Abstract
We previously reported that five repeated pulses of 5-HT lead to down-regulation of the TM-apCAM isoform at the surface of Aplysia sensory neurons (SNs). We here examined whether apCAM down-regulation is required for 5-HT-induced long-term facilitation. We also analyzed the role of the cytoplasmic and extracellular domains by overexpressing various apCAM mutants by DNA microinjection. When TM-apCAM was up-regulated in SNs by DNA microinjection, five pulses of 5-HT failed to produce either synaptic facilitation or an enhancement of synaptic growth, suggesting that down-regulation of apCAM is required for 5-HT-induced EPSP enhancement and new varicosity formation. However, disrupting the extracellular domain function of overexpressed apCAM with a specific antibody restored 5-HT-induced excitatory postsynaptic potential increase but not synaptic growth. The overexpression of the MAP Kinase mutant of TM-apCAM, which is not internalized by 5-HT, inhibited new varicosity formation, but did not inhibit excitatory postsynaptic potential increase. Deletion mutants containing only the cytoplasmic portion of apCAM blocked 5-HT-induced synaptic growth but not excitatory postsynaptic potential increase. Thus, our data suggest that TM-apCAM may act as a suppressor of both synaptic-strength enhancement in pre-existing synapses and of new synaptic varicosity formation in the nonsynaptic region, via different mechanisms.
The synaptic connections between the sensory (SN) and motor neurons (MN) of Aplysia undergo short-term and long-term facilitation in in vitro culture in response to either a brief single or repeated pulses of 5-HT (Clark and Kandel 1984; Frost et al. 1985; Montarolo et al. 1986; Rayport and Schacher 1986; Glanzman et al. 1989a,b, 1990). Whereas short-term facilitation requires the modification of pre-existing proteins, long-term facilitation requires a cascade of gene activation and the growth of new synaptic connections (Montarolo et al. 1986; Castellucci et al. 1989; Glanzman et al. 1990; Bailey and Kandel 1993). Structural changes are generally correlated with long-term synaptic plasticity. Hippocampal long-term potentiation (LTP) is accompanied by morphological changes at the synapse, such as enlargements of the spine head or new synaptic sprouting (Yuste and Bonhoeffer 2001). Although such structural changes are known to be associated with long-term memory storage in Aplysia, little is known of the mechanisms that give rise to this structural plasticity.
Recent studies have revealed that apCAM (Aplysia cell adhesion molecule) is associated with structural changes during LTF. Using gold-conjugated monoclonal antibodies to apCAM, the apCAM at the sensory neuron membrane surface was found to be down-regulated through endocytosis by 5-HT (Bailey et al. 1992; Mayford et al. 1992). No such change was observed in postsynaptic cells. Application of 5-HT decreased the fasciculation of growth cones with SN neurites, and similarly, incubating isolated SNs or MN with anti-apCAM Ab or Fab fragments reduced the fasciculation of growth cones with homologous neurites (Keller and Schacher 1990; Mayford et al. 1992; Peter et al. 1994). In addition, preincubation of the sensory to motor neuron synapse with anti-apCAM Ab 4E8 blocked 5-HT-induced LTF (Zhu et al. 1995), showing that apCAM function is necessary for LTF. Taken together, these results show that apCAM is important for the fasciculation of growth cones with SN neurites and influences the formation of new synaptic connections in response to 5-HT.
apCAM exists as three isoforms in Aplysia: a transmembrane (TM-apCAM) and two GPI-linked isoforms (GPI-apCAM [small form and large form]). By selectively overexpressing TM-apCAM and GPI-apCAM in Aplysia SNs, we found that only the TM-apCAM was specifically internalized at the surface membrane of SNs by 5-HT (Bailey et al. 1997). Although apCAM is shown to be involved in the expression of LTF, the role of apCAM down-regulation is not clear. Is the down-regulation of TM-apCAM in SNs necessary for LTF? How does down-regulation of TM-apCAM in SNs contribute to the formation of LTF? How do the cytoplasmic and extracellular domains of apCAM regulate LTF? Furthermore, it is not clear whether apCAM is purely associated with structural changes or whether this molecule may be involved in functional modulations at pre-existing synapses. In the present study, we adopted overexpression strategy to address these questions and used various mutant forms of apCAM. By analyzing the effects of apCAM overexpression on 5-HT-induced long-term functional and structural changes at the synapse, we investigated the role of apCAM during the formation of LTF.
RESULTS
Expressions of HA-Epitope-Tagged and GFP-Fused apCAM Constructs in SN
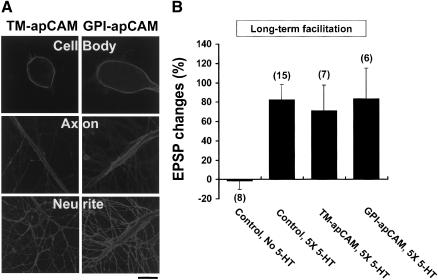
For the expression experiment, we individually tagged TM-apCAM and the large and short forms of GPI-apCAM with the HA epitope (YPYDVPDYA) derived from hemagglutinin of the influenza virus (Fig. 1A; Bailey et al. 1997). Similarly, we overexpressed deletion mutants, which had the entire cytoplasmic tail (Δcyto) or the most of the extracellular Ig domains from the second to the fifth Ig domains (Δextra) of TM-apCAM deleted. To further examine the function of the cytoplasmic tail of apCAM, we fused the entire cytoplasmic tail with GFP and constructed an MAP kinase mutant form (MAP kinase mutant [T→A]). We overexpressed these constructs by microinjection in SNs after first subcloning them into the neuronal expression vector pNEXδ (Kaang et al. 1993). To monitor the ectopic expression of these various recombinant apCAM constructs in SN, we used immunofluorescence with the Cy3-conjugated anti-HA antibody, for the HA-tagged constructs, and green fluorescence, for the GFP-fused construct (GFP-CYTO). We found that, using this technique, the apCAM constructs were successfully overexpressed in SN or MN. Immunofluorescence was detected on the surface of the cell body and on the neurites of SN (Figs. 3A and 6A, below) and MN (Fig. 4A, below). In the case of the GFP-fused constructs (GFP-CYTO), GFP fluorescence was detected in the cytosol of SNs, which indicated that the GFP-fused cytoplasmic tails of TM-apCAM were successfully overexpressed in the cytosol of SNs (Fig. 6A, below). Taken together, these results suggest that the various recombinant apCAM constructs were successfully overexpressed in Aplysia neurons. In addition, elevated TM-apCAM mRNA levels were detected by RT-PCR in SNs microinjected with HA-tagged TM-apCAM (Fig. 3B, below).
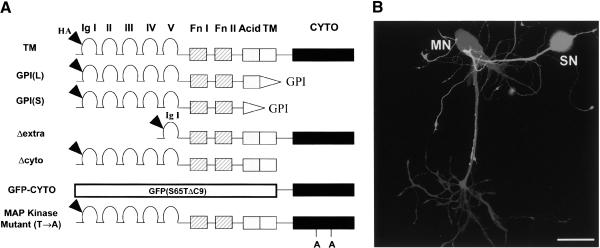
Figure 1.
Microinjected apCAM DNA constructs. (A) Schematic diagram showing the domain organization of various apCAMs DNA constructs used in this study. [TM] HA-tagged TM-apCAM; [GPI(L)] HA-tagged GPI-apCAM (large form); [GPI(S)] HA-tagged GPI-apCAM (small form); [Δextra] HA-tagged TM-apCAM lacking the extracellular domain except for Ig I; [Δcyto] HA-tagged TM-apCAM lacking the cytoplasmic tail; [GFP-CYTO] TM-apCAM having only the cytoplasmic tail fused with GFP (S65TΔC9); [MAP kinase mutant (T→A)] HA-tagged TM-apCAM not having two putative MAP kinase phosphorylation sites (T889A, T923A); [Ig] immunoglobulin domain of the C-2 type; [Fn] fibronectin type III; [TM] transmembrane domain; [GPI] glycosylphosphoinositol attachment signal; [Acid] glutamate-rich domain; [HA] hemagglutinin epitope tag; [GFP (S65TΔC9)] a variant of green fluorescent protein (Kim and Kaang 1998); [CYTO] cytoplasmic tail. (B) Coculture of sensory neuron (SN) isolated from the pleaural ganglia and the motor neuron LFS (MN) isolated from the abdominal ganglia Aplysia kurodai after 5 d of culture. Scale bar, 100 μm.
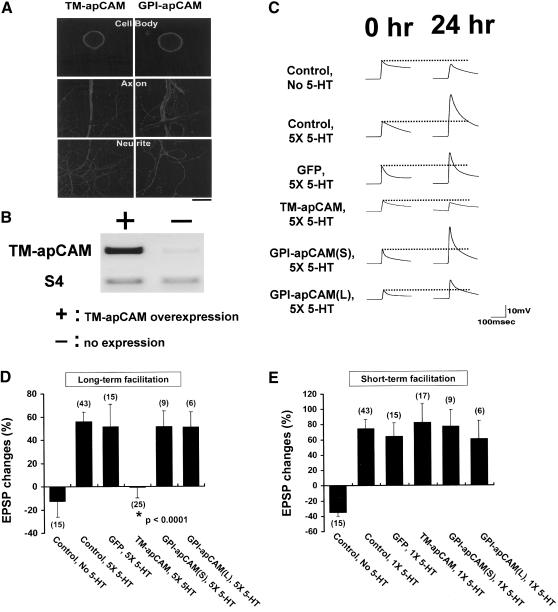
Figure 3.
The effects of apCAM overexpression in SN on long-term and short-term functional synaptic changes. (A) Confocal microscopic pictures showing subcellular localization of apCAM isoform proteins overexpressed in SN cocultured with target MN. Cy3-conjugated anti-HA antibody was used to detect the expression of apCAM proteins on the surface of the cell body, main axon and neurites of SNs. Scale bar, 50 μm. (B) Semiquantitative RT-PCR result showing the mRNA level of TM-apCAM in TM-apCAM overexpressing (+ lane) or nonexpressing (-lane) SNs. The amount of apCAM mRNA was quantified and normalized versus S4 mRNA. The expression level of TM-apCAM mRNA in TM-apCAM overexpressing SNs was much higher than that of the nonexpressing control SNs. (C) Representative EPSP traces before and 24 h after 5-HT treatment. TM-apCAM or GPI-apCAM (small and large forms) was expressed by DNA microinjection with GFP. After checking apCAM expression by GFP fluorescence, initial EPSP was measured. (D) Mean percentage changes in EPSP amplitude showing the effect of apCAM overexpression in SNs on LTF when SNs were microinjected with the TM-apCAM or GPI-apCAM [small and large forms (GPI-apCAM(S) and GPI-apCAM(L)]. The overexpression of TM-apCAM in SNs blocked 5-HT-induced long-term functional synaptic changes. The EPSP amplitude change of cells overexpressing TM-apCAM was comparable to that of the control cells (CTL; no injection and no 5-HT treatment), but it was significantly different from that of both the 5-HT-treated control cells and the cells overexpressing either GFP or GPI-apCAM. A one-way analysis of variance and Duncan's multiple range test were used to determine the significance of EPSP changes (F = 6.29, dF = 5, p < 0.0001). Each bar represents the mean ± SEM. (E) Group data showing the effects of apCAM overexpression on short-term facilitation. Neither the overexpression of TM-apCAM nor that of GPI-apCAM in SNs had any effect on short-term facilitation. One-way analysis of variance and Duncan's multiple range test were used to determine the significance of EPSP changes (F = 5.66, dF = 5, p < 0.0001). Each bar represents the mean ± SEM.
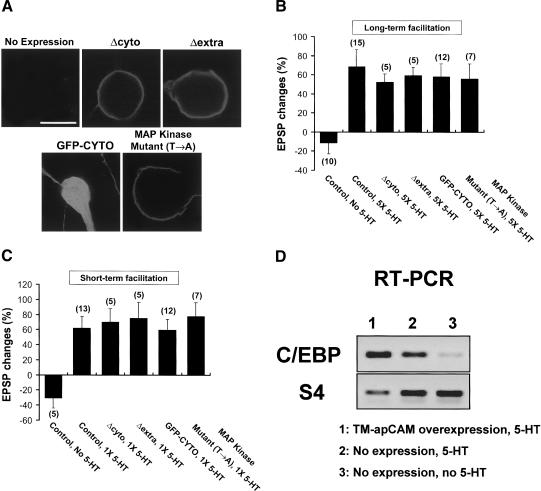
Figure 6.
The effect of the overexpression of apCAM deletion mutants in SNs on long-term and short-term synaptic changes. (A) Confocal images showing successful overexpression of recombinant apCAM mutants. Scale bar, 30 μm. (B) Histogram showing the mean percentage changes of EPSP amplitude measured from the cells overexpressing the apCAM mutant forms [Δcyto, Δextra, GFP-CYTO, and MAP kinase mutant (T→A)] after exposure to five pulses of 5-HT treatment. Enhancement of EPSP amplitude was normally induced by 5-HT treatment when these mutant forms [Δcyto, Δextra, GFP-CYTO, and MAP kinase mutant (T→A)] were overexpressed in SNs compared with control cells not expressing apCAM DNA constructs. One-way analysis of variance and Duncan's multiple range test were used to determine the significance of EPSP changes (F = 3.58, dF = 5, p < 0.08). Each bar represents the mean ± SEM. (C) Mean percentage changes of EPSP showing the effect of the overexpression of apCAM mutant forms on short-term facilitation. The overexpression of apCAM mutant forms had no effect on short-term facilitation. The EPSP amplitude was normally increased by a single pulse of 5-HT treatment in cells overexpressing apCAM mutant forms. One-way analysis of variance and Duncan's multiple range test were used to determine the significance of EPSP changes (F = 3.75, dF = 5, p < 0.007). Each bar represents the mean ± SEM. (D) Semiquantitative RT-PCR showing the expression levels of C/EBP and S4 mRNA in TM-apCAM overexpressing SNs or in nonexpressing SN after 5-HT treatment. C/EBP mRNA was normally induced by 5-HT treatment both in TM-apCAM overexpressing SNs and in nonexpressing SN versus the untreated controls.
Figure 4.
The effects of apCAM overexpression in MNs on long-term functional synaptic changes. (A) Images showing the overexpression of each apCAM isoform in MNs. Each apCAM isoform was successfully expressed in the surface membrane of the cell body, axon, and neurites. Scale bar, 50 μm. (B) Group data showing the effects of apCAM overexpression in MNs on 5-HT-induced long-term functional synaptic changes. The EPSP amplitude was normally increased in cells overexpressing each apCAM construct 24 h after 5-HT treatment. One-way analysis of variance and Duncan's multiple range test were used to determine the significance of EPSP changes (F = 3.66, dF = 3, p < 0.03). Each bar represents the mean ± SEM.
The Overexpression of TM-apCAM and GPI-apCAM in SN Had No Effect on Basal Synaptic Transmission
Modification in the expression level of cell adhesion molecule can alter basal synaptic efficacy during development process or synaptic plasticity in adults (Dityateva et al. 2000; Polo-Parada et al. 2001; Biederer et al. 2002). Before examining the effect of apCAM overexpression on long-term facilitation, we checked whether apCAM overexpression in SNs itself had any effect on basal synaptic transmission in sensori-motor synapses. For this purpose, we first measured the amplitude of EPSP evoked in post-synaptic MNs by extracellular stimulation on presynaptic SNs. Next, we microinjected each apCAM DNA constructs subcloned into pNEXδ in SN with the GFP DNA construct, which was used for the expression marker in these experiments. Cells successfully expressing apCAMs were checked by GFP fluorescence, and the amplitude of EPSP was remeasured 24 h after microinjection. The overexpression of the TM-apCAM isoform and GPI-apCAM isoform in SNs did not affect basal synaptic transmission. Although the size of EPSP was slightly increased, there were no statistically significant changes in EPSP amplitude before and after the overexpression of TM-apCAM and GPI-apCAM in SN (11.6% ± 11.2%, n = 7; and 11.6% ± 10.0%, n = 6, respectively) compared with nonexpressing control cells (14.9% ± 13.5%, n = 12; p > 0.9, one-way analysis of variance and Duncan's multiple range test). All these data showed that the overexpression of apCAMs, TM-apCAM and GPI-apCAM isoform, in SNs did not influence basal synaptic transmission in sensori-motor synapses.
Effect of 5-HT on the Expression Level of Ectopic TM-apCAM
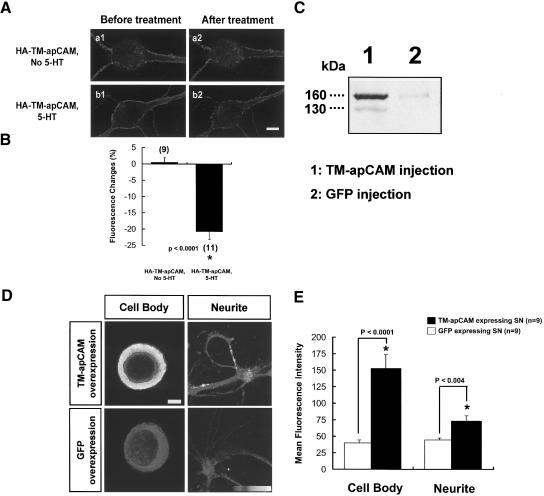
In this study, we intended to overexpress TM-apCAM in SNs by microinjecting recombinant (HA-epitope-tagged) TM-apCAM DNA. However, HA-TM-apCAM overexpressed in SN is internalized by 5-HT treatment as shown by previous electron microscopic analysis (Bailey et al. 1997). Thus, we determined the expression levels of TM-apCAM after 5-HT treatment in TM-apCAM overexpressing SN, or in GFP overexpressing SN as a control. First, we examined the 5-HT-induced internalization of HA-TM-apCAM by immunocytochemical analysis. 5-HT reduced the surface peak fluorescence in HA-TM-apCAM overexpressing SN (-20.8% ± 2.4%, n = 11; Fig. 2A[b],B), whereas its surface fluorescence was unchanged (0.5% ± 1.5%, n = 9) in the absence of 5-HT treatment (Fig. 2A[a],B), confirming that recombinant HATM-apCAM is normally internalized by 5-HT treatment. Next, we examined the expression levels of TM-apCAM after 5-HT treatment in SNs expressing recombinant TM-apCAM or GFP immunocytochemically using a specific antibody, for endogenous and ectopic TM-apCAM. Thus, we generated a polyclonal anti-apCAMC Ab, antibody against the cytoplasmic domain of TM-apCAM to detect both recombinant and endogenous TM-apCAM molecules. In Western blot analysis (Fig. 2C), protein extracts from Aplysia abdominal ganglion neurons expressing HA-TM-apCAM (Fig. 2C, lane 1) or GFP (Fig. 2C, lane 2) were probed with anti-apCAMC Ab. TM-apCAM molecules migrated slower (∼160 kDa) than expected for a molecular mass of ∼102 kDa, probably because of a glutamate-rich domain and an unidentified post-translational modification, as previously reported (Mayford et al. 1992; Bailey et al. 1997). Next, immunocytochemistry with anti-apCAMC Ab was used to determine how much TM-apCAM remained after 5-HT treatment in SN overexpressing recombinant TM-apCAM. After 5-HT treatment, we found that immunofluorescence intensities of TM-apCAM were higher in TM-apCAM overexpressing SN (152.8 ± 20.8, n = 9 in cell bodies; 73.1 ± 7.9, n = 9 in neurites, respectively) than in GFP overexpressing SN (40.0 ± 4.9, n = 9 in cell bodies; 44.7 ± 2.8, n = 9 in neurites, unpaired t-test; p < 0.0001 and p < 0.004, respectively; Fig. 2D,E). This result shows that expression levels of TM-apCAM in SN ectopically overexpressing recombinant TM-apCAM remained high compared with GFP overexpressing control SN even after TM-apCAM down-regulation by 5-HT treatment.
Figure 2.
Expression levels of TM-apCAM in isolated cultured SNs overexpressing HA-TM-apCAM after repetitive 5-HT treatment. (A) Surface membrane fluorescence images of live SNs overexpressing HA-tagged TM-apCAM before (a1, b1) and 1 h after 5-HT treatment (a2, b2). All images were obtained by confocal microscopy (Radiance2000; BioRad). Surface membrane fluorescence was reduced (-20.8% ± 2.4%, n = 11) after 5-HT treatment in HA-tagged TM-apCAM (HA-TM-apCAM) overexpressing SNs (b1, b2). However, the surface fluorescence of cells expressing HA-tagged TM-apCAM was unchanged (0.5% ± 1.5 %, n = 9) in the absence of 5-HT treatment (a1, a2). Scale bar, 10 μm. (B) Mean percentage changes of HA-tagged TM-apCAM immunofluorescence by 5-HT treatment. The unpaired student t-test was used to determine the significance of surface membrane fluorescence changes (p < 0.0001). Each bar represents the mean ± SEM. (C) Western blot analysis of HA-tagged TM-apCAM expressed in Aplysia neurons by anti-apCAMC Ab antibody against the cytoplasmic domain of TM-apCAM (see Materials and Methods). Recombinant (lane 1) and endogenous (lane 2) TM-apCAM molecules migrated slower (∼160 kDa) than expected for a molecular mass of ∼102 kDa, which was reported previously (Mayford et al. 1992; Bailey et al. 1997). A minor band (∼130 kDa) was also observed, which represents a differently modified TM-apCAM. (D) Confocal microscopic images showing immunocytochemical staining by anti-apCAMC Ab. SNs were fixed and immunostained after 5-HT treatment using anti-apCAMC Ab. Fluorescence intensities of cell bodies and neuritic processes were higher in SNs overexpressing TM-apCAM than in the control SNs overexpressing GFP. Scale bar, 10 μm. (E) Bar graph representing mean fluorescence intensity of each experimental group (TM-apCAM overexpressing SN and GFP overexpressing control SN) after 5-HT treatment. We examined the fluorescence intensity in two different regions (cell body, neuritic processes) of each SN. The fluorescence intensity of TM-apCAM overexpressing SN was significantly higher than that of GFP overexpressing SN (unpaired student t-test; p < 0.0001 in cell body, p < 0.004 in neuritic processes, respectively). Bars represent means ± SEM.
The Overexpression of TM-apCAM, But Not of GPI-apCAM in SN Blocked 5-HT-Induced Long-Term Functional Synaptic Changes
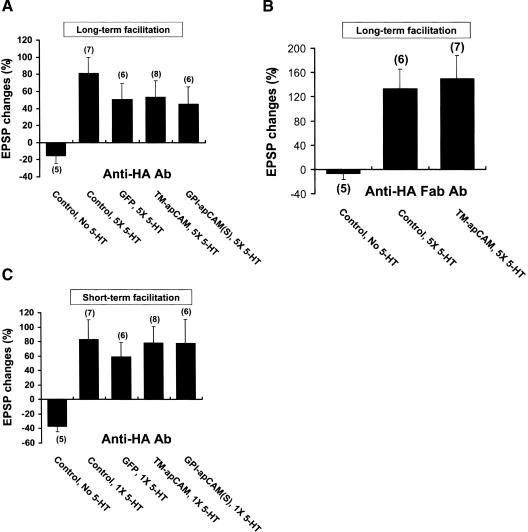
Next, we examined whether apCAM overexpression can affect 5-HT-induced long-term facilitation. To examine this, the initial EPSP was measured 24 h after apCAM overexpression, and the cultures were given five pulses of 5-HT. Then, 24 h later, we measured the degree of facilitation. As shown in Figure 3, the overexpression of TM-apCAM blocked 5-HT-induced long-term facilitation (Fig. 3C,D). Cells overexpressing TM-apCAM, which were then exposed to five pulses of 5-HT, showed no increase in synaptic potential amplitude 24 h after 5-HT treatment (-0.6% ± 9.2%, n = 25; Fig. 3C,D). On the other hand, the overexpression of either the large or the small forms of GPI-apCAM in SNs had no effect on 5-HT-induced long-term facilitation. The EPSP amplitude increased 24 h after 5-HT treatment in the SNs overexpressing large and small forms of GPI-apCAM (51.2% ± 12.9%, n = 6; and 51.3% ± 15.2%, n = 9, respectively; Fig. 3C,D). The control cultures not exposed to 5-HT showed no increase in the strength of their synaptic connections 24 h later (-12.8% ± 13.3%, n = 15; Fig. 3C,D). The five pulses of 5-HT produced a normal long-term facilitation in the SNs expressing only GFP (51.6% ± 18.9%, n = 15) and in the nonexpressing cells (55.9% ± 8.4%, n = 43; Fig. 3C,D). A one-way analysis of variance and Duncan's multiple range test were used to determine the significance of EPSP changes (F = 6.29, dF = 5, p < 0.0001). Short-term facilitation, however, was not affected by the overexpression of TM-apCAM. Short-term facilitation was normally produced by a brief (5 min) pulse of 5-HT in the presence of the overexpression of TM-apCAM in SNs (82.5% ± 24.8%, n = 17), and this was comparable to that shown by the nonexpressing or the GFP overexpressing cells (74.7% ± 12.0%, n = 43; and 64.5% ± 17.3%, n = 15, respectively; Fig. 3E). One-way analysis of variance and Duncan's multiple range test were used to determine the significance of EPSP changes (F = 5.66, dF = 5, p < 0.0001). These data show that the overexpression of TM-apCAM, but not GPI-apCAM, in SN inhibits 5-HT-induced long-term functional synaptic changes. Furthermore, these results strongly suggest that down-regulation of TM-apCAM is required for the expression of long-term functional synaptic changes in response to 5-HT treatment.
TM-apCAM Overexpression in Target MN Did Not Block 5-HT-Induced Long-Term Functional Synaptic Changes
apCAMs, both TM-apCAM and GPI-apCAM isoforms, are expressed in target MNs as well as in SNs. To explore the role of postsynaptic apCAM in long-term synaptic changes, we investigated the effect of apCAM overexpression in target MNs on 5-HT-induced long-term facilitation. For this experiment, we used the same protocol as in the experiment examining the effect of apCAM overexpression in SNs on long-term functional synaptic changes. Overexpression of apCAM in the MNs (Fig. 4A) did not affect 5-HT-induced synaptic facilitation. When TM-apCAM and GPI-apCAM were overexpressed in the MNs, long-term facilitation was normally induced by 5-HT treatment (70.7% ± 26.8%, n = 7; and 83.0% ± 32.4%, n = 6, respectively). EPSP amplitude normally increased in nonexpressing control cells 24 h after 5-HT treatment (82.3% ± 16.1%, n = 15), whereas the EPSP amplitude did not significantly change 24 h after initial measurement in control cells that had not received 5-HT treatment (-1.2% ± 8.9%, n = 8; Fig. 4B). One-way analysis of variance and Duncan's multiple range test were used to determine the significance of EPSP changes (F = 3.66, dF = 3, p < 0.03). This result indicates that TM-apCAM overexpression in SN, but not in MN selectively can block 5-HT-induced long-term functional synaptic changes.
Blockade of 5-HT-Enhanced Synaptic Strength by Ectopically Expressed TM-apCAM Is Recovered by anti-HA Antibody Recognizing the First IgG Domain of apCAM
TM-apCAM consists of an extracellular domain, which is involved in homophilic and heterophilic interactions, and a cytoplasmic tail that interacts with other proteins for signal transduction (Mayford et al. 1992; Bailey et al. 1997). To determine whether extracellular domain function of the overexpressed apCAMs contributes to the blocking effect of TM-apCAM overexpression on long-term facilitation, we attempted to inhibit the extracellular domain function of recombinant TM-apCAMs using an antibody against the HA epitope tag that was inserted into the first IgG domain of apCAM. We found that we could recover the blocking effect of TM-apCAM overexpression on the long-term facilitation with anti-HA Ab. The EPSP amplitude normally increased (53.4% ± 19.0%, n = 8) 24 h after 5-HT was administered to anti-HA Ab-treated SN overexpressing TM-apCAM (Fig. 5A). Similarly, the EPSP amplitude also increased (45.2% ± 20.0%, n = 6) 24 h after 5-HT treatment when the SN overexpressing the GPI-apCAM was incubated with anti-HA Ab (Fig. 5A). These increases in EPSP amplitude were comparable to those of nonexpressing SN (81.4% ± 18.4%, n = 7) or GFP overexpressing SN (50.5% ± 18.6%, n = 6; Fig. 5A). One-way analysis of variance and Duncan's multiple range test were used to determine the significance of EPSP changes (F = 3.26, dF = 4, p < 0.05). Antibody treatment itself can induce internalization of cell adhesion molecules from surface membrane by antibody cross-linking (Schaefer et al. 1999, 2002). To rule out this possibility, we took advantage of Fab fragmented anti-HA antibody. Preincubation of Fab fragmented anti-HA antibody also reversed the inhibition of long-term facilitation by TM-apCAM overexpression (149.5% ± 38.5%, n = 7). EPSP amplitude normally increased in nonexpressing control cells in response to 5-HT treatment (133.7% ± 31.7%, n = 6) compared with control cells not exposed to 5-HT (-6.7% ± 9.9%, n = 5; Fig. 5B). One-way analysis of variance and Duncan's multiple range test were used to determine the significance of EPSP changes (F = 6.43, dF = 2, p < 0.01). Finally, anti-HA Ab treatment had no effect on short-term facilitation. The EPSP amplitude was increased by a brief pulse of 5-HT when TM-apCAM and GPI-apCAM were overexpressed (78.4% ± 22.7%, n = 8; and 77.6% ± 33.2%, n = 6, respectively), and this was comparable to the short-term facilitation observed in noninjected control SN or GFP overexpressing SN (83.0% ± 26.9%, n = 7; and 59.0% ± 19.7%, n = 6, respectively; Fig. 5C). EPSP amplitude decreased in noninjected control SN in the absence of 5-HT (-26.6% ± 5.2%, n = 5). One-way analysis of variance and Duncan's multiple range test were used to determine the significance of EPSP changes (F = 3.46, dF = 4, p < 0.05). These results show that the inhibition of 5-HT-induced long-term facilitation by TM-apCAM overexpression is mediated by extracellular domain function of overexpressed TM-apCAM.
Figure 5.
Treatment of anti-HA Ab, which recognizes the extracellular domain of overexpressed TM-apCAM, released the inhibition of LTF by TM-apCAM overexpression. (A) Mean percentage changes in EPSP amplitude showing the effect of apCAM overexpression on LTF when anti-HA antibody was pretreated before 5-HT treatment. The blocking effect of TM-apCAM overexpression on LTF was removed by anti-HA antibody pretreatment. The EPSP amplitude changes of cells overexpressing TM-apCAM or GPI-apCAM were not significantly different from those of cells overexpressing only GFP or nonexpressing control cells. One-way analysis of variance and Duncan's multiple range test were used to determine the significance of EPSP changes (F = 3.26, dF = 4, p < 0.05). Each bar represents the mean ± SEM. (B) Mean percentage changes of EPSP showing the effect of Fab fragmented anti-HA antibody treatment on long-term functional synaptic changes in cells overexpressing TM-apCAM. We used Fab fragmented anti-HA antibody to avoid nonspecific effect such as antibody cross-linking. One-way analysis of variance and Duncan's multiple range test were used to determine the significance of EPSP changes (F = 6.43, dF = 2, p < 0.01). Each bar represents the mean ± SEM. (C) Mean percentage changes of EPSP showing the effect of apCAM overexpression on short-term facilitation. Treatment of anti-HA antibody had no effect on short-term facilitation. The EPSP was increased by 5-HT treatment in cells overexpressing GFP, TM-apCAM, and GPI-apCAM. One-way analysis of variance and Duncan's multiple range test were used to determine the significance of EPSP changes (F = 3.46, dF = 4, p < 0.05). Each bar represents the mean ± SEM.
Both the Extracellular Domain and the Cytoplasmic Domain Are Necessary for the Inhibition of Long-Term Facilitation by TM-apCAM Overexpression
If extracellular domain function is important for the inhibition of long-term facilitation by TM-apCAM overexpression, then what is the role of the cytoplasmic domain in this process? Is the extracellular domain function on its own sufficient to inhibit long-term facilitation? To address these questions, we used mutant constructs (Δcyto, Δextra, and GFP-CYTO) of TM-apCAM. First, we constructed TM-apCAM without its cytoplasmic tail (Δcyto) and successfully expressed it in the surface membrane of SN (Fig. 6A) to examine whether the extracellular domain of TM-apCAM in itself can inhibit 5-HT-induced long-term functional synaptic changes. This construct may behave in the same way as GPI-apCAM, which also lacks the cytoplasmic tail (Fig. 1A). The overexpression of Δcyto had no effect on long-term facilitation. The EPSP amplitude increased 24 h after exposure to five pulses of 5-HT treatment in the presence of Δcyto overexpression in SN (52.1% ± 8.7%, n = 5; Fig. 6B). Short-term facilitation was also not affected by Δcyto overexpression (70.1% ± 17.5%, n = 5; Fig. 6C). This result shows that the extracellular domain alone is not sufficient for the inhibition of long-term EPSP increase, suggesting that the cytoplasmic tail function of overexpressed TM-apCAM is necessary in the inhibition of long-term facilitation.
Then, can the cytoplasmic domain of TM-apCAM in itself inhibit long-term EPSP increase? To test this possibility, we generated a mutant construct of TM-apCAM (Δextra) lacking all of the extracellular Ig domains, except the first IgG, and overexpressed this construct in SN (Fig. 6A). Cells overexpressing Δextra showed a normal EPSP amplitude increase 24 h after 5-HT treatment (59.2% ± 8.7%, n = 5; Fig. 6B) and normal short-term facilitation (75.3% ± 21.1%, n = 6; Fig. 6C). To further confirm the role of the TM-apCAM cytoplasmic tail in the inhibition of long-term EPSP increase, we fused the cytoplasmic tail with GFP (GFPCYTO) and expressed it in the cytosol of SN (Fig. 6A). The truncated cytoplasmic tail (GFP-CYTO and Δextra) may act as dominant-negative proteins by interfering with the interaction between TM-apCAM and some binding proteins involved in long-term synaptic changes. However, similarly in the case of Δextra, the overexpression of this fusion protein (GFP-CYTO) in the cytosol of SN did not interrupt 5-HT-induced long-term functional synaptic changes. The amplitude of the EPSP increased 24 h after 5-HT treatment (57.9% ± 13.5%, n = 12; Fig. 6B). In 5-HT-treated control cells not expressing apCAM mutant constructs, the amplitude of EPSP was normally increased (68.2% ± 18.2%, n = 15), but not in the no-5-HT-treated control cells (-15.1% ± 18.8%, n = 5; Fig. 6B). Short-term facilitation was also not affected by GFP-CYTO overexpression (59.8% ± 14.1%, n = 12). The EPSP amplitude increased by 5-HT treatment in nonexpressing control cells (61.8% ± 15.5%, n = 13; Fig. 6C). The amplitude of EPSP was slightly decreased in control cells not receiving 5-HT treatment (-30.6% ± 13.4%, n = 5). These results clearly show that the cytoplasmic domain in itself does not inhibit the 5-HT-induced long-term EPSP increase, and that both the extracellular domain and the cytoplasmic domain are required for the inhibition of long-term increase of EPSP amplitude by TM-apCAM overexpression.
The MAP Kinase Phosphorylation Sites Are Essential for the Inhibition of Long-Term Functional Synaptic Changes by TM-apCAM Overexpression
The cytoplasmic tail of TM-apCAM contains two putative MAP kinase phosphorylation sites that were reported to be required for the 5-HT-induced down-regulation of TM-apCAM at the surface membrane of SN (Bailey et al. 1997). Then what is the role of these MAP kinase phosphorylation sites in the long-term functional synaptic changes evoked by 5-HT treatment? To examine this issue, we overexpressed a MAP kinase mutant (T→A) construct, mutated by replacing two threonine residues (889 and 923) with alanine, and investigated its effect on long-term functional synaptic changes. We found that 5-HT treatment normally induced long-term functional synaptic changes in the MAP kinase mutant (T→A) overexpressing SN. The EPSP amplitude increased 24 h after five pulses of 5-HT treatment in the MAP kinase mutant (T→A) overexpressing SN (55.6% ± 16.0%, n = 7; Fig. 6B). In the 5-HT-treated control SN, which did not express the mutant DNA, the EPSP amplitude was normally increased (68.2% ± 18.2%, n = 15), but in the untreated control SN, the EPSP amplitude slightly decreased (-11.4% ± 10.4%, n = 10; Fig. 6B). Short-term facilitation was also not affected by MAP kinase mutant (T→A) overexpression (77.3% ± 18.5%, n = 7). The EPSP amplitude increased in the 5-HT-treated control SN (61.8% ± 15.5%, n = 13). The EPSP amplitude slightly decreased in the no-5-HT-treated control SN (-30.6% ± 13.4%, n = 5; Fig. 6C). These results suggest that TM-apCAM overexpression inhibits the 5-HT-induced long-term functional synaptic changes through the mechanisms mediated by MAP kinase phosphorylation sites of TM-apCAM.
TM-apCAM Overexpression Does Not Affect the 5-HT-Activated Signal Cascades That Lead to C/EBP Induction
Next, we examined whether TM-apCAM overexpression in SN could interfere with the transcriptional activation of C/EBP in response to 5-HT treatment, because C/EBP induction is an essential step for the induction of long-term synaptic facilitation. C/EBP is an immediate early gene induced by CREB when multiple pulses of 5-HT are treated to SNs. Antibody treatment or RNA interference against C/EBP was found to block the induction of long-term facilitation (Alberini et al. 1994; Lee et al. 2001). By semiquantitative RT-PCR, the amount of C/EBP mRNA was measured in SNs overexpressing TM-apCAM after 5-HT treatment. S4 ribosomal protein, which is constitutively expressed in SNs, was used as a control. C/EBP was induced normally by fivefold and fourfold immediately after 5-HT treatment, both in SNs overexpressing TM-apCAM and in control SNs not expressing TM-apCAM, respectively, versus the untreated control (Fig. 6D). This result shows that TM-apCAM overexpression in SNs does not interfere with the induction of C/EBP mRNA in response to 5-HT treatment. Therefore, TM-apCAM overexpression in SNs may inhibit the long-term increase of synaptic strength by affecting the downstream events of C/EBP mRNA induction or the C/EBP-independent steps of long-term facilitation.
TM-apCAM Overexpression in SN Inhibits the Increase in the Number of SN Varicosities in Response to 5-HT Treatment
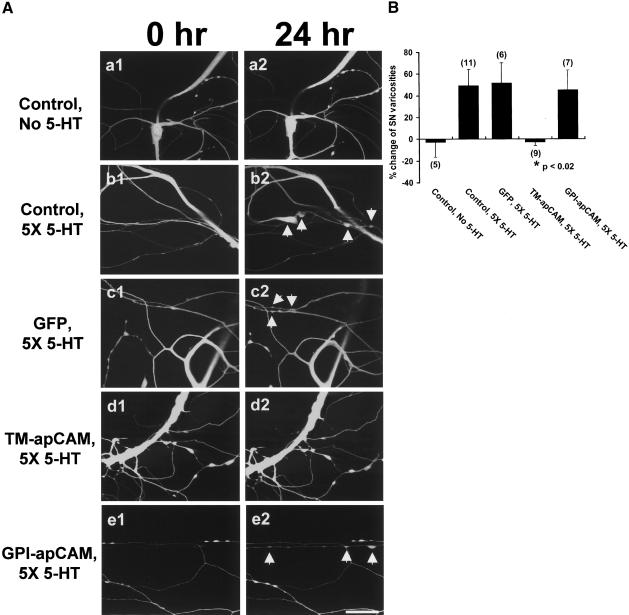
Long-term memory storage involves new synaptic growth, and 5-HT-induced long-term facilitation is associated with the growth of new synaptic connections between the SN and their target MN (Bailey and Chen 1983, 1988). Then, what is the role of apCAM down-regulation in this new synapse formation evoked by 5-HT treatment? To answer this question, we overexpressed the HA-epitope-tagged apCAM isoforms in SN and examined changes in the number of SN varicosities contacting the major axon of the MN after 5-HT treatment. The overexpression of TM-apCAM blocked new varicosity formation associated with long-term facilitation (Fig. 7A,B). When TM-apCAM was overexpressed, the number of SN varicosities contacting the major axon of MN was not increased (-2.6% ± 3.3%, n = 9; Fig. 7A[d],B), and this was not significantly different from control cells not exposed to 5-HT treatment (-2.9% ± 13.4%, n = 5; Fig. 7A[a],B). In contrast, the number of varicosities of GPI-apCAM overexpressing SN was normally increased 24 h after 5-HT treatment (45.1% ± 18.2%, n = 7; Fig. 7A[e],B). Similarly, the number of varicosities of GFP overexpressing or nonexpressing SN was also increased by 5-HT treatment (51.7% ± 18.7%, n = 6; 49.0 ± 15.2%, n = 11, respectively; Fig. 7A[c],A[b],B). One-way analysis of variance and Duncan's multiple range test were used to determine the significance of varicosity number changes (F = 3.56, dF = 4, p < 0.02). These data strongly suggest that TM-apCAM down-regulation in SN is necessary for 5-HT-induced long-term structural changes.
Figure 7.
The effect of apCAM overexpression in SNs on structural changes associated with 5-HT-induced LTF. (A) Confocal fluorescence images of SN varicosities contacting a major axon of target MN before (a1, b1, c1, d1, e1) and 24 h after 5-HT treatment (a2, b2, c2, d2, e2). When TM-apCAM was overexpressed in SNs, the number of varicosities did not increase 24 h after 5-HT treatment (d1, d2). The number of varicosities was increased in control cells not expressing (b1, b2) or expressing GFP alone (c1, c2) or in the cell expressing the GPI-linked isoform (GPI-apCAM; e1, e2) by exposure to five pulses of 5-HT. Arrows indicate newly formed varicosities after 24 h. Scale bar, 30 μm. (B) Mean percentage changes of varicosity numbers in cells overexpressing apCAM isoforms by 5-HT treatment. The overexpression of TM-apCAM in SN blocked the structural modifications associated with LTF. One-way analysis of variance and Duncan's multiple range test were used to determine the significance of varicosity number changes (F = 3.56, dF = 4, p < 0.02). Each bar represents the mean ± SEM.
The Overexpression of TM-apCAM Constructs Containing the Cytoplasmic Domain (TM-apCAM, Δextra, GFP-CYTO, MAP Kinase Mutant) in SN Blocks 5-HT-Induced Structural Modifications
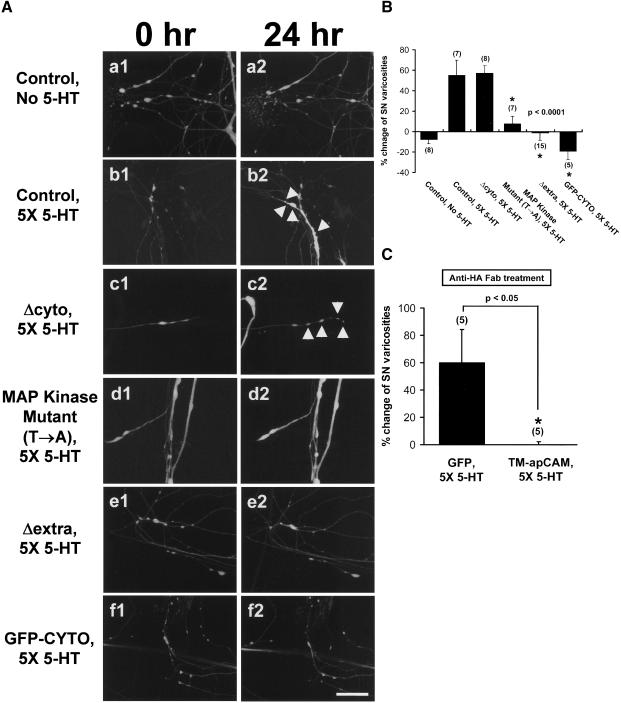
Next, by what mechanisms does TM-apCAM inhibit new varicosity formation? To address this issue, we asked what was the role of extracellular domain and cytoplasmic domain of TM-apCAM in 5-HT-induced structural synaptic changes. To answer this question, we used apCAM mutant constructs (Δcyto, Δextra, GFP-CYTO, MAP kinase mutant [T→A]) and examined the effects of their overexpression on new varicosity formation during LTF. Δcyto overexpression in SN had no effect on 5-HT-induced new varicosity formation in response to 5-HT treatment. The number of varicosities was significantly increased 24 h after 5-HT treatment in cells overexpressing Δcyto (57.3% ± 7.3%, n = 8; Fig. 8A[c],B) and this was not significantly different from 5-HT-treated nonexpressing control cells (55.1% ± 14.7%, n = 7; Fig. 8A[b],B). However, the overexpression of apCAM mutant constructs lacking MAP kinase phosphorylation sites (MAP kinase mutant [T→A]) or containing only the cytoplasmic domain (Δextra, GFP-CYTO) inhibited the increase of varicosity number 24 h after 5-HT treatment. The number of varicosities contacting the main axon of target MNs was unchanged or only slightly reduced in cells overexpressing the MAP kinase mutant (T→A), Δextra, or GFP-CYTO 24 h after 5-HT treatment (7.6% ± 7.6%, n = 7; -1.3% ± 7.1%, n = 15; -19.2% ± 8.1%, n = 5, respectively; Fig. 8A[d],A[e],A[f],B). Varicosity number was also slightly decreased in control cells not exposed to 5-HT treatment (-7.7% ± 4.0%, n = 8; Fig. 8A[a],B). One-way analysis of variance and Duncan's multiple range test were used to determine the significance of varicosity number changes (F = 12.88, dF = 5, p < 0.0001). Therefore, overexpressed TM-apCAM may inhibit 5-HT-induced new varicosity formation by a mechanism independent of MAP kinase phosphorylation. Taken together, these results show that cytoplasmic domain function of TM-apCAM is essential for the inhibition of 5-HT-induced long-term structural changes, irrespective of its being phosphorylated by MAP kinase.
Figure 8.
The effect of overexpression of apCAM deletion mutants in SNs on structural changes associated with 5-HT-induced LTF. (A) Confocal microscopic images of SN varicosities contacting a major axon of target MN before (a1, b1, c1, d1, e1, f1) and 24 h after 5-HT treatment (a2, b2, c2, d2, e2, f2). The overexpression of apCAM mutant forms containing mutations of MAP kinase phosphorylation sites or containing the cytoplasmic domain (Δextra, GFP-CYTO) in SNs inhibited normal increase of varicosity number 24 h after 5-HT treatment (d1, d2; e1, e2; f1, f2). The number of varicosities was normally increased in cells expressing Δcyto (c1, c2) or control cells not expressing apCAM DNA constructs (b1, b2) by exposure to five pulses of 5-HT. Arrows indicate newly formed varicosities after 24 h. Scale bar, 30 μm. (B) Mean percentage changes of varicosity numbers in cells overexpressing apCAM mutant forms by 5-HT treatment. One-way analysis of variance and Duncan's multiple range test were used to determine the significance of varicosity number changes (F = 12.88, dF = 5, p < 0.0001). Each bar represents the mean ± SEM. (C) Bar graph showing the mean percentage changes of varicosity number in TM-apCAM or GFP overexpressing SNs cocultured with target MNs 24 h after 5-HT treatment. Treatment of Fab fragmented anti-HA Ab before 5-HT treatment could not relieve the inhibiting effect of TM-apCAM overexpression on increased varicosity. The unpaired student t-test was used to determine the significance of varicosity number changes (p < 0.05). Bars represent means ± SEM.
Blockade of 5-HT-Induced Synaptic Growth by TM-apCAM Overexpression Is Not Recovered by anti-HA Antibody Recognizing the First IgG Domain of Overexpressed apCAM
Blocking the extracellular domain function with a specific antibody, anti-HA Ab recognizing the first IgG domain of overexpressed apCAM, recovered the inhibition of 5-HT-induced long-term synaptic efficacy increase (Fig. 5). Thus, we asked whether antibody treatment also recovers the TM-apCAM-mediated inhibition of 5-HT-induced new varicosity formation. To address this question, we incubated sensory-motor coculture cells with Fab fragmented anti-HA Ab before 5-HT treatment and examined the changes in the numbers of SN varicosities contacting major axons of target MNs. Antibody treatment was found not to recover the inhibition of synaptic growth by TM-apCAM overexpression, but to recover the inhibition of increased synaptic efficacy. The number of varicosities in SN overexpressing TM-apCAM was not increased by 5-HT treatment irrespective of treating with Fab fragmented anti-HA Ab (0.0% ± 2.2%, n = 5; Fig. 8C). Fab fragmented anti-HA Ab treatment alone did not affect the normal increase in SN varicosities induced by 5-HT. In control experiments, the number of varicosities was significantly increased by 5-HT treatment in SNs overexpressing GFP (60.0% ± 24.5%, n = 5; Fig. 8C).
This result indicates that the inhibition of 5-HT-induced synaptic growth by TM-apCAM overexpression does not require the involvement of the extracellular domain. This is consistent with the finding that the overexpression of the cytoplasmic domain (Δextra, GFP-CYTO, MAP kinase mutant [T→A]) blocks the increase in the number of varicosities (Fig. 8), but not increased synaptic efficacy (Fig. 6).
DISCUSSION
The aim of this study was to elucidate the physiological role of apCAM during LTF by examining the effect of apCAM overexpression on 5-HT-induced LTF. Our findings show that transmembrane apCAM down-regulation is required for 5-HT-induced long-term EPSP enhancement and varicosity formation and that these processes occur via different mechanisms.
apCAM was markedly up-regulated in Aplysia sensory neurons microinjected with the HA-TM-apCAM DNA construct (Fig. 3A,B). Although overexpressed HA-TM-apCAM is normally internalized from the surface membrane by 5-HT (Fig. 2A,B), the expression level of total TM-apCAM in cells overexpressing HA-TM-apCAM was significantly higher than in cells overexpressing control GFP, even after 5-HT treatment (Fig. 2D,E), indicating that significant levels of TM-apCAM remain on the membrane after 5-HT treatment. In other words, the down-regulation of apCAM does not go to completion on the membrane despite some internalization. In parallel with this incomplete down-regulation of apCAM, both aspects of LTF (i.e., EPSP increase and varicosity formation) were found to be inhibited. Therefore, it is reasonable to conclude that LTF inhibition results from TM-apCAM overexpression, and that the down-regulation of TM-apCAM is required for LTF. These results are entirely consistent with a previously described defasciculation model (Bailey et al. 1997), if it is presumed that overexpressed TM-apCAM is involved in neuritic fasciculation through its adhesive extracellular domains, and by doing so inhibits the defasciculation process. However, this model cannot explain all of our data. For example, the overexpression of the MAP kinase (T→A) mutant, which is not internalized by 5-HT, would be expected to increase fasciculation through intact adhesive extracellular domains. The overexpression of this mutant inhibits new varicosity (Fig. 8A,B), but does not inhibit EPSP enhancement (Fig. 6B). In addition, the overexpression of Δextra and GFP-CYTO also inhibits new varicosity formation (Fig. 8A,B), but do not inhibit EPSP enhancement (Fig. 6B). These experiments demonstrate that LTF lasting 24 h can be induced without new varicosity formation under certain experimental conditions, as has been shown by others (Sun and Schacher 1998; Casadio et al. 1999). However, it is possible that LTF lasting more than 72 h after 5-HT may be blocked by the overexpression of these apCAM mutants, because new varicosity formation is essential for LTF lasting 72 h after 5-HT (Casadio et al. 1999). The different roles of apCAM during EPSP enhancement and varicosity formation suggest a revision to the previous model of apCAM function. Thus, we propose that TM-apCAM acts as a suppressor of both synaptic strength enhancement in pre-existing synapses, and of new synaptic varicosity formation in the nonsynaptic region, via different mechanisms.
How then does TM-apCAM inhibit the 5-HT-induced synaptic strengthening of pre-existing synapses? TM-apCAM does not seem to affect the 5-HT-activated intracellular signaling cascade, because TM-apCAM overexpression has no effect on short-term facilitation or the induction of C/EBP mRNA by 5-HT-activated signaling. However, it is possible that TM-apCAM blocks the downstream events of C/EBP induction and C/EBP-independent processes during 5-HT-activated signaling, because it is known that cell adhesion molecules, like NCAM and L1, can affect intracellular signal transduction mechanisms and second messenger systems (Schuch et al. 1989; Doherty et al. 1991; Klinz et al. 1995). Thus, for the suppression of EPSP increase during LTF, we suggest that TM-apCAM down-regulation may be a synaptic tag or that it may allow a synaptic tag to form at preexisting synapses (Martin and Kosik 2002). According to the synaptic tagging hypothesis (Frey and Morris 1997), synapse-strengthening gene product(s) produced in a cell body are ubiquitously present in the cell, and are captured only at specific synapses that have been tagged by previous synaptic activity. Our hypothesis is based on the presumption that TM-apCAM acts as a stabilizer of synaptic efficacy in the resting state by stabilizing the synaptic structure. This stabilizer should be removed to allow newly synthesized proteins and synaptic vesicles to be incorporated at pre-existing synapses or even at nonfunctional empty synapses (Kim et al. 2003). Our data also suggest that intact, structurally complete apCAM is required as a stabilizer, because the overexpression of intact TM-apCAM inhibits EPSP enhancement during LTF formation; moreover, this inhibition was blocked by antibodies binding to the extracellular domain. In addition, we found that the overexpression of the cytoplasmic domain alone had no effect on EPSP enhancement (Table 1), and that MAP kinase phosphorylation sites seem to be important for inhibiting EPSP enhancement by TM-apCAM, because the MAP kinase (T→A) mutant overexpression did not inhibit EPSP enhancement (Fig. 6B). This type of synaptic stability regulated by apCAM may undergo a remodeling process via apCAM down-regulation during the course of LTF to accommodate new synaptic proteins synthesized either from the cell body or locally at synapses.
Table 1.
Summary of the Effects of Various Recombinant apCAMs' Overexpression on 5-HT-Induced Long-Term Functional (EPSP) and Structural (Varicosity) Changes
| Long-term facilitation
|
||
|---|---|---|
| Overexpression | EPSP ↑ | Varicosity ↑ |
| In sensory neuron | ||
| TM-apCAM | No | No |
| GPI(S)-apCAM | Yes | Yes |
| GPI(L)-apCAM | Yes | N.D.a |
| Δcyto | Yes | Yes |
| Δextra | Yes | No |
| GFP-CYTO | Yes | No |
| MAP kinase mutant (T→A) | Yes | No |
| TM-apCAM, anti-HA Ab | Yes | No |
| In motor neuron | ||
| TM-apCAM | Yes | N.D. |
| GPI(S)-apCAM | Yes | N.D. |
Only full-length TM-apCAM overexpression in presynaptic SNs inhibited the 5-HT-induced increase of EPSP amplitude. However, overexpression of other apCAM mutant constructs had no effect on the increase of EPSP amplitude. The overexpression of DNA constructs containing the cytoplasmic domain of TM-apCAM (TM-apCAM, Δextra, GFP-CYTO, MAP kinase mutant (T→A), TM-apCAM, antiHA Ab) inhibited new varicosity formation by 5-HT treatment.
N.D.: Not determined.
Given that the down-regulation of apCAM is important for LTF, how does apCAM regulate 5-HT-induced structural modifications or varicosity formation? The cytoplasmic domain of TM-apCAM seems to act as a suppressor of new varicosity formation at nonsynaptic sites because the overexpressed cytoplasmic domains (Δextra and GFP-CYTO) inhibit varicosity formation and could inhibit the internalization of endogenous TM-apCAM in a dominant-negative manner. In addition, TM-apCAM overexpression also blocked varicosity formation, and high expression levels of ectopic TM-apCAM are probably sufficient to maintain the neuritic fasciculation even in the presence of 5-HT. All of these manipulations can inhibit defasciculation and eventually inhibit the neurite outgrowth that is necessary to find a new synaptic target, which is consistent with the defasciculation hypothesis.
However, this possibility is weakened by the fact that the overexpression of GPI-apCAM or Δcyto, which are devoid of the cytoplasmic domain, and which are not internalized by 5-HT, helps with fascicle formation but does not inhibit new varicosity formation (Figs. 7A,B and 8A,B). Thus, the internalization of TM-apCAM is required for varicosity formation, but not to achieve defasciculation, rather to remove the suppressive function attributed to the cytoplasmic domain of TM-apCAM. Consistent with this, all apCAM constructs containing the cytoplasmic domains (TM-apCAM, Δextra, and GFP-CYTO) suppressed varicosity formation. It is not known how the cytoplasmic domain of TM-apCAM suppresses synaptogenesis in the nonsynaptic region. The cytoplasmic domain may suppress new varicosity formation at nonsynaptic sites by stabilizing the cytoskeletal structure by interaction with cytoskeletal proteins.
Varicosity formation seems to be more easily suppressed by apCAM than EPSP enhancement. For example, MAP kinase phosphorylation sites in the cytoplasmic tail do not need to be intact to suppress varicosity formation by TM-apCAM (Fig. 8A,B), which is in contrast to the lack of effectiveness of this mutant at synaptic sites (Fig. 6B). Furthermore, the intact extracellular portion of TM-apCAM is required to suppress EPSP enhancement at pre-existing synapses.
Although the correlation between TM-apCAM down-regulation and 5-HT-induced new varicosity formation has been described previously (Bailey et al. 1992; Mayford et al. 1992; Zhu et al. 1995), our result provides the first evidence of a causal relationship between TM-apCAM down-regulation and new varicosity formation. The identification and characterization of the functions of proteins interacting with cytoplasmic domain of TM-apCAM would help to further demonstrate the mechanistic role of the cytoplasmic domain.
Then, what is the role of the defasciculation caused by TM-apCAM down-regulation during LTF? Although we are presently unable to address the role of the defasciculation caused by TM-apCAM, because we focused on the down-regulation of TM-apCAM and not on the resulting defasciculation, reduced adhesiveness at synaptic or nonsynaptic sites by TM-apCAM down-regulation may facilitate LTF formation. It is possible that defasciculation by apCAM down-regulation may be particularly important for the synaptic growth involved in long-lasting LTF at 72 h after 5-HT treatment or during the development of the nervous system.
Taken together, our data suggest that the down-regulation of TM-apCAM is required to promote both EPSP enhancement in pre-existing synapses and varicosity formation in nonsynaptic regions. Our results also indicate that the cytoplasmic domain of apCAM may have a suppressive function, which must be removed to allow LTF formation. However, the mode of action of apCAM may differ at pre-existing synapses and nonsynaptic regions. Thus, our study shows that the role of apCAM molecules during LTF formation is not straightforward, and shows that they are functionally more diverse than previously believed, and that this diversity ranges from signal regulation to varicosity stabilization. We believe that the elucidation of the modes of action of apCAM is crucial to our further understanding of the mechanisms underlying long-term memory formation.
MATERIALS AND METHODS
DNA Constructions
For construction of the Flag-tagged transmembrane isoform of apCAM (Flag-TM-apCAM), amplification from HA-apCAM-TM (Bailey et al. 1997) with a sense primer 1 (5′-tcccaagCTTCCTGA ATGCAACACT-3′; the HindIII site is italicized) and an antisense primer 1 (3′-CCCGGAGACGTTGGAGGCTGATGTTCCTGCTGC TACTGTTCC-5′; the Flag-epitope coding sequence is italicized) generated a 0.25-kb fragment (denaturation for 1 min at 94°C, annealing for 1 min at 48°C, extension for 1 min at 72°C, 20 cycles). Next, a second fragment (0.33 kb) was produced by amplification (the same PCR condition) from the same template (HA-TM-apCAM) with a sense primer 2 (5′-CCGACTACAAGGAC GACGATGACAAGGACCAGGTCACGCTCAA-3′; the Flag-epitope coding sequence is italicized) and an antisense primer 2 (3′-CTCACACTCCACCTCTCC-5′; denaturation for 1 min at 94°C, annealing for 1 min at 48°C, extension for 1 min at 72°C, 20 cycles). These two overlapping fragments were mixed, and recombinant PCR (denaturation for 1 min at 94°C, annealing for 1 min at 48°C, extension for 1 min at 72°C, 30 cycles) was performed with the sense primer 1 and antisense primer 2 to generate a 0.47-kb fragment; this product was then digested with HindIII and TfiI to release a recombinant fragment (0.37 kb) containing the N-terminal portion of Flag-tagged apCAM. HA-TM-apCAM was digested with BamHI and TfiI to generate a 1.71-kb fragment. The remaining region (3.52 kb) for Flag-TM-apCAM was produced by cutting HA-TM-apCAM with HindIII and BamHI. These three fragments (0.37 kb, 1.71 kb, and 3.52 kb), encompassing the whole sequence of the transmembrane isoform of apCAM and pNEXδ, a neuronal expression vector (Kaang et al. 1993), were ligated to generate the Flag-tagged transmembrane isoform of apCAM.
For deletion of the extracellular domain, except for the first IgG domain of HA-apCAM-TM (Δ extra), the transmembrane and cytoplasmic tail regions of HA-apCAM-TM were amplified by PCR (30 cycles of denaturation for 1 min at 94°C, annealing for 1 min at 43°C, extension for 1 min at 72°C) from HA-apCAM-TM with a sense primer 3 (5′-AACTGCAGAACCACCAATGTGGGTA AAGAAGAAGGTGA-3′; the BstXI site is italicized) and an antisense primer 3 (3′-GGTCAACCAGACCACAG-5′), generating 0.69 kb. This fragment was cut with BstXI and KpnI and ligated into BstXI-KpnI-linearized pNEX.
For generation of the cytoplasmic tail of TM-apCAM fused with GFP (S65TΔC9; GFP-CYTO), the cytoplasmic tail of TM-apCAM was amplified by PCR (denaturation for 1 min at 94°C, annealing for 1 min at 46°C, extension for 1 min at 72°C, 30 cycles) from the HA-tagged transmembrane isoform of apCAM with a sense primer 4 (5′-ACCACCATGGACTACAAGGAC GACGATGACAAGCCAGAGCTGGTGGA-3′; the Flag-epitope coding sequence is italicized) and an antisense primer 3 (3′-GGTCAACCAGACCACAG-5′), generating 0.49 kb. This fragment was cut with NcoI and KpnI, and ligated into NcoI-KpnI-linearized pNEXδ, thus creating Flag-tagged TM-apCAM containing only the cytoplasmic tail. This DNA construct (pNEXδ-CYTO) was digested with HindIII at a single site existing in the 5′ upstream region. The GFP (S65TΔC9; Kim and Kaang 1998) fragment was produced by cutting the previously constructed pNEXδ-S65TΔC9-lacZ (7 kb) with HindIII. The HindIII-linearized GFP (S65TΔC9) fragment was then ligated into HindIII-cut pNEXδ-CYTO.
For HA-tagged TM-apCAM and GPI-apCAM, the transmembrane mutant lacking the entire cytoplasmic tail (Δcyto), and the MAP kinase mutant construct (MAP kinase mutant [T→A]), we used HA-tagged DNA constructs as described by Bailey et al. (1997).
Cell Culture
Cell culture was performed as described previously (Schacher and Proshansky 1983). Aplysia kurodai was purchased from a local supplier in Pusan, South Korea and maintained in recirculating seawater tanks at 14°C before use. The nervous system of A. kurodai is similar to that of Aplysia californica (Lim et al. 1997). Ganglia were dissected from A. kurodai (70-100 g) and incubated for 1.5-2 h at 34°C in 1% protease (type IX; Sigma) dissolved in an equal volume of isotonic L15 and artificial seawater (ASW: 460 mM NaCl, 10 mM KCl, 11 mM CaCl2, 55 mM MgCl2, and 10 mM HEPES at pH 7.6), and washed several times with ASW. Pleural SNs were isolated from the pleural ganglion and cocultured with identified motor cell LFS isolated from the abdominal ganglia and maintained for 4 d at 18°C in an incubator. For measurement of apCAM immunofluorescence changes induced by 5-HT, SNs were isolated from pleural ganglia and plated in a culture dish. Cultures were allowed to grow for 5 d and used for experiments. Only the sensory-motor coculture whose initial EPSP was >3 mV was used for the subsequent experiments.
Microinjection
The microinjection of the various DNA constructs (1 mg/mL DNA) used in this study, which were dissolved in a buffer containing 0.1% fast green, 10 mM Tris-Cl (pH 7.3), and 100 mM NaCl, was performed into Aplysia cultured SNs by applying positive air pressure, as described previously (Kaang et al. 1992; Kaang 1996a,b). Microinjected cells were incubated for 18-24 h at 18°C and used for electrophysiological measurement, immunocytochemistry, and the imaging of structural changes.
Electrophysiology
Experiments were performed on cultures 4 d after plating. Motor neurons cocultured with SNs were impaled with sharp microelectrodes (10-20 MΩ) filled with 2 M K-acetate, 0.5 M KCl, and 10 mM K-HEPES and hyperpolarized to -80 to -90 mV to prevent the cell from firing action potentials. Intracellular signals were amplified using an Axoclamp 2B (Axon Instrument). Synaptic potentials were evoked in the LFS motor cell by stimulating each sensory cell with a brief (0.5 msec) depolarizing pulse using an extracellular electrode placed near the cell body of an SN. Synaptic potentials were recorded before and 24 h after the onset of five pulses of 5-HT (10 μM) treatment to measure the long-term change. To examine short-term change, EPSP was evoked in motor cell with an interstimulus interval (ISI) of 15 min. A brief (5 min) pulse of 5-HT (10 μM) was applied for preparation 10 min after the initial EPSP measurement. To block the adhesion activity of overexpressed apCAMs, monoclonal anti-HA Ab (BabCO) or Fab fragmented monoclonal anti-HA Ab (BabCO) was treated 0.5 ∼ 1 h prior to the initial EPSP measurement. For the baseline recording experiment, the initial EPSP was measured 1 h before DNA microinjection at 4 d after coculture and the second EPSP 24 h after microinjection. Data were stored on VCR recorder tapes using a digital data recorder (Model VR-10B; Instrutech Corp.).
Immunocytochemistry
SNs cocultured with LFS MNs were microinjected with each apCAM isoform and mutant form DNA construct. The cultures were incubated for 18-24 h after microinjection, and fixed with 4% paraformaldehyde in phosphate-buffered saline. After blocking nonspecific binding by preincubating cells with 3% BSA in phosphate-buffered saline, the cells were incubated with the anti-HA Ab 12CA5 (1′ Ab), diluted 1:1000 in blocking solution, for 1 h and incubated with the Cy3-conjugated anti-mouse IgG (2′ Ab), diluted 1:100 in blocking solution, for 1 h. For the experiment to measure the expression level of TM-apCAM after 5-HT treatment, cultured SNs that overexpress TM-apCAM or GFP were fixed with 4% paraformaldehyde and permeabilized by incubating with 0.1% Triton X-100 for 10 min. After blocking with 3% BSA, SNs were incubated with anti-apCAMC Ab (1′ Ab; 1:100) for 2 h and then incubated with (Cy3-conjugated anti-mouse IgG; 2′ Ab) for 2 h. Immunofluorescence was observed under a confocal microscope (Radiance2000; BioRad). For immunocytochemistry of live SNs in culture, cultures were rinsed first with perfusion medium consisting of a 1:1 mixture of isotonic L15 and ASW. Cultures were incubated for 40 min with the anti-HA Ab 12CA5 (1′ Ab; 1:100) and rinsed with perfusion medium. Cultures were incubated for 40 min with the Cy3-conjugated anti-mouse IgG (2′ Ab; 1:100). Then cultures were rinsed with perfusion medium and fluorescence images were taken under the confocal microscope (Radiance2000; BioRad). Immediately after the cells were viewed, each culture was treated with either control solution (isotonic L15 plus ASW) or 10 μM 5-HT in control solution for 1 h. Cultures were rinsed and the same view areas were examined.
Western Blotting
Mouse anti-apCAMC antibodies were raised against a GST-fusion apCAMC that was constructed by subcloning the entire cytoplasmic tail of apCAM cDNA into a pGEX-KG vector. The resulting fusion protein was purified from overexpressed Escherichia coli extract using GST-agarose beads (Sigma). Purified GST-apCAMC proteins were used to immunize a BALB/c mouse, and anti-GST antibodies were removed from the resulting mouse sera by using GST-overexpressed E. coli lysate, according to the previously reported method (Gruber and Zingales 1995), and the remaining antibodies were purified by protein-A affinity chromatography (Protein-A Sepharose 4 Fast Flow; Amersham Biosciences). Western blotting was performed with abdominal ganglionic lysates. Each ganglion containing 7-11 neurons expressing HA-TM-apCAM or GFP was lysated in 50 μL of boiling SDS gel sample buffer. Then 20 μL of each lysate was subjected to 7% SDS-PAGE, and proteins were subsequently transferred onto a Hybond ECL filter (Amersham) by semidry electrophoretic transfer. The filter was incubated with anti-apCAMC Ab, diluted 1:1000, followed by horseradish peroxidase-conjugated goat anti-mouse IgG Ab (Pierce), diluted 1:2000. Immunoreactivity was detected using a chemiluminescence system (ECL; Amersham).
RT-PCR Analysis
RT-PCR was performed as previously described (Schacher et al. 1999). Five sensory cells were harvested and transferred to 200 μL of Trizol reagent (Invitrogen). Total RNA was isolated by following the manufacturer's instructions, and genomic or injected plasmid DNA was removed using 1 U of RNase-free DNase I (Promega). DNase I was heat inactivated at 65°C with 2.5 mM EDTA. Total RNA was primed with random hexamers and reverse-transcribed with 100 U of superscript II RT (Invitrogen). For PCR analysis, 2 μL of twofold dilutions of the cDNA was used (35 cycles), with gene-specific primers, and the products were visualized by agarose gel electrophoresis. Gene-specific primers had the following sequences: apCAM: 5′-TTCCTGAATGCAACACT-3′ and 5′-CCCCGCCCAGGATGTCA-3′; C/EBP: 5′-TACTCTCA ACCTTCCCTCAAGC-3′ and 5′-TGACAAATGAACAAAATG CACA-3′; S4: 5′-GACCCTCTGGTGAAGGTGAA-3′ and 5′-TGGACAGCTTCACACCTTTG-3′.
Dye Injection and Imaging Structural Changes
After recording the amplitude of the EPSP on both days, the fluorescent dye 5(6)-carboxyfluorescein (6% in 0.44 M KOH at pH 7.0; Molecular Probes) was injected into the sensory cell with 0.5-nA hyperpolarizing current pulses (500 msec at 1 Hz) for 2-5 min (Glanzman et al. 1989b; Schacher and Montarolo 1991). Phase contrast and fluorescence images of the same view areas along the major axons of the motor cell were taken both before and 24 h after the 5-HT treatments under the confocal microscope (Radiance2000; BioRad). To minimize differences in imaged structures that might have arisen because of differences in the extent of dye filling, the light intensities used on the second day were adjusted to match the intensity of the stored images taken on the first day. In some cells that expressed enough GFP proteins to image varicosities, we used GFP fluorescence to monitor the changes of varicosity numbers.
Quantification of Structural Changes
Counts of varicosity number were obtained from fluorescent images of SN neurites contacting the proximal major axon of a motor cell. Previous studies have indicated that this portion of the motor cell axon is the most favorable substrate for the growth of SN neurites that form varicosities with transmitter release sites (Glanzman et al. 1989b, 1990; Schacher et al. 1990). Because the axon of LFS is a relatively thick structure, four to six focal planes were often required to image all of the labeled neurites and varicosities in a given view area. Structures that were slightly elongated spheres connected by narrow neuritic necks greater than or equal to 2 μm were counted as varicosities (Bailey and Chen 1983, 1988). Counts of varicosities were performed in a blind manner.
Quantification of Fluorescence Intensity
Two different areas (cell bodies and neurites) of SNs were quantified for fluorescence intensity. We randomly selected five 8-μm2 regions in each area and measured the mean fluorescence intensity. The mean value of the fluorescence intensity was calculated in five regions in each area, and this was defined as the mean fluorescence intensity, which was determined using Laser-Pix 4.0 software (BioRad).
Acknowledgments
We thank Craig Bailey for valuable comments on a previous version of the manuscript. This work was supported by the Korea Ministry of Science and Technology under National Research Laboratory Program (M1-0104-00-0140) and Neurobiology Research Program (M1-0108-00-0075) and BRC-Frontier Program (M103KV010012 03K2201 01210). E.R.K. is supported by the Howard Hughes Medical Institute. J.-H.H., C.-S.L., and Y.-S. L. were supported by a BK21 Research Fellowship from the Korea Ministry of Education and Human Resources Development.
The publication costs of this article were defrayed in part by payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 USC section 1734 solely to indicate this fact.
Article published online ahead of print. Article and publication date are at http://www.learnmem.org/cgi/doi/10.1101/lm.61104.
References
- Alberini, C.M., Ghirardi, M., Metz, R., and Kandel E.R. 1994. C/EBP is an immediate-early gene required for the consolidation of long-term facilitation in Aplysia. Cell 76: 1099-1114. [DOI] [PubMed] [Google Scholar]
- Bailey, C.H. and Chen, M. 1983. Morphological basis of long-term habituation and sensitization in Aplysia. Science 220: 91-93. [DOI] [PubMed] [Google Scholar]
- ____. 1988. Long-term memory in Aplysia modulates the total number of varicosities of single identified sensory neurons. Proc. Natl. Acad. Sci. 85: 2373-2377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey, C.H. and Kandel, E.R. 1993. Structural changes accompanying memory storage. Annu. Rev. Physiol. 55: 397-426. [DOI] [PubMed] [Google Scholar]
- Bailey, C.H., Chen, M., Keller, F., and Kandel, E.R. 1992. Serotonin-mediated endocytosis of apCAM: An early step of learning-related synaptic growth in Aplysia. Science 256: 645-649. [DOI] [PubMed] [Google Scholar]
- Bailey, C.H., Kaang, B.-K., Chen, M., Martin, K.C., Lim, C.-S., Casadio, A., and Kandel, E.R. 1997. Mutation in the phosphorylation sites of MAP Kinase blocks learning-related internalization of apCAM in Aplysia sensory neurons. Neuron 18: 913-924. [DOI] [PubMed] [Google Scholar]
- Biederer, T., Sara, Y., Mozhayeva, M., Atasoy, D., Liu, X., Kavalali, E.T., and Südhof, T.C. 2002. SynCAM, a synaptic adhesion molecule that drives synapse assembly. Science 297: 1525-1531. [DOI] [PubMed] [Google Scholar]
- Casadio, A., Martin, K.C., Giustetto, M., Zhu, M., Chen, M., Bartsch, D., Bailey, C.H., and Kandel, E.R. 1999. A transient, neuron-wide form of CREB-mediated long-term facilitation can be stabilized at specific synapses by local protein synthesis. Cell 99: 221-237. [DOI] [PubMed] [Google Scholar]
- Castellucci, V.F., Blumenfeld, H., Goelet, P., and Kandel, E.R. 1989. Inhibitor of protein synthesis blocks long-term behavioral sensitization in the isolated gill withdrawal reflex of Aplysia. J. Neurobiol. 20: 1-9. [DOI] [PubMed] [Google Scholar]
- Clark, G.A. and Kandel, E.R. 1984. Branch-specific heterosynaptic facilitation in Aplysia siphon sensory cells. Proc. Natl. Acad. Sci. 81: 2577-2581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dityateva, A., Dityateva, G., and Schachner, M. 2000. Synaptic strength as a function of post-versus presynaptic expression of the neural cell adhesion molecule NCAM. Neuron 26: 207-217. [DOI] [PubMed] [Google Scholar]
- Doherty, P., Ashton, S.V., Moore, S.E., and Walsh, F.S. 1991. Morphoregulatory activities of NCAM and N-cadherin can be accounted for by G protein-dependent activation of L- and N-type neuronal Ca2+ channels. Cell 67: 21-33. [DOI] [PubMed] [Google Scholar]
- Frey, U. and Morris, R.G. 1997. Synaptic tagging and long-term potentiation. Nature 385: 533-536. [DOI] [PubMed] [Google Scholar]
- Frost, W.N., Castellucci, V.F., Hawkins, R.D., and Kandel, E.R. 1985. Monosynaptic connections from the sensory neurons of the gill- and siphon-withdrawal reflex in Aplysia participate in the storage of long-term memory for sensitization. Proc. Natl. Acad. Sci. 82: 8266-8269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glanzman, D.L., Mackey, S.L., Hawkins, R.D., Dyke, A., Lloyd, P.E., and Kandel, E.R. 1989a. Depletion of serotonin in the nervous system of Aplysia reduces the behavioral enhancement of gill withdrawal as well as the heterosynaptic facilitation produced by tail shock. J. Neurosci. 9: 4200-4213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glanzman, D.L., Kandel, E.R., and Schacher, S. 1989b. Identified target motor neuron regulates neurite outgrowth and synapse formation of Aplysia sensory neurons in vitro. Neuron 3: 441-450. [DOI] [PubMed] [Google Scholar]
- Glanzman, D.L., Kandel, E.R., and Schacher, S. 1990. Target-dependent structural changes accompanying long-term synaptic facilitation in Aplysia neurons. Science 249: 799-802. [DOI] [PubMed] [Google Scholar]
- Gruber, A. and Zingales, B. 1995. Alternative method to remove antibacterial antibodies from antisera used for screening of expression libraries. Biotechniques 19: 28. [PubMed] [Google Scholar]
- Kaang, B.-K. 1996a. Neuronal expression of reporter genes in the intact nervous system of Aplysia. Mol. Cells 6: 285-295. [Google Scholar]
- ____. 1996b. Parameters influencing ectopic gene expression in Aplysia neurons. Neurosci. Lett. 221: 29-32. [DOI] [PubMed] [Google Scholar]
- Kaang, B.-K., Pfaffinger, P.J., Grant, S.G.N., Kandel, E.R., and Furukawa, Y. 1992. Overexpression of an Aplysia shaker K+ channel gene modifies the electrical properties and synaptic efficacy of identified Aplysia neurons. Proc. Natl. Acad. Sci. 89: 1133-1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaang, B.-K., Kandel, E.R., and Grant, S.G.N. 1993. Activation of cAMP-responsive genes by stimuli that produce long-term facilitation in Aplysia sensory neurons. Neuron 10: 427-435. [DOI] [PubMed] [Google Scholar]
- Keller, F. and Schacher, S. 1990. Neuron-specific membrane glycoproteins promoting neurite fasciculation in Aplysia californica. J. Cell Biol. 111: 2637-2650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim, H.-K. and Kaang, B.-K. 1998. Truncated green fluorescent protein mutants and their expression in Aplysia neurons. Brain Res. Bull. 47: 35-41. [DOI] [PubMed] [Google Scholar]
- Kim, J.-H., Udo, H., Li, H.-L., Youn, T.Y., Chen, M., Kandel, E.-R., and Bailey, C.-H. 2003. Presynaptic activation of silent synapses and growth of new synapses contribute to intermediate and long-term facilitation in Aplysia. Neuron 40: 151-165. [DOI] [PubMed] [Google Scholar]
- Klinz, S.G., Schachner, M., and Maness, P.F. 1995. L1 and N-CAM antibodies trigger protein phosphatase activity in growth cone-enriched membranes. J. Neurochem. 65: 84-95. [DOI] [PubMed] [Google Scholar]
- Lee, J.A., Kim, H.K., Kim, K.-H., Han, J.-H., Lee, Y.-S., Lim, C.-S., Chang, D.-J., Kubo, T., and Kaang, B.-K. 2001. Overexpression of and RNA interference with the CCAAT enhancer-binding protein on long-term facilitation of Aplysia sensory to motor synapses. Learn. Mem. 8: 220-226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim, C.-S., Chung, D.-Y., Kaang, B.-K. 1997. Partial anatomical and physiological characterization and dissociated cell cultue of the nervous system of the marine mollusc Aplysia kurodai. Mol. cells 7: 399-407. [PubMed] [Google Scholar]
- Martin, K.C. and Kosik, K.S. 2002. Synaptic tagging—Who's it? Nat. Rev. Neurosci. 3: 813-820. [DOI] [PubMed] [Google Scholar]
- Mayford, M., Barzilai, A., Keller, F., Schacher, S., and Kandel, E.R. 1992. Modulation of NCAM-related adhesion molecule with long-term synaptic plasticity in Aplysia. Science 256: 638-644. [DOI] [PubMed] [Google Scholar]
- Montarolo, P.G., Goelet, P., Castellucci, V.F., Morgan, J., Kandel, E.R., and Schacher, S. 1986. A critical period for macromolecular synthesis in long-term heterosynaptic facilitation in Aplysia. Science 234: 1249-1254. [DOI] [PubMed] [Google Scholar]
- Peter, N., Aronoff, B., Wu, F., and Schacher, S. 1994. Decrease in growth cone-neurite fasciculation by sensory or motor cells in vitro accompanies down-regulation of Aplysia cell adhesion molecules by neurotransmitters. J. Neurosci. 14: 1413-1421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polo-Parada, L., Bose, C.M., and Landmesser, L.T. 2001. Alterations in transmission, vesicle dynamics, and transmitter release machinery at NCAM-deficient neuromuscular junctions. Neuron 32: 815-828. [DOI] [PubMed] [Google Scholar]
- Rayport, S.G. and Schacher, S. 1986. Synaptic plasticity in vitro: Cell culture of identified Aplysia neurons mediating short-term habituation and sensitization. J. Neurosci. 6: 759-763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schacher, S. and Montarolo, P.G. 1991. Target-dependent structural changes in sensory neurons of Aplysia accompany long-term heterosynaptic inhibition. Neuron 6: 679-690. [DOI] [PubMed] [Google Scholar]
- Schacher, S. and Proshansky, E. 1983. Neurite regeneration by Aplysia neurons in dissociated cell culture: Modulation by Aplysia hemolymph and the presence of the initial axonal segment. J. Neurosci. 3: 2403-2413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schacher, S., Montarolo, P.G., and Kandel, E.R. 1990. Selective short- and long-term effects of serotonin small cardioactive peptide and titanic stimulation on sensorimotor synapses of Aplysia in culture. J. Neurosci. 10: 3286-3294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schacher, S., Wu, F., Panyko, J.D., Sun, Z.Y., and Wang, D. 1999. Expression and branch-specific export of mRNA are regulated by synapse formation and interaction with specific postsynaptic targets. J. Neurosci. 19: 6338-6347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaefer, A.W., Kamiguchi, H., Wong, E.V., Beach, C.M., Landreth, G., and Lemmon, V. 1999. Activation of the MAPK signal cascade by the neural cell adhesion molecule L1 requires L1 internalization. J. Biol. Chem. 274: 37965-37973. [DOI] [PubMed] [Google Scholar]
- Schaefer, A.W., Kamei, Y., Kamiguchi, H., Wong, E.V., Rapoport, I., Kirchhausen, T., Beach, C.M., Landreth, G., Lemmon, S.K., and Lemmon, V. 2002. L1 endocytosis is controlled by a phosphorylation-dephosphorylation cycle stimulated by outside-in signaling by L1. J. Cell Biol. 157: 1223-1232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuch, U., Lohse, M.J., and Schachner, M. 1989. Neural cell adhesion molecules influence second messenger systems. Neuron 3: 13-20. [DOI] [PubMed] [Google Scholar]
- Sun, Z.Y. and Schacher, S. 1998. Binding of serotonin to receptors at multiple sites is required for structural plasticity accompanying long-term facilitation of Aplysia sensorimotor synapses. J. Neurosci. 18: 3991-4000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuste, R. and Bonhoeffer, T. 2001. Morphological changes in dendritic spines associated with long-term synaptic plasticity. Annu. Rev. Neurosci. 24: 1071-1089. [DOI] [PubMed] [Google Scholar]
- Zhu, H., Wu, F., and Schacher, S. 1995. Changes in expression and distribution of Aplysia cell adhesion molecules can influence synapse formation and elimination in vitro. J. Neurosci. 15: 4173-4183. [DOI] [PMC free article] [PubMed] [Google Scholar]