expression of tTFs induces and maintains the activation or repression of downstream targets (such as Dichaete and Grainyhead), allowing aging postembryonic neuroblasts to acquire specific tTF codes that regulate the timely death of postembryonic neuroblasts (Maurange, Cheng et al. 2008). The establishment of neuronal identity and neuronal number is thus tightly linked to the tTFs. The recurring implementation of tTFs in establishing neuronal identity in multiple contexts may also regulate the aging of neuroblasts and end of their divisions.
Figure 4. Neuroblast tTFs control apoptosis in both neuroblasts and neurons.
(A) Abdominal neuroblasts have to transit trough the tTFs to schedule apoptosis. The tTF Castor downregulates the expression of Dichaete that inhibits precocious RHG activation and upregulates Grh. Grh installs the competence to respond to a pulse of AbdA that triggers apoptosis.
(B) Neuroblasts tTFs controls Notch mediated neuronal apoptosis. Two pathways determine the binary life-or-death fate of neurons. tOPC neuroblasts tTFs control both the identity of the neurons produced at each time window and their survival by specifying the death of NotchON neurons in a first phase and of NotchOFF neurons in a second phase. The red arrows indicate the dying neurons.
Polycomb group genes are required for postembryonic neuroblasts survival and their removal promotes Hox gene expression and postembryonic neuroblast apoptosis in the central brain, as well as in the ventral nerve cord, thorax and abdomen (Bello, Holbro et al. 2007). How are Polycomb genes regulated in postembryonic neuroblasts and what are their target genes? One possibility is that they act downstream of the tTF series, which could trigger the timely expression of AbdA. Another possibility is that, like in the embryo, AbdA expression is dependent on signaling from the postembryonic neuroblast progeny, which can act by regulating Polycomb gene expression. Supporting this hypothesis is the fact that, like in the embryo, overexpression of NotchICD in larval neuroblasts triggers AbdA expression and promotes apoptosis (Arya, Sarkissian et al. 2015).
A conundrum arises however, since at the end of embryogenesis, most of the abdominal neuroblasts die after a pulse of AbdA (see previous section) although they have not transited through the full temporal series and established the D− Grh+ Cas− AbdA+ code. Interestingly, ectopic expression of either AbdA or the thoracic Hox genes Antp and Ubx proteins can induce apoptosis of both thoracic and abdominal postembryonic neuroblasts. This suggests that all Hox genes have the ability to induce postembryonic neuroblast apoptosis (Bello, Hirth et al. 2003). Hox dependent neuroblast apoptosis is also observed in the gnathal segments in the embryo and early larvae (Kuert, Hartenstein et al. 2014). From the initial 80 neuroblasts, 24 are observed in the stage 16 embryo, 19 in the 1st instar larvae and 14 in the 3rd instar larvae. Unlike abdominal neuroblasts, thoracic embryonic and postembryonic neuroblasts do not express any Hox gene, and therefore do not undergo apoptosis. Could this expression be dependent on the amount of signaling given by post-embryonic neuroblast progeny as is observed in embryonic abdominal neuroblasts? A closer inspection and comparison of the lineages of thoracic, apoptotic and non-apoptotic abdominal and gnathal neuroblasts might uncover a relationship between the amount/type of progeny/signaling with neuroblast Hox gene expression and PCD activation.
The 4 posterior segments of the embryonic abdomen constitute the caudal region: A set of 4 postembryonic neuroblasts, 2 per side, exhibits sex-specific proliferation. In the female these postembryonic neuroblasts stop proliferating in the mid-third instar larvae but the same neuroblasts continue dividing in the male during the larval and pupal stages producing a sex specific lineage (Truman and Bate 1988). Such specification is regulated by the activity of the gene doublesex (dsx), which encodes a transcription factor controlling male or female differentiation (Taylor and Truman 1992). Interestingly, dsx plays a dual role in sex-specific neuroblast fate: the Dsx female isoform promotes neuroblast PCD while the male isoform is required for neuroblast survival (Birkholz, Vef et al. 2013) (Figure 3). Neuroblast survival is also partially dependent on the activity of Abdominal-B (AbdB) but the mechanisms by which this gene and other factors regulate neuroblast sex specific survival remain to be clarified.
Head
In the central brain 8 additional neuroblasts, known as type II neuroblasts, can be found. Type II neuroblasts divide asymmetrically, self-renewing and producing an intermediate neural progenitor (INP), which will divide 3-5 times to self-renew and produce a GMC (Boone and Doe 2008). Both type I and type II neuroblasts in the central brain stop dividing by a terminal symmetric division. The same is also true for INPs (Maurange, Cheng et al. 2008, Weng and Cohen 2015).
Optic lobe neuroblasts start to be produced during the larval stages and neurogenesis lasts until early pupa (Choksi, Southall et al. 2006, Egger, Gold et al. 2010, Ngo, Wang et al. 2010, Yasugi, Sugie et al. 2010, Lanet, Gould et al. 2013, Neriec and Desplan 2016). Neuroblasts originate from the inner (IPC) and outer proliferation center (OPC), two neuroepithelial crescents that generate most of the neurons and glia populating the lamina, medulla, lobula and lobula plate neuropiles (Li, Erclik et al. 2013, Viktorin, Riebli et al. 2013, Bertet, Li et al. 2014, Apitz and Salecker 2015). OPC neuroblasts terminate proliferation by terminally differentiating (Li, Erclik et al. 2013) (Figure 3) but no studies have yet addressed how IPC neuroblasts proliferation is stopped.
Mushroom body (MB) neuroblasts are the longest active neural stem cells in the fly; they start proliferating in the embryo and continue uninterrupted until mid pupa, when they stop proliferating through apoptosis (Siegrist, Haque et al. 2010) (Figure 3). Curiously, in trans-heterozygous flies for Df(3l)H99 and XR38 (a deletion that removes rpr and the NBRR), MB neuroblasts persist until later stages and keep proliferating, producing neurons that are incorporated into the adult mushroom body structure. Interestingly, however, even in the absence of this RHG activity, mushroom body neuroblasts are still later eliminated. This happens because prior to elimination, a reduction of insulin/PI3Kinase signaling triggers neuroblast autophagy. In this way a fail-safe mechanism to ensure MB-neuroblasts elimination by promoting neuroblast autophagy is installed. In the absence of FOXO, RHG dependent apoptosis is delayed, suggesting that FOXO regulates both the timing of RHG dependent apoptosis and the autophagy fail-safe mechanism. It furthers suggests that the timing of elimination of MB neuroblasts depends on the nutritional status of the animal (see also (Speder, Liu et al. 2011).
4. Apoptosis in neurons
4.1 Embryonic apoptosis
Many neurons are destined to die, unless they receive intrinsic or extrinsic survival signals. A characteristic example is that of pioneer neurons and the midline glia in stage 13 embryos. The segmentally repeated early differentiating neurons pCC, MP1, dMP2 and vMP2 extend their axons to delineate the major axonal tracts that span the embryo on either side of the midline (Bate and Grunewald 1981, Jacobs and Goodman 1989, Hidalgo and Brand 1997). These pioneer neurons express two EGF ligands, Vein, a homolog of the vertebrate Neuregulin, and Spitz, which promotes the survival of the neighboring midline glial cells that help fasciculate the axons and form the longitudinal bundles and the commissures that cross the midline in every segment (Hidalgo, Kinrade et al. 2001, Bergmann, Tugentman et al. 2002). Glial cells compete for EGF ligand, and those that fail to activate the EGFR pathway express the pro-apoptotic gene hid, triggering apoptosis (Zhou, Hashimi et al. 1995, Luer and Technau 2009). In turn, midline glial cells regulate the survival of follower neurons that will constitute the longitudinal axon tracts, forming a feedback loop between neurons and glia (Booth, Kinrade et al. 2000).
After forming the longitudinal axonal pathways, some of the pioneer neurons die in a Hox-dependent manner. Posterior MP1 and dMP2 neurons survive to larval stages, due to the AbdB mediated repression of grim and reaper. However, their anterior counterparts undergo PCD, which can be rescued by mis-expression of AbdB (Miguel-Aliaga and Thor 2004). Interestingly, the surviving posterior dMP2 neurons assume a different role during larval stages, as they “trans-differentiate” into ilp-7 expressing neuroendocrine neurons that innervate the hindgut (Miguel-Aliaga, Thor et al. 2008). Similarly, MP1 neurons become pdf-expressing peptidergic interneurons at larval stages (Wheeler, Kearney et al. 2006). The exact opposite apoptotic pattern can be observed in the MP3 grasshopper pioneer neurons. Thoracic MP3 neurons survive, while those in abdominal segments A3-A6 become obsolete after guiding the follower neurons, and undergo apoptosis (Truman, Thorn et al. 1992). The contribution of Hox genes has not been shown rigorously in this case.
Contrary to the anti-apoptotic role that AbdB plays in the midline pioneer neurons, it is capable of exerting a pro-apoptotic role in other contexts during embryonic neuronal development. For example, the Capability neuropeptide-expressing Va neurons survive only in anterior abdominal segments A2-A4, while they undergo AbdB-driven apoptosis in the posterior segments (Suska, Miguel-Aliaga et al. 2011).
Most of the neuronal cell death that is observed during Drosophila embryonic development occurs during stages 14-17, when the ventral nerve cord condenses by almost a quarter of its length (Page and Olofsson 2008). In parallel, a massive apoptotic wave spanning stage 13 to stage 17 decreases the glial cells number by almost 75% (Zhou, Hashimi et al. 1995, Dong and Jacobs 1997, Bergmann, Tugentman et al. 2002), although the extent of glial death in the later stages has been challenged (Rogulja-Ortmann, Luer et al. 2007). Initially, apoptotic cell death is observed uniformly in the ventral nerve cord, gradually being restricted to the anterior and posterior termini. By stage 17, more than half of neuronal apoptosis occurs in the posterior-most segments A6-A8 (Page and Olofsson 2008). Forcing neurons, glia, or neuroblasts to survive during this extensive phase of apoptosis by expressing the baculovirus protein p35, which acts as a broad caspase inhibitor and interrupts programmed cell death, inhibits condensation of the VNC, but does not appear to have detrimental effects for the survival of the adult fly (Page and Olofsson 2008) (discussed further in Section 5.2).
Apart from their role in neuronal survival, glial cells act as the macrophages of the CNS by removing neuronal debris. They are activated after neuronal PCD (Sonnenfeld and Jacobs 1995, Freeman, Delrow et al. 2003, Mergliano and Minden 2003). A number of phagocytic receptors, such as Draper and Six-microns-under (Simu), are expressed in embryonic glia and participate in the phagocytosis of apoptotic neurons (Kurant, Axelrod et al. 2008, Kurant 2011).
Apoptotic death occurs both in irregular and in segmentally repeated patterns. One lineage that has been studied extensively for its very stereotyped apoptosis is that of the ventral nerve chord neuroblast 7-3 (Lundell, Lee et al. 2003, Karcavich and Doe 2005, Rogulja-Ortmann, Renner et al. 2008, Lee, Sehgal et al. 2013). In this lineage, the first GMC gives rise to the EW1 serotonergic interneuron and the GW motoneuron; the second GMC generates the EW2 serotonergic interneuron and a neuron that undergoes PCD; the third GMC generates the EW3 corazonin-positive (vCrz) interneuron and its apoptotic sibling. All these binary cell fate or survival decisions are mediated by the asymmetric inheritance of Numb in one of the neuronal progeny, which inhibits Notch signaling in this cell. If the sibling of EW3 that undergoes PCD is forced to survive, it also differentiates as a vCrz interneuron (Novotny, Eiselt et al. 2002, Lundell, Lee et al. 2003, Karcavich and Doe 2005, Lee, Sehgal et al. 2013).
Neurons that do not undergo apoptosis following a Notch-mediated binary cell fate decision are often subject to Hox-mediated, segment-dependent apoptosis. In the case of the ventral nerve cord neuroblast NB7-3, the GW motorneuron that was generated by the first GMC, undergoes apoptosis in segments T3-A7. Ubx, which is expressed in these segments, is necessary and sufficient to induce reaper and kill the motorneurons (Rogulja-Ortmann, Luer et al. 2007, Rogulja-Ortmann, Renner et al. 2008).
4.2 Larval neuronal apoptosis
During larval stages, the extent of neuronal apoptosis is very restricted compared to the embryo. Most of the neurons that undergo PCD in the larva die immediately after the division of the GMC. Hemilineages in which half of the neurons die in a Notch-dependent manner are produced extensively by central brain neuroblasts. The 100 neuroblasts that populate each brain hemisphere are responsible for the production of about 100,000 neurons (Chiang, Lin et al. 2011). An example of such binary choices is observed in the four engrailed-expressing neuroblast lineages of the central brain, MC1, MC2, AC, and PC. GMCs produced by the MC1 neuroblasts give rise to two different neurons, while AC and PC neuroblasts generate only one neuronal type that survives (NotchOFF) and one that dies via apoptotis (NotchON). Contrary, in the case of the MC2 neuroblasts, the NotchON progeny survives while the NotchOFF undergoes apoptosis (Kumar, Bello et al. 2009). A similar mode of neurogenesis where one of the two progeny of the GMC dies in a Notch-dependent manner can be observed in more than 25% of the central brain lineages (Lin, Lai et al. 2010, Yu, Kao et al. 2010, Lovick, Kong et al. 2016), in some lineages of the optic lobe (Bertet, Li et al. 2014), as well as the ventral nerve cord (Truman, Moats et al. 2010, Baek, Enriquez et al. 2013). It is important to note the essential contribution of the tTFs in regulating neuronal death. In the tips of the OPC of the developing optic lobes, tTFs expression in the neuroblasts modulate whether their NotchON or NotchOFF neuronal progeny die in a specific temporal window. More specifically, they regulate the expression of pro-apoptotic genes to trigger the apoptosis of NotchON (reaper) or NotchOFF (hid) neurons (Bertet, Li et al. 2014) (Figure 4B). The same regulation might occur in hemilineages of the central brain and of the VNC.
4.3 Pupal neuronal apoptosis
Metamorphosis has given holometabolous insects the opportunity to inhabit different environments at different life stages. However, this comes at a cost, as these insects need to develop two different nervous systems to accommodate the different needs of the larval and adult life stages. During metamorphosis, larval neurons can be largely divided into two categories: those that die and those that prune and remodel their dendrites and axons to acquire a new function in the adult nervous system (e.g. mushroom body neurons – (Lee, Lee et al. 1999)). Neurons undergo apoptosis in two waves. Larval neurons that are not retained in the adult and are not needed for ecdysis, the molting of the fly's exoskeleton, undergo PCD a few hours after pupal formation. The neurons that participate in ecdysis die after metamorphosis within 24 hours after eclosion (Kimura and Truman 1990, Tissot and Stocker 2000).
20-hydroxyecdysone is a steroid hormone that regulates metamorphosis and ecdysis in arthropods and plays a central role in the control of apoptosis of larval neurons. Three ecdysone pulses regulate larval to pupal to adult transitions: a ‘late larval pulse’ at puparium formation, a small ‘prepupal pulse’ 10 hours after puparium formation (APF), and a long ‘pupal pulse’ starting 24 hours APF that decreases gradually until eclosion (Handler 1982). Ecdysone acts through its hormonal receptors, EcR-A, EcR-B1, and EcR-B2 to differentially regulate grim and reaper expression in different neurons and induce apoptosis (Robinow, Draizen et al. 1997, Draizen, Ewer et al. 1999).
Soon after the late larval pulse of ecdysone (6h apf), Corazonin-expressing peptidergic interneurons (vCrz) undergo apoptosis (Choi, Lee et al. 2006). PCD of vCrz neurons is triggered by ecdysone via its receptors, EcR-B1 and EcR-B2, and is mediated by reaper but surprisingly not DIAP1. After the small prepupal pulse, RP2 motorneurons in abdominal segments A2-A7 are eliminated; this process also requires the B isoforms of the ecdysone receptor and reaper, and is also independent of DIAP1 activity (Winbush and Weeks 2011). Interestingly, although RP2 motorneurons in A1 and aCC motorneurons in A2-A7 express EcR-B isoforms during the prepupal pulse, they survive into the pupal stage (Winbush and Weeks 2011). These two observations suggest an unknown additional layer of PCD regulation besides ecdysone.
A number of optic lobe neurons also die in late larval and pupal stages in a complex spatiotemporal pattern (Togane, Ayukawa et al. 2012). Spatially, two distinct clusters of cells undergo apoptosis in the lamina, four clusters in the medulla and one cluster in the lobula plate and the region of T2/T3/C neurons between the lobula plate cortex and the medulla rim. Temporally, neuronal apoptosis spans the entire pupal stage in the optic lobe, with most of the affected optic lobe neurons dying soon after the prepupal pulse in an ecdysone/EcR-B1-dependent process (Hara, Hirai et al. 2013).
While ecdysone triggers apoptosis in vCrz, RP2 neurons and several optic lobe neurons, it is required for the survival of other neurons during the long pupal pulse. Approximately 300 ventral nerve cord neurons, termed type II neurons, express high levels of the ecdysone receptor EcR-A during metamorphosis. These neurons remain alive until eclosion and degenerate once ecdysone levels fall (Robinow, Talbot et al. 1993, Robinow, Draizen et al. 1997). Their apoptosis can be reversed by the administration of ecdysone or by decapitation. Ecdysone treatment inhibits the expression of reaper, while decapitation leads to the accumulation of low levels of reaper that are not sufficient to trigger apoptosis (Robinow, Talbot et al. 1993, Robinow, Draizen et al. 1997). Ecdysone acts on type II neurons by inhibiting the expression of reaper and grim, preventing neurons from undergoing PCD. At the same time, CCAP-expressing neurons die in an identical ecdysone decline-dependent manner (Draizen, Ewer et al. 1999, Lee, Kikuno et al. 2013).
Survival and apoptosis of different neurons during pupal stages can also be sex-specific. fruitless is alternatively spliced in males and females, and a specific male isoform acting in male neurons is necessary for the specification of sex-specific neuronal circuits and for male courtship behavior (Demir and Dickson 2005). fruitless is involved in the selective apoptosis of the male-specific mAL neurons in the female, which is mainly driven by reaper (Kimura, Ote et al. 2005).
5. Emerging ideas
5.1 Regulation of apoptosis
If there is one rule for PCD during neurodevelopment, it is that there is no rule. PCD emerges under different circumstances, and may be driven by different regulators. Apoptosis can be triggered by both intrinsic and extrinsic signals, and may utilize one or a combination of the aforementioned pro-apoptotic genes, reaper, hid, grim and sickle.
The intrinsic regulators can be either temporal factors, which signal timely apoptosis of neuroblasts or neurons, or spatial signals that instruct cell fate based on their location. The most widely used temporal signals in the CNS operate in neuroblasts - as neuroblasts progress through the tTFs, competence to terminally differentiate or die is installed. However, not all neuroblasts have the same fate, which is to a large extent defined by their location and is under Hox regulation. The same is observed in neurons - the survival of the midline pioneer neurons, MP1 and dMP2, depends on the expression of AbdB (Miguel-Aliaga and Thor 2004). An additional level of PCD regulation relies on a Notch-dependent binary cell fate decision in the hemilineages that are generated from GMCs. In many cases during larval neurogenesis of adult neurons, one of the two neuronal progeny of the GMC undergoes Notch-dependent apoptosis, which in some cases results in the NotchON neuron or in other cases the NotchOFF neuron dying. Finally, sex-specific PCD regulates both neuroblast and neuronal fate.
To trigger apoptosis, temporal, spatial and Notch-dependent signals have to activate the expression of one or more of the pro-apoptotic genes, reaper, hid, grim or sickle. Their expression pattern differs widely during neural development, although three of them (reaper, hid, and grim) are able to trigger apoptosis when mis-expressed. What is the individual role of these four genes? What is the selective advantage of having four different apoptotic regulators?
reaper and/or grim are required for almost all of the CNS apoptosis occurring in the embryonic stages, as well as for most of the postembryonic neuronal PCD, which is mostly mediated by ecdysone (White, Grether et al. 1994, Chen, Nordstrom et al. 1996, Miguel-Aliaga and Thor 2004). hid, on the other hand, which is widely used in non-neuronal PCD (Jiang, Baehrecke et al. 1997), is expressed more sparsely in embryonic neuronal tissues, and not exclusively in neurons that are destined to die. hid seems to be responsible for the death of midline glia, in collaboration with reaper (Zhou, Hashimi et al. 1995, Zhou, Schnitzler et al. 1997). reaper and grim are needed to trigger apoptosis of neuroblasts in the abdominal neuromeres (Tan, Yamada-Mabuchi et al. 2011). grim appears to regulate apoptosis of neuropeptide-expressing neurons, such as the vCrz (in collaboration with reaper) (Choi, Lee et al. 2006, Lee, Sehgal et al. 2013), Capability-expressing Va neurons (Suska, Miguel-Aliaga et al. 2011), and CCAP-expressing neurons (Lee, Kikuno et al. 2013). It is also important to highlight that even if one of the RHG genes is not expressed in an apoptotic cell, it is often sufficient to trigger apoptosis if mis-expressed in this cell; e.g. although hid and reaper are responsible for midline glia apoptosis, ectopic expression of grim, which is not expressed in these cells, can induce their apoptosis (Wing, Zhou et al. 1998).
Another difference between the RHG genes is that their expression is regulated by different environmental conditions and by distinct signaling molecules. For example, the different ecdysone receptors have specific effects on the expression of reaper. Moreover, reaper expression is also regulated by the p53/DNA damage pathway (Brodsky, Nordstrom et al. 2000, Jiang, Lamblin et al. 2000, Ollmann, Young et al. 2000), while hid expression is regulated by the EGFR and the Ras/MAPK pathway (Bergmann, Agapite et al. 1998, Kurada and White 1998). A plausible model that could explain the existence of four genes with similar downstream functions is that they are partially redundant and accumulation of pro-apoptotic activity is what irreversibly triggers apoptosis. By integrating information from different sources that differentially control each of them, the pro-apoptotic genes “compute” whether a cell needs to undergo apoptosis. This secures cell death efficiency, timing, and specificity.
5.2 Forcing dying cells to survive
At different developmental stages, up to 30% of the cells of the CNS are dying. What happens when a cell that is supposed to die is forced to survive? This is a fascinating question, albeit slightly arbitrary in its interpretation, as “living dead” cells are normally not encountered in nature. There are two ways to approach this question. One is to assess neuronal identity and connectivity in mutants that lack RHG genes. In this case, the neuronal apoptotic program cannot even begin. A second method is to overexpress an exogenous protein, the baculovirus p35 protein, to prevent death after the death cascade has started. It has been reported that p35-expressing rescued neuroblasts appear larger than normal, probably because the apoptotic cascade has already initiated (Cenci and Gould 2005). Third, one can force neuroblasts to survive, and assess the identity of neurons that should have never been produced. Although the interpretation of cell identity might be confusing in this case, as the neuroblasts have escaped PCD and, potentially, cell cycle control, “immortal” neuroblasts and their progeny represent a great model for studying neural tumors (Narbonne-Reveau, Lanet et al. 2016).
Embryos homozygous for the H99 deletion, where cell death is inhibited, display an enlarged VNC often merged with the epidermis (Page and Olofsson 2008), and die as embryos. However, inhibiting apoptosis in neurons is mostly tolerated in flies. Some innervation defects are observed (Jiang and Reichert 2012), but, in general, neurons that survive manage to form connections and participate in a hyperplastic nervous system (White, Grether et al. 1994, Zhou, Hashimi et al. 1995, Draizen, Ewer et al. 1999, Rogulja-Ortmann, Luer et al. 2007). For example, if the death of the anterior pioneer neuron dMP2 is prevented by overexpression of p35, the “undead” neuron seems to follow the fate of its posterior counterpart and differentiate into neuroendocrine neurons, although the presence of AbdB appears necessary for specific aspects of terminal differentiation (Miguel-Aliaga and Thor 2004). Apart from neurons, supernumerary midline glia in H99 embryos also seem to incorporate normally in the midline (Dong and Jacobs 1997).
In many hemilineages neurons are eliminated even before differentiating. The reason why this happens is not understood. Perhaps the generation of these neurons is part of a conserved neurogenic program that included the generation of sibling neurons that may no longer be needed but may have been necessary in ancestors that lived in different environments and under different selective pressures. Another possibility is that the generation of more neurons than needed favors fast adaptation in the face of different environmental pressures. PCD may be activated or repressed in different neurons of different lineages, facilitating a rapid remodeling of the neuronal circuitry
Another question that arises is what the identity of “living dead” neurons. In the case of the hemilineages, where GMCs give rise to one living and one dead neuron, what identity will the dead neuron acquire if it is forced to survive? Will it be the same as its sister cell? The answer to this question is case-dependent. For example, the second GMC from embryonic NB7-3 gives rise to neuron EW2 and EW2sib, which undergoes cell death. If EW2sib is forced to survive, it expresses similar markers to its sibling EW2 (i.e., Kr and Zfh2), although this does not necessarily indicate identical fates (Karcavich and Doe 2005). Similarly, for neuroblasts NB2-1, NB2-2a, NB2-4a, NB2-5, NB3-1a, NB3-2, NB3-5, NB4-4, NB5-1, NB5-4a, NB5-5, NB6-1, and NB7-1, the additional neurons observed in H99 embryos that should have died project in an identical manner to their sibling cells. In contrast, in neuroblasts lineages NB4-2, NB5-3, NB7-2 andNB7-4, H99 embryos show additional cells but also irregular axonal projections that are not observed in wild-type flies (Rogulja-Ortmann, Luer et al. 2007, Kumar, Bello et al. 2009, Lovick, Kong et al. 2016).
Inhibiting apoptosis in neuroblasts compared to neurons should have a larger effect given that they continue to proliferate. However, this does not seem to affect the nervous system massively. Eliminating rpr and grim in the abdominal neuromeres leads to a block in neuroblast PCD, which in turn gives rise to large numbers of neurons that are incorporated seemingly normally into the abdominal adult nervous system (Tan, Yamada-Mabuchi et al. 2011). It is interesting to note that the fly hatches with an enlarged abdominal nervous system, which seems to introduce a physical constrain for copulation (Peterson, Carney et al. 2002).
There are several questions that remain unanswered. Although “undead” supernumerary neurons and glia seem to incorporate relatively normally in the nervous system, it has not been assessed how this affects circuit function and behavior. Moreover, although “undead” neuroblasts initially continue to proliferate, they haven't been reported to persist to adulthood. There have been reports of caspase-independent cell engulfment, which is not affected in H99 mutants or p35 expressing cells and recapitulates the cell death pattern (Mergliano and Minden 2003).
5.3 Cell cycle exit or apoptosis
Under wild-type conditions, the life of a neuroblast ends by one of two mechanisms: the neuroblast either divides terminally to give rise to two neurons or glia, or it undergoes apoptosis. What is the functional significance of the two mechanisms? Do they represent different solutions to the same problem (elimination of the neuroblast) or do they lead to different end products? One possibility is that neuroblasts undergoing apoptosis produce a signal necessary for neuronal maturation or axonal targeting, in a manner similar to what is observed in imaginal disc cells where Wg and other molecules induce compensatory proliferation when undergoing apoptosis (Ryoo, Gorenc et al. 2004, Wells, Yoshida et al. 2006).
Embryos mutant for prospero, a gene that promotes cell cycle exit, display an increase in reaper-mediated apoptosis (Li and Vaessin 2000). Conversely, in embryonic abdominal neuroblasts that are eliminated by PCD, the time that neuroblasts proliferate is expanded in prospero mutants, indicating a potential cell cycle exit before PCD occurs (Li and Vaessin 2000, Bello, Hirth et al. 2003). It is therefore possible that both mechanisms are employed in order for the animal to avoid over-proliferating (oncogenic) cells. As already discussed, mushroom body neuroblasts have a fail-safe mechanism that assures neuroblast removal in the absence of RHG activity.
When a neuroblast undergoes apoptosis, its proliferative capacity is lost. On the other hand, neuroblasts that terminally divide may be able to pass this capacity to their progeny, leaving open the opportunity for later cell divisions. The terminal division of neuroblasts often gives rises to two glial cells, such as, for example, in the OPC of the developing optic lobe (Li, Erclik et al. 2013). The same has been observed in vertebrate systems; for example, Müller glia are generated in the latest temporal window by the retinal progenitor cells after the production of retinal ganglion cells, interneurons, cones, and rods (Cepko 2014). Moreover, Müller glial cells have been shown to retain proliferative capacity and to be able to divide and produce both Müller glial cells and other retinal neurons (Moshiri, Close et al. 2004, Bernardos, Barthel et al. 2007, Gallina, Palazzo et al. 2015). Therefore, it is possible that in the case of neuroblast terminal division, the capacity to proliferate is transferred by the neuroblast to one (or both) of its two progeny, which are usually glial cells. In favor of this argument, glial cells in multiple contexts have been shown to maintain mitotic activity (Doetsch 2003). This capacity may reveal itself immediately by the amplification of the glial population, or later upon activation in adulthood (Guo, Zhang et al. 2014, Duan, Liu et al. 2015, Sammut, Cook et al. 2015). This difference in proliferative capacity may be mediated by the different levels of Prospero inherited by the terminally dividing neuroblast to its progeny. Prospero has been shown to influence proliferative capacity according to its expression levels; absence of Prospero leads to proliferation, low levels of Prospero induce quiescence, while high levels of Prospero promote differentiation (Lai and Doe 2014).
6. Conclusion
The Drosophila nervous system has been an instrumental model system for understanding the mechanisms of programmed cell death, as well as its developmental significance. With the complete identification of all fly neuroblasts, and most of their lineages, a comprehensive comparison between homologous lineages will lead to a thorough understanding of how multiple developmental cues are integrated for the regulation of PCD. Finally, this knowledge lays the foundation for studying apoptosis during neural development in other insects and arthropods. This may eventually allow us to understand how and under which selective pressure the occurrences and mechanisms for regulation of PCD evolved over time.
Acknowledgements
We thank Cédric Maurange, Laura Quintana, Anthony Rossi, Vilaiwan Fernandes, Jens Rister, and Erin Barnhart for critical reading and suggestions on the manuscript. Our research on Drosophila neurodevelopment is supported by NIH EY13012. Support for FPT was provided by NYU Abu Dhabi grant G-1205C to CD. NK was supported by an EMBO long-term fellowship (365-2014) and a post-doctoral HFSP fellowship (LT000122/2015-L).
References
- Abdelwahid E, Yokokura T, Krieser RJ, Balasundaram S, Fowle WH, White K. Mitochondrial disruption in Drosophila apoptosis. Dev Cell. 2007;12(5):793–806. doi: 10.1016/j.devcel.2007.04.004. [DOI] [PubMed] [Google Scholar]
- Abrams JM, White K, Fessler LI, Steller H. Programmed cell death during Drosophila embryogenesis. Development. 1993;117(1):29–43. doi: 10.1242/dev.117.1.29. [DOI] [PubMed] [Google Scholar]
- Allan DW, Thor S. Transcriptional selectors, masters, and combinatorial codes: regulatory principles of neural subtype specification. Wiley Interdiscip Rev Dev Biol. 2015;4(5):505–528. doi: 10.1002/wdev.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almeida MS, Bray SJ. Regulation of post-embryonic neuroblasts by Drosophila Grainyhead. Mech Dev. 2005;122(12):1282–1293. doi: 10.1016/j.mod.2005.08.004. [DOI] [PubMed] [Google Scholar]
- Apitz H, Salecker I. A region-specific neurogenesis mode requires migratory progenitors in the Drosophila visual system. Nat Neurosci. 2015;18(1):46–55. doi: 10.1038/nn.3896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arya R, Sarkissian T, Tan Y, White K. Neural stem cell progeny regulate stem cell death in a Notch and Hox dependent manner. Cell Death Differ. 2015;22(8):1378–1387. doi: 10.1038/cdd.2014.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baek M, Enriquez J, Mann RS. Dual role for Hox genes and Hox co-factors in conferring leg motoneuron survival and identity in Drosophila. Development. 2013;140(9):2027–2038. doi: 10.1242/dev.090902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bangs P, Franc N, White K. Molecular mechanisms of cell death and phagocytosis in Drosophila. Cell Death Differ. 2000;7(11):1027–1034. doi: 10.1038/sj.cdd.4400754. [DOI] [PubMed] [Google Scholar]
- Bangs P, White K. Regulation and execution of apoptosis during Drosophila development. Developmental Dynamics. 2000;218(1):68–79. doi: 10.1002/(SICI)1097-0177(200005)218:1<68::AID-DVDY6>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- Bate CM, Grunewald EB. Embryogenesis of an insect nervous system II: a second class of neuron precursor cells and the origin of the intersegmental connectives. J Embryol Exp Morphol. 1981;61:317–330. [PubMed] [Google Scholar]
- Baumgardt M, Karlsson D, Terriente J, Diaz-Benjumea FJ, Thor S. Neuronal subtype specification within a lineage by opposing temporal feed-forward loops. Cell. 2009;139(5):969–982. doi: 10.1016/j.cell.2009.10.032. [DOI] [PubMed] [Google Scholar]
- Bayraktar OA, Doe CQ. Combinatorial temporal patterning in progenitors expands neural diversity. Nature. 2013;498(7455):449–455. doi: 10.1038/nature12266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bello B, Holbro N, Reichert H. Polycomb group genes are required for neural stem cell survival in postembryonic neurogenesis of Drosophila. Development. 2007;134(6):1091–1099. doi: 10.1242/dev.02793. [DOI] [PubMed] [Google Scholar]
- Bello BC, Hirth F, Gould AP. A pulse of the Drosophila Hox protein Abdominal-A schedules the end of neural proliferation via neuroblast apoptosis. Neuron. 2003;37(2):209–219. doi: 10.1016/s0896-6273(02)01181-9. [DOI] [PubMed] [Google Scholar]
- Berger C, Urban J, Technau GM. Stage-specific inductive signals in the Drosophila neuroectoderm control the temporal sequence of neuroblast specification. Development. 2001;128(17):3243–3251. doi: 10.1242/dev.128.17.3243. [DOI] [PubMed] [Google Scholar]
- Bergmann A, Agapite J, McCall K, Steller H. The Drosophila gene hid is a direct molecular target of Ras-dependent survival signaling. Cell. 1998;95(3):331–341. doi: 10.1016/s0092-8674(00)81765-1. [DOI] [PubMed] [Google Scholar]
- Bergmann A, Tugentman M, Shilo BZ, Steller H. Regulation of cell number by MAPK-dependent control of apoptosis: a mechanism for trophic survival signaling. Dev Cell. 2002;2(2):159–170. doi: 10.1016/s1534-5807(02)00116-8. [DOI] [PubMed] [Google Scholar]
- Bernardos RL, Barthel LK, Meyers JR, Raymond PA. Late-stage neuronal progenitors in the retina are radial Muller glia that function as retinal stem cells. J Neurosci. 2007;27(26):7028–7040. doi: 10.1523/JNEUROSCI.1624-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertet C, Li X, Erclik T, Cavey M, Wells B, Desplan C. Temporal patterning of neuroblasts controls Notch-mediated cell survival through regulation of Hid or Reaper. Cell. 2014;158(5):1173–1186. doi: 10.1016/j.cell.2014.07.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhat KM. Segment polarity genes in neuroblast formation and identity specification during Drosophila neurogenesis. Bioessays. 1999;21(6):472–485. doi: 10.1002/(SICI)1521-1878(199906)21:6<472::AID-BIES4>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- Birkholz O, Rickert C, Nowak J, Coban IC, Technau GM. Bridging the gap between postembryonic cell lineages and identified embryonic neuroblasts in the ventral nerve cord of Drosophila melanogaster. Biol Open. 2015;4(4):420–434. doi: 10.1242/bio.201411072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birkholz O, Vef O, Rogulja-Ortmann A, Berger C, Technau GM. Abdominal-B and caudal inhibit the formation of specific neuroblasts in the Drosophila tail region. Development. 2013;140(17):3552–3564. doi: 10.1242/dev.096099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boone JQ, Doe CQ. Identification of Drosophila type II neuroblast lineages containing transit amplifying ganglion mother cells. Dev Neurobiol. 2008;68(9):1185–1195. doi: 10.1002/dneu.20648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Booth GE, Kinrade EF, Hidalgo A. Glia maintain follower neuron survival during Drosophila CNS development. Development. 2000;127(2):237–244. doi: 10.1242/dev.127.2.237. [DOI] [PubMed] [Google Scholar]
- Bossing T, Udolph G, Doe CQ, Technau GM. The embryonic central nervous system lineages of Drosophila melanogaster. I. Neuroblast lineages derived from the ventral half of the neuroectoderm. Dev Biol. 1996;179(1):41–64. doi: 10.1006/dbio.1996.0240. [DOI] [PubMed] [Google Scholar]
- Brennecke J, Hipfner DR, Stark A, Russell RB, Cohen SM. bantam encodes a developmentally regulated microRNA that controls cell proliferation and regulates the proapoptotic gene hid in Drosophila. Cell. 2003;113(1):25–36. doi: 10.1016/s0092-8674(03)00231-9. [DOI] [PubMed] [Google Scholar]
- Britton JS, Edgar BA. Environmental control of the cell cycle in Drosophila: nutrition activates mitotic and endoreplicative cells by distinct mechanisms. Development. 1998;125(11):2149–2158. doi: 10.1242/dev.125.11.2149. [DOI] [PubMed] [Google Scholar]
- Brodsky MH, Nordstrom W, Tsang G, Kwan E, Rubin GM, Abrams JM. Drosophila p53 binds a damage response element at the reaper locus. Cell. 2000;101(1):103–113. doi: 10.1016/S0092-8674(00)80627-3. [DOI] [PubMed] [Google Scholar]
- Brody T, Odenwald WF. Programmed transformations in neuroblast gene expression during Drosophila CNS lineage development. Dev Biol. 2000;226(1):34–44. doi: 10.1006/dbio.2000.9829. [DOI] [PubMed] [Google Scholar]
- Campos-Ortega JA. Genetic mechanisms of early neurogenesis in Drosophila melanogaster. J Physiol Paris. 1994;88(2):111–122. doi: 10.1016/0928-4257(94)90022-1. [DOI] [PubMed] [Google Scholar]
- Cashio P, Lee TV, Bergmann A. Genetic control of programmed cell death in Drosophila melanogaster. Semin Cell Dev Biol. 2005;16(2):225–235. doi: 10.1016/j.semcdb.2005.01.002. [DOI] [PubMed] [Google Scholar]
- Cenci C, Gould AP. Drosophila Grainyhead specifies late programmes of neural proliferation by regulating the mitotic activity and Hox-dependent apoptosis of neuroblasts. Development. 2005;132(17):3835–3845. doi: 10.1242/dev.01932. [DOI] [PubMed] [Google Scholar]
- Cepko C. Intrinsically different retinal progenitor cells produce specific types of progeny. Nat Rev Neurosci. 2014;15(9):615–627. doi: 10.1038/nrn3767. [DOI] [PubMed] [Google Scholar]
- Chell JM, Brand AH. Nutrition-responsive glia control exit of neural stem cells from quiescence. Cell. 2010;143(7):1161–1173. doi: 10.1016/j.cell.2010.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen P, Nordstrom W, Gish B, Abrams JM. grim, a novel cell death gene in Drosophila. Genes Dev. 1996;10(14):1773–1782. doi: 10.1101/gad.10.14.1773. [DOI] [PubMed] [Google Scholar]
- Chen P, Rodriguez A, Erskine R, Thach T, Abrams JM. Dredd, a novel effector of the apoptosis activators reaper, grim, and hid in Drosophila. Dev Biol. 1998;201(2):202–216. doi: 10.1006/dbio.1998.9000. [DOI] [PubMed] [Google Scholar]
- Chiang AS, Lin CY, Chuang CC, Chang HM, Hsieh CH, Yeh CW, Shih CT, Wu JJ, Wang GT, Chen YC, Wu CC, Chen GY, Ching YT, Lee PC, Lin CY, Lin HH, Wu CC, Hsu HW, Huang YA, Chen JY, Chiang HJ, Lu CF, Ni RF, Yeh CY, Hwang JK. Three-dimensional reconstruction of brain-wide wiring networks in Drosophila at single-cell resolution. Curr Biol. 2011;21(1):1–11. doi: 10.1016/j.cub.2010.11.056. [DOI] [PubMed] [Google Scholar]
- Choi YJ, Lee G, Park JH. Programmed cell death mechanisms of identifiable peptidergic neurons in Drosophila melanogaster. Development. 2006;133(11):2223–2232. doi: 10.1242/dev.02376. [DOI] [PubMed] [Google Scholar]
- Choksi SP, Southall TD, Bossing T, Edoff K, de Wit E, Fischer BE, van Steensel B, Micklem G, Brand AH. Prospero acts as a binary switch between self-renewal and differentiation in Drosophila neural stem cells. Dev Cell. 2006;11(6):775–789. doi: 10.1016/j.devcel.2006.09.015. [DOI] [PubMed] [Google Scholar]
- Christich A, Kauppila S, Chen P, Sogame N, Ho SI, Abrams JM. The damage-responsive Drosophila gene sickle encodes a novel IAP binding protein similar to but distinct from reaper, grim, and hid. Curr Biol. 2002;12(2):137–140. doi: 10.1016/s0960-9822(01)00658-3. [DOI] [PubMed] [Google Scholar]
- Claveria C, Albar JP, Serrano A, Buesa JM, Barbero JL, Martinez AC, Torres M. Drosophila grim induces apoptosis in mammalian cells. Embo j. 1998;17(24):7199–7208. doi: 10.1093/emboj/17.24.7199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Claveria C, Caminero E, Martinez AC, Campuzano S, Torres M. GH3, a novel proapoptotic domain in Drosophila Grim, promotes a mitochondrial death pathway. Embo j. 2002;21(13):3327–3336. doi: 10.1093/emboj/cdf354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clavier A, Rincheval-Arnold A, Colin J, Mignotte B, Guenal I. Apoptosis in Drosophila: which role for mitochondria? Apoptosis. 2016;21(3):239–251. doi: 10.1007/s10495-015-1209-y. [DOI] [PubMed] [Google Scholar]
- Clavier A, Ruby V, Rincheval-Arnold A, Mignotte B, Guenal I. The Drosophila retinoblastoma protein, Rbf1, induces a Debcl- and Drp1-dependent mitochondrial apoptosis. J Cell Sci. 2015;128(17):3239–3249. doi: 10.1242/jcs.169896. [DOI] [PubMed] [Google Scholar]
- Cleary MD, Doe CQ. Regulation of neuroblast competence: multiple temporal identity factors specify distinct neuronal fates within a single early competence window. Genes Dev. 2006;20(4):429–434. doi: 10.1101/gad.1382206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demir E, Dickson BJ. fruitless splicing specifies male courtship behavior in Drosophila. Cell. 2005;121(5):785–794. doi: 10.1016/j.cell.2005.04.027. [DOI] [PubMed] [Google Scholar]
- Doe CQ. Molecular markers for identified neuroblasts and ganglion mother cells in the Drosophila central nervous system. Development. 1992;116(4):855–863. doi: 10.1242/dev.116.4.855. [DOI] [PubMed] [Google Scholar]
- Doe CQ, Technau GM. Identification and cell lineage of individual neural precursors in the Drosophila CNS. Trends Neurosci. 1993;16(12):510–514. doi: 10.1016/0166-2236(93)90195-r. [DOI] [PubMed] [Google Scholar]
- Doetsch F. The glial identity of neural stem cells. Nat Neurosci. 2003;6(11):1127–1134. doi: 10.1038/nn1144. [DOI] [PubMed] [Google Scholar]
- Dong R, Jacobs JR. Origin and differentiation of supernumerary midline glia in Drosophila embryos deficient for apoptosis. Dev Biol. 1997;190(2):165–177. doi: 10.1006/dbio.1997.8688. [DOI] [PubMed] [Google Scholar]
- Dormand EL, Brand AH. Runt determines cell fates in the Drosophila embryonic CNS. Development. 1998;125(9):1659–1667. doi: 10.1242/dev.125.9.1659. [DOI] [PubMed] [Google Scholar]
- Dorstyn L, Colussi PA, Quinn LM, Richardson H, Kumar S. DRONC, an ecdysone-inducible Drosophila caspase. Proc Natl Acad Sci U S A. 1999;96(8):4307–4312. doi: 10.1073/pnas.96.8.4307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorstyn L, Read SH, Quinn LM, Richardson H, Kumar S. DECAY, a novel Drosophila caspase related to mammalian caspase-3 and caspase-7. J Biol Chem. 1999;274(43):30778–30783. doi: 10.1074/jbc.274.43.30778. [DOI] [PubMed] [Google Scholar]
- Doumanis J, Quinn L, Richardson H, Kumar S. STRICA, a novel Drosophila melanogaster caspase with an unusual serine/threonine-rich prodomain, interacts with DIAP1 and DIAP2. Cell Death Differ. 2001;8(4):387–394. doi: 10.1038/sj.cdd.4400864. [DOI] [PubMed] [Google Scholar]
- Draizen TA, Ewer J, Robinow S. Genetic and hormonal regulation of the death of peptidergic neurons in the Drosophila central nervous system. J Neurobiol. 1999;38(4):455–465. doi: 10.1002/(sici)1097-4695(199903)38:4<455::aid-neu2>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- Duan CL, Liu CW, Shen SW, Yu Z, Mo JL, Chen XH, Sun FY. Striatal astrocytes transdifferentiate into functional mature neurons following ischemic brain injury. Glia. 2015;63(9):1660–1670. doi: 10.1002/glia.22837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egger B, Chell JM, Brand AH. Insights into neural stem cell biology from flies. Philos Trans R Soc Lond B Biol Sci. 2008;363(1489):39–56. doi: 10.1098/rstb.2006.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egger B, Gold KS, Brand AH. Notch regulates the switch from symmetric to asymmetric neural stem cell division in the Drosophila optic lobe. Development. 2010;137(18):2981–2987. doi: 10.1242/dev.051250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans EK, Kuwana T, Strum SL, Smith JJ, Newmeyer DD, Kornbluth S. Reaper-induced apoptosis in a vertebrate system. Embo j. 1997;16(24):7372–7381. doi: 10.1093/emboj/16.24.7372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraser AG, McCarthy NJ, Evan GI. drICE is an essential caspase required for apoptotic activity in Drosophila cells. Embo j. 1997;16(20):6192–6199. doi: 10.1093/emboj/16.20.6192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman MR, Delrow J, Kim J, Johnson E, Doe CQ. Unwrapping glial biology: Gcm target genes regulating glial development, diversification, and function. Neuron. 2003;38(4):567–580. doi: 10.1016/s0896-6273(03)00289-7. [DOI] [PubMed] [Google Scholar]
- Gallina D, Palazzo I, Steffenson L, Todd L, Fischer AJ. Wnt/betacatenin-signaling and the formation of Muller glia-derived progenitors in the chick retina. Dev Neurobiol. 2015 doi: 10.1002/dneu.22370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goyal G, Fell B, Sarin A, Youle RJ, Sriram V. Role of mitochondrial remodeling in programmed cell death in Drosophila melanogaster. Dev Cell. 2007;12(5):807–816. doi: 10.1016/j.devcel.2007.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green P, Hartenstein AY, Hartenstein V. The embryonic development of the Drosophila visual system. Cell Tissue Res. 1993;273(3):583–598. doi: 10.1007/BF00333712. [DOI] [PubMed] [Google Scholar]
- Grether ME, Abrams JM, Agapite J, White K, Steller H. The head involution defective gene of Drosophila melanogaster functions in programmed cell death. Genes Dev. 1995;9(14):1694–1708. doi: 10.1101/gad.9.14.1694. [DOI] [PubMed] [Google Scholar]
- Grosskortenhaus R, Robinson KJ, Doe CQ. Pdm and Castor specify late-born motor neuron identity in the NB7-1 lineage. Genes Dev. 2006;20(18):2618–2627. doi: 10.1101/gad.1445306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo Z, Zhang L, Wu Z, Chen Y, Wang F, Chen G. In vivo direct reprogramming of reactive glial cells into functional neurons after brain injury and in an Alzheimer's disease model. Cell Stem Cell. 2014;14(2):188–202. doi: 10.1016/j.stem.2013.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haining WN, Carboy-Newcomb C, Wei CL, Steller H. The proapoptotic function of Drosophila Hid is conserved in mammalian cells. Proc Natl Acad Sci U S A. 1999;96(9):4936–4941. doi: 10.1073/pnas.96.9.4936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Handler AM. Ecdysteroid titers during pupal and adult development in Drosophila melanogaster. Dev Biol. 1982;93(1):73–82. doi: 10.1016/0012-1606(82)90240-8. [DOI] [PubMed] [Google Scholar]
- Hara Y, Hirai K, Togane Y, Akagawa H, Iwabuchi K, Tsujimura H. Ecdysone-dependent and ecdysone-independent programmed cell death in the developing optic lobe of Drosophila. Dev Biol. 2013;374(1):127–141. doi: 10.1016/j.ydbio.2012.11.002. [DOI] [PubMed] [Google Scholar]
- Hartenstein V, Campos-Ortega JA. Early neurogenesis in wild-type Drosophila melanogaster. Wilhelm Roux's archives of developmental biology. 1984;193(5):308–325. doi: 10.1007/BF00848159. [DOI] [PubMed] [Google Scholar]
- Harvey NL, Daish T, Mills K, Dorstyn L, Quinn LM, Read SH, Richardson H, Kumar S. Characterization of the Drosophila caspase, DAMM. J Biol Chem. 2001;276(27):25342–25350. doi: 10.1074/jbc.M009444200. [DOI] [PubMed] [Google Scholar]
- Hay BA, Wassarman DA, Rubin GM. Drosophila homologs of baculovirus inhibitor of apoptosis proteins function to block cell death. Cell. 1995;83(7):1253–1262. doi: 10.1016/0092-8674(95)90150-7. [DOI] [PubMed] [Google Scholar]
- Hidalgo A, Brand AH. Targeted neuronal ablation: the role of pioneer neurons in guidance and fasciculation in the CNS of Drosophila. Development. 1997;124(17):3253–3262. doi: 10.1242/dev.124.17.3253. [DOI] [PubMed] [Google Scholar]
- Hidalgo A, Kinrade EF, Georgiou M. The Drosophila neuregulin vein maintains glial survival during axon guidance in the CNS. Dev Cell. 2001;1(5):679–690. doi: 10.1016/s1534-5807(01)00074-0. [DOI] [PubMed] [Google Scholar]
- Hipfner DR, Weigmann K, Cohen SM. The bantam gene regulates Drosophila growth. Genetics. 2002;161(4):1527–1537. doi: 10.1093/genetics/161.4.1527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isshiki T, Pearson B, Holbrook S, Doe CQ. Drosophila neuroblasts sequentially express transcription factors which specify the temporal identity of their neuronal progeny. Cell. 2001;106(4):511–521. doi: 10.1016/s0092-8674(01)00465-2. [DOI] [PubMed] [Google Scholar]
- Ito K, Hotta Y. Proliferation pattern of postembryonic neuroblasts in the brain of Drosophila melanogaster. Dev Biol. 1992;149(1):134–148. doi: 10.1016/0012-1606(92)90270-q. [DOI] [PubMed] [Google Scholar]
- Jacobs JR, Goodman CS. Embryonic development of axon pathways in the Drosophila CNS. I. A glial scaffold appears before the first growth cones. J Neurosci. 1989;9(7):2402–2411. doi: 10.1523/JNEUROSCI.09-07-02402.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang C, Baehrecke EH, Thummel CS. Steroid regulated programmed cell death during Drosophila metamorphosis. Development. 1997;124(22):4673–4683. doi: 10.1242/dev.124.22.4673. [DOI] [PubMed] [Google Scholar]
- Jiang C, Lamblin AF, Steller H, Thummel CS. A steroid-triggered transcriptional hierarchy controls salivary gland cell death during Drosophila metamorphosis. Mol Cell. 2000;5(3):445–455. doi: 10.1016/s1097-2765(00)80439-6. [DOI] [PubMed] [Google Scholar]
- Jiang Y, Reichert H. Programmed cell death in type II neuroblast lineages is required for central complex development in the Drosophila brain. Neural Dev. 2012;7:3. doi: 10.1186/1749-8104-7-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jimenez F, Campos-Ortega JA. Defective neuroblast commitment in mutants of the achaete-scute complex and adjacent genes of D. melanogaster. Neuron. 1990;5(1):81–89. doi: 10.1016/0896-6273(90)90036-f. [DOI] [PubMed] [Google Scholar]
- Jones G, Jones D, Zhou L, Steller H, Chu Y. Deterin, a new inhibitor of apoptosis from Drosophila melanogaster. J Biol Chem. 2000;275(29):22157–22165. doi: 10.1074/jbc.M000369200. [DOI] [PubMed] [Google Scholar]
- Jovanovic M, Hengartner MO. miRNAs and apoptosis: RNAs to die for. Oncogene. 2006;25(46):6176–6187. doi: 10.1038/sj.onc.1209912. [DOI] [PubMed] [Google Scholar]
- Jürgens G. Segmental organisation of the tail region in the embryo of Drosophila melanogaster. Roux's archives of developmental biology. 1987;196(3):141–157. doi: 10.1007/BF00376308. [DOI] [PubMed] [Google Scholar]
- Kaiser WJ, Vucic D, Miller LK. The Drosophila inhibitor of apoptosis D-IAP1 suppresses cell death induced by the caspase drICE. FEBS Lett. 1998;440(1-2):243–248. doi: 10.1016/s0014-5793(98)01465-3. [DOI] [PubMed] [Google Scholar]
- Karcavich R, Doe CQ. Drosophila neuroblast 7-3 cell lineage: a model system for studying programmed cell death, Notch/Numb signaling, and sequential specification of ganglion mother cell identity. J Comp Neurol. 2005;481(3):240–251. doi: 10.1002/cne.20371. [DOI] [PubMed] [Google Scholar]
- Karlsson D, Baumgardt M, Thor S. Segment-specific neuronal subtype specification by the integration of anteroposterior and temporal cues. PLoS Biol. 2010;8(5):e1000368. doi: 10.1371/journal.pbio.1000368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufman TC, Lewis R, Wakimoto B. Cytogenetic Analysis of Chromosome 3 in DROSOPHILA MELANOGASTER: The Homoeotic Gene Complex in Polytene Chromosome Interval 84a-B. Genetics. 1980;94(1):115–133. doi: 10.1093/genetics/94.1.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura K, Ote M, Tazawa T, Yamamoto D. Fruitless specifies sexually dimorphic neural circuitry in the Drosophila brain. Nature. 2005;438(7065):229–233. doi: 10.1038/nature04229. [DOI] [PubMed] [Google Scholar]
- Kimura KI, Truman JW. Postmetamorphic cell death in the nervous and muscular systems of Drosophila melanogaster. J Neurosci. 1990;10(2):403–401. doi: 10.1523/JNEUROSCI.10-02-00403.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohwi M, Doe CQ. Temporal fate specification and neural progenitor competence during development. Nat Rev Neurosci. 2013;14(12):823–838. doi: 10.1038/nrn3618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kornbluth S, White K. Apoptosis in Drosophila: neither fish nor fowl (nor man, nor worm). J Cell Sci. 2005;118(Pt 9):1779–1787. doi: 10.1242/jcs.02377. [DOI] [PubMed] [Google Scholar]
- Kuert PA, Hartenstein V, Bello BC, Lovick JK, Reichert H. Neuroblast lineage identification and lineage-specific Hox gene action during postembryonic development of the subesophageal ganglion in the Drosophila central brain. Dev Biol. 2014;390(2):102–115. doi: 10.1016/j.ydbio.2014.03.021. [DOI] [PubMed] [Google Scholar]
- Kumar A, Bello B, Reichert H. Lineage-specific cell death in postembryonic brain development of Drosophila. Development. 2009;136(20):3433–3442. doi: 10.1242/dev.037226. [DOI] [PubMed] [Google Scholar]
- Kurada P, White K. Ras promotes cell survival in Drosophila by downregulating hid expression. Cell. 1998;95(3):319–329. doi: 10.1016/s0092-8674(00)81764-x. [DOI] [PubMed] [Google Scholar]
- Kurant E. Keeping the CNS clear: glial phagocytic functions in Drosophila. Glia. 2011;59(9):1304–1311. doi: 10.1002/glia.21098. [DOI] [PubMed] [Google Scholar]
- Kurant E, Axelrod S, Leaman D, Gaul U. Six-microns-under acts upstream of Draper in the glial phagocytosis of apoptotic neurons. Cell. 2008;133(3):498–509. doi: 10.1016/j.cell.2008.02.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurusu M, Nagao T, Walldorf U, Flister S, Gehring WJ, Furukubo-Tokunaga K. Genetic control of development of the mushroom bodies, the associative learning centers in the Drosophila brain, by the eyeless, twin of eyeless, and Dachshund genes. Proc Natl Acad Sci U S A. 2000;97(5):2140–2144. doi: 10.1073/pnas.040564497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacin H, Truman JW. Lineage mapping identifies molecular and architectural similarities between the larval and adult Drosophila central nervous system. Elife. 2016;5 doi: 10.7554/eLife.13399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai SL, Doe CQ. Transient nuclear Prospero induces neural progenitor quiescence. Elife. 2014;3 doi: 10.7554/eLife.03363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanet E, Gould AP, Maurange C. Protection of neuronal diversity at the expense of neuronal numbers during nutrient restriction in the Drosophila visual system. Cell Rep. 2013;3(3):587–594. doi: 10.1016/j.celrep.2013.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leaman D, Chen PY, Fak J, Yalcin A, Pearce M, Unnerstall U, Marks DS, Sander C, Tuschl T, Gaul U. Antisense-mediated depletion reveals essential and specific functions of microRNAs in Drosophila development. Cell. 2005;121(7):1097–1108. doi: 10.1016/j.cell.2005.04.016. [DOI] [PubMed] [Google Scholar]
- Lee G, Sehgal R, Wang Z, Nair S, Kikuno K, Chen CH, Hay B, Park JH. Essential role of grim-led programmed cell death for the establishment of corazonin-producing peptidergic nervous system during embryogenesis and metamorphosis in Drosophila melanogaster. Biol Open. 2013;2(3):283–294. doi: 10.1242/bio.20133384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee GG, Kikuno K, Nair S, Park JH. Mechanisms of postecdysis-associated programmed cell death of peptidergic neurons in Drosophila melanogaster. J Comp Neurol. 2013;521(17):3972–3991. doi: 10.1002/cne.23387. [DOI] [PubMed] [Google Scholar]
- Lee T, Lee A, Luo L. Development of the Drosophila mushroom bodies: sequential generation of three distinct types of neurons from a neuroblast. Development. 1999;126(18):4065–4076. doi: 10.1242/dev.126.18.4065. [DOI] [PubMed] [Google Scholar]
- Lewis EB. A gene complex controlling segmentation in Drosophila. Nature. 1978;276(5688):565–570. doi: 10.1038/276565a0. [DOI] [PubMed] [Google Scholar]
- Li L, Vaessin H. Pan-neural Prospero terminates cell proliferation during Drosophila neurogenesis. Genes Dev. 2000;14(2):147–151. [PMC free article] [PubMed] [Google Scholar]
- Li X, Chen Z, Desplan C. Temporal patterning of neural progenitors in Drosophila. Curr Top Dev Biol. 2013;105:69–96. doi: 10.1016/B978-0-12-396968-2.00003-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Erclik T, Bertet C, Chen Z, Voutev R, Venkatesh S, Morante J, Celik A, Desplan C. Temporal patterning of Drosophila medulla neuroblasts controls neural fates. Nature. 2013;498(7455):456–462. doi: 10.1038/nature12319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin N, Zhang C, Pang J, Zhou L. By design or by chance: cell death during Drosophila embryogenesis. Apoptosis. 2009;14(8):935–942. doi: 10.1007/s10495-009-0360-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin S, Lai SL, Yu HH, Chihara T, Luo L, Lee T. Lineage-specific effects of Notch/Numb signaling in post-embryonic development of the Drosophila brain. Development. 2010;137(1):43–51. doi: 10.1242/dev.041699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovick JK, Kong A, Omoto JJ, Ngo KT, Younossi-Hartenstein A, Hartenstein V. Patterns of growth and tract formation during the early development of secondary lineages in the Drosophila larval brain. Dev Neurobiol. 2016;76(4):434–451. doi: 10.1002/dneu.22325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luer K, Technau GM. Single cell cultures of Drosophila neuroectodermal and mesectodermal central nervous system progenitors reveal different degrees of developmental autonomy. Neural Dev. 2009;4:30. doi: 10.1186/1749-8104-4-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundell MJ, Lee HK, Perez E, Chadwell L. The regulation of apoptosis by Numb/Notch signaling in the serotonin lineage of Drosophila. Development. 2003;130(17):4109–4121. doi: 10.1242/dev.00593. [DOI] [PubMed] [Google Scholar]
- Maurange C, Cheng L, Gould AP. Temporal transcription factors and their targets schedule the end of neural proliferation in Drosophila. Cell. 2008;133(5):891–902. doi: 10.1016/j.cell.2008.03.034. [DOI] [PubMed] [Google Scholar]
- Maurange C, Gould AP. Brainy but not too brainy: starting and stopping neuroblast divisions in Drosophila. Trends Neurosci. 2005;28(1):30–36. doi: 10.1016/j.tins.2004.10.009. [DOI] [PubMed] [Google Scholar]
- McCarthy JV, Dixit VM. Apoptosis induced by Drosophila reaper and grim in a human system. Attenuation by inhibitor of apoptosis proteins (cIAPs). J Biol Chem. 1998;273(37):24009–24015. doi: 10.1074/jbc.273.37.24009. [DOI] [PubMed] [Google Scholar]
- McGinnis W, Krumlauf R. Homeobox genes and axial patterning. Cell. 1992;68(2):283–302. doi: 10.1016/0092-8674(92)90471-n. [DOI] [PubMed] [Google Scholar]
- Meeusen SL, Nunnari J. How mitochondria fuse. Curr Opin Cell Biol. 2005;17(4):389–394. doi: 10.1016/j.ceb.2005.06.014. [DOI] [PubMed] [Google Scholar]
- Meier P, Silke J, Leevers SJ, Evan GI. The Drosophila caspase DRONC is regulated by DIAP1. Embo j. 2000;19(4):598–611. doi: 10.1093/emboj/19.4.598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mergliano J, Minden JS. Caspase-independent cell engulfment mirrors cell death pattern in Drosophila embryos. Development. 2003;130(23):5779–5789. doi: 10.1242/dev.00824. [DOI] [PubMed] [Google Scholar]
- Miguel-Aliaga I, Thor S. Segment-specific prevention of pioneer neuron apoptosis by cell-autonomous, postmitotic Hox gene activity. Development. 2004;131(24):6093–6105. doi: 10.1242/dev.01521. [DOI] [PubMed] [Google Scholar]
- Miguel-Aliaga I, Thor S, Gould AP. Postmitotic specification of Drosophila insulinergic neurons from pioneer neurons. PLoS Biol. 2008;6(3):e58. doi: 10.1371/journal.pbio.0060058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moshiri A, Close J, Reh TA. Retinal stem cells and regeneration. Int J Dev Biol. 2004;48(8-9):1003–1014. doi: 10.1387/ijdb.041870am. [DOI] [PubMed] [Google Scholar]
- Narbonne-Reveau K, Lanet E, Dillard C, Foppolo S, Chen CH, Parrinello H, Rialle S, Sokol NS, Maurange C. Neural stem cell-encoded temporal patterning delineates an early window of malignant susceptibility in Drosophila. Elife. 2016;5 doi: 10.7554/eLife.13463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nassif C, Noveen A, Hartenstein V. Embryonic development of the Drosophila brain. I. Pattern of pioneer tracts. J Comp Neurol. 1998;402(1):10–31. [PubMed] [Google Scholar]
- Nassif C, Noveen A, Hartenstein V. Early development of the Drosophila brain: III. The pattern of neuropile founder tracts during the larval period. J Comp Neurol. 2003;455(4):417–434. doi: 10.1002/cne.10482. [DOI] [PubMed] [Google Scholar]
- Neriec N, Desplan C. From the Eye to the Brain: Development of the Drosophila Visual System. Curr Top Dev Biol. 2016;116:247–271. doi: 10.1016/bs.ctdb.2015.11.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ngo KT, Wang J, Junker M, Kriz S, Vo G, Asem B, Olson JM, Banerjee U, Hartenstein V. Concomitant requirement for Notch and Jak/Stat signaling during neuro-epithelial differentiation in the Drosophila optic lobe. Dev Biol. 2010;346(2):284–295. doi: 10.1016/j.ydbio.2010.07.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noveen A, Daniel A, Hartenstein V. Early development of the Drosophila mushroom body: the roles of eyeless and dachshund. Development. 2000;127(16):3475–3488. doi: 10.1242/dev.127.16.3475. [DOI] [PubMed] [Google Scholar]
- Novotny T, Eiselt R, Urban J. Hunchback is required for the specification of the early sublineage of neuroblast 7-3 in the Drosophila central nervous system. Development. 2002;129(4):1027–1036. doi: 10.1242/dev.129.4.1027. [DOI] [PubMed] [Google Scholar]
- Ollmann M, Young LM, Di Como CJ, Karim F, Belvin M, Robertson S, Whittaker K, Demsky M, Fisher WW, Buchman A, Duyk G, Friedman L, Prives C, Kopczynski C. Drosophila p53 is a structural and functional homolog of the tumor suppressor p53. Cell. 2000;101(1):91–101. doi: 10.1016/S0092-8674(00)80626-1. [DOI] [PubMed] [Google Scholar]
- Olson MR, Holley CL, Gan EC, Colon-Ramos DA, Kaplan B, Kornbluth S. A GH3-like domain in reaper is required for mitochondrial localization and induction of IAP degradation. J Biol Chem. 2003;278(45):44758–44768. doi: 10.1074/jbc.M308055200. [DOI] [PubMed] [Google Scholar]
- Orme M, Meier P. Inhibitor of apoptosis proteins in Drosophila: gatekeepers of death. Apoptosis. 2009;14(8):950–960. doi: 10.1007/s10495-009-0358-2. [DOI] [PubMed] [Google Scholar]
- Page DT, Olofsson B. Multiple roles for apoptosis facilitating condensation of the Drosophila ventral nerve cord. Genesis. 2008;46(2):61–68. doi: 10.1002/dvg.20365. [DOI] [PubMed] [Google Scholar]
- Pearson BJ, Doe CQ. Regulation of neuroblast competence in Drosophila. Nature. 2003;425(6958):624–628. doi: 10.1038/nature01910. [DOI] [PubMed] [Google Scholar]
- Peterson C, Carney GE, Taylor BJ, White K. reaper is required for neuroblast apoptosis during Drosophila development. Development. 2002;129(6):1467–1476. doi: 10.1242/dev.129.6.1467. [DOI] [PubMed] [Google Scholar]
- Poulson DF. Histogenesis, organogenesis and differentiation in the embryo of Drosophila melanogaster Meigen. Biology of Drosophila. 1950:168–274. [Google Scholar]
- Prokop A, Bray S, Harrison E, Technau GM. Homeotic regulation of segment-specific differences in neuroblast numbers and proliferation in the Drosophila central nervous system. Mech Dev. 1998;74(1-2):99–110. doi: 10.1016/s0925-4773(98)00068-9. [DOI] [PubMed] [Google Scholar]
- Prokop A, Technau GM. The origin of postembryonic neuroblasts in the ventral nerve cord of Drosophila melanogaster. Development. 1991;111(1):79–88. doi: 10.1242/dev.111.1.79. [DOI] [PubMed] [Google Scholar]
- Pronk GJ, Ramer K, Amiri P, Williams LT. Requirement of an ICE-like protease for induction of apoptosis and ceramide generation by REAPER. Science. 1996;271(5250):808–810. doi: 10.1126/science.271.5250.808. [DOI] [PubMed] [Google Scholar]
- Reichert H, Bello B. Hox genes and brain development in Drosophila. Adv Exp Med Biol. 2010;689:145–153. doi: 10.1007/978-1-4419-6673-5_11. [DOI] [PubMed] [Google Scholar]
- Robinow S, Draizen TA, Truman JW. Genes that induce apoptosis: transcriptional regulation in identified, doomed neurons of the Drosophila CNS. Dev Biol. 1997;190(2):206–213. doi: 10.1006/dbio.1997.8696. [DOI] [PubMed] [Google Scholar]
- Robinow S, Talbot WS, Hogness DS, Truman JW. Programmed cell death in the Drosophila CNS is ecdysone-regulated and coupled with a specific ecdysone receptor isoform. Development. 1993;119(4):1251–1259. doi: 10.1242/dev.119.4.1251. [DOI] [PubMed] [Google Scholar]
- Rogulja-Ortmann A, Luer K, Seibert J, Rickert C, Technau GM. Programmed cell death in the embryonic central nervous system of Drosophila melanogaster. Development. 2007;134(1):105–116. doi: 10.1242/dev.02707. [DOI] [PubMed] [Google Scholar]
- Rogulja-Ortmann A, Renner S, Technau GM. Antagonistic roles for Ultrabithorax and Antennapedia in regulating segment-specific apoptosis of differentiated motoneurons in the Drosophila embryonic central nervous system. Development. 2008;135(20):3435–3445. doi: 10.1242/dev.023986. [DOI] [PubMed] [Google Scholar]
- Rogulja-Ortmann A, Technau GM. Multiple roles for Hox genes in segment-specific shaping of CNS lineages. Fly (Austin) 2008;2(6):316–319. doi: 10.4161/fly.7464. [DOI] [PubMed] [Google Scholar]
- Ryoo HD, Gorenc T, Steller H. Apoptotic cells can induce compensatory cell proliferation through the JNK and the Wingless signaling pathways. Dev Cell. 2004;7(4):491–501. doi: 10.1016/j.devcel.2004.08.019. [DOI] [PubMed] [Google Scholar]
- Sammut M, Cook SJ, Nguyen KC, Felton T, Hall DH, Emmons SW, Poole RJ, Barrios A. Glia-derived neurons are required for sex-specific learning in C. elegans. Nature. 2015;526(7573):385–390. doi: 10.1038/nature15700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmid A, Chiba A, Doe CQ. Clonal analysis of Drosophila embryonic neuroblasts: neural cell types, axon projections and muscle targets. Development. 1999;126(21):4653–4689. doi: 10.1242/dev.126.21.4653. [DOI] [PubMed] [Google Scholar]
- Schmidt H, Rickert C, Bossing T, Vef O, Urban J, Technau GM. The embryonic central nervous system lineages of Drosophila melanogaster. II. Neuroblast lineages derived from the dorsal part of the neuroectoderm. Dev Biol. 1997;189(2):186–204. doi: 10.1006/dbio.1997.8660. [DOI] [PubMed] [Google Scholar]
- Siegrist SE, Haque NS, Chen CH, Hay BA, Hariharan IK. Inactivation of both Foxo and reaper promotes long-term adult neurogenesis in Drosophila. Curr Biol. 2010;20(7):643–648. doi: 10.1016/j.cub.2010.01.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skeath JB. At the nexus between pattern formation and cell-type specification: the generation of individual neuroblast fates in the Drosophila embryonic central nervous system. Bioessays. 1999;21(11):922–931. doi: 10.1002/(SICI)1521-1878(199911)21:11<922::AID-BIES4>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- Skeath JB, Carroll SB. Regulation of proneural gene expression and cell fate during neuroblast segregation in the Drosophila embryo. Development. 1992;114(4):939–946. doi: 10.1242/dev.114.4.939. [DOI] [PubMed] [Google Scholar]
- Skeath JB, Thor S. Genetic control of Drosophila nerve cord development. Curr Opin Neurobiol. 2003;13(1):8–15. doi: 10.1016/s0959-4388(03)00007-2. [DOI] [PubMed] [Google Scholar]
- Song Z, McCall K, Steller H. DCP-1, a Drosophila cell death protease essential for development. Science. 1997;275(5299):536–540. doi: 10.1126/science.275.5299.536. [DOI] [PubMed] [Google Scholar]
- Sonnenfeld MJ, Jacobs JR. Macrophages and glia participate in the removal of apoptotic neurons from the Drosophila embryonic nervous system. J Comp Neurol. 1995;359(4):644–652. doi: 10.1002/cne.903590410. [DOI] [PubMed] [Google Scholar]
- Sousa-Nunes R, Yee LL, Gould AP. Fat cells reactivate quiescent neuroblasts via TOR and glial insulin relays in Drosophila. Nature. 2011;471(7339):508–512. doi: 10.1038/nature09867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Speder P, Liu J, Brand AH. Nutrient control of neural stem cells. Curr Opin Cell Biol. 2011;23(6):724–729. doi: 10.1016/j.ceb.2011.08.004. [DOI] [PubMed] [Google Scholar]
- Sprecher SG, Urbach R, Technau GM, Rijli FM, Reichert H, Hirth F. The columnar gene vnd is required for tritocerebral neuromere formation during embryonic brain development of Drosophila. Development. 2006;133(21):4331–4339. doi: 10.1242/dev.02606. [DOI] [PubMed] [Google Scholar]
- Srinivasula SM, Datta P, Kobayashi M, Wu JW, Fujioka M, Hegde R, Zhang Z, Mukattash R, Fernandes-Alnemri T, Shi Y, Jaynes JB, Alnemri ES. sickle, a novel Drosophila death gene in the reaper/hid/grim region, encodes an IAP-inhibitory protein. Curr Biol. 2002;12(2):125–130. doi: 10.1016/s0960-9822(01)00657-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suska A, Miguel-Aliaga I, Thor S. Segment-specific generation of Drosophila Capability neuropeptide neurons by multi-faceted Hox cues. Dev Biol. 2011;353(1):72–80. doi: 10.1016/j.ydbio.2011.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan Y, Yamada-Mabuchi M, Arya R, St Pierre S, Tang W, Tosa M, Brachmann C, White K. Coordinated expression of cell death genes regulates neuroblast apoptosis. Development. 2011;138(11):2197–2206. doi: 10.1242/dev.058826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor BJ, Truman JW. Commitment of abdominal neuroblasts in Drosophila to a male or female fate is dependent on genes of the sex-determining hierarchy. Development. 1992;114(3):625–642. doi: 10.1242/dev.114.3.625. [DOI] [PubMed] [Google Scholar]
- Thomenius M, Freel CD, Horn S, Krieser R, Abdelwahid E, Cannon R, Balasundaram S, White K, Kornbluth S. Mitochondrial fusion is regulated by Reaper to modulate Drosophila programmed cell death. Cell Death Differ. 2011;18(10):1640–1650. doi: 10.1038/cdd.2011.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tissot M, Stocker RF. Metamorphosis in drosophila and other insects: the fate of neurons throughout the stages. Prog Neurobiol. 2000;62(1):89–111. doi: 10.1016/s0301-0082(99)00069-6. [DOI] [PubMed] [Google Scholar]
- Togane Y, Ayukawa R, Hara Y, Akagawa H, Iwabuchi K, Tsujimura H. Spatio-temporal pattern of programmed cell death in the developing Drosophila optic lobe. Dev Growth Differ. 2012;54(4):503–518. doi: 10.1111/j.1440-169X.2012.01340.x. [DOI] [PubMed] [Google Scholar]
- Truman JW, Bate M. Spatial and temporal patterns of neurogenesis in the central nervous system of Drosophila melanogaster. Dev Biol. 1988;125(1):145–157. doi: 10.1016/0012-1606(88)90067-x. [DOI] [PubMed] [Google Scholar]
- Truman JW, Moats W, Altman J, Marin EC, Williams DW. Role of Notch signaling in establishing the hemilineages of secondary neurons in Drosophila melanogaster. Development. 2010;137(1):53–61. doi: 10.1242/dev.041749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Truman JW, Taylor BJ, Awad TA. Formation of the adult nervous system. 1993:1245–1275. [Google Scholar]
- Truman JW, Thorn RS, Robinow S. Programmed neuronal death in insect development. J Neurobiol. 1992;23(9):1295–1311. doi: 10.1002/neu.480230917. [DOI] [PubMed] [Google Scholar]
- Tsuji T, Hasegawa E, Isshiki T. Neuroblast entry into quiescence is regulated intrinsically by the combined action of spatial Hox proteins and temporal identity factors. Development. 2008;135(23):3859–3869. doi: 10.1242/dev.025189. [DOI] [PubMed] [Google Scholar]
- Udolph G, Luer K, Bossing T, Technau GM. Commitment of CNS progenitors along the dorsoventral axis of Drosophila neuroectoderm. Science. 1995;269(5228):1278–1281. doi: 10.1126/science.7652576. [DOI] [PubMed] [Google Scholar]
- Udolph G, Prokop A, Bossing T, Technau GM. A common precursor for glia and neurons in the embryonic CNS of Drosophila gives rise to segment-specific lineage variants. Development. 1993;118(3):765–775. doi: 10.1242/dev.118.3.765. [DOI] [PubMed] [Google Scholar]
- Urbach R, Jussen D, Technau GM. Gene expression profiles uncover individual identities of gnathal neuroblasts and serial homologies in the embryonic CNS of Drosophila. Development. 2016;143(8):1290–1301. doi: 10.1242/dev.133546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urbach R, Schnabel R, Technau GM. The pattern of neuroblast formation, mitotic domains and proneural gene expression during early brain development in Drosophila. Development. 2003;130(16):3589–3606. doi: 10.1242/dev.00528. [DOI] [PubMed] [Google Scholar]
- Urbach R, Technau GM. Molecular markers for identified neuroblasts in the developing brain of Drosophila. Development. 2003;130(16):3621–3637. doi: 10.1242/dev.00533. [DOI] [PubMed] [Google Scholar]
- Viktorin G, Riebli N, Reichert H. A multipotent transit-amplifying neuroblast lineage in the central brain gives rise to optic lobe glial cells in Drosophila. Dev Biol. 2013;379(2):182–194. doi: 10.1016/j.ydbio.2013.04.020. [DOI] [PubMed] [Google Scholar]
- Wells BS, Yoshida E, Johnston LA. Compensatory proliferation in Drosophila imaginal discs requires Dronc-dependent p53 activity. Curr Biol. 2006;16(16):1606–1615. doi: 10.1016/j.cub2006.07.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weng R, Cohen SM. Control of Drosophila Type I and Type II central brain neuroblast proliferation by bantam microRNA. Development. 2015;142(21):3713–3720. doi: 10.1242/dev.127209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wheeler SR, Kearney JB, Guardiola AR, Crews ST. Single-cell mapping of neural and glial gene expression in the developing Drosophila CNS midline cells. Dev Biol. 2006;294(2):509–524. doi: 10.1016/j.ydbio.2006.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wheeler WM. Neuroblasts in the arthropod embryo. Journal of Morphology. 1891;4(3):337–343. [Google Scholar]
- White K, Grether ME, Abrams JM, Young L, Farrell K, Steller H. Genetic control of programmed cell death in Drosophila. Science. 1994;264(5159):677–683. doi: 10.1126/science.8171319. [DOI] [PubMed] [Google Scholar]
- White K, Kankel DR. Patterns of cell division and cell movement in the formation of the imaginal nervous system in Drosophila melanogaster. Dev Biol. 1978;65(2):296–321. doi: 10.1016/0012-1606(78)90029-5. [DOI] [PubMed] [Google Scholar]
- White K, Tahaoglu E, Steller H. Cell killing by the Drosophila gene reaper. Science. 1996;271(5250):805–807. doi: 10.1126/science.271.5250.805. [DOI] [PubMed] [Google Scholar]
- Winbush A, Weeks JC. Steroid-triggered, cell-autonomous death of a Drosophila motoneuron during metamorphosis. Neural Dev. 2011;6:15. doi: 10.1186/1749-8104-6-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wing JP, Karres JS, Ogdahl JL, Zhou L, Schwartz LM, Nambu JR. Drosophila sickle is a novel grim-reaper cell death activator. Curr Biol. 2002;12(2):131–135. doi: 10.1016/s0960-9822(01)00664-9. [DOI] [PubMed] [Google Scholar]
- Wing JP, Zhou L, Schwartz LM, Nambu JR. Distinct cell killing properties of the Drosophila reaper, head involution defective, and grim genes. Cell Death Differ. 1998;5(11):930–939. doi: 10.1038/sj.cdd.4400423. [DOI] [PubMed] [Google Scholar]
- Yaffe MP. Dynamic mitochondria. Nat Cell Biol. 1999;1(6):E149–150. doi: 10.1038/14101. [DOI] [PubMed] [Google Scholar]
- Yasugi T, Sugie A, Umetsu D, Tabata T. Coordinated sequential action of EGFR and Notch signaling pathways regulates proneural wave progression in the Drosophila optic lobe. Development. 2010;137(19):3193–3203. doi: 10.1242/dev.048058. [DOI] [PubMed] [Google Scholar]
- Yoo SJ, Huh JR, Muro I, Yu H, Wang L, Wang SL, Feldman RM, Clem RJ, Muller HA, Hay BA. Hid, Rpr and Grim negatively regulate DIAP1 levels through distinct mechanisms. Nat Cell Biol. 2002;4(6):416–424. doi: 10.1038/ncb793. [DOI] [PubMed] [Google Scholar]
- Yu HH, Kao CF, He Y, Ding P, Kao JC, Lee T. A complete developmental sequence of a Drosophila neuronal lineage as revealed by twin-spot MARCM. PLoS Biol. 2010;8(8) doi: 10.1371/journal.pbio.1000461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou L, Hashimi H, Schwartz LM, Nambu JR. Programmed cell death in the Drosophila central nervous system midline. Curr Biol. 1995;5(7):784–790. doi: 10.1016/s0960-9822(95)00155-2. [DOI] [PubMed] [Google Scholar]
- Zhou L, Schnitzler A, Agapite J, Schwartz LM, Steller H, Nambu JR. Cooperative functions of the reaper and head involution defective genes in the programmed cell death of Drosophila central nervous system midline cells. Proc Natl Acad Sci U S A. 1997;94(10):5131–5136. doi: 10.1073/pnas.94.10.5131. [DOI] [PMC free article] [PubMed] [Google Scholar]