Abstract
CD36 is a multifunctional immuno-metabolic receptor with many ligands. One of its physiological functions in the heart is the high-affinity uptake of long-chain fatty acids (FAs) from albumin and triglyceride rich lipoproteins. CD36 deletion markedly reduces myocardial FA uptake in rodents and humans. The protein is expressed on endothelial cells and cardiomyocytes and at both sites is likely to contribute to FA uptake by the myocardium. CD36 also transduces intracellular signaling events that influence how the FA is utilized and mediate metabolic effects of FA in the heart. CD36 mediated signaling regulates AMPK activation in a way that adjusts oxidation to FA uptake. It also impacts remodeling of myocardial phospholipids and eicosanoid production, effects exerted via influencing intracellular calcium (iCa2+) and the activation of phospholipases. Under excessive FA supply CD36 contributes to lipid accumulation, inflammation and dysfunction. However, it is also important for myocardial repair after injury via its contribution to immune cell clearance of apoptotic cells. This review describes recent progress regarding the multiple actions of CD36 in the heart and highlights those areas requiring future investigation.
Keywords: fatty acid, uptake, signaling, mitochondria, AMPK, LKB1
1. Introduction: Diverse pathways coordinate energy regulation
At the heart of the cell's most basic functions is the need for energy and this is particularly true of the cardiomyocyte. Numerous cellular pathways are normally dedicated to energy procurement or usage and to regulating interactions with the extracellular environment and other cells in order to maintain energy homeostasis. The surface receptor cluster of differentiation 36 (CD36) has a regulatory role in energy metabolism through its ability to recognize long chain fatty acids (FAs), promoting accumulation of intracellular free FA (FFAs) [2]. In addition CD36-mediated signaling facilitates, in a cell type or context dependent manner, FA storage or usage [3]. CD36 binds multiple ligands and resides within transmembrane or membrane-associated functional protein clusters [4-6]. Ligand interaction with CD36 can differentially induce changes in protein-protein interactions within these molecular clusters and consequently alter downstream signaling. This way FAs induce CD36-mediated signals that influence FA oxidation [1] and/or raise cytosolic calcium, triggering production of mediators that impact behavior of distant cells [7-9]. Some CD36 ligands, e.g. extracellular matrix components, influence cell-environment responses and processes such as cell adhesion/migration and angiogenesis [5] that indirectly relate to tissue energetics [10]. The protein is also a receptor for modified lipid moieties [11], contributing to uptake of oxidized low density lipoproteins (oxLDL), [12] or to apoptotic cell clearance [13, 14]. It functions as a co-receptor for Toll-like Receptors (TLRs) in recognizing pathogen-associated lipids and facilitating the acute inflammation that is part of the innate immune response [15]. But it also contributes to inflammation resolution [13]. This review will focus on only those CD36-mediated processes that appear to affect heart metabolism and function and will attempt to highlight controversy and biology that require future investigation.
2. Heart FA uptake: Metabolic flexibility and the evolving role of CD36
Chronically active muscles including the heart utilize large amounts of ATP, much of which is acquired from circulating lipids. Predominantly this includes FFAs associated with albumin as well as FFAs released from very low density lipoproteins (VLDL) or from intestinally derived chylomicrons via lipoprotein lipase (LpL)-mediated enzymatic cleavage of the triacylglycerol (TG) ester bonds [2, 16]. During fasting, as circulating insulin decreases, FFA levels markedly increase in the bloodstream reflecting activation within adipocytes of two intracellular lipases, adipose TG lipase (ATGL) and hormone sensitive lipase (HSL). Uptake of FFAs by tissues increases in parallel with the rise in FFA levels. In particular, muscle and heart reduce their glucose use and rely primarily on FA catabolism for energy.
Cellular uptake of FFA by cardiomyocytes
FFAs can rapidly move across the plasma membrane [17] but whether this process is sufficient to provide the high rates of FA uptake necessary for cells active in FA metabolism has been questioned [18]. Studies in many mammalian cell types using FA complexed to albumin at increasing molecular ratios to vary concentration of unbound FA, yielded evidence for two distinct pathways of FA uptake, a low capacity saturable component and a high capacity non-saturable component. The saturable pathway has kinetics consistent with protein-facilitation: saturability, high affinity for long chain FAs (Km around 10 nanomolar within the concentration range of unbound FA in the circulation) and sensitivity to protein modifying agents [19]. The non-saturable pathway would operate at high ratios of FA: albumin and unbound FA concentrations above those found in the circulation, for example such as occurs with hydrolysis of TG-rich lipoproteins by LpL along the capillary surface or following hydrolysis of dietary TG by pancreatic lipase in the small intestinal lumen [20, 21].
In cardiomyocytes, high affinity saturable FA uptake supporting a protein mediated process has been described [22-24]. One study that monitored appearance of intracellular FAs as a function of exogenous FA levels suggested that FA uptake is an energy consuming process that can occur against the FA concentration gradient [25].
Heart FA uptake from albumin and TG rich lipoproteins
CD36 was identified as a cellular FA transporter in 1993 based on work with isolated adipocytes [26], and physiological relevance of this function, especially as related to heart metabolism, has been documented by numerous studies [2, 16, 27]. CD36 was shown to traffic between the cell surface and intracellular compartments and is recruited to the sarcolemma by insulin and AMPK in a vesicle-mediated process [27, 28] controlled by the Akt substrate 160 (AS160) Rab GTPase-activating protein (RabGAP AS160) and its target GTPase, Rab8a [29].
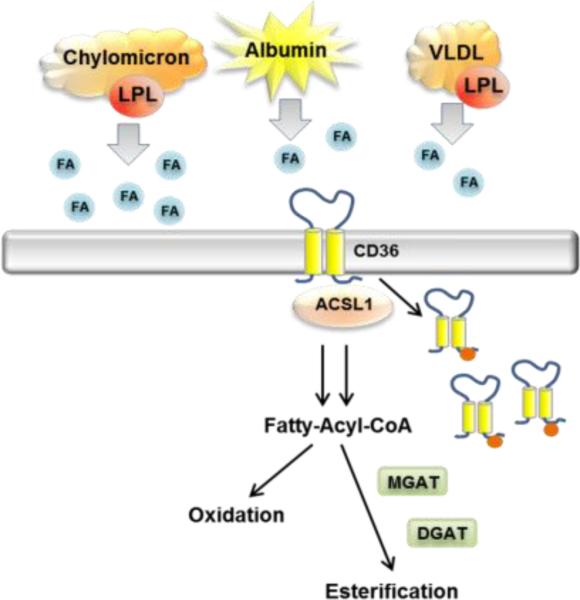
Uptake of FFAs into the heart has been studied using isolated hearts and whole animals. Several lines of evidence support the hypothesis that CD36 modulates cellular FFA transfer and accumulation in vivo. When CD36 was knocked out in mice the phenotype included increased circulating levels of FFAs and a 50-80% reduction in heart uptake of the slowly oxidized palmitate analog 123I-BMIPP (15-(p-iodophenyl)-3-(R, S)-methyl pentadecanoic acid) or of iodinated (15-(p-iodophenyl) pentadecanoic acid (IPPA), both injected intravenously. Reduced tracer accumulation was also observed in skeletal muscle and adipose tissues but not in liver [30]. Activity of LpL in plasma was not altered but that of heart tissue was increased indicating no defect in hydrolysis of TG from lipoproteins in CD36 deficiency. When myocardial FA uptake from VLDL and chylomicrons was compared in mice lacking CD36, heart LpL or both, LpL was found important for uptake from both particle types while CD36 was only important for FA uptake from VLDL (Fig. 1). In the case of chylomicrons, the effect of CD36 deletion on FA uptake was negligible [31]. Since lipolysis of chylomicron TG would result in especially high local release of FFAs, these findings are consistent with CD36 facilitation of a high affinity low capacity process that is quantitatively marginal at very high FA levels.
Fig. 1. Model for heart FFA uptake.
FFAs carried by albumin or generated from VLDL lipolysis by LpL enter the heart via CD36 while FFAs generated from chylomicrons enter mostly via non-CD36-mediated mechanisms. FFAs induce CD36 internalization possibly via promoting its ubiquitination (red tags). The FAs might internalize within CD36-rich vesicles. LpL also creates remnant particles that bind to lipoprotein receptors for uptake of core lipids.
In humans there are data to support existence of a higher affinity uptake process, possibly facilitated by CD36 at lower circulating FFA levels in humans [32]. In individuals with CD36 deficiency (2-6% prevalence in Asians and African Americans), positron emission tomography (PET) studies usually in fasted subjects showed that myocardial accumulation of 123I-BMIPP was undetectable while liver accumulation tended to increase [33-35]. A recent PET study examined [11C]-palmitate uptake as a function of plasma FFA concentration by heart, muscle and adipose tissue in individuals with either partial or total CD36 deficiency. Two experimental protocols were used to create conditions with low (breakfast plus glucose intake) and high (overnight fast) circulating FAs. The findings indicated that CD36 is important for normal myocardial FFA uptake at both low and high circulating FFA levels while it is only important for muscle and adipose tissue uptake at low FFA levels [36]. Thus the human myocardium but not adipose tissue or muscle appears dependent on the CD36-mediated pathway for FFA uptake under both fed and fasted states.
Cardiac metabolic flexibility
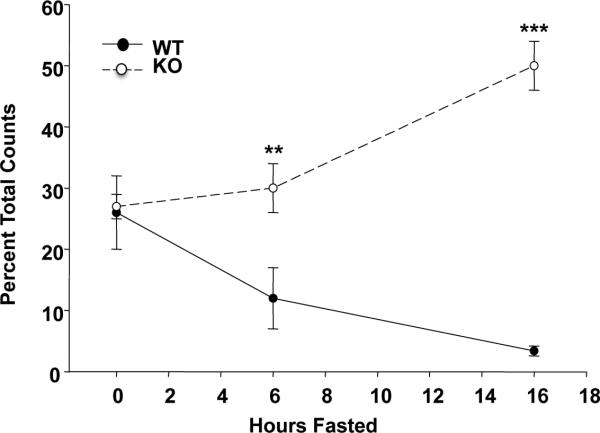
A feature that is characteristic of the CD36 deficient myocardium in rodents [37, 38] and humans [39] is its increased reliance on glucose metabolism. The myocardium of the Cd36 deficient mouse does not reduce its glucose utilization during fasting as occurs with the CD36 sufficient heart (Fig. 2). There is good evidence to support the interpretation that CD36 is important to the ability of the heart and muscle to accomplish substrate switching. This ability reflects the function of the protein in cellular FA uptake [2] and its role in regulating the activation of FOXO1 [38] and AMPK (see following sections), both important metabolic sensors. The increased reliance of the CD36 deficient heart on glucose and possibly ketones would help preserve myocardial energy [40]. Under baseline physiologic conditions heart function is normal and might even be improved in aged mice that are protected from excess lipid accumulation [41]. Based on its role in FFA uptake, targeting CD36 has been used to reduce the myocardial accumulation of intracellular lipids and the associated oxidative toxicity often observed in the diabetic heart. Cd36 deficiency effectively reversed the myocyte TG accumulation and cardiac dysfunction of the MHC-PPARalpha mouse with cardiac specific overexpression of PPARalpha [42] and a similar rescue was observed with deficiency of cardiac LpL [43].
Fig. 2. Cardiac metabolic flexibility is lost in Cd36KO mice.
2-[18F]fluorodeoxyglucose (FDG) uptake by the heart is rapidly reduced by fasting in WT mice but not in Cd36KO mice. Hearts were harvested for counting at 0, 6 and 16h after start of fasting. **p<0.001
Tolerance to ischemia/reperfusion was studied in isolated working Cd36−/− hearts and the seemingly contradictory outcomes observed with one study showing impairement [44] while better recovery was observed in the second study [45] are instructive. In the latter study which used glucose and high insulin in the perfusate, Cd36 deficiency led to reduced injury presumably because high glucose utilization requires less oxygen to create ATP from glucose rather than FFAs. In contrast, with more limited glucose delivery due to no insulin inclusion Cd36 deficiency was deleterious, but injury was reduced when short chain FFAs were supplied as an alternative energy source [44]. Which of these experiments mimic in vivo actions of CD36 in animals or humans is currently unclear and likely to depend on the nutritional and/or activity state. Endogenous TG stores in the Cd36−/− heart are low and are markedly depleted after an overnight fast when stores in the CD36 sufficient heart are normally doubled [1, 46]. The inability of the Cd36−/− myocardium to accomplish the metabolic adpatation of switching to more FA use during fasting makes the mouse susceptible to death when the fast exceeds 20hrs [47] or when it is combined with a brief cold exposure [48].
CD36 and endothelial transport
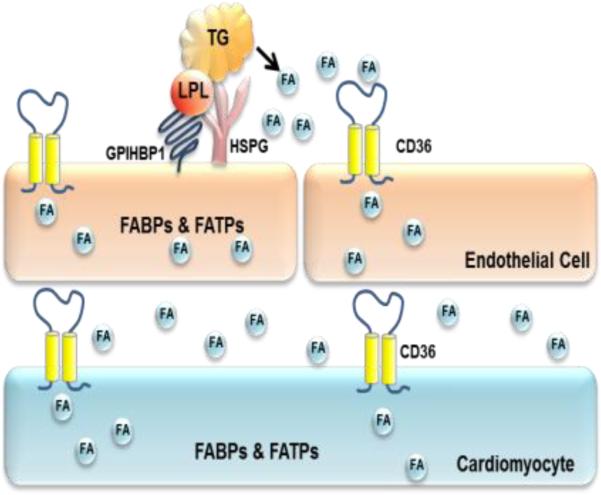
The most obvious phenotype uncovered with CD36 deficiency is an increase in circulating FFAs levels, reduced FFA uptake into heart, skeletal muscle and adipose tissues, and enhanced cardiac glucose oxidation. While these observations support the importance of CD36 in parenchymal FFA uptake, the rapidity of normal blood FFA clearance is consistent with a defect in the first tissue cells required for removal of circulating FFAs. CD36 is highly expressed in capillary endothelial cells of the same tissues noted to have uptake defects in Cd36−/− mice: heart, skeletal muscle, and adipose tissue [49], including brown adipose. In the heart CD36 is more abundant on endothelial cells as compared to cardiomyocytes (Fig. 3). CD36 is less abundant in large blood vessels and very low in endothelial cells from testes and brain [50].
Fig. 3. Heart CD36 is likely to function in FFA transfer both at the level of endothelial cells and cardiomyocytes.
FABP: FA binding protein, FATP: FA transport protein, HSPG: Heparan sulfate proteoglycan, GPIHBP1: glycosylphosphatidylinositol anchored high density lipoprotein binding protein 1.
How do FFAs cross the endothelial barrier in non-fenestrated capillaries?
Is this process regulated or do FFAs in the blood diffuse non-selectively out of the vasculature? There are at least four options for this process (Fig. 3). 1) FFAs associated with albumin could cross the endothelial barrier between cells. During intravascular lipolysis, there is evidence that the endothelial barrier is altered and becomes more permeable [51, 52] and the rapid local clearance of chylomicron-derived FFAs might involve movement between endothelial cells. 2) FFAs might also partition in the membrane bilayer and be transported into cells by lateral movement in an “interfacial continuum” of cell membranes. When chylomicron lipolysis was allowed to proceed in non-albumin-containing buffers, lamellar structures continuous with membrane leaflets were identified in the capillary endothelium and extracellular space [53]. 3) FFA/albumin complexes might be internalized into intracellular vesicles that could transfer FFAs largely unmetabolized across the endothelial barrier [54]. 4) Receptors such as CD36 could be required to transfer FFAs into and through endothelial cells, especially when the concentration of FFAs is low (Fig. 3). Whether the FFAs remain associated with CD36, are CoA modified or are carried by an intracellular FFA binding protein is unknown but it is likely that endothelial CD36 plays a role in tissue FA uptake. Endothelial cell deletion of PPARgamma, a major regulator of CD36 expression, reduces by ~80% endothelial CD36 mRNA and protein and associates with dyslipidemia (high blood TG and FFA levels) that is similar to that of Cd36−/− mice [55] possibly reflecting loss of endothelial CD36.
The observations that cardiomyocyte-specific Cd36 deletion alters heart metabolism [56] and that changes in CD36 expression modulate cardiomyocyte FFA uptake [27] are consistent with a role of cardiomyocyte CD36 in handling of FFAs after their transendothelial release.
3. How does CD36 facilitate FA uptake?
Two concepts are likely basic to the function of CD36 as a FA translocase in the myocardium. First, CD36 is a high affinity receptor for long chain FAs (Km of 5-10nM) suitable for the low levels of unbound FFAs in the circulation [57]. As FFA levels increase, CD36 will continue to function well if metabolism is efficient in removing the FA that is taken up. At FA levels that exceed metabolic capacity, CD36 becomes dysfunctional as shown by its lack of internalization and persistence at the sarcolemma [27], which might reflect altered posttranslational modification of the protein. The heart has enormous capacity for FA catabolism, rapidly channeling ~80% of FA uptake into oxidation [16] and this large capacity explains why the effect of CD36 deletion on reducing FA uptake is particularly dramatic in the heart [30, 34, 36].
Second, sarcolemmal CD36 targets the FA to specific metabolic sites and consequently influences FA utilization. This is based on studies showing that CD36 function is required for the adaptive upregulation of mitochondrial FA oxidation [46, 58, 59] and on the evidence for a role of CD36 mediated signaling in many aspects of cellular FA processing [3]. This regulation fails at persistently high FA levels as recently shown [60] and as illustrated by the finding that increases in sarcolemmal CD36, which are indicative of dysfunction in its trafficking, are observed very early with high fat feeding; these changes precede abnormalities of insulin signaling, glucose or lipid metabolism by about 2 weeks [61]. Interestingly, the evidence points to association of the early CD36 dysfunction observed with high fat feeding with an increase of postabsorptive insulin levels [60, 61]. Treatment of isolated rat cardiomyocytes with high insulin for 48h results in insulin resistance with persistent relocation of CD36 to the plasma membrane while inclusion of omega-3 polyunsaturated FA in the medium prevents this relocation and the onset of insulin resistance [62]. Posttranslational regulation of CD36 includes its ubiquitination, with opposite effects of FA and insulin [63], its glycosylation which was found altered in the heart of the spontaneously hypertensive rat [64] as well as its acetylation, palmitoylation, etc [3]. A better knowledge of the various factors that post-translationally regulate CD36 is needed and would improve our understanding of why its cellular trafficking becomes dysfunctional.
Novel insight into how CD36 might function in facilitating FA uptake has been recently generated by two key findings: The first one is the elucidation of the crystal structure of the CD36 family member LIMP-2 [65], and the second one is the demonstration that CD36 resides within or in proximity of molecular complexes of key metabolic proteins [1, 66].
LIMP-2 structure showed the existence of a large tunnel that traverses the entire length of the molecule and that is predicted by homology modeling to be present in CD36. This tunnel would function to deliver the lipid ligand to the outer leaflet of the bilayer [65]. Modeling of CD36 structure suggests that the FA binding cavity on the surface of the protein [67] provides access to the intramolecular tunnel [3]. Conceivably CD36 would deliver FA via its tunnel to the bilayer and to FA processing enzymes associated with the inner leaflet. Alternatively the protein might internalize together with the FA directing its intracellular trafficking [1, 68]. Both pathways are likely to occur and might contribute to the different fates of internalized FA. There is strong evidence for CD36 induced internalization with oxLDL binding and recent reports indicate that the FA binding site on CD36 involves amino acid residues important for oxLDL uptake. CD36-mediated oxLDL uptake is also suppressed by the FA uptake inhibitor sulfosuccinimidyl oleate (SSO) [67]. Furthermore, FA binding to CD36 increases oxLDL uptake suggesting that both ligands might internalize together and it was postulated that the FA opens up the protein conformation facilitating oxLDL binding [69]. There is evidence that FA binding induces CD36 internalization, although it does not always result in measurable changes in membrane CD36 content since the protein is constantly recycling between intracellular stores and the cell surface. Addition of FA associates with CD36 ubiquitination in C2C12 muscle cells [63] and enterocytes [60]. In myotubes, FA induce internalization of CD36 into vesicles rich in the AMPK kinase LKB1 within 15 min of FA addition [1], but whether these vesicles contain the FA remains to be determined. Overall the data available so far suggest that CD36 recognizes the FA at low concentration and targets it to a metabolic fate that is likely to depend on the cell type and cellular context. In the heart CD36 would primarily target the FA to beta oxidation as discussed in the following section.
4. Sarcolemmal CD36 targets FAs to metabolic sites
It has been well documented that CD36 is important for optimal FA oxidation in muscle and heart tissues. CD36 overexpression in muscle enhances by >3 fold the response of FA oxidation to contraction [58] while Cd36 deletion reduces FA oxidation during exercise and exercise duration [59, 70]. In the isolated working heart, the deletion reduces FA oxidation by 40 and 60%, respectively at low (0.4mM) and high (1.2 mM) perfusate FA [45]. While the CD36 sufficient working heart upregulates palmitate oxidation as workload is increased (from 5 to 35mmHg) this is not observed in the CD36 deficient heart [47].
CD36 association with mitochondria
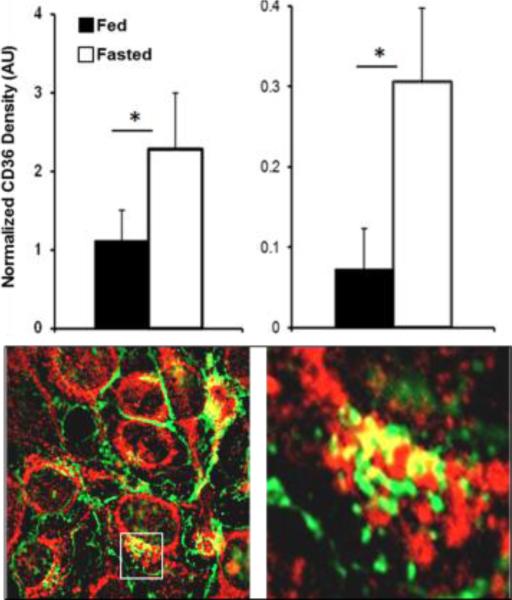
It has been proposed that the importance of CD36 for FA oxidation might relate to its functioning in transfer of FAs into the mitochondria. Presence of CD36 in purified mitochondria and co-immunoprecipitation (co-IP) of CD36 with carnitine palmitoyltransferase I (CPTI) were reported. In addition increased mitochondrial CD36 content paralleled upregulation of FA oxidation [70-72]. Association of CD36 with mitochondria could not be observed in one study that used both biochemical fractionation and imaging and tested co-localization of CD36 with the mitochondrial protein MitoNeet [73]. This discrepancy might reflect the possibility that CD36 association with the mitochondria is dynamically regulated and could reflect a CD36 pool in mitochondria-associated membranes. Our own observations identify CD36 in isolated heart mitochondria and show a fasting-induced increase in mitochondrial CD36 content that parallels the increase in sarcolemmal CD36 (Fig. 4, Top) In Hela cells expressing CD36 we observe areas where it co-localizes with the outer mitochondrial voltage-dependent anion channel (VDAC) (Samovski D, unpublished observations) (Fig. 4, Bottom).
Fig. 4. CD36 association with Top mitochondria.
Top: Hearts of 16h fasted mice have higher CD36 content in sarcolemma (left) and in isolated mitochondria (right), *p<0.05. Bottom: Hela cells expressing CD36 (green) show areas of CD36 co-localization with the mitochondrial anion channel VDAC (red), (40X, Axiovert 200M) (Samovski D, unpublished data).
CD36 association with mitochondria in skeletal muscle might be regulated by Ca2+ flux via the Cav1.1 channel which activates CaMKII and eNOS. The Cav1.1/CaMKII/eNOS pathway was shown to influence both FA oxidation by muscle fibers and CD36 proximity to mitochondrial proteins such as CPT1 and long-chain acyl-CoA synthetase 1 (ACSL1) [66]. It is not clear if this pathway would apply to the heart, however altered regulation of cytosolic calcium is observed in CD36 deficient cardiomyocytes as described below. The association between ACSL1 and CD36, documented using the ligand proximity assay, is particularly interesting since ACSL1 has been reported to target the FA to mitochondrial oxidation in the myocardium [74].
CD36 also targets FFA to esterification and/or to packaging into lipoproteins as reviewed elsewhere [3]. Recent studies in HEK293 cells with or without CD36 expression showed that intracellular FA and diglycerides (DG) decrease more rapidly in cells expressing the protein as levels of TGs increase. The authors proposed that CD36 enhances FA esterification by a mechanism that does not involve faster FA transfer across the membrane [69]. A similar lipid profile is observed in hearts of the CD36−/− mouse administered 123I-BMIPP; lower label recovery in FA and TG while label in DG is increased [30].
CD36 regulates AMPK activation by influencing assembly of pathway proteins
The role of CD36 in enhancing FA oxidation appears linked to CD36 AMPK inter-regulation. AMPK functions as an important energy sensor and metabolic regulator in the heart and when activated from its normally quiescent state by rising energy demand, it functions to upregulate nutrient uptake and catabolism [75]. AMPK enhances FA oxidation by reducing malonyl-CoA levels via phosphorylation and inactivation of acetyl-CoA carboxylase (ACC) [76]. AMPK activation induces sarcolemmal recruitment of CD36 and [27] on the flip side CD36 regulates AMPK activation as recently reported in work that included myotubes [1]. CD36 was shown important for coordinating the dynamic protein interactions within a molecular complex consisting of the CD36 partner tyrosine kinase Fyn, the AMPK kinase LKB1 and AMPK. CD36 expression maintains AMPK quiescent by allowing Fyn to access and phosphorylate LKB1, promoting its nuclear sequestration away from AMPK (Fig. 5). Palmitate binding to CD36 within minutes activates AMPK via its ability to dissociate Fyn from the complex as CD36 is internalized into LKB1-rich vesicles (Fig. 5, inset). Thus while CD36 keeps AMPK inhibited, palmitate binding acts to reverse this inhibition activating AMPK (Fig. 6). This dual effect would serve to adjust the capacity for FA oxidation to match FA availability and explains earlier observations in cardiomyocytes where both sarcolemmal CD36 recruitment and AMPK activation were shown important for FA oxidation [77, 78]. Furthermore, dysregulation of AMPK signaling [79] or CD36 deletion [38, 47] associates with metabolic inflexibility evidenced by a diminished capacity to adjust FA oxidation to FA availability. In summary, similar to its effects on innate immunity or inflammation, the metabolic effects of CD36 involve its ability to function within different molecular complexes of functional proteins.
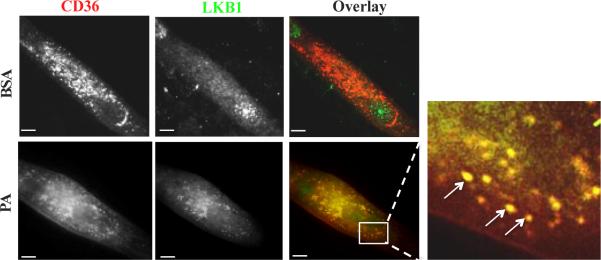
Fig. 5. CD36 inhibits LKB1 and this is reversed by palmitic acid.
Top: LKB1 (green) is mostly in the nucleus in CD36 (red) expressing myotubes (BSA control). Bottom: Palmitic acid (300 uM, 2:1 with BSA, for 15 min) relocates LKB1 to cytosolic CD36-positive vesicular structures (inset, arrows point to overlap between CD36 and LKB1). From Samovski D et al. 2015 [1]
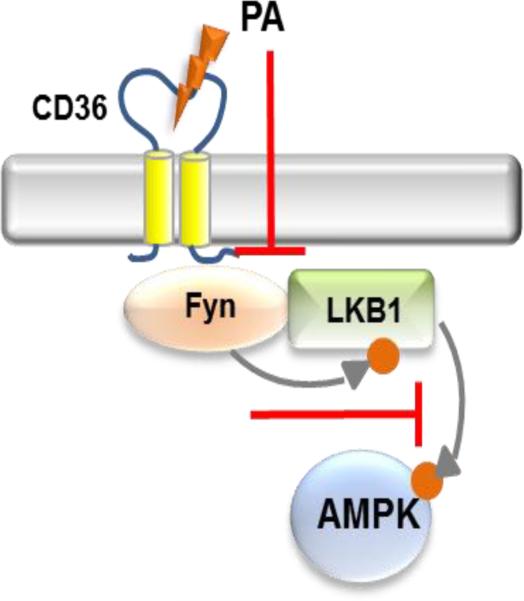
Fig. 6. AMPK regulation.
CD36 is in a molecular complex in proximity to Fyn, LKB1 and AMPK. CD36 suppresses AMPK keeping it quiescent by allowing Fyn inhibition of LKB1. This inhibition is reversed by CD36 ligation of palmitic acid (PA), which activates LKB1 and AMPK by dissociating Fyn.
5. CD36 regulates cytosolic calcium and myocardial phospholipid metabolism
There is evidence for involvement of CD36 in the regulation of store operated Ca2+ channels (SOC) and for activation of Ca2+ dependent phospholipases A2 that cycle polyunsaturated FA into phospholipids [7, 8, 80]. Membrane SOCs (Orai protein multimers) are activated by the ER Ca2+ sensor STIM1 following ER Ca2+ depletion in response to inositol 1,4,5-trisphosphate (IP3) or as a result of inhibition of the Sarco/Endoplasmic Reticulum Calcium ATPase (SERCA). SOC calcium entry replenishes ER Ca2+ and can lead to a sustained increase in cytosolic Ca2+ that drives or modulates many basic functions in non-excitable and also in excitable cells [81]. SOC Ca2+ is important for myocardial health because of its contribution to the replenishment of Ca2+ stores and to the long term maintenance of cellular Ca2+ levels. SOC function also modulates excitation coupled gene transcription and abnormal SOC signaling and is implicated in hypertrophic cardiac remodeling for example in response to pressure overload [82].
Knockdown of CD36 in cardiomyocytes delays clearance of cytosolic Ca2+. Consistent with this, Cd36 deficient hearts have a marked compensatory increase in levels of SERCA2 mRNA and protein and display altered expression of a number of Ca2+ homeostasis genes. Fasting the mice for 20 hours prolongs Ca2+ transients and action potential but these electrical changes are more pronounced in Cd36 deficient hearts resulting in conduction anomalies such as severe atrioventricular block and bradycardia that can result in the animal's death [47]. Myocardial remodeling is apparent with significant changes in Ca2+ dependent phospholipid metabolism involving FA cycling and acyl composition that reflect influence of CD36 in regulating phospholipase activation [8]. The Cd36 deficient genotype in the heart associates with increases in the amount of lysophospholipids and in the content of phospholipid species containing DHA [47]. In addition it is characterized by increases in the content of several classes of eicosanoids, including prostaglandins and the hydroxy (HETE) and epoxy (EET) derivatives (Unpublished observations).
6. CD36 and cardiovascular disease; role in inflammation and its resolution
CD36 has been characterized as a multi-ligand receptor capable of inducing macrophage inflammation and foam cell formation. As noted above, among its ligands is oxLDL and uptake of oxLDL is often viewed as the source of cholesterol that accumulates in arterial wall macrophages [83]. CD36 also appears to form a complex with TLR4 [84]. Finally, CD36 was reported to be important for the nucleation and accumulation of cholesterol crystals within macrophages resulting in lysosomal disruption and activation of the NLRP3 inflammasome [85], possible atherogenic events.
Despite these potentially atherogenic processes, the in vivo evidence of a role of CD36 to promote vascular disease is less secure. In part, the complexity and redundancy of the inflammatory response may limit the ability to detect the contribution of a single molecule in knockout mice whose lesions are driven by supra-physiologic levels of circulating apolipoprotein B-containing cholesterol particles. Initial studies performed crossing Cd36−/− mice with apoE deficient mice showed a reduction of atherosclerosis [30] while other studies found that Cd36 loss did not affect atherosclerosis generation [86]. A possible reason for these differences is the use of different lines of knockout mice and breeding issues, e.g. if another atherosclerosis altering allele is close to CD36. A more likely explanation is that scavenger receptors, including CD36, have dual roles and could have either pro- or anti-atherosclerotic effects; more macrophage lipid removal would reduce disease while in other situations macrophage lipid uptake could increase foam cell formation and inflammation [87].
The role of macrophage CD36 in mounting acute inflammation for host defense against pathogens is well established [15, 88]. In low glucose, LpL and presumably FFA supply to macrophages enable these cells to maintain phagocytosis, a condition that might mimic what occurs in an abscess cavity [89]. However, macrophages can be both inflammatory as well as assist with inflammation resolution when alternatively activated, a process that is induced by lipid uptake. It was noted in earlier work that macrophage-mediated lipolysis of VLDL liberates ligands for PPARgamma which reduces the inflammatory state of the macrophage [90]. More recently, the conversion of macrophages to the alternatively activated anti-inflammatory phenotype was shown to require CD36-mediated lipoprotein uptake followed by lysosomal hydrolysis [91]. In addition to its role in mounting inflammation, there is good evidence that CD36 is important for resolving inflammation by contributing to clearance of apoptotic neutrophils, which promotes tissue repair [13]. In the heart CD36 may be important for resolution of cardiac inflammation after injury by contributing to monocyte clearance of apoptotic cells [14, 92]. These dual actions of CD36 in helping mount and resolve inflammation would be consistent with the concept that normally inflammation is tightly controlled, being self-limited and programing its own resolution [93, 94].
7. Summary and future directions
As with many biological processes, those mediated by CD36 are complex and include modulation of FA metabolism, calcium homeostasis, and cellular inflammation. Our tools of using non-physiologic in vitro systems and gross genetic modifications of mice may fail to identify all the players involved in physiologic processes that often have redundancy or are highly context dependent. Insight has been recently obtained regarding the role of CD36 in the heart both under homeostatic or pathological conditions but many questions remain unanswered. What role does endothelial CD36 play in myocardial FA uptake and is its function altered in disease? What are the posttranslational modifications responsible for the early dysfunctional CD36 persistence at the sarcolemma under conditions of hyperinsulinemia? Can the lipid abnormalities be improved by preventing or reversing these modifications? Would enhancing CD36 expression in the myocardium be beneficial in helping resolve injury after infarct and how will this impact metabolic reprograming? In addition to these questions there are directions that are emerging where much more needs to be done. The action of CD36 to coordinate protein interactions within molecular complexes of metabolic proteins opens a new perspective regarding its metabolic functions. Identifying the molecular partners and interactions that define each regulatory effect would provide great insight into potential therapeutic targets. It is highly likely that the ability of CD36 to regulate cellular metabolism is not limited to lipid uptake and utilization. Finally much progress is needed in translating research approaches to humans where common variants in the CD36 gene impact its level often in a tissue specific manner [95]. The human gene is complex having multiple promoters and transcripts with tissue or cell specific distribution [96, 97] and understanding how these transcripts are regulated is likely to have relevance to myocardial metabolism. Also exploring potential epigenetic influences on the gene of nutrients and other environmental factors might provide insight into the etiology of metabolic dysfunction and the associated cardiovascular complications.
Highlights.
Importance of CD36 for cardiac metabolic flexibility
CD36 maintains AMPK quiescent
Fatty acid uptake signals to upregulate fatty acid oxidation
Cytosolic calcium and myocardial phospholipid metabolism
CD36 contributes to inflammation and its resolution in the heart
Acknowledgments
Research conducted in the authors’ laboratory was funded by National Institutes of Health (NIH) grants R01-DK-033301 (NAA), HL45095 (IJG) and by Nutrition and Obesity Research Center grant P30-DK-056341 at Washington University.
Abbreviations
- CD36
cluster of differentiation 36
- FFA
unesterified fatty acids
- TG
triglycerides
- LpL
Lipoprotein lipase
- VLDL
very low density lipoproteins
- 123I-BMIPP
15-(piodophenyl)-3-(R, S)-methyl pentadecanoic acid
- AMPK
adenosine monophosphate-activated protein kinase
- LKB1
serine–threonine liver kinase B1
- SERCA
SR/ER calcium ATPase
- CaMK
calmodulin kinase
- CPTI
carnitine palmitoyltransferase I
- ACSL1
Acyl-CoE A synthase for long chain FA 1
- oxLDL
oxidized low density lipoproteins
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Samovski D, Sun J, Pietka T, Gross RW, Eckel RH, Su X, Stahl PD, Abumrad NA. Regulation of AMPK activation by CD36 links fatty acid uptake to beta-oxidation. Diabetes. 2015;64:353–359. doi: 10.2337/db14-0582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Goldberg IJ, Eckel RH, Abumrad NA. Regulation of fatty acid uptake into tissues: lipoprotein lipase- and CD36-mediated pathways. Journal of lipid research. 2009;50(Suppl):S86–90. doi: 10.1194/jlr.R800085-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pepino MY, Kuda O, Samovski D, Abumrad NA. Structure-function of CD36 and importance of fatty acid signal transduction in fat metabolism. Annual review of nutrition. 2014;34:281–303. doi: 10.1146/annurev-nutr-071812-161220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Canton J, Neculai D, Grinstein S. Scavenger receptors in homeostasis and immunity. Nature reviews. Immunology. 2013;13:621–634. doi: 10.1038/nri3515. [DOI] [PubMed] [Google Scholar]
- 5.Lawler PR, Lawler J. Molecular basis for the regulation of angiogenesis by thrombospondin-1 and -2. Cold Spring Harb Perspect Med. 2012;2:a006627. doi: 10.1101/cshperspect.a006627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Silverstein RL, Li W, Park YM, Rahaman SO. Mechanisms of cell signaling by the scavenger receptor CD36: implications in atherosclerosis and thrombosis. Transactions of the American Clinical and Climatological Association. 2010;121:206–220. [PMC free article] [PubMed] [Google Scholar]
- 7.Dramane G, Abdoul-Azize S, Hichami A, Vogtle T, Akpona S, Chouabe C, Sadou H, Nieswandt B, Besnard P, Khan NA. STIM1 regulates calcium signaling in taste bud cells and preference for fat in mice. The Journal of clinical investigation. 2012;122:2267–2282. doi: 10.1172/JCI59953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kuda O, Jenkins CM, Skinner JR, Moon SH, Su X, Gross RW, Abumrad NA. CD36 protein is involved in store-operated calcium flux, phospholipase A2 activation, and production of prostaglandin E2. The Journal of biological chemistry. 2011;286:17785–17795. doi: 10.1074/jbc.M111.232975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sundaresan S, Shahid R, Riehl TE, Chandra R, Nassir F, Stenson WF, Liddle RA, Abumrad NA. CD36-dependent signaling mediates fatty acid-induced gut release of secretin and cholecystokinin. FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 2013;27:1191–1202. doi: 10.1096/fj.12-217703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Clark MG, Rattigan S, Barrett EJ. Nutritive blood flow as an essential element supporting muscle anabolism. Current opinion in clinical nutrition and metabolic care. 2006;9:185–189. doi: 10.1097/01.mco.0000222097.90890.c2. [DOI] [PubMed] [Google Scholar]
- 11.Podrez EA, Poliakov E, Shen Z, Zhang R, Deng Y, Sun M, Finton PJ, Shan L, Gugiu B, Fox PL, Hoff HF, Salomon RG, Hazen SL. Identification of a novel family of oxidized phospholipids that serve as ligands for the macrophage scavenger receptor CD36. The Journal of biological chemistry. 2002;277:38503–38516. doi: 10.1074/jbc.M203318200. [DOI] [PubMed] [Google Scholar]
- 12.Park YM. CD36, a scavenger receptor implicated in atherosclerosis. Experimental & molecular medicine. 2014;46:e99. doi: 10.1038/emm.2014.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ballesteros I, Cuartero MI, Pradillo JM, de la Parra J, Perez-Ruiz A, Corbi A, Ricote M, Hamilton JA, Sobrado M, Vivancos J, Nombela F, Lizasoain I, Moro MA. Rosiglitazone-induced CD36 up-regulation resolves inflammation by PPARgamma and 5-LO-dependent pathways. Journal of leukocyte biology. 2014;95:587–598. doi: 10.1189/jlb.0613326. [DOI] [PubMed] [Google Scholar]
- 14.Novak ML, Thorp EB. Shedding light on impaired efferocytosis and nonresolving inflammation. Circulation research. 2013;113:9–12. doi: 10.1161/CIRCRESAHA.113.301583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stuart LM, Deng J, Silver JM, Takahashi K, Tseng AA, Hennessy EJ, Ezekowitz RA, Moore KJ. Response to Staphylococcus aureus requires CD36-mediated phagocytosis triggered by the COOH-terminal cytoplasmic domain. The Journal of cell biology. 2005;170:477–485. doi: 10.1083/jcb.200501113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lopaschuk GD, Ussher JR, Folmes CD, Jaswal JS, Stanley WC. Myocardial fatty acid metabolism in health and disease. Physiological reviews. 2010;90:207–258. doi: 10.1152/physrev.00015.2009. [DOI] [PubMed] [Google Scholar]
- 17.Hamilton JA. New insights into the roles of proteins and lipids in membrane transport of fatty acids. Prostaglandins, leukotrienes, and essential fatty acids. 2007;77:355–361. doi: 10.1016/j.plefa.2007.10.020. [DOI] [PubMed] [Google Scholar]
- 18.Kampf JP, Kleinfeld AM. Is membrane transport of FFA mediated by lipid, protein, or both? An unknown protein mediates free fatty acid transport across the adipocyte plasma membrane. Physiology (Bethesda) 2007;22:7–14. doi: 10.1152/physiol.00011.2006. [DOI] [PubMed] [Google Scholar]
- 19.Su X, Abumrad NA. Cellular fatty acid uptake: a pathway under construction. Trends in endocrinology and metabolism: TEM. 2009;20:72–77. doi: 10.1016/j.tem.2008.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Abumrad NA, Davidson NO. Role of the gut in lipid homeostasis. Physiological reviews. 2012;92:1061–1085. doi: 10.1152/physrev.00019.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Berk PD, Stump DD. Mechanisms of cellular uptake of long chain free fatty acids. Molecular and cellular biochemistry. 1999;192:17–31. [PubMed] [Google Scholar]
- 22.Schwenk RW, Luiken JJ, Bonen A, Glatz JF. Regulation of sarcolemmal glucose and fatty acid transporters in cardiac disease. Cardiovascular research. 2008;79:249–258. doi: 10.1093/cvr/cvn116. [DOI] [PubMed] [Google Scholar]
- 23.Sorrentino D, Stump D, Potter BJ, Robinson RB, White R, Kiang CL, Berk PD. Oleate uptake by cardiac myocytes is carrier mediated and involves a 40-kD plasma membrane fatty acid binding protein similar to that in liver, adipose tissue, and gut. The Journal of clinical investigation. 1988;82:928–935. doi: 10.1172/JCI113700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stremmel W. Fatty acid uptake by isolated rat heart myocytes represents a carrier-mediated transport process. The Journal of clinical investigation. 1988;81:844–852. doi: 10.1172/JCI113393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Carley AN, Kleinfeld AM. Fatty acid (FFA) transport in cardiomyocytes revealed by imaging unbound FFA is mediated by an FFA pump modulated by the CD36 protein. The Journal of biological chemistry. 2011;286:4589–4597. doi: 10.1074/jbc.M110.182162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Abumrad NA, el-Maghrabi MR, Amri EZ, Lopez E, Grimaldi PA. Cloning of a rat adipocyte membrane protein implicated in binding or transport of long-chain fatty acids that is induced during preadipocyte differentiation. Homology with human CD36, The Journal of biological chemistry. 1993;268:17665–17668. [PubMed] [Google Scholar]
- 27.Glatz JF, Luiken JJ, Bonen A. Membrane fatty acid transporters as regulators of lipid metabolism: implications for metabolic disease. Physiological reviews. 2010;90:367–417. doi: 10.1152/physrev.00003.2009. [DOI] [PubMed] [Google Scholar]
- 28.Schwenk RW, Dirkx E, Coumans WA, Bonen A, Klip A, Glatz JF, Luiken JJ. Requirement for distinct vesicle-associated membrane proteins in insulin- and AMP-activated protein kinase (AMPK)-induced translocation of GLUT4 and CD36 in cultured cardiomyocytes. Diabetologia. 2010;53:2209–2219. doi: 10.1007/s00125-010-1832-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Samovski D, Su X, Xu Y, Abumrad NA, Stahl PD. Insulin and AMPK regulate FA translocase/CD36 plasma membrane recruitment in cardiomyocytes via Rab GAP AS160 and Rab8a Rab GTPase. J Lipid Res. 2012;53:709–717. doi: 10.1194/jlr.M023424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Coburn CT, Knapp FF, Jr., Febbraio M, Beets AL, Silverstein RL, Abumrad NA. Defective uptake and utilization of long chain fatty acids in muscle and adipose tissues of CD36 knockout mice. The Journal of biological chemistry. 2000;275:32523–32529. doi: 10.1074/jbc.M003826200. [DOI] [PubMed] [Google Scholar]
- 31.Bharadwaj KG, Hiyama Y, Hu Y, Huggins LA, Ramakrishnan R, Abumrad NA, Shulman GI, Blaner WS, Goldberg IJ. Chylomicron- and VLDL-derived lipids enter the heart through different pathways: in vivo evidence for receptor- and non-receptor-mediated fatty acid uptake. J Biol Chem. 2010;285:37976–37986. doi: 10.1074/jbc.M110.174458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Carpentier AC, Frisch F, Brassard P, Lavoie F, Bourbonnais A, Cyr D, Giguere R, Baillargeon JP. Mechanism of insulin-stimulated clearance of plasma nonesterified fatty acids in humans. American journal of physiology. Endocrinology and metabolism. 2007;292:E693–701. doi: 10.1152/ajpendo.00423.2006. [DOI] [PubMed] [Google Scholar]
- 33.Hirano K, Kuwasako T, Nakagawa-Toyama Y, Janabi M, Yamashita S, Matsuzawa Y. Pathophysiology of human genetic CD36 deficiency. Trends Cardiovasc Med. 2003;13:136–141. doi: 10.1016/s1050-1738(03)00026-4. [DOI] [PubMed] [Google Scholar]
- 34.Tanaka T, Nakata T, Oka T, Ogawa T, Okamoto F, Kusaka Y, Sohmiya K, Shimamoto K, Itakura K. Defect in human myocardial long-chain fatty acid uptake is caused by FAT/CD36 mutations. J Lipid Res. 2001;42:751–759. [PubMed] [Google Scholar]
- 35.Yoshizumi T, Nozaki S, Fukuchi K, Yamasaki K, Fukuchi T, Maruyama T, Tomiyama Y, Yamashita S, Nishimura T, Matsuzawa Y. Pharmacokinetics and metabolism of 123I-BMIPP fatty acid analog in healthy and CD36-deficient subjects. Journal of nuclear medicine : official publication, Society of Nuclear Medicine. 2000;41:1134–1138. [PubMed] [Google Scholar]
- 36.Hames KC, Vella A, Kemp BJ, Jensen MD. Free fatty acid uptake in humans with CD36 deficiency. Diabetes. 2014;63:3606–3614. doi: 10.2337/db14-0369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hajri T, Ibrahimi A, Coburn CT, Knapp FF, Jr., Kurtz T, Pravenec M, Abumrad NA. Defective fatty acid uptake in the spontaneously hypertensive rat is a primary determinant of altered glucose metabolism, hyperinsulinemia, and myocardial hypertrophy. The Journal of biological chemistry. 2001;276:23661–23666. doi: 10.1074/jbc.M100942200. [DOI] [PubMed] [Google Scholar]
- 38.Nahle Z, Hsieh M, Pietka T, Coburn CT, Grimaldi PA, Zhang MQ, Das D, Abumrad NA. CD36-dependent regulation of muscle FoxO1 and PDK4 in the PPAR delta/beta-mediated adaptation to metabolic stress. The Journal of biological chemistry. 2008;283:14317–14326. doi: 10.1074/jbc.M706478200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fukuchi K, Nozaki S, Yoshizumi T, Hasegawa S, Uehara T, Nakagawa T, Kobayashi T, Tomiyama Y, Yamashita S, Matsuzawa Y, Nishimura T. Enhanced myocardial glucose use in patients with a deficiency in long-chain fatty acid transport (CD36 deficiency) Journal of nuclear medicine : official publication, Society of Nuclear Medicine. 1999;40:239–243. [PubMed] [Google Scholar]
- 40.Nakatani K, Watabe T, Masuda D, Imaizumi M, Shimosegawa E, Kobayashi T, Sairyo M, Zhu Y, Okada T, Kawase R, Nakaoka H, Naito A, Ohama T, Koseki M, Oka T, Akazawa H, Nishida M, Komuro I, Sakata Y, Hatazawa J, Yamashita S. Myocardial energy provision is preserved by increased utilization of glucose and ketone bodies in CD36 knockout mice. Metabolism: clinical and experimental. 2015;64:1165–1174. doi: 10.1016/j.metabol.2015.05.017. [DOI] [PubMed] [Google Scholar]
- 41.Koonen DP, Febbraio M, Bonnet S, Nagendran J, Young ME, Michelakis ED, Dyck JR. CD36 expression contributes to age-induced cardiomyopathy in mice. Circulation. 2007;116:2139–2147. doi: 10.1161/CIRCULATIONAHA.107.712901. [DOI] [PubMed] [Google Scholar]
- 42.Yang J, Sambandam N, Han X, Gross RW, Courtois M, Kovacs A, Febbraio M, Finck BN, Kelly DP. CD36 deficiency rescues lipotoxic cardiomyopathy. Circulation research. 2007;100:1208–1217. doi: 10.1161/01.RES.0000264104.25265.b6. [DOI] [PubMed] [Google Scholar]
- 43.Duncan JG, Bharadwaj KG, Fong JL, Mitra R, Sambandam N, Courtois MR, Lavine KJ, Goldberg IJ, Kelly DP. Rescue of cardiomyopathy in peroxisome proliferator-activated receptor-alpha transgenic mice by deletion of lipoprotein lipase identifies sources of cardiac lipids and peroxisome proliferator-activated receptor-alpha activators. Circulation. 2010;121:426–435. doi: 10.1161/CIRCULATIONAHA.109.888735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Irie H, Krukenkamp IB, Brinkmann JF, Gaudette GR, Saltman AE, Jou W, Glatz JF, Abumrad NA, Ibrahimi A. Myocardial recovery from ischemia is impaired in CD36-null mice and restored by myocyte CD36 expression or medium-chain fatty acids. Proc Natl Acad Sci U S A. 2003;100:6819–6824. doi: 10.1073/pnas.1132094100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kuang M, Febbraio M, Wagg C, Lopaschuk GD, Dyck JR. Fatty acid translocase/CD36 deficiency does not energetically or functionally compromise hearts before or after ischemia. Circulation. 2004;109:1550–1557. doi: 10.1161/01.CIR.0000121730.41801.12. [DOI] [PubMed] [Google Scholar]
- 46.Trent CM, Yu S, Hu Y, Skoller N, Huggins LA, Homma S, Goldberg IJ. Lipoprotein lipase activity is required for cardiac lipid droplet production. Journal of lipid research. 2014;55:645–658. doi: 10.1194/jlr.M043471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pietka TA, Sulkin MS, Kuda O, Wang W, Zhou D, Yamada KA, Yang K, Su X, Gross RW, Nerbonne JM, Efimov IR, Abumrad NA. CD36 protein influences myocardial Ca2+ homeostasis and phospholipid metabolism: conduction anomalies in CD36-deficient mice during fasting. The Journal of biological chemistry. 2012;287:38901–38912. doi: 10.1074/jbc.M112.413609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Putri M, Syamsunarno MR, Iso T, Yamaguchi A, Hanaoka H, Sunaga H, Koitabashi N, Matsui H, Yamazaki C, Kameo S, Tsushima Y, Yokoyama T, Koyama H, Abumrad NA, Kurabayashi M. CD36 is indispensable for thermogenesis under conditions of fasting and cold stress. Biochemical and biophysical research communications. 2015;457:520–525. doi: 10.1016/j.bbrc.2014.12.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Greenwalt DE, Scheck SH, Rhinehart-Jones T. Heart CD36 expression is increased in murine models of diabetes and in mice fed a high fat diet. The Journal of clinical investigation. 1995;96:1382–1388. doi: 10.1172/JCI118173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Nolan DJ, Ginsberg M, Israely E, Palikuqi B, Poulos MG, James D, Ding BS, Schachterle W, Liu Y, Rosenwaks Z, Butler JM, Xiang J, Rafii A, Shido K, Rabbany SY, Elemento O, Rafii S. Molecular signatures of tissue-specific microvascular endothelial cell heterogeneity in organ maintenance and regeneration. Developmental cell. 2013;26:204–219. doi: 10.1016/j.devcel.2013.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Eiselein L, Wilson DW, Lame MW, Rutledge JC. Lipolysis products from triglyceride-rich lipoproteins increase endothelial permeability, perturb zonula occludens-1 and F-actin, and induce apoptosis. American journal of physiology. Heart and circulatory physiology. 2007;292:H2745–2753. doi: 10.1152/ajpheart.00686.2006. [DOI] [PubMed] [Google Scholar]
- 52.Rutledge JC, Mullick AE, Gardner G, Goldberg IJ. Direct visualization of lipid deposition and reverse lipid transport in a perfused artery : roles of VLDL and HDL. Circulation research. 2000;86:768–773. doi: 10.1161/01.res.86.7.768. [DOI] [PubMed] [Google Scholar]
- 53.Scow RO, Blanchette-Mackie EJ. Endothelium, the dynamic interface in cardiac lipid transport. Molecular and cellular biochemistry. 1992;116:181–191. doi: 10.1007/BF01270586. [DOI] [PubMed] [Google Scholar]
- 54.Ring A, Pohl J, Volkl A, Stremmel W. Evidence for vesicles that mediate long-chain fatty acid uptake by human microvascular endothelial cells. Journal of lipid research. 2002;43:2095–2104. doi: 10.1194/jlr.m200285-jlr200. [DOI] [PubMed] [Google Scholar]
- 55.Kanda T, Brown JD, Orasanu G, Vogel S, Gonzalez FJ, Sartoretto J, Michel T, Plutzky J. PPARgamma in the endothelium regulates metabolic responses to high-fat diet in mice. The Journal of clinical investigation. 2009;119:110–124. doi: 10.1172/JCI36233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Nagendran J, Pulinilkunnil T, Kienesberger PC, Sung MM, Fung D, Febbraio M, Dyck JR. Cardiomyocyte-specific ablation of CD36 improves post-ischemic functional recovery. Journal of molecular and cellular cardiology. 2013;63:180–188. doi: 10.1016/j.yjmcc.2013.07.020. [DOI] [PubMed] [Google Scholar]
- 57.Richieri GV, Kleinfeld AM. Unbound free fatty acid levels in human serum. Journal of lipid research. 1995;36:229–240. [PubMed] [Google Scholar]
- 58.Ibrahimi A, Bonen A, Blinn WD, Hajri T, Li X, Zhong K, Cameron R, Abumrad NA. Muscle-specific overexpression of FAT/CD36 enhances fatty acid oxidation by contracting muscle, reduces plasma triglycerides and fatty acids, and increases plasma glucose and insulin. J Biol Chem. 1999;274:26761–26766. doi: 10.1074/jbc.274.38.26761. [DOI] [PubMed] [Google Scholar]
- 59.McFarlan JT, Yoshida Y, Jain SS, Han XX, Snook LA, Lally J, Smith BK, Glatz JF, Luiken JJ, Sayer RA, Tupling AR, Chabowski A, Holloway GP, Bonen A. In vivo, fatty acid translocase (CD36) critically regulates skeletal muscle fuel selection, exercise performance, and training-induced adaptation of fatty acid oxidation. The Journal of biological chemistry. 2012;287:23502–23516. doi: 10.1074/jbc.M111.315358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Buttet M, Poirier H, Traynard V, Gaire K, Tran TT, Sundaresan S, Besnard P, Abumrad NA, Niot I. Deregulated Lipid Sensing by Intestinal CD36 in Diet-Induced Hyperinsulinemic Obese Mouse Model. PloS one. 2016;11:e0145626. doi: 10.1371/journal.pone.0145626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Bonen A, Jain SS, Snook LA, Han XX, Yoshida Y, Buddo KH, Lally JS, Pask ED, Paglialunga S, Beaudoin MS, Glatz JF, Luiken JJ, Harasim E, Wright DC, Chabowski A, Holloway GP. Extremely rapid increase in fatty acid transport and intramyocellular lipid accumulation but markedly delayed insulin resistance after high fat feeding in rats. Diabetologia. 2015;58:2381–2391. doi: 10.1007/s00125-015-3691-8. [DOI] [PubMed] [Google Scholar]
- 62.Franekova V, Angin Y, Hoebers NT, Coumans WA, Simons PJ, Glatz JF, Luiken JJ, Larsen TS. Marine omega-3 fatty acids prevent myocardial insulin resistance and metabolic remodeling as induced experimentally by high insulin exposure, American journal of physiology. Cell physiology. 2015;308:C297–307. doi: 10.1152/ajpcell.00073.2014. [DOI] [PubMed] [Google Scholar]
- 63.Smith J, Su X, El-Maghrabi R, Stahl PD, Abumrad NA. Opposite regulation of CD36 ubiquitination by fatty acids and insulin: effects on fatty acid uptake. The Journal of biological chemistry. 2008;283:13578–13585. doi: 10.1074/jbc.M800008200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lauzier B, Merlen C, Vaillant F, McDuff J, Bouchard B, Beguin PC, Dolinsky VW, Foisy S, Villeneuve LR, Labarthe F, Dyck JR, Allen BG, Charron G, Des Rosiers C. Post-translational modifications, a key process in CD36 function: lessons from the spontaneously hypertensive rat heart. Journal of molecular and cellular cardiology. 2011;51:99–108. doi: 10.1016/j.yjmcc.2011.04.001. [DOI] [PubMed] [Google Scholar]
- 65.Neculai D, Schwake M, Ravichandran M, Zunke F, Collins RF, Peters J, Neculai M, Plumb J, Loppnau P, Pizarro JC, Seitova A, Trimble WS, Saftig P, Grinstein S, Dhe-Paganon S. Structure of LIMP-2 provides functional insights with implications for SR-BI and CD36. Nature. 2013;504:172–176. doi: 10.1038/nature12684. [DOI] [PubMed] [Google Scholar]
- 66.Georgiou DK, Dagnino-Acosta A, Lee CS, Griffin DM, Wang H, Lagor WR, Pautler RG, Dirksen RT, Hamilton SL. Ca2+ Binding/Permeation via Calcium Channel, CaV1.1, Regulates the Intracellular Distribution of the Fatty Acid Transport Protein, CD36, and Fatty Acid Metabolism. The Journal of biological chemistry. 2015 doi: 10.1074/jbc.M115.643544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kuda O, Pietka TA, Demianova Z, Kudova E, Cvacka J, Kopecky J, Abumrad NA. Sulfo-N-succinimidyl oleate (SSO) inhibits fatty acid uptake and signaling for intracellular calcium via binding CD36 lysine 164: SSO also inhibits oxidized low density lipoprotein uptake by macrophages. The Journal of biological chemistry. 2013;288:15547–15555. doi: 10.1074/jbc.M113.473298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Siddiqi S, Saleem U, Abumrad NA, Davidson NO, Storch J, Siddiqi SA, Mansbach CM., 2nd A novel multiprotein complex is required to generate the prechylomicron transport vesicle from intestinal ER. Journal of lipid research. 2010;51:1918–1928. doi: 10.1194/jlr.M005611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Xu S, Jay A, Brunaldi K, Huang N, Hamilton JA. CD36 enhances fatty acid uptake by increasing the rate of intracellular esterification but not transport across the plasma membrane. Biochemistry. 2013;52:7254–7261. doi: 10.1021/bi400914c. [DOI] [PubMed] [Google Scholar]
- 70.Yoshida Y, Jain SS, McFarlan JT, Snook LA, Chabowski A, Bonen A. Exercise- and training-induced upregulation of skeletal muscle fatty acid oxidation are not solely dependent on mitochondrial machinery and biogenesis. The Journal of physiology. 2013;591:4415–4426. doi: 10.1113/jphysiol.2012.238451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Bezaire V, Bruce CR, Heigenhauser GJ, Tandon NN, Glatz JF, Luiken JJ, Bonen A, Spriet LL. Identification of fatty acid translocase on human skeletal muscle mitochondrial membranes: essential role in fatty acid oxidation. American journal of physiology. Endocrinology and metabolism. 2006;290:E509–515. doi: 10.1152/ajpendo.00312.2005. [DOI] [PubMed] [Google Scholar]
- 72.Campbell SE, Tandon NN, Woldegiorgis G, Luiken JJ, Glatz JF, Bonen A. A novel function for fatty acid translocase (FAT)/CD36: involvement in long chain fatty acid transfer into the mitochondria. J Biol Chem. 2004;279:36235–36241. doi: 10.1074/jbc.M400566200. [DOI] [PubMed] [Google Scholar]
- 73.Jeppesen J, Mogensen M, Prats C, Sahlin K, Madsen K, Kiens B. FAT/CD36 is localized in sarcolemma and in vesicle-like structures in subsarcolemma regions but not in mitochondria. Journal of lipid research. 2010;51:1504–1512. doi: 10.1194/jlr.M003756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Ellis JM, Mentock SM, Depetrillo MA, Koves TR, Sen S, Watkins SM, Muoio DM, Cline GW, Taegtmeyer H, Shulman GI, Willis MS, Coleman RA. Mouse cardiac acyl coenzyme a synthetase 1 deficiency impairs Fatty Acid oxidation and induces cardiac hypertrophy. Molecular and cellular biology. 2011;31:1252–1262. doi: 10.1128/MCB.01085-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Hardie DG. AMP-activated protein kinase: the guardian of cardiac energy status. The Journal of clinical investigation. 2004;114:465–468. doi: 10.1172/JCI22683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Hardie DG, Schaffer BE, Brunet A. AMPK: An Energy-Sensing Pathway with Multiple Inputs and Outputs. Trends in cell biology. 2015 doi: 10.1016/j.tcb.2015.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Angin Y, Schwenk RW, Nergiz-Unal R, Hoebers N, Heemskerk JW, Kuijpers MJ, Coumans WA, van Zandvoort MA, Bonen A, Neumann D, Glatz JF, Luiken JJ. Calcium signaling recruits substrate transporters GLUT4 and CD36 to the sarcolemma without increasing cardiac substrate uptake. American journal of physiology. Endocrinology and metabolism. 2014;307:E225–236. doi: 10.1152/ajpendo.00655.2013. [DOI] [PubMed] [Google Scholar]
- 78.Habets DD, Coumans WA, Voshol PJ, den Boer MA, Febbraio M, Bonen A, Glatz JF, Luiken JJ. AMPK-mediated increase in myocardial long-chain fatty acid uptake critically depends on sarcolemmal CD36. Biochemical and biophysical research communications. 2007;355:204–210. doi: 10.1016/j.bbrc.2007.01.141. [DOI] [PubMed] [Google Scholar]
- 79.Ruderman NB, Carling D, Prentki M, Cacicedo JM. AMPK, insulin resistance, and the metabolic syndrome. The Journal of clinical investigation. 2013;123:2764–2772. doi: 10.1172/JCI67227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Gilbertson TA, Khan NA. Cell signaling mechanisms of oro-gustatory detection of dietary fat: advances and challenges. Progress in lipid research. 2014;53:82–92. doi: 10.1016/j.plipres.2013.11.001. [DOI] [PubMed] [Google Scholar]
- 81.Prakriya M, Lewis RS. Store-Operated Calcium Channels. Physiological reviews. 2015;95:1383–1436. doi: 10.1152/physrev.00020.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Ruhle B, Trebak M. Emerging roles for native Orai Ca2+ channels in cardiovascular disease. Current topics in membranes. 2013;71:209–235. doi: 10.1016/B978-0-12-407870-3.00009-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Goyal T, Mitra S, Khaidakov M, Wang X, Singla S, Ding Z, Liu S, Mehta JL. Current Concepts of the Role of Oxidized LDL Receptors in Atherosclerosis. Current atherosclerosis reports. 2012 doi: 10.1007/s11883-012-0228-1. [DOI] [PubMed] [Google Scholar]
- 84.Stewart CR, Stuart LM, Wilkinson K, van Gils JM, Deng J, Halle A, Rayner KJ, Boyer L, Zhong R, Frazier WA, Lacy-Hulbert A, El Khoury J, Golenbock DT, Moore KJ. CD36 ligands promote sterile inflammation through assembly of a Toll-like receptor 4 and 6 heterodimer. Nature immunology. 2010;11:155–161. doi: 10.1038/ni.1836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Sheedy FJ, Grebe A, Rayner KJ, Kalantari P, Ramkhelawon B, Carpenter SB, Becker CE, Ediriweera HN, Mullick AE, Golenbock DT, Stuart LM, Latz E, Fitzgerald KA, Moore KJ. CD36 coordinates NLRP3 inflammasome activation by facilitating intracellular nucleation of soluble ligands into particulate ligands in sterile inflammation. Nature immunology. 2013;14:812–820. doi: 10.1038/ni.2639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Moore KJ, Kunjathoor VV, Koehn SL, Manning JJ, Tseng AA, Silver JM, McKee M, Freeman MW. Loss of receptor-mediated lipid uptake via scavenger receptor A or CD36 pathways does not ameliorate atherosclerosis in hyperlipidemic mice. The Journal of clinical investigation. 2005;115:2192–2201. doi: 10.1172/JCI24061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.van Berkel TJ, Out R, Hoekstra M, Kuiper J, Biessen E, van Eck M. Scavenger receptors: friend or foe in atherosclerosis? Current opinion in lipidology. 2005;16:525–535. doi: 10.1097/01.mol.0000183943.20277.26. [DOI] [PubMed] [Google Scholar]
- 88.Hoebe K, Georgel P, Rutschmann S, Du X, Mudd S, Crozat K, Sovath S, Shamel L, Hartung T, Zahringer U, Beutler B. CD36 is a sensor of diacylglycerides. Nature. 2005;433:523–527. doi: 10.1038/nature03253. [DOI] [PubMed] [Google Scholar]
- 89.Yin B, Loike JD, Kako Y, Weinstock PH, Breslow JL, Silverstein SC, Goldberg IJ. Lipoprotein lipase regulates Fc receptor-mediated phagocytosis by macrophages maintained in glucose-deficient medium. The Journal of clinical investigation. 1997;100:649–657. doi: 10.1172/JCI119576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Chawla A, Lee CH, Barak Y, He W, Rosenfeld J, Liao D, Han J, Kang H, Evans RM. PPARdelta is a very low-density lipoprotein sensor in macrophages. Proceedings of the National Academy of Sciences of the United States of America. 2003;100:1268–1273. doi: 10.1073/pnas.0337331100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Huang SC, Everts B, Ivanova Y, O'Sullivan D, Nascimento M, Smith AM, Beatty W, Love-Gregory L, Lam WY, O'Neill CM, Yan C, Du H, Abumrad NA, Urban JF, Jr., Artyomov MN, Pearce EL, Pearce EJ. Cell-intrinsic lysosomal lipolysis is essential for alternative activation of macrophages. Nature immunology. 2014;15:846–855. doi: 10.1038/ni.2956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Driscoll WS, Vaisar T, Tang J, Wilson CL, Raines EW. Macrophage ADAM17 deficiency augments CD36-dependent apoptotic cell uptake and the linked anti-inflammatory phenotype. Circulation research. 2013;113:52–61. doi: 10.1161/CIRCRESAHA.112.300683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Serhan CN, Chiang N, Dalli J. The resolution code of acute inflammation: Novel pro-resolving lipid mediators in resolution. Seminars in immunology. 2015;27:200–215. doi: 10.1016/j.smim.2015.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Serhan CN, Dalli J, Colas RA, Winkler JW, Chiang N. Protectins and maresins: New pro-resolving families of mediators in acute inflammation and resolution bioactive metabolome. Biochimica et biophysica acta. 2015;1851:397–413. doi: 10.1016/j.bbalip.2014.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Love-Gregory L, Sherva R, Schappe T, Qi JS, McCrea J, Klein S, Connelly MA, Abumrad NA. Common CD36 SNPs reduce protein expression and may contribute to a protective atherogenic profile. Hum Mol Genet. 2011;20:193–201. doi: 10.1093/hmg/ddq449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Andersen M, Lenhard B, Whatling C, Eriksson P, Odeberg J. Alternative promoter usage of the membrane glycoprotein CD36. BMC Mol Biol. 2006;7:8. doi: 10.1186/1471-2199-7-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Pietka TA, Schappe T, Conte C, Fabbrini E, Patterson BW, Klein S, Abumrad NA, Love-Gregory L. Adipose and muscle tissue profile of CD36 transcripts in obese subjects highlights the role of CD36 in fatty acid homeostasis and insulin resistance. Diabetes care. 2014;37:1990–1997. doi: 10.2337/dc13-2835. [DOI] [PMC free article] [PubMed] [Google Scholar]