Abstract
Background and aims
Previous results of the AIM-HIGH trial showed that baseline levels of the conventional lipid parameters were not predictive of future cardiovascular (CV) outcomes. The aims of this secondary analysis were to examine the levels of cholesterol in high density lipoprotein (HDL) subclasses (HDL2-C and HDL3-C), small dense low density lipoprotein (sdLDL-C), and LDL triglyceride (LDL-TG) at baseline, as well as the relationship between these levels and CV outcomes.
Methods
Individuals with CV disease and low baseline HDL-C levels were randomized to simvastatin plus placebo or simvastatin plus extended release niacin (ERN), 1,500 to 2,000 mg/day, with ezetimibe added as needed in both groups to maintain an on-treatment LDL-C in the range of 40 to 80 mg/dL. The primary composite endpoint was death from coronary disease, nonfatal myocardial infarction, ischemic stroke, hospitalization for acute coronary syndrome, or symptom-driven coronary or cerebrovascular revascularization. HDL-C, HDL3-C, sdLDL-C and LDL-TG were measured at baseline by detergent-based homogeneous assays. HDL2-C was computed by the difference between HDL-C and HDL3-C. Analyses were performed on 3,094 study participants who were already on statin therapy prior to enrollment in the trial. Independent contributions of lipoprotein fractions to CV events were determined by Cox proportional hazards modeling.
Results
Baseline HDL3-C was protective against CV events (HR: 0.84, p=0.043) while HDL-C, HDL2-C, sdLDL-C and LDL-TG were not event-related (HR: 0.96, p=0.369; HR: 1.07, p=0.373; HR: 1.05, p=0.492; HR: 1.03, p=0.554, respectively).
Conclusions
The results of this secondary analysis of the AIM-HIGH Study indicate that levels of HDL3-C, but not other lipoprotein fractions, are predictive of CV events, suggesting that the HDL3 subclass may be primarily responsible for the inverse association of HDL-C and CV disease.
Keywords: Cardiovascular risk, Homogeneous assays, HDL3-cholesterol, HDL2-cholesterol, Small dense LDL, LDL-triglyceride
INTRODUCTION
The Atherothrombosis Intervention in Metabolic Syndrome with Low HDL/High Triglycerides and Impact on Global Health Outcomes (AIM-HIGH) trial was a randomized, double-blind clinical trial assessing the effect of extended release niacin (ERN) added to intensive statin-based low density lipoprotein cholesterol (LDL-C) lowering in patients with established cardiovascular (CV) disease and low levels of high density lipoprotein cholesterol (HDL-C) and elevated triglycerides (1). The trial was stopped prematurely because the addition of ERN to intensive LDL-lowering therapy during a median three year follow up period failed to further reduce incident CV events compared with LDL-lowering therapy alone (1).
In a secondary analysis, it was found that the parameters of the classic lipid profile, LDL-C, HDL-C or non-HDL-C at baseline, were not predictive of clinical CV events in either the placebo or ERN group (2). This finding raises the possibility that non-lipoprotein-related effects of niacin might have influenced such events in AIM-HIGH (2). In contrast, levels of lipoprotein(a) at baseline were predictive of CV events (3). One explanation for this lack of predictive power of the traditional lipid parameters may relate to the fact that most participants were already on a statin prior to being enrolled in the trial or that these standard lipoprotein measurements were not specific enough in this setting. In this new secondary analysis, we evaluated whether more specific lipoprotein parameters that can be analyzed with methods available in general clinical laboratories, i.e., HDL subclasses, small dense LDL-C (sdLDL-C) and LDL triglyceride (LDL-TG), were predictive of CV outcomes. Recent reports indicate an association between lower HDL3-C and increased risk for clinical events in patients with significant coronary heart disease (CHD) (4) or carotid artery disease (5). These findings lend support to our hypothesis that HDL3-C would more accurately reflect the athero-protective properties of HDL than does total HDL-C.
Other novel lipid measures and their relationships to CV risk have been described recently. For example, cholesterol in small dense LDL particles (sdLDL-C) has been related to risk of coronary events in patients with CHD and low HDL-C (6). In addition, sdLDL-C levels have consistently predicted incident CHD in an urban Japanese cohort (7), a biracial American cohort (8), and in normoglycemic, non-diabetic participants from a multi-ethnic study of atherosclerosis (9). Elevated sdLDL-C has also been shown to predict future CV events in patients with stable CHD (10). Since elevated LDL-TG levels have been associated with CHD and systemic inflammation (11), this measurement is also of interest. The AIM-HIGH trial provides a unique opportunity to examine these parameters in a dyslipidemic population with established CV disease.
PATIENTS AND METHODS
Study population
Demographic, laboratory parameters, and clinical characteristics of the AIM-HIGH cohort, along with LDL-lowering therapy design have been previously described (1). Participants had established stable atherosclerotic CV disease with HDL <40 mg/dL for men, <50 mg/dL for women, high triglyceride (150 to 400 mg/dL), and LDL-C <180 mg/dL (adjusted for lipid lowering). At the study entry, all subjects initially received simvastatin 40 mg daily, plus ERN at doses increasing weekly from 500 mg to 2000 mg/day. Subjects tolerating at least 1500 mg ERN were randomized to 1:1 to ERN or matching placebo tablets. The subgroup for this study included 3,094 participants who had sufficient sample available for measurement of the lipoprotein particles of interest out of the 3,196 participants who were receiving statin therapy prior to the initiation of the trial. Based on information collected at the screening visit, the 3,094 participants were taking at least six different statins. Based on the reported doses, 61.8% were considered to be on moderate-intensity treatment and 35.1% on high-intensity treatment (12). Events occurred in 15.4% of participants who were on moderate treatment prior to the trial and 17.3% of those on intensive treatment. Analyses of the lipoprotein parameters for this study were performed on plasma collected at baseline for 2,632 men and 462 women. The primary composite outcome was death from CHD or the occurrence of nonfatal myocardial infarction, ischemic stroke, hospitalization for acute coronary syndrome or cerebrovascular revascularization.
Laboratory methods
Blood samples for lipid analysis were collected from fasting participants into EDTA-containing vacutainers and processed using standardized conditions. Plasma samples were aliquoted into 2 mL cryovials and shipped on dry ice to the central laboratory. Determination of total HDL-C and HDL3-C concentrations in plasma samples was performed by fully automated detergent-based homogeneous methods (Denka Seiken, Tokyo, Japan) on a Roche Modular P chemistry analyzer (Roche Diagnostics Inc., Indianapolis, IN). The intra- and inter-assay coefficients of variation (CVs) on quality control samples with low and high HDL-C levels were 1.18% and 1.16%, respectively, and 3.49% and 2.68%, respectively. The intra- and inter-assay CVs on quality control samples with low and high HDL3-C levels were 1.18% and 3.49%, respectively, and 0.99% and 3.46%, respectively. HDL2-C was estimated by subtracting HDL3-C from total HDL-C. Values of homogeneous HDL3-C and calculated HDL2-C were in close agreement with cholesterol values measured in HDL3 and HDL2 fractions separated by ultracentrifugation in the density range 1.125–1.210 kg/L and 1.063–1.125 kg/L, respectively (13). Additionally, the assay validation showed good linearity and precision (14).
Determination of sdLDL-C concentration was performed by a fully automated homogeneous method (Denka Seiken, Tokyo, Japan) on a Roche Modular P chemistry analyzer (Roche Diagnostics Inc., Indianapolis, IN) as previously described (15). Results of the assay validation showed an excellent agreement between the values obtained by this homogeneous method and those obtained by density gradient ultracentrifugation (16). The intra- and inter-assay CVs on quality control samples with low and high sdLDL-C levels were 1.11% and 0.75%, respectively, and 4.65% and 4.46%, respectively. Concentration of triglycerides in LDL (LDL-TG) was determined by the LDL-TG-EX homogeneous method (Denka Seiken, Tokyo, Japan) on a Roche Modular P chemistry analyzer (Roche Diagnostics Inc., Indianapolis, IN). The intra- and inter-assay CVs on quality control samples with low and high LDL-TG levels were 1.57% and 1.06%, respectively, and 2.38% and 1.60%, respectively. The estimation of the glomerular filtration rate (eGFR) was performed by the CKD-EPI formula (17)
Statistical analysis
Baseline distributions were examined using histograms overall and by primary event status (observed or unobserved) for each parameter. Descriptive statistics including the mean, standard deviation (SD), median, and quartiles were calculated for each parameter. The t-test was used to compare baseline distributions by primary event status. Correlation between parameters was assessed using Pearson’s correlation coefficient. Cox Proportional Hazards regression was used to estimate the independent relationship between each parameter and CV events; these models included gender, history of diabetes, eGFR, BMI and randomization assignment as fixed covariates. Each lipoprotein parameter was modeled individually, and any parameter that showed a statistically significant relationship with events (p<0.05) was also parameterized using baseline quartiles. Unadjusted Kaplan-Meier curves showing the event-free distribution by baseline quartiles were constructed. A multivariate lipoprotein analysis was performed by including all four continuous parameters in a Cox Proportional Hazards model including fixed effects of gender, history of diabetes, eGFR, BMI and randomization assignment. Thus, subgroup analyses by treatment were not performed. There were no formal type-I error adjustments, and the results of this secondary analysis should be interpreted in the context of hypothesis generation. All reported confidence intervals and p-values are two-tailed. A p value <0.05 defined significance.
RESULTS
The baseline demographic, clinical characteristics and lipoprotein parameters of this AIM-HIGH cohort are shown in Table 1.
Table 1.
Baseline demographics and clinical characteristics.
| Total (N=3094) |
CV event | p -Value | |||
|---|---|---|---|---|---|
| No (N=2587) | Yes (N=507) | ||||
| Gender | Female | 462 (14.9%) | 402 (15.5%) | 60 (11.8%) | 0.032* |
| Male | 2632 (85.1%) | 2185 (84.5%) | 447 (88.2%) | ||
| Race | White | 2855 (92.2%) | 2390 (92.4%) | 465 (91.7%) | |
| Black/African American | 100 (3.2%) | 72 (2.8%) | 28 (5.5%) | ||
| Asian | 40 (1.3%) | 38 (1.5%) | 2 (0.4%) | ||
| Other | 99 (3.2%) | 87 (3.4%) | 12 (2.4%) | ||
| History of myocardial infarction | 1766 (57.1%) | 1469 (56.8%) | 297 (58.6%) | 0.461 | |
| Coronary artery bypass graft | 1136 (36.7%) | 925 (35.8%) | 211 (41.6%) | 0.013* | |
| Percutaneous coronary intervention | 1919 (62.0%) | 1595 (61.7%) | 324 (63.9%) | 0.34 | |
| Stroke or cerebrovascular disease | 655 (21.2%) | 519 (20.1%) | 136 (26.8%) | <0.001* | |
| Peripheral vascular disease | 401 (13.0%) | 320 (12.4%) | 81 (16.0%) | 0.027* | |
| Metabolic syndrome | 2471 (79.9%) | 2061 (79.7%) | 410 (81.0%) | 0.047* | |
| History of diabetes | 1062 (34.3%) | 853 (33.0%) | 209 (41.2%) | <0.001* | |
| History of hypertension | 2212 (71.5%) | 1824 (70.5%) | 388 (76.5%) | 0.006* | |
| Age (years) | Mean ± SD | 63.8 ± 8.72 | 63.7 ± 8.73 | 64.4 ± 8.67 | 0.076 |
| BMI | Mean ± SD | 31.2 ± 5.37 | 31.1 ± 5.31 | 31.6 ± 5.65 | 0.062 |
| HbA1c, % | Mean ± SD | 5.98 ± 0.80 | 5.97 ± 0.78 | 6.07 ± 0.85 | 0.010* |
| eGFR, mL/min/1.73 m2 | Mean ± SD | 77.5 ± 18.5 | 77.8 ± 18.3 | 75.99 ± 19.71 | 0.047* |
| HDL-C, mg/dL (mmol/L) | Mean ± SD | 34.9 ± 5.6 (0.4 ± 0.1) | 35.0 ± 5.7 (0.4 ± 0.1) | 34.3 ± 5.5 (0.4 ± 0.1) | 0.015* |
| HDL3-C, mg/dL (mmol/L) | Mean ± SD | 17.4 ± 2.9 (0.5 ± 0.1) | 17.5 ± 2.9 (0.5 ± 0.1) | 17.1 ± 2.8 (0.4 ± 0.1) | 0.006* |
| HDL2-C, mg/dL (mmol/L) | Mean ± SD | 21.3 ± 3.6 (0.6 ± 0.1) | 21.3 ± 3.7 (0.6 ± 0.1) | 20.9 ± 3.5 (0.5 ± 0.1) | 0.035* |
| LDL-C, mg/dL (mmol/L) | Mean ± SD | 71.1 ± 18.1 (0.8 ± 0.2) | 70.9 ± 18.2 (0.8 ± 0.2) | 71.8 ± 17.5 (0.8 ± 0.2) | 0.293 |
| sdLDL, mg/dL (mmol/L) | Mean ± SD | 32.7 ± 12.8 (0.9 ± 0.3) | 32.6 ± 12.8 (0.9 ± 0.3) | 32.9 ± 12.7 (0.9 ± 0.3) | 0.61 |
| TG, mg/dL (mmol/L) | Mean ± SD | 179.6 ± 65.8 (2.0 ± 0.7) | 179.3 ± 65.5 (2.0 ± 0.7) | 181.0 ± 67.2 (2.1 ± 0.8) | 0.593 |
| LDL-TG, mg/dL (mmol/L) | Mean ± SD | 21.2 ± 6.1 (0.2 ± 0.1) | 21.1 ± 6.1 (0.2 ± 0.1) | 21.4 ± 6.1 (0.2 ± 0.1) | 0.358 |
Patients were predominantly white (92%) and 85% of this cohort were men. More than half of the patients had a history of myocardial infarction, about one third had a history of diabetes, and more than 71% had a history of hypertension. The mean HDL3-C value was 17.4 mg/dl (0.45 mmol/L), slightly lower than the mean HDL2-C value of 21.3 mg/dl (0.55 mmol/L). The mean sdLDL-C value was 32.7 mg/dl (0.85 mmol/L) while the LDL-TG was 21.2 mg/dl (0.24 mmol/L (Table 1).
The distribution of each lipoprotein parameter was approximately symmetric, with the sdLDL values slightly skewed to the right. A modest correlation (r=0.59, p<0.001) was observed between sdLDL-C and LDL-TG concentrations. Approximately 48±20% of LDL-C was sdLDL in this cohort while, on average, LDL TG represented 13±5% of the total plasma triglyceride.
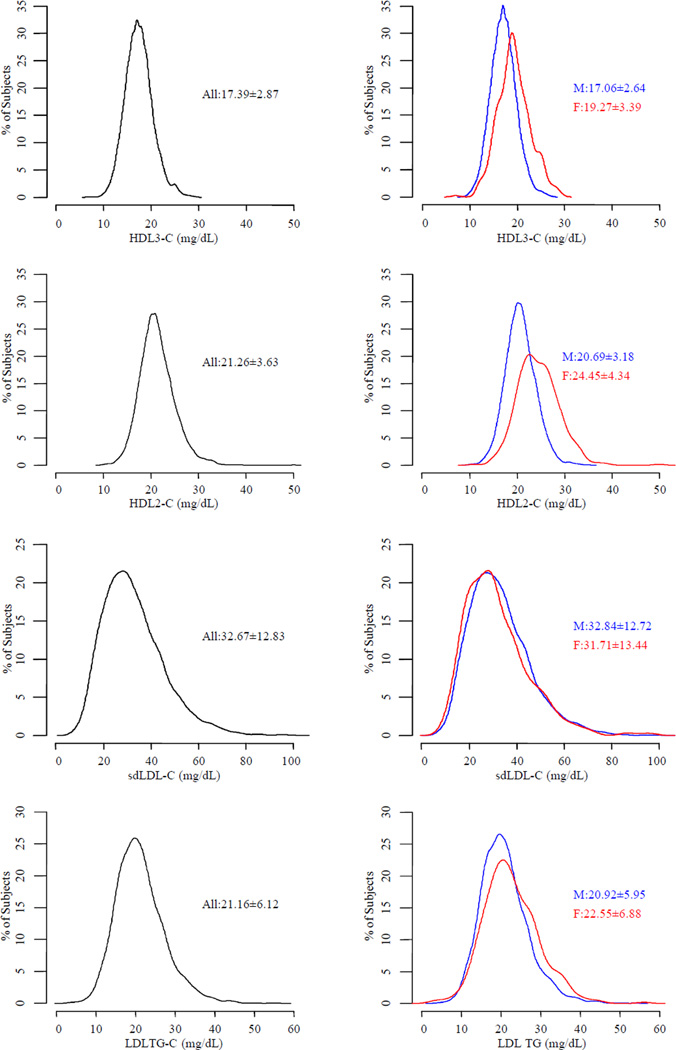
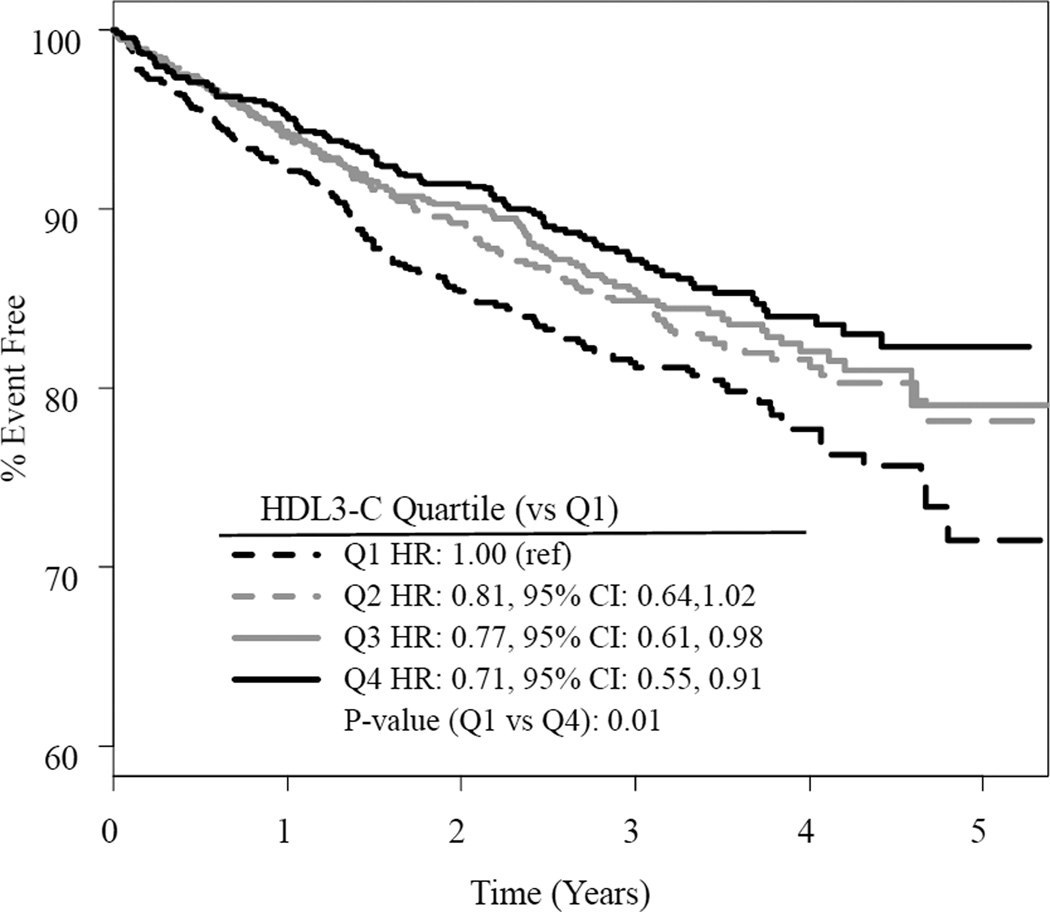
There were 507 participants who experienced a primary outcome. Results did not differ by treatment assignment, nor was there evidence of interaction between treatment and any baseline lipoprotein. A greater proportion of those having a CV event during the trial were male and had a coronary artery bypass graft, stroke or cerebrovascular disease, peripheral artery disease, history of diabetes, and history of hypertension compared to those without an event (Table 1). Mean levels of HDL3-C, HDL2-C, and total HDL-C were lower in those with events than in those without events (p=0.006, 0.035, and 0.015, respectively). The distribution of HDL3-C among those having a primary outcome was shifted to slightly lower levels compared to those without a primary outcome (Figure 1). Baseline levels of HDL3-C were inversely predictive of CV events. Compared to the lowest quartile, the highest quartile was about 1.4 times less likely to experience a CV event (HR: 0.73, 95% CI: 0.56, 0.95, p=0.017, Figure 2).
Fig. 1. The overall lipoprotein distribution is summarized on the left and in those without (blue) and with events (red) on the right.
The median (25th and 75th percentile) of each parameter is displayed as text.
Fig. 2. Proportions based on Kaplan-Meier estimates, shown separately by quartile of HDL3-C but pooled across randomization assignments.
Hazard ratios are derived from Cox Proportional Hazards models including treatment, gender, BMI, eGFR and history of diabetes. Pair-wise comparisons of the highest three quartiles to the lowest quartile are displayed.
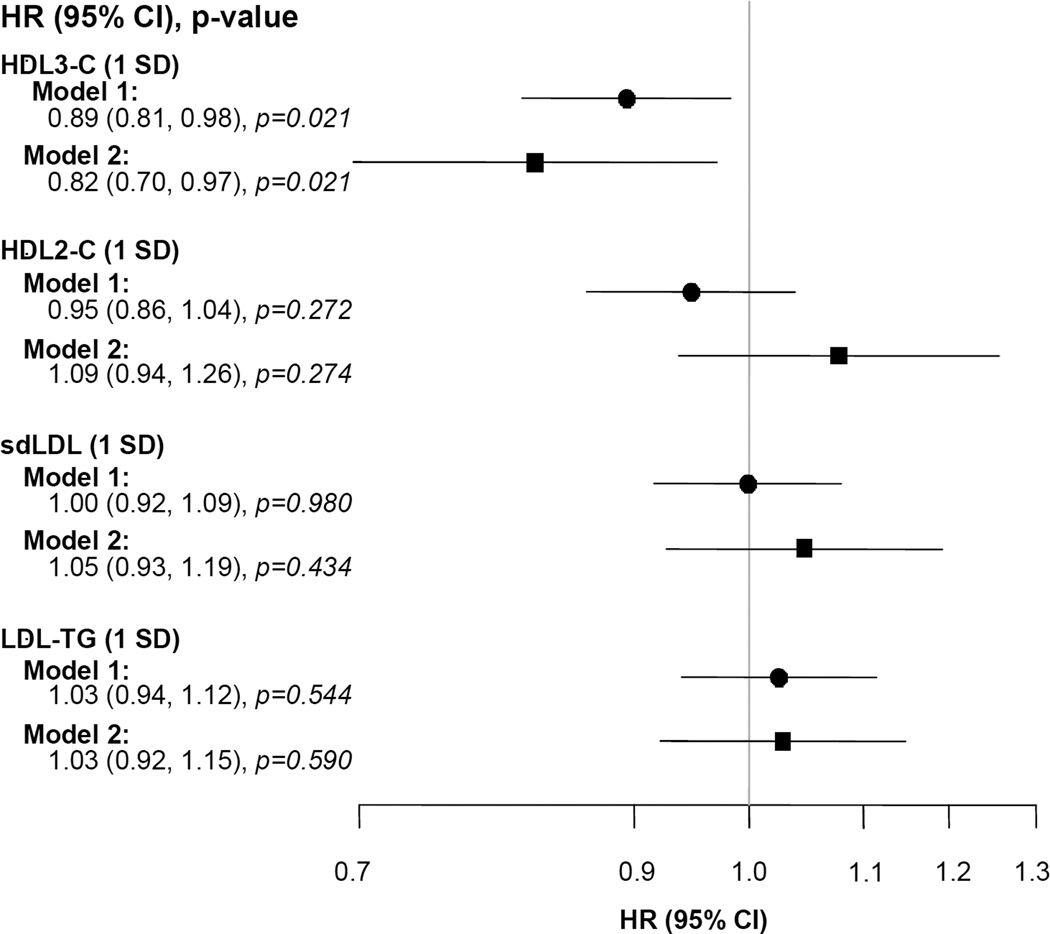
Baseline total HDL-C did not predict events (HR: 0.96, 95% CI 0.87–1.05, p=0.369). However, a one standard deviation increase in HDL3-C was significantly associated with a 9% reduction in CV event risk (HR: 0.91, 95% CI: 0.82–1.00, p=0.044). Multivariate analysis also indicated that HDL3-C was inversely related to CV events (HR: 0.84, 95% CI: 0.71–0.99, p=0.043), but neither HDL2-C (HR: 1.07, 95% CI: 0.92–1.24, p=0.373), sdLDL (HR: 1.05, 95% Cl: 0.92–1.19, p=0.492) nor LDL-TG (HR: 1.03, 95% Cl 0.92–1.16), p=0.554) predicted CV events; Figure 3). The proportional hazard assumption was evaluated and satisfied (p=0.69) for this model.
Fig. 3. Cox proportional hazards models for the primary composite outcome as a function of lipoprotein parameters/baseline standard deviation as continuous variables.
Model 1 includes treatment group, gender, history of diabetes, BMI, eGFR and single lipoprotein parameters. Model 2 includes treatment group, gender, history of diabetes, BMI, eGFR and the four lipoprotein sub-fraction parameters simultaneously.
DISCUSSION
Historically, HDL has been divided in HDL2 and HDL3 subclasses based on their relative hydrated density in ultracentrifugation, which is primarily determined by the lipid to protein ratio. Higher levels of cholesterol in both these lipoprotein subclasses have been associated with lower risk of CV disease in some studies, but not in others (18). Considering that the increase of HDL-C in the cohort receiving ERN did not result in clinical benefit, we wanted to evaluate whether baseline HDL2-C or HDL3-C levels were predictive of CV events. Interestingly, we found that higher baseline HDL3-C levels, but not HDL2-C levels, were predictive of fewer adverse CV events in this cohort receiving intensive LDL-C lowering therapy with statins. One explanation for this finding could be that this denser HDL3-C subclass containing smaller HDL particles may better reflect the cholesterol efflux capacity and antioxidant, anti-inflammatory, anti-thrombotic, and anti-apoptotic properties of small, dense HDL particles than does HDL2-C (19, 20).
A wealth of epidemiological data has shown that HDL-C levels are inversely related to CV risk (21). These observations have suggested that HDL-C is athero-protective. However, as HDL-C only reflects the average cholesterol content of a complex heterogeneous mixture of lipoprotein particles, it may not adequately capture the athero-protective anti-inflammatory, antioxidant, and cholesterol efflux properties of HDL. In the AIM-HIGH Trial, HDL-C was measured in patients with established CV disease who were randomized to niacin or placebo while receiving intensive LDL-C lowering therapy. After a two-year follow up, the patients randomized to niacin had significantly higher HDL-C, but this change did not lead to fewer CV events compared to the placebo group. Thus, raising HDL-C did not reduce CV events in this cohort, which suggests that HDL-C is not causally related to CVD. A Mendelian randomization study, evaluating a polymorphism that increased HDL-C levels without altering the risk of myocardial infarction, also failed to establish a causal link between HDL-C and CVD (22). These findings confirm that total HDL-C level does not necessarily reflect the athero-protective properties of HDL. Interestingly, one study published this year concluded that smaller, denser HDL3-C levels are primarily responsible for the inverse association between HDL-C and incident CHD (23) and the other concluded that the small and medium size HDL particle measurements improve mortality risk prediction (24). These studies lend further support to the concept that the smaller, denser HDL subpopulation is superior to total HDL in risk prediction.
Although a number of studies have suggested that sdLDL-C measurement is useful for identifying risk for CVD (6–9), sdLDL-C levels failed to predict CV events in this cohort with established CV disease. The reason for the negative findings is not completely clear, but may be due to the fact that all patients were taking statins, which generally lower sdLDL-C (25). There was also variability in the time of day when patients were sampled, which could also be a contributing factor; sdLDL-C has been shown to exhibit circadian change, with highest values in the morning followed by a steady decrease during the day (26). In the Framingham Offspring Study (27), women with CHD had higher sdLDL-C concentrations than controls, but men did not exhibit this difference.
Little is known about the clinical usefulness of LDL-TG levels, probably due to the complexity of the analytical methods used for their determination. In a study (11) where levels of LDL-TG were measured after fractionation of LDL by equilibrium density-gradient centrifugation, it was found that alteration of the LDL metabolism characterized by high LDL-TG was correlated with CHD and systemic low-grade inflammation. LDL-TG failed to predict CV events in our cohort. The differences between the previous study (11) and our study in relation to LDL-TG and CV outcomes could be related to the fact that the earlier study excluded patients taking lipid lowering drugs while in AIM-HIGH the study participants were taking statins which normalize LDL metabolism and reduce systemic inflammation.
Detergent-based homogeneous assays for HDL3-C, HDL2-C, sdLDL-C and LDL-TG are simple and convenient methods to be used in clinical laboratories as they do not require separation of lipoproteins by ultracentrifugation or dedicated instrumentation. Additionally, the values are highly precise on automated analyzers. Further application of these methods to large scale clinical studies should clarify whether these lipoprotein parameters are superior markers of CVD and provide additional information to the conventional lipoprotein measurements.
Some limitations to the current analysis should be recognized. The AIM-HIGH cohort was predominantly composed of white men, and therefore our findings cannot be generalized to women or other ethnic groups. Secondly, the measurements of lipoprotein subclasses at baseline do not address the relationship of the changes in these parameters with outcome. Third, a single baseline measurement may not reflect lifetime exposure to these risk factors. Fourth, the convenient homogeneous methods for the determination of these lipoprotein measures used here may not measure the same population of lipoprotein particles as other approaches. Therefore, the observed risk associations may vary depending on the characteristics of the population and on the specific methodology used to measure these lipoprotein subclasses.
In conclusion, this secondary analysis from the AIM-HIGH Study found that levels of HDL3-C, but not HDL-C, HDL2-C, sdLDL, or LDL-TG, predict CV events in patients with metabolic dyslipidemia. This finding lends support to the hypothesis that HDL3 subclass may be primarily responsible for the inverse association between HDL-C and CV disease.
Acknowledgments
FUNDING
The Atherothrombosis Intervention in Metabolic syndrome with low high-density lipoprotein/high triglycerides: Impact on Global Health (AIM-HIGH) study was supported by the National Heart, Lung, and Blood Institute (U01 HL081616 and U01 HL081649) and by an unrestricted grant from AbbVie, Inc. AbbVie donated the extended-release niacin, the matching placebo, and the ezetimibe; Merck donated the simvastatin. Neither of these companies had any role in the oversight or design of the study or in the analysis or interpretation of the data.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
CONFLICT OF INTEREST
Dr Santica Marcovina is a consultant to Denka Seiken Co Ltd and MedTest Dx for Lp(a) standardization. Dr Kevin O’Brien receives grant support from Sanofi. April Slee and Drs John Albers and Jerome Fleg have no conflicts of interest to disclose.
AUTHOR CONTRIBUTIONS
All authors have made substantial contributions to the conception and design, and/or acquisition of data, and/or analysis and interpretation of data; have participated in drafting the manuscript (or revising it critically for important intellectual content); and have given final approval of the manuscript to be submitted for publication.
DISCLAIMER
The content of this manuscript is the responsibility of the authors and does not necessarily reflect the views of the National Institutes of Health or the Department of Health and Human Services.
Contributor Information
John J Albers, Email: jja@uw.edu.
April Slee, Email: AprilS@axioresearch.com.
Jerome L Fleg, Email: flegj@nhlbi.nih.gov.
Kevin D O’Brien, Email: cardiac@uw.edu.
Santica M Marcovina, Email: smm@uw.edu.
REFERENCES
- 1.AIM-HIGH Investigators. Boden WE, Probstfield JL, Anderson T, Chaitman BR, Desvigne-Nickens P, Koprowicz K, McBride R, Teo K, Weintraub W. Niacin in patients with low HDL cholesterol levels receiving intensive statin therapy. New Engl J Med. 2011;365(24):2255–2267. doi: 10.1056/NEJMoa1107579. [DOI] [PubMed] [Google Scholar]
- 2.Guyton JR, Slee AE, Anderson T, Fleg JL, Goldberg RB, Kashyap ML, Marcovina SM, Nash SD, O'Brien KD, Weintraub WS, Xu P, Zhao XQ, Boden WE. Relationship of lipoproteins to cardiovascular events: the AIM-HIGH Trial (Atherothrombosis Intervention in Metabolic Syndrome with Low HDL/High Triglycerides and Impact on Global Health Outcomes) J Am Coll Cardio. 2013;62(17):1580–1584. doi: 10.1016/j.jacc.2013.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Albers JJ, Slee A, O'Brien KD, Robinson JG, Kashyap ML, Kwiterovich PO, Jr, Xu P, Marcovina SM. Relationship of apolipoproteins A-1 and B, and lipoprotein(a) to cardiovascular outcomes: the AIM-HIGH trial (Atherothrombosis Intervention in Metabolic Syndrome with Low HDL/High Triglyceride and Impact on Global Health Outcomes) J Am Coll Cardiol. 2013;62(17):1575–1579. doi: 10.1016/j.jacc.2013.06.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Martin SS, Khokhar AA, May HT, Kulkarni KR, Blaha MJ, Joshi PH, Toth PP, Muhlestein JB, Anderson JL, Knight S, Li Y, Spertus JA, Jones SR. Lipoprotein Investigators Collaborative (LIC). HDL cholesterol subclasses, myocardial infarction, and mortality in secondary prevention: the Lipoprotein Investigators Collaborative. Eur Heart J. 2015;36(1):22–30. doi: 10.1093/eurheartj/ehu264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kim DS, Burt AA, Rosenthal EA, Ranchalis JE, Eintracht JF, Hatsukami TS, Furlong CE, Marcovina S, Albers JJ, Jarvik GP. HDL-3 is a superior predictor of carotid artery disease in a case-control cohort of 1725 participants. J Am Heart Assoc. 2014;3(3):e000902. doi: 10.1161/JAHA.114.000902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Williams PT, Zhao XQ, Marcovina SM, Brown BG, Krauss RM. Levels of cholesterol in small LDL particles predict atherosclerosis progression and incident CHD in the HDL-Atherosclerosis Treatment Study (HATS) PLoS One. 2013;8(2):e56782. doi: 10.1371/journal.pone.0056782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Arai H, Kokubo Y, Watanabe M, Sawamura T, Ito Y, Minagawa A, Okamura T, Miyamato Y. Small dense low-density lipoproteins cholesterol can predict incident cardiovascular disease in an urban Japanese cohort: the Suita study. J Atheroscler Thromb. 2013;20(2):195–203. doi: 10.5551/jat.14936. [DOI] [PubMed] [Google Scholar]
- 8.Hoogeveen RC, Gaubatz JW, Sun W, Dodge RC, Crosby JR, Jiang J, Couper D, Virani SS, Kathiresan S, Boerwinkle E, Ballantyne CM. Small dense low-density lipoprotein-cholesterol concentrations predict risk for coronary heart disease: the Atherosclerosis Risk In Communities (ARIC) study. Arterioscler Thromb Vasc Biol. 2014;34(5):1069–1077. doi: 10.1161/ATVBAHA.114.303284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tsai MY, Steffen BT, Guan W, McClelland RL, Warnick R, McConnell J, Hoefner DM, Remaley AT. New automated assay of small dense low-density lipoprotein cholesterol identifies risk of coronary heart disease: the Multi-ethnic Study of Atherosclerosis. Arterioscler Thromb Vasc Biol. 2014;34(1):196–201. doi: 10.1161/ATVBAHA.113.302401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nishikura T, Koba S, Yokota Y, Hirano T, Tsunoda F, Shoji M, Hamazaki Y, Suzuki H, Itoh Y, Katagiri T, Kobayashi Y. Elevated small dense low-density lipoprotein cholesterol as a predictor for future cardiovascular events in patients with stable coronary artery disease. J Atheroscler Thromb. 2014;21(8):755–767. doi: 10.5551/jat.23465. [DOI] [PubMed] [Google Scholar]
- 11.März W, Scharnagl H, Winkler K, Tiran A, Nauck M, Boehm BO, Winkelmann BR. Low-density lipoprotein triglycerides associated with low-grade systemic inflammation, adhesion molecules, and angiographic coronary artery disease: the Ludwigshafen Risk and Cardiovascular Health study. Circulation. 2004;110(19):3068–3074. doi: 10.1161/01.CIR.0000146898.06923.80. [DOI] [PubMed] [Google Scholar]
- 12.Amsterdam EA, Wenger NK, Brindis RG, Casey DE, Jr, Ganiats TG, Holmes DR, Jr, Jaffe AS, Jneid H, Kelly RF, Kontos MC, Levine GN, Liebson PR, Mukherjee D, Peterson ED, Sabatine MS, Smalling RW, Zieman SJ. 2014 ACC/AHA guideline for the management of patients with non–ST-elevation acute coronary syndromes: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2014;130:e344–e426. doi: 10.1161/CIR.0000000000000134. [DOI] [PubMed] [Google Scholar]
- 13.Ito Y, Satoh N, Ishii T, Kumakura J, Hirano T. Development of a homogenous assay for measurement of high-density lipoprotein-subclass cholesterol. Clin Chim Acta. 2014;427:86–93. doi: 10.1016/j.cca.2013.09.009. [DOI] [PubMed] [Google Scholar]
- 14.Ashmaig ME, Gupta S, McConnell JP, Warnick GR. Validation of a novel homogeneous assay for HDL3-C measurement. Clin Chim Acta. 2013;425:37–41. doi: 10.1016/j.cca.2013.07.007. [DOI] [PubMed] [Google Scholar]
- 15.Ito Y, Fujimura M, Ohta M, Hirano T. Development of a new homogeneous assay for quantification of small dense LDL cholesterol. Clin Chem. 2011;57(1):57–65. doi: 10.1373/clinchem.2010.149559. [DOI] [PubMed] [Google Scholar]
- 16.Albers JJ, Kennedy H, Marcovina SM. Evaluation of a new homogenous method for detection of small dense LDL cholesterol: comparison with the LDL cholesterol profile obtained by density gradient ultracentrifugation. Clin Chim Acta. 2011;412(7–8):556–561. doi: 10.1016/j.cca.2010.12.003. [DOI] [PubMed] [Google Scholar]
- 17.Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF, III, Feldman HI, Kusek JW, Eggers P, Van Lente F, Greene T, Coresh J for the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) A New Equation to Estimate Glomerular Filtration Rate. Ann Intern Med. 2009;150(9):604–612. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Superko HR, Pendyala L, Williams PT, Momary KM, King SB, 3rd, Garrett BC. High-density lipoprotein subclasses and their relationship to cardiovascular disease. J Clin Lipidol. 2012;6(6):496–523. doi: 10.1016/j.jacl.2012.03.001. [DOI] [PubMed] [Google Scholar]
- 19.Du XM, Kim MJ, Hou L, Le Goff W, Chapman MJ, Van Eck M, Curtiss LK, Burnett JR, Cartland SP, Quinn CM, Kockx M, Kontush A, Rye KA, Kritharides L, Jessup W. HDL particle size is a critical determinant of ABCA1-mediated macrophage cellular cholesterol export. Circ Res. 2015;116(7):1133–1142. doi: 10.1161/CIRCRESAHA.116.305485. [DOI] [PubMed] [Google Scholar]
- 20.Camont L, Lhomme M, Rached F, Le Goff W, Nègre-Salvayre A, Salvayre R, Calzada C, Lagarde M, Chapman MJ, Kontush A. Small, dense high-density lipoprotein-3 particles are enriched in negatively charged phospholipids: relevance to cellular cholesterol efflux, antioxidative, antithrombotic, anti-inflammatory, and antiapoptotic functionalities. Arterioscler Thromb Vasc Biol. 2013;33(12):2715–2723. doi: 10.1161/ATVBAHA.113.301468. [DOI] [PubMed] [Google Scholar]
- 21.Hovingh GK, Rader DJ, Hegele RA. HDL re-examined. Curr Opin Lipidol. 2015;26(2):127–132. doi: 10.1097/MOL.0000000000000161. [DOI] [PubMed] [Google Scholar]
- 22.Voight BF, Peloso GM, Orho-Melander M, Frikke-Schmidt R, Barbalic M, Jensen MK, Hindy G, Hólm H, Ding EL, Johnson T, Schunkert H, Samani NJ, Clarke R, et al. Plasma HDL cholesterol and risk of myocardial infarction: a mendelian randomization study. Lancet. 2012;380(9841):572–580. doi: 10.1016/S0140-6736(12)60312-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Joshi PH, Toth PP, Lirette ST, Griswold ME, Massaro JM, Martin SS, Blaha MJ, Kulkarni KR, Correa A, D’Agustino RB, Sr, Jones SR Lipoprotein Investigators Collaborative (LIC) Study Group. Association of high-density lipoprotein subclasses and incident coronary heart disease: The Jackson Heart and Framingham Offspring Cohort Studies. Eur J Prev Cardiol. 2016;23(1):41–49. doi: 10.1177/2047487314543890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McGarrah RW, Craig DM, Haynes C, Dowdy ZE, Shah SH, Kraus WE. High-Density Lipoprotein Subclass Measurements Improve Mortality Risk Prediction, Discrimination and Reclassification in a Cardiac Catheterization Cohort. Atherosclerosis. 2016;246:229–235. doi: 10.1016/j.atherosclerosis.2016.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hirayama S, Miida T. Small dense LDL: An emerging risk factor for cardiovascular disease. Clin Chim Acta. 2012;414:215–224. doi: 10.1016/j.cca.2012.09.010. [DOI] [PubMed] [Google Scholar]
- 26.Hirayama S, Soda S, Ito Y, Matsui H, Ueno T, Fukushima Y, Ohmura H, Hanyu O, Aizawa Y, Miida T. Circadian change of serum concentration of small dense LDL-Cholesterol in type 2 diabetic patients. Clin Chim Acta. 2010;411(3–4):253–257. doi: 10.1016/j.cca.2009.11.017. [DOI] [PubMed] [Google Scholar]
- 27.Ai M, Otokozawa S, Asztalos BF, Ito Y, Nakajima K, White CC, Cupples LA, Wilson PW, Schaefer EJ. Small dense low density lipoprotein cholesterol and coronary heart disease: results from the Framingham Offspring Study. Clin Chem. 2010;56(6):967–976. doi: 10.1373/clinchem.2009.137489. [DOI] [PMC free article] [PubMed] [Google Scholar]