Abstract
Recent studies have demonstrated that anti-staphylococcal beta-lactam antibiotics, like nafcillin, render methicillin-resistant Staphylococcus aureus (MRSA) more susceptible to killing by innate host defense peptides (HDPs), such as cathelicidin LL-37. We compared the effects of growth in 1/4 minimum inhibitory concentration (MIC) of nafcillin or vancomycin on LL-37 killing of 92 methicillin-susceptible S. aureus (MSSA) isolates. For three randomly selected strains among these, we examined the effects of nafcillin, vancomycin, daptomycin, or linezolid on LL-37 killing and autolysis. Growth in the presence of sub-inhibitory nafcillin significantly enhanced LL-37 killing of MSSA compared to vancomycin and antibiotic-free controls. Nafcillin also reduced MSSA production of the golden staphylococcal pigment staphyloxanthin in 39% of pigmented strains vs. 14% for vancomycin. Among antibiotics tested, only nafcillin resulted in significantly increased MSSA autolysis. These studies point to additional mechanisms of anti-staphylococcal activity of nafcillin beyond direct bactericidal activity, properties that vancomycin and other antibiotic classes do not exhibit. The ability of nafcillin to enhance sensitivity to innate host defense peptides may contribute to its superior effectiveness against MSSA as suggested by studies comparing clinical outcomes to vancomycin treatment.
Keywords: Nafcillin, Vancomycin, Innate immunity, Bacteremia, S. aureus, Antimicrobial peptides
Introduction
Recent increases in methicillin resistance in Staphylococcus aureus causing bacteremia (SAB) has expanded the use of vancomycin as an empiric as well as directed antimicrobial therapy. While this is medically and microbiologically acceptable practice, recent studies suggest that empiric therapy with vancomycin for SAB, including those caused by isolates exhibiting methicillin resistance (MRSA), did not result in decreased mortality.[1,2] Additionally, vancomycin used to treat methicillin-susceptible S. aureus (MSSA) bacteremia was associated with at least a three-fold increase in all-cause and infection-related mortality when compared to treatment with anti-staphylococcal beta-lactams.[3-5] Even when not initiated empirically, the transition from vancomycin to an anti-staphylococcal beta-lactam after the identification of MSSA in blood cultures may still reduce mortality.[5] The risk for treatment failures and, most importantly, patient death, underscores the importance of using beta-lactams, such as nafcillin, as empiric or tailored therapy for MSSA bacteremia.[3,5-7]
The human innate immune response plays an important role in the first-line of defense against infection. In particular, LL-37 belongs to the cathelicidin family of host defense peptides (HDPs) that are prevalent in human skin and neutrophils, and possesses broad spectrum antimicrobial activity against both Gram-negative and Gram-positive bacteria, including S. aureus.[8-10] We and others have recently demonstrated that administered antibiotics have important pharmacodynamic interactions with LL-37 and other HDPs, the most striking of which was the enhanced killing of MRSA by LL-37 when exposed to beta-lactams.[11-13] Synergy with LL-37 was not demonstrated for the anti-MRSA antibiotics vancomycin, linezolid, or daptomycin against MRSA. Conversely, other antibiotics, traditionally classified as bacteriostatic by virtue of their in vitro activity, antagonize the antibacterial activities of LL-37.[14] Strikingly, the “immunity-sensitizing” activity of beta-lactams extends to Gram-positive pathogens that were beta-lactam resistant, such as ampicillin (and daptomycin) promoting LL-37 sensitivity in vancomycin-resistant strains of Enterococcus faecium.[15,16]
In the present study, we sought to explore this phenomenon of antibiotic-induced sensitization to MSSA interaction with LL-37, with specific focus on comparing nafcillin to vancomycin. We hypothesized that nafcillin could better sensitize S. aureus to LL-37 killing than vancomycin; therefore, partially explaining the observed worse outcomes and longer bacteremia in patients with MSSA in which vancomycin treatment was continued beyond the initial empiric indication.[12]
Materials and Methods
Bacterial Isolates and Antibiotic Susceptibility Testing
The MSSA strains studied were obtained from a previously published retrospective clinical study, which was approved by the institutional review board of the University of Maryland, Baltimore.[5] All isolates were collected from hospitalized subjects with bacteremia and exposed to vancomycin, either empirically (i.e., within 24 h before or after blood culture collection) or 24 h after culture collection to hospital discharge. Isolates were stored in cryovials at −70°C. Susceptibility testing to nafcillin and vancomycin was performed using microbroth dilution method in RPMI + 5% LB, the same conditions employed for subsequent LL-37 killing assays. In addition, vancomycin susceptibility was evaluated using Epsilometer test (E-test, AB BIODISK, La Balme Les Grottes, France; bioMerieux, Durham, NC) according to manufacturer's instructions. Other relevant clinical and microbiological data surrounding these isolates including daptomycin and linezolid susceptibility testing results, staphylococcal protein A (spa) gene sequence typing, patient antibiotic therapy, and clinical outcomes (i.e., 30-day all-cause mortality and post-infection hospital days) have been previously published.[5]
Antimicrobial Peptide Killing Assays
Human cathelicidin LL-37 (net charge +6 at pH 7.5) purchased from AnaSpec, Inc. (Fremont, CA) was the HDP evaluated in this study due to the correlation of relative LL-37 resistance with S. aureus virulence and its emergence as a biomarker predicting outcome among hemodialysis patients highly vulnerable to invasive S. aureus infection.[17,12,18] The concentration of LL-37 selected for the killing assays was 8 μM based on pilot studies that determined conditions allowing baseline bacterial survival prior to antibiotic sensitization. Given that these were bacteremia isolates, a relatively higher concentration was required compared to what would have been used to test clinical S. aureus isolates from other sites (e.g., skin, sputum). Bacteria were grown to the stationary phase for 14 - 16 h at 37°C in 5 mL of antibiotic-free LB broth or broth containing 0.25 × minimum inhibitory concentration (MIC) of nafcillin or vancomycin, washed in PBS, resuspended in fresh PBS to OD600 nm of 0.5, serially diluted to the range of 10 4 CFU/mL, and subjected to LL-37 killing assays as previously described in a final concentration in the range of 103 CFU/mL [19,13]. At 0 and 2h exposure time-points, six 10 μL samples for each time-point and antibiotic were plated on tryptic soy agar (TSA) plates and colonies enumerated after 24 h. The mean percentage survival (± SD) was quantified and expressed in relation to cfus at time 0. In addition, the survival ratio was determined by (% survival with antibiotic) ÷ (% survival without antibiotic). In the subset of pigmented isolates, the color of the colonies was recorded by visual inspection as white, yellow or golden pre- and post-antibiotic sensitization.
Effects of MSSA Treatment Option Antibiotics on Bacterial Autolysis and LL-37 killing
Three bacterial strains were randomly selected from the group and subjected to autolysis assays after growth to stationary phase (14-16 hr) in the absence or presence of subinhibitory concentrations (approximately 0.25 × MIC) of antibiotics that clinicians would consider in treating MSSA bacteremia: nafcillin (beta-lactam), vancomycin (glycopeptide), linezolid (oxazolidinone), and daptomycin (lipopeptide). Bacteria were washed once in PBS and the sample split into 2 parts, one of which was subjected to LL-37 killing assays as described above, and the other part resuspended in PBS/0.2% Triton X-100 to an OD600 nm of approximately 0.7-0.8 in a plastic cuvette (duplicate samples). Cuvettes were sealed with parafilm, placed in 37°C shaker (200 RPM), and OD 600nm recorded at 1, 2, and 6 hrs. Results are expressed at % OD600 of baseline reading at time 0.
Statistical Analysis
All data was entered into an Excel database and reformatted for analysis in SPSS version 21 (IBM, Chicago, Illinois). Statistical significance was set a priori at p < 0.05. A paired t-test was used to detect for significant differences in percent survival within the same isolate that was unsensitized versus exposed to antibiotics prior to experimentation with the LL-37 killing assay. The McNemar test was used to detect for significant differences in isolate color within the same isolate that was unsensitized versus exposed to post-sensitization. Classification and regression tree (CART) were conducted to identify factors contributing to survival ratio for nafcillin < 0.2 (i.e., at least 80% killing) and conversion to white color with exposure to nafcillin.
Results
Antibiotic Susceptibility
A total of 92 unique clinical bloodstream MSSA isolates were available for in vitro experimentation. Relevant clinical and microbiological data have been extensively characterized and published. All 92 subjects from which these isolates were obtained were exposed to vancomycin at some point during hospitalization—7 received vancomycin 24-hr before positive cultures, 52 within 24-hr of culture collection, and 83 were on vancomycin 24-hr after positive cultures. All isolates were susceptible to nafcillin, daptomycin and linezolid per CLSI standards (Table 1). Over 70% of the MSSA strains had vancomycin MIC ≥ 1.5, with 4 isolates notably at MIC = 3.0.
Table 1.
Antibiotic Susceptibility
| Minimum Inhibitory Concentration (mcg/mL) | No. (%) N = 92 | |
|---|---|---|
| Nafcillin | 0.0625 | 2 (2.2) |
| 0.125 | 17 (19) | |
| 0.250 | 48 (52) | |
| 0.500 | 21 (23) | |
| 1.00 | 4 (4) | |
| Vancomycin | 0.500 | 1 (1) |
| 0.750 | 4 (4) | |
| 1.00 | 21 (23) | |
| 1.50 | 44 (48) | |
| 2.00 | 18 (20) | |
| 3.00 | 4 (4) | |
| Daptomycin | 0.050 | 2 (2) |
| 0.090 | 1 (1) | |
| 0.130 | 6 (7) | |
| 0.190 | 4 (4) | |
| 0.250 | 15 (16) | |
| 0.380 | 22 (24) | |
| 0.500 | 20 (22) | |
| 0.750 | 19 (21) | |
| 1.00 | 3 (3) | |
| Linezolid | 0.130 | 1 (1) |
| 0.190 | 1 (1) | |
| 0.250 | 10 (11) | |
| 0.380 | 21 (23) | |
| 0.500 | 24 (26) | |
| 0.750 | 22 (24) | |
| 1.00 | 13 (14) | |
LL-37 Killing Assays
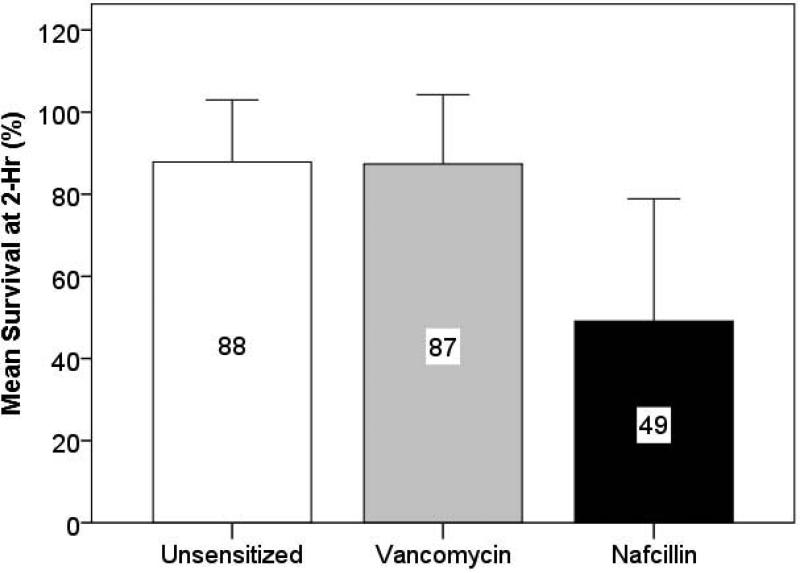
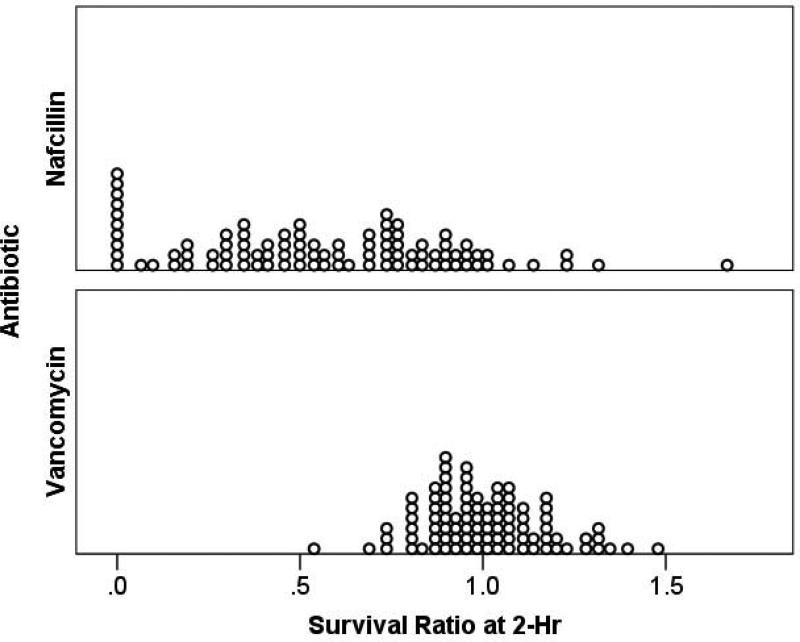
In LL-37 killing assays, the mean percent MSSA survival at 2-hr for the group of isolates was significantly lower for nafcillin-mediated sensitization when compared to the unsensitized or vancomycin-sensitized conditions performed in parallel (49% vs 87-88%, p<0.001) (Fig. 1A). Compared to the unsensitized status, the mean (± standard deviation, [interquartile range]) survival ratio at 2-hr was significantly lower for nafcillin than vancomycin (−0.56 ± 0.35 [0.32–0.83] and 1.00 ± 0.17 [0.89–1.11], respectively, p < 0.001). The individual strain results are depicted in scatterplot format in Fig. 1B. The ratio of the percent survival in LL-37 of nafcillin-exposed to unexposed (antibiotic-free) conditions for individual strains is shown in the top half; the ratio of the percent survival in LL-37 of vancomycin-exposed to unexposed (antibiotic-free) conditions is in the lower half. In these data, a ratio of <1.0 denotes strains that are rendered more vulnerable by the antibiotic exposure to LL-37 killing, while strains with ratio >1.0 are rendered more resistant to LL-37 killing. Notably for most strains, growth in nafcillin resulted in a ratio <1.0, whereas growth in vancomycin resulted in approximately half of the strains exhibiting increased resistance to LL-37 killing.
Figure 1A. Percent Survival of Methicillin-Susceptible Staphylococcus aureus Bacteremic Isolates p = 0.767 p < 0.001a.
Figure 1A. Mean percent survival in 8 μM LL-37 at 2 hours of 92 MSSA clinical bloodstream isolates after growth in antibiotic-free LB (unsensitized), or LB containing ¼ MIC of vancomycin or nafcillin. Growth in vancomycin showed no change from baseline, whereas nafcillin showed a significant reduction in mean percent survival of the group.
a P-values were statistically significant for nafcillin-unsensitized and nafcillin-vancomycin comparisons.
Error bars represent standard deviation.
Figure 1B. Survival Ratio of Methicillin-Susceptible Staphylococcus aureus Bacteremic Isolates.
Figure 1B. Scatterplot depicting the % survival ratio of nafcillin-sensitized (top) or vancomycin-sensitized (bottom) to unsensitized individual MSSA strains. Sensitization involved growing the bacteria in ¼ MIC of the respective antibiotic prior to LL-37 killing assay. A ratio < 1.0 represents strains that showed enhanced LL-37 killing by antibiotic exposure; a ratio > 1.0 denotes strains that were rendered more resistant to LL-37 killing by antibiotic exposure.
Effect of Antibiotic Exposure on Pigment
The post-antibiotic sensitization color was recorded for 49 of the 92 isolates with notable pigmentation at baseline. Of these 49 strains, 44 (90%) were yellow or golden as unsensitized (Table 2). Significantly more strains that were sensitized to nafcillin, as compared to vancomycin, converted from yellow/golden to white color after antibiotic sensitization (17 [39%] versus 6 [14%] of 44 total yellow or golden isolates, p < 0.001).
Table 2.
Color After Antibiotic Sensitization
| Antibiotic | Color | No. (%) N = 49 |
|---|---|---|
| Unsensitized | Yellow | 16 (32) |
| Golden | 28 (57) | |
| Yellow or Golden | 44 (90) | |
| Nafcillina | Yellow | 21 (40) |
| Golden | 17 (32) | |
| Yellow or Golden | 38 (73) | |
| Vancomycina | Yellow | 20 (42) |
| Golden | 23 (48) | |
| Yellow or Golden | 43 (90) |
Seventeen (39%) isolates sensitized to nafcillin and 6 (14%) sensitized to vancomycin converted to the white color after antibiotic sensitization (p < 0.001).
Using CART, it was observed that isolates that converted to the white color with nafcillin sensitization, as opposed to those without change in color, had a significantly higher survival ratio of < 0.2 (24% versus 14%, p = 0.043). In addition, a higher proportion of isolates with USA type 100 or 800, in contrast to USA 200 or 300, converted to white with nafcillin exposure (32% versus 0, p = 0.012).
Effect of Anti-staphylococcal Antibiotics on Autolysis
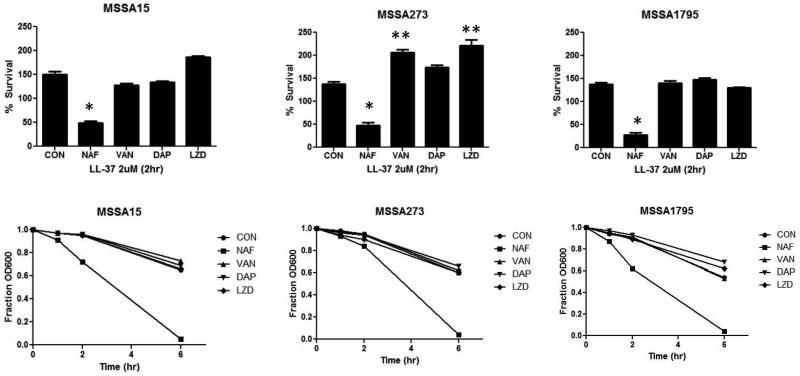
The dramatic differences in LL-37 sensitization of nafcillin versus vancomycin led us to inquire what the effects of LL-37 sensitization would be among other antimicrobials that clinicians might consider using to treat MSSA bacteremia. For three randomly selected strains, nafcillin was the only antibiotic that significantly enhanced LL-37 killing and markedly increased autolysis (Fig. 2). For one of the strains (MSA273, middle panel of Fig. 2), growth in linezolid, daptomycin and vancomycin increased resistance to LL-37 killing compared to control.
Figure 2. Effects of Sub-inhibitory Anti-staphylococcal Antibiotics on LL-37 Killing and Autolysis.
Effects of growth of 3 MSSA isolates to late logarithmic phase in ¼ MIC of nafcillin (NAF), vancomycin (VAN), daptomycin (DAP), or linezolid (LZD) on LL-37 killing (top panels) and autolysis (bottom panels). * P<0.05 compared to control (CON; decreased survival); **P<0.05 compared to control (increased survival)
Discussion
It has been appreciated for a long time that beta-lactam antibiotics offer significant clinical advantages over glycopeptides in the treatment of serious S. aureus infections. Most have attributed these differences to the increased potent bactericidal activity of nafcillin and other anti-staphylococcal beta-lactams over glycopeptides.[23] However, skepticism is increasing regarding whether the in vitro killing activity of antibiotics truly allows useful categorization of antibiotics into ‘bactericidal’ versus ‘bacteriostatic’ in a manner that translates directly into clinical outcome. In one case in particular, daptomycin achieves remarkable (sometimes 4 log10 CFU reduction in 4 hours) killing in vitro under standard CLSI killing assays, but does not offer any difference in shortening the duration of MSSA or MRSA bacteremia over standard therapy.[24]
Our recent work has shown that antibiotics that are administered to patients have additional mechanisms of action beyond their direct effect on inhibiting growth or killing through their pharmacodynamic interaction with HDPs, like cathelicidin LL-37. Specifically, we have demonstrated that beta-lactams, like nafcillin, markedly increase innate immune-mediated killing of MRSA.[19,13] Ampicillin and ceftaroline provide similar immunity-boosting activity against ampicillin- and vancomycin-resistant Enterococcus faecium.[16,15] Conversely, bacteriostatic antibiotics, like erythromycin and chloramphenicol, antagonize the effects of LL-37.[14] These findings raise the possibility that the traditional reliance on ‘bactericidal’ antibiotics for the treatment of meningitis or endocarditis may in part represent a surrogate selection for for antibiotics that have synergy with (as opposed to antagonizing) innate HDPs.
In this study, we examined the ability of vancomycin versus nafcillin to enhance killing by cathelicidin LL-37. We observed that nafcillin sensitized clinical MSSA bloodstream isolates to LL-37 killing, whereas vancomycin did not (Fig. 1A). Interestingly, vancomycin treatment actually reduced the activity of LL-37 killing for approximately half of the strains, as shown by the survival ratio of sensitized: unsensitized > 1 (Fig. 1B). Thus, nafcilin, not only exhibit bactericidal activity against S. aureus, but they also display favorable pharmacodynamic interactions with antimicrobials of the innate immunity, effectively boosting their activity, a property that vancomycin does not share.
Another finding was the qualitative reduction in pigment observed for 39% of MSSA isolates sensitized to nafcillin, compared to only 14% for vancomycin sensitization. Staphyloxanthin can contribute to S. aureus innate immune resistance and pathogenicity, as shown by studies where deletion of the staphyloxanthin biosynthesis enzyme CrtM renders S. aureus more susceptible to whole blood and neutrophil killing, with consequentially reduced virulence in murine skin abscess and systemic infection.[25] Beta-lactam effects on pigment production in S. aureus merit further characterization as it may reflect a modulation of virulence factor expression and possible third benefit of beta-lactam anti-staphylococcal activity in vivo. Further investigation is warranted to explore the quantitative effect of LL-37 antibiotic sensitization on staphyloxanthin and membrane fluidity.
Finally, based on our preliminary data indicating that genetic or pharmacological manipulation of Gram-positive bacteria that results in enhanced LL-37 activity is frequently accompanied by increased autolysis, we found pronounced increases in autolysis induced by nafcillin [26]. Our finding paralleled the consistent increase in sensitization to LL-37 killing by nafcillin, which was not observed for vancomycin, daptomycin, or linezolid. For one of the strains, growth in the other antibiotics actually resulted in reduced LL-37 killing (Fig. 2).
Cefazolin shares LL-37 sensitizing ability for MRSA and is known to possess similar clinical efficacy with increased tolerability compared to nafcillin in treating MSSA bacteremia.[7,5,13] Empiric combination therapy using vancomycin with either nafcillin or cefazolin may improve clinical outcomes in patients with staphylococcal bacteremia. In the case of MSSA, vancomycin monotherapy and de-escalation from vancomycin to definitive beta-lactam therapy are inferior to initial beta-lactam treatment. In the case of MRSA, vancomycin treatment failures are being observed with increasing vancomycin E-test MIC.[27] To date, clinical trials have not been conducted to evaluate the risk and benefits of empiric combination therapy for staphylococcal bacteremia, especially for MSSA.
We wish to point out several interpretive limitations of this study. First, susceptibility testing of LL-37 was not conducted since the concentration of LL-37 used in the killing assays was derived from pilot studies that allowed baseline bacterial survival prior to antibiotic sensitization. The concentration of LL-37 used was lower than in vivo physiological concentrations and selected for their ability to discriminate different MSSA isolates. However, the concentration of LL-37 used was a little higher than non-inflamed body fluids.[28] Second, other HDPs were not explored, but should be in future investigations since bloodstream bacteria are exposed to multiple HDPs in vivo. Lastly, all MSSA isolates evaluated in this study were collected from hospitalized subjects who were treated with vancomycin. Due to the lack of MSSA isolates obtained from subjects treated with nafcillin, associating the “immunity-sensitizing” property of nafcillin to clinical outcomes was not possible.
In summary, these findings show that anti-staphylococcal antibiotic therapy in vivo extends much beyond the heavily relied upon susceptibility assays and kill curves in standard bacterial media. There are potentially unappreciated and unexplored properties of pharmacologic antibiotics through added antibacterial effects, such as pharmacodynamic boosting of the innate immunity by HDPs and even virulence factor attenuation. They also explain, at least in part, the superiority of beta-lactam antibiotics over vancomycin as anti-staphylococcal therapy for serious infections.
Acknowledgments
Funding:
Funding for this research was provided by U54 HD071600-01 09/26/2011-06/30/2016 the National Institute of Child Health and Human Development on Developmental and Translational Pharmacology of Pediatric Antimicrobial Therapy (J.L., G.S. and V.N.), K23AI089978 from the National Institute of Allergy And Infectious Diseases (J.L.), and the Great Lakes Regional Center for Excellence in Biodefense and Emerging Infectious Disease Research (AI057153, V.N.). No funding bodies had any role in study design, data collection and analysis, decision to publish, or preparation of the manuscript
Footnotes
Compliance with Ethical Standards
Conflict of Interest:
G.S. has received speaking honoraria from Cubist, Forest, and Novartis Pharmaceuticals, consulting fees from Cubist and Forest Pharmaceuticals, and research grant support from Forest Pharmaceuticals.
Ethical Approval :
The bacterial strains studied were obtained from a previously published retrospective clinical study, which was approved by the institutional review board of the University of Maryland, Baltimore.
Informed consent:
Informed consent was not necessary for the conduct of this in vitro study.
REFERENCES
- 1.Schweizer ML, Furuno JP, Harris AD, Johnson JK, Shardell MD, McGregor JC, Thom KA, Sakoulas G, Perencevich EN. Empiric antibiotic therapy for Staphylococcus aureus bacteremia may not reduce in-hospital mortality: a retrospective cohort study. PloS one. 2010;5(7):e11432. doi: 10.1371/journal.pone.0011432. doi:10.1371/journal.pone.0011432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Holmes NE, Turnidge JD, Munckhof WJ, Robinson JO, Korman TM, O'Sullivan MV, Anderson TL, Roberts SA, Gao W, Christiansen KJ, Coombs GW, Johnson PD, Howden BP. Antibiotic choice may not explain poorer outcomes in patients with Staphylococcus aureus bacteremia and high vancomycin minimum inhibitory concentrations. The Journal of infectious diseases. 2011;204(3):340–347. doi: 10.1093/infdis/jir270. doi:10.1093/infdis/jir270. [DOI] [PubMed] [Google Scholar]
- 3.Kim SH, Kim KH, Kim HB, Kim NJ, Kim EC, Oh MD, Choe KW. Outcome of vancomycin treatment in patients with methicillin-susceptible Staphylococcus aureus bacteremia. Antimicrobial agents and chemotherapy. 2008;52(1):192–197. doi: 10.1128/AAC.00700-07. doi:10.1128/AAC.00700-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McConeghy KW, Bleasdale SC, Rodvold KA. The empirical combination of vancomycin and a beta-lactam for Staphylococcal bacteremia. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2013;57(12):1760–1765. doi: 10.1093/cid/cit560. doi:10.1093/cid/cit560. [DOI] [PubMed] [Google Scholar]
- 5.Schweizer ML, Furuno JP, Harris AD, Johnson JK, Shardell MD, McGregor JC, Thom KA, Cosgrove SE, Sakoulas G, Perencevich EN. Comparative effectiveness of nafcillin or cefazolin versus vancomycin in methicillin-susceptible Staphylococcus aureus bacteremia. BMC infectious diseases. 2011;11:279. doi: 10.1186/1471-2334-11-279. doi:10.1186/1471-2334-11-279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Stryjewski ME, Szczech LA, Benjamin DK, Jr., Inrig JK, Kanafani ZA, Engemann JJ, Chu VH, Joyce MJ, Reller LB, Corey GR, Fowler VG., Jr. Use of vancomycin or first-generation cephalosporins for the treatment of hemodialysis-dependent patients with methicillin-susceptible Staphylococcus aureus bacteremia. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2007;44(2):190–196. doi: 10.1086/510386. doi:10.1086/510386. [DOI] [PubMed] [Google Scholar]
- 7.Lee S, Choe PG, Song KH, Park SW, Kim HB, Kim NJ, Kim EC, Park WB, Oh MD. Is cefazolin inferior to nafcillin for treatment of methicillin-susceptible Staphylococcus aureus bacteremia? Antimicrobial agents and chemotherapy. 2011;55(11):5122–5126. doi: 10.1128/AAC.00485-11. doi:10.1128/AAC.00485-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nijnik A, Hancock RE. The roles of cathelicidin LL-37 in immune defences and novel clinical applications. Current opinion in hematology. 2009;16(1):41–47. doi: 10.1097/moh.0b013e32831ac517. [DOI] [PubMed] [Google Scholar]
- 9.Vandamme D, Landuyt B, Luyten W, Schoofs L. A comprehensive summary of LL-37, the factotum human cathelicidin peptide. Cellular immunology. 2012;280(1):22–35. doi: 10.1016/j.cellimm.2012.11.009. doi:10.1016/j.cellimm.2012.11.009. [DOI] [PubMed] [Google Scholar]
- 10.Wong JH, Ye XJ, Ng TB. Cathelicidins: peptides with antimicrobial, immunomodulatory, anti-inflammatory, angiogenic, anticancer and procancer activities. Current protein & peptide science. 2013;14(6):504–514. doi: 10.2174/13892037113149990067. [DOI] [PubMed] [Google Scholar]
- 11.McGillivray SM, Tran DN, Ramadoss NS, Alumasa JN, Okumura CY, Sakoulas G, Vaughn MM, Zhang DX, Keiler KC, Nizet V. Pharmacological inhibition of the ClpXP protease increases bacterial susceptibility to host cathelicidin antimicrobial peptides and cell envelope-active antibiotics. Antimicrobial agents and chemotherapy. 2012;56(4):1854–1861. doi: 10.1128/AAC.05131-11. doi:10.1128/AAC.05131-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Midorikawa K, Ouhara K, Komatsuzawa H, Kawai T, Yamada S, Fujiwara T, Yamazaki K, Sayama K, Taubman MA, Kurihara H, Hashimoto K, Sugai M. Staphylococcus aureus susceptibility to innate antimicrobial peptides, beta-defensins and CAP18, expressed by human keratinocytes. Infection and immunity. 2003;71(7):3730–3739. doi: 10.1128/IAI.71.7.3730-3739.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sakoulas G, Okumura CY, Thienphrapa W, Olson J, Nonejuie P, Dam Q, Dhand A, Pogliano J, Yeaman MR, Hensler ME, Bayer AS, Nizet V. Nafcillin enhances innate immune-mediated killing of methicillin-resistant Staphylococcus aureus. Journal of molecular medicine. 2014;92(2):139–149. doi: 10.1007/s00109-013-1100-7. doi:10.1007/s00109-013-1100-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kristian SA, Timmer AM, Liu GY, Lauth X, Sal-Man N, Rosenfeld Y, Shai Y, Gallo RL, Nizet V. Impairment of innate immune killing mechanisms by bacteriostatic antibiotics. FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 2007;21(4):1107–1116. doi: 10.1096/fj.06-6802com. doi:10.1096/fj.06-6802com. [DOI] [PubMed] [Google Scholar]
- 15.Sakoulas G, Bayer AS, Pogliano J, Tsuji BT, Yang SJ, Mishra NN, Nizet V, Yeaman MR, Moise PA. Ampicillin enhances daptomycin- and cationic host defense peptide-mediated killing of ampicillin- and vancomycin-resistant Enterococcus faecium. Antimicrobial agents and chemotherapy. 2012;56(2):838–844. doi: 10.1128/AAC.05551-11. doi:10.1128/AAC.05551-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sakoulas G, Rose W, Nonejuie P, Olson J, Pogliano J, Humphries R, Nizet V. Ceftaroline Restores Daptomycin Activity against Daptomycin-Nonsusceptible Vancomycin-Resistant Enterococcus faecium. Antimicrobial agents and chemotherapy. 2014;58(3):1494–1500. doi: 10.1128/AAC.02274-13. doi:10.1128/AAC.02274-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gombart AF, Bhan I, Borregaard N, Tamez H, Camargo CA, Jr., Koeffler HP, Thadhani R. Low plasma level of cathelicidin antimicrobial peptide (hCAP18) predicts increased infectious disease mortality in patients undergoing hemodialysis. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2009;48(4):418–424. doi: 10.1086/596314. doi:10.1086/596314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ouhara K, Komatsuzawa H, Kawai T, Nishi H, Fujiwara T, Fujiue Y, Kuwabara M, Sayama K, Hashimoto K, Sugai M. Increased resistance to cationic antimicrobial peptide LL-37 in methicillin-resistant strains of Staphylococcus aureus. The Journal of antimicrobial chemotherapy. 2008;61(6):1266–1269. doi: 10.1093/jac/dkn106. doi:10.1093/jac/dkn106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dhand A, Bayer AS, Pogliano J, Yang SJ, Bolaris M, Nizet V, Wang G, Sakoulas G. Use of antistaphylococcal beta-lactams to increase daptomycin activity in eradicating persistent bacteremia due to methicillin-resistant Staphylococcus aureus: role of enhanced daptomycin binding. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2011;53(2):158–163. doi: 10.1093/cid/cir340. doi:10.1093/cid/cir340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cosgrove SE, Sakoulas G, Perencevich EN, Schwaber MJ, Karchmer AW, Carmeli Y. Comparison of mortality associated with methicillin-resistant and methicillin-susceptible Staphylococcus aureus bacteremia: a meta-analysis. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2003;36(1):53–59. doi: 10.1086/345476. doi:10.1086/345476. [DOI] [PubMed] [Google Scholar]
- 21.Mascitti KB, Edelstein PH, Fishman NO, Morales KH, Baltus AJ, Lautenbach E. Prior vancomycin use is a risk factor for reduced vancomycin susceptibility in methicillin-susceptible but not methicillin-resistant Staphylococcus aureus bacteremia. Infection control and hospital epidemiology : the official journal of the Society of Hospital Epidemiologists of America. 2012;33(2):160–166. doi: 10.1086/663708. doi:10.1086/663708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Han JH, Mascitti KB, Edelstein PH, Bilker WB, Lautenbach E. Effect of reduced vancomycin susceptibility on clinical and economic outcomes in Staphylococcus aureus bacteremia. Antimicrobial agents and chemotherapy. 2012;56(10):5164–5170. doi: 10.1128/AAC.00757-12. doi:10.1128/AAC.00757-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fowler VG, Jr., Boucher HW, Corey GR, Abrutyn E, Karchmer AW, Rupp ME, Levine DP, Chambers HF, Tally FP, Vigliani GA, Cabell CH, Link AS, DeMeyer I, Filler SG, Zervos M, Cook P, Parsonnet J, Bernstein JM, Price CS, Forrest GN, Fatkenheuer G, Gareca M, Rehm SJ, Brodt HR, Tice A, Cosgrove SE, Endocarditis Sa, Bacteremia Study G Daptomycin versus standard therapy for bacteremia and endocarditis caused by Staphylococcus aureus. The New England journal of medicine. 2006;355(7):653–665. doi: 10.1056/NEJMoa053783. doi:10.1056/NEJMoa053783. [DOI] [PubMed] [Google Scholar]
- 24.Rose WE, Leonard SN, Sakoulas G, Kaatz GW, Zervos MJ, Sheth A, Carpenter CF, Rybak MJ. daptomycin activity against Staphylococcus aureus following vancomycin exposure in an in vitro pharmacodynamic model with simulated endocardial vegetations. Antimicrobial agents and chemotherapy. 2008;52(3):831–836. doi: 10.1128/AAC.00869-07. doi:10.1128/AAC.00869-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liu GY, Nizet V. Color me bad: microbial pigments as virulence factors. Trends in microbiology. 2009;17(9):406–413. doi: 10.1016/j.tim.2009.06.006. doi:10.1016/j.tim.2009.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sakoulas G, Eliopoulos GM, Fowler VG, Jr., Moellering RC, Jr., Novick RP, Lucindo N, Yeaman MR, Bayer AS. Reduced susceptibility of Staphylococcus aureus to vancomycin and platelet microbicidal protein correlates with defective autolysis and loss of accessory gene regulator (agr) function. Antimicrobial agents and chemotherapy. 2005;49(7):2687–2692. doi: 10.1128/AAC.49.7.2687-2692.2005. doi:10.1128/AAC.49.7.2687-2692.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.McConeghy KW, Bleasdale SC, Rodvold KA. The Empirical Combination of Vancomycin and a Beta-Lactam for Staphylococcal Bacteremia. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2013 doi: 10.1093/cid/cit560. doi:10.1093/cid/cit560. [DOI] [PubMed] [Google Scholar]
- 28.Byfield FJ, Wen Q, Leszczynska K, Kulakowska A, Namiot Z, Janmey PA, Bucki R. Cathelicidin LL-37 peptide regulates endothelial cell stiffness and endothelial barrier permeability. Am J Physiol Cell Physiol. 2011;300(1):C105–112. doi: 10.1152/ajpcell.00158.2010. doi:10.1152/ajpcell.00158.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]