Abstract
Objectives
1) Investigate the impact of electrode type and surgical approach on scalar electrode location in a large patient cohort; and 2) examine the relation between electrode location and postoperative audiologic performance.
Setting
Tertiary academic hospital.
Patients
220 post-lingually deafened adults undergoing cochlear implant (CI).
Main Outcome Measures
Primary outcome measures of interest were scalar electrode location and postoperative audiologic performance.
Results
In 68% of implants, electrodes were observed to be located solely in the scala tympani (ST). Multivariate analysis demonstrated perimodiolar(PM) and Mid-scala(MS) electrodes were 22.4 (95%CI:6.3–80.0, p<0.001) and 55.0 (95%CI:9.7–312.8, p<0.001) times more likely to have at least one electrode in the scala vestibuli (SV) compared to lateral wall(LW) electrodes, respectively. Compared to cochleostomy(C), round window(RW) and extended round window(ERW) approaches demonstrated 70% reduction in SV insertion (OR 0.28,95%CI:0.1–0.8, p=0.01; ERW (OR O.28,95%CI:0.1–0.7, p=0.005). Examining postoperative audiometric performance, CNC score increased 0.6% with every 10° increase in angular insertion depth beyond the group minimum of 208° (Coefficient 0.0006,95%CI:0.0001–0.001, p=0.03). SV insertion was associated with a 12% decrease in CNC score (Coefficient -0.12,95%CI:-0.22- -0.02, p=0.02). CNC score decreased 0.3% for every 1 year increase in age (Coefficient -0.003,95%CI:-0.006- -0.0006), p=0.02).
Conclusions
Electrode design and surgical approach were predictors of scalar electrode location. Specifically, LW electrodes showed higher rates of ST insertion compared to PM or MS. RW and ERW approaches showed higher rates of ST insertion when compared to C. In regards to performance, ST insertion, younger age, and greater angular insertion depth were predictors of improved CNC scores.
Keywords: Cochlear implant, cochlear implantation, lateral wall, perimodiolar, mid-scala, electrode location, scalar translocation, angular insertion depth, insertion depth, angular depth of insertion, speech perception, audiologic outcomes, CNC score, AzBio score, intrascalar location
INTRODUCTION
Over the past few decades, cochlear implantation (CI) has become the standard of care for hearing rehabilitation in patients with bilateral profound sensorineural hearing loss. As the indications for CI have expanded to include younger patients and individuals with greater degrees of residual hearing, increasing emphasis has been placed on atraumatic surgery and the preservation of cochlear structure. Scala tympani (ST) insertions minimize intracochlear trauma and are therefore widely accepted as the preferred location for electrode placement. In contrast, electrode array insertions with any contact in the scala vestibuli (SV) are associated with injury to the basilar membrane, Reissner’s membrane, and the Organ of Corti.1
Recent research has focused on identification of factors that impact scalar electrode position. Electrode design has been identified as a potentially important factor, with lateral wall (LW) electrodes entering SV less frequently than perimodiolar (PM) electrodes.2, 3 Evidence also suggests that surgical approach influences scalar position. Round window (RW) and extended round window (ERW) insertions have been shown to be associated with lower rates of SV insertion when compared to cochleostomy (C) insertions.2 Besides electrode design and surgical approach, differences in cochlea size and depth of insertion may also influence intracochlear electrode location. Given that cochlear morphology is variable, it follows that a smaller cochlear volume and/or greater depth of electrode insertion may predispose to interscalar electrode translocation.
Perhaps more important than the reduction of cochlear trauma associated with ST insertion, recent studies suggest that postoperative audiometric performance is better when all electrodes are positioned within the ST.2, 4–6 Maintenance of residual hearing has also been shown to be superior in patients with electrodes positioned entirely within the ST.7, 8
Given the above, the primary objectives of the current study were as follows: 1) investigate the impact of electrode type and surgical approach on electrode location in a large cohort of patients, while controlling for cochlear volume and depth of insertion; and 2) examine the relation between electrode location and postoperative audiometric performance. We hypothesized that both surgical approach and electrode design would impact scalar electrode location.2 Additionally, we suspected that electrodes located entirely within the ST would be associated with improved speech perception postoperatively.
MATERIALS AND METHODS
Patient Selection
Post-lingually deafened adult patients undergoing CI were eligible for inclusion and consenting patients underwent both preoperative and postoperative temporal bone computed tomography (CT). We retrospectively reviewed a prospectively acquired database, and selected patients for whom information regarding electrode type, surgical approach, cochlear volume, and electrode location was available. Institutional review board approval was obtained.
Demographics and Operative Characteristics
Patient demographics (age, gender, race), type of electrode (PM, LW, Mid-scala [MS]), and surgical approach (traditional C, ERW, RW) were retrospectively recorded. PM and MS electrode arrays were inserted using an advance off-stylet technique. ERW approaches entailed opening the round window membrane and enlarging it by drilling the anterior-inferior margin. Implants from all 3 FDA-approved device manufacturers (MED-EL [ME] GmbH Innsbruck, Austria, Cochlear Americas [CA] Sydney, Australia, and Advanced Bionics [AB] Corporation Stafa, Switzerland) were used.
Cochlear Volume
In order to segment intracochlear anatomy (ST and SV) in pre-op CT images, an active shape model was created with micro CT (µCT) scans of the cochlea acquired ex-vivo. The model was then fitted to the CT images and was used to estimate the position of the anatomical structures. Voxels, enclosed by ST and SV, were determined using segmented meshes. These voxels were automatically counted and cochlear volume was calculated by multiplying number of enclosed voxels by voxel size.
Electrode Location
The primary outcome measure of interest was scalar electrode location. Methodology for radiographically determining electrode location in relation to ST and SV has been previously reported. Briefly, an automated method described above is used to identify the ST and SV. Each contact on the electrode array was localized in post-operative CT using either semi-automated or fully automated approaches we developed.9, 10 This approach for determining scalar location of each contact has been validated using cadaveric models.11 For convention in this report, electrode arrays with any contact in the SV were termed SV insertion. Conversely, if all intracochlear electrode contacts were located outside of the SV, the insertion was characterized as ST insertion. Angular insertion depth of the electrode array was automatically measured as the maximum angular depth of all of the contacts using the coordinate system defined by Verbist et al.12
Postoperative Audiometric Performance
The results of postoperative consonant-nucleus-consonant (CNC) word and AzBio sentence scores performed between 12–16 months postoperatively were recorded. Testing was performed in quiet, with a unilateral CI in the test ear. Speech recognition performance was assessed using recorded stimuli at a calibrated presentation level of 60 dBA. Patients tested outside the 12–16 month postoperative period were excluded from these analyses to limit bias introduced by varying duration of CI use.
Statistical Analysis
Both univariate analyses and multivariate analyses were performed. For univariate comparisons, Fisher’s exact test, chi-square tests, two-tailed t-tests, and Mann-Whitney tests were used to compare parametric and non-parametric continuous data, as appropriate. Analysis of variance (ANOVA) with post-hoc comparison analysis was performed when indicated. Pearson or Spearman tests were performed to assess correlations of parametric and non-parametric variables. Two multivariate regression analyses were performed to identify independent predictors of primary outcome measures of interest: 1) scalar electrode location and 2) CNC scores. Electrode type, surgical approach, cochlear volume, and angular insertion depth were included in the multivariate analyses because controlling for these variables was felt to be clinically relevant. Other covariates were included in the multivariate analysis if the p<0.10 on univariate analysis. All analyses were performed with GraphPad Prism 6.0 (GraphPad Software, Inc., La Jolla, CA) or STATA 12MP (StataCorp LP, College Station, TX).
RESULTS
Demographics and Operative Characteristics
The 184 patients (220 implants) included were predominantly male (58.7%), Caucasian (93.0%), and had a mean age at the time of CI of 60.2 years. In order of frequency, electrode types implanted were PM (52.3%), LW (41.3%), and MS (6.4%). The details regarding manufacturer and model for LW electrodes are listed in Table 1. Approaches used were RW (38.7%), ERW (34.5%), and C (26.8%).
Table 1.
Details regarding manufacturer and electrode array for electrodes included in this study.
| Electrode Array | n (%) | |
|---|---|---|
| MED-EL | Flex 28 | 28 (12.7%) |
| Standard | 17 (7.7%) | |
| Flex 24 | 4 (1.8%) | |
| Combi 40+ | 1 (0.5%) | |
| Medium | 1 (0.5%) | |
| COCHLEAR AMERICAS | Contour Advance | 115 (52.3%) |
| Slim Straight | 19 (8.6%) | |
| ADVANCED BIONICS | 1J | 21 (9.5%) |
| Mid-Scala | 14 (6.4%) | |
Cochlear Volume
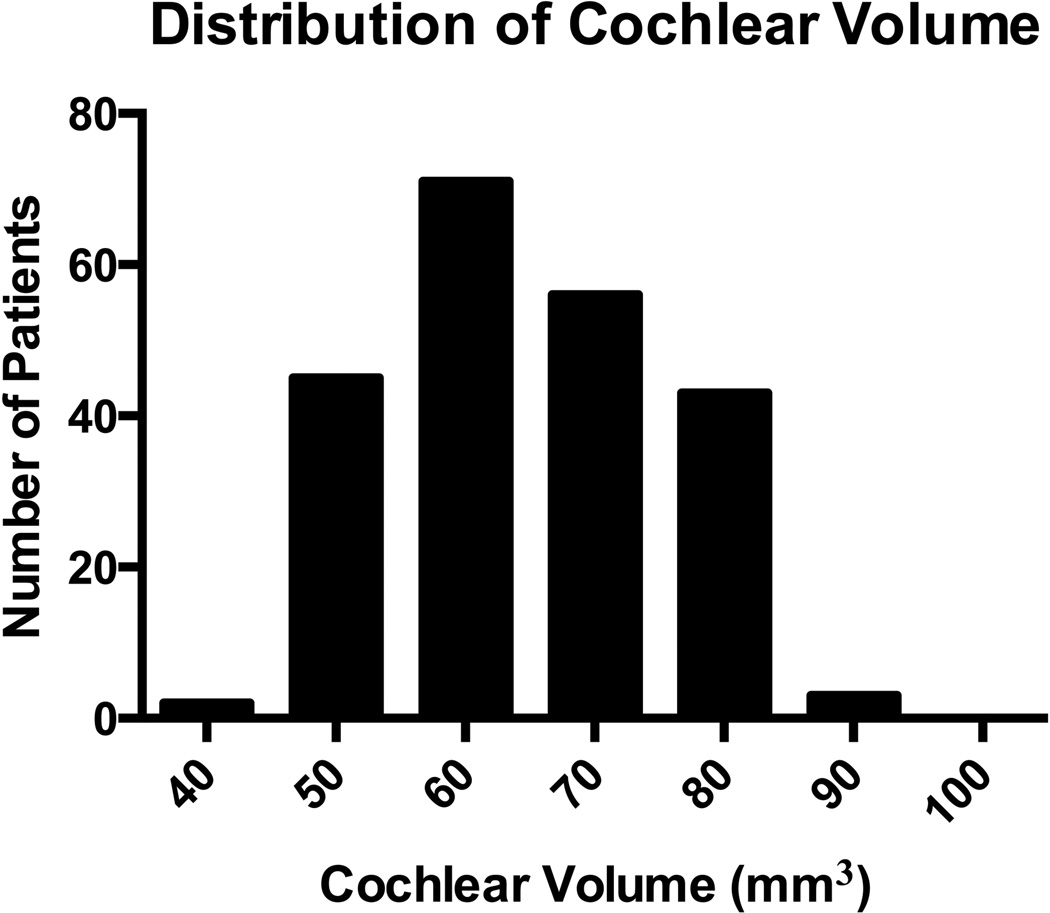
Figure 1 shows the cochlear volume distribution among included patients. Mean cochlear volume was 64.7 ± 10.5 mm3 (range 42.3–90.2 mm3).
Figure 1.
Distribution of cochlear volume amongst all enrolled patients.
Angular Insertion Depth
The mean angular insertion depth in the entire cohort was 420 ± 99° (range 208.1 – 714.8°). No differences in angular depth of insertion were found in relation to age, gender, race, or cochlear volume (p>0.05). Differences in angular insertion depth were observed as a function of electrode type (p<0.0001). Post-hoc analysis demonstrated greater angular insertion depths for LW electrodes (469 ± 117°) compared to PM electrodes (384 ± 59°)(p<0.0001). Angular insertion depth for MS (393 ± 95°) did not significantly differ from either LW or PM (p>0.05). Angular insertion depth also varied depending on approach (p=0.01). Greater angular insertion depths were observed for RW insertions (450 ± 114°) compared to ERW insertions (396.7 ± 78°)(p=0.02). No difference in insertion depth between C (403 ± 83°), and either RW or ERW approaches, was apparent (p>0.05).
Electrode Location
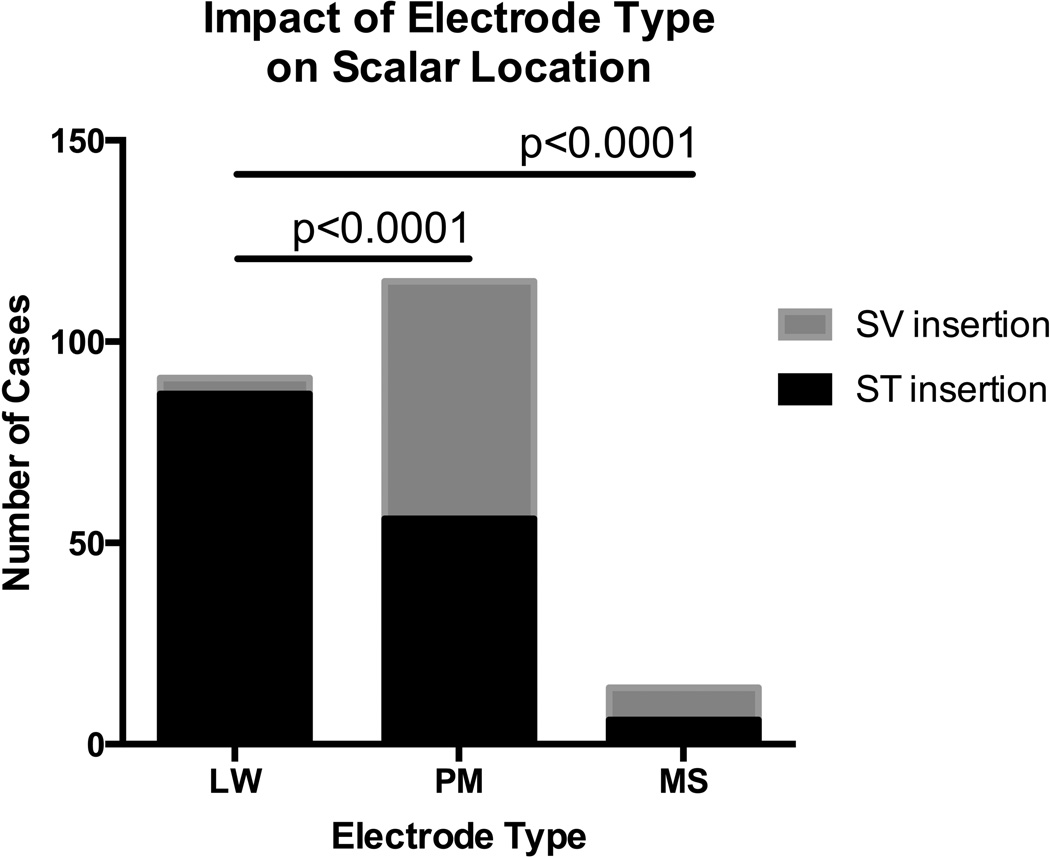
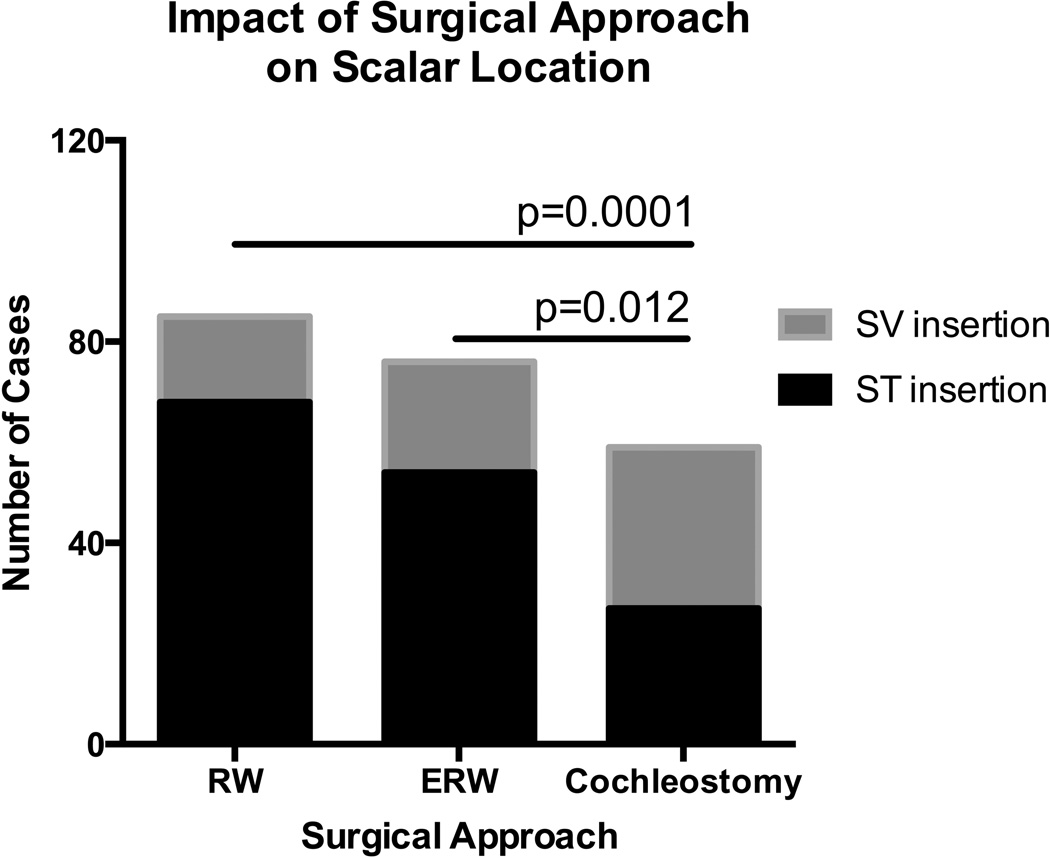
Scala tympani insertion was observed in 68% of implants; the remaining 32% of electrode arrays had at least one contact located in the SV. Univariate analyses of covariate associations with electrode location are shown in Table 2. In regards to electrode type, higher rates of ST insertion were observed with LW (95.6%) electrodes compared to either PM (48.7%) or MS (42.9%) electrodes (p<0.0001)(Figure 2). With respect to surgical approach, RW (80.0%) and ERW (71.1%) approaches were more likely to result in ST insertion when compared to C (45.8%)(p=0.0001 and 0.012, respectively)(Figure 3). ST insertions were also associated with greater angular insertion depths compared to SV insertions (p=0.02).
Table 2.
Univariate analysis of variables that impact scalar electrode location. Scala tympani insertions were defined as having all electrode contacts within the scala tympani. Conversely, electrode arrays with any contact in the scala vestibuli were characterized as scala vestibuli insertions.
| Scala tympani insertion |
Scala vestibule insertion |
p value | |
|---|---|---|---|
| N= 149 | N= 71 | ||
| Mean ± SD | Mean ± SD | ||
| Age | 60.1 ± 16.2 | 60.4 ± 13.9 | 0.90 |
| Cochlear Volume (mm3) | 64.8 ± 11.0 | 64.7 ± 9.1 | 0.94 |
| Angular Insertion Depth (°) | (438 ± 111°) | (385 ± 52°) | 0.02 |
| n (%) | n (%) | ||
| Gender | |||
| Male | 84 (56.4%) | 41 (57.7%) | 0.96 |
| Female | 65 (43.6%) | 30 (42.3%) | |
| Race | |||
| Caucasian | 137 (91.9%) | 65 (91.5%) | 0.92 |
| Other | 12 (8.1%) | 6 (8.5%) | |
| Type of Electrode | |||
| Lateral wall | 87 (58.4%) | 4 (5.6%) | <0.0001 |
| Perimodiolar | 56 (37.6%) | 59 (83.1%) | |
| Mid Scala | 6 (4.0%) | 8 (11.3%) | |
| Surgical Approach | |||
| Round window | 68 (45.6%) | 17 (23.9%) | <0.0001 |
| Extended round window | 54 (36.2%) | 22 (31.0%) | |
| Cochleostomy | 27 (18.1%) | 32 (45.1%) | |
Figure 2.
Higher rates of scala tympani insertion were observed for lateral wall electrodes when compared to both perimodiolar and Mid-Scala electrodes. LW- lateral wall, PM- perimodiolar, MS- Mid-Scala, ST- scala tympani, SV- scala vestibuli.
Figure 3.
Round window and extended round window surgical approaches were associated with higher rates of scala tympani insertion when compared to cochleostomy. RW- round window, ERW- extended round window, ST- scala tympani, SV- scala vestibuli.
Multivariate logistic regression analysis was then performed which demonstrated that electrode type and surgical approach were independently predictive of scalar electrode location. PM and MS electrodes were 22.4 (95%CI 6.3–80.0, p<0.001) and 55.0 (95%CI: 9.7–312.8, p<0.001) times more likely to have at least one electrode contact in the SV when compared to LW electrodes, respectively (Table 3). When compared to C approaches, RW and ERW approaches demonstrated approximately 70% reduction in SV insertion (RW OR 0.29, 95%CI 0.1–0.8, p=0.01; ERW 0.28, 95%CI 0.1–0.7, p=0.006).
Table 3.
Multivariate logistic regression analyzing factors that impact scalar electrode location. When controlling for cochlear volume and angular insertion depth, electrode type and surgical approach were independently predictive of scalar electrode location.
| Scala Vestibuli Insertion | Odds Ratio | 95% Confidence Interval | p value |
|---|---|---|---|
| Cochlear Volume (mm3) | 0.98 | 0.95 – 1.02 | 0.39 |
| Angular Insertion Depth (°) | 0.99 | 0.99 – 1.00 | 0.26 |
| Electrode Type | |||
| Lateral Wall | Reference | ||
| Perimodiolar | 22.40 | 6.28 – 79.96 | <0.001 |
| Mid Scala | 54.96 | 9.66 – 312.82 | <0.001 |
| Surgical Approach | |||
| Cochleostomy | Reference | ||
| Round Window | 0.29 | 0.11 – 0.77 | 0.01 |
| Extended Round Window | 0.28 | 0.12 – 0.69 | 0.006 |
Cochlear Implant Performance
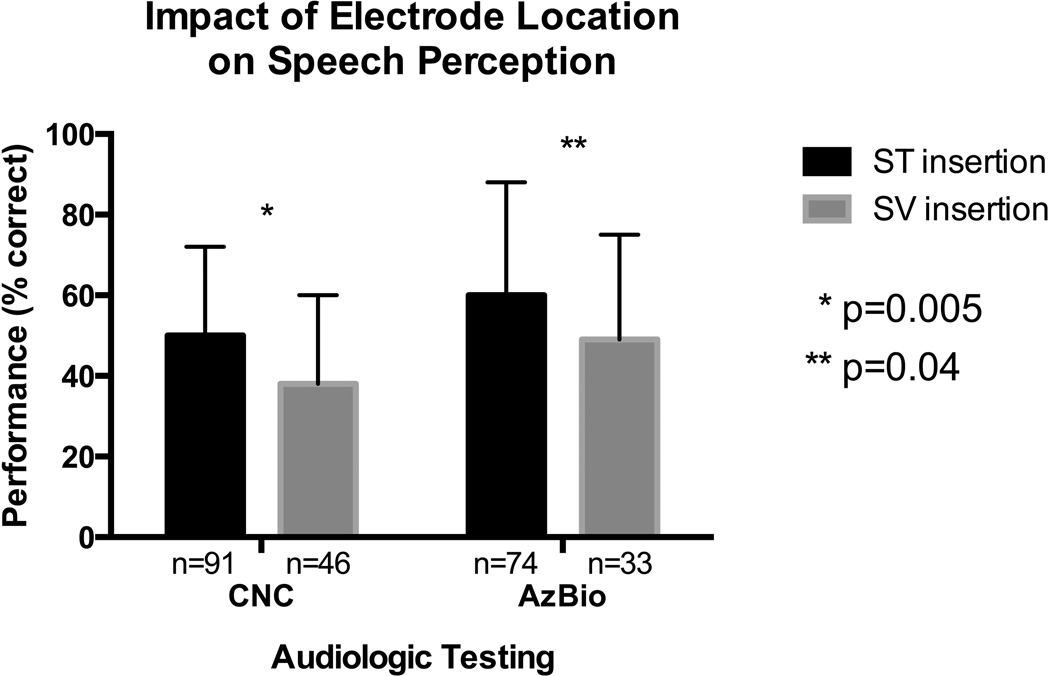
Audiologic outcomes were assessed 12–16 months (mean 13.1 ± 1.0 months) postoperatively. CNC scores were available for 137 patients with a mean postoperative score of 46.6% ± 23.0. In this subset of patients, distribution of electrode type (PM- 54.8%, LW- 39.4%, MS- 5.8%) and rate of ST insertion (66.4%) were similar to that observed in the entire cohort. In univariate analysis, CNC scores were not significantly associated with the following variables: age, gender, race, electrode type, surgical approach, and cochlear volume (p>0.05). When examining the impact of scalar electrode location of performance, the average CNC score was significantly higher for ST insertions when compared to SV insertions (50.5% vs. 38.9%; p=0.005)(Figure 4). A significant positive correlation was observed between angular insertion depth and CNC scores (r=0.23, p=0.006).
Figure 4.
Better performance on CNC and AzBio testing was noted for ST insertions when compared to SV insertions at 12–16 months postoperatively. ST- scala tympani, SV- scala vestibuli, CNC- consonant-nucleus-consonant.
AzBio scores were available for 107 patients at 12–16 months, with a mean score of 57.1% ± 28.1. In univariate analysis, AzBio scores were not significantly associated with the following variables: gender, race, electrode type, surgical approach, angular insertion depth, and cochlear volume. An inverse relation between age and AzBio scores was evident (r=−0.29, p=0.003). Similar to that observed with CNC performance, AzBio scores were statistically better with ST insertions than SV insertions (60.5% vs. 49.6%; p=0.04)(Figure 4).
Multivariate linear regression was then used to identify factors independently associated with postoperative CNC performance (Table 4). CNC score decreased 0.3% for each increase in age of 1 year (Coefficient -0.003, 95% CI: -0.006 - -0.0006, p=0.02). CNC score increased 0.6% with every 10° increase in angular insertion depth (Coefficient 0.0006, 95% CI: 0.0002-0.001, p=0.009). Lastly, SV insertion was associated a 12% decrease in CNC score (Coefficient -0.12, 95% CI: -0.22 - -0.03, p=0.01).
Table 4.
Multivariate linear regression analyzing factors that independently predict CNC performance at 12–16 months postoperatively. CNC- consonant-nucleus-consonant.
| CNC Score | Coefficient | 95% Confidence Interval | p value |
|---|---|---|---|
| Age | −0.003 | −0.006 – −0.0006 | 0.02 |
| Cochlear Volume (mm3) | −0.002 | −0.006 – −0.001 | 0.23 |
| Angular Insertion Depth (°) | 0.0006 | 0.0002 – 0.001 | 0.009 |
| Electrode Type | |||
| Lateral Wall | Reference | ||
| Perimodiolar | 0.07 | −0.03 – 0.17 | 0.18 |
| Mid Scala | 0.11 | −0.06 – 0.28 | 0.20 |
| Surgical Approach | |||
| Cochleostomy | Reference | ||
| Round Window | 0.003 | −0.10 – 0.10 | 0.95 |
| Extended Round Window | 0.08 | −0.02 – 0.19 | 0.12 |
| Scala Vestibuli Insertion | −0.12 | −0.22 – −0.03 | 0.01 |
DISCUSSION
This study was primarily designed to identify variables that impact scalar electrode position while controlling for cochlear volume and angular insertion depth. The data presented herein shows that LW electrodes, and both RW and ERW approaches are independent predictors of ST insertion. With 220 implants included in these analyses, the current study is the largest to date examining factors that influence CI scalar electrode position in vivo. In addition, we sought to examine the relation between the scalar electrode position and postoperative speech perception performance during a defined time period postoperatively, specifically 12–16 months after surgery. Our results indicate that younger age at the time of CI, having all electrode contacts within the ST, and greater angular insertion depth are associated with better postoperative speech perception.
Cochlear Volume and Angular Insertion Depth
Variability in human cochlea size has been previously demonstrated.13 We elected to examine cochlea volume, as it represents the entire three-dimensional space in which CI electrodes reside. While we suspected that smaller cochlear volumes would predispose to interscalar translocation and thus higher rates of SV insertion, no relation between cochlear volume and scalar electrode location was evident.
The finding that angular insertion depth was not associated with scalar electrode position in our multivariate model deserves mention. Insertion depth, whether measured by linear insertion length or angular insertion depth, is a descriptor of intracochlear electrode array position. We chose to specifically use an angular measurement as it accounts for variance in both cochlea size and linear insertion depth. As expected, LW electrodes demonstrated greater mean angular insertion depths than either PM or MS electrode designs. When controlling for electrode design and surgical approach, multivariate analysis revealed no association between angular insertion depth and electrode scalar location.
In contrast to this finding, other studies suggest that a deeper insertion impacts scalar position and intracochlear trauma. Finley et al. suggested that insertion depth impacts scalar position and number of electrodes in the SV.5 However, all of the 14 patients in the study had at least one electrode located within the SV. For this reason, conclusions regarding the impact of depth of insertion on rates of ST versus SV insertion cannot be made. Adunka et al. studied insertion of MED-EL devices through C approaches in 21 temporal bones.14 While deeper insertions were associated with increased trauma, deeper insertions in this analysis were all characterized by intentional, forceful insertion beyond the point at which electrode resistance was encountered. In fact, forceful insertion was continued until basal electrode buckling made further advancement impossible. However, when controlling for a soft technique, deep insertions did not result in increased traumatization. This latter finding, which is congruent with our results, is likely most consistent with current clinical practice in which surgeons aim to prevent electrodes from buckling by inserting them no further than the point of first resistance. Lastly, Radeloff et al. showed that interscalar translocation into the SV was associated with greater angular insertion depths for PM electrodes inserted via C approaches in 18 temporal bones.15 No statistically significant relation between angular depth of insertion and electrode location was evident for 28 LW electrodes. Perhaps the fact that this study was performed in cadaveric specimens accounts for differences when compared to our findings.
Impact of Electrode Type and Surgical Approach on Scalar Electrode Location
Both electrode type and surgical approach were found to be independent predictors of scalar electrode location. It should be emphasized that ST insertion was achieved in almost all cases when LW electrodes were implanted (95.6%). Conversely, these rates were considerably lower for PM (48.7%) and MS (42.9%) electrode arrays. The superiority of LW electrodes in this regard is further highlighted by multivariate analysis showing that PM and MS electrodes were 22 and 55 times as likely, respectively, to have at least one electrode contact within the SV when compared to LW electrodes. One explanation for this finding is that the flexible mechanical properties of LW electrodes used in this study make it very unlikely for the electrode tip to penetrate through the basilar and Reissner’s membranes.16 Other studies have examined the impact of LW and PM electrode array designs on ST insertion, with similar results favoring the LW design.2, 3
To our knowledge, this is the first study examining rates of ST insertion with MS electrodes in a clinical setting. It should be emphasized that the MS group was considerably smaller than either the LW or PM groups, therefore definitive conclusions regarding electrode location outcomes for MS arrays cannot be made. Nevertheless, we observed that 8 of 14 (57.1%) MS arrays had electrodes located in SV, which was comparable to PM designs but statistically worse than LW. Frisch et al. and Hassepass et al. have examined the position of MS electrodes in cadaveric temporal bones, with a combined SV electrode position rate of 7%.17, 18 In light of these discrepancies between our clinical findings and those of cadaveric studies, in addition to the small number of MS implants included in our study, further investigation of the MS electrode design and its position within the scala is needed.
Our data also show that surgical approach is an independent predictor for scalar electrode location. Specifically, RW and ERW approaches demonstrated an approximate 70% reduction in rate of having an electrode contact located in SV when compared to C approaches. With nearly twice the number of implants included in the present cohort, this finding is consistent with prior studies that have demonstrated higher rates of SV insertion with C approaches.2 Despite variability in cochleostomy location that is likely present amongst even experienced surgeons and may contribute to higher rates of electrode locations outside the ST, the authors feel the current multivariate results convincingly support the use of either a RW or ERW approach.
In summary, structural damage can be inferred when an electrode translocates scala or is inserted directly into the SV. In the context of the data herein, the authors feel that strong consideration should be given to the use of LW electrodes, implanted via either RW or ERW approaches when possible. Given the small number of MS patients in this study, further studies are needed to definitively determine the relation between the MS design and scalar electrode location.
Postoperative Speech Perception Performance
Ultimately, postoperative speech understanding is of greatest interest to both clinicians and patients. Other groups have demonstrated that SV placement adversely impacts performance on postoperative monosyllabic word score testing when compared to ST insertions.2, 5, 6 The results of this study corroborate this finding, and show that electrode placement entirely within the ST is an independent predictor of better performance on CNC testing at 12–16 months postoperatively. Several possible mechanisms may explain why SV placement impacts outcomes. For one, electrode translocation into the SV damages the basilar membrane and Reissner’s membrane, and likely injures the osseous spiral lamina and spiral ligament.19 Holden et al. further postulate that while monopolar-coupled electrodes located in the ST likely stimulate ganglion cells in the immediate scalar turn, if such electrodes are positioned within the SV they are more likely to stimulate ganglion cells in the next, more-apical turn.6 This may contribute to cross-turn stimulation, resulting in pitch confusion and diminished speech perception. Taken together, every effort should be undertaken to achieve positioning of electrodes within the ST to maximize optimal clinical outcomes.
Interestingly, multivariate analysis also identified that angular insertion depth was an independent predictor for improved CNC word score performance postoperatively. The rationale for this finding is that deeper insertions of electrode arrays distribute coverage over the entire length of the cochlea, including the apical region, which in turn extends the range of pitch percepts obtainable and may confer speech perception benefit. It should be noted the positive linear correlation herein is modeled for insertion depths beyond the minimal insertion angle for this cohort (208°), and it does not represent the relationship across the entire spectrum insertion angles. While there is no consensus in the literature regarding insertion depth and speech perception performance, other investigators have similarly shown that greater insertion depths result in favorable outcomes.20–22 The most compelling evidence in support of the positive correlation between angular depth of insertion and performance was reported by Buchman et al.23 In a prospective study of 13 adults randomized to receive either standard or medium-length MED-EL electrode arrays, a trend towards better speech perception with longer electrodes (and greater angular insertion depth) was observed (p=0.07). Interim analysis of their data was performed, retrospectively adding 6 additional subjects that were not part of the prospective protocol, and statistical significance was reached; given the apparent benefit conferred by longer electrodes, the study was discontinued by the IRB for ethical reasons.
Contrary to these findings, Van der Marel et al. reported no relation between depth of insertion and speech perception in 100 post-lingual adults.24 All patients received a LW electrode from a single manufacturer, therefore less variance in insertion depth may explain why their study failed to find any relation between depth and performance. Lastly, Finley et al. and Holden et al. demonstrated a negative correlation between insertion depth and postoperative word recognition.5
Younger age at the time of CI was also found to correlate with improved post-operative CNC performance. This was not entirely surprising, yet other studies examining the relation between age and postoperative speech perception have yielded conflicting results. Green et al. and Leung et al. did not demonstrate a correlation between age at CI and post-implant audiologic performance, while other groups have shown that younger patients perform significantly better.5, 25–27 One possible explanation supporting this latter notion is that diminished cognition, specifically central auditory processing abilities, may contribute to poorer performance in older patients.6 The discrepant results amongst studies analyzing postoperative performance reinforce the notion that multiple factors likely influence speech understanding after CI, and not all studies can account for variability introduced by each factor.6 Further analysis of factors that limit speech understanding will be important for both patient counseling prior to CI and continued modification of rehabilitative strategies after CI.
While the current study is the largest to date examining factors that influence scalar electrode location, there are certainly limitations. Given the fact that patients were not randomized to either electrode type or surgical approach, the study is subject to selection bias. Currently, randomized prospective studies are ongoing to assess these relations. In regards to postoperative speech perception analysis, variables not included in our multivariate analysis due to the retrospective nature of the study may influence postoperative speech perception and confound our findings. These include, but are not limited to, cognitive status, duration of deafness, preoperative performance, patient motivation, and programming strategies. Given the fact that all patients were post-lingual adults who failed an appropriate hearing aid trial, we feel that variability introduced by inability to control for factors such as duration of deafness is minimized.
CONCLUSION
This study examined factors that impact intracochlear electrode position and postoperative speech perception in 220 ears undergoing CI. The results demonstrate that surgical approach and electrode type are independent predictors of ST insertion in vivo. Specifically, LW electrodes are associated with higher rates of ST insertion compared to PM and MS electrodes. In addition, RW and ERW approaches resulted in higher rates of ST insertion when compared to C approaches. In regards to postoperative speech perception, ST insertion, greater angular insertion depths, and younger age positively correlated with improved monosyllabic word scores 12–16 months after CI. These data suggest that part of the variability in speech recognition after CI can be accounted for by factors related to intracochlear electrode position.
Acknowledgments
FINANCIAL MATERIAL AND SUPPORT: The project was supported by grants R01DC008408, R01DC014462, and R01DC014037 from the National Institute on Deafness and Other Communication Disorders and UL1TR000445 from the National Center for Advancing Translational Sciences. The content is solely the responsibility of the authors and does not represent the official views of these institutes.
IRB APPROVAL: This study was approved by the Vanderbilt IRB #090155.
Footnotes
CONFLICTS OF INTEREST/DISCLOSURES: Dr. Wanna is a consultant for MED-EL, Advanced Bionics, Oticon Medical, and Cochlear Americas. Dr. Rivas is a consultant for MED-EL, Advanced Bionics, Cochlear Americas, Stryker and Grace Medical. Dr. Labadie is a consultant for Advanced Bionics, Medtronic, and Ototronix.
This paper will be presented at Combined Otolaryngology Spring Meetings: May 18–22 in Chicago, Illinois and won the AOS 2016 Resident Research Award.
REFERENCES
- 1.Adunka O, Kiefer J, Unkelbach MH, Radeloff A, Gstoettner W. Evaluating cochlear implant trauma to the scala vestibuli. Clin Otolaryngol. 2005;30(2):121–127. doi: 10.1111/j.1365-2273.2004.00935.x. [DOI] [PubMed] [Google Scholar]
- 2.Wanna GB, Noble JH, Carlson ML, et al. Impact of electrode design and surgical approach on scalar location and cochlear implant outcomes. Laryngoscope. 2014;124(Suppl 6):S1–S7. doi: 10.1002/lary.24728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Boyer E, Karkas A, Attye A, Lefournier V, Escude B, Schmerber S. Scalar localization by cone-beam computed tomography of cochlear implant carriers: a comparative study between straight and periomodiolar precurved electrode arrays. Otol Neurotol. 2015;36(3):422–429. doi: 10.1097/MAO.0000000000000705. [DOI] [PubMed] [Google Scholar]
- 4.Aschendorff A, Kromeier J, Klenzner T, Laszig R. Quality control after insertion of the nucleus contour and contour advance electrode in adults. Ear Hear. 2007;28(2 Suppl):75S–79S. doi: 10.1097/AUD.0b013e318031542e. [DOI] [PubMed] [Google Scholar]
- 5.Finley CC, Holden TA, Holden LK, et al. Role of electrode placement as a contributor to variability in cochlear implant outcomes. Otol Neurotol. 2008;29(7):920–928. doi: 10.1097/MAO.0b013e318184f492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Holden LK, Finley CC, Firszt JB, et al. Factors affecting open-set word recognition in adults with cochlear implants. Ear Hear. 2013;34(3):342–360. doi: 10.1097/AUD.0b013e3182741aa7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wanna GB, Noble JH, Gifford RH, et al. Impact of Intrascalar Electrode Location, Electrode Type, and Angular Insertion Depth on Residual Hearing in Cochlear Implant Patients: Preliminary Results. Otol Neurotol. 2015;36(8):1343–1348. doi: 10.1097/MAO.0000000000000829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nordfalk KF, Rasmussen K, Hopp E, Greisiger R, Jablonski GE. Scalar position in cochlear implant surgery and outcome in residual hearing and the vestibular system. Int J Audiol. 2014;53(2):121–127. doi: 10.3109/14992027.2013.854413. [DOI] [PubMed] [Google Scholar]
- 9.Zhao Y, Dawant BM, Labadie RF, Noble JH. Automatic localization of cochlear implant electrodes in CT. Med Image Comput Comput Assist Interv. 2014;17(Pt 1):331–338. doi: 10.1007/978-3-319-10404-1_42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Noble JH, Dawant BM. Automatic graph-based localization of cochlear implant electrodes in CT. Lecture Notes in Computer Science - Proceedings of MICCAI. 2015;9350:152–159. doi: 10.1007/978-3-319-24571-3_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schuman TA, Noble JH, Wright CG, Wanna GB, Dawant B, Labadie RF. Anatomic verification of a novel method for precise intrascalar localization of cochlear implant electrodes in adult temporal bones using clinically available computed tomography. Laryngoscope. 2010;120(11):2277–2283. doi: 10.1002/lary.21104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Verbist BM, Skinner MW, Cohen LT, et al. Consensus panel on a cochlear coordinate system applicable in histologic, physiologic, and radiologic studies of the human cochlea. Otol Neurotol. 2010;31(5):722–730. doi: 10.1097/MAO.0b013e3181d279e0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Erixon E, Hogstorp H, Wadin K, Rask-Andersen H. Variational anatomy of the human cochlea: implications for cochlear implantation. Otol Neurotol. 2009;30(1):14–22. doi: 10.1097/MAO.0b013e31818a08e8. [DOI] [PubMed] [Google Scholar]
- 14.Adunka O, Kiefer J. Impact of electrode insertion depth on intracochlear trauma. Otolaryngol Head Neck Surg. 2006;135(3):374–382. doi: 10.1016/j.otohns.2006.05.002. [DOI] [PubMed] [Google Scholar]
- 15.Radeloff A, Mack M, Baghi M, Gstoettner WK, Adunka OF. Variance of angular insertion depths in free-fitting and perimodiolar cochlear implant electrodes. Otol Neurotol. 2008;29(2):131–136. doi: 10.1097/MAO.0b013e318157f0ea. [DOI] [PubMed] [Google Scholar]
- 16.Hochmair I, Hochmair E, Nopp P, Waller M, Jolly C. Deep electrode insertion and sound coding in cochlear implants. Hear Res. 2015;322:14–23. doi: 10.1016/j.heares.2014.10.006. [DOI] [PubMed] [Google Scholar]
- 17.Frisch CD, Carlson ML, Lane JI, Driscoll CL. Evaluation of a new mid-scala cochlear implant electrode using microcomputed tomography. Laryngoscope. 2015 doi: 10.1002/lary.25347. [DOI] [PubMed] [Google Scholar]
- 18.Hassepass F, Bulla S, Maier W, et al. The new mid-scala electrode array: a radiologic and histologic study in human temporal bones. Otol Neurotol. 2014;35(8):1415–1420. doi: 10.1097/MAO.0000000000000412. [DOI] [PubMed] [Google Scholar]
- 19.Skinner MW, Holden TA, Whiting BR, et al. In vivo estimates of the position of advanced bionics electrode arrays in the human cochlea. Ann Otol Rhinol Laryngol Suppl. 2007;197:2–24. [PubMed] [Google Scholar]
- 20.Skinner MW, Ketten DR, Holden LK, et al. CT-derived estimation of cochlear morphology and electrode array position in relation to word recognition in Nucleus-22 recipients. J Assoc Res Otolaryngol. 2002;3(3):332–350. doi: 10.1007/s101620020013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yukawa K, Cohen L, Blamey P, Pyman B, Tungvachirakul V, O'Leary S. Effects of insertion depth of cochlear implant electrodes upon speech perception. Audiol Neurootol. 2004;9(3):163–172. doi: 10.1159/000077267. [DOI] [PubMed] [Google Scholar]
- 22.Hochmair I, Arnold W, Nopp P, Jolly C, Muller J, Roland P. Deep electrode insertion in cochlear implants: apical morphology, electrodes and speech perception results. Acta Otolaryngol. 2003;123(5):612–617. [PubMed] [Google Scholar]
- 23.Buchman CA, Dillon MT, King ER, Adunka MC, Adunka OF, Pillsbury HC. Influence of cochlear implant insertion depth on performance: a prospective randomized trial. Otol Neurotol. 2014;35(10):1773–1779. doi: 10.1097/MAO.0000000000000541. [DOI] [PubMed] [Google Scholar]
- 24.van der Marel KS, Briaire JJ, Verbist BM, Muurling TJ, Frijns JH. The influence of cochlear implant electrode position on performance. Audiol Neurootol. 2015;20(3):202–211. doi: 10.1159/000377616. [DOI] [PubMed] [Google Scholar]
- 25.Friedland DR, Runge-Samuelson C, Baig H, Jensen J. Case-control analysis of cochlear implant performance in elderly patients. Arch Otolaryngol Head Neck Surg. 2010;136(5):432–438. doi: 10.1001/archoto.2010.57. [DOI] [PubMed] [Google Scholar]
- 26.Green KM, Bhatt Y, Mawman DJ, et al. Predictors of audiological outcome following cochlear implantation in adults. Cochlear Implants Int. 2007;8(1):1–11. doi: 10.1179/cim.2007.8.1.1. [DOI] [PubMed] [Google Scholar]
- 27.Leung J, Wang NY, Yeagle JD, et al. Predictive models for cochlear implantation in elderly candidates. Arch Otolaryngol Head Neck Surg. 2005;131(12):1049–1054. doi: 10.1001/archotol.131.12.1049. [DOI] [PubMed] [Google Scholar]